Significance
Nonpolio enteroviruses are responsible for a high burden of neurological and other diseases and exhibit a peak in summer every year, but drivers of their seasonality are not clearly understood. We find that the seasonal pattern of enterovirus cases in the United States has a spatial structure comparable with that of prevaccination poliomyelitis. The average monthly distribution of cases is more flat in the south and has a more pronounced peak that occurs later toward the north, with the peak for poliomyelitis occurring approximately 1 month later than that for nonpolio enteroviruses. We find that climate, but not demography, is likely to explain this seasonality and identify the dew point temperature as a strong predictor of the intensity of enterovirus transmission.
Keywords: enterovirus, poliomyelitis, seasonality, climate, United States
Abstract
Nonpolio enteroviruses are diverse and common viruses that can circulate year-round but tend to peak in summer. Although most infections are asymptomatic, they can result in a wide range of neurological and other diseases. Many serotypes circulate every year, and different serotypes predominate in different years, but the drivers of their geographical and temporal dynamics are not understood. We use national enterovirus surveillance data collected by the US Centers for Disease Control and Prevention during 1983−2013, as well as demographic and climatic data for the same period, to study the patterns and drivers of the seasonality of these infections. We find that the seasonal pattern of enterovirus cases is spatially structured in the United States and similar to that observed for historical prevaccination poliomyelitis (1931−1954). We identify latitudinal gradients for the amplitude and the timing of the peak of cases, meaning that those are more regularly distributed all year-round in the south and have a more pronounced peak that arrives later toward the north. The peak is estimated to occur between July and September across the United States, and 1 month earlier than that for historical poliomyelitis. Using mixed-effects models, we find that climate, but not demography, is likely to drive the seasonal pattern of enterovirus cases and that the dew point temperature alone explains ∼30% of the variation in the intensity of transmission. Our study contributes to a better understanding of the epidemiology of enteroviruses, demonstrates important similarities in their circulation dynamics with polioviruses, and identifies potential drivers of their seasonality.
Human enteroviruses (Enterovirus genus) are a large group of viruses of more than 100 serotypes that includes polioviruses, coxsackieviruses A and B, echoviruses, and other serotypes (1). Enteroviruses cause a broad spectrum of illnesses (including meningitis, encephalitis, paralysis, myocarditis, respiratory illness, and rash), and their burden has become increasingly apparent in recent years (2, 3), making them to be increasingly recognized as a significant cause of neurological and respiratory diseases.
The most studied enteroviruses are polioviruses, which cause poliomyelitis and are the subject of intensive eradication efforts (4). More recently, there has been an increasing number of studies on enterovirus A71 (EV-A71) and coxsackieviruses A16 (CV-A16) and A6 (CV-A6), which have been responsible for large outbreaks of hand-foot-and-mouth disease (HFMD) in Southeast Asia during the last 10 to 20 y, resulting in severe complications in thousands of cases (5). In China alone, the total number of HFMD cases has exceeded 1 million annually (5). Although not associated with HFMD outbreaks of the same size, EV-A71 has also been responsible for clusters of severe neurological illnesses in Europe (6, 7) and the United States (8). In 2014, enterovirus D68 (EV-D68) was implicated in an outbreak of severe respiratory illness in the United States (9) and subsequently was seen in other regions of the world (10, 11). In addition, potential associations between EV-D68 and cases of acute flaccid myelitis are currently being studied (12). Despite the clinical importance of enterovirus infections, little is known about the mechanisms underlying their spatial and temporal dynamics.
Enteroviruses are detected year-round but tend to peak in summer. However, the seasonal determinants of their transmission dynamics remain unknown. Elucidating the drivers of seasonal patterns in infectious disease can provide a powerful probe for clarifying their epidemic dynamics (13). Here, we analyze enterovirus surveillance data from the United States collected between 1983 and 2013 (Materials and Methods) to describe the seasonality of these infections across the country and further assess the contribution of demographic and climatic factors to the observed patterns. As a comparison, we perform the same analysis on historical poliomyelitis cases during the prevaccination era of 1931−1954 (Materials and Methods). We find that the seasonal pattern of enterovirus cases exhibits a well-defined geographical structure across the United States very similar to that of historical poliomyelitis, and find that measures of humidity, such as the dew point temperature, are strong predictors of the intensity of enterovirus transmission.
Results
Description of Nonpolio Enterovirus Cases.
During the period 1983−2013, a total of 27,858 nonpolio enterovirus cases were reported from the contiguous United States, including 57 different serotypes. Case age was available for the period 2000−2013, during which time it was reported in 84% of cases. Among those, 34% had an age <1 y. The specimen type was reported for 71% of cases, and among those, the most commonly reported types were cerebrospinal fluid (55%), throat swab (29%), and stool (15%).
Seasonal Patterns of Incidence.
