Abstract
Previous studies have found that children show rapid and significant improvements in their ability to remember individual items and the contextual details that surround these items (i.e., episodic memory) during early childhood. Encoding processes have been suggested to contribute to the development of episodic memory, however, few studies have investigated encoding processes. The goal of the current study was to examine age- and performance-related effects on encoding in children between 4 and 8 years of age using event-related potentials (ERPs). Results revealed effects of both age and performance on encoding, as indexed by the ERP response. However, the nature of these effects differed between subsequent recognition and subsequent recollection, as well as for the two ERP components (i.e., Nc, LSW) examined. These findings are important as they contribute empirical evidence that encoding processes show developmental change across early childhood. In addition, these findings highlight the importance of controlling for performance differences in future studies examining developmental changes in episodic memory.
Keywords: encoding, episodic memory, age-related differences, performance-related differences, event-related potentials
The ability to remember improves dramatically during childhood. Previous studies have consistently shown that memory for individual items and contextual details that surround these items develops across childhood. Interestingly, improvements in children’s memory for contextual details are quite substantial during early childhood (before 8 years of age, e.g., Bauer et al., 2012; Drummey & Newcombe, 2002; Riggins, 2014; Riggins & Rollins, 2015; Sluzenski, Newcombe, & Kovacs, 2006). For example, Riggins (2014) used a cohort-sequential design to examine developmental changes in children’s memory for individual items and binding in children who were 4, 6, or 8 years of age and followed them for 3 years. Each year, the experimenters asked children to learn and remember novel facts and their sources and then recall them after a week delay. Results revealed increases in both memory for individual items (facts or sources) and correct fact/source combinations between 4 and 10 years. Item memory improved in a linear fashion but memory for fact/source combinations showed accelerated rates of change between 5 and 7 years. The authors concluded that early childhood might be a period of particularly rapid change in the ability to remember contextual details.
However, why such changes in memory occur or what stage/stages of the memory process contributes to these effects (i.e., encoding, consolidation, storage or retrieval) remains unclear. Most previous studies on episodic memory development have focused on retrieval or storage processes (Bauer, 2005; Chechile & Ehrensbeck, 1983; Chechile, Richman, Topinka, & Ehrensbeck, 1981; Marshall, Drummey, Fox, & Newcombe, 2002; Riggins & Rollins, 2015; Riggins, Rollins, & Graham, 2013; Wickelgren, 1975). Overall, these studies suggest that storage improves significantly with age in childhood, whereas only modest developments occur in retrieval process (see Howe & O’Sullivan, 1997, for review). Although encoding processes have been suggested to contribute to the maturation of episodic memory, few studies have investigated the developmental trajectories of encoding (see Bauer, 2007).
The lack of studies examining encoding may be due to the fact that encoding is largely an “unobservable” process, making it difficult to study using traditional behavioral measures (see Bauer, 2007). In adults, most studies of encoding have focused on how memory performance is affected by manipulating encoding levels, encoding strategies, or attention during encoding (e.g., Craik & Lockhart, 1972; Naveh-Benjamin, Brav, & Levy, 2007; Naveh-Benjamin, Guez, & Marom, 2003; Wegesin, Jacobs, Zubin, Ventura, & Stern, 2000). These studies indicate that deeper encoding, use of strategies, and full attention promote better episodic memory performance. Similar studies have been conducted in older children, and have examined the use of deliberate strategies during encoding such as rehearsal (Flavell, Beach, & Chinsky, 1966), elaboration (Pressley, 1982), and organization (Flavell, Friedrichs, & Hoyt, 1970; Kee & Bell, 1981; Schwenck, Bjorklund, & Schneider, 2009). These studies show age-related improvements in memory when deliberate strategies were used versus when they were not. Based on these findings, it has been suggested that the early development of episodic memory is related to increases in the use of deliberate strategies (i.e., memory improves with age because children are better at using strategies during encoding, Schwenck et al., 2009). Because it is difficult to have infants and young children use encoding strategies, behavioral studies in these groups often control for potential differences in encoding by using “learn to criterion” methods in order to study the other processes of memory, such as retrieval (e.g., Brainerd & Reyna, 1995; Howe & Courage, 1997). Thus, developmental changes in encoding remain essentially unexplored in infants and young children (see Bauer et al., 2006 for a novel attempt to bridge this gap in infancy).
Compared to behavioral approaches, neural methodologies (e.g., fMRI, ERP) allow for examination of the neural correlates of encoding by comparing neural responses recorded during encoding to stimuli that are subsequently remembered versus subsequently forgotten (termed subsequent recognition effects) or/and by comparing stimuli that are subsequently remembered with contextual details and stimuli subsequently remembered without contextual details (termed subsequent recollection effects). These methods have proven useful for exploring questions not only regarding which neural regions are involved in the encoding process, but also whether they change as a function of age. In adults, studies using fMRI suggest that structures in the medial temporal lobe (MTL) and the prefrontal cortex (PFC) are important for encoding items and their contextual information (Blumenfeld & Ranganath, 2007; Davachi, Mitchell, & Wagner, 2003; Henson, Rugg, Shallice, Josephs, & Dolan, 1999; Prince, Daselaar, & Cabeza, 2005; Ranganath et al., 2004). In school-aged children and adolescents, fMRI studies of encoding have shown developmental changes in MTL and PFC, as well as in the connectivity between these regions (Güler & Thomas, 2013; Ghetti, DeMaster, Yonelinas, & Bunge, 2010; Menon, Boyett-Anderson, & Reiss, 2005; Ofen et al., 2007). Increased activation in the PFC has been suggested to relate to more extensive use of appropriate strategies and cognitive control in older children (see Ofen, 2012). However, due to demands of the scanning environment, fMRI has not yet examined age-related changes in encoding in early childhood.
Event-related potentials (ERPs), which measure brain activity related to the formation of memories in temporal units that match the speed of cognitive processes (i.e., milliseconds), have also been widely used to study the spatiotemporal dynamics of encoding in adults (e.g., Angel, Isingrini, Bouazzaoui, & Fay, 2013). Analysis of ERP amplitudes has contributed to our knowledge of subsequent recognition effects and subsequent recollection effects in adults. ERP studies have suggested that subsequent recognition effects are faster and may rely more on familiarity processes and frontal regions, whereas subsequent recollection effects are more prolonged and are thought to require recollection processes and parietal regions (Yonelinas, 2002).
