Abstract
Background
Malignant melanoma is a class of malignant tumors derived from melanocytes. lncRNAs have been considered as pro-/anti-tumor factors in progression of cancers. The function of lncRNA TUG1 on growth of melanoma was investigated in this study.
Material/Methods
The TUG1 and miR-129-5p expression were examined via qRT-PCR. The protein expression was investigated by Western blotting assay. Luciferase reporter assay was used to assess if lncRNA TUG1 can bind to miR-129-5p and if miR-129-5p can target AEG1 mRNA. CCK-8 and apoptosis assay were used to detect cell growth and apoptosis. The metastasis of melanoma cells was detected by wound-healing and Transwell assays. The effects of TUG1 on growth of melanoma in vivo and cell chemoresistance were investigated via xenograft animal experiment and CCK-8 assay.
Results
The expression of TUG1 and AEG1 was elevated and the miR-129-5p level was decreased in melanoma specimens and cell lines. Downregulation of either TUG1 or AEG1 suppressed cell growth and metastasis. miR-129-5p can bind directly to AEG1 and TUG1 can directly sponge miR-129-5p. Inhibition of TUG1 expression suppressed the expression of Bcl-2, MMP-9, and cyclin D1, and raised the level of cleaved caspase3 by modulating AEG1 level in melanoma cells. Inhibition of TUG1 reduced the growth of tumors in vivo and improved the chemosensitivity of A375 cells to cisplatin and 5-FU.
Conclusions
Reduction of TUG1 level suppressed cell growth and metastasis by regulating AEG1 expression mediated by targeting miR-129-5p. Suppression of lnc TUG1 may be a promising therapeutic strategy in the treatment of malignant melanoma.
MeSH Keywords: Melanoma; MicroRNAs; RNA, Long Noncoding
Background
Malignant melanoma is a malignant tumor originating from melanocytes and has a very poor prognosis [1]. Recent research shows that its incidence has clearly increased [2]. Although remarkable advances have been recently achieved in the treatment of tumors, the prognosis of metastatic melanoma remains unsatisfactory [3]. Accordingly, investigating novel targets is an important challenge in malignant melanoma therapy.
Long noncoding RNAs, a class of transcribed RNA sequence, has more than 200 nucleotides [4]. They seem to be mRNAs in structure, but do not encode proteins [5]. LncRNAs have been considered to exert essential functions in tumorigenesis and procession of a few malignant tumors [6]. Taurine-upregulated gene1 (TUG1) is a transcript upregulated by taurine [7]. A number of reports emphasized that long noncoding RNA TUG1 can enhance the growth and metastasis of pancreatic cancer cells, osteosarcoma cells, and bladder cancer cells [8–10]. However, the effects of TUG1 on melanoma development and the underlying mechanism involved are unknown.
MicroRNAs (miRNAs) are a series of 19–25 nucleotides noncoding RNA molecules [11] and are involved in the initiation and progression of a variety of malignant tumors by binding directly to the targeted mRNA, leading to translation inhibition and/or RNA degradation [12]. Some studies have reported that miR-129-5p level is downregulated in many malignant tumors [13]. Nevertheless, the expression and action of miR-129-5p in malignant melanoma are not clearly understood.
Astrocyte-elevated gene-1 (AEG-1) is encoded on chromosome 8q22 [14], and upregulation of AEG1 was confirmed in various malignant tumors [15,16]. In addition, aberrant AEG1 expression is correlated with a poor clinical prognosis and chemoresistance in patients with tumors [17–19]. Increasing evidence shows that the level of AEG1 is elevated in malignant melanoma, and silencing of AEG1 significantly inhibits the proliferation and metastasis of melanoma cells [20]. These results indicate that AEG1 has an important role in tumorigenesis and progression of malignant melanoma.
In this article, we investigated the TUG1, miR-129-5p, and AEG1 levels in melanoma specimens and cell lines, and analyzed the underlying regulatory mechanism in the development of melanoma. We found that long noncoding RNA TUG1 promotes tumor growth and metastasis through the TUG1/miR-129-5p/AEG1 axis in malignant melanoma.
Material and Methods
Samples and cell lines
A total of 48 patients with melanoma were enrolled. Paired melanoma specimens and adjacent normal specimens were obtained by surgical resection from Hubei provincial Hospital of Traditional Chinese Medicine. The tumor tissues were confirmed as malignant melanoma by at least 2 pathologists and the tissues were stored in liquid nitrogen until use. The Ethics Committee of Hubei provincial Hospital of Traditional Chinese Medicine approved the investigation, and written informed consents were obtained from the enrolled patients. A375, WM35, SK-MEL-5, and SK-MEL-2 cell lines were obtained from the cell bank of Wuhan University, and incubated in DMEM with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco, USA) in an atmosphere of 5% CO2 at 37°C. Human epidermal melanocytes (HEMa-LP) were obtained from Cascade Biologists (UK) and were incubated in HMGS-2 medium in the same condition as in melanoma cell lines.
Plasmid construction and transfection
miR-129-5p mimics, miR-129-5p inhibitors, sh-TUG1, pcDNA-TUG1, sh-AEG1, and pcDNA-AEG1 were purchased from GenePharma (Shanghai, China). PcDNA3.1 was used to generate the corresponding plasma. Transfection to A375 cells was performed with Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s guidelines.
