Abstract
Background
miR-23b overexpression can promote cardiomyocyte apoptosis and reduce cell growth under hypoxic conditions, suggesting that miR-23b acts as a biomarker for ST-elevation myocardial infarction (STEMI). The aim of this study was to investigate the effect of miR-23b on STEMI patients.
Material/Methods
We enrolled 80 eligible patients with STEMI and 60 control subjects. Blood samples were obtained at 6 h, 12 h, 24 h, 48 h, 3 days, and 7 days after the onset of symptoms. Another blood sample was collected before and after percutaneous coronary intervention (PCI). The samples were used for real-time quantitative PCR analysis. A Siemens Immulite2000 detector (Germany) was used for cTnI detection, and the serum CK-MB content was detected by electrochemical luminescence method.
Results
The expression level of miR-23b was increased in patients with STEMI (P<0.05). No significance difference was observed among risk factors, although the clinical data was comparable (P>0.05). The level of miR-23b in STEMI patients after PCI was lower (P<0.05). The ROC curve of plasma miR-23b showed a separation, with an AUC of 0.809 (95%CI, 0.737–0.936, P<0.05), compared to CK-MB with an AUC of 0.753 (95%CI, 0.707–0.896) and cTnI with an AUC of 0.783 (95%CI, 0.723–0.917).
Conclusions
The present study reveals that miR-23b is a useful biomarker of STEMI, providing a novel insight for the diagnosis for STEMI.
MeSH Keywords: Anterior Wall Myocardial Infarction; Creatine Kinase, MB Form; MicroRNAs; Troponin I
Background
Acute myocardial infarction (AMI) is a severe cardiovascular disease caused by acute artery occlusion, characterized by high mortality and poor prognosis [1]. The diagnosis of patients with ST-elevation myocardial infarction (STEMI) mainly depends on clinical manifestation and myocardial enzyme changes, among which the level of troponins (cTns) is widely regarded as the criterion standard [2]. However, previous studies show that cTns is significantly increased in the treatment of various diseases [3,4]. Given that the specificity of cTns for STEMI is not sufficient, it is of great importance to seek more targeted STEMI diagnostic markers with higher sensitivity and specificity.
MicroRNAs are a class of highly conservative non-coding small RNAs (about 21–24 nucleotides) that regulate gene expression in signal pathways, which play an important role in maintaining the homeostasis [5]. It regulates post-transcriptional gene silencing, promotes mRNA degradation or inhibits the transcription by recognizing the 3′UTR of target mRNA, thus controlling protein expression level, which participates in a series of reactions such as cell differentiation, metabolism, proliferation, and apoptosis [6].
He et al. found that miR-23b overexpression promotes cardiomyocyte apoptosis and reduces cell growth under hypoxic conditions [7], suggesting that miR-23b is a biomarker for STEMI. Thus, we aimed to investigate the level of miR-23b in STEMI patients and to characterize miR-23b as a novel biomarker for STEMI.
Material and Methods
Patients
We enrolled 80 eligible patients with STEMI and 60 control subjects from January 2015 to January 2017 at The First Hospital of Hebei Medical University. All STEMI patients were first diagnosed by percutaneous coronary intervention (PCI). All participants provided informed consent, and this study was approved by the Ethics Committee of The First Hospital of Hebei Medical University.
Samples collection
Blood samples were obtained at 6 h, 12 h, 24 h, 48 h, 3 days, and 7 days after the onset of symptoms, and another blood sample was collected at 24 h before and after PCI. The samples were centrifuged at 10 000 g for 15 min within 2 h of collection, followed by plasma separation and freezing at −80°C.