Examination of age differences in subsequent recognition and subsequent recollection effects has also been used to understand changes in encoding during aging by comparing encoding in younger versus older adults (Cansino, Trejo-Morales, & Hernández-Ramos, 2010; Friedman, Nessler, & Johnson, 2007; Friedman & Trott, 2000; Nessler, Johnson Jr, Bersick, & Friedman, 2006). For example, Cansino et al. (2010) used ERPs to study changes in subsequent recollection in young, middle-aged, and older adults. In all three groups, the authors observed larger amplitudes for items that were subsequently remembered with contextual details than items that were subsequently remembered without details. However, the onset of this difference was delayed across age groups, suggesting differences in the speed and/or efficiency of encoding between groups. In addition, the middle-aged and older adults showed a more pervasive subsequent recollection effect at the posterior leads than did the young group, despite similar responses at frontal and central leads, suggesting that different neural resources were contributing to subsequent recollection effects in younger and older adults.
To the best of our knowledge, only two studies to date have used ERP to examine age-related encoding differences in children (Rollins & Riggins, 2013, 2017). For example, Rollins & Riggins (2013) examined both subsequent recognition effects and subsequent recollection effects in 6-year-old children and adults. The results revealed few age differences in subsequent recollection ERP effects as measured by a subjective recollection task (i.e., the remember/know task). In contrast, subsequent recognition effects were detected in both children and adults but these effects differed between age groups. Specifically, subsequent recognition effects were observed later in children, the direction of the difference in ERP amplitudes was different in children and adults, and topographic differences were observed between age groups. These findings suggested that developmental changes do occur in encoding between 6 years of age and adulthood. Rollins and Riggins (2017) further examined the development of subsequent subjective recollection in children (6–8 years), adolescents (11–13 years), and adults. These results indicated no age-related differences in ERP effects associated with recollection at encoding (although differences were observed at retrieval). Based on these two studies, it is still unclear if and when encoding processes change during childhood particularly because only one group of children was involved in both studies. Moreover, since older groups were better at remembering contextual details associated with the items than younger groups in both previous studies, the contribution of age versus performance to these effects also remains unclear.
Recent memory development studies have shown that it is important to tease apart age-related differences from performance-related differences (Church, Petersen, & Schlaggar, 2010; Duarte, Ranganath, Trujillo, & Knight, 2006; Paz-Alonso, Gallego, & Ghetti, 2013; Sastre, Wendelken, Lee, Bunge, & Ghetti, 2016). For example, Sastre et al. (2016) carried out an fMRI study to investigate how age and performance differences contributed to differences in retrieval in school-aged children and adults. They found that during retrieval, high performing 10- to 11-year-olds showed whole hippocampus activation similar to low performing adults, but only high performing adults showed activation in the hippocampal head. Similarly, Duarte et al. (2006) carried out an ERP study to examine the contribution of age and performance on recollection effects (comparing ‘remember’ vs ‘know’ trials) and familiarity-based recognition effects (comparing ‘know’ vs ‘miss’ trials) in younger and older adults. ERP results indicated that old-high performing adults showed different familiarity-based recognition effects but similar recollection effects as young adults, whereas old-low performing adults showed differences in both recollection and familiarity-based recognition. These findings suggest that both age and performance are important factors to consider when investigating age-related changes in memory and its neural substrates.
The goal of the current study was to investigate age- and performance-related differences in encoding across early childhood (4–8 years of age). Using an intentional objective source memory task, subsequent recognition effects were examined by comparing subsequent hit and miss trials and subsequent recollection effects were examined by comparing subsequent source correct and incorrect trials. ERP methodology was used to measure the encoding processing because it is relatively easy to implement in young children compared to other methods (e.g., fMRI). The present study focused on two temporally-distinct ERP components: negative component (Nc) and late slow wave (LSW). Nc is thought to index attention that is modulated by memory, whereas LSW is related to memory updating (DeBoer, Scott, & Nelson, 2005, 2007). The amplitude of Nc and LSW were examined to test age and performance related differences as well as their interaction. Although previous ERP studies did not provide much information on the developmental trajectories of encoding across early childhood (Rollins & Riggins, 2013, 2017), previous behavioral and fMRI studies clearly indicate that source memory develops significantly between 5–7 years (e.g., Riggins, 2014; Riggins, Geng, Blankenship, & Redcay, 2016) that performance levels affect neural response underlying source memory (e.g., Sastre et al., 2016). Therefore, we predicted that differences in ERP responses associates with encoding would be observed between age and performance groups in the current study.
Method
Participants
Children were recruited from a major metropolitan area through the use of both a University maintained database of families interested in participating in research and the distribution of recruitment flyers. Children were screened to ensure they were not more than three weeks premature and had no diagnoses for any neurological conditions, developmental delays, or disabilities, which were the exclusion criteria for the study.
Participants included 117 4- to 8-year-old children (M = 6.45 years, SD = 1.43, 61 males, 56 females). A total of 104 children provided usable behavioral data (4 were excluded due to technical issues, 7 were excluded due to poor behavioral performance, and 2 did not complete the testing session). A total of 86 children provided usable ERP data for testing subsequent recognition effects (6 were excluded due to technical issues, 13 were excluded due to poor ERP data quality, 3 did not complete the ERP testing session, and 7 were removed due to poor behavioral performance, and 2 were excluded due to fewer than 10 trials in at least one condition, see justification below). Among these 86 children, 73 also provided usable data for testing subsequent recollection effects (14 were not included because they had fewer than 10 trials in at least one of these conditions).
Stimuli
A total of 160 images of animals and objects determined to be age appropriate were selected from the Bank of Standardized Stimuli (BOSS, Brodeur, Dionne-Dostie, Montreuil, & Lepage, 2010). To ensure any observed effects were not the result of the items themselves, all 160 possible items were randomly divided into 4 sets of 40 items and then counterbalanced across participants. During encoding, participants saw 120 stimuli (40 per character block) paired to one of three different character sources. The 40 stimuli not paired with a character were presented as new items during the retrieval phase of the study. Two versions of the task were created with the 4 sets of 40 items, with sets differentially assigned to the three characters or as new at retrieval. Character sources were selected to represent characters who were well-known to children (i.e., The Little Mermaid, SpongeBob, and Mickey Mouse) and one of the characters was selected as a typically female-preferred character, one was a typically male-preferred character, and one was a character typically liked equally by males and females. To further highlight the differences between the characters, items were presented to the left of two characters (Mickey Mouse and The Little Mermaid) and to the right of one character (SpongeBob). To simplify the counterbalancing process, the order of the character-blocks were the same for all children and task version was counterbalanced. Item presentation order was randomized within block by the presentation software, Eprime (Psychology Software Tools, Pittsburgh, PA). All stimuli were presented at eye level on a computer screen at a distance of 100 cm.
