Significance
Antibody blockade of the B7-H1/PD-1 interaction induces regression of advanced human cancers in some patients, while recurrence occurs in others due to tumor escape from T cell attack. Here, we describe a possible mechanism of tumor escape from therapy caused by biased stimulation of T cells to dominant antigen. Based on this finding, we tested a split immunization approach to prevent cancer recurrence in mouse tumor models. These findings may help design approaches for combination cancer immunotherapies.
Keywords: B7-H1, PD-1, dominant antigen, cytolytic T cells, subdominant antigen
Abstract
Induced B7-H1 expression in the tumor microenvironment initiates adaptive resistance, which impairs immune functions and leads to tumor escape from immune destruction. Antibody blockade of the B7-H1/PD-1 interaction overcomes adaptive resistance, leading to regression of advanced human cancers and survival benefits in a significant fraction of patients. In addition to cancer cells, B7-H1 is expressed on dendritic cells (DCs), but its role in DC functions is less understood. DCs can present multiple antigens (Ags) to stimulate dominant or subdominant T cell responses. Here, we show that immunization with multiple tumor Ag-loaded DCs, in the absence of B7-H1, vastly enhances cytotoxic T lymphocyte (CTL) responses to dominant Ag. In sharp contrast, CTL responses to subdominant Ag were paradoxically suppressed, facilitating outgrowth of tumor variants carrying only subdominant Ag. Suppressed CTL responses to subdominant Ag are largely due to the loss of B7-H1–mediated protection of DCs from the lysis of CTL against dominant Ag. Therefore, B7-H1 expression on DCs may help maintain the diversity of CTL responses to multiple tumor Ags. Interestingly, a split immunization approach, which presents dominant and subdominant Ags with different DCs, promoted CTL responses to all Ags and prevented tumor escape in murine tumor models. These findings have implications for the design of future combination cancer immunotherapies.
Polyclonal T cell responses against a broad spectrum of antigens (Ag) and Ag epitopes are desirable, as they may prevent the emergence of bacterial and viral/tumor variants. However, T cell clones do not respond to Ag stimulation equally, and a hierarchy of T cell responses to diverse antigenic epitopes is naturally present; only a few T cell clones are favorably activated and expand during normal T cell responses to multiple Ags, a phenomenon called “immune dominance.” Epitopes that elicit strong T cell responses are termed “dominant epitopes,” and less-favored epitopes are called “subdominant” (1, 2). Multiple mechanisms generate immune dominance. The affinity of T cell epitopes to cognate MHC molecules is critical, as high-affinity epitopes may out-compete MHC binding, leading to preferential presentation. The life span of MHC–peptide complexes, T cell competition for dendritic cells (DCs), the availability of T cell epitopes or repertoires, and the presence of regulatory T cells all contribute to the dominance of a particular T cell epitope/clone (1, 2). Costimulation by B7-1/CD28 and 4-1BB promotes stimulatory capabilities of subdominant epitopes and alters the composition of T cell repertoires (3, 4). Owing to genetic instability and rapid growth, tumor cells display heterogenic tumor Ags. Therefore, a dominant T cell response may be less favorable because it eliminates only a portion of tumor cells and allows the emergence of tumor variants, a potential cause of cancer recurrence (5, 6).
B7-H1 (PD-L1, CD274) and its counterreceptor PD-1 play an important role in the control of T cell responses and the attributes of tumor immunity (7–9). B7-H1 is an inducible cell-surface protein in the B7-CD28 family (10, 11). Upon interacting with PD-1, B7-H1 delivers a negative signal and inhibits T cell responses (10, 12). B7-H1 is mainly induced by IFN-γ, which is mainly released by infiltrating effector T cells in the tumor microenvironment and impairs T cell responses to tumors, a mechanism called “adaptive resistance” (8, 13–16). mAb-mediated blockade of the B7-H1/PD-1 interaction enhances T cell responses to cancer Ags and suppresses tumor growth in both mouse models and human T cell–tumor coculture systems (13–15). In addition to being a ligand, B7-H1 also acts as an antiapoptotic receptor on tumor cells and is required for resistance to CTL-mediated lysis (17). These early findings established the foundation for the subsequent development of antibody-mediated B7-H1/PD-1 blockade therapy (anti-PD therapy) in human cancers. Anti-PD therapy is now FDA-approved for ≥10 different advanced human cancers, including melanoma, lung cancer, kidney cancer, bladder cancer, head and neck cancer, gastric cancer, liver cancer, and Hodgkin’s lymphoma (8, 9).
While the B7-H1/PD-1 interaction suppresses effector T cell function, its role in activating naive T cells is less understood. As on other host cells, B7-H1 can be induced on DCs and macrophages (10). Myeloid-derived DCs from ovarian cancer patients stimulate T cells to produce more IFN-γ and IL-2 and less IL-10 during B7-H1 blockade (18), and B7-H1 on DCs also induces and maintains T cell anergy in vitro (19). Although antigen-presenting cells (APCs) from the lymphoid organs of naive mice express negligible amounts of B7-H1, its expression can be up-regulated quickly in lymphoid organs before activated T cells exit, leading to a partial suppression of T cell responses (20). These studies support a possible negative-regulatory role for B7-H1 on APCs during the priming of T cell responses to Ag. During tumor progression, multiple Ags are continuously released and presented by APCs to multiple T cell clones. It is unknown whether the B7-H1/PD-1 pathway affects immunodominance and, if so, what its role is in the emergence of tumor variants; these are the questions we address in this study.
Results
B7-H1 Has Distinct Effects on CTL in Response to Dominant vs. Subdominant Ag.