Real-time quantitative PCR analysis
The total RNA was isolated from the plasma by use of TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocols, then we assessed the quality and quantity of RNA using NanoDrop (NanoDrop Products, Wilmington, Del., USA). cDNA was synthesized from 50 ng of total RNA using an miRCURY LNA Universal cDNA synthesis kit (Exiqon, Vedbaek, Denmark). The expression level of miR-23b in plasma was determined by qRT-PCR, which was carried out in triplicate reactions with the SYBR Green PCR SuperMix Kit (TransGen Biotech, Beijing, China). The primer of miR-23b was ATCACATTGCCAGGGATTA.
CK-MB, cTnI detection
The Siemens Immulite2000 detector (Germany) was used for cTnI detection and the serum CK-MB content was detected by electrochemical luminescence method. Results were subjected to statistical analyses.
Statistical analysis
The data were analyzed using SPSS17.0 software and GraphPad. Normal distribution measurement data was calculated as the mean ±SD, and the t test was used for comparison between variable groups. Categorical variables were compared using the chi-square test. The correlation analysis of continuous variables was based on the Spearman test correlation method. The diagnostic efficacy of the plasma mir-23b content was analyzed with the ROC curve, and the area under the ROC curve (AUC) was calculated. P<0.05 was regarded as statistically significant.
Results
The expression level of miR-23b was increased in patients with STEMI
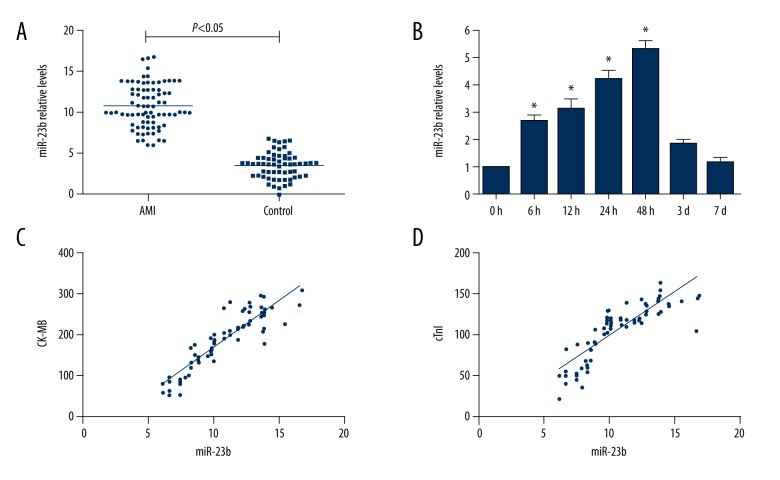
In this study, we selected 80 STEMI patients and 60 healthy volunteers. The baseline clinical characteristics of the patients and control groups are shown in Table 1. There were no significant differences between STEMI patients and controls in the baseline clinical data, including age, sex, blood pressure, body mass index, and other biochemical parameters. Plasma miR-23b expression levels detected immediately after admission were higher in the STEMI group than in the control group (Figure 1A, P<0.05). Blood samples obtained from STEMI patients at 0 h, 6 h, 12 h, 24 h, 48 h, 3 days, and 7 days were analyzed to investigate changes in miR-23b in STEMI patients. As presented in Figure 1B, the concentrations of miR-23b in STEMI patients were significantly elevated after the onset of symptoms, and achieved a peak at 48 h and nearly returned to the control levels after 7 days. CK-MB and cTnI concentrations were also detected, and were found to be positively correlated with miR-23b. Moreover, the results show that the relative miR-23b levels in STEMI patients were positively correlated with the serum concentrations of CK-MB (r=0.905, P<0.05) and cTnI (r=0.849, P<0.05) (Figure 1C, 1D).
Table 1.