Procedure
Prior to data collection, the University Institutional Review Board approved all procedures. Parents or guardians provided informed consent for all participants. Participants over the age of 7 years provided written assent. After the study was complete, participants received monetary compensation, a small gift, and a certificate with a picture of their brain waves. Participants were enrolled in a larger study on memory and brain development. The focus of this report is the ERP paradigm, which consisted of collection of ERP data during memory encoding, followed by behavioral data collection for the retrieval portion of the task. During testing, parents completed questionnaires regarding demographics and children’s behavior.
Participants first completed a short training and practice to ensure they understood the task. The training session introduced the child to both the encoding and retrieval portions of the task. For encoding, the Experimenter first showed a picture of a character alone on the screen and identified the character by name. Then the Experimenter sequentially presented two items next to the character and verbally labeled each item. The child was told that it was important to remember both the item and the character. This was done for each of the 3 characters, which resulted in a total of 6 paired items. Immediately following encoding training, the child was sequentially shown each of the 6 old items and 3 new items. For each item they were asked to identify whether it was old or new (item memory), and for old items, with which character the item had previously been presented (source memory). During this retrieval training period, the Experimenter corrected inaccurate responses. Following training, the child practiced both the encoding and retrieval portions of the paradigm. During encoding practice, each character was paired with 5 different items and children were instructed to observe and remember which items went with which characters. During retrieval practice, inaccurate responses were not corrected. Children were required to make item and source memory judgments on the 15 old items and 5 new items and obtain an accuracy score of 80% or higher before proceeding. If children did not pass with the required accuracy, the Experimenter explained the task rules again and participants were asked to complete another practice session with different stimuli. Following successful completion of the practice, participants were fitted with a stretchy Lycra cap appropriate for their head circumference containing 64 recording sensors.
Encoding
EEG was recorded during encoding. Participants were instructed to observe and remember which items went with which characters. No deliberate strategy to accomplish this was recommended. Children were also asked to remain as still as possible and informed that they would get a short break, lasting approximately 30 seconds to 1 minute, between each character block. Within each character-block only one character was presented and paired with 40 items that “belonged to them,” resulting in 120 trials across all character blocks. Each individual trial consisted of the presentation of an item with the character (1500ms) followed by a fixation cross which remained on the screen during the inter-stimulus interval (1000–3000ms, with an average of 2000ms).
Retrieval
The retrieval portion of the task began approximately 15 minutes after the conclusion of the encoding portion. This delay was to ensure that working memory did not drive performance on the task and to allow for the inclusion of a brief snack and removal of the cap and electrodes. Children were instructed to respond “yes” if the item presented was one they had seen during encoding, and “no” if the item presented was new. If children indicated seeing the item previously, they were then asked to indicate to which of the three characters the item belonged. Items were presented on the screen until children identified them as being old or new. If the item was identified as old, the three characters remained on the screen until children indicated which character they believed the item “belonged to”. Children gave all answers verbally and responses were recorded by the Experimenter.
Variables of interest included the following: old items that the children accurately recalled as old (referred to as ‘hits’), and new items that the children accurately recalled as new (referred to as ‘correct rejections’), new items incorrectly identified as old and paired with a source (referred to as ‘false alarms’), and old items incorrectly rejected as new items (referred to as ‘misses’). Stimuli accurately recalled as old were further categorized as ‘source correct’ if the child correctly recognized the character with whom the item was presented, or ‘source incorrect’ if the child correctly identified an item as old but attributed the item to the incorrect character.
EEG Data Recording and Analysis
During encoding, EEG was continuously recorded from 64 Ag/AgCI electrodes as well as two vertical and two horizontal electrooculogram channels at a sampling rate of 512 Hz using a BioSemi Active 2 EEG recording system. Preprocessing and data reduction were carried out using Brain Electrical Source Analysis (BESA) software (MEGIS Software GmbH, Gräfelfing, Germany) and EEGlab (Delorme & Makeig, 2004). Bad channels were interpolated and ocular artifacts were corrected using BESA. A maximum of 8 bad channels (<12.5% channels) were allowed for inclusion in the dataset. The other preprocessing steps, including filtering, segmentation, artifact rejection, and re-referencing to the average, were performed in EEGlab. The continuous EEG data were filtered with a low pass of 0.1 Hz and a high pass of 30 Hz and then segmented into epochs starting 100 ms before and ending 1500 ms after stimuli onset. Segments were rejected if any artifact was detected or if the maximum amplitude > 200 μV or if the minimum amplitude < −200 μV. The averaged data of all segments for each channel were re-referenced to the average potential of all channels.
Waveform Analysis
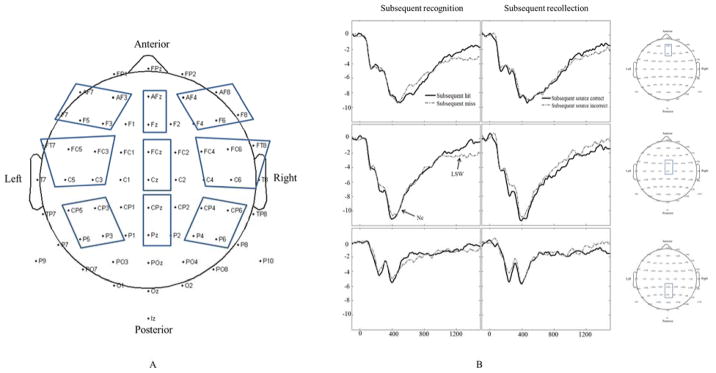
ERPs were averaged for hit, miss, source correct, and source incorrect trials. We selected 9 regions of interest for further analyses based on previous studies (e.g., Czernochowski, Mecklinger, Johansson, & Brinkmann, 2005; Marshall et al., 2002; Rollins & Riggins, 2013, 2017) and included the following leads: left frontal (AF3, AF7, F3, F5, F7), middle frontal (AFz, Fz), right frontal (AF4, AF8, F4, F6, F8), left central (FT7, FC5, FC3, C3, C5), middle central (FCz, Cz), right central (FC4, FC6, FT8, C4, C6), left posterior (CP3, CP5, P3, P5), middle posterior (CPz, Pz), and right posterior (CP4, CP6, P4, P6), see Figure 1. The average of all channels in each region was computed to quantify the ERP response. This study focused on two ERP components: Nc and LSW. The average amplitude of Nc was calculated between 250–700 ms after the onset of stimuli. Nc is negative-going in frontal-central leads but positive-going in posterior leads due to polarity inversion. The average amplitude of LSW was calculated between 1100–1500 ms. LSW is positive-going in frontal-central leads but negative-going in posterior leads due to polarity inversion. Time windows were selected based on previous ERP studies in children (Marshall et al., 2002; Rollins & Riggins, 2013).