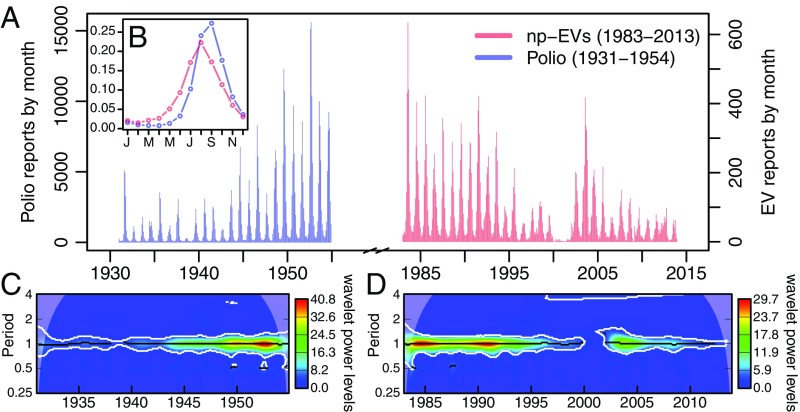
The monthly number of nonpolio enterovirus cases exhibits an annual peak in the United States that occurs in summer (Fig. 1A). A similar seasonal pattern is apparent for reported poliomyelitis incidence in the prevaccination period (Fig. 1A), although the peak for poliomyelitis was slightly later than that for nonpolio enterovirus cases (Fig. 1B). The wavelet analysis of the two time series of cases shows a clear band at 1 y (Fig. 1 C and D), confirming that the long-term temporal variation in the incidence of cases is dominated by the 1-y periodic component (annual seasonality). The interruption of this band for the nonpolio enterovirus cases between 2000 and 2001 corresponds to the introduction of molecular typing, which led to a temporary drop in the reporting of cases.
Fig. 1.
Annual summer seasonality of nonpolio enterovirus and historical poliomyelitis cases in the United States. (A) Time series of the monthly number of nonpolio enterovirus cases (np-EVs; red) and poliomyelitis cases (Polio; blue) in the United States during the periods 1983–2013 and 1931–1954, respectively. (B, shown as Inset) Average monthly distribution of cases within the year for nonpolio enterovirus (red) and poliomyelitis (blue) based on all cases reported. (C and D) Average wavelet power of the two time series in A: poliomyelitis (C) and nonpolio enteroviruses (D).
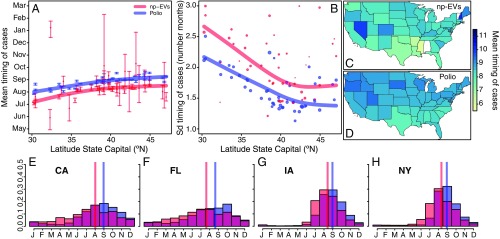
We characterized the seasonal pattern of incidence in each state, both for nonpolio enterovirus cases (all serotypes combined) and for poliomyelitis, by summarizing the average monthly distribution of cases for the years 1983−2013 and 1931−1954, respectively (Fig. 2 E–H and SI Appendix, Fig. S1). When including all states with at least 50 cases reported over the entire time periods, the mean timing of cases (i.e., mean month in which cases occurred) of enterovirus ranged between July in Texas (7.05; 95% CI, 6.96 to 7.14) and September in Colorado (9.33; 95% CI, 8.98 to 9.64), and between August in Texas (7.83; 95% CI, 7.80 to 7.86) and October in South Dakota (9.61; 95% CI, 9.56 to 9.67) for poliomyelitis. For both groups, the mean month in which cases occurred increased from south to north (Fig. 2 A, C, and D), with poliomyelitis cases approximately 1 mo later than nonpolio enterovirus cases (Fig. 2A). The dispersion of cases throughout the year (measured as the SD from the mean) decreased from south to north, meaning that the distribution of the monthly average incidence of cases was flatter in the south and showed an increasing peak toward the north, with poliomyelitis cases showing a more peaked distribution compared with nonpolio enterovirus cases (Fig. 2B).
Fig. 2.
Description of the monthly distribution of cases within the year per state. (A) Mean timing of cases of nonpolio enterovirus (np-EVs; red) and poliomyelitis (Polio; blue) per state as a function of the latitude of its capital city. The 95% CIs of the mean were obtained by bootstrapping 1,000 times. (B) SD of the timing of cases per state as a function of the latitude of its capital city. In A and B, the lines are cubic splines weighted by the total number of cases reported in each state. The size of the points in B indicates the number of total cases in each state. A sensitivity analysis of A and B data using the latitude of the state’s center of population showed a similar pattern (SI Appendix, Fig. S5). (C and D) US maps showing the mean timing of cases per state. (E–H) Monthly distribution of cases of enterovirus (red) and poliomyelitis (blue) within the year in four different states: California (CA), Florida (FL), Iowa (IA), and New York (NY). In E–H, the vertical lines indicate the estimated mean timing of cases in each state.
