The shoot apical meristem (SAM), at the tip of the plant stem, has two roles; it is the source of the new cells that are needed for stem growth, and it is the site of small cellular outgrowths, called leaf primordia (LP), that develop into the leaves (1). These LPs occur in predictable positions, with the site of the next primordium (I1) being specified by the location of the most recently appearing primordia (e.g., P1, P2). The ordered arrangement of LPs around the circumference of the SAM is known as phyllotaxy (2). A major question in plant development is what events occur at the I1 position, so that the primordium develops at this spot rather than elsewhere on the SAM. Two recent papers now provide some of the answers. In this issue of PNAS, Pien et al. (3) have demonstrated that a localized induction in the SAM of the wall-loosening protein expansin is sufficient to induce a primordium and set into motion all of the events needed to produce a mature leaf. Reinhardt et al. (4) obtained similar results, but used instead a localized application of the plant hormone auxin. Together, these two papers are starting to answer some of the main questions about LP formation.
Expansins are a family of small (25–27 kDa) proteins that are localized in the cell wall (5). They have no known enzymatic activity (6), but have the ability to break hydrogen bonds between cell wall polysaccharides when activated by an acidic environment (7). The expansins are primarily localized in the expanding regions of plants and are believed to be responsible for the cell wall loosening that is required for plant cell expansion (5). In 1997 Fleming et al. (8) applied a cucumber expansin to the surface of tomato SAMs, and a primordia-looking outgrowth developed at that spot. This outgrowth did not result in a recognizable leaf, however. They believed that the problem was that the expansin did not penetrate beyond the outer (L1) layer of the SAM (9); it was well known (1) that the LPs are formed from cells from least three cell layers in the SAM (the L2 and L3 layers in addition to the L1).
Pien et al. (3) have used an imaginative approach to overcome the penetration problem. They introduced a cucumber expansin gene into tobacco plants combined with the tetracycline-inducible promoter system. Application of anhydrotetracycline (Ahtet) induced the expression of expansin in those cells to which Ahtet was applied. When Ahtet was applied to a small region of the SAM expansin was induced in L1–L3 layers. Induction of expansion at the I2 location, where a LP would normally arise only after one appeared at the I1 location, resulted in a LP at that spot. An up-regulation of expansin, then, is sufficient to cause a leaf to be formed at the SAM, even at a spot where its development would normally be strongly inhibited. When LPs form in vivo, there can also be an up-regulation of expansin. In tomato apices (10) one expansin gene, LeExp18, is up-regulated in LP coincident with the origin of the primordium. Likewise, in deep-water rice, the OsEXP1 expansin gene is expressed primarily in the youngest LP (11). The LP, once induced by Ahtet, continued to develop into a complete, normal leaf, even though the expansin was induced for only the first day (3). This finding indicates that initiating a LP is sufficient to set into motion the complete developmental pathway leading to a mature leaf. Some targeted signal that activates these specific expansin genes in a particular set of SAM cells could be controlling the location of leaf formation. However, the subsequent growth of the leaf still is influenced by expansin. When Ahtet was applied to one side of a P2 primordium, there was a subsequent increase in the size of the leaf blade on that side.
But expansin is not the only exogenous factor that can induce the formation of LPs. The auxin hormone indoleacetic acid (IAA) has a similar effect. The site of IAA synthesis in plants is uncertain, but is believed to be in young leaves and perhaps the apical meristem (12). IAA then moves by polar auxin transport (PAT), a process that involves symmetrical uptake of IAA into cells coupled with asymmetrical efflux of auxin from only one end of the cell (13). This results in a one-directional movement of auxin. Inhibitors of PAT such as naphthylphthalamic acid or 2,4,5-triiodobenzoic acid are known to alter the phyllotaxy (14) or to completely prevent the formation of LPs (4). Mutants of Arabidopsis that are blocked in PAT, such as pin1, likewise fail to produce LPs (15). Reinhardt et al. (4) found that application of IAA to a localized position on naphthylphthalamic acid-treated tomato stem apices that normally would form no LPs, resulted in the induction of LPs that subsequently grew into fairly normal leaves. If IAA was applied to the I2 position on normal apices, a primordium would appear there, instead of the normal I1 position.