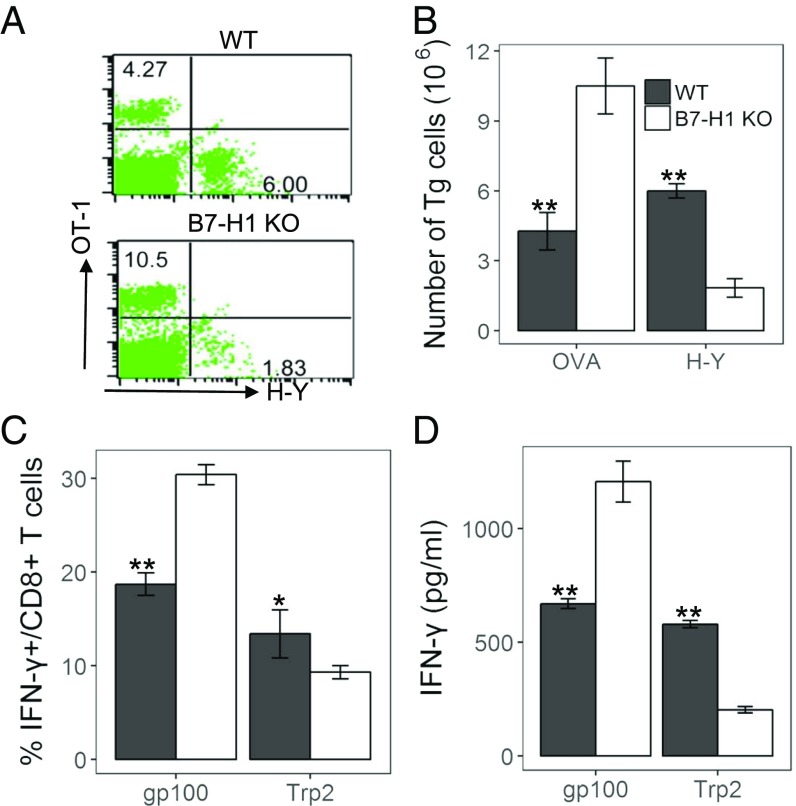
We tested the effects of B7-H1 deficiency on cytotoxic T lymphocyte (CTL) responses to hierarchical Ags using the chicken ovalbumin (OVA) epitope OVA257–264 (SIINFEKL, H-2Kb), which is dominant over male histocompatibility Ag H-Y738–746 (KCSRNRQYL, H-2Db) (21). In this model, naive OT-1 or H-Y T cell clones were purified from respective transgenic (Tg) mice and transferred into C57BL/6 (B6) mice. Mice were subsequently immunized with bone marrow (BM)-derived DCs purified from WT or B7-H1–KO male/OVA-Tg mice. Five days later, T cells were harvested and enumerated by OT-1 tetramer and anti–H-Y T cell receptor (TCR) mAb staining. Immunization with B7-H1–KO/OVA-Tg DCs significantly enhanced proliferation of OT-1 CTLs versus WT/OVA-Tg DCs (KO =10.5% vs. WT = 4.27%). In contrast, immunization with B7-H1–KO/OVA-Tg DCs significantly reduced H-Y CTLs (KO =1.83% vs. WT = 6%) (Fig. 1 A and B). We examined endogenous CTL responses against OVA and H-Y peptides by immunizing mice with DCs without T cell transfer. Similarly, B7-H1–KO/OVA-Tg DC immunization produced more IFN-γ+CD8+ CTLs to OVA peptide but fewer IFN-γ+CD8+ CTLs to H-Y, as shown via intracellular staining (Fig. S1A) and further validated using ELISA of IFN-γ (Fig. S1B). Our results suggest that B7-H1 on DCs has distinct effects on CTL responses to different Ag by promoting subdominant CTLs while suppressing dominant CTL responses.
Fig. 1.
B7-H1 on DCs has an opposite effect on T cell responses to dominant and subdominant Ags. (A and B) Naive OT-1 and H-Y T cells were transferred into mice and immunized with DCs purified from WT or B7-H1–KO male/OVA-Tg mice. After 5 d, spleen cells were analyzed by flow cytometry. (A and B) Representative plots (A) and absolute number (B) of OT-1 and H-Y T cells in spleens. (C and D) B6 mice were immunized s.c. with gp100 and TRP2 coloaded WT or B7-H1–KO DCs. At day 8, spleen cells were restimulated with the peptide in vitro. (C) IFN-γ+CD8+ T cells were counted. (D) Concentrations of IFN-γ in cultured supernatants detected by ELISA. Results represent at least three experiments unless otherwise stated. *P < 0.05 and **P < 0.01, determined by Student’s t test. Error bars indicate SD.
To validate this unexpected finding, we tested an additional Ag system with a pair of peptides: gp10025–33 (KVPRNQDWL, H-2Db) and TRP2181–188 (VYDFFVWL, H-2Kb). Previous studies showed that gp100 peptide is dominant to TRP2 peptide for inducing CTL responses (22–24). B6 mice were immunized with BM-derived DCs from either WT or B7-H1–KO mice loaded with equal concentrations of gp100 and TRP2 peptides. Spleen cells were harvested after 7 d to examine CTL responses. Mice immunized with gp100 and TRP2 coloaded with B7-H1–KO DCs had elevated IFN-γ+CD8+ T cells to gp100 peptide versus WT DC peptides (from 18.7 to 30.4%), while IFN-γ+CD8+ T cells to TRP2 peptides had decreased levels (from 13.7 to 9.7%) (Fig. 1C). A similar result was also obtained via IFN-γ ELISA (Fig. 1D). The effect of B7-H1–blocking mAbs on CTL responses to different Ags upon DC immunization was also tested. Mice were immunized by WT DCs pulsed with gp100 and TRP2 as described previously and were treated with a B7-H1 mAb (clone 10B5) (13–15). The proliferation of T cells in response to gp100 was significantly enhanced by 10B5, whereas the response to TRP2 was significantly reduced (Fig. S1C), a result similar to immunization with B7-H1–KO DCs. Collectively, our findings show that B7-H1 has distinct effects on CTL responses to dominant vs. subdominant Ags.
