Abstract
Aging is the most significant risk factor associated with chronic disease in humans. The accumulation of genetic damage throughout life leads to a variety of biological aberrations, including disrupted protein homeostasis, metabolic dysfunction, and altered cellular signaling. Such changes ultimately result in cellular senescence, death, or transformation to uncontrolled proliferation, thereby compromising human health. Events contributing to age-dependent physiological decline also occur in the context of hormonal and metabolic changes, affecting interconnected cellular networks. This complexity often confounds the development of effective treatments for aging and age-related diseases. In contrast to monotherapy and polypharmacology, an innovative systems pharmacology approach can identify synergistic combinations of drugs that modulate distinct mechanistic nodes within a network, minimizing off-target side effects and enabling better therapeutic outcomes. G protein-coupled receptors (GPCRs) are particularly good targets for the application of systems pharmacology, because they activate different signal transduction pathways that can culminate in a common response. Here, we describe a systems pharmacology strategy for the treatment of age-related macular degeneration (AMD), a multifactorial chronic disease of the eye. By considering the retina as part of a large, interconnected network, systems pharmacology will enable the identification of combination therapies targeting GPCRs to help restore genomic, proteomic, and endocrine homeostasis. Such an approach can be advantageous in providing drug regimens for the treatment of AMD, while also having broader ramifications for ameliorating adverse effects of chronic, age-related disease in humans.
Keywords: age-related macular degeneration, retina, eye, photoreceptors, GPCR
Advanced age is the number one risk factor for most cancers, cardiovascular diseases, and neurodegenerative disorders. However, little is known about the multiple and complex etiologies underlying the array of human age-related diseases. While normal aging may increase the likelihood of a disease occurring, genetic components can modulate both its progression and severity. Thus, the success of a therapeutic intervention also can vary markedly between individuals. Even monogenic diseases in their early phases, when the biological system can still cope with the mutation of a key gene, can worsen over time as genetic and environmental adverse factors accumulate. Moreover, treatment of age-related diseases can be further complicated when they are chronic due to the cumulative toxicity and side effects associated with different therapies.
Age-related macular degeneration (AMD) is an example of a chronic, polygenic disease in which susceptibility, progression, and severity are determined by multiple genetic variants as well as by environmental and lifestyle factors. Successful prevention and/or treatment of AMD require more information about the molecular etiology of the disease and whether involved pathways are similarly implicated in other human aging diseases. Then, could a truly novel therapeutic approach, once established in vision research, be extended to develop therapies that improve human lifespan and health? Vision research has the potential to lead medical sciences into a new era of therapeutic development, just as it has done in areas of basic science.
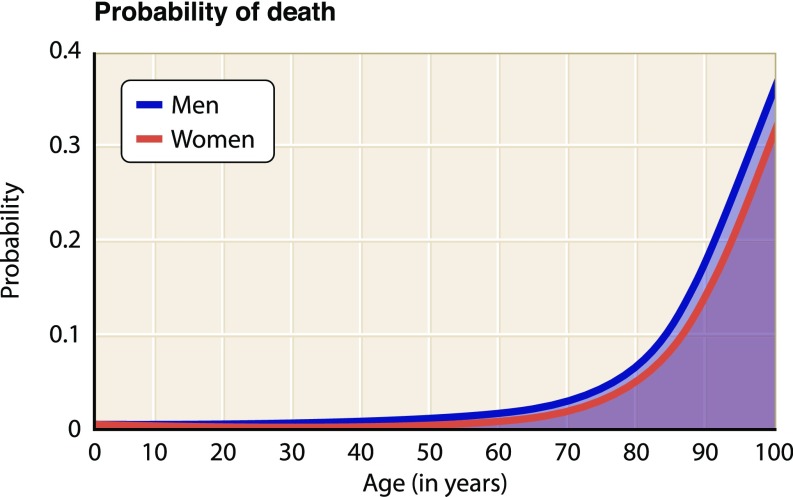
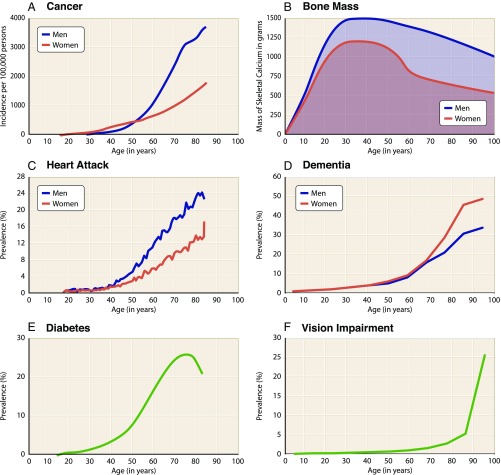