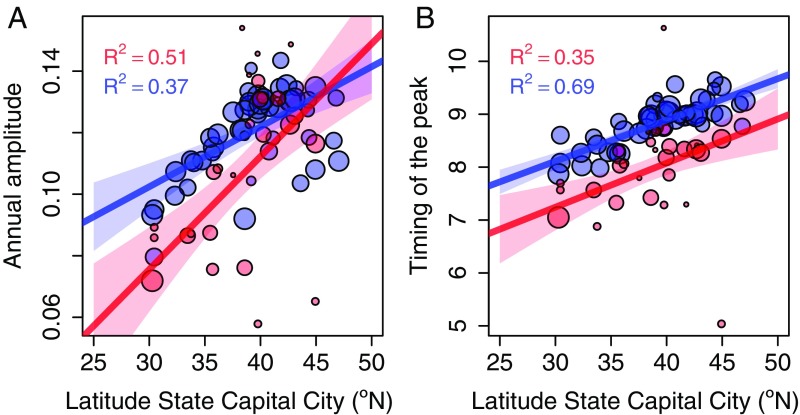
We further characterized the seasonal curves by estimating the amplitude and the timing of the peak for each year of data in each state (Materials and Methods). Both the amplitude and the timing of the peak (measured as the amplitude and phase of the annual component) showed a latitudinal gradient for enterovirus cases (regression analysis of amplitude: R2 = 0.51, r = 0.03, P < 0.001; peak: R2 = 0.35, r = 0.08, P < 0.001) and for poliomyelitis cases (amplitude: R2 = 0.37, r = 0.02, P < 0.001; peak: R2 = 0.69, r = 0.08, P < 0.001) (Fig. 3 and SI Appendix, Fig. S2). The latitudinal gradients of the timing of the peak for enterovirus and poliomyelitis had a very similar slope (Fig. 3B), but the peak for nonpolio enteroviruses was estimated to occur nearly 1 mo earlier than that for poliomyelitis, as shown by the estimated difference on the intercept (σ = 0.892, P < 0.001) in the joint regression for enterovirus and poliomyelitis (SI Appendix, Table S1). A similar analysis showed that the latitudinal gradient for the amplitude was more pronounced for enterovirus cases than for poliomyelitis (P = 0.036), as indicated by a steeper slope (Fig. 3A and SI Appendix, Table S2). However, when including in the regressions only those states with data for both enterovirus and poliomyelitis cases, this difference in the latitudinal gradients for the amplitude was not apparent (P = 0.257, SI Appendix, Fig. S3 and Table S4).
Fig. 3.
Latitudinal gradients in the seasonal pattern of nonpolio enterovirus (red) and poliomyelitis (blue) cases. (A) Gradients for the annual amplitude. (B) Gradients for the timing of the peak. The dots are mean values per state of the seasonal characteristics (amplitude and timing of the peak) weighted by the number of cases informing that value each year. The size of the dots indicates the number of years informing the mean value of each state. The lines are the estimated linear regressions weighted by the number of years informing each value, and the shades are the 95% CIs of the estimated linear regression by minimizing the least squares. A sensitivity analysis of A and B data using the latitude of the state’s center of population showed a similar pattern (SI Appendix, Fig. S4).
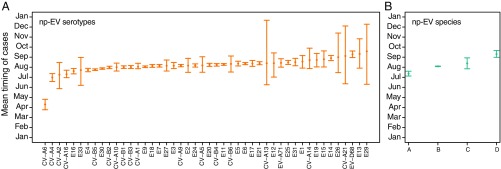
The causes of a peak occurring slightly earlier for nonpolio enteroviruses compared with historical poliomyelitis remain unclear, but it could be that some enterovirus serotypes systematically circulate earlier than others. To further explore this hypothesis, we estimated the mean timing of cases across the United States by serotype and by species (Fig. 4) and found small, but not significant, differences, except for CV-A6 that ranked first with an estimated mean timing of cases in April (4.30; 95% CI, 3.77 to 4.80). EV-D68 ranked among the last ones, with an estimated mean timing of cases in September (9.28; 95% CI, 8.97 to 9.61). When looking at the mean timing of cases by species, species A ranked first (7.37; 95% CI, 7.13 to 7.60) and species D ranked last (9.31; 95% CI, 8.98 to 9.66), with species B (8.09; 95% CI, 8.06 to 8.12) and C (8.38; 95% CI, 7.85 to 8.93) having similar mean estimates. However, the estimates for species A, C, and D were informed by a small number of cases (983, 88, and 114, respectively) compared with B (23,680), and most species D cases were EV-D68. Interestingly, the estimated mean timing of cases for species C enterovirus (which includes polioviruses) occurred relatively late, as did historical poliomyelitis, but the low number of nonpolio cases of species C informing that estimate was too small to conclude that both (polio and nonpolio species C) had a similar mean timing of cases that was later than that for species B.
Fig. 4.
Mean timing of cases by nonpolio enterovirus (np-EV) serotype (A) and species (B) with their 95% CIs.
Role of Demography and Climate.
