Abstract
Background
The present study sought to assess the renal and liver protective effect of N-acetylcysteine through NH3 and urea metabolism in patients with chronic obstructive pulmonary disease who were scheduled for coronary artery bypass grafting surgery.
Material/Methods
Patients with chronic obstructive pulmonary disease (COPD) who were scheduled for coronary artery bypass grafting were divided into 2 groups so as to receive (Group 1, n=35) or not receive (Group 2, n=35) 900 mg/day of n-acetylcysteine for 7 days before the operation starting from their admission to the service by a pulmonologist with the purpose of treating COPD until the day of surgery. Both groups were subjected to the same anesthesia protocol. Blood samples were taken preoperatively, within the first 15th minute following cessation of the cardiopulmonary bypass, at postoperative 24th hour, and at postoperative 48th hour. Blood tests included ammonia (NH3), lactate, blood urea nitrogen, creatinine, aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), troponin I (Tn I), and creatinine kinase-muscle brain (CKMB).
Results
There was a significant difference between the groups’ NH3 and lactate levels after cardiopulmonary bypass, postoperative 24th hour, and postoperative 48th hour (respectively, NH3: 39.0±8.8 vs. 55.4±19.6 and 40.1±8.4 vs. 53.2±20.2 mcg/dl, lactate: 1.7±0.9 vs. 2.1±1.2 and 1.2±0.5 vs. 1.8±1.4 mmol/L; p<0.01). Creatinine and BUN levels in Group 2 were found to be significantly higher at the postoperative 48th hour compared to the levels of Group 1 (P<0.05).
Conclusions
N-acetylcysteine pretreatment appears to improve renal and hepatic functions through regulation of ammonia and nitrogen metabolism and reduction of lactate in patients with chronic obstructive pulmonary disease who undergo coronary artery bypass grafting surgery. We found that N-acetylcysteine improved kidney and/or liver functions.
MeSH Keywords: Acetylcysteine; Coronary Artery Bypass; Pulmonary Disease, Chronic Obstructive
Background
Chronic obstructive pulmonary disease (COPD) is among the leading causes of death worldwide, with an estimated prevalence of 9% to 10% in the general population [1]. However, COPD was reported to be far more prevalent among patients scheduled for coronary artery bypass grafting (CABG) and it has also been suggested that declined respiratory function is the main predictor of mortality in patients undergoing CABG [2,3].
Postoperative organ failure is an important predictor for early mortality in patients undergoing on-pump coronary artery bypass grafting [4]. Activation of the inflammatory cascade during extracorporeal circulation and development of ischemia-reperfusion injury are known to be responsible for failure of various organ and systems including pulmonary, liver and renal dysfunction [5]. Patients with COPD more tend to have several comorbidities after CABG since these patients already have several comorbidities before the operation [6]. It was also demonstrated that COPD severity is correlated with the postoperative occurrence risk of occurrence of severe organ and system dysfunction [7].
Recently, there has been a trend towards the protection of organs against deleterious effects of the cardiopulmonary bypass that occurs during cardiac surgery [8]. N-acetylcysteine, which has long been used as the antidote to acetaminophen, has become used due to its presumed protection against ischemia-reperfusion injury in tissues [9]. Based on its free radical scavenger properties and maintenance of cellular glutathione status, its use has increasingly become generalized in many areas, with varying evidence. Although studies failed to demonstrate renal protective effect of perioperative use of N-acetylcysteine, its use was demonstrated to preserve pulmonary functions in patients undergoing CABG [10,11]. The present study sought to investigate the potential renal and hepatic protective effect of n-acetylcysteine through NH3 and urea metabolism in patients with chronic obstructive pulmonary disease undergoing coronary artery bypass grafting surgery.
Material and Methods
The Institutional Ethics Board of Erzincan University approved the study (ID; 44495147-181). This prospective, randomized study included 70 patients who had been diagnosed with COPD at a chest diseases clinic, had a FEV1 (volume taken at the first second of forced expiration) of 50% to 80%, and who were scheduled for CABG. The diagnosis of COPD was confirmed by reviewing the patients’ counseling charts, radiography reports, and spirometer testing results archived by the Department of Chest Disease. Patients with a history of renal or hepatic failure and those with abnormal findings in kidney or liver functional parameters, as well as in the tests showing liver cell and cholestatic damage in the preoperative laboratory assessment, were excluded from the study. Patients were divided into 2 groups to receive (Group 1, n=35) or not receive (Group 2, n=35) 900 mg/day of n-acetylcysteine for 7 days until the day of surgery. Both groups were subjected to the same anesthesia protocol. Blood samples were taken preoperatively, within the first 15th minute following cessation of the cardiopulmonary bypass, at postoperative 24th hour, and at postoperative 48th hour. Blood tests included ammonia (NH3), lactate, blood urea nitrogen (BUN), creatinine, aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), troponin I (TnI), and creatinine kinase-muscle brain (CKMB).