A common observation supported by statistical data is that, between the ages of about 20 and 50 y old, human health is in its most stable state and is associated with a relatively low death rate (Fig. 1). During this period, human mortality rises only slightly, with males more affected than females. However, beginning around the seventh decade of life, mortality rates increase exponentially. The number of significant illnesses also increases linearly from about the fifth decade of human life. For example, cancer is initially more prevalent in women, but after about 60 y of age, it becomes more than twice as likely in men (Fig. 2A) (1). Changes in bone mass and density also occur with age, peaking during the reproductive years and then declining significantly over time in both sexes but more severely in women (Fig. 2B), which result in osteoporosis, associated bone fractures, and postural deformities (2). In the United States, cardiovascular disease is the leading cause of death, exhibiting a dramatically increased prevalence with age (Fig. 2C) (3). Similarly, the prevalence of dementia (4), diabetes (5), and visual impairment (6) also increases markedly with age (Fig. 2 D–F). The molecular biology of aging is complex, involving physiological perturbations that can be grouped into three distinct categories: loss of protein homeostasis, metabolic dysfunction, and altered cellular signaling. Collectively, these changes lead inescapably to cell senescence, apoptosis, and other forms of programmed cell death (7) or to uncontrollable cellular transformation and growth (8), compromising organismal viability (summarized extensively in ref. 9).
Fig. 1.
Death rates for men and women in the United States based on data from the US Social Security Administration. Probability of death begins to increase exponentially after the sixth decade of life (https://www.ssa.gov/).
Fig. 2.
Frequencies and changes of human debilitating conditions with age. Graphs show data for (A) cancer (1), (B) bone mass (2), (C) cardiovascular disease (3), (D) dementia (4), (E) diabetes (5), and (F) vision impairment (6). These changes are representative of physiological decline occurring in the latter decades of life.
Protein Homeostasis, Aging, and AMD
A common feature of aging is the accumulation of genetic damage throughout life. Over time, genomic stability is continuously challenged by exogenous and endogenous threats, resulting in various types of genetic lesions (Fig. S1) (9). Single-cell whole-genome sequencing has revealed the accumulation of somatic mutations with age, exhibiting age-related, region-related, and disease-related molecular signatures (10). With advanced age, the somatic maintenance processes that normally repair DNA become less efficient and genetic lesions accumulate, resulting in the production of aberrant protein products that compromise cellular viability (11). Loss of protein homeostasis can also be aggravated by environmental stress, which causes cellular proteins to unfold (12). With an age-associated decline of repair mechanisms to manage misfolded proteins, they tend to aggregate, thereby impairing cellular function (Fig. S2) (9). Amyloid beta (Aβ) serves as an example of an aggregate-prone family of proteins that are highly toxic and clinically implicated in neurodegenerative disorders, such as Alzheimer’s disease, the most common type of dementia (13). The recent finding that Aβ is also elevated in aging retina and may be a key factor in AMD pathology has opened up new perspectives about the potential etiology and therapeutic approaches for treating this debilitating blinding disease (14). Expression systems also are attenuated with age; coding and noncoding genes are transcribed at reduced rates, affecting the levels of different and important signaling pathway components (15). Thus, it now may be possible to reestablish homeostatic levels of gene and gene product activities reflective of a younger system to stem the age-related decline of an organism. Even in severe monogenic diseases, cells can manage insults before they irreversibly deteriorate. Therefore, the challenge is to support this youthful resilience through pharmacological interventions as the organism ages.