It is not too surprising that auxin and expansin might have similar effects on LP initiation. Enlargement of plant cells is constrained by a resistant cell wall composed of cellulose microfibrils, crosslinked by other polysaccharides such as xyloglucans (16). The wall prevents the osmotic uptake of water into the cells until the wall is loosened; i.e., until crosslinks in the wall are cleaved (5). In many cases where auxin promotes cell enlargement, it does so by inducing cells to excrete protons into the cell walls, and the lowered apoplastic pH activates expansins that break the crosslinks between the cellulose microfibrils (17). The hydrostatic pressure of the cell contents, the turgor pressure, then expands the loosened walls. The rate of cell enlargement can depend on both the amount of expansin present and the apoplastic pH. This is shown by results obtained with tobacco BY2 cells (18). Addition of expansin caused these cells to expand, indicating that the endogenous expansin was nonoptimal. But expansion also was induced when the walls were acidified by the fungal toxin fusicoccin., indicating that the apoplastic pH was also suboptimal. Most cells may have suboptimal levels of both expansin and wall pH and therefore limited potential for enlargement; such a situation may exist in the SAM. But if there is an increase in either expansin or a lowering of the apoplastic pH in response to auxin the set of cells may grow and form a LP.
In meristematic regions in plants growth consists of an increase in volume coupled with division, so as to result in additional cells of approximately the size of the original cells. There has long been a controversy as to whether growth starts by an increase in cell size, which triggers division, or whether division occurs first, followed by restoration of the original cell size (19). The fact that the first visible sign of a new LP is a periclinal division in the L1 or L2 layer has suggested that division comes first (1). However, γirradiated wheat seeds, which could not undergo any cell division, upon germination formed one new primordium at the SAM by the bulging out of a set of L1 cells (20). Both the location and size of the primordium were similar to that of the untreated plants. This finding supports the idea that cell enlargement is the first step in primordium formation. The results of Pien et al. provide additional support for this position. Expansins are well known to be involved in cell enlargement, but are not known to promote mitosis without cell enlargement (5). The fact that LPs can be induced by added expansins provides strong support for the idea that the initial step in primordium formation is enhanced expansion of a specific set of cells in the SAM.
The SAM consists of two zones. The cells of the central zone (CZ), which occupy the center of the SAM, are undifferentiated and divide to produce cells in the peripheral zone (PZ), a ring of cells below the CZ (21). Cells in the CZ are unable to form primordia. LPs only occur in the PZ. The Reinhardt et al. paper (4) shows that any PZ cell has the capacity to participate in LP formation, because LPs formed on the naphthylphthalamic acid-treated apices wherever auxin was applied. But what determines LP location normally? A series of surgical experiments have shown that the existing primordia inhibit the cells around them, and that the next LP, at I1, occurs where this inhibitory effect in minimal (1). A hotly debated issue is the identity of the inhibitory influence of the existing primordia.
One possibility is that the inhibitor produced by existing LPs is a flavonoid (22), which inhibits PAT. In some fashion PAT would seem to be involved in LP formation, because inhibition of PAT by either naphthylphthalamic acid (4) or the pin1 mutant (15) can completely block LP formation. Polarly transported auxin is not needed for the maintenance of the CZ, or the progression of cells from the CZ to the peripheral zone, because both processes occur normally in the pin1 mutants (23). PIN1, itself, is up-regulated in developing LPs (23). The initiation of a LP may simply require that sufficient auxin accumulates in a set of cells so as to set off the necessary cell enlargement. This accumulation of auxin requires active PAT, which will occur only when the level of the PAT inhibitor from the existing primordia drops below some threshold. The site of the minimum of the inhibitor will have a maximum of PAT and will accumulate auxin. In a feed-forward loop, as auxin accumulates it induces more PIN1 and thus more auxin accumulation. As yet unknown is whether in LP formation auxin acts simply by causing cell wall acidification, or whether it is also capable of up-regulating the endogenous expansin gene.
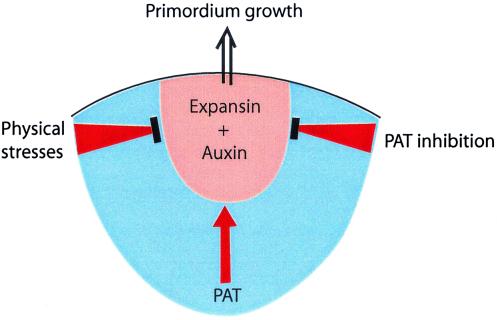
An alternative to the concept of a chemical inhibitor of LP formation is the idea that the location of the next LP is determined by the physical interactions between the cells. The ability of any cell to enlarge will depend to a considerable extent on the forces exerted on it by its neighbors. For example, an epidermal cell at I1 will be compressed by the cells below in L2 and L3, whereas the external wall will be under tension because of the curvature of the apex (24). Green and coworkers (24, 25) have espoused the idea that the location of I1 is located at the site of maximum shear stress and minimum of tension. There can be little doubt that the physical forces play some role, but the demonstration (3, 4) that LPs can be induced in normally inappropriate positions by either expansin or auxin is difficult to reconcile with this theory. It is more likely that the site of LP initiation requires a “hot-spot” of auxin coupled with the compatible set of stresses on the cells (Fig. 1).
Figure 1.
