Abstract
13C-Metabolic flux analysis (13C-MFA) is a powerful model-based analysis technique for determining intracellular metabolic fluxes in living cells. It has become a standard tool in many labs for quantifying cell physiology, e.g. in metabolic engineering, systems biology, biotechnology, and biomedical research. With the increasing number of 13C-MFA studies published each year, it is now ever more important to provide practical guidelines for performing and publishing 13C-MFA studies so that quality is not sacrificed as the number of publications increases. The main purpose of this paper is to provide an overview of good practices in 13C-MFA, which can eventually be used as minimum data standards for publishing 13C-MFA studies. The motivation for this work is two-fold: (1) currently, there is no general consensus among researchers and journal editors as to what minimum data standards should be required for publishing 13C-MFA studies; as a result, there are great discrepancies in terms of quality and consistency; and (2) there is a growing number of studies that cannot be reproduced or verified independently due to incomplete information provided in these publications. This creates confusion, e.g. when trying to reconcile conflicting results, and hinders progress in the field. Here, we review current status in the 13C-MFA field and highlight some of the shortcomings with regards to 13C-MFA publications. We then propose a checklist that encompasses good practices in 13C-MFA. We hope that these guidelines will be a valuable resource for the community and allow 13C-flux studies to be more easily reproduced and accessed by others in the future.
Keywords: Minimum data standards, fluxomics, metabolism, stable isotopes, good practices in 13C-MFA
1. INTRODUCTION
Over the past 20 years, 13C metabolic flux analysis (13C-MFA) has emerged as an accurate and reliable method for measuring intracellular metabolic fluxes (Crown and Antoniewicz, 2013). In vivo fluxes provide valuable information on cellular physiology that can be applied for engineering metabolic and regulatory pathways, improving cellular phenotypes, and understanding mechanisms of disease. As such, 13C-MFA has found numerous applications in a range of research areas, including in metabolic engineering (Reed et al., 2010; Stephanopoulos, 1999), systems biology (Niklas and Heinzle, 2012), biotechnology (Ahn and Antoniewicz, 2012; Boghigian et al., 2010), and biomedical research (Hiller and Metallo, 2013). 13C-MFA offers significant advantages over alternative approaches for determining metabolic fluxes such as flux balance analysis (FBA) and stoichiometric MFA. Notably, 13C-MFA can accurately determine fluxes of metabolic cycles and parallel pathways (Ahn and Antoniewicz, 2013; Klein and Heinzle, 2012), compartment-specific fluxes (Kruger et al., 2012; O'Grady et al., 2012; Wahrheit et al., 2011), stereochemistry-specific fluxes (Crown et al., 2011), and reversible reactions (Yoo et al., 2008). After two decades of research and development, 13C-MFA has reached a high level of maturity; the experimental, analytical and computational approaches have been standardized (Antoniewicz et al., 2007b; Wiechert et al., 2001), and several advanced software packages are available for designing and analyzing tracer experiments (Quek et al., 2009; Weitzel et al., 2013).
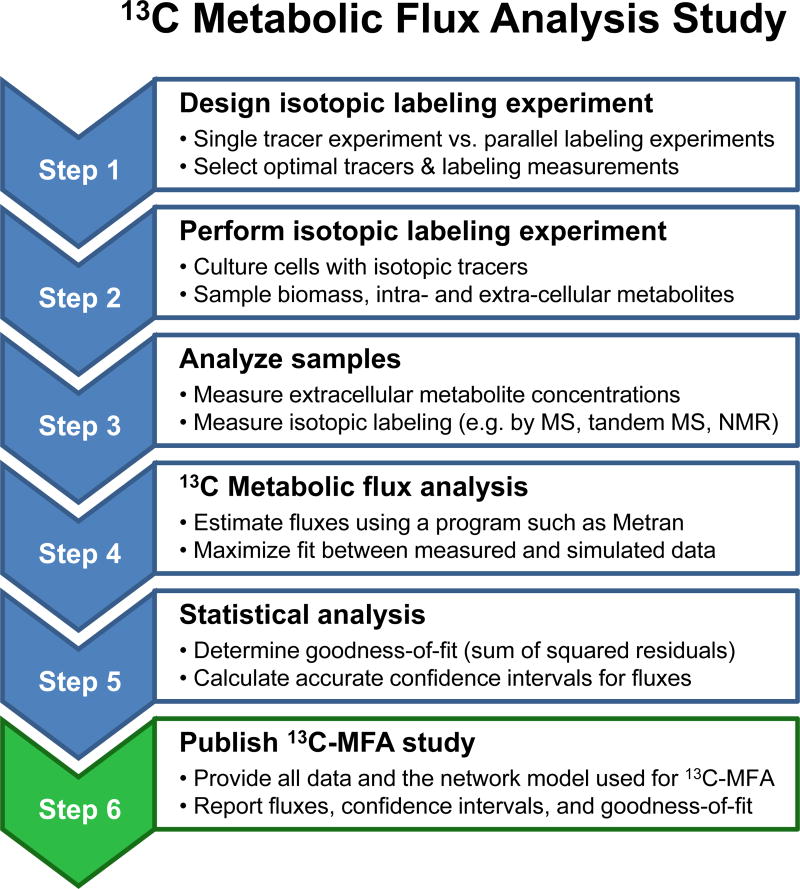
Given the advantages of 13C-MFA and the availability of easy-to-use software tools, the number of studies utilizing 13C-MFA has steadily increased over the past decade. At the same time, it is important to recognize that performing 13C-MFA studies is not a trivial task (Crown and Antoniewicz, 2013). As outlined in Figure 1, 13C-MFA studies consist of the following steps: designing and performing isotopic labeling experiments (Antoniewicz, 2013a; Crown et al., 2012), measuring isotopic labeling (Antoniewicz et al., 2007a), estimating fluxes by least-squares regression (Wiechert et al., 2001), and determining statistical properties including goodness-of-fit and confidence intervals of fluxes (Antoniewicz et al., 2006). With this process of 13C-MFA in mind, it is valuable to reflect on the historical development of the field. In the early days of 13C-MFA, only a handful of groups worked in this area and these groups could be considered experts. The researchers were heavily focused on all aspects of 13C-MFA; they were very meticulous about the details of the entire flux analysis process, from protocol development and data quality to model construction and model validation. The information provided in these studies was often sufficient to reproduce the reported analysis results. Now that 13C-MFA is used by a much wider audience with diverse scientific backgrounds, it is critical that the quality of 13C-MFA studies is maintained at a high level. In this paper, we reviewed current status in the 13C-MFA field and concluded that only about 30% of the studies examined were found to be acceptable. Thus, there is a clear need for practical guidelines that encourage good practices in 13C-MFA.