Figure 1.
Clusters selected for statistical analysis (A) and examples of ERP waveforms selected from midline clusters for each condition (B)
As recommended by DeBoer and colleagues, participants with fewer than 10 trials in any condition were excluded from ERP data analysis (DeBoer et al., 2005, 2007). Participants contributed an average of 49.3 hit trials (SD = 19.65, range= 12–95), 47.8 miss trials (SD = 20.50, range= 11–95), 26.7 source correct trials (SD = 10.28, range= 10–53), and 28.3 source incorrect trials (SD = 11.23, range= 10–68). Age was significantly related to the number of subsequent hit trials and the number of subsequent source correct trials (r = .23, p = .037; r = .26, p = .026). The measure of subsequent recognition, dprime, was related to the number of subsequent hit trials and the number of subsequent miss trials (r = .49, p < .001; r = −.31, p = .003). Source correct percentage was related to the number of subsequent source correct trials and the number of subsequent source incorrect trials (r = .55, p < .001; r = −.55, p < .001). Although average amplitude was used as the dependent measure and has been shown to be robust to differences in trials numbers between conditions (Luck, 2014), the number of trials for each condition was included as covariates in the current study to account for its possible influence on amplitude.
Statistical Analysis
IBM SPSS Statistics 20 (IBM Corp., Chicago, IL) was used for testing the relations of age to behavioral performance: subsequent recognition effects (i.e., dprime) and subsequent recollection effects (i.e., correct source percentages). Repeated-measures ANOVAs were conducted on mean amplitudes for epochs associated with the Nc and LSW components (see Figure 1) using a 2 Condition (hit, miss OR source correct, source incorrect) × 3 Coronal Plane (frontal, central, posterior) × 3 Sagittal Plane (left, middle, right) x Age x Performance design. In this design, Age and Performance were between-group factors and included as continuous variables. Condition, Coronal Plane, and Sagittal Plane were within-subject factors. Only main effects and interactions involving Condition, Age, and/or Performance are reported and examined. The Greenhouse-Geisser correction was applied if the assumption of sphericity was violated.
If any interaction involving Age or Performance was observed, follow-up analyses were performed by grouping children by age or performance. Age groups were created via a median split on age in months to form young or old groups (80.11 months for subsequent recognition, 80.28 months for subsequent recollection). Performance groups were created by a separate median split on the dependent variable of interest to form low or high performing groups, i.e., dprime (median was 1.94) and source correct percentage (median was 0.47).
Results
Behavioral Results
Recognition memory, indexed by dprime (mean = 1.80, SD = .71, range = 0.07–3.20; the difference between the z-transforms of hit rate and false alarm rate), was significantly related to age (mean = 6.61, SD = 1.39, range = 4.04–8.97; r = 0.53, p < .001). Source memory, indexed by source correct percentage out of hits (mean = 0.49, SD = 0.11, range = 0.24–0.75), was marginally significantly related to age (mean = 6.65, SD = 1.40, range = 4.04–8.97; r = 0.23, p = .055).
Subsequent Recognition Effect
Nc Component (250–700 ms)
The Omnibus ANOVA revealed a 2-way interaction between Condition × Coronal Plane (F (2, 160) = 3.92, p = .04, η2 = 0.05). Follow-up analyses indicated that Nc was more negative going for subsequent hits versus subsequent misses in frontal and central regions (F (1, 80) = 5.86, p = .018, η2 = 0.07; F (1, 80) = 23.76, p < .001, η2 = 0.23). There was no difference between the subsequent hit and subsequent miss conditions in the posterior region.
LSW Component (1100–1500 ms)
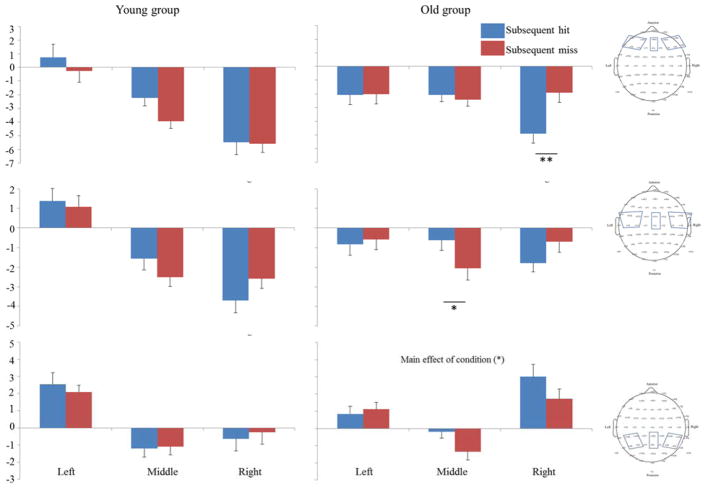
The Omnibus ANOVA revealed a Condition × Coronal Plane × Sagittal Plane × Age interaction (F (4, 320) = 2.87, p = .046, η2 = .035). In order to follow up this interaction, we split the data first by Age (using a median split, see Figure 2) and then by Condition. Follow-up analyses for each Age Group revealed, in older children, there was 3-way interaction between Condition × Coronal Plane × Sagittal Plane (F (4, 156) = 8.60, p < .001, η2 = 0.18). In both frontal and central regions, there was 2-way interaction between Condition × Sagittal Plane (F (2, 78) = 5.10, p = .02, η2 = 0.12; F (2, 78) = 3.81, p = .033, η2 = 0.09). In the frontal region, the 2-way interaction was driven by a more positive going waveform for subsequent misses compared to subsequent hits in right hemisphere (F (1, 39) = 13.3, p = .001, η2 = 0.25). In the central region, the 2-way interaction was driven by a more positive going waveform for subsequent hits compared to subsequent misses in midline leads (F (1, 39) = 4.32, p = .04, η2 = 0.10). In the posterior region, there was a main effect of Condition (F (1, 39) = 6.30, p = .016, η2 = 0.14), such that a more negative going waveform was observed for subsequent misses compared to subsequent hits. In the younger group, there was no main effects or interactions of interest.