RNA extraction and quantitative real-time PCR
TRIzol reagent was used to extract total RNA (Thermo Fisher Scientific, USA) from tissues and cells. The miR-129-5p cDNA was synthesized using the TaqMan miRNA reverse transcription kit. The mRNA level of miR-129-5p was quantified by qRT-PCR using the TaqMan human MiRNA assay kit. The cDNA synthesis of TUG1 and AEG1 were performed with the SYBR PrimeScript RT-polymerase (TaKaRa), and quantitative PCR was performed with the SYBR PrimeScript RT-PCR kit (TaKaRa). U6 and β-actin were used as the endogenous control. The 2-ΔΔCT method was utilized to measure the relative fold difference. TUG1-F: 5′-CAAGAAACAGCAACACCAGAAG-3′, TUG1-R: 5′-TAAGGTCCCCATTCAAGTCAGT-3′; miR-129-5p-F: 5-GGGGG CTTTTTGCGGTCTGG-3, miR-129-5p-R 5-AGTGCGTGTCG TGGAGTC-3; AEG1-F: 5-AAATAGCCAGCCTATCAAGACTC-3, AEG1-R: 5-TTCAGACTTGGTCTGTGAAGGAG-3; β-actin-F: 5′-TGG ACTTCGAGCAGGAAATGG-3′, β-actin-R: 5′-ACGTCGCACTT CATGATCGAG-3′; U6-F: 5-CTCGCTTCGGCAGCACA-3, U6-R: 5-AACGCTTCACGAATTTGCGT-3.
Western blot analysis
Tumor tissues and cells were washed with PBS and lysed in RIPA buffer, then total proteins were extracted according to the manufacturer’s protocol. A BCA protein kit was used to detect the concentration of proteins. The proteins were separated using 10% SDS-PAGE assay and moved to a PVDF membrane. The membranes were blocked with 5% milk protein in TBST and incubated with primary antibodies at 4°C overnight, followed by incubation with secondary antibody for 1 h at room temperature before being detected by an enhanced chemiluminescence system. The following antibodies as primary antibody were applied to detect the targeting proteins expression: anti-AEG1 (Rabbit 1: 500; MyBioSource, USA), anti-Bcl-2 (1: 1000; Abcam, UK), anti-MMP-9 (1: 1000; Abcam, UK), anti-cleaved caspase 3 (1: 500; Abcam, UK), anti-cyclin D1 (1: 2000; Abcam, UK) and anti-β-actin (1: 2000; Abcam, UK). The corresponding HRP-conjugated antibody (1: 3000; Abcam, UK) was used as the secondary antibody.
CCK-8 assay
Melanoma cell proliferation was examined by CCK-8 assay according to the manufacture’s protocol. After transfection, A375 cells were cultured in 96-well plates with the same conditions. At 12, 24, 48, and 72 h, 90 μl of fresh culture media and 10 μl CCK-8 solution were added to each sample. Subsequently, transfected A375 cells were cultured at 37°C for 2 h, and the value was measured using a microplate reader at 450 nanometers.
Transwell chamber assay
A375 cells were cultured for 48 h after transfection. Transwell assay was performed to determine the cell invasion activity. The Transwell chamber membrane was coated with matrigel. We added 2×105 cells in the upper chamber with serum-free medium. DMEM with 10% FBS was put into the lower chamber. After incubating for 48 h, swabs were used to wipe off the cells in the upper chamber, and cells in lower chamber were fixed and stained with 0.5% crystal violet. Cells counting was performed using a microscope.
Wound-healing assay
After transfection, cells were cultured in appropriate conditions for 48 h. Wounds were created using a plastic scriber to scratch the cell monolayer. Cells were then washed and cultured in DMEM with 10% FBS for 48 h. The migration activity was recorded using a microscope at 0 and 48 h, and determined by subtracting the final wound width from the initial wound width.
Flow cytometry
Cells were cultured and washed with PBS. Next, cells were resuspended in binding buffer and stained with Annexin V-FITC and PI. FACS analysis was performed according to the manufacturer’s guidelines. Cell cycle analysis was performed using propidium iodide cell cycle detection kits (BD Biosciences).
Luciferase assay
The putative binding sequences of TUG1-WT, TUG1-MUT, AEG1-WT, and AEG1-MUT were cloned into the pMIR-REPORT vectors. miR-129-5p mimics, miR-129-5p mimics+pcDNA-control, and miR-129-5p+pcDNA-TUG1 or miR-control were transfected into A375 cells with reporter plasmids using Lipofectamine 2000 (Invitrogen). After 36 h, the luciferase activities were determined using the Dual-Luciferase Reporter Assay System (Promega).
Chemosensitivity assay
At 48 h after transfection, A375 cells were incubated in fresh medium, and then placed into 96-well plates. Various doses of cisplatin or 5-FU were added into the cell culture for 72 h. Next, the cell proliferation was checked using CCK-8 assay.
Tumor growth in vivo
After transfection, A375 cells were transplanted into nude mice (25–30 g, 6 weeks old, n=6), mice were sacrificed on days 5, 10, 15, 20, and 25, and the weight and volume of tumor tissues were measured and recorded.