Organismal Effects of Intercellular Communication Breakdown
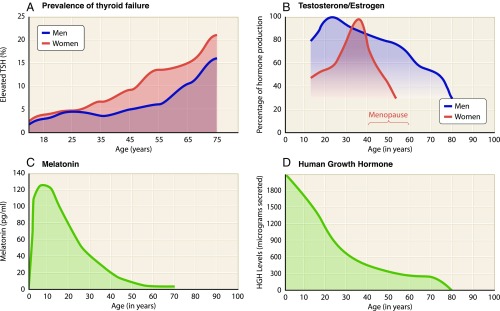
The fragility of human life results from natural senescence, spontaneous mutations, environmental insults, and other contributing factors too numerous to list here. In addition, these processes occur with altered intercellular signaling on a background of hormonal changes. Production of all hormones is dramatically dysregulated with age, including amino acid derivatives, eicosanoids, peptide hormones, and steroids. Four examples are illustrated in Fig. 3. The prevalence of thyroid dysfunction, resulting in either hypothyroidism or hyperthyroidism, increases by more than twofold between the third and sixth decades of life (Fig. 3A) (16). Such disruptions to endocrine homeostasis affect all organs of the body. Production of testosterone and estrogen peaks during the reproductive years but declines thereafter (Fig. 3B), with decreased levels implicated in age-associated reduction of bone and lean body mass (17, 18). Melatonin production exhibits a similar pattern, peaking during adolescence and declining thereafter (Fig. 3C). Indeed, decreased melatonin levels have been associated with deterioration of circadian rhythms, suppressed immunocompetence, and lowered sleep efficacy, predisposing to neurodegenerative disease in advanced age (19). Similar changes have been observed for human growth hormone (Fig. 3D), implicating dysregulation of the hypothalamic–pituitary–somatotropic (HPS) axis in advanced age (20). These are simply four examples derived from a much longer list of hormones exhibiting substantial age-related changes. Globally, such alterations to intercellular signaling manifest debilitating effects at the organismal level and modify the course of age-related disease, regardless of its initial etiology. Organ dysfunction may initially involve a local insult, which becomes exacerbated on a background of disrupted endocrine homeostasis. Therefore, to develop therapies for organ-specific dysfunction, alterations in intercellular communication at the organismal level demand consideration.
Fig. 3.
Age-dependent changes in endocrine homeostasis. Human data were compiled for (A) prevalence of thyroid failure (16), (B) testosterone/estrogen production (17, 18), (C) melatonin production (19), and (D) human growth hormone production (20). Thyroid dysfunction increases with age alongside decreased production of testosterone, estrogen, melatonin, and growth hormone.
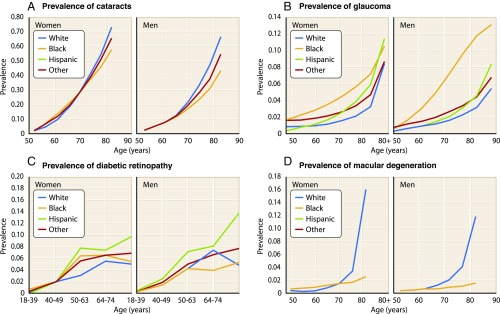
For AMD, age is the key factor determining the development of this debilitating disease (Fig. 4D). The exponentially increasing prevalence of AMD after the sixth decade of life mirrors the mortality rate shown in Fig. 1, with similar trends observed for other common age-associated eye disorders, such as cataracts, glaucoma, and diabetic retinopathy (Fig. 4 A–C) (6). Unfortunately, no specific etiology of AMD has yet been elucidated, although more than 20 million US citizens are affected. AMD is the most common cause of blindness in the elderly, resulting from degeneration of highly concentrated photoreceptor cells (cones) located in the central retina, a region known as the fovea of the macula (21). In the “dry” (nonexudative) form characterized clinically by blurring of central vision, AMD pathogenesis involves a thinning macula secondary to drusen deposition between the retinal pigmented epithelium (RPE) and choroid. Drusen is composed of a variety of extracellular lipids and proteins common to other age-associated disorders, such as atherosclerosis, suggesting that similar pathologic processes may be involved (22). Specifically, the identification of proinflammatory complement proteins in drusen has led to the hypothesis that dry AMD is at least in part the result of an immune signaling defect (23). In the less common “wet” (exudative) form of the disease, aberrant angiogenesis and subsequent hemorrhage lead to severe vision loss. A significant number of treatments for AMD are being developed, albeit without much success aside from anti-VEGF therapies for the wet form of this disease (24–26).
Fig. 4.
Prevalence of age-associated eye disorders in the US population according to age, sex, and race (6). Age is the primary risk factor for developing (A) cataracts, (B) glaucoma, (C) diabetic retinopathy, and (D) AMD.