Figure 1.
Workflow for 13C metabolic flux analysis.
The main purpose of this paper is to provide such guidelines, which can eventually serve as the basis for establishing minimum standards for publishing 13C-MFA studies. The motivation for this work is two-fold: (1) currently, there is no general consensus among researchers and journal editors as to what minimum data standards should be required for publishing 13C-MFA studies. This has resulted in great discrepancies in terms of quality and consistency; and (2) increasing number of studies are published that cannot be reproduced or verified independently due to incomplete information provided in these studies. This hinders progress in the field and can often generate confusion, e.g. when trying to reconcile conflicting reports. In the end, we hope that the guidelines provided in this paper will serve as useful checklist for researchers and journal editors to enhance the quality of 13C-MFA publications in the future.
2. GOOD PRACTICES IN 13C-MFA
2.1. The need for minimum standards for 13C-MFA
The ability to reproduce results is the underpinning of the scientific process. In the case of 13C-MFA, this means reproducing not only the experimental data but also the computational results, i.e. the estimated fluxes, goodness-of-fit, and confidence intervals of fluxes. 13C-MFA differs from other omics techniques, such as genomics, transcriptomics, proteomics, and metabolomics in that 13C-MFA provides dynamic information on the flow of matter in biological systems. However, isotopic labeling measurements do not directly measure fluxes; instead the labeling data must be analyzed using a model to extract flux information (Wiechert et al., 2001). As a consequence, the accuracy of the flux analysis results depends both on the accuracy of the measurement set and the accuracy of the model used to interpret the data. Much work has been done in ensuring that isotopic labeling measurements by MS, tandem MS and NMR are accurate (Antoniewicz, 2013b; Antoniewicz et al., 2007a; Antoniewicz et al., 2011; Choi et al., 2012; Szyperski, 1995). In contrast, there are still uncertainties regarding the models employed in 13C-MFA. Even for well-studied organisms such as E. coli there are numerous (slightly different) metabolic network models used by different research groups (Antoniewicz et al., 2007c; Schaub et al., 2008; Toya et al., 2010), and these models are continually updated (Leighty and Antoniewicz, 2012). To promote more clarity and consistency in the field, it would be valuable to establish good practices for performing and publishing 13C-MFA studies. This would ensure that other researchers can reproduce the reported 13C-MFA results using the original data set and the original model, or repeat the flux analysis calculations at a later time using an updated model if needed.
Thus, the primary objective of establishing good practices in 13C-MFA is to promote clarity and reproducibility. In the long term, this may also have additional positive outcomes, such as establishing data standards for databases for depositing isotopic labeling data, 13C-MFA models, and 13C-flux analysis results (Chelliah et al., 2013). Through such databases, flux studies could be more easily shared with other researchers and consistency between different studies could be enhanced. Universal data standards in other omics fields have already greatly facilitated distribution of research results, opened new research directions in systems biology, and increased accountability (Brazma, 2009; Brazma et al., 2001; Brazma et al., 2006; Ioannidis et al., 2009; Klipp et al., 2007; Kohl, 2011). The guidelines we propose for 13C-MFA are shown in Table 1 and are described in more detail next. We have divided the guidelines into seven categories: (1) experiment description; (2) metabolic network model; (3) external flux data; (4) isotopic labeling data; (5) flux estimation; (6) goodness-of-fit; and (7) flux confidence intervals.
Table 1.