Statistical analysis
SPSS15.0 was used for statistical analyses. The t test was used to analyze comparisons between 2 groups, multiple comparisons were analyzed by one-way analysis of variance, the Fisher exact probability test was used to analyze the relationship of the lnc TUG1 and the clinical characteristics, and Kaplan-Meier plots were used to evaluate the function of lnc TUG1 in melanoma prognosis. The association between 2 quantitative was assessed by Pearson correlation analyses. Data are represented as mean ±SD, and P<0.05 was regarded as a statistically significant difference.
Results
The expressions of TUG1 and AEG1 were elevated, but the level of miR-129-5p was reduced in melanoma specimens and cell lines
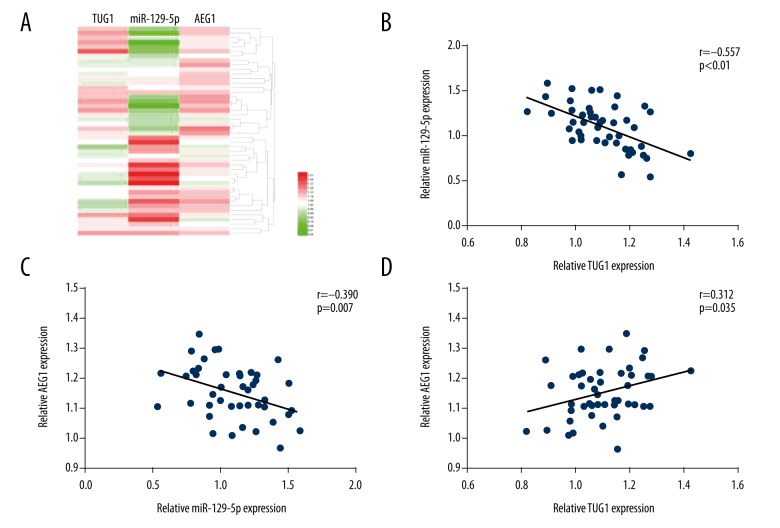
The levels of TUG1, miR-129-5p, and AEG1 were determined by qRT-PCR and Western blot assay in melanoma tissues and cell lines. The results show that the level of TUG1 and AEG1 was elevated compared to paired non-tumor tissues or HEMa-LP cells. Moreover, the miR-129-5p level was remarkably reduced in melanoma tissues and cell lines (Figure 1A–1H). Patients enrolled were divided into a high lnc TUG1 expression group (n=24) and a low lnc TUG1 expression group (n=24) according to mean level of lnc TUG1. There was no significant association between the lnc TUG1 level and age or sex in individuals with melanoma, but the overexpression of lnc TUG1 was obviously correlated with the TNM stage and distant migration of malignant melanoma (Table 1). Furthermore, the high expression of lnc TUG1 was closely related with decreased overall survival (Figure 1I). Pearson correlation analysis was used to confirm the positive association between TUG1 and AEG1. The miR-129-5p level was negatively associated with the level of TUG1 and AEG1 (Figure 2).
Figure 1.
The relative expression of TUG1, miR-129-5p, and AEG1 in melanoma tissues and cell lines. (A, B). The relative TUG1 level was increased in melanoma specimens and cell lines. (C, D). Low expression of miR-129-5p was identified in melanoma tissues and cell lines. (E–H). The protein level of AEG1 was increased in melanoma tissues and cell lines. (I). High TUG1 expression was related with decreased overall survival. * p<0.05 compared with HEMa-LP cells. # p<0.05 compared with non-tumor tissues.
Table 1.
Relationship between the clinical feature and lnc TUG1 level in patients with melanoma.
| Characteristics | No. of patients N=48 |
lnc TUG1 | P value | |
|---|---|---|---|---|
| High expression | Low expression | |||
| Age (years) | ||||
| ≤40 | 21 | 9 | 12 | 0.561 |
| >40 | 27 | 15 | 12 | |
| Sex | ||||
| Male | 23 | 11 | 12 | 1.000 |
| Female | 25 | 13 | 12 | |
| TNM stage | ||||
| I+II | 17 | 2 | 15 | 0.000* |
| III+IV | 31 | 22 | 9 | |
| Distant metastasis | ||||
| No | 18 | 3 | 15 | 0.001* |
| Yes | 30 | 21 | 9 | |
Figure 2.
The relationship among TUG1, miR-129-5p, and AEG1 in melanoma tissues. (A) The heat map shows that the TUG1 and AEG1 levels were negatively related with the level of miR-129-5p. (B–D) Pearson correlation analysis was used to assess the correlations among TUG1, miR-129-5p, and AEG1 in melanoma tissues.
Downregulation of TUG1inhibited cells growth and metastasis, arrested cell cycle, and enhanced cell apoptosis
Sh-TUG1 was transfected into A375 cells for detection of the effect of TUG1 on A375 cells. CCK8 assay showed that downregulation of TUG1 inhibited cell proliferation. Flow cytometry confirmed that low expression of TUG1 promoted cell apoptosis and arrested cell cycle at the G0/G1 phase. Wound healing assay and Transwell chamber assay identified that sh-TUG1 suppressed the migration and invasion of A375 cells (Figures 3, 4). In addition, inhibition of AEG1 expression produced similar results. Importantly, the overexpression of miR-129-5p suppressed cell proliferation, invasion, migration, and induced cell apoptosis (Figure 5).
