Introduction
Adherence is a critical component of any treatment plan. To effectively achieve the desired result of a therapy intervention, the patient must participate in the recommended treatment, often independently without direct clinical supervision. Poor adherence to clinical recommendations may render evidence-based interventions ineffective, ultimately causing immense financial burden on the health-care system as a whole.(1–4) Patient non-adherence has been studied and discussed within a multitude of healthcare-related professions(5–9), but most often with a focus on medication adherence.(1, 10, 11) While there is an extensive literature base on adherence, the best methodology for improving patient adherence has yet to be definitively identified,(12, 13) and likely varies amongst patients and interventions.
Within the field of dysphagia, there are a variety of approaches that may be used to manage swallowing impairment. Treatments for dysphagia may include diet modifications, such as thickening liquids, changes in head posture that may improve safety of the swallow, and exercise programs targeting muscular adaptations.(14, 15) These approaches may be complex and time consuming to patients and their families, making adherence to these recommendations challenging. Almost all of these interventions require the patient to alter established patterns of behavior. The results of patients not adhering to dysphagia recommendations may be serious and can induce a higher risk for penetration or aspiration events. As a result, nonadherence to these recommendations could increase the likelihood of developing negative health outcomes, including malnutrition,(16) dehydration, and aspiration related pneumonia,(17) that increase mortality and decrease quality of life.(15, 18)
Family members or the healthcare team are often responsible, in part, for the execution of dysphagia treatment that may ultimately contribute to the overall quality of adherence, either in a positive or negative manner.(19) However, in cases where the patient is cognitively intact and able to make cognizant decisions, it is ultimately the patient’s responsibility to participate in management of their dysphagia. To what degree patients participate is also the individual’s choice, making the study of factors that contribute to patient-specific adherence with recommendations of the utmost importance.
Eating and drinking are critical for hydration and nutritional intake. People with dysphagia eat less food overall.(20) Beyond this, eating and dining have strong social and emotional connections. Altering these longstanding behavioral patterns across all social contexts can be complex and difficult for patients and their families. Given these complexities, issues that contribute to patient adherence with dysphagia-related recommendations must be studied directly and cannot be inferred from the general health adherence literature. Additionally, when evaluating current treatments and designing new interventions for dysphagia, adherence will substantially influence the results of a study and must be quantified to truly determine treatment efficacy. To effectively design studies focused on improving patient adherence to dysphagia management strategies, it is first necessary to gain an understanding of what is known about adherence to dysphagia-related recommendations. The aim of this systematic review was to address this gap in knowledge by systematically evaluating current knowledge regarding patient adherence to dysphagia recommendations. This review lays the groundwork for future studies and provides a benchmark of what is currently understood on this topic.
Methods
Inclusion and Exclusion Criteria
Overall, the goal of this review was to examine what is known about patient adherence to recommendations in dysphagia management. Methodology in preparing this review were rigorous, guided by the literature,(21) and in compliance with AMSTAR guidelines (A Measurement Tool to Assess the Methodological Quality of Systematic Reviews).(22) The development of our research question was formulated using the PICO framework: Population, Intervention, Comparison, Outcomes.(21)
Population: All populations of patients who were diagnosed with dysphagia or were being treated for swallowing-related impairments were included in this review. This incorporated a variety of etiologies, including patients with stroke, head and neck cancer, Parkinson disease, or other diagnoses where patients experience dysphagia. We excluded studies examining patient populations with advanced conditions causing moderately or severely reduced cognition (e.g. severe traumatic brain injury, advanced stage dementia, late stage Parkinson disease, etc.) or individuals that may require assistance to fully adhere to recommendations. Additionally, studies were excluded that examined only interventions for gastroesophageal reflux disease (GERD), laryngopharyngeal reflux (LPR), or other gastrointestinal issues.