B7-H1 Protects DCs from CTL-Mediated Lysis.
To explore the mechanism underlying this differential effect, we examined B7-H1 expression on DCs before and after immunization. No significant changes in B7-H1 expression were found (Fig. S2). Next, we excluded several possibilities, including differential expression of H-2 alleles on WT vs. B7-H1–KO DCs, peptide competition for H-2 alleles, and a possible role for regulatory T cells. Flow cytometry analysis revealed similar levels of H-2Db and H-2Kb on WT and B7-H1–KO DCs (Fig. S3). Dominant and subdominant epitopes were presented in different H-2 alleles, and therefore peptide competition for access to the same H-2 alleles could be excluded. The influence of regulatory T cells was also ruled out, because there was no difference in regulatory T cells from mice immunized with WT or B7-H1–KO DCs (Fig. S4). Gene-expression profiling via RNA sequencing showed that WT and B7-H1–KO DCs did not have major differences in gene expression (Fig. S5).
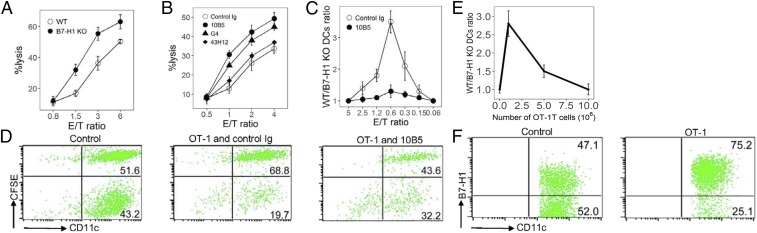
Specific CTLs eliminated APCs which presented cognate Ags after the initial priming event (25). Previously, we reported that B7-H1 protected tumor cells from lysis using tumor-specific CD8+ CTLs (17). To test whether B7-H1 protects DCs from CTL lysis to allow better stimulation of subdominant CTLs, we first determined the susceptibility of OVA-loaded DCs to OT-1 CTL lysis in a carboxyfluorescein succinimidyl ester (CFSE)-DAPI killing assay in vitro. WT DCs were consistently 15–25% less sensitive than B7-H1–KO DCs to CTL lysis at various effector cell-to-target cell (E/T) ratios in many experiments; a representative result is presented in Fig. 2A. Similar results were also obtained using gp100-specific CTLs against WT or B7-H1–KO DCs loaded with gp100 peptides (Fig. S6, Left). B7-H1–mediated resistance is not restricted to peptide-specific CTLs, because allo-CTL (H-2Kd) lysed significantly more B7-H1–KO DCs than WT DCs (H-2Kb) in a CFSE-DAPI killing assay (Fig. S6, Right). The inclusion of an anti-murine B7-H1 mAb (clone 10B5) or an anti-murine PD-1 mAb (clone G4) eliminated the resistance of WT DCs to CTL lysis while control Ig did not (Fig. 2B). Our results indicate a role for the B7-H1/PD-1 axis, because both 10B5 and G4 mAbs block the binding of B7-H1 to PD-1 (14, 15). 43H12, an anti-murine B7-H1 mAb which blocks B7-H1/B7-1 but not the B7-H1/PD-1 interaction (26), did not affect CTL lysis, excluding an effect on this axis. Our findings indicate that B7-H1 expression could protect DCs from CTL lysis and this protection is dependent on the B7-H1/PD-1 interaction.
Fig. 2.
B7-H1–KO DCs are more susceptible to CTL lysis. (A) WT or B7-H1–KO DCs were loaded with 3 μM OVA peptides, CFSE-labeled, and cocultured with activated OT-1 cells at the indicated E/T ratio for 4 h. Cells were stained with DAPI and analyzed by flow cytometry. (B) WT DCs were cocultured with OT-1 cells in 10 µg/mL 10B5, G4, 43H12, or control Ig. (C and D) Ratios of WT/B7-H1–KO DCs (C) and representative plots (at E/T = 1:2) (D) after OT-1 cells were incubated with an equal mixture of OVA peptide-pulsed WT DCs (with CFSE) or B7-H1–KO DCs (without CFSE) with 10B5 or control Ig for 4 h in vitro. (E and F) activated OT-1 cells were transferred into mice with equal OVA peptide-pulsed WT and B7-H1–KO DCs. Twenty-four hours later, cells were collected, and DCs were counted by staining with mAbs to CD11c and B7-H1. Results are shown as ratios of WT/B7-H1–KO DCs (E) and representative plots of DCs with the transfer of 1 × 106 OT-1 cells (F) in ascetic fluid.
When Ag-loaded WT and B7-H1–KO DCs were mixed together and exposed to CTLs in vitro, KO DCs were preferentially lysed. OVA peptide-loaded WT and B7-H1 WT DCs were labeled with or without CFSE, mixed in equal numbers, and cocultured with activated OT-1 T cells for 4 h in a range of E/T ratios (1.2–0.3), the number of WT DCs remained significantly higher than the number of B7-H1–KO DCs (Fig. 2C). The data for the E/T ratio = 0.6 are shown in Fig. 2D. When DCs without OVA served as the control, the number of WT DCs was similar to the number of B7-H1–KO DCs (Fig. 2D, control), indicating that recognition of Ag is required. Inclusion of 10B5 in the culture largely decreased the number of WT DCs in the coculture (Fig. 2D, OT-1 and 10B5), supporting a role for B7-H1 in the protection of DCs from CTL lysis. Finally, we examined the effect of B7-H1 on DC apoptosis after exposure to CTL. Purified DCs from WT or B7-H1–KO mice were pulsed with gp100 peptides and cocultured with activated gp100-specific TCR Tg CTLs for 4 h. As expected, B7-H1–KO DCs had significantly more apoptosis than WT DCs as shown by increased annexin V staining (Fig. S7). These results indicate that a lack of B7-H1 causes CTL-mediated DC apoptosis.