Figure 2.
Mean (SE) of LSW amplitude for each cluster in each Age Group (* p < .05, ** p < .01)
Second, we examined the effects for subsequent hit and subsequent miss conditions separately. For the subsequent hit condition, there were main effects of Coronal plane and Sagittal plane as well as the interaction between them (F (2, 160) = 28.23, p < .001, η2 = 0.26; F (2, 160) = 9.90, p = .001, η2 = 0.11; F (4, 320) = 21.13, p < .001, η2 = 0.21), but no effects involving Age (Coronal Plane × Sagittal Plane × Age, p > .18). For the subsequent miss condition, there were main effects of Coronal Plane and Sagittal Plane (F (2, 160) = 37.06, p < .001, η2 = 0.32; F (2, 160) = 9.99, p < .001, η2 = 0.11), significant interactions between Sagittal Plane × Age (F (2, 160) = 10.95, p < .001, η2 = 0.12) and between Coronal Plane × Sagittal Plane × Age (F (4, 320) = 3.43, p = .023, η2 = 0.04). Follow-up analyses indicated that in left and right hemisphere, age was significantly related to LSW amplitude in frontal, central, and posterior regions (left frontal: r = −.311, p = .004; left central: r = −.371, p < .001; left posterior: r = −.23, p = .034; right frontal: r = .32, p = .003; right central: r = .29, p = .007; right posterior: r = .28, p = .008).
Subsequent Recollection Effect
Nc Component (250–700 ms)
The Omnibus ANOVA revealed 2-way interactions between Sagittal Plane × Age and between Coronal Plane × Age (F (2, 134) = 12.10, p < .001, η2 = 0.15; F (2, 134) = 5.05, p = .019, η2 = 0.07). Follow-up analyses revealed that in frontal and posterior regions, age was significantly related to Nc amplitude (F (1, 67) = 7.66, p = .007, η2 = 0.10; F (1, 67) = 14.63, p < .001, η2 = 0.18). In both left and right hemispheres, age was also significantly related to Nc amplitude (F (1, 67) = 5.09, p = .027, η2 = 0.07; F (1, 67) = 6.02, p = .017, η2 = 0.08).
LSW Component (1100–1500 ms)
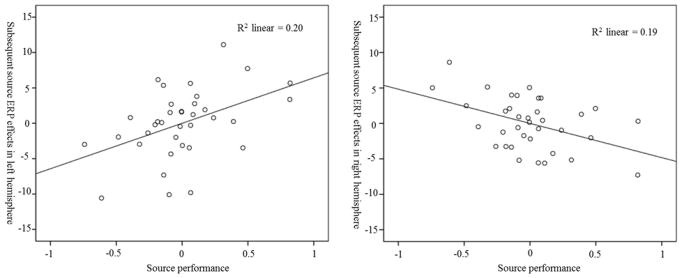
The Omnibus ANOVA revealed a 4-way interaction between Condition × Sagittal Plane × Age × Source Performance (F (2, 134) = 3.75, p = .039, η2 = 0.05). We further tested the interaction in each Age Group and found a significant 3-way Condition × Sagittal Plane × Source interaction in older children (F (2, 64) = 6.92, p = .005, η2 = 0.18). Follow-up analyses in each hemisphere indicated that the interaction between Condition × Source Performance was significant in both left and right hemisphere (F (1, 32) = 10.58, p = .003, η2 = 0.25; F (1, 32) = 11.33, p = .002, η2 = 0.26). Performance on the source memory task (source correct percentage out of hits) was positively related to differences in LSW amplitude between source correct and source incorrect trials in the left hemisphere, but negatively related to the difference between source correct and source incorrect trials in the right hemisphere, see Figure 3. In younger children, there was no significant main effect of Condition or any interaction involving Condition.
Figure 3.
Scatter plots for the correlations between source performance and subsequent source ERP effects in older children in both left and right hemispheres. Source performance represents normalized source correct percentage. Subsequent source ERP effects refer to the LSW amplitude difference between subsequent source correct trials and subsequent source incorrect trials.
Second, we further tested the 4-way interaction in each Performance Group and found the significant interaction between Hemisphere × Age in the high performing group (F (2, 62) = 4.30, p = .033, η2 = 0.12). Follow-up analyses indicated that there was hemisphere difference in the younger high-performing children, (F (2, 28) = 6.20, p = .019, η2 = 0.31), but not the older high-performing children (p > 0.26). Specifically, LSW amplitude in left hemisphere was greater than that in midline and right hemisphere leads. In the low performing group, there were no significant effects involving Condition or Age.
Finally, we further tested the 4-way interaction in each condition. In the source correct Condition, there were interaction between Hemisphere × Age and between Hemisphere × Performance (F (2, 134) = 4.33, p = .03, η2 = 0.06; F (2, 134) = 6.54, p = .007, η2 = 0.09). Follow-up analyses indicated that in left hemisphere, both age and performance were related to LSW amplitude (F (1, 67) = 4.77, p = .032, η2 = 0.07; F (1, 67) = 12.83, p = .001, η2 = 0.16); in right hemisphere, only age was related to LSW amplitude (F (1, 67) = 4.52, p = .037, η2 = 0.06). There were no significant effects for the incorrect source Condition.
Discussion
The goal of the current study was to examine age- and performance-related effects on encoding in children between 4 and 8 years of age using ERPs. Results revealed effects of both age and performance on encoding, as indexed by the ERP response. However, the nature of these effects differed between subsequent recognition and subsequent recollection, as well as for the two ERP components (i.e., Nc, LSW). In short, for subsequent recognition memory, age effects were observed in LSW; for subsequent recollection memory, age effects were observed in the Nc and both age and performance effects were observed in LSW. Therefore, future studies should consider both age and performance differences in encoding when examining developmental changes in episodic memory.