Intervention: Any swallowing intervention, recommendation, or therapy regimen to either improve swallow function or reduce risk of penetration or aspiration was included (e.g., exercise based therapies for the tongue, pharynx, or other swallowing muscles, postural changes, or diet modifications). Excluded were any therapies that were solely clinician-administered, such as treatments that can only be provided in an outpatient clinic or on an inpatient basis. Included interventions were required to have a component of self-delivery to allow examination of patient adherence. As such, papers focusing on staff or caregiver adherence were not included.
Comparison: Contrasting and comparing adherence rates between studies identified in review search.
Outcomes: Outcomes included proportions, percentages, or rates of adherence as reported in individual studies. Papers that did not report a percentage, proportion, or other numerical quantification of participant adherence to swallow intervention/recommendation(s) were not included in this review.
Databases and Search Terms
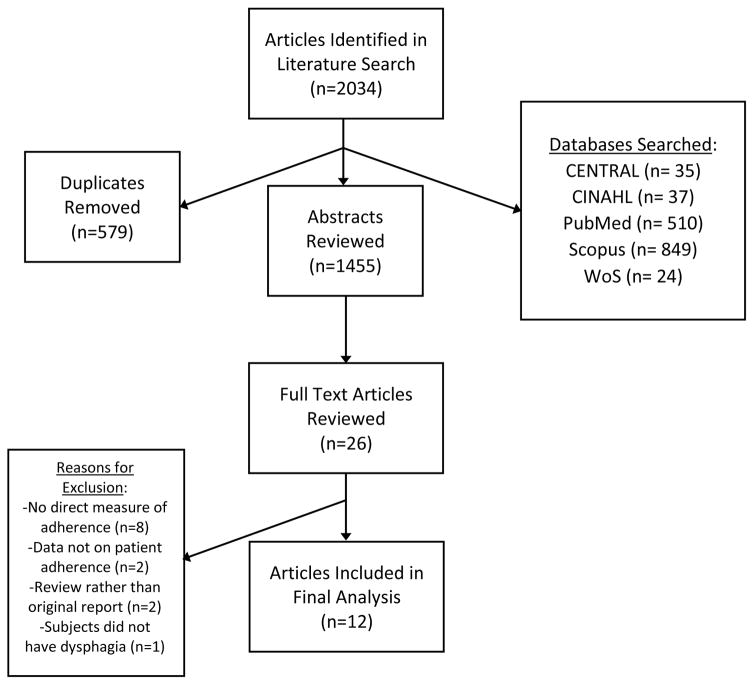
Five databases were searched (PubMed, Scopus, CINAHL, CENTRAL, and Web of Science) using terms developed by the first author (BNK) and a librarian (SJ) to capture all articles related to adherence, dysphagia, swallowing, or deglutition (For MeSH terms, please see Appendix). Our initial search was conducted in September of 2016, and an updated search was performed in April of 2017 to include the most recent publications in this review (for full list of citations, please visit http://go.wisc.edu/4e5xzf). The total number of abstracts identified from both searches, after de-duplication, was 2034 (Figure 1.) Grey literature (e.g. unpublished data, non-peer reviewed electronic sources)(23) and abstract-only texts were not included.
Figure 1.
Abstract Review and Data Abstraction
Abstract, full text review, and data abstraction of articles written in English were performed by the first two authors (BNK and CKB) and with discrepancies settled by a third reviewer (NRP).(21) Reasons for exclusion of articles included: no direct measure of adherence (no objective data), exclusive focus on healthcare provider or family member adherence, review of literature rather than original report (Figure 1). During data abstraction, each article was assigned a quality rating between 1–5 according to the JAMA Quality Rating Scale for Studies and Other Evidence (www.jamanetwork.com). After final review, 12 articles were included in the final review and data abstraction (Figure 1). The following data were abstracted from each article:
Population studied, sample size (n), and age range
Study design (quality rating from JAMA)
Type of recommendation
Method for recording adherence
Main findings of adherence to specific recommendations
Barriers and facilitators to adherence (if identified; Table 2).
Table 2.