An hypothesis to explain the location where a new LP arises. This occurs by the growth in volume of a set of cells that have sufficient expansin and auxin, and where the physical stresses exerted by neighboring cells are permissive. Existing primordia influence the location by generating incompatible physical stresses close to themselves, and by release of inhibitors of PAT. At a sufficient distance from existing primordia the PAT inhibitors would be low enough to permit auxin to move, by PAT, to the cells that will form the primordium.
Footnotes
See companion article on page 11812.
References
- 1.Steeves T A, Sussex I M. Patterns in Plant Development. 2nd Ed. Cambridge, U.K.: Cambridge Univ. Press; 1989. [Google Scholar]
- 2.Jean R V. Phyllotaxis: A Systemic Study in Plant Morphogenesis. Cambridge, U.K.: Cambridge Univ. Press; 1994. [Google Scholar]
- 3.Pien S, Wyrzykowska J, McQueen-Mason S, Smart C, Fleming A. Proc Natl Acad Sci USA. 2001;98:11812–11817. doi: 10.1073/pnas.191380498. . (First Published September 18, 2001; 10.1073/pnas.191380498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reinhardt D, Mandel T, Kuhlemeier C. Plant Cell. 2000;12:507–518. doi: 10.1105/tpc.12.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cosgrove D J. Plant Physiol. 1998;118:333–339. doi: 10.1104/pp.118.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McQueen-Mason S, Cosgrove D J. Plant Physiol. 1995;107:87–100. doi: 10.1104/pp.107.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McQueen-Mason S, Cosgrove D J. Proc Natl Acad Sci USA. 1994;91:6574–6578. doi: 10.1073/pnas.91.14.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleming A J, McQueen-Mason S, Mandel T, Kuhlemeier C. Science. 1997;276:1415–1418. [Google Scholar]
- 9.Fleming A J, Caderas D, Wehrli E, McQueen-Mason S, Kuhlemeier C. Planta. 1999;208:166–174. [Google Scholar]
- 10.Reinhardt D, Wittwer F, Mandel T, Kuhlemeir C. Plant Cell. 1988;10:1427–1437. doi: 10.1105/tpc.10.9.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho H-T, Kende H. Plant J. 1998;15:805–812. doi: 10.1046/j.1365-313x.1998.00258.x. [DOI] [PubMed] [Google Scholar]
- 12.Bandurski R S, Cohen J D, Slovin J P, Reinecke D M. In: Plant Hormones: Physiology, Biochemistry, and Molecular Biology. 2nd Ed. Davies P J, editor. Dordrecht, the Netherlands: Kluwer; 1995. pp. 39–65. [Google Scholar]
- 13.Lomax T L, Muday G K, Rubery P H. In: Plant Hormones: Physiology, Biochemistry, and Molecular Biology. 2nd Ed. Davies P J, editor. Dordrecht, the Netherlands: Kluwer; 1995. pp. 509–530. [Google Scholar]
- 14.Meicenheimer R D. Am J Bot. 1981;68:1139–1154. [Google Scholar]
- 15.Gälweiler L, Guan C, Müller A, Wisman E, Mendgen K, Yephremov A, Palme K. Science. 1998;282:2226–2230. doi: 10.1126/science.282.5397.2226. [DOI] [PubMed] [Google Scholar]
- 16.Carpita N C, Gibeaut D M. Plant J. 1993;3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 17.Rayle D L, Cleland R E. Plant Physiol. 1992;99:1271–1274. doi: 10.1104/pp.99.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Link B M, Cosgrove D J. Plant Physiol. 1998;118:907–916. doi: 10.1104/pp.118.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobs T. Plant Cell. 1997;9:1021–1029. doi: 10.1105/tpc.9.7.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foard D E. Can J Bot. 1971;49:1601–1603. [Google Scholar]
- 21.Bowman J L, Eshed Y. Trends Plant Sci. 2000;5:110–115. doi: 10.1016/s1360-1385(00)01569-7. [DOI] [PubMed] [Google Scholar]
- 22.Brown D E, Rashotte A M, Murphy A S, Normanly J, Tague B W, Peer W A, Taiz L, Muday G K. Plant Physiol. 2001;126:524–535. doi: 10.1104/pp.126.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vernoux T, Kronenberger J, Grandjean O, Laufs P, Traas J. Development (Cambridge, UK) 2000;127:5157–5165. doi: 10.1242/dev.127.23.5157. [DOI] [PubMed] [Google Scholar]
- 24.Selker J M L, Steucek G L, Green P B. Dev Biol. 1992;153:29–43. doi: 10.1016/0012-1606(92)90089-y. [DOI] [PubMed] [Google Scholar]
- 25.Green P B. Am J Bot. 1999;86:1059–1076. [PubMed] [Google Scholar]