As a first step to explore whether there was evidence that any demographic [population size, population density, number of live births, and birthrates (SI Appendix, Figs. S6–S9)] or climatic factors [temperature, precipitation, dew point temperature, potential evaporation, pressure, relative humidity, and specific humidity (SI Appendix, Figs. S10–S16)] could explain the spatial structure of the seasonal patterns of enterovirus cases, we conducted univariable linear regressions for the amplitude and the timing of the peak (Materials and Methods and SI Appendix, Tables S6 and S7). We found that the annual range of the dew point temperature was the strongest predictor of the amplitude (R2 = 0.35), and that latitude was the strongest predictor of the timing of the peak (R2 = 0.26), closely followed by the annual minimum of specific humidity (R2 = 0.24). A larger annual range between the maximum and the minimum dew point was associated with a larger amplitude (r = 0.003, P < 0.001), and a higher minimum value of the specific humidity was associated with an earlier peak (r = −0.305, P < 0.001). Although some demographic variables were statistically significantly associated with the amplitude and the timing of the peak, climatic factors explained more of the variance (SI Appendix, Tables S6 and S7). Birthrates performed better than the other demographic factors in explaining both the variance in amplitude (22% vs. <10%) and the timing of the peak (11% vs. <7%), but this was still less than several climatic variables. Higher birthrates were associated with an earlier peak and smaller amplitude (SI Appendix, Fig. S19).
The incidence of cases is driven by changes in the efficiency of virus transmission, the prevalence of immunity in the population, and the number of prevalent infections (“mass action”). Incidence generally lags transmission efficiency because of the time taken for infection to establish in the population and the incubation period. However, transmission is more likely to be directly affected by climatic variables. We therefore controlled for the number of prevalent infections in the population by estimating the case reproduction number (Materials and Methods) and regressed these estimates against climatic and demographic variables using linear mixed-effects models that included state as a random effect and accounted for temporal autocorrelation (Materials and Methods). Exploratory plots to assess the relationship between the intensity of transmission and each climatic variable (SI Appendix, Figs. S23 and S24) suggested that temperature, dew point, potential evaporation, and/or specific humidity would have an important role in the statistical analysis. The best individual predictor of the intensity of transmission was the dew point (Fig. 5A and SI Appendix, Table S8), followed by temperature and specific humidity (SI Appendix, section S3). The final model (SI Appendix, Table S13) included three variables: dew point, potential evaporation, and pressure (which was treated as a categorical variable) and had estimated marginal (M) and conditional (C) R2 values [sensus (14)] of 0.47 and 0.55, respectively, meaning that it explained a relatively large amount of the variance of the intensity of transmission and that the random effects captured only a small amount of it (the difference between R2C and R2M was small). No demographic variables were retained in the final model (SI Appendix, Table S8). The best model with only one fixed effect was dew point, which after accounting for autoregressive errors (SI Appendix, Table S14), had an R2M value of 0.23 and a R2C value of 0.29, indicating that dew point alone was an important predictor of the intensity of enterovirus transmission.
Fig. 5.
Dew point temperature and intensity of transmission. (A) Monthly estimates of the case reproduction number (log10) against monthly mean of dew point. Each color corresponds to a state. The lines are cubic splines for the observations of each state. (B) Effect of dew point (DP) on poliovirus survival. Experimental studies conducted in the early 1960s studied the influence of relative humidity on the survival of poliovirus in aerosols (15). We used data in ref. 15 and an approximation of the dew point based on temperature, relative humidity, and pressure to look at those results for different values of the dew point. This approximation was based on the so-called Magnus formula (39), accounting for an improvement of the saturation water vapor pressure in ref. 40 and using the constants in ref. 41. The lower and upper bounds of the intervals for the dew point were computed using the lower and upper values reported for temperature and relative humidity.
Discussion
We have described the seasonality of enterovirus cases across the United States using national surveillance data collected over three decades (1983−2013). We have shown that enterovirus cases peak in summer, with a relatively flat seasonal profile of incidence in the south and a more pronounced peak that arrives later toward the north. This spatial structure is very similar to that observed for historical poliomyelitis, although the peak of enterovirus cases was estimated to occur approximately 1 mo earlier than the peak of historical poliomyelitis. The reason for this shift in the peak remains unclear, but could be explained by an earlier peak for species A and B enteroviruses compared with species C (which includes poliovirus). Our analyses of the mean timing of cases by serotype and by species have revealed small, but not conclusive, differences, except for CV-A6, which ranked first with a mean timing of cases in April. An alternative explanation for the later peak of poliomyelitis could be that fecal-oral transmission in the polio period accounted for more transmission compared with the enterovirus period, where the majority of the transmission is likely to be respiratory. The survival of the virus in the environment (e.g., sewage) is longer (measured in weeks) than in droplets or aerosols (measured in hours or days), allowing for longer periods of fecal-oral transmission compared with the respiratory route.