Barriers and facilitators identified in systematic review.
| Barriers | Facilitators |
|---|---|
|
| |
Shinn et al 2013;
Leiter & Windsor 1996;
Low et al 2001;
Shim, Oh, Han 2013;
Cnossen et al 2014;
Wall et al 2017
Results
Twelve articles that recorded patient adherence to dysphagia-related recommendations were included.
Populations Studied
In this review, nine of the twelve studies included head and neck cancer patients with dysphagia.(24–32) The remaining three studies included patients who were diagnosed with dysphagia of varied etiologies.(17, 33, 34) These etiologies were the following: stroke, hemilaryngectomy, cerebral spinal surgery;(33) Parkinson disease, airway malignancy, Huntington disease, motor neuron disease, Alzheimer’s disease, muscular dystrophy, tetraplegia, poliomyelitis;(17) brain lesion, deconditioning, unspecified neuromuscular or neurodegenerative disease, local structural lesion;(34) and cardiovascular accident.(17, 33) Studies were conducted in various settings, with all but one(17) in a hospital-based setting. Of these hospital based studies, many were administering outpatient treatments,(24–32)one included both inpatient and outpatients(34), and one with just inpatients.(33) The one study not conducted in a hospital setting incorporated a variety of settings including patients living at home, in institutional or rehab settings and hospital inpatients.(17)
The number of subjects in the relevant studies ranged from 6 to 497 (median = 78). One study(17) reported an age range and divided mean between two groups, another study(34) only reported mean age, and two others reported mean age and standard deviation rather than range and average age.(31, 32) One study(27) did not report an age range or mean. Including all studies that reported the age range and mean,(24–26, 28–30, 33) the collective age range for this review was 21–94, and the mean was 60.4 years.
Study Design
Two studies(31, 32) used a randomized control design, earning the highest JAMA rating of 1. One study(29) used a prospective cohort study design with a JAMA rating of 2. Four studies(17, 25, 27, 34) completed retrospective studies earning a JAMA score of 3. Five studies(24, 26, 28, 30, 33) earned a JAMA quality rating score of 4 and used the following designs: prospective cohort,(24) case-series(26, 28, 33) and pilot study.(30)
Types of Recommendations
Most of the studies identified in this search (8/12) reported adherence to swallowing strength-based exercise regimens.(24–26, 28–32) Three studies tracked adherence for use of diet modifications/compensatory strategies.(17, 33, 34) One study did not specify the details of therapy interventions.(27)
Recording of Adherence
Nine studies recorded adherence based on self-report using journals, logs, checklists and diaries.(17, 25, 26, 28–32, 34) One study reported data retrieved from the SwallowIT® application and clinician tracked adherence data.(32) Two studies(24, 33) reported patient adherence data obtained by SLP documentation and observation.
Adherence Findings
Two methods were used to report adherence in the studies that qualified for inclusion in this review: 1) calculating an “average adherence rate” to recommendations using either actual observations of behavior or tallied exercise logs,(30, 32, 33) or 2) dividing participants into groups based on level of adherence, with each “adherence group” then expressed as a percentage of the total number of participants in the study.(17, 24–29, 31, 34) An example of “grouped adherence” reporting is found in a paper by Hutcheson and colleagues(25) where participants who performed exercises 4 or less times a day were considered “partially” adherent, and those who performed exercises 4 or more times a day were considered “fully” adherent. We calculated three pooled adherence rates based on these methods of reporting:
The pooled adherence for the three studies(30, 32, 33) that calculated an “average adherence rate” was 51.3% (SD= 35.3%).