Serum levels of BUN, AST, ALT, ALP, and CKMB were measured using an Olympus AU2700TM Chemistry ImmunoAnalyser Device (Tokyo - JAPAN). Lactate levels were measured with Beckman Coulter lactate reagent (Beckman Coulter, Inc., USA) using an Olympus AU2700TM Chemistry ImmunoAnalyser Device in plasma samples obtained with sodium fluoride/potassium oxalate tubes. Serum TnI levels of the patients were measured with a Siemens Advia Centaur XP immunoassay device (Deerfield, IL, USA). Serum NH3 levels were measured with infinity ammonia reagent (ThermoFisher Scientific-Middletown, USA) using an Olympus AU400 Chemistry Analyzer device (Tokyo, JAPAN) in plasma samples obtained with EDTA-containing tubes.
Statistical analyses
Statistical analyses were performed using SPSS (Statistical Package for the Social Sciences) version 20.0. Descriptive statistics are reported as mean ± standard deviation for continuous variables and as frequency and percentage for categorical variables. Parameters with a normal distribution were compared using the unpaired t test, whereas parameters not demonstrating normal distribution were compared using the Mann-Whitney test. A p value of less than 0.05 was considered as statistically significant.
Results
Comparison of baseline characteristics between the 2 groups is demonstrated in Table 1. No significant difference was found between the 2 groups in terms of age (43±15 vs. 41±17 years, p>0.05), body mass index (BMI) (27±3.2 vs. 27±3.0, p>0.05), waist circumference (91±9 vs. 94±8 cm, p>0.05), tobacco or alcohol use, and laboratory findings (p>0.05). Also, there was no significant difference between the groups regarding the seriousness of COPD (FEV1) (P>0.005) (Table 1).
Table 1.
Patient characteristics and analysis results grouped to according to received N-acetylcysteine treatment.
| All patients | NAC in treat | No treat | P values* | |
|---|---|---|---|---|
| N | 70 | 35 | 35 | – |
| Male, (%) | 40 (57) | 20 (57) | 20 (57) | – |
| Female, (%) | 15 (43) | 15 (43) | 15 (43) | – |
| Age, year | 42±16 | 43±15 | 41±17 | a0.6715 |
| BMI, kg/m2 | 26.9±3.1 | 27.1±3.2 | 26.6±3.0 | a0.4437 |
| WC, cm | 93±9 | 91±9 | 94±8 | a0.1536 |
| Cigarette use, (%) | 25 (36) | 12 (34) | 13 (37) | b0.8383 |
| Alcohol use, (%) | 17 (24) | 7 (20) | 10 (29) | b0.5293 |
| Glucose, mg/dl | 98±16 | 99±15 | 98±17 | a0.7719 |
| Leukocyte, 103/ul | 7.6±1.3 | 7.5±1.4 | 7.7±1.2 | a0.5326 |
| Hematocrit,% | 43,4±5.8 | 43.7±5.3 | 43.0±6.4 | a0.6049 |
| Grade of COPD (FEV1) | 66.1±7.7 | 66.9±7.8 | 65.3±7.5 | a0.3874 |
Comparison between NAC in treat and no treat.
Unpaired t test;
Mann-Whitney Test,
BMI – Body mass index; WC – waist circumference; COPD – chronic obstructive pulmonary disease; FEV1 – volume taken at the first second of forced expiration. Glucose, Leukocyte, and hematocrit values belong to the preoperative period.