The clinical information of two groups.
| Variable | STEMI (n=80) | Control (n=60) | P value |
|---|---|---|---|
| Male | 46 (57.5%) | 35 (58.3%) | 0.921 |
| Age | 62.83±7.52 | 63.15±7.32 | 0.723 |
| BMI (kg/m2) | 24.09±2.45 | 24.06±2.35 | 0.855 |
| Risk factors | |||
| Hypertension | 50 (62.5%) | 32 (53.3%) | 0.276 |
| Diabetes mellitus | 31 (38.8%) | 19 (31.7%) | 0.387 |
| Dyslipidemia | 48 (60.0%) | 31 (51.7%) | 0.325 |
| Smoking | 39 (48.8%) | 24 (40.0%) | 0.303 |
Figure 1.
miR-23b expression level in AMI and healthy controls. (A) Plasma level of miR-23b in AMI is significantly elevated compared with control group. (B) Plasma level of miR-23b in AMI patients at the indicated time point. Data are presented as the mean ±SD; P<0.05 versus health control. (C) Correlation between miR-23b and CK-MB in plasma from AMI patients. (D) The correlation between miR-23b and cTn in plasma from AMI patients.
The level of miR-23b in sub-groups of patients with STEMI
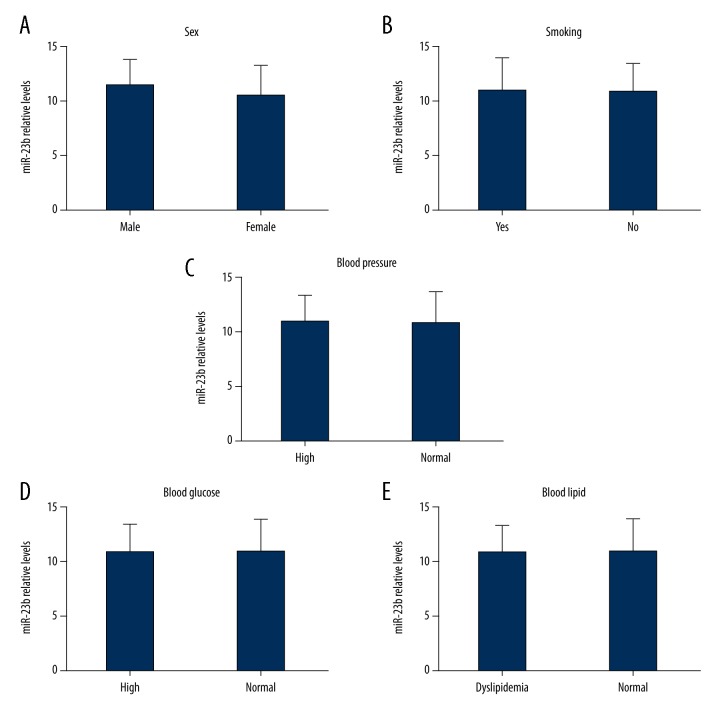
The main risk factors of acute cardiac infarction are age, smoking, blood pressure, blood glucose, and blood lipid. Thus, we investigated the expression of miR-23b in STEMI patients under different conditions. No significant difference was observed in different risk factors, although the clinical data were comparable (P>0.05; Figure 2).
Figure 2.
No significant differences were observed in sex, smoking, blood pressure, blood glucose, and blood lipid in patients with STEMI.
The level of miR-23b in STEMI patients after PCI
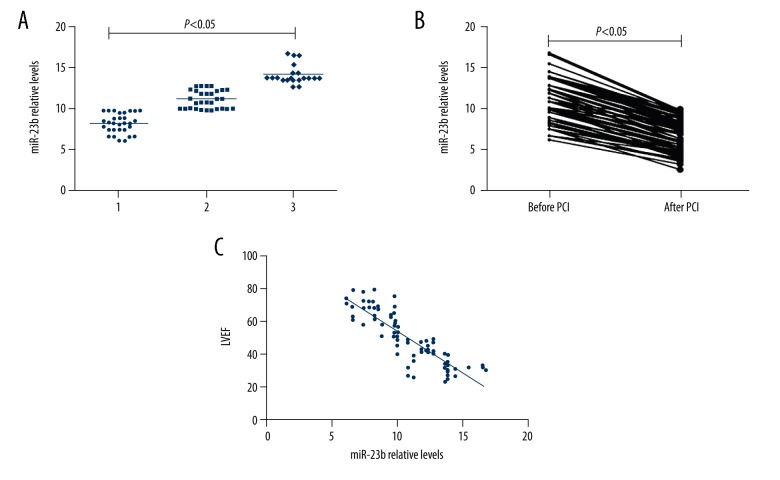
Plasma miR-23b level in the before PCI group was significantly higher than in the control group. As shown in Figure 3A, miR-23b levels were significantly higher in STEMI patients with 2- and 3-branch lesions (P<0.05). The expression of miR-23b in STEMI patients was significantly downregulated after PCI (P<0.05; Figure 3B). Furthermore, correlation analysis revealed that miR-23b levels were negatively correlated with left ventricular ejection fraction (r=−0.861, P<0.05; Figure 3C).