Is it possible that systemic changes need to be considered when attempting to develop successful treatments? For example, reduced insulin-like growth factor (IGF) signaling in long-lived rodent models leads to HPS axis-associated improvements in endocrine homeostasis and metabolic function (27, 28). Of particular interest, increased IGF signaling has been shown to have a stimulatory effect on VEGF-dependent choroidal neovascularization, a hallmark of wet AMD (29). Studies of hypothyroid (Thrb−/−) mice have elucidated the role of 3,3′,5-triiodothyronine (T3) acting through the TRβ2 nuclear thyroid hormone receptor in determining expression patterns and survival of cone photoreceptor subpopulations (30), with hypothyroidism linked to photoreceptor preservation and excessive T3 exposure linked to accelerated deterioration of cones (31). Epidemiological studies have provided further proof of concept by showing a positive association in humans between hyperthyroidism and an increased risk of incident AMD (32). This newly discovered association between thyroid hormone status and AMD suggests that T3 is an important determinant of susceptibility to retinal degeneration in humans. Like IGF, thyroid hormone exerts important physiological effects by acting through intracellular second messenger signaling pathways (33). Therefore, interventions that attenuate age-related changes in T3 and/or IGF signaling could be used to protect cone cells and thus, preserve eyesight. Such an approach serves as an example of a systemic strategy to treat AMD.
Systems Pharmacology Approach to AMD Therapy
Mechanistic insights into disease pathogenesis are critical for optimal therapeutic development. However, many common disorders are caused by a combination of genetic, environmental, and unidentified factors and therefore, are naturally complex (34). In addition, molecular changes associated with a given disorder also are dynamic, involving multilayered network changes as a disease progresses. Cellular interactions and cross-talk between interconnected subcellular pathways add yet another layer of depth to an already complex scenario (35). Thus, to achieve optimal efficacy, multifaceted treatments will likely be required. Combinations of drugs that therapeutically modulate multiple mechanistic nodes within a network may enable better outcomes than treating a disease with a single drug (36).
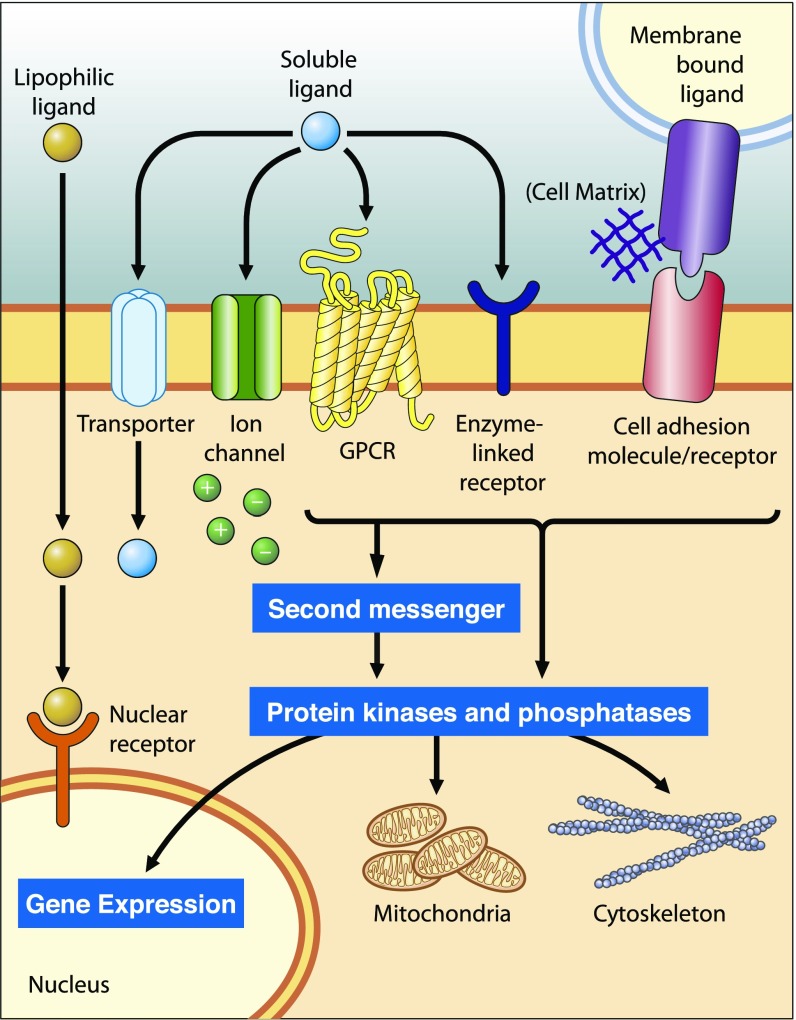
Perhaps the best way to find an effective treatment for a complex disease is to stabilize networks between different signaling pathways that intersect (Fig. 5). Transporters of small molecules, ion channels, G protein-coupled receptors (GPCRs), receptor kinases/phosphatases, and other entities affect second messenger levels that, in turn, support gene expression, metabolic function, and cellular structure (37). In the eye, the visual pigment rhodopsin is a prototypical GPCR that modulates the levels of cGMP and calcium (second messengers), critical for phototransduction and the overall viability of photoreceptor cells (38). Similar regulation of cyclic nucleotide signaling by GPCRs has been shown in regions of the brain (39), making GPCRs an attractive target for a systems pharmacology approach to drug treatment.