Serum AST, ALT, ALP, CK-MB, and TnI levels demonstrated no significant difference between the 2 groups at any of the 4 measurement time points (preoperative, during cardiopulmonary bypass, 24th postoperative hour, and 48th postoperative hour) (p>0.05) (Table 2). Serum NH3 levels of the 2 groups were also similar in the preoperative period (37.4±9.09 vs. 41.4±11.46 mcg/dl for Group 1 and 2, respectively, unpaired t test p>0.05) whereas the differences in NH3 levels were significant after cardiopulmonary bypass (37.8±10.26 vs. 47.0±14.57, for Group 1 and 2, respectively, p<0.05, unpaired t test), at 24th hour (39.0±8.80 vs. 55.4±19.64, for Group 1 and 2, respectively, p<0.05, unpaired t test) and at 48th hour (40.1±8.44 vs. 53.2±20.15, for Group 1 and 2, respectively, p<0.05, unpaired t test) (Figure 1). Serum BUN levels demonstrated no significant difference in the preoperative period (17.6±4.99 vs. 18.6±4.72, for Group 1 and 2, respectively, p>0.05) and after cardiopulmonary bypass (17.1±4.61 vs. 19.6±8.56, for Group 1 and 2, respectively, p>0.05) whereas the differences were significant at 24th postoperative hour (18.7±4.36 vs. 24.4±9.52, for Group 1 and 2, respectively, p<0.05) and 48th postoperative hour (19.8±5.42 vs. 24.9±10.90, for Group 1 and 2, respectively, p<0.05) (Figure 2). Lactate levels at 24th and 48th postoperative hours were significantly higher in Group 1 when compared to Group 2 (p<0.05) (Figure 3). Creatinine levels at the 48th postoperative hour were significantly higher in Group 2 when compared to Group 1 (p<0.05).
Table 2.
Comparison of study parameters between two groups. Blood samples were taken at four different time points.
| Variable | All patients n=70 | Group 1 (N-acetylcysteine) n=35 | Group 2 (Controls) n=35 | P values* |
|---|---|---|---|---|
| Creatinin, mg/dl | ||||
| Preop | 0.97±0.23 | 0.94±0.20 | 1.01±0.26 | a0.2412 |
| After bypass | 1.00±0.24 | 1.00±0.23 | 0.99±0.25 | a0.8598 |
| At the 24th hour after surgery | 1.11±0.29 | 1.09±0.24 | 1.13±0.33 | a0.5708 |
| At the 48th hour after surgery | 1.17±0,32 | 1.08±0.31 | 1.25±0,30 | a0.0237 |
|
| ||||
| BUN, mg/dl | ||||
| Preop | 18±4.9 | 18±5.0 | 18±4.6 | a0.4479 |
| After bypass | 18±7.0 | 17±4.6 | 19±5.7 | a0.2019 |
| At the 24th hour after surgery | 22±8.0 | 19±4.4 | 23±6.2 | a0.0010 |
| At the 48th hour after surgery | 22±9.0 | 20±5.4 | 23±6.9 | a0.0287 |
|
| ||||
| AST, IU/L | ||||
| Preop | 25±12 | 26±13 | 23±10 | a0.3360 |
| After bypass | 35±20 | 36±16 | 34±23 | a0.5599 |
| At the 24th hour after surgery | 45±31 | 41±17 | 48±41 | b0.8555 |