The pooled adherence rate for studies that used “grouped adherence”: was 51.9% (SD= 19.67, median=57%), however this rate combines participants who were “fully” adherent with those who were “partially” adherent.(17, 24–29, 31, 34)
For three studies of the studies that reported “grouped adherence”, they also specified a sub-group with either “high” or “full” levels of adherence. For these studies, the pooled average of high levels of adherence was 21.9% (SD= 7.8%).(24, 25, 28)
Adherence Barriers/Facilitators/Support
Six of the studies identified potential barriers to adherence.(17, 24, 28, 32, 33) The most frequently reported barriers were difficulty of the task(24, 28, 32, 34) and fatigue.(24, 28, 32) Only two studies(28, 33) reported potential facilitators of improved adherence. These included both internal factors relating to the participants themselves (high motivation, social support, psychological well being and increased physical condition) and external factors relating to the environment or other variables (having written instructions about exercises and eating alone). Additionally, two studies(28, 30) reported providing weekly phone calls to their subjects to encourage participation in treatment.
Discussion
In our systematic review of the literature, we identified twelve full text articles that reported and discussed patient adherence to recommendations in the treatment of dysphagia (Table 1). Of the papers included in our review, some examined adherence as a main outcome, while others considered adherence as a secondary aim or in conjunction with the main outcome of interest. The first article we identified was published in 1996.(33) In 2016, three papers on this topic were published, which suggests that interest in reporting and understanding adherence may be increasing. The average adherence rate to dysphagia recommendations from studies that reported an overall level of average patient adherence ranged between 21.9% for those patients considered to be “fully adherent” to 52% for those with “average adherence”. When comparing these averages to the average adherence reported in an extensive review of 569 healthcare-related adherence studies(35) (24.8% adherence rate, range 4.6%–100%), it appears that adherence to dysphagia recommendations is similar or higher than average. However, with only twelve studies identified in our search, it is abundantly clear that there is a lot more to learn about adherence to dysphagia recommendations within our field.
Table 1.
Results of full text abstraction after systematic review. For specific barriers and facilitators reported, please see Table 2.
| Author(s) Year |
Study Population (n) |
Age Range (mean; +/−) |
Design (JAMA Quality Rating) |
Recommendations | Adherence Tracking |
Adherence Findings | Barriers/ Facilitators Identified? |
|---|---|---|---|---|---|---|---|
| Leiter & Windsor 1996 | Dysphagia, varied etiologies (n=8) | 69–80 (72) | Case-Series (4) | Swallowing Recommendations (Posturing, bolus size, swallow + cough, alternating food/liquid) | Observation | 36% Average Adherence | Yes |
| Low et al 2001 | Dysphagia, varied etiologies (n=140) | 55–94 (77.4 & 79)a | Retrospective Cohort (3) | Swallowing Recommendations (modified foods/liquids, techniques) | Self-Report | 67% Adherent w/swallow recs 84% Adherent w/liquid recs |
Yes |
| Starmer et al 2011 | HNC (n=118) | <60 years | Retrospective Cohort (3) | Participation in SLP Therapy (before, during, after HNC tx) | Records Reviewed | 80% Adherence (multidisciplinary) 17% Adherence (outside referral) |
No |
| Shinn et al 2013 | HNC (n=19) | 31–79 (57) | Prospective Cohort (4) | Exercise | Documented by SLP | 32% Partially Adherent 13% Fully Adherent |
Yes |
| Hutcheson et al 2013 | HNC (n=497) | 38–80 (56) | Retrospective Observational (3) | Exercise | Self-Report | 32% Partially Adherent 26% Fully Adherent |
No |
| Duarte et al 2013 | HNC (n=85) | 22–91 (60) | Case-Series (4) | Exercise | Self-Report | 67% Adherent | No |
| Shim, Oh, & Han 2013 | Dysphagia, varied etiologies (n=62) | (64.1) | Retrospective Chart Review (3) | Thickeners | Self-Report | 57% Adherence (90% of Inpatients 41% of Outpatients) Adherent)3 |
Yes |
| Cnossen et al 2014 | HNC (n=33, 64% participated in exercise program) | 21–77 (60) | Case-Series (4) | Exercise | Self-Report | 42% Low Adherence 30% Moderate Adherence 27% High Adherence |
Yes |
| Cnossen et al 2016 | HNC (n=50) | 40–77 (61) | Prospective Cohort Study (2) | Exercise | Self-Report (diary) | 70% Adherence at 6-weeks 38% Adherence at 12 weeks |