Figure 3.
(A) Plasma miR-23b level at indicated time points after PCI. (B) Plasma miR-23b levels in AMI patients after PCI were significantly lower than in the before PCI group (P<0.05). (C) Correlations between plasma miR-23b levels and LVEF in STEMI patients (r=−0.861, P<0.05).
The plasma miR-23b is a sensitive biomarker for STEMI
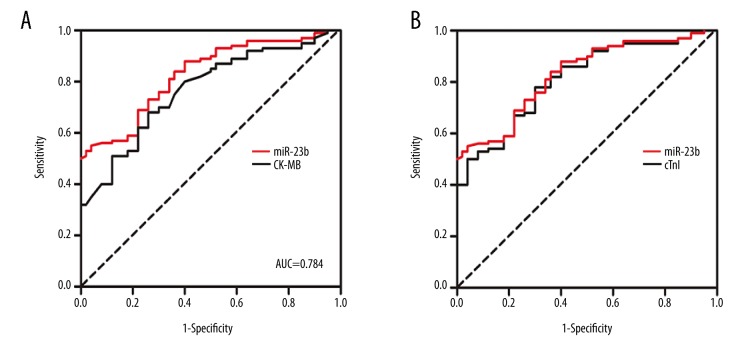
To evaluate the potential role of miR-23b as a biomarker for STEMI, ROC analysis was performed to determine the suitability of miR-23b. The ROC curve of plasma miR-23b showed a separation, with an AUC of 0.809 (95%CI, 0.737–0.936, P<0.05), compared to CK-MB with an AUC of 0.753 (95%CI, 0.707–0.896) and cTnI with an AUC of 0.783 (95%CI, 0.723–0.917) (Figure 4). Taken together, these data suggest that miR-23b is a sensitive and specific biomarker for STEMI.
Figure 4.
ROC analysis of miR-23b level as STEMI prediction. (A) The ROC curve of plasma miR-23b showed a separation, with an AUC of 0.809 (95%CI, 0.737–0.936, P<0.05), compared to CK-MB with an AUC of 0.753 (95%CI, 0.707–0.896). (B) The ROC curve of plasma miR-23b showed a separation, with an AUC of 0.809 (95%CI, 0.737–0.936, P<0.05), compared to cTnI with an AUC of 0.783 (95%CI, 0.723–0.917).
Discussion
Some microRNAs are highly expressed in the cardiovascular system and play an important role in the regulation of cardiovascular development, as well as being involved in the pathogenesis of related diseases [8–12]. It has been reported that some microRNAs may be potential biomarkers for the diagnosis of AMI, such as miR-1, miR-133, miR-208, and miR-499 [13,14]. High-throughput miRNA profiling shows overexpression of miR-23b in failing human myocardium [15]. miR-23b was found to be highly expressed in the heart and is considered to be a modulator in many cancers and other biological processes [16].
Major specific serum cardiac markers include troponins and CK-MB. An elevation in cardiac markers is required for the diagnosis of AMI [17]. Although troponins are more specific and sensitive in the diagnosis of myocardial injury compared to CK-MB [18], elevated troponins and CK-MB have been detected in many other diseases [4,19].