Good practices in publishing 13C-MFA studies
| Category | Minimum information | Recommended additional information |
|---|---|---|
| 1. Experiment description |
|
|
| 2. Metabolic network model |
|
|
| 3. External flux data |
|
|
| 4. Isotopic labeling data |
|
|
| 5. Flux estimation |
|
|
| 6. Goodness-of-fit |
|
|
| 7. Flux confidence intervals |
|
|
2.2. Experiment description
An accurate and complete description of the tracer experiment is needed to correctly interpret the analysis results and compare and contrast the results with other literature data. As a good practice, we recommend that the following information is provided. First, information regarding the source of cells, medium, and isotopic tracers, including details about the specific strain used, medium supplements added (e.g. serum, yeast extract, inhibitors, growth factors, etc.), and manufacturer’s specification of the isotopic purity of tracers. Second, information regarding the execution of the isotopic labeling experiment, including how cells were grown, how/when tracers were introduced, how long the labeling period was, how metabolism was quenched, how cells were harvested, and how metabolites were extracted. Third, information about the measurements performed and the analytical techniques used, including when the samples were collected, what metabolites were measured, how the growth rate and external rates were determined, and what methods were used to measure isotopic labeling. In particular, it is important that the description of MS and NMR measurement conditions is complete. As additional information, we also recommend describing how the isotopic labeling experiment was designed. For example, how were the isotopic tracers and the labeling measurements selected. It is well known that the design of the tracer experiment determines for a large part the accuracy with which fluxes can be estimated (Crown and Antoniewicz, 2012; Metallo et al., 2009; Nargund and Sriram, 2013; Walther et al., 2013). Thus, providing insight into the design of the experiment can be valuable as it may help other researchers to better design their own experiments (Antoniewicz, 2013a).
2.3. Metabolic network model
The metabolic network model is at the center of all flux analysis studies. The model defines which fluxes will be considered in the analysis, and perhaps more importantly, which reactions will be excluded from the analysis, i.e. are considered insignificant (although this is not always clearly stated). As a good practice, we recommend listing all reactions in table form, as the information is then presented in a clear, concise manner, all substrates and products are explicitly stated, and atom transitions can easily be defined. Presentation of network stoichiometry in a figure, or as a stoichiometry matrix, is acceptable as long as no reactions are omitted. As additional information, we also recommend including a list of balanced and non-balanced metabolites, so it is clear which metabolites are assumed to be at a metabolic (pseudo) steady-state, and which cofactors (e.g. ATP, NADH, and NADPH) are additionally balanced. In addition to defining the network stoichiometry, definition of the atom transitions is needed for 13C-MFA. Since atom transitions are well known for central carbon metabolism it is not critical that these are always included. However, we do recommend specifying atom transitions for less common metabolic pathways, reactions that can have alternative stereochemistries (e.g. Re and Si-citrate synthase) (Crown et al., 2011), and amino acid pathways, which can vary between different organisms (e.g. isoleucine, threonine, methionine, and lysine). Finally, as a summary of the model, we recommend listing the number of reactions, the number of fluxes, the number of balanced and non-balanced metabolites, and the number of free fluxes (i.e. the rank of the null space of the stoichiometry matrix).
2.4. External flux data
The external flux data are the net cellular uptake and excretion rates of metabolites. This is the primary data used in stoichiometric MFA for calculating fluxes, and is also used in 13C-MFA. External rates are determined by observing changes in extracellular metabolite concentrations over time during a cell culture (Leighty and Antoniewicz, 2011). As a good practice, we recommend listing all of the calculated external rates in a table, expressed either as absolute rates (mol/h), biomass specific rates (mol/g/h), or as yields (e.g. mol of product produced /mol of substrate consumed). As additional information, we also recommend providing all of the primary measurements that were used to calculate the external rates, i.e. the measured cell densities and metabolite concentrations over time. Preferably, the time-course data should be presented in a table form. In the case of a figure, the reader must estimate slopes of curves, which can result in inaccurate rate estimates. In addition, it is valuable to check carbon and electron balances using the calculated external rates to evaluate overall data consistency, if possible (van der Heijden et al., 1994), assuming gas rates such as O2 consumption and CO2 production rates were also measured.
2.5. Isotopic labeling data
The use of isotopic labeling data is the distinguishing feature between 13C-MFA and stoichiometric MFA. Labeling measurements carry valuable information about relative intracellular fluxes. Reliable isotopomer measurements are critical for determining accurate metabolic flux distributions by 13C-MFA. As a good practice, we recommend listing all measured isotopomer data in table form. For MS data, the raw (i.e. uncorrected) mass isotopomer distributions should be listed. It is not recommend to list only MS data that was corrected for natural isotope abundances, since there is still no agreement in the community which correction method should be used and different correction algorithms provide different corrected values (Fernandez et al., 1996; Lee et al., 1991; van Winden et al., 2002; Wahl et al., 2004). For NMR measurements, the relative areas of fine spectra and fractional enrichments of specific carbon atoms should be listed. For all isotopic labeling measurements it is important to provide the measurement precision (either assumed or measured), as this information is used for weighing of the residuals in least-squares regression and for determining the goodness-of-fit (Antoniewicz et al., 2006). As additional information, we also recommend providing clear descriptions of all labeling measurements to avoid any confusion. For example, for GC-MS data, we recommend identifying the metabolite name, derivatization method used (e.g. TBDMS, TMS), and the mass-to-charge ratio (m/z) of the measured fragments. For less common derivativation methods the atomic formulae of the fragments should also be listed. Lastly, we also recommend directly measuring the isotopic purity of the purchased tracers, as these can differ somewhat from the manufacturer’s specifications, and directly measuring tracer composition in the medium to account for any potential dilutions (e.g. from the inoculum), and to validate the composition of tracer mixtures (e.g. [1-13C]glucose + [U-13C]glucose (Antoniewicz, 2013c)).