No |
| Krisciunas et al 2016 | HNC (n=153) | 61.9 (+/− 9.6) | Randomized Control Trial (1) | Exercise + Electrical Stimulation | Self-Report (checklist) | 54% Adherent | No |
| Wall et al 2016 | HNC (n=71) | 59.50 (+/− 6.15) | Randomized Control Trial (1) | Exercise (prophylactic) | Self-Report (log-books), SwallowIT, Clinician tracked | 27% Average Adherence | Yes |
| Hajdu et al 2017 | HNC (n=6) | 42–67 (57) | Pilot Study (4) | Exercise | Self-Report (logbook, weekly phone calls) | 92% Average Adherence | No |
This study reported means for two groups, the first number (77.4) represents average age of surviving subjects, the second number represents average age of subjects who died. +/− Represents standard deviation for the mean age that was reported in two studies
Patient Populations
Dysphagia treatment serves diverse populations across the lifespan. The acute or chronic nature of the primary diagnoses of patients with dysphagia, or comorbid conditions are likely to be factors impacting the degree of adherence to a specific therapy or recommendation.(4, 36) Thus, consideration should be given to the primary diagnoses of patients identified in our review.
Of the twelve papers identified in this review, a majority (9/12) had a focus on adherence with recommendations in patients with head and neck cancer. For patients with head and neck cancer, symptoms impacting swallowing may fluctuate throughout treatment,(37, 38) which may contribute to changes in adherence. In fact, one of the studies in our review showed how adherence to exercise decreased throughout treatment, from 70% at 6-weeks of intensity-modulated radiation therapy to only 38% adherence at 12 weeks.(29) The severity of swallowing deficits may also depend on the approach used during tumor intervention (e.g. chemoradiation therapy vs. intensity modulated chemoradiation therapy vs. surgical intervention).(39, 40) These competing factors are variable and are likely to impact the overall severity of impaired swallowing,(41–43) making this a challenging population to study. Consequently, adherence rates from a head and neck cancer population are unique and cannot be readily generalized to other dysphagia-related conditions because of these distinct features.
Five out of nine head and neck cancer studies in this review had a prophylactic treatment component. Prophylactic exercises are prescribed in an attempt to strengthen multiple muscle groups involved in swallowing before chemoradiation treatment with the goal of preventing or diminishing the devastating effects radiation has on swallowing function.(44, 45) Prophylactic management of any kind is administered before the onset of symptoms. We postulate that adherence to this type of proactive treatment may vary based (1) on the treatment type or recommendation; or (2) whether or not the person is currently experiencing symptoms from their diagnos(es) or condition(s). These additional factors may affect generalizability of this information to other types of treatment for dysphagia.
The three studies identified in our review that included etiologies other than head and neck cancer examined at least 16 different primary diagnoses. As discussed with head and neck cancer, these conditions are complex and contain their own characteristics and related complications with swallowing. While the importance and impact of the research presented here regarding head and neck cancer populations cannot be overstated, continued focused work to determine the factors influencing adherence across other patient groups is needed.
Tracking Adherence
The method used to track adherence is an important consideration. The quality of data collected depends on a reliable approach to track and measure adherence. In all but three of the twelve studies examined, a self-report approach for obtaining adherence-related data was used. Of the three studies using different measures to track adherence, one video-recorded patients eating and directly observed whether they were adherent to recommendations,(33) another used record revies ability to perform the exercises.(24) Although this third approach could be considered a measure of knowledge or ability to perform the exercises rather than truly reflecting adherence, the authors justify this measurement saying, “It was not possible for participants to demonstrate competency to the speech pathologist at later follow-up appointments if they had not practiced regularly at home” (p. 1708).