We investigated the dynamics of miR-23b in patients with STEMI, finding that the level of miR-23b was significantly elevated in plasma of STEMI patients compared to healthy controls. Moreover, the level of miR-23b achieved a peak at 48 h and the correlation analysis revealed the positive association between miR-23b and CK-MB or cTnI.
Male sex [20], smoking [21], hypertension [22], hyperglycemia [23], and dyslipidemia [24] were the main risk factors associated with progression of AMI, but the differences were not statistically significant, perhaps because of the small sample size.
The ROC curve of plasma miR-23b showed a separation compared to CK-MB and cTns, which are commonly considered the criterion standard biomarkers for AMI, suggesting it is possible that miR-23b is another diagnostic biomarker. However, based on our data, we could not determine if miR-23b is released at the time of symptom onset, nor could we determine the details of the microenvironment within the release; many microRNAs show a restricted expression pattern temporally and spatially [25]. Further studies of molecular mechanisms are needed to thoroughly characterize the role of mir-23b in STEMI.
Conclusions
The present study reveals the biomarker of miR-23b in STEMI, providing a novel insight for the diagnosis for STEMI.
Footnotes
Conflict of interest
None.
Source of support: None
References
- 1.Antman EM, Hand M, Armstrong PW, et al. 2007 Focused Update of the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: Developed in collaboration With the Canadian Cardiovascular Society endorsed by the American Academy of Family Physicians: 2007 Writing Group to Review New Evidence and Update the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction, Writing on Behalf of the 2004 Writing Committee. Circulation. 2008;117:296–329. doi: 10.1161/CIRCULATIONAHA.107.188209. [DOI] [PubMed] [Google Scholar]
- 2.Jaffe AS, Ravkilde J, Roberts R, et al. It’s time for a change to a troponin standard. Circulation. 2000;102:1216–20. doi: 10.1161/01.cir.102.11.1216. [DOI] [PubMed] [Google Scholar]
- 3.Giannitsis E, Katus HA. Cardiac troponin level elevations not related to acute coronary syndromes. Nat Rev Cardiol. 2013;10:623–34. doi: 10.1038/nrcardio.2013.129. [DOI] [PubMed] [Google Scholar]
- 4.Rosjo H, Varpula M, Hagve TA, et al. Circulating high sensitivity troponin T in severe sepsis and septic shock: Distribution, associated factors, and relation to outcome. Intensive Care Med. 2011;37:77–85. doi: 10.1007/s00134-010-2051-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Condorelli G, Latronico MV, Dorn GW., 2nd microRNAs in heart disease: Putative novel therapeutic targets? Eur Heart J. 2010;31:649–58. doi: 10.1093/eurheartj/ehp573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rigoutsos I, Furnari F. Gene-expression forum: Decoy for microRNAs. Nature. 2010;465:1016–17. doi: 10.1038/4651016a. [DOI] [PubMed] [Google Scholar]
- 7.He W, Che H, Jin C, Ge S. Effects of miR-23b on hypoxia-induced cardiomyocytes apoptosis. Biomed Pharmacother. 2017;96:812–17. doi: 10.1016/j.biopha.2017.09.148. [DOI] [PubMed] [Google Scholar]
- 8.Lorenzen JM, Batkai S, Thum T. Regulation of cardiac and renal ischemia-reperfusion injury by microRNAs. Free Radic Biol Med. 2013;64:78–84. doi: 10.1016/j.freeradbiomed.2013.06.044. [DOI] [PubMed] [Google Scholar]