Fig. 5.
Cellular signaling networks. Cellular signaling receptors differ in their mechanisms of activation and signal transmission, subcellular localization, and ligand binding (37). Interconnected signaling pathways converge on common effector molecules, which modulate gene expression, mitochondrial functionality, and cytoskeletal structure. Adapted with permission from ref. 37.
Age-related metabolic dysfunction manifests at both cellular and organismal levels. At the cellular level, mitochondria are tightly regulated by second messengers and effector molecules (Fig. 5), and mitochondrial dysfunction has been shown to aggravate general aging as well as the AMD phenotype (21, 40, 41). At the organismal level, intestinal dysbiosis has been implicated in driving the AMD phenotype through a gut–retina axis (42). In conjunction with the finding that commensal bacteria influence human physiology by producing metabolites that interact with GPCRs (43), it is, therefore, conceivable that exogenous GPCR ligands could mimic the protective effect conferred by specific microbial metabolites. Moreover, synergies between different signaling pathways and their culmination into specific cellular nodes can lower the drug levels required to treat chronic diseases. Along with key hormonal adjustments, could this be a feasible strategy to treat complex human diseases? A systems pharmacology approach can be used to repurpose drugs already used clinically to develop combined treatment strategies for optimal management of complex human disorders.
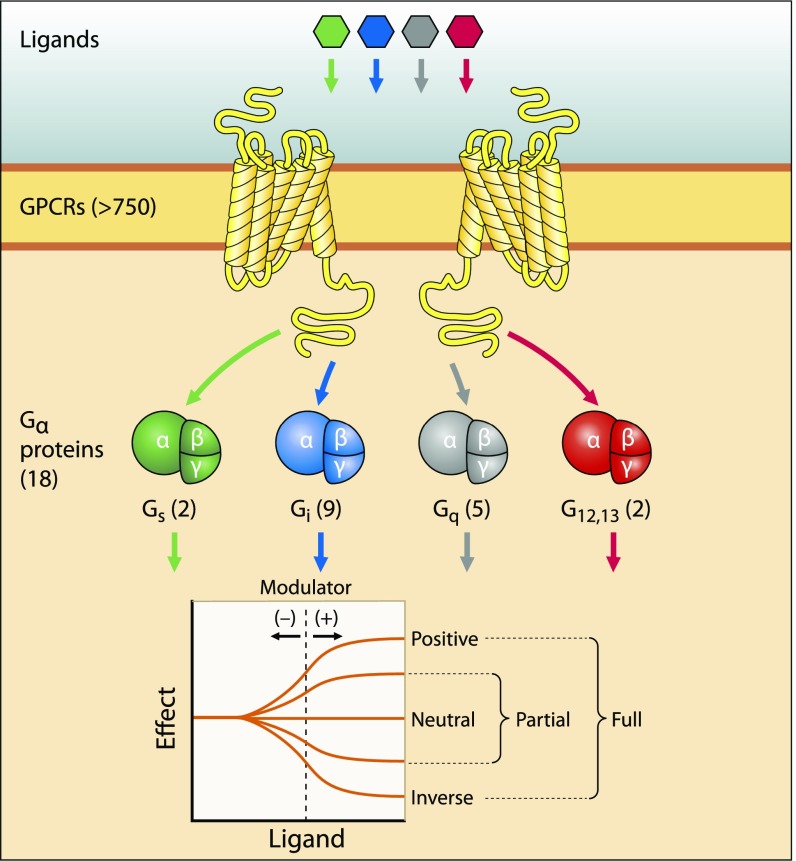
GPCRs provide an excellent example for the application of a systems pharmacology approach (44), because many different signal transduction pathways can converge on a common second messenger or effector molecule (Fig. 6). Next generation sequencing initially provided a comprehensive database of transcripts expressed in mouse and human retinas (45, 46). Detailed analyses then suggested interconnected signaling components involving several GPCRs, major GPCR effector enzymes, and NADPH oxidase subunits that contribute to retinal physiology as well as to pathological conditions. Combination pharmacological interventions were then designed to address possible implications of these interconnected signaling mechanisms in a mouse model of retinal degenerative disease. Using mice as the animal model to study AMD comes with inherent constraints. Given the differing topography between mouse and human retinas, such as the absence of a macula in mice, light-induced degeneration serves only as an approximate model of stress-induced photoreceptor/RPE toxicity, similar in nature to the accumulation of various stressors with age precipitating the AMD phenotype. Indeed, these mice exhibit an age-associated increase in susceptibility to light-induced retinopathy (47). Interestingly, when administered at individually subeffective doses, combined treatment with Bromocriptine, Metoprolol, and Doxazosin resulted in morphological and functional protection of photoreceptor cells by modifying distinct GPCR signaling pathways that culminated in a common response (48). The synergistic effect of these Food and Drug Administration-approved, repurposed drugs was elicited by modulation of somatic maintenance pathways and intercellular signaling. These results not only provided a proof of concept supporting the involvement of these signaling components in retinal degeneration, but more importantly, they laid the groundwork for additional