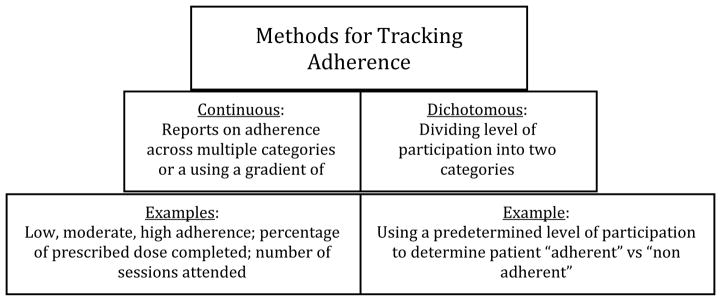
These varied approaches to tracking and measuring adherence raise the question of what method is best for determining how well a patient adheres to recommendations. Unfortunately, the best method for tracking adherence still remains to be definitely decided, although experts who have studied adherence offer some advice.(4) It has been recommended that adherence be recorded using multiple approaches so that data between sources can be triangulated for the most accurate view of patient adherence.(4) In general, there are two broad domains encompassing measurement and reporting of adherence: 1) “Continuous” measurement is defined as “offer[ing] three or more ordered response categories, or is based on multiple adherence criteria, or uses a reliable, validated continuous measure”; or, 2) “Dichotomous” measurement, defined as “involv[ing] two categories (adherent vs. not adherent), sometimes based on a percentage determined by the researchers,” (p. 803).(4) (Figure 2.) By combining approaches for adherence tracking methodology through continuous and dichotomous strategies and comparing data using multiple sources, the most complete picture of adherence is likely to be established.
Figure 2.
Self-report of adherence alone can introduce bias into a data set given that individuals are likely to over-report participation.(46) In a study comparing self-report measures (interview, diary, questionnaire) with “nonself-report measures” (pill count, plasma drug concentration, electronic monitors, etc), researchers found that certain self-report measures were more concordant with nonself-report measures.(47) For example, interviews had a significantly lower association with nonself-report measures than did diaries or questionnaires, indicating that not all self-report measures will provide the same accuracy.(47) Although there are many ways to use self-reported adherence, it is still unknown which measure is best for tracking home exercise programs.(48) Recently, devices used regularly in swallowing therapy, such as the SwallowStrong® device and the Iowa Oral Performance Instrument (IOPI), have been designed to include objective reporting of patient adherence.(49–51) Use of such devices gives potential for multiple, more accurate measures of adherence within dysphagia therapy. We conclude and encourage that future adherence research in dysphagia include multiple, both continuous and dichotomous measures of adherence wherever possible.
Barriers and Facilitators
Of the twelve articles included in this review, half of them attempted to identify barriers and or facilitators to adherence (Table 2). The method by which this information was obtained varied among the papers and included: phone interview sometime after completion of the study,(24, 34) immediate informal interview upon trial completion,(33) statistical comparison of characteristics of patients who were adherent and those who were not,(17) weekly phone interviews during treatment,(28) and daily log-book entries or clinician-led questions after completion of a session.(32) Barriers identified are likely to vary depending upon the specific treatment modality. For example, “dissatisfaction with texture or taste” was related to a diet or liquid modification recommendation while “difficulty in performing exercises” was specific to an exercise regimen. However, several barriers identified in our review could relate to a variety of treatments, including depression, questioning of motivation or relevance, therapy buy-in, forgetting to complete tasks, or living at home. Some barriers, if corrected, could serve as facilitators. For example, negative social implications of following recommendations could be a barrier, but having a social support network that encourages completion of recommendations could serve as a facilitator. Similarly, overall decreased physical condition could reduce adherence with a therapy regimen, but improved or increased physical condition might recover adherence. One paper excluded from our review(52) sought to identify barriers and facilitators using a unique approach by informally interviewing exclusively nonadherent patients. Patient interviews using sound qualitative methodology could be a useful approach to gain more specific information on barriers and facilitators to adherence in future studies.