The similarities found in the mean timing of cases across nonpolio enterovirus serotypes, regardless of those being associated with different clinical outcomes, affecting different age groups, and having different transmission pathways, suggest the existence of external common mechanisms across serotypes underlying their seasonal dynamics. Our findings strongly suggest that climatic factors may be among these mechanisms but do not support a major role for demography. Among the climatic variables evaluated, we have identified the dew point temperature as the main predictor of the intensity of enterovirus transmission. The dew point is the temperature to which air must be cooled at constant pressure for saturation to occur; that is, for air water vapor to condense to form liquid water (or in other words, to have a relative humidity of 100%). As such, the dew point depends on temperature and humidity. It is not surprising then that models with temperature or specific humidity as the only fixed effect followed the dew point as the best univariable models. Dew point was clearly the best single predictor, but disentangling the effect of dew point, temperature, and specific humidity was not possible due to the high correlation among these climatic variables, and it could be that the dew point worked as a summary measure of the combined effect of the other two. Nevertheless, our results suggest that the amount of water vapor in the air directly affects the intensity of enterovirus transmission.
Laboratory experiments conducted in the 1960s found an effect of relative humidity on the survival of poliovirus in aerosols when temperature was maintained constant (15). Although we did not find an association between the intensity of enterovirus transmission and relative humidity, we could approximate the dew point during those experiments (Fig. 5B), and our results are consistent with those findings. Just after spraying, the survival of poliovirus was high for values of the dew point above 10 °C, and for longer time periods (including hours), an important proportion of viral particles was recovered for dew point values above 13 °C (15). At the population level, we found that the intensity of enterovirus transmission increased with increasing dew point, and the case reproduction number was around 1 for values of the dew point between approximately 0 °C and 12 °C (Fig. 5A). That being said, enteroviruses can also be transmitted by the fecal-oral route, meaning that virus survival in media other than aerosols, like sewage, could be important, although in places with good sanitation such as the United States, the respiratory route may account for most of the transmission events.
We also found that the annual range between the maximum and minimum dew point was the best individual climatic predictor of the amplitude of incidence, and the annual minimum dew point was ranked second for the timing of the peak. The last point might imply the existence of a threshold of the dew point under which transmission barely occurs, and thereby, transmission might “start” later in states with a lower minimum dew point, consequently resulting in a later peak.
In the United States, some populations in the western states live at high altitudes. The final model for the intensity of enterovirus transmission included pressure, which is strongly inversely correlated with altitude, perhaps suggesting that altitude was associated with a lower intensity of transmission.
Although based on our findings, demographic variables seem to have little if any role in driving the seasonal pattern of enteroviruses, it could be that births have an important role in the long-term dynamics of individual enterovirus serotypes, which are likely to depend, at least partly, on changes in population immunity and numbers of susceptible children.
A previous study in China (5) found that HFMD (mainly associated with EV-A71 and CV-A16) exhibited a latitudinal gradient in the annual amplitude similar to what we found for nonpolio enterovirus and poliomyelitis cases in the United States. However, that study also found that HFMD in China had an annual peak of cases around June in the north, but two peaks each year in the south (a big one around May and a smaller one around October). This semiannual pattern was not observed for nonpolio enterovirus cases (or poliomyelitis) in the United States. However, EV-A71 and CV-A16 represent a very small proportion of cases captured by the US surveillance system, making a direct comparison difficult. Nonetheless, although Xing et al. (5) reported very small associations between individual climatic variables and the timing of the peak of HFMD, they found that climatic factors were the main predictors of the two main epidemiological regions of HFMD in China, in agreement with our conclusions that climate is likely to drive the seasonal pattern of enterovirus diseases.
Enterovirus surveillance is passive in the United States and has been subject to important changes in detection and typing methods during the last 20 y. As a consequence, the data for enterovirus cases used here are sparse, meaning that only some states report cases every year; most did not consistently report over the study period, and states may even inconsistently report during the year. Moreover, enterovirus reports may more closely reflect outbreak-driven testing, rather than endemic circulation. Despite these limitations, the similarities that we have found in the amplitude and the timing of the peak with historical poliomyelitis support the results for nonpolio enteroviruses.
The role of climatic factors was not tested for poliomyelitis in the 1931−1954 period. The climatic data available for that period [e.g., through the National Center for Environmental Prediction I (NCEP I) dataset global reanalysis, which starts only in 1948] is of lower quality and provided through a coarse grid of ∼250 km at the lowest latitudes in the United States and, therefore, does not capture climate variability with a sufficient spatial resolution. Moreover, some important variables are not available in that dataset (e.g., relative humidity and dew point).
Two major questions remain. The first is whether the latitudinal gradients described here imply traveling waves (16) of enteroviruses from south to north in the United States, or whether, on the contrary, these viruses have more local dynamics and perhaps persist all year-round everywhere. Analyses of viral sequence data from the same season and from consecutive seasons collected in different states could help answer this question. The second question concerns the long-term nonseasonal dynamics of individual enterovirus serotypes. Despite a peak of cases of nonpolio enterovirus (all serotypes combined) being observed every year, some serotypes have longer, relatively regular multiannual cycles (1, 17). The contribution of population immunity, cross-immunity (18), and viral evolution to these diverse patterns is not currently understood.