therapeutic development by optimizing the choice of pharmacological agents to be tested (45, 49), possibly along with hormonal augmentation. Using a holistic systems pharmacology approach to developing therapeutics for AMD ultimately aims to ameliorate the multifactorial disease by modulating distinct, interconnected pathways involved in pathogenesis. This unbiased approach involves relevant integrated systems without prior knowledge about which is more dominant in the progression of disease—photoreceptors, RPE, or choriocapillaris (50). By boosting mechanisms of somatic maintenance and mitigating detrimental changes to intercellular signaling and metabolic function, the goal is to improve the health of photoreceptors in the retina along with RPE and choriocapillaris function.
Fig. 6.
GPCR signalosome: components and actions. GPCRs constitute a large family of transmembrane proteins that transduce extracellular stimuli into intracellular signaling responses. Ligand binding to GPCRs leads to activation of heterotrimeric G alpha (Gα) proteins, which have various downstream effects on intracellular processes. Drug action on GPCRs was reviewed extensively in ref. 35.
Conclusions
Recent clinical trials aimed at developing treatments for AMD have focused mainly on single pathways. These include modulator compounds targeting components of the visual cycle (24), blockers of immunological complement system activation (25), and progrowth receptor inhibitors (26), none of which have achieved desirable results. To improve outcomes, it thus could be necessary to consider the retina as part of a large interconnected system that requires the application of systems pharmacology. One of the aims of a systems pharmacology approach is to attenuate and/or compensate for age-related epigenetic changes that result in altered transcription profiles (9). Thus, targeting subcellular signaling pathways involved in somatic maintenance may be an effective strategy to restore the epigenome to a younger, healthier state. In conjunction with restoring protein homeostasis in the eye, drugs that also act to reestablish normal intercellular signaling and metabolic function by select global endocrine regulators offer the potential to optimize outcomes in maintaining the variability of cells and their structures as well as interneuron connectivity. Systems pharmacology also will help elucidate how known genotypes of AMD lead to disease. Many of the genes associated with AMD, including age-related maculopathy susceptibility, ATP-binding cassette transporter, and apolipoprotein E, already have been implicated in various metabolic disorders (23, 51). Others, such as amyloid precursor protein (14) and complement factor H (23), suggest that AMD pathogenesis also involves disrupted protein homeostasis and altered immune signaling, respectively. By studying the efficacy of GPCR-modifying compounds in attenuating age-related disruptions to protein homeostasis, metabolic function, and cellular signaling, systems pharmacology will provide insight into the molecular mechanisms involved in aggravating or ameliorating the AMD phenotype. As the molecular underpinnings of disparate human diseases become better understood, an innovative combination of systems pharmacology, rapid high-throughput screening, and iterative chemistry could enable the development of broad-ranging mechanism-based therapies to combat chronic, age-related diseases by reestablishing the transcriptome of younger and healthier cells.