According to the World Health Organization 2003 comprehensive report on adherence, the main barriers to adherence to patient-specific interventions in healthcare involve decreased education and skills in self-management, decreased motivation, and a lack of support to incur behavioral change.(53) While these barriers don’t align one-for-one with the barriers identified in this review, many have similar themes such as low motivation and decreased self-management to complete a task (denial, task difficult, remembering). Other reasons for non-adherence identified in areas of health care include forgetfulness, substance abuse, fear of disclosure, work and family responsibilities(54), lack of interest, lack of time, medical conditions, and family priorities.(55) Facilitators have been identified in other areas too, including flexibility in program timing, home-based exercises, exercises that are easy to perform,(55) feeling of self-worth, seeing positive effects of medications, understanding the need of adherence, and use of reminder tools.(54) While some of these barriers and facilitators discussed from other realms of healthcare could apply to patients with dysphagia, it is easy to identify those that would not apply in dysphagia management. For example, from the HIV literature the number of pills required and negative side effects of drug therapy would not apply, or from the osteoporosis literature, fear of falling or injury and reduced mobility would not be barriers necessarily affecting someone with dysphagia. Because dysphagia diagnoses are so broad and may involve one or more comorbidities and complex therapy approaches, more work is needed to identify barriers and facilitators relevant to patients with dysphagia.
Other Considerations
The papers in this review cover many topics related to adherence. However, there are several areas that have yet to be explored. One specific topic that has received little attention is the relationship between dose of behavioral or exercise therapy and patient adherence. One study in our review(31) discussed this specifically, saying that there is a “clear lack of consensus regarding optimal dose of swallow therapy and therefore acceptable compliance rates” (p. 331). This becomes apparent as we examine the varied doses in this review, the wide range of therapies available, and the diverse definitions of adherence. Dose frequency is cited by the World Health Organization as one of the top barriers to adherence in therapy interventions.(53) Thus, finding the minimum effective dose necessary to induce physiological change is a critical component in development of treatment recommendations that maximize patient adherence.(53, 56) Further exploration is needed to determine effects of dose on physiological and biological components of exercise interventions.
Another topic not covered in this review is external influences on patient adherence, such as health-systems related factors.(57) In this review, we only included studies that reported a quantifiable measure of patient-specific adherence. However, there were several studies we uncovered that explored other persons and factors that may influence patient adherence including speech-language pathologists,(58) nursing staff,(59) and the healthcare team.(60) Rosenvinge and Starke (2005) conducted a study where they trained health care team members and observed patient adherence to dysphagia recommendations before and after training.(60) They demonstrated an improvement in adherence with recommendations for fluid consistencies, amounts given, and safe swallow guidelines after a staff education and training intervention to improve understanding of dysphagia recommendations.(60) Further, the level of caregiver involvement and support is important to consider when examining adherence in patients with cognitive impairment, where there is reliance on a caregiver to carry out treatment plans.
In addition to the external factors influencing patient adherence, internal patient-specific factors such as self-efficacy must also be considered.(61) Classically identified as a critical component of social cognitive theory,(62) self-efficacy is the expectation of how one will be able perform a “specific task”.(63) In lay terms, self-efficacy is the level of self-confidence someone has about their own ability to successfully complete a task,(63, 64) in this case the “specific task” would be follow-through with recommendations for management of dysphagia. In fact, both self-efficacy and “expectation of outcome” were both found to predict adherence to an exercise-based program in a classic study,(65) where self-efficacy was even more so predictive than expectations alone. Improving self-efficacy through educational interventions can be beneficial to improving adherence in patients with chronic disease.(66) This idea of enhancing self-efficacy in populations of patients who have dysphagia requires further study and should be examined in future work focused on in improving adherence.(61)
Limitations
Other factors to consider in adherence research are the innate limitations that come with the nature of this work. Many of the studies examined in this review acknowledged and discussed these limitations, and they should be considered and addressed in future research. First, studies examining adherence to therapy regimens might have an inherently biased sample: those who agreed to participate in an intervention study may be more motivated than those who declined inclusion, and thus may demonstrate higher adherence than the general population. One article in our review(29) mentioned this as a limitation, stating that head and neck cancer patients in their study could have been more motivated to complete the exercises, hence why they enrolled in the study. Several papers cited measuring adherence via self-report(17, 28, 29, 32, 34) and having a small sample size(17, 28, 31–34) as limitations.