Figure 3.
The downregulation of TUG1 inhibited the proliferation of A375 cells, promoted A375 cells apoptosis, and arrested cell cycle at the G0/G1 phase. (A) Inhibition of TUG1 reduced the growth of A375 cells. (B, D) Low expression of TUG1 enhanced cell apoptosis. (C, E) Silencing of TUG1 arrested cell cycle at the G0/G1 phase. * p<0.05 compared with NC group and sh-control group.
Figure 4.
Silencing of TUG1 suppressed the metastasis of A375 cells. (A) Inhibition of TUG1 alleviated the invasive ability of A375 cells. (B) Reduced expression of TUG1 depressed the migratory capability of A375 cells. * p<0.05 compared with NC group and sh-control group.
Figure 5.
The effects of miR-129-5p and AEG1 on cell proliferation and metastasis. (A) Upregulation of miR-129-5p enhanced cell apoptosis and suppressed the proliferation and metastasis ability of A375 cells. (B) Silencing of AEG1 promoted A375 cells apoptosis and alleviated the proliferative, invasive, and migratory ability of A375 cells. * p<0.05 compared with NC group and miR-control group. # p<0.05 compared with NC group and sh-control group.
miR-129-5p and AEG1 mediated the function of TUG1 on the growth and metastasis of A375 cells
To further investigate the association among miR-129-5p, AEG1, and TUG1, we transfected sh-control, sh-TUG1, and sh-TUG1+miR-129-5p inhibitors into A375 cells, respectively. The level of miR-129-5p was elevated in the sh-TUG1 group compared with the sh-control group and sh-TUG1+miR-129-5p inhibitors group (Figure 6A). sh-TUG1 suppressed the growth and metastasis of A375 cells, and inducing cell apoptosis, whereas the cotransfection of miR-129-5p inhibitor abrogated the suppressive function of sh-TUG1 on the proliferation and metastasis ability of A375 cells (Figure 7B). Similarly, sh-control, sh-TUG1, and sh-TUG1+ pcDNA-AEG1 were transfected into A375 cells. sh-TUG1 reduced the expression of AEG1 compared with the sh-control group (Figure 6E), the cotransfection of sh-TUG1 with pcDNA-AEG1 partially recovered the expression of AEG1, and alleviated the inhibitory ability of sh-TUG1 on the growth and metastasis of A375 cells in comparison with sh-TUG1 group (Figure 7A). Conversely, the transfection of pcDNA-TUG1 enhanced the growth, invasion, and migration of A375 cells, but sh-AEG1 and miR-129-5p mimics alleviated the promoting function of pcDNA-TUG1 in A375 cells (Figure 7C, 7D). Therefore, the role of TUG1 in growth and metastasis of A375 cells was mediated, in part at least, by miR-129-5p and AEG1.
Figure 6.
The interaction among TUG1, miR-129-5p, and AEG1 in A375 cells. (A, B) Silencing of TUG1 promoted the expression of miR-129-5p, but conversely, upregulation of TUG1 depressed the expression of miR-129-5p. (C) The transfection of miR-129-5p mimics suppressed the expression of AEG1 in A375 cells, but the cotransfection of miR-129-5p mimics and pcDNA-TUG1 reversed the inhibiting function of miR-129-5p on the expression of AEG1 in A375 cells. (D) High expression of TUG1 enhanced AEG1 expression, but the cotransfection of miR-129-5p mimics with pcDNA-TUG1 alleviated the promoting function of pcDNA-TUG1 on the expression of AEG1 in A375 cells. (E) Silencing of TUG1 distinctly inhibited the expression of AEG1, but miR-129-5p inhibitors attenuated the inhibitory function of sh-TUG1 on the expression of AEG1 in A375 cells. * p<0.05 compared with NC group and sh-control group. # p<0.05 compared with sh-TUG1 group. $ p<0.05 compared with NC group and pcDNA-control group. & p<0.05 compared with pcDNA-TUG1 group. @ p<0.05 compared with NC group and miR-control group. % p<0.05 compared with miR-129-5p mimics group.
Figure 7.