Supplementary Material
Acknowledgments
We thank Leslie T. Webster Jr. for valuable comments on this manuscript. This research was supported, in part, by NIH Grants EY009339, EY027283, and EY024864. K.P. is the John H. Hord Professor of Pharmacology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1721033115/-/DCSupplemental.
References
- 1.Sapra KT, Park PS, Palczewski K, Muller DJ. Mechanical properties of bovine rhodopsin and bacteriorhodopsin: Possible roles in folding and function. Langmuir. 2008;24:1330–1337. doi: 10.1021/la702299z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 3.Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics–2011 update: A report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Department of Health and Human Services . National Diabetes Statistics Report, 2017. Centers for Disease Control and Prevention; Atlanta: 2017. [Google Scholar]
- 6.Friedman DS. Vision Problems in the U.S.: Prevalence of Adult Vision Impairment and Age-Related Eye Disease in America. National Eye Institute; Bethesda, MD: 2008. [Google Scholar]
- 7.Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Blagosklonny MV. Aging: ROS or TOR. Cell Cycle. 2008;7:3344–3354. doi: 10.4161/cc.7.21.6965. [DOI] [PubMed] [Google Scholar]
- 9.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lodato MA, et al. Aging and neurodegeneration are associated with increased mutations in single human neurons. Science. 2017;359:555–559. doi: 10.1126/science.aao4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, et al. A conserved NAD+ binding pocket that regulates protein-protein interactions during aging. Science. 2017;355:1312–1317. doi: 10.1126/science.aad8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem. 2009;78:959–991. doi: 10.1146/annurev.biochem.052308.114844. [DOI] [PubMed] [Google Scholar]
- 13.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 14.Ratnayaka JA, Serpell LC, Lotery AJ. Dementia of the eye: The role of amyloid beta in retinal degeneration. Eye (Lond) 2015;29:1013–1026. doi: 10.1038/eye.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wood SH, Craig T, Li Y, Merry B, de Magalhães JP. Whole transcriptome sequencing of the aging rat brain reveals dynamic RNA changes in the dark matter of the genome. Age (Dordr) 2013;35:763–776. doi: 10.1007/s11357-012-9410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khattak RM, Ittermann T, Nauck M, Below H, Völzke H. Monitoring the prevalence of thyroid disorders in the adult population of Northeast Germany. Popul Health Metr. 2016;14:39. doi: 10.1186/s12963-016-0111-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Baltimore Longitudinal Study of Aging Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 18.Roshan S, Nader S, Orlander P. Review: Ageing and hormones. Eur J Clin Invest. 1999;29:210–213. doi: 10.1046/j.1365-2362.1999.00436.x. [DOI] [PubMed] [Google Scholar]
- 19.Karasek M. Melatonin, human aging, and age-related diseases. Exp Gerontol. 2004;39:1723–1729. doi: 10.1016/j.exger.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Bartke A. Growth hormone and aging: A challenging controversy. Clin Interv Aging. 2008;3:659–665. doi: 10.2147/cia.s3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron. 2012;75:26–39. doi: 10.1016/j.neuron.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14:835–846. [PubMed] [Google Scholar]
- 23.Guillonneau X, et al. On phagocytes and macular degeneration. Prog Retin Eye Res. 2017;61:98–128. doi: 10.1016/j.preteyeres.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Kiser PD, Palczewski K. Retinoids and retinal diseases. Annu Rev Vis Sci. 2016;2:197–234. doi: 10.1146/annurev-vision-111815-114407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leung E, Landa G. Update on current and future novel therapies for dry age-related macular degeneration. Expert Rev Clin Pharmacol. 2013;6:565–579. doi: 10.1586/17512433.2013.829645. [DOI] [PubMed] [Google Scholar]
- 26.Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K. SEVEN-UP Study Group Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: A multicenter cohort study (SEVEN-UP) Ophthalmology. 2013;120:2292–2299. doi: 10.1016/j.ophtha.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 27.Holzenberger M, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 28.Ortega-Molina A, et al. Pten positively regulates brown adipose function, energy expenditure, and longevity. Cell Metab. 2012;15:382–394. doi: 10.1016/j.cmet.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Lambooij AC, et al. Insulin-like growth factor-I and its receptor in neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2003;44:2192–2198. doi: 10.1167/iovs.02-0410. [DOI] [PubMed] [Google Scholar]