| At the 48th hour after surgery | 48±35 | 42±22 | 55±44 | a0.1297 |
|
| ||||
| ALT, IU/L | ||||
| Preop | 24±14 | 24±16 | 25±11 | a0.9520 |
| After bypass | 26±17 | 25±18 | 28±16 | b0.1923 |
| At the 24th hour after surgery | 32±23 | 30±23 | 33±23 | b0.3384 |
| At the 48th hour after surgery | 29±20 | 30±25 | 29±15 | b0. 6217 |
|
| ||||
| ALP, IU/L | ||||
| Preop | 78±28 | 75±31 | 82±24 | a0.3525 |
| After bypass | 72±23 | 69±26 | 75±19 | a0.2440 |
| At the 24th hour after surgery | 78±29 | 78±35 | 78±23 | a0.9612 |
| At the 48th hour after surgery | 71±25 | 69±20 | 74±29 | a0.3422 |
|
| ||||
| CK-MB, IU/L | ||||
| Preop | 17±8 | 18±8 | 17±8 | b0.6510 |
| After bypass | 37±28 | 37±24 | 36±32 | a0.8774 |
| At the 24th hour after surgery | 53±61 | 52±28 | 54±82 | b0.0954 |
| At the 48th hour after surgery | 40±56 | 32±19 | 48±77 | b0.2131 |
|
| ||||
| TnI, ng/ml | ||||
| Preop | 0.33±0.91 | 0.38±1.20 | 0.28±0.47 | b0.4557 |
| After bypass | 3.76±7.09 | 3.58±5.15 | 3.93±8.68 | b0.6427 |
| At the 24th hour after surgery | 6.85±9.43 | 5.05±5.80 | 8.64±11.84 | b0.6724 |
| At the 48th hour after surgery | 8.87±10.90 | 7.15±7.06 | 10.59±13.61 | a0.1887 |
|
| ||||
| NH3, mcg/dl | ||||
| Preop | 39.4±10.5 | 37.4±9.1 | 41.4±11.5 | a0.1146 |
| After bypass | 42.4±13.3 | 37.8±10.3 | 47.0±14.5 | a0.0031 |
| At the 24th hour after surgery | 47.2±17.2 | 39.0±8.8 | 55.4±19.6 | a<0.0001 |
| At the 48th hour after surgery | 46.6±16.7 | 40.1±8.4 | 53.2±20.2 | a0.0007 |
|
| ||||
| Lactate, mmol/L | ||||
| Preop | 1.0±0.4 | 0.9±0.3 | 1.0±0.4 | a0.2655 |
| After bypass | 1.0±0.3 | 0.9±0.3 | 1.0±0.4 | a0.0828 |
| At the 24th hour after surgery | 1.7±0.9 | 1.4±0.5 | 2.1±1.2 | a0.0018 |
| At the 48th hour after surgery | 1.5±1.1 | 1.2±0.5 | 1.8±1.4 | b0.0083 |
Comparison was based on
unpaired t test;
Mann-Whitney Test.
Figure 1.
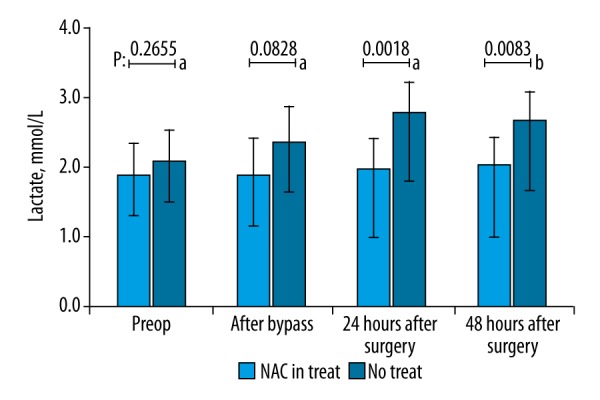
Comparison of the serum NH3 levels between groups with regard to samples taken at preoperative period, after cardiopulmonary bypass, at 24th postoperative hour, and at 48th postoperative hour. NH3 levels demonstrated no difference preoperatively whereas patients in N-acetylcysteine group had significantly lower NH3 levels in all other measurement time points (p<0.05, unpaired t test).
Figure 2.
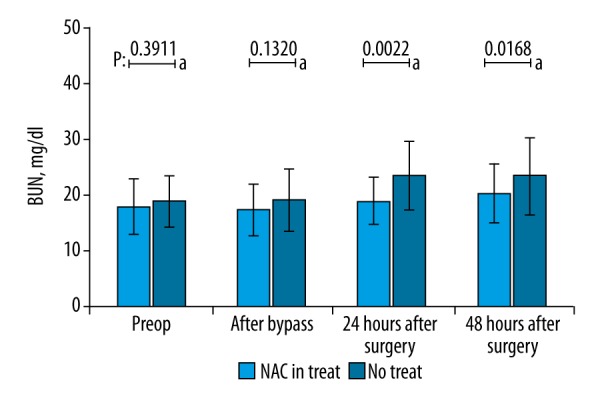
Comparison of BUN levels at 4 consecutive time points. No significant difference was found in the preoperative period and after cessation of cardiopulmonary bypass, whereas patients in N-acetylcysteine group had significantly lower BUN levels at the 24th and 48th postoperative hours (p<0.05, unpaired t test).
Figure 3.