2.6. Flux estimation
In 13C-MFA, flux estimation is accomplished by solving a large multi-parameter optimization problem, where the objective is to find flux values that best fit the observed extracellular rates and isotopic labeling data (Antoniewicz et al., 2006; Wiechert et al., 2001). In the early days of 13C-MFA, researchers often had to write their own program code to perform this analysis. In recent years, however, several software packages have become available, such as Metran, OpenFlux, and 13CFLUX that can be used for flux estimation (Quek et al., 2009; Weitzel et al., 2013; Yoo et al., 2008). The output of these programs consists, at a minimum, of the estimated fluxes for all reactions in the model and possibly a few other model parameters (e.g. dilution parameters such as D- and G-values (Kharroubi et al., 1992; Leighty and Antoniewicz, 2012)). As a good practice, we recommend describing what program was used for flux estimation and listing all of the estimated metabolic fluxes in a table. Fluxes can be presented as biomass specific fluxes (mol/g/h), or as fluxes normalized to substrate uptake rate (e.g. mol/100 mol glucose). Because flux estimation is inherently a non-linear optimization problem, there can be many local suboptimal solutions. In general, there is no guarantee that a solution is the global optimal solution. One common approach to address this issue is to restart flux estimation several times with random initial values for the fluxes. In our experience, restarting 13C-MFA at least 10 times with random values is an effective approach to search for the global solution.
2.7. Goodness-of-fit
After estimating the metabolic fluxes, the goodness of the fit must be assessed to determine if the fit is statistically acceptable. An acceptable fit gives credibility to the estimated fluxes and the conclusions drawn from these results. In practice, goodness-of-fit is evaluated by calculating the sum of squared residual (SSR), i.e. the weighted squared differences between the measured and simulated data (Antoniewicz et al., 2006). Assuming that the model is correct and the data are without gross measurement errors, the minimized weighted SSR is a stochastic variable with a χ2-distribution. The number of degrees of freedom is equal to the number of fitted measurements n minus the number of estimated independent parameters p. The acceptable range of SSR values is between χ2α/2(n-p) and χ21-α/2(n-p), where α is a certain chosen threshold value, for example 0.05 for 95% confidence interval. As a good practice, we recommend performing the χ2-statistical test for SSR. It is important to indicate what measurement precisions were used to calculate the SSR value. If the SSR value falls within the statistically acceptable range the fit is accepted. If the SSR is outside acceptable bounds, the network model and the measurement data set must be reassessed until an acceptable SSR value is obtained. As additional information, we also recommend listing in a table the experimental and fitted measurements to allow easy comparison.
2.8. Flux confidence intervals
Determining accurate confidence intervals for estimated metabolic fluxes is just as important as estimating the flux values themselves (Antoniewicz et al., 2006). Confidence intervals reflect the certainty with which the fluxes were determined. This information is critical, for example, to determine the statistical significance of changes in fluxes between different experimental conditions or strains. In the early days of 13C-MFA, confidence intervals of fluxes were determined by linearizing the isotopomer equations near the optimal solution and applying linear statistics (Wiechert et al., 1997). However, because isotopomer equations are inherently non-linear, more accurate methods were developed for 13C-MFA (Antoniewicz et al., 2006). Currently, the two preferred methods for determining accurate (nonlinear) confidence intervals of fluxes are based on evaluating the sensitivity of SSR to flux variations (Antoniewicz et al., 2006; Kleijn et al., 2006), or using Monte Carlo simulations. As a good practice, we recommend describing what method was used to determine confidence intervals of fluxes and listing confidence intervals (commonly 95% confidence intervals) for all estimated metabolic fluxes. As additional information, we also recommend providing an indication of the nonlinear confidence intervals, for example, by listing both 68% and 95% confidence intervals (Antoniewicz et al., 2006).
3. REVIEW OF CURRENT STATUS IN THE FIELD
3.1. Literature search
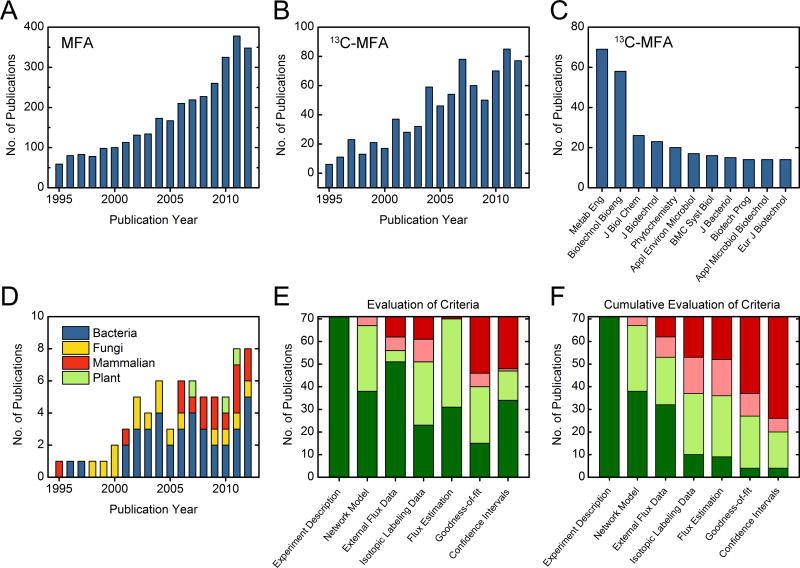
To evaluate current trends in 13C-MFA publications, we conducted a literature search in Web of Science using the search terms “metabolic flux analysis” and “13C or isotope or isotopic or isotopomer” (Fig. 2A&B). This resulted in about 800 papers. The top two journals that published 13C-MFA studies were Metabolic Engineering (MBE) and Biotechnology and Bioengineering(B&B) (Fig. 2C). Next, we searched articles in MBE and B&B that contained the search terms ‘metabolic flux’ or ‘flux analysis’ or ‘fluxes’. This second, more exhaustive search resulted in about 700 papers in MBE and B&B. All of the papers were then inspected individually and divided into one of the following categories: 13C-MFA, MFA, FBA, and other. Out of the 700 papers, 71 were experimental 13C-MFA papers and about 50 were theoretical or simulation-based 13C-MFA studies. We estimated that the subset of 71 experimental 13C-MFA papers represented about 20% of papers published in the 13C-MFA field. These papers covered a diverse set of organisms, including bacteria, fungi, mammalian cells, and plants (Fig. 2D). The papers were then evaluated according to the seven categories described in the previous sections (see also Supplementary Data).