The causes of seasonal variation in the incidence of infectious diseases remains an open question that has been dominated by research on influenza (19–22) and other winter viruses like respiratory syncytial virus (23). The results in this paper bring evidence to this field for another group of pathogens. The physical and chemical properties that affect virus survival in different media (droplets, aerosols, water, waste, and surfaces) have been extensively reviewed before for enteroviruses and other enteric and airborne viruses (24–26). In addition to virus survival or epidemiological studies, where possible, environmentally controlled transmission studies (mainly in animal models) (27, 28) will be a key tool to further elucidate the mechanisms through which environmental factors determine the seasonal patterns of different viral diseases.
In the context of enterovirus epidemiology, these findings constitute a first step toward a better understanding of the drivers of their transmission dynamics and their related spatial and temporal epidemic patterns. Future work must focus on understanding the diversity of circulation patterns among the different serotypes.
Materials and Methods
Nonpolio Enterovirus Data.
Enterovirus detections reported to the US Centers for Disease Control and Prevention (CDC) through the National Enterovirus Surveillance System (NESS) between January 1983 and December 2013 were used. NESS is a voluntary, passive surveillance system that includes reports from US laboratories with the capacity to type enteroviruses. A detailed description of NESS can be found in ref. 17. The reports from NESS contain information on the state, month, and year of the sample collection and were used to obtain the monthly incidence of nonpolio enterovirus cases (Fig. 1A). The analyses were restricted to the data from the contiguous United States, which excludes Alaska, Hawaii, and all offshore territories. Data for the period 1983−1999 were reported by patient, whereas data for 2000−2013 were reported by specimen. For the latter, because more than one enterovirus detection could be reported per patient, we used the patient identification number to avoid counting a patient multiple times. The detection of an enterovirus (particularly, in a nonsterile site) does not imply that this is the etiological agent of the clinical symptoms. For simplicity, here we use “enterovirus cases” to refer to patients reported with a positive sample.
Polio Data.
The number of poliomyelitis cases reported in the contiguous United States per state and month between January 1931 and December 1954 were available from a previous publication (29) (Fig. 1A). The total number of poliomyelitis cases reported during this period was 433,743.
Demographic Data.
The population size by state and year from 1983 to 2013 was extracted from the United States Census Bureau (https://www.census.gov), and the population density was obtained dividing those numbers by the land area. The annual number of live births per state was extracted from the Vital Statistics Data maintained by the CDC (30). The crude annual birthrate was obtained by dividing the annual number of live births by the annual population size (SI Appendix, Figs. S6–S9).
Climate Data.
We extracted climate data from the North American Regional Reanalysis (NARR) dataset (31), which covers a period starting in 1979 until present and provides data through a high-resolution grid of ∼32 km at the lowest latitude. We extracted monthly estimates from the NARR Monthly Means dataset for the following variables: air temperature, accumulated precipitation, dew point temperature, potential evaporation, vapor pressure, relative humidity, and specific humidity (SI Appendix, Figs. S10–S16 and Table S5). For each state, we used the value of the variables in the grid closest to its capital.
Wavelets.
We performed a wavelet analysis of the time series of enterovirus and poliomyelitis cases to detect and quantify the periodicity of these two time series. This method is particularly adapted to nonstationary data (32). Here, we applied the Morlet wavelet, as has been classically done for the analysis of epidemiological data (32). This analysis was performed using the “WaveletComp” R package (33).
Distribution of Cases Within the Year: Mean Timing and Dispersion.
We estimated the mean and SD of the timing of cases from the average monthly distribution of cases in each state obtained using the total number of cases reported over the entire periods of the study. Both statistics (mean and SD) were computed using circular statistics through the “circular” R package (34). Circular statistics, contrary to arithmetic statistics, are adapted to data that are best represented in a circle rather than in a line, as it is the case for time in months within a year. Note that the date of cases was given in months (entire values), and therefore the estimates of the mean timing of cases are biased toward the beginning of the months.
Characteristics of Seasonal Patterns of Incidence.
To describe the seasonal pattern of incidence in each state and each year, we fitted a seasonal model with four harmonic terms to the proportion of cases reported each month (for each year of data) and we extracted the amplitude of the annual and semiannual components, the timing of the peak (measured as the phase of the annual component), and the relative contribution of the semiannual component. Details are in SI Appendix, section S1.
Latitudinal Gradients for the Amplitude and the Timing of the Peak.
For each state, we took the mean value of the amplitude and timing of the peak of cases weighted by the number of cases informing that value each year. We tested for the presence of latitudinal gradients on those characteristics by fitting linear regression models separately for enteroviruses and poliomyelitis, weighted by the number of years informing the value of each state and with the latitude of the state’s capital city as independent variable. A sensitivity analysis using the latitude of the state’s center of population (35) provided similar results (SI Appendix, Fig. S5). We also tested for differences between enteroviruses and poliomyelitis in their latitudinal gradients of the seasonal characteristics (SI Appendix, section S2).
Univariable Linear Models for the Amplitude and the Timing of the Peak.
We conducted univariable linear models for the amplitude and the timing of the peak of cases using climatic and demographic factors, as well as latitude, longitude, and elevation, as covariables. We used the following annual summary statistics for the climatic variables obtained from the monthly data: minimum, maximum, median, and range.