We next evaluated the role of B7-H1 in DC survival in vivo. B6 mice were injected with OT-1 cells and subsequently injected i.p. with an equal mixture of OVA-pulsed WT and B7-H1–KO DCs. Cells were collected from the peritoneal cavity after 24 h, and DCs were enumerated by B7-H1 and CD11c mAb staining. Transfer of 106 OT-1 CTLs significantly decreased B7-H1–KO DCs (Fig. 2E), as seen by a significant increase in the WT DC/B7-H1–KO DC ratio (Fig. 2F). Together, our results indicate that B7-H1 expression confers DC resistance to CTL-mediated lysis in vitro and in vivo.
Accelerated Elimination of B7-H1–Deficient DCs by Dominant CTLs Prevents Priming of Subdominant CTLs.
Rapid elimination of B7-H1− DCs by activated CTLs may prevent subsequent priming of T cell clones which need more time to be stimulated. Thus, it is possible that dominant T cells may recognize and lyse DCs to prevent subsequent priming of subdominant T cells, which may take longer to be activated than dominant T cells (25). Therefore, a time lag in the priming of dominant vs. subdominant T cells may explain our observations. To test this possibility, first we compared the kinetics of OT-1 and H-Y T cell division in vitro. Naive T cells were labeled with CFSE and stimulated with OVA or H-Y peptide-pulsed WT DCs, respectively. After 4 h, these T cells were collected using microbeads and transferred to a second well without DCs. On day 4, all OT-1 T cells had proliferated and CFSE was diluted, whereas most H-Y T cells had not yet divided (Fig. S10A, Upper). The H-Y T cells, however, were not intrinsically defective in proliferation, because when OT-1 and H-Y T cells were stimulated with anti-CD3 mAb, both proceeded similarly through six to seven rounds of division on day 4 (Fig. S10A, Lower). We also tested division kinetics of dominant vs. subdominant T cells in vivo. Mice were first injected with CFSE-labeled Thy1.1 naive T cells and subsequently were immunized with gp100- or TRP2-pulsed DC2.4 (27). At different time points, the division of T cells in the draining lymph nodes (LNs) was analyzed using mAbs to Thy1.1/CD8 and CFSE dilutions. Thy1.1+CD8+T cells from gp100/DC-immunized mice had detectable CFSE dilutions on day 2, whereas T cells from TRP2/DC-immunized mice started proliferation on day 3 with a 1-d lag (Fig. S10B). Our results suggest that subdominant T cells take longer to become activated than dominant T cells in vitro and in vivo.
With a time lag for subdominant T cell activation, dominant T cells may lyse DCs and make Ag unavailable for subdominant T cells. DCs’ increased susceptibility to CTL lysis in the absence of B7-H1 would accelerate this process. Then, we examined whether the presence of activated T cells prevents subsequent priming of newly arrived T cells in vivo. After activation of gp100-specific T cells by immunization with WT or B7-H1–KO DC-gp100 peptides for 5 d, mice were injected with CFSE-labeled Thy1.1 naive T cells from gp100 TCR Tg mice. T cell activation and division from draining LNs was assayed by detecting CFSE dilutions. As a control, CFSE-labeled naive gp100 Tg T cells were transferred at the time of DC immunization. In the B7-H1–KO DC-immunized mice, 66.5% of transferred T cells underwent division, at a significantly greater rate than the 50.8% in the WT DC-immunization group. indicating that the absence of B7-H1 on DCs enhanced the initial T cell response to gp100 Ag. In contrast, when new, naive T cells were transferred on day 5, only 33.8% of the T cells divided in the B7-H1–KO DC-immunized mice, significantly less than the 41.7% in the WT DC-immunized mice (Fig. S10 C and D). Therefore, our results indicate that immunization with Ag-pulsed B7-H1–KO DCs, although stimulating a strong initial T cell response, prevents the activation of newly recruited T cells due to an accelerated loss of DCs.
B7-H1 Ablation Leads to Accelerated Elimination of Tumors That Carry Dominant Ag but to the Emergence of Tumors That Carry Subdominant Ag.