Age effects were observed for both subsequent recognition and subsequent recollection. Age effects imply developmental differences as a function of age, which may possibly arise due to maturation. We focus on LSW first because it is thought to reflect memory updating, and has been previously shown to be related to recognition and episodic memory and sensitive to age during this developmental period (DeBoer et al., 2005, 2007; Marshall et al., 2002; Riggins et al., 2013). Age differences were present in LSW amplitude for the subsequent recognition effect. Specifically, in older but not younger children, there was difference in LSW amplitude between subsequently remembered items and subsequently forgotten items. Age differences were also observed in LSW for the subsequent recollection effect. Specifically, older but not younger children showed differences in LSW amplitude between source correct and source incorrect conditions and significant correlations between source memory performance and amplitude differences between these conditions.
Condition differences in the LSW response were observed only in older children. In younger children, regardless of their level of performance on the task, subsequent recognition effects and subsequent recollection effects were not present. It may be that these effects are not present in younger children or, at the very least, are more difficult to detect in younger compared to older children. It is possible that younger children have more variability in their behavioral or neural responses or that brain-behavior associations are still developing. Future research examining these, and other possibilities, is greatly needed.
Although ERPs do not allow us to definitively pinpoint what is changing as a function of age, we can speculate based on the condition driving the age differences. First, for subsequent recognition effects measured by LSW, the age difference found in the interaction was not driven by the hit condition but rather by the miss condition. This might suggest that when memory is successful in younger and older children, the underlying neural processes are similar but reasons for unsuccessful memory formation differ between younger and older children. Perhaps this is due to differences in attention, cognitive control, monitoring, persistence, or executive function.
Second, for subsequent recollection effects measured by LSW, age effects on source memory performance were present for the source correct condition but not the source incorrect condition. This result suggests that there may be differences in the underlying neural substrates for successful formation of source memories between age groups or performance groups. Furthermore, these subsequent recollection effects were further qualified by performance, suggesting it may not be maturation alone that accounts for differences in the ERP response; performance may play a role as well. Specifically, the subsequent recollection effect was correlated with the amplitude difference between source correct and incorrect trials in older children but not in younger children. This finding might suggest that only after children reach certain age or maturation level does performance begin to relate to the neural responses reflecting subsequent recollection effects.
The interaction of age and performance is consistent with Sastre et al. (2016), suggesting that both age and performance differences contributed to neural differences underlying episodic memory (but the retrieval process) between school-aged children and adults. This interpretation is also consistent with literature examining the neural substrates (e.g., hippocampus) of episodic memory in children, which suggests changes in hippocampal structure between 4–8 years of age (Lavenex & Lavenex, 2013; Riggins, Blankenship, Mulligan, Rice, & Redcay, 2015) and changes in hippocampal function within this age range, including connectivity with cortical regions (Blankenship, Redcay, Dougherty, & Riggins, 2017; Riggins et al., 2016). Finally, in older children, the correlation between source performance and condition differences in LSW amplitude was positive in left hemisphere but negative in right hemisphere. This finding is partially consistent with a previous study (Riggins et al., 2013) which indicated the same postive correlation in the left hemisphere but no significant correlation in the right hemisphere in children between the ages of 5 and 6 years. As ERP methodology has low spatial resolution and few previous studies examined the neural underpinning of encoding for children between 4–8 years, it is hard to determine why such hemisphere differences exist. This is a question that would be important to address in future research.
In summary, age-related differences were observed for both subsequent recognition and subsequent recollection. These effects imply age-related differences in encoding. Evidence to this effect is important because it is the first, to our knowledge, to suggest developmental changes occur in encoding processes during early childhood. These findings provide the first empirical evidence to support the contributions of encoding processes to the development of episodic memory (Bauer, 2007), as previous studies have mainly focused on storage or retrieval (e.g., Marshall et al., 2002; Riggins et al., 2013; Rollins & Riggins, 2017).
The Nc associated with subsequent recollection, results also revealed relations with age. However, this effect did not interact with condition (i.e., subsequent source correct and source incorrect trials). Thus, it may not reflect age differences in memory processes specifically. It is possible it reflects the development or maturation of other cognitive abilities (i.e., attention) that apply similarly to successful and unsuccessful memory formation. This interpretation would be consistent with previous research suggesting the Nc reflects obligatory attention that can be modulated by memory (DeBoer et al., 2005, 2007). The results may imply that children across age and performance groups were able to investigate different amount of attention resource to process items subsequently remembered and the ones subsequently forgotten.
The current study has several limitations. First, we have a focused age range, which was by design in order to examine neural correlates of memory improvements previously documented in this age range. However, this restricted age range does not allow us to generalize beyond this range to make connections with infant or adult ERP literature. Second, age and performance were correlated making it difficult to tease the effects apart entirely. Third, the sample in this study is cross-sectional, limiting conclusions made about the developmental time course of the encoding process. Future work should utilize a longitudinal sample to better understand the developmental trajectory of encoding processes and how it relates to improvements in episodic memory. Fourth, the objective source memory task in the current study may have contamination due to guesses. Future studies may use a subjective source memory task to reduce the influence of such contamination, but it is well known that subjective recollection tasks require additional metacognitive abilities that are still developing in early childhood. Finally, the ERP methodology used in the current study has low spatial resolution making all references to possible neural substrates associated with effects speculative. Future investigations could use methods with greater spatial resolution to examine activation of specific regions (e.g., hippocampus) during encoding.
Despite these limitations, this study represents an initial investigation into the neural correlates underlying age-related changes in encoding across early childhood using ERPs. Specifically, both age- and performance-related differences were investigated. For subsequent recognition, results suggested age effects on encoding. For subsequent recollection, results showed that both age and performance influenced encoding. These findings are important as they contribute empirical evidence that encoding processes show developmental change across early childhood. These findings also highlight the importance of examining both age and performance differences in future studies examining developmental changes in episodic memory. Finally, developmental differences in encoding may contribute to effects in previous research that have identified early childhood as a period of particularly rapid change in the ability to remember contextual details.
Acknowledgments
Thank you to the members of the Neurocognitive Development Lab, especially Jennifer Sloane, Marissa Clark, and Elizabeth Mulligan, for helping with data collection. This work was supported by NICHD under Grant HD079518; and University of Maryland.