Estimation of the Case Reproduction Number.
We estimated the monthly case reproduction number in each state to quantify the intensity of transmission over time using the methods described in ref. 36 and implemented in the “epiEstim” R package. For the serial interval we assumed a gamma distribution, with mean equal to 1.1 mo and SD equal to 0.2 mo based on data for poliovirus (37).
Mixed-Effects Models of the Intensity of Enterovirus Transmission.
We modeled the (log10-transformed) case reproduction number with linear mixed-effects models, where state was a random effect (random intercept), climatic and demographic variables were fixed effects, and we accounted for autocorrelated errors. The models were implemented using the “nlme” R package (38). Model selection was performed using a bottom-up strategy, although a top-down process gave the same final model. Diagnostic plots were used to check that the final model did not violate the assumptions of linear regression (SI Appendix, Fig. S27). To measure the goodness of fit, we used the marginal and conditional R2 for mixed-effects models introduced in ref. 14. See SI Appendix, section S3 for a full description.
Supplementary Material
Acknowledgments
We thank Isobel M. Blake and Juliette Paireau for helpful discussions. M.P.-S. is funded by the Wellcome Trust (Grant 106073/Z/14/Z). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1721159115/-/DCSupplemental.
References
- 1.Pallansch MA, et al. Enteroviruses: Polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. In: Knipe DM, Howley P, editors. Fields Virology. 6th Ed. Vol 2. Lippincott Williams & Wilkins; Philadelphia: 2013. pp. 490–530. [Google Scholar]
- 2.Martin NG, et al. Hospital admissions for viral meningitis in children in England over five decades: A population-based observational study. Lancet Infect Dis. 2016;16:1279–1287. doi: 10.1016/S1473-3099(16)30201-8. [DOI] [PubMed] [Google Scholar]
- 3.Rao CD, Yergolkar P, Shankarappa KS. Antigenic diversity of enteroviruses associated with nonpolio acute flaccid paralysis, India, 2007-2009. Emerg Infect Dis. 2012;18:1833–1840. doi: 10.3201/eid1811.111457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Polio Eradication Initiative 2013 Fact File: Polio eradication and endgame strategic plan 2013–2018. Available at polioeradication.org/wp-content/uploads/2016/07/PEESP_EN_A4.pdf. Accessed March 27, 2017.
- 5.Xing W, et al. Hand, foot, and mouth disease in China, 2008-12: An epidemiological study. Lancet Infect Dis. 2014;14:308–318. doi: 10.1016/S1473-3099(13)70342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassel C, et al. Transmission patterns of human enterovirus 71 to, from and among European countries, 2003 to 2013. Euro Surveill. 2015;20:30005. doi: 10.2807/1560-7917.ES.2015.20.34.30005. [DOI] [PubMed] [Google Scholar]
- 7.Launes C, et al. Utility of FilmArray meningitis/encephalitis panel during outbreak of brainstem encephalitis caused by enterovirus in Catalonia in 2016. J Clin Microbiol. 2016;55:336–338. doi: 10.1128/JCM.01931-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pérez-Vélez CM, et al. Outbreak of neurologic enterovirus type 71 disease: A diagnostic challenge. Clin Infect Dis. 2007;45:950–957. doi: 10.1086/521895. [DOI] [PubMed] [Google Scholar]
- 9.Midgley CM, et al. Severe respiratory illness associated with enterovirus D68 - Missouri and Illinois, 2014. MMWR Morb Mortal Wkly Rep. 2014;63:798–799. [PMC free article] [PubMed] [Google Scholar]
- 10.Dyrdak R, et al. Outbreak of enterovirus D68 of the new B3 lineage in Stockholm, Sweden, August to September 2016. Euro Surveill. 2016;21:30403. doi: 10.2807/1560-7917.ES.2016.21.46.30403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuffenecker I, et al. Epidemiological and clinical characteristics of patients infected with enterovirus D68, France, July to December 2014. Euro Surveill. 2016;21 doi: 10.2807/1560-7917.ES.2016.21.19.30226. [DOI] [PubMed] [Google Scholar]
- 12.Greninger AL, et al. A novel outbreak enterovirus D68 strain associated with acute flaccid myelitis cases in the USA (2012-14): A retrospective cohort study. Lancet Infect Dis. 2015;15:671–682. doi: 10.1016/S1473-3099(15)70093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grassly NC, Fraser C. Seasonal infectious disease epidemiology. Proc Biol Sci. 2006;273:2541–2550. doi: 10.1098/rspb.2006.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol. 2013;4:133–142. [Google Scholar]
- 15.Harper GJ. Airborne micro-organisms: Survival tests with four viruses. J Hyg (Lond) 1961;59:479–486. doi: 10.1017/s0022172400039176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grenfell BT, Bjørnstad ON, Kappey J. Travelling waves and spatial hierarchies in measles epidemics. Nature. 2001;414:716–723. doi: 10.1038/414716a. [DOI] [PubMed] [Google Scholar]
- 17.Khetsuriani N, Lamonte-Fowlkes A, Oberst S, Pallansch MA. Centers for Disease Control and Prevention Enterovirus surveillance–United States, 1970-2005. MMWR Surveill Summ. 2006;55:1–20. [PubMed] [Google Scholar]