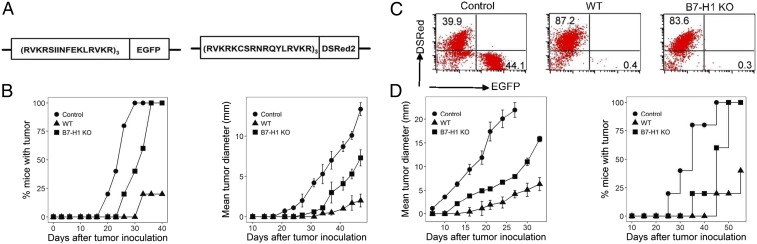
Advanced human cancers are highly heterogenic in their Ag expression due to genetic instability. An unparalleled expansion of the T cell repertoire in the absence of B7-H1 may shape a focused T cell response to dominant Ag and spare tumor cells expressing only subdominant Ag. We developed a tumor model to monitor emerging tumor variants in the absence of B7-H1. EL4 tumor cells were transfected to express fusion minigenes encoding either OVA peptide/EGFP (green fluorescence) or H-Y peptide/DSRed (red fluorescence) (Fig. 3A). B6 mice in groups of five were injected with OT-1 and H-Y T cells and subsequently were immunized with purified DCs from male WT/OVA-Tg or male B7-H1–KO/OVA-Tg mice as described above. Seven days after the final immunizations, mice were inoculated intradermally (i.d.) by an equal mixture of EL4-OVA/EGFP and EL4-HY/DSRed tumor cells to determine if differential elimination of these tumors occurs. OVA Tg DCs from WT mice effectively prevented EL4 tumor outgrowth in 80% of mice (8/10) (Fig. 3B, Left); the remaining two mice had significantly smaller tumors (Fig. 3B, Right). In contrast, immunizing with B7-H1–KO/OVA-Tg DCs was less effective than with WT DCs; all tumors eventually grew, albeit tumor growth was delayed compared with PBS-treated controls (Fig. 3B, Left). Tumors were resected from each group at day 50; cells were pooled and analyzed for EGFP and DSRed. In the control, unimmunized mice, the ratio of EL4-OVA/EGFP and EL4-HY/DSRed tumor cells was comparable (44.1% vs. 39.9%), indicating an equal growth rate and/or elimination of EL4-OVA/EGFP and EL4-HY/DSRed tumor cells. In mice immunized with male WT OVA Tg DCs, 8 of 10 mice did not develop palpable tumors, indicating both EL4-OVA/EGFP and EL4-HY/DSRed tumor cells were eliminated. In the remaining two tumors, EL4-HY/DSRed cells comprised 87.2%, while EL4-OVA/EGFP cells were 0.4%. This result indicates that EL4 cells carrying the subdominant H-Y epitope could escape T cell immunity in a small fraction of mice. Importantly, tumors from mice immunized with male B7-H1–KO/OVA-Tg DCs were also predominantly comprised of EL4- HY/DSRed cells (Fig. 3C). In addition to evaluating tumor composition at day 50, we repeated the experiment and examined tumor composition at 12 d and 30 d after inoculation, respectively. In the control group without DC immunization, EL4-OVA/EGFP and EL4-HY/DSRed tumor cells stayed comparable at both day 12 and day 30. In mice immunized with WT DCs, the percent of EL4-HY/DSRed cells increased from 64.8% at day 12 to 76.3% at day 30. However, tumors from mice immunized with B7-H1–KO DCs were predominantly comprised of EL4-HY/DSRed cells from day 12 (Fig. S8). T cells were obtained from spleens at day 8 and were stained for Fas and granzyme B expression. OT-1 T cells from the B7-H1–KO DC-immunized mice had higher levels of Fas and granzyme B than OT-1 cells from WT DC-immunized mice. In contrast, H-Y T cells from B7-H1–KO DC-immunized mice had lower levels of Fas and granzyme B compared with H-Y T cells from WT DC-immunized mice (Fig. S9).
Fig. 3.
DC immunization in the absence of B7-H1 eliminated tumors carrying dominant Ag but encouraged the emergence of tumors carrying subdominant Ag. (A) Schematic representation of vector design for pEGFP-N1 with the insertion of a minigene encoding OVA peptide (Left) and pDSRed-N1 with the insertion of a minigene encoding H-Y peptide (Right). (B) B6 mice were immunized twice at days −14 and −7 with BM-derived DCs from WT or B7-H1–KO male/OVA-Tg mice. Mice were challenged i.d. with an equal mixture of 5 × 106 EL4-OVA/EGFP and EL4-HY/DSRed. Tumor growth is presented as a percentage of incidence (Left) and as mean tumor diameter (Right). (C) Tumors were surgically excised at day 50, and cells were analyzed by flow cytometry for EGFP and DSRed. (D) B6 mice were immunized s.c. with WT or B7-H1–KO DCs coloaded with gp100 and TRP2 and were inoculated i.d. with B16 cells. Mean tumor diameters (Left) and the mortality (Right) were recorded.
We validated these findings in another mouse model of B16 melanoma that naturally expresses both gp100 and TRP2 Ag. B6 mice were immunized with WT or B7-H1–KO BM-derived DCs loaded with equal concentrations of gp100 and TRP2 peptides. Seven days later, mice were challenged by i.d. inoculation of B16 melanoma cells. As expected, mice without immunization rapidly developed progressively growing tumors, while those immunized with WT DC peptides significantly suppressed tumor growth. Mice immunized with B7-H1–KO DC peptides initially developed smaller tumor nodules than control but eventually developed progressively growing tumors (Fig. 3D). Collectively, our findings indicate that loss of B7-H1 on DCs decreases T cell responses to subdominant Ag and results in accelerated growth of tumors with subdominant Ag.
A Split Immunization Strategy to Prevent Tumor Variant Escape.