miR-129-5p and AEG1 mediated the function of TUG1 on the growth and metastasis of A375 cells. (A) The cotransfection of sh-TUG1 with pcDNA-AEG1 alleviated the inhibitory ability of sh-TUG1 on the proliferation and metastasis of A375 cells in comparison with sh-TUG1 group. (B) The cotransfection of miR-129-5p inhibitor enhanced the proliferation and metastasis activity of A375 cells transfected with sh-TUG1 in comparison with sh-TUG1 group. (C, D) miR-129-5p mimics or sh-AEG1 abrogated the growth, migration, and invasion of A375 cells transfected with pcDNA-TUG1. * p<0.05 compared with sh-control group. # p<0.05 compared with sh-TUG1 group. $ p<0.05 compared with pcDNA-control group. & Compared with pcDNA-TUG1 group
TUG1 directly sponged miR-129-5p in A375 cells
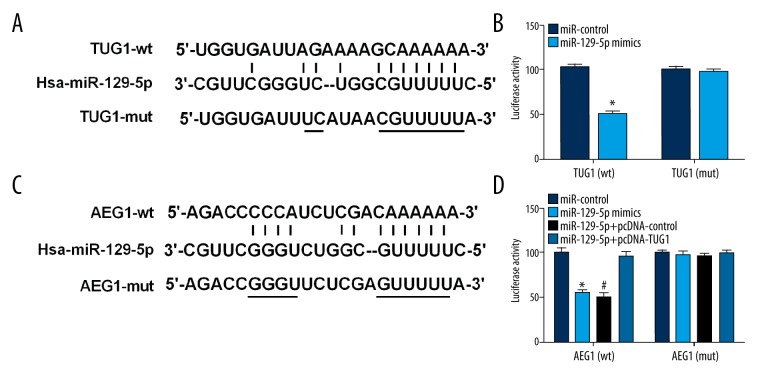
Bioinformatics analysis showed miR-129-5p may be a target of TUG1. Luciferase assay was performed to ascertain whether TUG1 can regulate miR-129-5p expression by acting as a molecular sponge, showing that miR-129-5p dramatically alleviated the luciferase activity of TUG1-wt, but had no significant effect on that of TUG1-mut (Figure 8A, 8B).
Figure 8.
TUG1 regulated the expression of AEG1 via acting as a ceRNA of miR-129-5p. (A) miR-129-5p and its predicted binding sequence for WT in TUG1. (B) miR-129-5p inhibited luciferase reporter activities of TUG1 in wild type, but did not affect that of TUG1 in mutated type. (C) miR-129-5p and its predicted binding sites for WT in AEG1. (D) The luciferase reporter activities were presented in these different conditions. * p<0.05 compared with miR-control group. # p<0.05 compared with miR-129-5p+pcDNA-TUG1 group.
miR-129-5p directly targeted AEG1 in A375 cells
Bioinformatics analysis showed that miR-129-5p can bind directly to 3′-UTR-AEG1. Luciferase assay showed that miR-129-5p obviously suppressed the luciferase activity of AEG1-wt, but did not significantly affect the luciferase expression of AEG1-mut. Furthermore, the cotransfection of miR-129-5p and pcDNA-TUG1 did not have a significant influence on the luciferase activity in AEG1-wt and AEG1-mut (Figure 8C, 8D).
TUG1 can modulate AEG1 expression by targeting miR-129-5p in A375 cells
The transfection of sh-TUG1 or pcDNA-TUG1 regulated the expression of miR-129-5p. The upregulation of TUG1 dramatically decreased the level of miR-129-5p and promoted AEG1 protein expression; however, the downregulation of TUG1 distinctly increased the level of miR-129-5p and inhibited AEG1 protein expression. Meanwhile, the cotransfection of miR-129-5p mimics with pcDNA-TUG1 remarkably abrogated the promotion effect of pcDNA-TUG1 on AEG1 protein expression. The cotransfection of miR-129-5p inhibitors with sh-TUG1 clearly alleviated the inhibitory function of sh-TUG1 on the expression of AEG1 (Figure 6).
TUG1 regulated the expression of proteins related with the proliferation and metastasis in A375 cells
Further studies were performed to investigate the reliable molecular mechanism of TUG1 in the development of malignant melanoma, the expressions of proteins associated with cell proliferation, apoptosis, and metastasis were detected by Western blotting in gain or loss experiments. These data manifested that the high expression of TUG1 distinctly enhanced the expression of Bcl-2, MMP-9, cyclin D1 and inhibited the expression of cleaved caspase 3 in A375 cells, but inhibition of AEG1 alleviated the effect of TUG1 on these protein expression (Figure 9A). Inversely, the low expression of TUG1 reduced the expression of Bcl-2, MMP-9, cyclin D1 and promoted the expression of cleaved caspase 3 in A375 cells, whereas the cotransfection of pcDNA-AEG1 alleviated the effect of sh-TUG1 on the expressions of Bcl-2, MMP-9, cyclin D1and cleaved caspase 3 (Figure 9B).
Figure 9.
The effect of TUG1 on the expression of proteins involved in cell proliferation, cell cycle, and cell apoptosis via regulating the AEG1 level. (A) Upregulation of TUG1 enhanced Bcl-2, MMP-9, and cyclin D1 expression, and depressed the expression of cleaved caspase 3. Sh-AEG1 plasmids reversed the function of pcDNA-TUG1 in these protein expressions. (B) Downregulation of TUG1 inhibited the expression of Bcl-2, MMP-9, and cyclin D1, and elevated the level of cleaved caspase 3, but the function of sh-TUG1 was abrogated by the cotransfection of pcDNA-AEG1 in A375 cells. * p<0.05 compared with pcDNA-control group. # p<0.05 compared with pcDNA-TUG1 group. $ p<0.05 compared with sh-control group. & p<0.05 compared with sh-TUG1 group.