- 30.Roberts MR, Srinivas M, Forrest D, Morreale de Escobar G, Reh TA. Making the gradient: Thyroid hormone regulates cone opsin expression in the developing mouse retina. Proc Natl Acad Sci USA. 2006;103:6218–6223. doi: 10.1073/pnas.0509981103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng L, Liu H, St Germain DL, Hernandez A, Forrest D. Deletion of the thyroid hormone-activating type 2 deiodinase rescues cone photoreceptor degeneration but not deafness in mice lacking type 3 deiodinase. Endocrinology. 2017;158:1999–2010. doi: 10.1210/en.2017-00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gopinath B, Liew G, Kifley A, Mitchell P. Thyroid dysfunction and ten-year incidence of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2016;57:5273–5277. doi: 10.1167/iovs.16-19735. [DOI] [PubMed] [Google Scholar]
- 33.Hönes GS, et al. Noncanonical thyroid hormone signaling mediates cardiometabolic effects in vivo. Proc Natl Acad Sci USA. 2017;114:E11323–E11332. doi: 10.1073/pnas.1706801115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunter DJ. Gene-environment interactions in human diseases. Nat Rev Genet. 2005;6:287–298. doi: 10.1038/nrg1578. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Palczewski K. Systems pharmacology links GPCRs with retinal degenerative disorders. Annu Rev Pharmacol Toxicol. 2016;56:273–298. doi: 10.1146/annurev-pharmtox-010715-103033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iyengar R. Complex diseases require complex therapies. EMBO Rep. 2013;14:1039–1042. doi: 10.1038/embor.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reiser J, Sever S, Faul C. Signal transduction in podocytes–spotlight on receptor tyrosine kinases. Nat Rev Nephrol. 2014;10:104–115. doi: 10.1038/nrneph.2013.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polans A, Baehr W, Palczewski K. Turned on by Ca2+! the physiology and pathology of Ca(2+)-binding proteins in the retina. Trends Neurosci. 1996;19:547–554. doi: 10.1016/s0166-2236(96)10059-x. [DOI] [PubMed] [Google Scholar]
- 39.Kelly MP. Cyclic nucleotide signaling changes associated with normal aging and age-related diseases of the brain. Cell Signal. 2018;42:281–291. doi: 10.1016/j.cellsig.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun N, Youle RJ, Finkel T. The mitochondrial basis of aging. Mol Cell. 2016;61:654–666. doi: 10.1016/j.molcel.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woo DK, Shadel GS. Mitochondrial stress signals revise an old aging theory. Cell. 2011;144:11–12. doi: 10.1016/j.cell.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 42.Rowan S, et al. Involvement of a gut-retina axis in protection against dietary glycemia-induced age-related macular degeneration. Proc Natl Acad Sci USA. 2017;114:E4472–E4481. doi: 10.1073/pnas.1702302114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen LJ, et al. Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature. 2017;549:48–53. doi: 10.1038/nature23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lagerström MC, Schiöth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7:339–357. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- 45.Chen Y, et al. Systems pharmacology identifies drug targets for Stargardt disease-associated retinal degeneration. J Clin Invest. 2013;123:5119–5134. doi: 10.1172/JCI69076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mustafi D, Maeda T, Kohno H, Nadeau JH, Palczewski K. Inflammatory priming predisposes mice to age-related retinal degeneration. J Clin Invest. 2012;122:2989–3001. doi: 10.1172/JCI64427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maeda A, Maeda T, Golczak M, Palczewski K. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J Biol Chem. 2008;283:26684–26693. doi: 10.1074/jbc.M804505200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y, et al. Synergistically acting agonists and antagonists of G protein-coupled receptors prevent photoreceptor cell degeneration. Sci Signal. 2016;9:ra74. doi: 10.1126/scisignal.aag0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Y, et al. Mechanism of all-trans-retinal toxicity with implications for stargardt disease and age-related macular degeneration. J Biol Chem. 2012;287:5059–5069. doi: 10.1074/jbc.M111.315432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhutto I, Lutty G. Understanding age-related macular degeneration (AMD): Relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol Aspects Med. 2012;33:295–317. doi: 10.1016/j.mam.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fritsche LG, et al. Age-related macular degeneration is associated with an unstable ARMS2 (LOC387715) mRNA. Nat Genet. 2008;40:892–896. doi: 10.1038/ng.170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.