References
- Angel L, Isingrini M, Bouazzaoui B, Fay S. Neural correlates of encoding processes predicting subsequent cued recall and source memory. NeuroReport. 2013;24(4):176–180. doi: 10.1097/WNR.0b013e32835d8452. [DOI] [PubMed] [Google Scholar]
- Bauer PJ. Developments in declarative memory: Decreasing susceptibility to storage failure over the second Year of Life. Psychological Science. 2005;16(1):41–47. doi: 10.1111/j.0956-7976.2005.00778.x. [DOI] [PubMed] [Google Scholar]
- Bauer PJ. Handbook of Child Psychology. John Wiley & Sons, Inc; 2007. Event memory. [Google Scholar]
- Bauer PJ, Doydum AO, Pathman T, Larkina M, Güler OE, Burch M. It’s all about location, location, location: Children’s memory for the “where” of personally experienced events. Journal of Experimental Child Psychology. 2012;113(4):510–522. doi: 10.1016/j.jecp.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer PJ, Wiebe SA, Carver LJ, Lukowski AF, Haight JC, Waters JM, Nelson CA. Electrophysiological Indexes of encoding and behavioral indexes of recall: Examining relations and developmental change late in the first year of life. Developmental Neuropsychology. 2006;29(2):293–320. doi: 10.1207/s15326942dn2902_2. [DOI] [PubMed] [Google Scholar]
- Blankenship SL, Redcay E, Dougherty LR, Riggins T. Development of hippocampal functional connectivity during childhood. Human Brain Mapping. 2017;38(1):182–201. doi: 10.1002/hbm.23353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C. Prefrontal cortex and long-term memory encoding: An integrative review of findings from neuropsychology and neuroimaging. The Neuroscientist. 2007;13(3):280–291. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- Brainerd CJ, Reyna VF. Learning rate, learning opportunities, and the development of forgetting. Developmental Psychology. 1995;31(2):251–262. doi: 10.1037/0012-1649.31.2.251. [DOI] [Google Scholar]
- Brodeur MB, Dionne-Dostie E, Montreuil T, Lepage M. The bank of standardized stimuli (BOSS), a new set of 480 normative photos of objects to be used as visual stimuli in cognitive research. PLoS ONE. 2010;5(5):e10773. doi: 10.1371/journal.pone.0010773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cansino S, Trejo-Morales P, Hernández-Ramos E. Age-related changes in neural activity during source memory encoding in young, middle-aged and elderly adults. Neuropsychologia. 2010;48(9):2537–2549. doi: 10.1016/j.neuropsychologia.2010.04.032. [DOI] [PubMed] [Google Scholar]
- Chechile RA, Ehrensbeck K. Long-term storage losses: A dilemma for multistore models. The Journal of General Psychology. 1983;109(1):15–30. doi: 10.1080/00221309.1983.9711505. [DOI] [PubMed] [Google Scholar]
- Chechile RA, Richman CL, Topinka C, Ehrensbeck K. A developmental study of the storage and retrieval of information. Child Development. 1981;52(1):251–259. doi: 10.2307/1129238. [DOI] [Google Scholar]
- Church JA, Petersen SE, Schlaggar BL. The “Task B problem” and other considerations in developmental functional neuroimaging. Human Brain Mapping. 2010;31(6):852–862. doi: 10.1002/hbm.21036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FIM, Lockhart RS. Levels of processing: A framework for memory research. Journal of Verbal Learning and Verbal Behavior. 1972;11(6):671–684. doi: 10.1016/S0022-5371(72)80001-X. [DOI] [Google Scholar]
- Czernochowski D, Mecklinger A, Johansson M, Brinkmann M. Age-related differences in familiarity and recollection: ERP evidence from a recognition memory study in children and young adults. [journal article] Cognitive, Affective, & Behavioral Neuroscience. 2005;5(4):417–433. doi: 10.3758/cabn.5.4.417. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: Distinct medial temporal lobe processes build item and source memories. Proceedings of the National Academy of Sciences. 2003;100(4):2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBoer T, Scott LS, Nelson CA. Event-related potentials in developmental populations. In: Handy T, editor. Methodological Handbook for Research Using Event-related Potentials. Cambridge: MA: The MIT Press; 2005. pp. 263–297. [Google Scholar]
- DeBoer T, Scott LS, Nelson CA. Methods for acquiring and analyzing infant event-related potentials. In: Hann Md., editor. Infant EEG and event-related potentials. New York: NY: Psychology Press; 2007. pp. 5–37. [Google Scholar]
- Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Drummey AB, Newcombe NS. Developmental changes in source memory. Developmental Science. 2002;5(4):502–513. doi: 10.1111/1467-7687.00243. [DOI] [Google Scholar]
- Duarte A, Ranganath C, Trujillo C, Knight RT. Intact recollection memory in high-performing older adults: ERP and behavioral evidence. Journal of Cognitive Neuroscience. 2006;18(1):33–47. doi: 10.1162/089892906775249988. [DOI] [PubMed] [Google Scholar]
- Flavell JH, Beach DR, Chinsky JM. Spontaneous verbal rehearsal in a memory task as a function of age. Child Development. 1966;37(2):283–299. doi: 10.2307/1126804. [DOI] [PubMed] [Google Scholar]
- Flavell JH, Friedrichs AG, Hoyt JD. Developmental changes in memorization processes. Cognitive Psychology. 1970;1(4):324–340. doi: 10.1016/0010-0285(70)90019-8. [DOI] [Google Scholar]
- Friedman D, Nessler D, Johnson R. Memory encoding and retrieval in the aging brain. Clinical EEG and Neuroscience. 2007;38(1):2–7. doi: 10.1177/155005940703800105. [DOI] [PubMed] [Google Scholar]
- Friedman D, Trott C. An event-related potential study of encoding in young and older adults. Neuropsychologia. 2000;38(5):542–557. doi: 10.1016/S0028-3932(99)00122-0. [DOI] [PubMed] [Google Scholar]
- Güler OE, Thomas KM. Developmental differences in the neural correlates of relational encoding and recall in children: An event-related fMRI study. Developmental Cognitive Neuroscience. 2013;3:106–116. doi: 10.1016/j.dcn.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S, DeMaster DM, Yonelinas AP, Bunge SA. Developmental differences in medial temporal lobe function during memory encoding. The Journal of Neuroscience. 2010;30(28):9548–9556. doi: 10.1523/JNEUROSCI.3500-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RNA, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: An event-related functional magnetic resonance imaging study. The Journal of Neuroscience. 1999;19(10):3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe ML, Courage ML. Independent paths in the development of infant learning and forgetting. Journal of Experimental Child Psychology. 1997;67(2):131–163. doi: 10.1006/jecp.1997.2395. [DOI] [PubMed] [Google Scholar]