- 18.Takahashi S, et al. Hand, foot, and mouth disease in China: Modeling epidemic dynamics of enterovirus serotypes and implications for vaccination. PLoS Med. 2016;13:e1001958. doi: 10.1371/journal.pmed.1001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deyle ER, Maher MC, Hernandez RD, Basu S, Sugihara G. Global environmental drivers of influenza. Proc Natl Acad Sci USA. 2016;113:13081–13086. doi: 10.1073/pnas.1607747113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaman J, Pitzer V, Viboud C, Lipsitch M, Grenfell B. Absolute humidity and the seasonal onset of influenza in the Continental US. PLoS Curr. 2009;2:RRN1138. doi: 10.1371/currents.RRN1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamerius JD, et al. Environmental predictors of seasonal influenza epidemics across temperate and tropical climates. PLoS Pathog. 2013;9:e1003194. doi: 10.1371/journal.ppat.1003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu H, et al. Characterization of regional influenza seasonality patterns in China and implications for vaccination strategies: Spatio-temporal modeling of surveillance data. PLoS Med. 2013;10:e1001552. doi: 10.1371/journal.pmed.1001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pitzer VE, et al. Environmental drivers of the spatiotemporal dynamics of respiratory syncytial virus in the United States. PLoS Pathog. 2015;11:e1004591. doi: 10.1371/journal.ppat.1004591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abad FX, Pintó RM, Bosch A. Survival of enteric viruses on environmental fomites. Appl Environ Microbiol. 1994;60:3704–3710. doi: 10.1128/aem.60.10.3704-3710.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sobsey MD, Meschke JS. 2003. Virus survival in the environment with special attention to survival in sewage droplets and other environmental media of fecal or respiratory origin (WHO, Geneva)
- 26.Yang W, Marr LC. Mechanisms by which ambient humidity may affect viruses in aerosols. Appl Environ Microbiol. 2012;78:6781–6788. doi: 10.1128/AEM.01658-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowen AC, Mubareka S, Steel J, Palese P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 2007;3:1470–1476. doi: 10.1371/journal.ppat.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaman J, Kohn M. Absolute humidity modulates influenza survival, transmission, and seasonality. Proc Natl Acad Sci USA. 2009;106:3243–3248. doi: 10.1073/pnas.0806852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez-Bakker M, King AA, Rohani P. Unraveling the transmission ecology of polio. PLoS Biol. 2015;13:e1002172. doi: 10.1371/journal.pbio.1002172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.US Department of Health and Human Services doi: 10.3109/15360288.2015.1037530. National Center for Health Statistics, Division of Vital Statistics, Natality public use data. Available at https://www.cdc.gov/nchs/data_access/vitalstatsonline.htm. Accessed October 25, 2016. [DOI] [PubMed]
- 31.Mesinger F, et al. North American regional reanalysis. Bull Am Meteorol Soc. 2006;87:343–360. [Google Scholar]
- 32.Cazelles B, Chavez M, Magny GC, Guégan JF, Hales S. Time-dependent spectral analysis of epidemiological time-series with wavelets. J R Soc Interface. 2007;4:625–636. doi: 10.1098/rsif.2007.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roesch A, Schmidbauer H. 2014 WaveletComp: Computational Wavelet Analysis (version 1.0). Available at https://rdrr.io/cran/WaveletComp/. Accessed October 7, 2015.
- 34.Agostinelli C, Lund U. 2013 R package ‘circular’: circular statistics (version 0.4-7). Available at https://r-forge.r-project.org/projects/circular. Accessed December 6, 2016.
- 35.US Department of Commerce, US Census Bureau; Centers of population Available at https://www.census.gov/geo/reference/centersofpop.html. Accessed July 3, 2017.
- 36.Cori A, Ferguson NM, Fraser C, Cauchemez S. A new framework and software to estimate time-varying reproduction numbers during epidemics. Am J Epidemiol. 2013;178:1505–1512. doi: 10.1093/aje/kwt133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grassly NC, et al. New strategies for the elimination of polio from India. Science. 2006;314:1150–1153. doi: 10.1126/science.1130388. [DOI] [PubMed] [Google Scholar]
- 38.Pinheiro J, et al. 2016 nlme: Linear and nonlinear mixed effects models (version 3.1-131). Available at https://cran.r-project.org/web/packages/nlme/index.html. Accessed October 26, 2016.
- 39.Lawrence GJ. The relationship between relative humidity and the dewpoint temperature in moist air: A simple conversion and applications. Bull Am Meteorol Soc. 2005;86:225–233. [Google Scholar]
- 40.Buck AL. New equations for computing vapor pressure and enhancement factor. J Appl Meteorol. 1981;20:1527–1532. [Google Scholar]
- 41.Bolton D. The computation of equivalent potential temperature. Mon Weather Rev. 1980;108:1046–1053. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.