Inhibition of TUG1 suppressed melanoma growth in an animal experiment and improved chemosensitivity in A375 cells
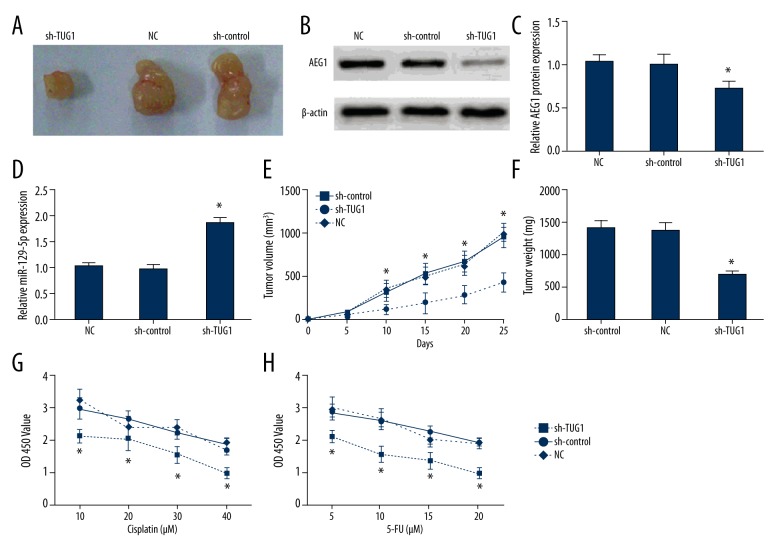
The role of TUG1 in melanoma was identified in nude mice models. The volume of tumors was clearly reduced in the sh-TUG1 group in comparison with those in the normal control group and sh-control group. The final weight of tumors was distinctly decreased in the sh-TUG1 group compared with the normal control group and sh-control group. The reduced AEG1 level and the raised miR-129-5p level were identified in the sh-TUG1 group compared with the NC group and sh-control group by Western blot assay and qRT-PCR. Accordingly, the anti-tumor function of sh-TUG1 on the melanoma growth was identified in vivo, and the transfection of sh-TUG1 reinforced chemosensitivity to cisplatin or 5-FU treatment of A375 cells (Figure 10).
Figure 10.
Inhibition of TUG1 suppressed melanoma growth and improved chemosensitivity in A375 cells. (A) The xenograft tumor tissues were represented in different groups at 25 days after inoculation. (B–D) The expression of AEG1 and miR-129-5p was detected in xenograft tumor tissues by Western blotting and qRT-PCR, respectively. (E) The transfection of sh-TUG1 decreased the tumor volume. (F) The transfection of sh-TUG1 reduced the tumor weight. (G, H) The transfection of sh-TUG1 reinforced chemosensitivity of A375 cells to cisplatin or 5-FU treatment.* p<0.05 compared with sh-control group and NC group.
Discussion
Melanoma is very dangerous malignant tumor, developing from melanin-producing cells called melanocytes [21]. Many reports pointed out that abnormal expression of lncRNAs play vital roles in the development of some malignant tumors and act as oncogene or anti-tumor roles, such as lncRNA MRCCAT1, which strengthened clear cell renal cell carcinoma proliferation and metastasis by suppressing the expression of NPR3 [22]. Upregulation of lnc CASC2 distinctly suppressed the growth of thyroid carcinoma cells [23]. Therefore, it was essential to uncover the specific regulation mechanism of lncRNA in melanoma to discover a useful therapeutic strategy. Dysregulation of TUG1 was found in different tumors; for example, TUG1 enhanced growth and metastasis pancreatic cancer by regulating the EMT pathway [8] and TUG1 promoted the development of osteosarcoma cells by targeting miR-153 [9]. However, some researchers found that TUG1 serves an anti-tumor role in glioma by inducing cell apoptosis [24]. At present, the expression and molecular mechanism of TUG1 in malignant melanoma remains unclear.
In this study, our data show that the level of TUG1 was remarkably increased in melanoma specimens and cell lines, and the TUG1 level was positively correlated with poor prognosis. Inhibition of TUG1 expression suppressed cell growth, arrested cell cycle at G0/G1 stage, and induced cell apoptosis. In addition, further studies showed that the functions of TUG1 on the proliferation and metastasis of A375 cells were mediated by AEG1 and miR-129-5p. These results verified that TUG1 exerted a pro-tumor function via regulating the miR-129-5p and AEG1 levels in melanoma.
Several studies had confirmed that lncRNAs can serve as a sponge to competitively target miRNA and regulate the function of miRNA [25]. Therefore, bioinformatics analysis was applied for detecting downstream targets, and miR-129-5p was considered a probable target of TUG1. Luciferase assay was conducted to further identify the correlation between TUG1 and miR-129-5p. As a result, miR-129-5p significantly inhibited the luciferase activity of TUG1-wt but did not affect that of TUG1-mut. It was confirmed that TUG1 can bind to miR-129-5p and regulate the function of miR-129-5p. Furthermore, bioinformatics analysis suggested that AEG1 may be a binding target of miR-129-5p. Similarly, it was found that miR-129-5p can directly target 3′-UTR-AEG1 by luciferase assay. Functionally, upregulation of TUG1 reduced the level of miR-129-5p and promoted AEG1 expression, but cotransfection of miR-129-5p mimics alleviated the promoting effect of TUG1 on AEG1 expression in A375 cells. Downregulation of TUG1 increased the level of miR-129-5p and decreased AEG1 expression, and the transfection of miR-129-5p mimics inhibited the protein expression of AEG1, but the cotransfection of pcDNA-TUG1 attenuated the suppressive function of miR-129-5p mimics. Together, it was evident that TUG1 regulated the development of malignant melanoma through miR-129-5p/AEG1 axis.