- Howe ML, O’Sullivan JT. What children’s memories tell us about recalling our childhoods: A review of storage and retrieval processes in the development of long-term retention. Developmental Review. 1997;17(2):148–204. doi: 10.1006/drev.1996.0428. [DOI] [Google Scholar]
- Kee DW, Bell TS. The development of organizational strategies in the storage and retrieval of categorical items in free-recall learning. Child Development. 1981;52(4):1163–1171. doi: 10.2307/1129502. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Lavenex BP. Building hippocampal circuits to learn and remember: Insights into the development of human memory. Behavioural Brain Research. 2013;254:8–21. doi: 10.1016/j.bbr.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Luck SJ. An introduction to the event-related potential technique. Cambridge MA: The MIT press; 2014. [Google Scholar]
- Marshall DH, Drummey AB, Fox NA, Newcombe NS. An event-related potential study of item recognition memory in children and adults. Journal of Cognition and Development. 2002;3(2):201–224. doi: 10.1207/s15327647jcd0302_4. [DOI] [Google Scholar]
- Menon V, Boyett-Anderson JM, Reiss AL. Maturation of medial temporal lobe response and connectivity during memory encoding. Cognitive Brain Research. 2005;25(1):379–385. doi: 10.1016/j.cogbrainres.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Brav TK, Levy O. The associative memory deficit of older adults: The role of strategy utilization. Psychology and Aging. 2007;22(1):202–208. doi: 10.1037/0882-7974.22.1.202. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Guez J, Marom M. The effects of divided attention at encoding on item and associative memory. Memory & Cognition. 2003;31(7):1021–1035. doi: 10.3758/bf03196123. [DOI] [PubMed] [Google Scholar]
- Nessler D, Johnson R, Jr, Bersick M, Friedman D. On why the elderly have normal semantic retrieval but deficient episodic encoding: A study of left inferior frontal ERP activity. NeuroImage. 2006;30(1):299–312. doi: 10.1016/j.neuroimage.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Ofen N. The development of neural correlates for memory formation. Neuroscience & Biobehavioral Reviews. 2012;36(7):1708–1717. doi: 10.1016/j.neubiorev.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofen N, Kao YC, Sokol-Hessner P, Kim H, Whitfield-Gabrieli S, Gabrieli JDE. Development of the declarative memory system in the human brain. Nature Neuroscience. 2007;10(9):1198–1205. doi: 10.1038/nn1950. doi: http://www.nature.com/neuro/journal/v10/n9/suppinfo/nn1950_S1.html. [DOI] [PubMed] [Google Scholar]
- Paz-Alonso PM, Gallego P, Ghetti S. Age differences in hippocampus-cortex connectivity during true and false memory retrieval. Journal of the International Neuropsychological Society. 2013;19(10):1031–1041. doi: 10.1017/S1355617713001069. [DOI] [PubMed] [Google Scholar]
- Pressley M. Elaboration and memory development. Child Development. 1982;53(2):296–309. doi: 10.2307/1128972. [DOI] [Google Scholar]
- Prince SE, Daselaar SM, Cabeza R. Neural correlates of relational memory: Successful encoding and retrieval of semantic and perceptual associations. The Journal of Neuroscience. 2005;25(5):1203–1210. doi: 10.1523/jneurosci.2540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D’Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42(1):2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Riggins T. Longitudinal investigation of source memory reveals different developmental trajectories for item memory and binding. Developmental Psychology. 2014;50(2):449–459. doi: 10.1037/a0033622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggins T, Blankenship SL, Mulligan E, Rice K, Redcay E. Developmental Differences in Relations Between Episodic Memory and Hippocampal Subregion Volume During Early Childhood. Child Development. 2015;86(6):1710–1718. doi: 10.1111/cdev.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggins T, Geng F, Blankenship SL, Redcay E. Hippocampal functional connectivity and episodic memory in early childhood. Developmental Cognitive Neuroscience. 2016;19:58–69. doi: 10.1016/j.dcn.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggins T, Rollins L. Developmental differences in memory during early childhood: Insights from event-related potentials. Child Development. 2015;86(3):889–902. doi: 10.1111/cdev.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggins T, Rollins L, Graham M. Electrophysiological investigation of source memory in early childhood. Developmental Neuropsychology. 2013;38(3):180–196. doi: 10.1080/87565641.2012.762001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins L, Riggins T. Developmental changes in memory encoding: insights from event-related potentials. Developmental Science. 2013;16(4):599–609. doi: 10.1111/desc.12072. [DOI] [PubMed] [Google Scholar]
- Rollins L, Riggins T. Age-related differences in subjective recollection: ERP studies of encoding and retrieval. Developmental Science. 2017:e12583. doi: 10.1111/desc.12583. n/a. [DOI] [PubMed] [Google Scholar]
- Sastre M, Wendelken C, Lee JK, Bunge SA, Ghetti S. Age- and performance-related differences in hippocampal contributions to episodic retrieval. Developmental Cognitive Neuroscience. 2016;19:42–50. doi: 10.1016/j.dcn.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenck C, Bjorklund DF, Schneider W. Developmental and individual differences in young children’s use and maintenance of a selective memory strategy. Developmental Psychology. 2009;45(4):1034–1050. doi: 10.1037/a0015597. [DOI] [PubMed] [Google Scholar]
- Sluzenski J, Newcombe NS, Kovacs SL. Binding, relational memory, and recall of naturalistic events: A developmental perspective. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2006;32(1):89–100. doi: 10.1037/0278-7393.32.1.89. [DOI] [PubMed] [Google Scholar]
- Wegesin DJ, Jacobs DM, Zubin NR, Ventura PR, Stern Y. Source memory and encoding strategy in normal aging. Journal of Clinical and Experimental Neuropsychology. 2000;22(4):455–464. doi: 10.1076/1380-3395(200008)22:4;1-0;ft455. [DOI] [PubMed] [Google Scholar]
- Wickelgren WA. Age and storage dynamics in continuous recognition memory. Developmental Psychology. 1975;11(2):165–169. doi: 10.1037/h0076457. [DOI] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46(3):441–517. doi: 10.1006/jmla.2002.2864. [DOI] [Google Scholar]