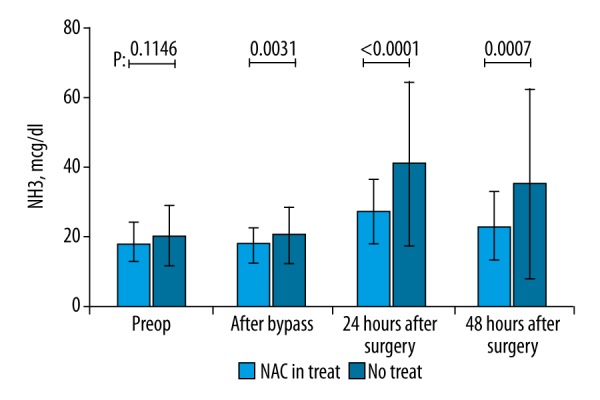
Comparison of serum lactate levels at four consecutive time points. No significant difference was found in the preoperative period and after cessation of cardiopulmonary bypass, whereas patients in N-acetylcysteine group had significantly lower lactate levels at the 24th and 48th postoperative hours (p<0.05, unpaired t test).
Discussion
There have been several studies demonstrating the beneficial effects of N-acetylcysteine on renal and hepatic functions [12–16]. N-acetylcysteine has begun to be used in a wide variety of clinical settings, including management of chronic periodontitis, treatment of acute pulmonary damage, palliation of peripheral neurotoxicity, to provide myocardial protection during extracorporeal circulation, and to prevent atrial fibrillation after CABG [17–20]. NAC has been reported to be beneficial due to its anti-oxidant and central anti-inflammatory properties. Moreover, past studies have proved that NAC, which is described with similar mechanisms in experimental animals, is useful in both non-acetaminophen- and non-acetaminophen-induced acute liver diseases [21,22]. However, high costs still prevent fully explaining the effects of NAC use in COPD patients who are recommended for integrated care [23].
A gradual increase in blood NH3 levels was found to be associated with cerebral edema, coma, and progressive decline in intellectual functions, while severe hyperammonemia may result in irreversible neurotoxicity and cellular necrosis in the central nervous system. Since glucose is the main source of energy that is utilized by the neurons of the central nervous system, the brain tissue cannot adapt quickly to use alternative energy compounds, such as free fatty acids, when the supply of the glucose to the neurons decreased. This is due to the fact that free fatty acids cannot pass through the blood-brain barrier. However, in situations where glucose supply to the central nervous system declines rapidly, brain tissue may utilize ketone bodies (e.g., hydroxybutyrate and acetoacetic acid) derived from fatty acid metabolism. One of the major causes of hyperammonium toxicity in the brain is that it inhibits the continuation of the TCA cycle and energy production by causing the consumption of the vital enzyme of the cycle, α-ketoglutarate. In addition, hyperammonemia reduces the glutamate transporter molecules by inducing the receptors of N-metil-D-aspartate (NMDA) and GABA, which results in an increase in the intracellular amount of GABA and glutamine. These changes alter cellular membrane permeability and increase intracranial pressure, leading to brain edema [24–27]. In our study, we found that N-acetylcysteine pretreatment was associated with a significant decrease in serum NH3 levels immediately after the cessation of cardiopulmonary bypass, and this decline was sustained up to the 48th postoperative hour. These findings suggest the preventive effect of N-acetylcysteine against the toxic effects of hyperammonemia after CABG.
Our finding that lactate levels were significantly lower in patients receiving N-acetylcysteine pretreatment may be attributed to increased microcirculation and oxygenation in liver and other tissues [28–31]. We also found that serum BUN levels at the 24th and 48th postoperative hours were significantly lower in patients receiving N-acetylcysteine when compared to controls, as evidence of the positive effects of N-acetylcysteine on renal functions. The lower creatinine levels we found at the 48th hour in patients who received N-acetylcysteine pretreatment compared to controls may support this finding. Renal protective mechanisms of N-acetylcysteine are likely to be same as those for liver tissue.
There have been several studies evaluating the tissue- and organ-protective effects of n-acetylcysteine in patients undergoing cardiac surgery on extracorporeal circulation. In one study, Fisher et al. [32] found that N-acetylcysteine treatment caused a significant decrease in serum creatinine in patients undergoing cardiac surgery. Sucu et al. [33] demonstrated that intravenous administration of N-acetylcysteine alleviated the pump-induced oxido-inflammatory response that developed during extracorporeal circulation. Şavluk et al. [13] demonstrated that intravenous N-acetylcysteine prophylaxis was associated with an improvement in renal functional tests in patients with moderate renal failure. These findings are in line with ours to some extent.