The AEG1 factor is highly overexpressed in many types of human cancers, including ovarian cancer [16], cervical carcinoma [26], hepatocellular carcinoma [27], and breast cancer [15]. AEG1 was found to participate in some important signal pathways, such as the PI3K/AKT signaling pathway [28] and the WNT signaling pathway [29]. Our study showed that the low expression of AEG1 suppressed the proliferation and metastasis of melanoma, which is identical to the results reported by Zhang [20]. To further study the underlying molecular mechanism, we investigated the expression of proteins related with cell proliferation and metastasis, cell cycle, and cell apoptosis. The data showed that upregulation of TUG1 elevated the levels of cyclin D1, MMP-9, and Bcl-2, but inhibited the activation of caspase 3. The cotransfection of sh-AEG1 with pcDNA-TUG1 reversed the outcomes induced by pcDNA-TUG1. On the other hand, downregulation of TUG1 reduced the level of cyclin D1, MMP-9, and Bcl-2, but raised the level of cleaved caspase 3. The pcDNA-AEG1 plasmids abrogated the effect of sh-TUG1 on these protein expressions. In the following experiments, sh-TUG1 was found to distinctly decrease the weight and volume of tumors and reinforced chemosensitivity of A375 cells to cisplatin or 5-FU treatment. The precise molecular mechanism of TUG1 was further explained in the development of melanoma.
Conclusions
The overexpression of TUG1 and AEG1 was identified in melanoma, but the level of miR-129-5p was decreased in melanoma tissues and cells lines. TUG1 and AEG1 can promote cell growth and metastasis, but miR-129-5p induced cell apoptosis and suppressed cell proliferation. TUG1 can competitively sponge miR-129-5p to modulate AEG1 expression. Downregulation of TUG1 inhibited the expression of cyclin D1, MMP-9, and Bcl-2, enhanced the activation of caspase 3 by regulating AEG1 level, strengthened the chemosensitivity of A375 cells to cisplatin or 5-FU treatment, and inhibited tumors growth in vivo. Collectively, these results show that downregulation of TUG1 suppressed cell growth, induced cell apoptosis, arrested cell cycle, and inhibited cell metastasis by regulating miR-129-5p/AEG1 axis in melanoma. This may be a potential new therapeutic strategy in treating malignant melanoma.
Footnotes
Source of support: Departmental sources
References
- 1.Volpe VO, Klufas DM, Hegde U, et al. The new paradigm of systemic therapies for metastatic melanoma. J Am Acad Dermatol. 2017;77(2):356–68. doi: 10.1016/j.jaad.2017.04.1126. [DOI] [PubMed] [Google Scholar]
- 2.Padrik P, Valter A, Valter E, et al. Trends in incidence and survival of cutaneous malignant melanoma in Estonia: A population-based study. Acta Oncol. 2017;56(1):52–58. doi: 10.1080/0284186X.2016.1243804. [DOI] [PubMed] [Google Scholar]
- 3.Svedman FC, Pillas D, Taylor A, et al. Stage-specific survival and recurrence in patients with cutaneous malignant melanoma in Europe – a systematic review of the literature. Clin Epidemiol. 2016;8:109–22. doi: 10.2147/CLEP.S99021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X, Cao Y, Gong X, et al. Long noncoding RNAs in head and neck cancer. Oncotarget. 2017;8:10726–40. doi: 10.18632/oncotarget.12960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McAninch D, Roberts CT, Bianco-Miotto T. Mechanistic insight into long noncoding RNAs and the placenta. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18071371. pii: E1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benetatos L, Voulgaris E, Vartholomatos G. The crosstalk between long noncoding RNAs and PI3K in cancer. Med Oncol. 2017;34(3):39. doi: 10.1007/s12032-017-0897-2. [DOI] [PubMed] [Google Scholar]
- 7.Niu Y, Ma F, Huang W, et al. Long noncoding RNA TUG1 is involved in cell growth and chemoresistance of small cell lung cancer by regulating LIMK2b via EZH2. Mol Cancer. 2017;16(1):5. doi: 10.1186/s12943-016-0575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin CF, Zhao FL. Long noncoding RNA TUG1 can promote proliferation and migration of pancreatic cancer via EMT pathway. Eur Rev Med Pharmacol Sci. 2017;21(10):2377–84. [PubMed] [Google Scholar]
- 9.Wang H, Yu Y, Fan S, et al. Knockdown of long noncoding RNA TUG1 inhibits the proliferation and cellular invasion of osteosarcoma cells by sponging MiR-153. Oncol Res. 2017 doi: 10.3727/096504017X14908298412505. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Q, Liu H, Cheng H, et al. Downregulation of long noncoding RNA TUG1 inhibits proliferation and induces apoptosis through the TUG1/miR-142/ZEB2 axis in bladder cancer cells. Onco Targets Ther. 2017;10:2461–71. doi: 10.2147/OTT.S124595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fazi F, Blandino G. MicroRNAs: Noncoding pleiotropic factors in development, cancer prevention and treatment. Microrna. 2013;2(2):81. doi: 10.2174/2211536611302020001. [DOI] [PubMed] [Google Scholar]