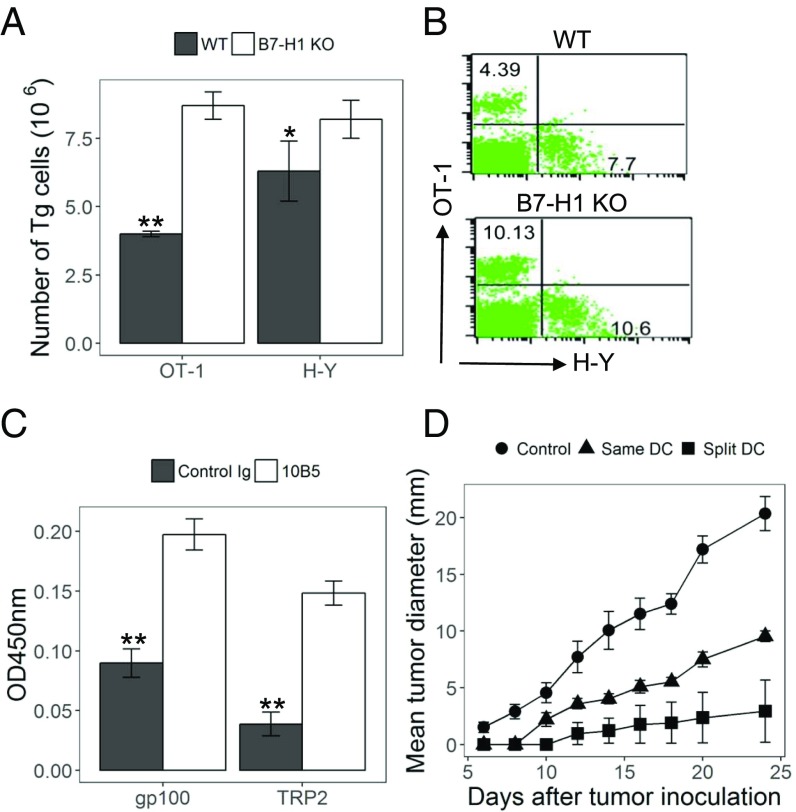
To prevent “premature” loss of DCs presenting subdominant Ag, we tested a split immunization strategy in which dominant and subdominant Ags were loaded onto different DCs and mixed together before immunization. Mice were first injected with OT-1 and H-Y T cells and immunized by an equal mixture of two separate sets of DCs, one from female OVA Tg mice (presenting dominant OVA Ag) and the other from male mice (presenting subdominant H-Y Ag). DCs from these strains on a B7-H1–KO background were also compared. As predicted, immunizations with separately loaded B7-H1–KO DCs stimulated the expansion of both subdominant H-Y T cells and dominant OT-1 T cells (Fig. 4 A and B). This contrasted with the suppressed H-Y T cell responses when both OVA and H-Y peptides were presented on the same B7-H1–KO DCs, as shown in Fig. 1. Similar results were obtained when mice were immunized by split loading of gp100 or TRP2 onto two separate sets of DCs and subsequently were treated with 10B5. In this split immunization setting, both dominant gp100 and subdominant TRP2 T cell responses were enhanced by 10B5 treatment, as indicated by CCK8 dye staining (Fig. 4C).
Fig. 4.
Split immunization with B7-H1 blockade prevents the loss of CTL responses to subdominant T cells and induces better tumor immunity. (A and B) Female B6 mice injected with purified naive OT-1 and H-Y cells were immunized with an equal mix of DCs from OVA Tg and WT male mice or with a mix of DCs from these strains on a B7-H1–KO background. Absolute number (A) and representative plots (B) of OT-1 and H-Y in spleens were analyzed at day 5. (C) Mice were immunized with an equal mix of WT BM DCs with gp100 or TRP2 peptides. Mice received 10B5 or control Ig i.p. concurrently with immunization. Spleen cells were obtained 8 d later and were restimulated with gp100 or TRP2 peptides for 72 h. Cell proliferation was shown by CCK8 dye staining. (D) Mice were immunized with B7-H1–KO DCs at day −7 and inoculated i.d. with B16 cells at day 0. DCs were pulsed with peptides in two ways: (i) DCs were loaded with a mix of 3 µM of gp100 and 3 µM of TRP2 peptides; (ii) DCs were divided into two tubes and pulsed separately with gp100 or TRP2 peptides (split DCs). After washing, DCs were mixed together for immunization. Tumor size is presented as the mean of tumor diameters.*P < 0.05 and **P < 0.01, determined by Student’s t test. Error bars indicate SD.
Finally, we tested this split immunization strategy in the B16 melanoma model. Mice were immunized with B7-H1–KO DCs, which were separately loaded with gp100 or TRP2 peptides. B7-H1–KO DCs were also loaded with both gp100 and TRP2 as the control. Seven days later mice were challenged with B16, and tumor sizes were monitored regularly. Mice with split immunization developed significantly smaller tumors than mice immunized by coloaded DCs (Fig. 4D), indicating that a potent immunity was generated by this strategy. Our results thus support the use of the split immunization strategy to enhance immunity and prevent tumor escape.
Discussion
Here, we present an unexpected finding that blockade of B7-H1 on DCs impairs T cell responses to subdominant Ag despite enhanced responses to dominant Ag. This effect impairs long-term control of tumor variants that carry subdominant Ag in our model. Exploiting this mechanism, we demonstrate that this paradoxical effect is at least partially explained by B7-H1–mediated protection from APC cytolysis, which uses dominant Ag to recognize T cells. Dominant T cells generally have faster responses than subdominant T cells to Ag stimulation; therefore, the B7-H1 blockade allows rapid expansion and activation of dominant responses, which would subsequently eliminate APCs and prevent activation of subdominant T cells. Based on these findings, we designed a split immunization strategy where these two types of Ag were presented by different APCs. In this setting, the effect of the B7-H1 blockade is maximized due to reinforcement of CTL responses to both dominant and subdominant Ag which prevent escape of tumor variants. These findings may explain the mechanism behind tumor recurrence in anti-PD therapy and help develop better strategies for future combination cancer immunotherapies.
Our findings uncover multifaceted physiological roles for B7-H1 as a controller of polyclonal T cell responses to Ag. First, B7-H1 on APCs suppresses fast-acting dominant T cells to restrain their responses to Ag. This effect may act via PD-1 to transmit inhibitory signals to T cells. While ample evidence indicates that anti-PD therapy acts largely to prevent interactions of tumor-associated B7-H1 and PD-1 on effector T cells, it is also evident that the B7-H1/PD-1 pathway plays a role in APC–T cell interactions which may occur in both lymphoid organs (18, 28) and the tumor microenvironment. Second, B7-H1 expression on APCs may facilitate the activation of slow-proliferating subdominant T cells. This effect is likely due to B7-H1 as a surviving receptor that protects APCs from CTL lysis. Arrays of tumor Ags are naturally presented by professional APCs to T cells; B7-H1 on APCs may thus shift the clonal composition of polyclonal T cell responses to these Ags.