We observed no difference between the groups with regard to smoking status and alcohol use, supporting that the difference in serum lactate and NH3 levels we found between groups could essentially be attributed to the effect of N-acetylcysteine.
In clinical practice, both cytosolic and mitochondrial enzyme AST and cytosolic enzyme ALT are aminotransferases that are used to evaluate hepatocellular damage. ALP, synthesized in bile duct epithelial cells, is a test used to evaluate cholestatic damage. ALT is relatively more specific to the liver. Aminotransferases are sensitive markers in liver diseases that accompany hepatic cell damage such as hepatitis [34]. The increase in serum aminotransferase level is associated with the passing of the in-cell enzyme to the serum due to hepatocellular necrosis. Briefly, ALT and AST are not the fundamental liver function tests. It has been reported that the hepatic metabolism of lactate is about 100 mmol per hour, and that this value can fall by 0.6 mmol/kg per hour if the patient has acute renal insufficiency [35]. Therefore, approximately 70% of the blood lactate clearance is performed by the liver, while the kidneys contribute by renal excretion. In this study, lactate levels were low due to the positive effect of N-acetyl cysteine on liver and kidney functions. However, no significant difference was found between the 2 groups in liver-associated aminotransferases. This is probably due to the fact that aminotransferases are the indicators of extreme cell damage that is caused by liver function. The lower incidence of ammonia in the same patient group was associated with the positive effect of ammonia metabolism, which is one of the major functions of the liver. This result also explains the decrease in lactate levels.
Another important finding of the present study is that use of blood urea levels seem to be more appropriate than using creatinine levels for early detection of the renal protective effects of N-acetylcysteine. This finding indicates that the actual protective effect of the N-acetylcysteine is caused by NH3 and urea metabolism. Supporting these, several studies suggested that blood creatinine levels might be misleading in early detection of renal impairment after cardiac surgery, and creatinine levels may even be as increased when renal functions are severely impaired [36–38].
The limitations of this study are the small number of patients and the exclusion of patients with abnormalities in liver and renal function tests.
Conclusions
In conclusion, our study demonstrated that N-acetylcysteine pretreatment has a stabilizing effect on NH3 and nitrogen metabolism in COPD patients undergoing CABG. Patients with COPD who were started on N-acetylcysteine before surgery tend to have lower lactic acid levels, indicating a protective effect of N-acetylcysteine against renal and hepatic tissue damage.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Bednarek M, Maciejewski J, Wozniak M, et al. Prevalence, severity and underdiagnosis of COPD in the primary care setting. Thorax. 2008;63(5):402–7. doi: 10.1136/thx.2007.085456. [DOI] [PubMed] [Google Scholar]
- 2.Fuster RG, Argudo JA, Albarova OG, et al. Prognostic value of chronic obstructive pulmonary disease in coronary artery bypass grafting. Eur J Cardiothorac Surg. 2006;29:202–9. doi: 10.1016/j.ejcts.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Medalion B, Katz MG, Cohen AJ, et al. Long-term beneficial effect of coronary artery bypass grafting in patients with COPD. Chest. 2004;125:56–62. doi: 10.1378/chest.125.1.56. [DOI] [PubMed] [Google Scholar]
- 4.Chang CH, Chen SW, Fan PC, et al. Sequential organ failure assessment score predicts mortality after coronary artery bypass grafting. BMC Surg. 2017;17(1):22. doi: 10.1186/s12893-017-0219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bronicki RA, Hall M. Cardiopulmonary bypass-induced inflammatory response: Pathophysiology and treatment. Pediatr Crit Care Med. 2016;17(8 Suppl 1):S272–78. doi: 10.1097/PCC.0000000000000759. [DOI] [PubMed] [Google Scholar]