Figure 2.
Evaluation of publication practices in 13C-MFA. (A) Annual number of publications on the topic of metabolic flux analysis (Web of Science search query “metabolic flux analysis” on 1/4/2013). (B) Annual number of publications on the topic of 13C metabolic flux analysis (Web of Science search query “metabolic flux analysis” and “13C or isotope or isotopic or isotopomer” on 1/4/2013). (C) Total number of 13C-MFA studies published in selected scientific journals. (D) Annual number of 13C-MFA publications in Metabolic Engineering (MBE) and Biotechnology and Bioengineering (B&B) and the organisms studied. (E) Evaluation of 13C-MFA publications in MBE and B&B according to the categories listed in Table 1. Color coding: dark green = satisfies good practices in 13C-MFA; light green = acceptable; light red = not recommended; dark red = not acceptable (see Supplementary data for details). (F) Cumulative evaluation of 13C-MFA publications in MBE and B&B.
3.2. Review of current status in 13C-MFA
All 71 experimental 13C-MFA papers provided sufficient experimental details, including details about the materials and strains used, the execution of the tracer experiment, and analytical methods used. The metabolic network model was also provided in all 71 papers: 38 papers presented the network in tabular form (good practice), 29 papers illustrated the network in a figure, and 4 papers referred to a previous publication where the model was described (Fig. 2E). External flux data and isotopic labeling data were also provided in a majority of the papers. Out of the 71 papers, 51 papers provided external flux data in a table (good practice), and 11 papers presented the data in the text or a figure; the remaining 9 papers did not report any external flux data. For isotopomer data, 51 papers provided the labeling measurements in a table (good practice), 10 in a figure (not recommended), and 10 papers did not provide any isotopic labeling data. All but one paper provided the estimated metabolic fluxes: 31 papers presented the estimated fluxes in a table (good practice), and 40 in a figure. As shown in Fig. 2E, most of the papers followed good practices in categories 1 through 5. However, more significant deficiencies were identified in the last two categories, i.e. “goodness-of-fit” and “flux confidence intervals”. Only a small fraction of the papers (15 papers) calculated the SSR value and determined if the fit was statistically acceptable (good practice); 25 papers presented only the simulated and experimental data in a table, and 6 papers showed this comparison in a figure (not recommended). Confidence intervals for the estimated fluxes were provided in 47 out of 71 papers: 34 papers provided the confidence intervals in a table (good practice), and 13 in a figure; the remaining 24 papers did not provide any confidence intervals for fluxes. Figure 2F summarizes our analysis of the current trends in 13C-MFA, for publications in the journals MBE and B&B.
4. DISCUSSION
In this paper, we have summarized good practices in 13C-MFA and provided a checklist for 13C-MFA studies that can enhance the quality of publications and ensure that flux analysis results can be independently verified (Table 1). After evaluating current status in the field, we concluded that only 30% of the studies examined were found to be acceptable based on our recommendations. On a whole, for the sampling of papers in MBE and B&B, the researchers did a satisfactory job in discussing the experimental conditions, presenting model stoichiometry, and displaying the estimated fluxes. Most papers were also sufficient in providing external flux data and isotopomer data. However, significant deficiencies were found in the analysis and reporting of the confidence intervals of estimated fluxes and evaluating the goodness-of-fit to ensure that the fit was statistically acceptable.
It is important to note that throughout the process of compiling these statistics we were generous in evaluating the papers. If anything, the statistics presented here overestimate the quality of published studies in 13C-MFA. For example, in the case of isotopomer data, we did not distinguish between papers that provided the complete listing of all isotopic measurements and papers that provided only a subset of the data. Similarly, for the estimated fluxes and confidence intervals, we treated papers that exhaustively listed all estimated fluxes and all confidence intervals the same as papers that provided only a single estimated flux and confidence interval. Going forward, it is important to place more emphasis on reporting the complete measurement data and complete analysis results, either in the body of the paper or as supplemental data, since partial information, whether it be isotopomer data, estimated fluxes, or confidence intervals, is not sufficient to fully verify the analysis results. While the minimum data standards proposed here were designed for reporting 13C-MFA studies, they can also be used as standards for reporting MFA studies without tracers, and isotopic non-stationary 13C-MFA studies (Noh et al., 2007; Young et al., 2011; Young et al., 2008).