Consistent with our findings, recent clinical studies suggest that anti-PD therapy may lead to a more focused T cell repertoire in cancer patients who respond to this treatment. Nakamura and coworkers (29) reported that diversity in the TCR-β repertoire in melanoma-infiltrating T cells had a tendency to decrease in responders compared with nonresponders after anti–PD-1 treatment. Riaz et al. (30) showed that anti–PD-1 mAb nivolumab treatment led to a more skewed TCR repertoire in melanoma biopsy specimens from patients who responded to this therapy. However, we had yet to determine if the focused TCR repertoire was associated with a loss in TCR recognizing subdominant Ags and subsequent recurrence of tumors, which we predict from our study. A recent mouse model study using viral SV40 large T Ag, however, showed that anti-PD therapy could promote epitope spreading by preventing fratricidal death of subdominant T cells (31). This finding is different from our results because we did not observe significant death of T cells recognizing dominant and subdominant Ags in our models. As mentioned previously, it is difficult now to identify dominant and subdominant Ags in cancer patients because multiple factors may determine the dominance of tumor Ags. Our study also may shed some light on a clinical observation in a small fraction of cancer patients that develop hyper-progression of disease after anti-PD therapy (32). It is tempting to speculate that the emergence of rapidly growing tumor variants during anti-PD therapy may be responsible for this observation. However, further analysis of recurrent tumors should provide valuable information in this regard. These clinical studies support our findings, and tumor models will be highly clinically relevant and provide insight for future studies.
Anti-PD therapy is broadly applied to treat advanced human cancers with significant survival benefits, but a fraction of patients who initially respond to anti-PD therapy develop acquired resistance (8, 9, 17, 33). Advanced human cancers are recognized as highly heterogenic, both genetically and immunologically, due to genetic instability, immunological selection, and editing (34, 35). Acquired resistance to anti-PD therapy may develop due to the emergence of tumor cells that have lost dominant Ags. Importantly, anti-PD therapy is the basis for combinatorial therapies with standard care (9). Anti-PD therapy is a new class of immunotherapy working mainly by preventing T cell dysfunction at the tumor site (7–9, 13, 16), and it also could enhance T cell priming in lymphoid organs (20). Our findings reveal an unexpected role for anti-PD therapy in modulating the composition of polyclonal T cell responses, and this unique feature warrants a careful design of combination therapies with tumor Ag-based immunization. This study supports a split immunization approach to prevent the emergence of dominant Ag-loss variants and to avoid cancer recurrence in the future.
Methods
Mice and Cell Lines.
C57BL/6 and BALB/c mice at age 4–6 wk were purchased from Jackson Laboratories or the Experimental Animal Center of Sun Yat-Sen University (Guangzhou, China). OT-1, H-Y, OVA, gp100 TCR Tg mice, and Thy1.1 and CD45.1xB6 mice were purchased from Jackson Laboratories (SI Methods). These strains were maintained in pathogen-free facilities at Sun Yat-Sen University, Johns Hopkins University, and Fujian Medical University. Experiments were conducted in accordance with NIH guidelines and were approved by the Institutional Animal Care and Use Committees of the respective universities.
Peptides and Antibodies.
OVA257–264, H-Y738–746, human gp10025–33, and murine TRP2181–188 peptides were synthesized by Life Technologies. For the mAbs used for analysis, see SI Methods. Fluorescence was detected by flow cytometry (BD FACSVerse) and analyzed using Cell Quest software (BD Biosciences).
DC Immunization and T Cell Transfer.
DCs were isolated from female or male (H-Y+) OVA Tg mice for immunization. Alternatively, gp100 and TRP2 or OVA and H-Y peptides were loaded on DCs in vitro. DCs were injected s.c., 100 µL per mouse. Antibodies were given i.p. at 200 µg per mouse. For the T cell transfer, 1 × 106 OT-1 and 5 × 106 H-Y T cells from the spleens and LNs of Tg mice were i.v. injected at 200 µL per mouse 1 d before the DC immunizations (SI Methods).
Murine Tumor Models.
Mice were immunized with DCs at weekly intervals. Seven days after final immunizations, tumor cells received i.d. injections. For the EL4 model, an equal mixture of 5 × 106 EL4-OVA/EGFP and 5 × 106 EL4-HY/DSRed cells were injected. For B16 melanoma, 3 × 105 cells (100 µL per mouse) were injected. Tumor growth was monitored. EL4 tumors were removed at day 12, 30, and 50; single-cell suspensions were analyzed (SI Methods).
T Cell Activity Assays.
T cell activity assays are described in SI Methods.
Statistical Analysis.
Statistical analysis is described in SI Methods.
Supplementary Material
Acknowledgments
We thank Beth Cadugan for editing; Mr. Sizheng Guo for technical support; and Dr. Weihua Xiao for the DC2.4 line. This study was supported by funds from Fujian Province Department of Science and Technology Research Programs (Grants 2014Y2001 and 2014Y4008); Guangdong Province Innovative Research Program Project (Grant 2011Y035); 863 Project grants to Sun Yat-Sen University; National Natural Science Foundation of China Grant 81702828; NIH Yale Specialized Program of Research Excellence (SPORE) in Skin Cancer Award P50 CA121974; Yale SPORE in Lung Cancer Award P50 CA196530; Yale Comprehensive Cancer Center Award P30 CA16359; and an endowed professorship from United Technologies Corporation.