- 12.Deb B, Uddin A, Chakraborty S. miRNAs and ovarian cancer: An overview. J Cell Physiol. 2017 doi: 10.1002/jcp.26095. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Wang Q, Yu J. MiR-129-5p suppresses gastric cancer cell invasion and proliferation by inhibiting COL1A1. Biochem Cell Biol. 2017 doi: 10.1139/bcb-2016-0254. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Yoo BK, Emdad L, Lee SG, et al. Astrocyte elevated gene-1 (AEG-1): A multifunctional regulator of normal and abnormal physiology. Pharmacol Ther. 2011;130(1):1–8. doi: 10.1016/j.pharmthera.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li M, Dai Y, Wang L, et al. Astrocyte elevated gene-1 promotes the proliferation and invasion of breast cancer cells by activating the Wnt/beta-catenin signaling pathway. Oncol Lett. 2017;13(4):2385–90. doi: 10.3892/ol.2017.5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Chen X, Tong M. Knockdown of astrocyte elevated gene-1 inhibited cell growth and induced apoptosis and suppressed invasion in ovarian cancer cells. Gene. 2017;616:8–15. doi: 10.1016/j.gene.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 17.Xu S, Gu G, Ni Q, et al. The expression of AEG-1 and Cyclin D1 in human bladder urothelial carcinoma and their clinicopathological significance. Int J Clin Exp Med. 2015;8(11):21222–28. [PMC free article] [PubMed] [Google Scholar]
- 18.Luo Y, Zhang X, Tan Z, et al. Astrocyte elevated gene-1 as a novel clinicopathological and prognostic biomarker for gastrointestinal cancers: A meta-analysis with 2999 patients. PLoS One. 2015;10(12):e0145659. doi: 10.1371/journal.pone.0145659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoo BK, Emdad L, Su ZZ, et al. Astrocyte elevated gene-1 regulates hepatocellular carcinoma development and progression. J Clin Invest. 2009;119(3):465–77. doi: 10.1172/JCI36460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Peng G, Wang Y, et al. Silencing of astrocyte elevated gene-1 inhibits proliferation and migration of melanoma cells and induces apoptosis. Clin Exp Pharmacol Physiol. 2017;44(7):815–26. doi: 10.1111/1440-1681.12767. [DOI] [PubMed] [Google Scholar]
- 21.Merkel EA, Gerami P. Malignant melanoma of sun-protected sites: A review of clinical, histological, and molecular features. Lab Invest. 2017;97(6):630–35. doi: 10.1038/labinvest.2016.147. [DOI] [PubMed] [Google Scholar]
- 22.Li JK, Chen C, Liu JY, et al. Long noncoding RNA MRCCAT1 promotes metastasis of clear cell renal cell carcinoma via inhibiting NPR3 and activating p38-MAPK signaling. Mol Cancer. 2017;16(1):111. doi: 10.1186/s12943-017-0681-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong X, Zhu H, Chen X. Low expression of long noncoding RNA CASC2 indicates a poor prognosis and promotes tumorigenesis in thyroid carcinoma. Biomed Pharmacother. 2017;93:391–97. doi: 10.1016/j.biopha.2017.06.063. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Zhang M, An G, et al. LncRNA TUG1 acts as a tumor suppressor in human glioma by promoting cell apoptosis. Exp Biol Med. 2016;241(6):644–49. doi: 10.1177/1535370215622708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao X, Zhou T, Guo S, et al. LncRNA MALAT1 sponges miR-204 to promote osteoblast differentiation of human aortic valve interstitial cells through up-regulating Smad4. Int J Cardiol. 2017;243:404–12. doi: 10.1016/j.ijcard.2017.05.037. [DOI] [PubMed] [Google Scholar]
- 26.Long M, Dong K, Gao P, et al. Overexpression of astrocyte-elevated gene-1 is associated with cervical carcinoma progression and angiogenesis. Oncol Rep. 2013;30(3):1414–22. doi: 10.3892/or.2013.2598. [DOI] [PubMed] [Google Scholar]
- 27.Srivastava J, Siddiq A, Emdad L, et al. Astrocyte elevated gene-1 promotes hepatocarcinogenesis: Novel insights from a mouse model. Hepatology. 2012;56(5):1782–91. doi: 10.1002/hep.25868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie Y, Zhong DW. AEG-1 is associated with hypoxia-induced hepatocellular carcinoma chemoresistance via regulating PI3K/AKT/HIF-1alpha/MDR-1 pathway. Excli J. 2016;15:745–57. doi: 10.17179/excli2016-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He W, He S, Wang Z, et al. Astrocyte elevated gene-1(AEG-1) induces epithelial-mesenchymal transition in lung cancer through activating Wnt/beta-catenin signaling. BMC Cancer. 2015;15:107. doi: 10.1186/s12885-015-1124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]