5. CONCLUSIONS
13C-Metabolic flux analysis has become a widely used technique in modern biological sciences for determining intracellular metabolic fluxes in complex biological systems. Many significant results have been derived from 13C-MFA studies and the number of publications in 13C-MFA is continually increasing. However, until now there have been no standards established for reporting 13C-MFA results. In this paper, we have proposed a first set of guidelines, which can act as minimum data standards for publishing 13C-MFA studies. The ultimate goal of establishing these standards is to ensure that flux analysis results can be independently verified. We believe that implementing these standards will increase clarity, reproducibility, accountability, and in the long term can lead to establishing databases and public repositories for 13C-MFA studies. In the short term, we hope that these guidelines will serve as a useful checklist for researchers and journal editors to enhance the quality of 13C-MFA publications.
Supplementary Material
Acknowledgments
This work was supported by the NSF CAREER Award (CBET-1054120) to MRA, and the NSF Graduate Fellowship to SBC.
ABBREVIATIONS
- B&B
Biotechnology and Bioengineering
- GC
gas chromatography
- MBE
Metabolic Engineering
- MFA
metabolic flux analysis
- MS
mass spectrometry
- NMR
nuclear magnetic resonance
- SSR
sum of squared residuals.
References
- Ahn WS, Antoniewicz MR. Towards dynamic metabolic flux analysis in CHO cell cultures. Biotechnol J. 2012;7:61–74. doi: 10.1002/biot.201100052. [DOI] [PubMed] [Google Scholar]
- Ahn WS, Antoniewicz MR. Parallel labeling experiments with [1,2-13C]glucose and [U-13C]glutamine provide new insights into CHO cell metabolism. Metab Eng. 2013;15:34–47. doi: 10.1016/j.ymben.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Antoniewicz MR. 13C Metabolic flux analysis: optimal design of isotopic labeling experiments. Curr Opin Biotechnol. 2013a doi: 10.1016/j.copbio.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Antoniewicz MR. Tandem mass spectrometry for measuring stable-isotope labeling. Curr Opin Biotechnol. 2013b;24:48–53. doi: 10.1016/j.copbio.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Antoniewicz MR. Using Multiple Tracers for 13C Metabolic Flux Analysis. Methods Mol Biol. 2013c;985:353–365. doi: 10.1007/978-1-62703-299-5_17. [DOI] [PubMed] [Google Scholar]
- Antoniewicz MR, Kelleher JK, Stephanopoulos G. Determination of confidence intervals of metabolic fluxes estimated from stable isotope measurements. Metab Eng. 2006;8:324–37. doi: 10.1016/j.ymben.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Antoniewicz MR, Kelleher JK, Stephanopoulos G. Accurate assessment of amino acid mass isotopomer distributions for metabolic flux analysis. Anal Chem. 2007a;79:7554–9. doi: 10.1021/ac0708893. [DOI] [PubMed] [Google Scholar]
- Antoniewicz MR, Kelleher JK, Stephanopoulos G. Elementary metabolite units (EMU): a novel framework for modeling isotopic distributions. Metab Eng. 2007b;9:68–86. doi: 10.1016/j.ymben.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniewicz MR, Kelleher JK, Stephanopoulos G. Measuring deuterium enrichment of glucose hydrogen atoms by gas chromatography/mass spectrometry. Anal Chem. 2011;83:3211–6. doi: 10.1021/ac200012p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniewicz MR, Kraynie DF, Laffend LA, Gonzalez-Lergier J, Kelleher JK, Stephanopoulos G. Metabolic flux analysis in a nonstationary system: fed-batch fermentation of a high yielding strain of E. coli producing 1,3-propanediol. Metab Eng. 2007c;9:277–92. doi: 10.1016/j.ymben.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boghigian BA, Seth G, Kiss R, Pfeifer BA. Metabolic flux analysis and pharmaceutical production. Metab Eng. 2010;12:81–95. doi: 10.1016/j.ymben.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Brazma A. Minimum Information About a Microarray Experiment (MIAME)--successes, failures, challenges. TheScientificWorldJournal. 2009;9:420–3. doi: 10.1100/tsw.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, Stoeckert C, Aach J, Ansorge W, Ball CA, Causton HC, Gaasterland T, Glenisson P, Holstege FC, Kim IF, Markowitz V, Matese JC, Parkinson H, Robinson A, Sarkans U, Schulze-Kremer S, Stewart J, Taylor R, Vilo J, Vingron M. Minimum information about a microarray experiment (MIAME)-toward standards for microarray data. Nat Genet. 2001;29:365–71. doi: 10.1038/ng1201-365. [DOI] [PubMed] [Google Scholar]
- Brazma A, Krestyaninova M, Sarkans U. Standards for systems biology. Nature reviews. Genetics. 2006;7:593–605. doi: 10.1038/nrg1922. [DOI] [PubMed] [Google Scholar]
- Chelliah V, Laibe C, Le Novere N. BioModels Database: a repository of mathematical models of biological processes. Methods Mol Biol. 2013;1021:189–99. doi: 10.1007/978-1-62703-450-0_10. [DOI] [PubMed] [Google Scholar]