- 9.Wahlquist C, Jeong D, Rojas-Munoz A, et al. Inhibition of miR-25 improves cardiac contractility in the failing heart. Nature. 2014;508:531–35. doi: 10.1038/nature13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian J, An X, Niu L. Role of microRNAs in cardiac development and disease. Exp Ther Med. 2017;13:3–8. doi: 10.3892/etm.2016.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matkovich SJ, Hu Y, Dorn GW., 2nd Regulation of cardiac microRNAs by cardiac microRNAs. Circ Res. 2013;113:62–71. doi: 10.1161/CIRCRESAHA.113.300975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gnecchi M, Pisano F, Bariani R. microRNA and cardiac regeneration. Adv Exp Med Biol. 2015;887:119–41. doi: 10.1007/978-3-319-22380-3_7. [DOI] [PubMed] [Google Scholar]
- 13.Wang F, Chen C, Wang D. Circulating microRNAs in cardiovascular diseases: From biomarkers to therapeutic targets. Front Med. 2014;8:404–18. doi: 10.1007/s11684-014-0379-2. [DOI] [PubMed] [Google Scholar]
- 14.Recchioni R, Marcheselli F, Olivieri F, et al. Conventional and novel diagnostic biomarkers of acute myocardial infarction: A promising role for circulating microRNAs. Biomarkers. 2013;18:547–58. doi: 10.3109/1354750X.2013.833294. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Wang Y, Liu W, van Wijnen AJ. Regulation and biological roles of the multifaceted miRNA-23b (MIR23B) Gene. 2018;642:103–9. doi: 10.1016/j.gene.2017.10.085. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda S, Pu WT. Expression and function of microRNAs in heart disease. Curr Drug Targets. 2010;11:913–25. doi: 10.2174/138945010791591304. [DOI] [PubMed] [Google Scholar]
- 17.Langorgen J, Ebbing M, Igland J, et al. The universal 2012 definition of myocardial infarction compared to the 2007 definition. Scand Cardiovasc J. 2016;50:201–5. doi: 10.1080/14017431.2016.1191664. [DOI] [PubMed] [Google Scholar]
- 18.Di Chiara A, Greco C, Savonitto S, et al. [Myocardial infarction redefined based on the consensus document of the ESC/ACC]. Ital Heart J Suppl. 2002;3:208–14. [in Italian] [PubMed] [Google Scholar]
- 19.Goodman SG, Steg PG, Eagle KA, et al. The diagnostic and prognostic impact of the redefinition of acute myocardial infarction: Lessons from the Global Registry of Acute Coronary Events (GRACE) Am Heart J. 2006;151:654–60. doi: 10.1016/j.ahj.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Hellerstein DK. Sexual activity and risk of myocardial infarction. JAMA. 1996;276:782. doi: 10.1001/jama.276.10.782b. author reply 783. [DOI] [PubMed] [Google Scholar]
- 21.Khawaja O, Al-Mallah M. The impact of public smoking ban on the incidence of myocardial infarction hospitalizations. Rev Cardiovasc Med. 2010;11:e121–29. doi: 10.3909/ricm0540. [DOI] [PubMed] [Google Scholar]
- 22.Perez P, Kimmoun A, Blime V, Levy B. Increasing mean arterial pressure in cardiogenic shock secondary to myocardial infarction: Effects on hemodynamics and tissue oxygenation. Shock. 2014;41:269–74. doi: 10.1097/SHK.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 23.Vihonen H, Tierala I, Kuisma M, et al. Ultra-acute increase in blood glucose during prehospital phase is associated with worse short-term and long-term survival in ST-elevation myocardial infarction. Scand J Trauma Resusc Emerg Med. 2014;22:30. doi: 10.1186/1757-7241-22-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aydin MU, Aygul N, Altunkeser BB, et al. Comparative effects of high-dose atorvastatin versus moderate-dose rosuvastatin on lipid parameters, oxidized-LDL and inflammatory markers in ST elevation myocardial infarction. Atherosclerosis. 2015;239:439–43. doi: 10.1016/j.atherosclerosis.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Cao L, Kong LP, Yu ZB, et al. microRNA expression profiling of the developing mouse heart. Int J Mol Med. 2012;30:1095–104. doi: 10.3892/ijmm.2012.1092. [DOI] [PubMed] [Google Scholar]