- 6.Angouras DC, Anagnostopoulos CE, Chamogeorgakis TP, et al. Postoperative and long-term outcome of patients with chronic obstructive pulmonary disease undergoing coronary artery bypass grafting. Ann Thorac Surg. 2010;89(4):1112–18. doi: 10.1016/j.athoracsur.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Saleh HZ, Mohan K, Shaw M, et al. Impact of chronic obstructive pulmonary disease severity on surgical outcomes in patients undergoing non-emergent coronary artery bypass grafting. Eur J Cardiothorac Surg. 2012;42(1):108–13. doi: 10.1093/ejcts/ezr271. [DOI] [PubMed] [Google Scholar]
- 8.Varghese D, Varghese B, Kelley K, et al. Prospective randomized study to evaluate changes in alpha-GST as a novel marker of hepatocellular necrosis in patients at high-risk of hepatic injury undergoing coronary revascularization with and without cardiopulmonary bypass. Ind J Thorac Cardiovasc Surg. 2007;23:63. [Google Scholar]
- 9.Bavarsad Shahripour R, Harrigan MR, Alexandrov AV. N-acetylcysteine (NAC) in neurological disorders: Mechanisms of action and therapeutic opportunities. Brain Behav. 2014;4(2):108–22. doi: 10.1002/brb3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mei M, Zhao HW, Pan QG, et al. Efficacy of N-acetylcysteine in preventing acute kidney injury after cardiac surgery: A meta-analysis study. J Invest Surg. 2018;31(1):14–23. doi: 10.1080/08941939.2016.1269853. [DOI] [PubMed] [Google Scholar]
- 11.Erdil N, Eroglu T, Akca B, et al. The effects of N-acetylcysteine on pulmonary functions in patients undergoing on-pump coronary artery surgery: A double-blind placebo-controlled study. Eur Rev Med Pharmacol Sci. 2016;20(1):180–87. [PubMed] [Google Scholar]
- 12.Dósa E, Heltai K, Radovits T, et al. Dose escalation study of intravenous and intra-arterial N-acetylcysteine for the prevention of oto- and nephrotoxicity of cisplatin with a contrast-induced nephropathy model in patients with renal insufficiency. Fluids Barriers CNS. 2017;14(1):26. doi: 10.1186/s12987-017-0075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savluk OF, Guzelmeric F, Yavuz Y, et al. N-acetylcysteine versus dopamine to prevent acute kidney injury after cardiac surgery in patients with preexisting moderate renal insufficiency. Braz J Cardiovasc Surg. 2017;32(1):8–14. doi: 10.21470/1678-9741-2016-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozaydin M, Peker T, Akcay S, et al. Addition of N-acetyl cysteine to carvedilol decreases the incidence of acute renal injury after cardiac surgery. Clin Cardiol. 2014;37(2):108–14. doi: 10.1002/clc.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nabi T, Nabi S, Rafiq N, Shah A. Role of N-acetylcysteine treatment in non-acetaminophen-induced acute liver failure: A prospective study. Saudi J Gastroenterol. 2017;23(3):169–75. doi: 10.4103/1319-3767.207711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teriaky A. The role of N-acetylcysteine in the treatment of non-acetaminophen acute liver failure. Saudi J Gastroenterol. 2017;23(3):131–32. doi: 10.4103/sjg.SJG_621_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alkadasi B, Abdulrab S, Gaafer S, et al. Effect of adjunctive use of systemic antioxidant therapy (N-acetylcysteine) on soluble receptor activator nuclear factor κB ligand levels in gingival crevicular fluid following surgical periodontal treatment for chronic periodontitis. J Oral Sci. 2017;59(4):519–26. doi: 10.2334/josnusd.16-0701. [DOI] [PubMed] [Google Scholar]
- 18.Yang LM, Zhao J, Wang HT, et al. [The protective effect of N-acetylcysteine on acute lung injury induced by PFIB inhalation]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2017;35(7):481–86. doi: 10.3760/cma.j.issn.1001-9391.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Zaki SM, Ahmed EM, Motawie AG, Abdel Fattah S. N-acetylcysteine versus progesterone on the cisplatin-induced peripheral neurotoxicity. Folia Morphol (Warsz) 2017 doi: 10.5603/FM.a2017.0090. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Soleimani A, Habibi MR, Hasanzadeh Kiabi F, et al. The effect of intravenous N-acetylcysteine on prevention of atrial fibrillation after coronary artery bypass graft surgery: A double-blind, randomized, placebo-controlled trial. controlled trial. Kardiol Pol. 2018;76(1):99–106. doi: 10.5603/KP.a2017.0183. [DOI] [PubMed] [Google Scholar]