- Choi J, Grossbach MT, Antoniewicz MR. Measuring complete isotopomer distribution of aspartate using gas chromatography/tandem mass spectrometry. Anal Chem. 2012;84:4628–32. doi: 10.1021/ac300611n. [DOI] [PubMed] [Google Scholar]
- Crown SB, Ahn WS, Antoniewicz MR. Rational design of 13C-labeling experiments for metabolic flux analysis in mammalian cells. BMC Syst Biol. 2012;6:43. doi: 10.1186/1752-0509-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crown SB, Antoniewicz MR. Selection of tracers for (13)C-Metabolic Flux Analysis using Elementary Metabolite Units (EMU) basis vector methodology. Metab Eng. 2012;14:150–161. doi: 10.1016/j.ymben.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crown SB, Antoniewicz MR. Parallel labeling experiments and metabolic flux analysis: past, present and future methodologies. Metab Eng. 2013;16:21–32. doi: 10.1016/j.ymben.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crown SB, Indurthi DC, Ahn WS, Choi J, Papoutsakis ET, Antoniewicz MR. Resolving the TCA cycle and pentose-phosphate pathway of Clostridium acetobutylicum ATCC 824: Isotopomer analysis, in vitro activities and expression analysis. Biotechnol J. 2011;6:300–5. doi: 10.1002/biot.201000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez CA, Des Rosiers C, Previs SF, David F, Brunengraber H. Correction of 13C mass isotopomer distributions for natural stable isotope abundance. J Mass Spectrom. 1996;31:255–62. doi: 10.1002/(SICI)1096-9888(199603)31:3<255::AID-JMS290>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Hiller K, Metallo CM. Profiling metabolic networks to study cancer metabolism. Curr Opin Biotechnol. 2013 doi: 10.1016/j.copbio.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Ioannidis JP, Allison DB, Ball CA, Coulibaly I, Cui X, Culhane AC, Falchi M, Furlanello C, Game L, Jurman G, Mangion J, Mehta T, Nitzberg M, Page GP, Petretto E, van Noort V. Repeatability of published microarray gene expression analyses. Nat Genet. 2009;41:149–55. doi: 10.1038/ng.295. [DOI] [PubMed] [Google Scholar]
- Kharroubi AT, Masterson TM, Aldaghlas TA, Kennedy KA, Kelleher JK. Isotopomer spectral analysis of triglyceride fatty acid synthesis in 3T3-L1 cells. Am J Physiol. 1992;263:E667–75. doi: 10.1152/ajpendo.1992.263.4.E667. [DOI] [PubMed] [Google Scholar]
- Kleijn RJ, van Winden WA, Ras C, van Gulik WM, Schipper D, Heijnen JJ. 13C-labeled gluconate tracing as a direct and accurate method for determining the pentose phosphate pathway split ratio in Penicillium chrysogenum. Appl Environ Microbiol. 2006;72:4743–54. doi: 10.1128/AEM.02955-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S, Heinzle E. Isotope labeling experiments in metabolomics and fluxomics. Wiley interdisciplinary reviews. Systems biology and medicine. 2012;4:261–72. doi: 10.1002/wsbm.1167. [DOI] [PubMed] [Google Scholar]
- Klipp E, Liebermeister W, Helbig A, Kowald A, Schaber J. Systems biology standards--the community speaks. Nat Biotechnol. 2007;25:390–1. doi: 10.1038/nbt0407-390. [DOI] [PubMed] [Google Scholar]
- Kohl M. Standards, databases, and modeling tools in systems biology. Methods Mol Biol. 2011;696:413–27. doi: 10.1007/978-1-60761-987-1_26. [DOI] [PubMed] [Google Scholar]
- Kruger NJ, Masakapalli SK, Ratcliffe RG. Strategies for investigating the plant metabolic network with steady-state metabolic flux analysis: lessons from an Arabidopsis cell culture and other systems. J Exp Bot. 2012;63:2309–23. doi: 10.1093/jxb/err382. [DOI] [PubMed] [Google Scholar]
- Lee WN, Byerley LO, Bergner EA, Edmond J. Mass isotopomer analysis: theoretical and practical considerations. Biol Mass Spectrom. 1991;20:451–8. doi: 10.1002/bms.1200200804. [DOI] [PubMed] [Google Scholar]
- Leighty RW, Antoniewicz MR. Dynamic metabolic flux analysis (DMFA): a framework for determining fluxes at metabolic non-steady state. Metab Eng. 2011;13:745–55. doi: 10.1016/j.ymben.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Leighty RW, Antoniewicz MR. Parallel labeling experiments with [U-(13)C]glucose validate E. coli metabolic network model for (13)C metabolic flux analysis. Metab Eng. 2012;14:533–541. doi: 10.1016/j.ymben.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Metallo CM, Walther JL, Stephanopoulos G. Evaluation of 13C isotopic tracers for metabolic flux analysis in mammalian cells. J Biotechnol. 2009;144:167–74. doi: 10.1016/j.jbiotec.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargund S, Sriram G. Designer labels for plant metabolism: statistical design of isotope labeling experiments for improved quantification of flux in complex plant metabolic networks. Molecular bioSystems. 2013;9:99–112. doi: 10.1039/c2mb25253h. [DOI] [PubMed] [Google Scholar]