Footnotes
Conflict of interest statement: Lieping Chen is a consultant/advisory board member/receives consulting fees from MedImmune, Pfizer, NextCure, Vcanbio, and GenomiCare and currently has sponsored research grants from Boehringer Ingelheim, Pfizer, and NextCure.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1722043115/-/DCSupplemental.
References
- 1.Yewdell JW, Bennink JR. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu Rev Immunol. 1999;17:51–88. doi: 10.1146/annurev.immunol.17.1.51. [DOI] [PubMed] [Google Scholar]
- 2.Yewdell JW. Confronting complexity: Real-world immunodominance in antiviral CD8+ T cell responses. Immunity. 2006;25:533–543. doi: 10.1016/j.immuni.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Johnston JV, et al. B7-CD28 costimulation unveils the hierarchy of tumor epitopes recognized by major histocompatibility complex class I-restricted CD8+ cytolytic T lymphocytes. J Exp Med. 1996;183:791–800. doi: 10.1084/jem.183.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halstead ES, Mueller YM, Altman JD, Katsikis PD. In vivo stimulation of CD137 broadens primary antiviral CD8+ T cell responses. Nat Immunol. 2002;3:536–541. doi: 10.1038/ni798. [DOI] [PubMed] [Google Scholar]
- 5.Cheuk E, et al. Human MHC class I transgenic mice deficient for H2 class I expression facilitate identification and characterization of new HLA class I-restricted viral T cell epitopes. J Immunol. 2002;169:5571–5580. doi: 10.4049/jimmunol.169.10.5571. [DOI] [PubMed] [Google Scholar]
- 6.Schreiber H, Wu TH, Nachman J, Kast WM. Immunodominance and tumor escape. Semin Cancer Biol. 2002;12:25–31. doi: 10.1006/scbi.2001.0401. [DOI] [PubMed] [Google Scholar]
- 7.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: Past, present, and future. J Clin Invest. 2015;125:3384–3391. doi: 10.1172/JCI80011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:328rv4. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freeman GJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong H, et al. Tumor-associated B7-H1 promotes T cell apoptosis: A potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 14.Hirano F, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 15.Strome SE, et al. B7-H1 blockade augments adoptive T cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501–6505. [PubMed] [Google Scholar]
- 16.Taube JM, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azuma T, et al. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111:3635–3643. doi: 10.1182/blood-2007-11-123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curiel TJ, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 19.Selenko-Gebauer N, et al. B7-H1 (programmed death-1 ligand) on dendritic cells is involved in the induction and maintenance of T cell anergy. J Immunol. 2003;170:3637–3644. doi: 10.4049/jimmunol.170.7.3637. [DOI] [PubMed] [Google Scholar]
- 20.Tsushima F, et al. Interaction between B7-H1 and PD-1 determines initiation and reversal of T cell anergy. Blood. 2007;110:180–185. doi: 10.1182/blood-2006-11-060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santori FR, Brown SM, Vukmanović S. Genomics-based identification of self-ligands with T cell receptor-specific biological activity. Immunol Rev. 2002;190:146–160. doi: 10.1034/j.1600-065x.2002.19011.x. [DOI] [PubMed] [Google Scholar]
- 22.Overwijk WW, et al. gp100/pmel 17 is a murine tumor rejection antigen: Induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188:277–286. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bloom MB, et al. Identification of tyrosinase-related protein 2 as a tumor rejection antigen for the B16 melanoma. J Exp Med. 1997;185:453–459. doi: 10.1084/jem.185.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowne WB, et al. Coupling and uncoupling of tumor immunity and autoimmunity. J Exp Med. 1999;190:1717–1722. doi: 10.1084/jem.190.11.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hermans IF, Ritchie DS, Yang J, Roberts JM, Ronchese F. CD8+ T cell-dependent elimination of dendritic cells in vivo limits the induction of antitumor immunity. J Immunol. 2000;164:3095–3101. doi: 10.4049/jimmunol.164.6.3095. [DOI] [PubMed] [Google Scholar]
- 26.Park JJ, et al. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T cell tolerance. Blood. 2010;116:1291–1298. doi: 10.1182/blood-2010-01-265975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shotorbani SS, et al. Over-expression of Hlx homeobox gene in DC2.4 dendritic cell enhances its maturation and antigen presentation. Cell Immunol. 2012;275:61–68. doi: 10.1016/j.cellimm.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 28.Perrot I, et al. Dendritic cells infiltrating human non-small cell lung cancer are blocked at immature stage. J Immunol. 2007;178:2763–2769. doi: 10.4049/jimmunol.178.5.2763. [DOI] [PubMed] [Google Scholar]
- 29.Inoue H, et al. Intratumoral expression levels of PD-L1, GZMA, and HLA-A along with oligoclonal T cell expansion associate with response to nivolumab in metastatic melanoma. OncoImmunology. 2016;5:e1204507. doi: 10.1080/2162402X.2016.1204507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riaz N, et al. Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell. 2017;171:934–949.e15. doi: 10.1016/j.cell.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Memarnejadian A, et al. PD-1 blockade promotes epitope spreading in anticancer CD8+ T cell responses by preventing fratricidal death of subdominant clones to relieve immunodomination. J Immunol. 2017;199:3348–3359. doi: 10.4049/jimmunol.1700643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato S, et al. Hyperprogressors after immunotherapy: Analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res. 2017;23:4242–4250. doi: 10.1158/1078-0432.CCR-16-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res. 2013;19:1021–1034. doi: 10.1158/1078-0432.CCR-12-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khong HT, Restifo NP. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat Immunol. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.