- 21.Bemeur C, Vaquero J, Desjardins P, Butterworth RF. N-acetyl cysteine attenuates cerebral complications of non-acetaminophen-induced acute liver failure in mice: antioxidant and anti-inflammatory mechanisms. Metab Brain Dis. 2010;25:241–49. doi: 10.1007/s11011-010-9201-2. [DOI] [PubMed] [Google Scholar]
- 22.James LP, McCullough SS, Lamps LW. Effect of N-acetyl cysteine on acetaminophen toxicity in mice: Relationship to reactive nitrogen and cytokine formation. Toxicol Sci. 2003;75:458–67. doi: 10.1093/toxsci/kfg181. [DOI] [PubMed] [Google Scholar]
- 23.Bandurska E, Damps-Konstańska I, Popowski P, et al. Impact of integrated care model (ICM) on direct medical costs in management of advanced chronic obstructive pulmonary disease (COPD) Med Sci Monit. 2017;23:2850–62. doi: 10.12659/MSM.901982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levi MS, Brimble MA. A review of neuroprotective agents. Curr Med Chem. 2004;11:2383–97. doi: 10.2174/0929867043364522. [DOI] [PubMed] [Google Scholar]
- 25.Lee JM, Zipfel GJ, Choi DW. The changing landscape of ischaemic brain injury mechanisms. Nature. 1999;399:7–14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
- 26.Kırış T, Gorgulu A. Eksitator aminoasitler ve eksitotoksisite. Turk Noroşirurji Dergisi. 2005;15:39–44. [Google Scholar]
- 27.Harvey RA, Ferrier DR. Amino Acids: Disposal of Nitrogen. 5th edition. Market St, Philadelphia, PA.: 2011. Lippincott’s illustrated reviews: Biochemistry; pp. 245–60. [Google Scholar]
- 28.Hjelle JJ, Brzeznicka EA, Klaassen CD. Comparison of the effects of sodium sulfate and N-acetylcysteine on the hepatotoxicity of acetaminophen in mice. J Pharmacol Exp Ther. 1986;236:526–34. [PubMed] [Google Scholar]
- 29.Jones AL. Mechanism of action and value of N-acetylcysteine in the treatment of early and late acetaminophen poisoning: a critical review. J Toxicol Clin Toxicol. 1998;36:277–85. doi: 10.3109/15563659809028022. [DOI] [PubMed] [Google Scholar]
- 30.Keays R, Harrison PM, Wendon JA, et al. Intravenous acetylcysteine in paracetamol induced fulminant hepatic failure: A prospective controlled trial. Br Med J. 1991;303:1026–29. doi: 10.1136/bmj.303.6809.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrison PM, Wendon JA, Gimson AE, et al. Improvement by acetylcysteine of hemodynamics and oxygen transport in fulminant hepatic failure. N Engl J Med. 1991;324:1852–57. doi: 10.1056/NEJM199106273242604. [DOI] [PubMed] [Google Scholar]
- 32.Fischer UM, Tossios P, Mehlhorn U. Renal protection by radical scavenging in cardiac surgery patients. Curr Med Res Opin. 2005;21(8):1161–64. doi: 10.1185/030079905X53289. [DOI] [PubMed] [Google Scholar]
- 33.Sucu N, Cinel I, Unlu A, et al. N-acetylcysteine for preventing pump-induced oxidoinflammatory response during cardiopulmonary bypass. Surg Today. 2004;34(3):237–42. doi: 10.1007/s00595-003-2699-8. [DOI] [PubMed] [Google Scholar]
- 34.Pratt DS, Kaplan MM. Laboratory tests. In: Schiff ER, Sorrell MF, Maddrey WC, editors. Schiff’s diseases of the liver. 8th ed. Vol. 1. Philadelphia: Lippencott-Raven; 1999. pp. 205–4. [Google Scholar]
- 35.Davenport A. Anionic bases for continuous forms of renal replacement therapy (CCRT) in the ICU. Intensive Care Med. 1999;25(11):1209–11. doi: 10.1007/s001340051047. [DOI] [PubMed] [Google Scholar]
- 36.Haase M, Bellomo R, Devarajan P, et al. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis. 2009;54(6):1012–24. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 37.Bjornsson TD. Use of serum creatinine concentrations to determine renal function. Clin Pharmacokinet. 1979;4:200–22. doi: 10.2165/00003088-197904030-00003. [DOI] [PubMed] [Google Scholar]
- 38.Xu SY, Carlson M, Engstrom A, et al. Purification and characterization of a human neutrophil lipocalin (HNL) from the secondary granules of human neutrophils. Scand J Clin Lab Invest. 1994;54:365–76. doi: 10.3109/00365519409088436. [DOI] [PubMed] [Google Scholar]