- Niklas J, Heinzle E. Metabolic flux analysis in systems biology of Mammalian cells. Adv Biochem Eng Biotechnol. 2012;127:109–32. doi: 10.1007/10_2011_99. [DOI] [PubMed] [Google Scholar]
- Noh K, Gronke K, Luo B, Takors R, Oldiges M, Wiechert W. Metabolic flux analysis at ultra short time scale: isotopically non-stationary 13C labeling experiments. J Biotechnol. 2007;129:249–67. doi: 10.1016/j.jbiotec.2006.11.015. [DOI] [PubMed] [Google Scholar]
- O'Grady J, Schwender J, Shachar-Hill Y, Morgan JA. Metabolic cartography: experimental quantification of metabolic fluxes from isotopic labelling studies. J Exp Bot. 2012;63:2293–308. doi: 10.1093/jxb/ers032. [DOI] [PubMed] [Google Scholar]
- Quek LE, Wittmann C, Nielsen LK, Kromer JO. OpenFLUX: efficient modelling software for 13C-based metabolic flux analysis. Microb Cell Fact. 2009;8:25. doi: 10.1186/1475-2859-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JL, Senger RS, Antoniewicz MR, Young JD. Computational approaches in metabolic engineering. J Biomed Biotechnol. 2010;2010:207414. doi: 10.1155/2010/207414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub J, Mauch K, Reuss M. Metabolic flux analysis in Escherichia coli by integrating isotopic dynamic and isotopic stationary 13C labeling data. Biotechnol Bioeng. 2008;99:1170–85. doi: 10.1002/bit.21675. [DOI] [PubMed] [Google Scholar]
- Stephanopoulos G. Metabolic fluxes and metabolic engineering. Metab Eng. 1999;1:1–11. doi: 10.1006/mben.1998.0101. [DOI] [PubMed] [Google Scholar]
- Szyperski T. Biosynthetically directed fractional 13C-labeling of proteinogenic amino acids. An efficient analytical tool to investigate intermediary metabolism. Eur J Biochem. 1995;232:433–48. doi: 10.1111/j.1432-1033.1995.tb20829.x. [DOI] [PubMed] [Google Scholar]
- Toya Y, Ishii N, Nakahigashi K, Hirasawa T, Soga T, Tomita M, Shimizu K. 13C-metabolic flux analysis for batch culture of Escherichia coli and its Pyk and Pgi gene knockout mutants based on mass isotopomer distribution of intracellular metabolites. Biotechnol Prog. 2010;26:975–92. doi: 10.1002/btpr.420. [DOI] [PubMed] [Google Scholar]
- van der Heijden RT, Romein B, Heijnen JJ, Hellinga C, Luyben KC. Linear constraint relations in biochemical reaction systems: II. Diagnosis and estimation of gross errors. Biotechnol Bioeng. 1994;43:11–20. doi: 10.1002/bit.260430104. [DOI] [PubMed] [Google Scholar]
- van Winden WA, Wittmann C, Heinzle E, Heijnen JJ. Correcting mass isotopomer distributions for naturally occurring isotopes. Biotechnol Bioeng. 2002;80:477–9. doi: 10.1002/bit.10393. [DOI] [PubMed] [Google Scholar]
- Wahl SA, Dauner M, Wiechert W. New tools for mass isotopomer data evaluation in (13)C flux analysis: mass isotope correction, data consistency checking, and precursor relationships. Biotechnol Bioeng. 2004;85:259–68. doi: 10.1002/bit.10909. [DOI] [PubMed] [Google Scholar]
- Wahrheit J, Nicolae A, Heinzle E. Eukaryotic metabolism: measuring compartment fluxes. Biotechnol J. 2011;6:1071–85. doi: 10.1002/biot.201100032. [DOI] [PubMed] [Google Scholar]
- Walther JL, Metallo CM, Zhang J, Stephanopoulos G. Optimization of (13)C isotopic tracers for metabolic flux analysis in mammalian cells. Metab Eng. 2013 doi: 10.1016/j.ymben.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzel M, Noh K, Dalman T, Niedenfuhr S, Stute B, Wiechert W. 13CFLUX2--high-performance software suite for 13C-metabolic flux analysis. Bioinformatics. 2013;29:143–5. doi: 10.1093/bioinformatics/bts646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiechert W, Mollney M, Petersen S, de Graaf AA. A universal framework for 13C metabolic flux analysis. Metab Eng. 2001;3:265–83. doi: 10.1006/mben.2001.0188. [DOI] [PubMed] [Google Scholar]
- Wiechert W, Siefke C, de Graaf AA, Marx A. Bidirectional reaction steps in metabolic networks: II. Flux estimation and statistical analysis. Biotechnol Bioeng. 1997;55:118–35. doi: 10.1002/(SICI)1097-0290(19970705)55:1<118::AID-BIT13>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Yoo H, Antoniewicz MR, Stephanopoulos G, Kelleher JK. Quantifying reductive carboxylation flux of glutamine to lipid in a brown adipocyte cell line. J Biol Chem. 2008;283:20621–7. doi: 10.1074/jbc.M706494200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JD, Shastri AA, Stephanopoulos G, Morgan JA. Mapping photoautotrophic metabolism with isotopically nonstationary (13)C flux analysis. Metab Eng. 2011;13:656–65. doi: 10.1016/j.ymben.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JD, Walther JL, Antoniewicz MR, Yoo H, Stephanopoulos G. An elementary metabolite unit (EMU) based method of isotopically nonstationary flux analysis. Biotechnol Bioeng. 2008;99:686–99. doi: 10.1002/bit.21632. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.