A limitation the authors noted while performing this review was lack of specificity when describing therapy interventions a lack of detail when describing methods of tracking adherence. It is often difficult to ascertain from the methods described what the specific intervention involved or what exact method of tracking was used. General rather than specific terms were used to describe adherence-tracking mechanisms, making it difficult to pinpoint the exact methodology used to record or measure adherence and reducing interpretability. More detailed descriptions of the interventions studied will be necessary to improve the design and execution of future work in this area.
Future Directions
The papers identified in this review provide a basis for future exploration of adherence to dysphagia recommendations. In addition, this systematic review identifies a gap in knowledge on this topic: there are very few studies in a large body of literature examining dysphagia interventions that actually account for and report on adherence. Further, within these studies that do measure and track for adherence, often it is difficult to discern the methodology used for recording adherence. Without these data, interpretation of outcomes and effectiveness of interventions are limited as the actual level of patient performance is unknown.
In designing future studies, we recommend consideration of the method of tracking, sample size, recruitment bias, external influences on patient adherence, and adequate characterization and description of the patient populations and treatment interventions. Further, including more detailed methodology and descriptions of interventions provided should bolster the external validity of findings. For behavioral and exercise interventions, identifying the minimal dose needed for biological and physiological change will be important for creating and modifying current interventions to maximize opportunities for patient adherence. Additional work must be done to identify the best methods of tracking adherence specific to dysphagia treatment as well. Qualitative methods may be useful in identifying barriers and facilitators to adherence by using patient-led interview to discuss reasons for adherence or nonadherence. Further, theoretical modeling of adherence has yet to be fully explored. Experts insist that the use of theoretical modeling in adherence research is critical, yet understudied.(67) An appropriate theoretical model for adherence to dysphagia therapy should be identified and explored to provide a better framework for understanding and improving adherence. The current state of a majority of research in our field often includes adherence as a secondary aim or afterthought to explain a lack of significant findings. Studies with primary aims to systematically quantify and interpret adherence to recommendations will be required.
Conclusions
Patient adherence is an important consideration in treatment development and implementation. Although dysphagia treatment is broad and complex, we only identified twelve studies reporting patient adherence data. We first conclude that very little is known or understood about patient adherence recommendations in the field of dysphagia research. Considering how important patient adherence is to the success of many of these recommendations, it is a critical topic that must be addressed in future studies to improve patient outcomes and quality of life. A majority of what is understood about adherence comes from other health disciplines. However, we must take on the challenge of directly studying adherence within our own field rather than assuming cross-over from other disciplines. We hope and anticipate that the information provided in this review will serve as a platform for the future study of adherence within dysphagia management.
Supplementary Material
Acknowledgments
Funding: This study was funded in part by the National Institute on Deafness and Other Communication Disorders (R01DC005935, R01DC008149, R01DC014358, T32-DC009401).
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.Vermeire E, Hearnshaw H, Van Royen P, Denekens J. Patient adherence to treatment: three decades of research. A comprehensive review. Journal of clinical pharmacy and therapeutics. 2001;26:331–342. doi: 10.1046/j.1365-2710.2001.00363.x. [DOI] [PubMed] [Google Scholar]
- 2.Donovan JL. Patient decision making: the missing ingredient in compliance research. International journal of technology assessment in health care. 1995;11:443–455. doi: 10.1017/s0266462300008667. [DOI] [PubMed] [Google Scholar]
- 3.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Medical care. 2005;43:521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 4.DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Medical care. 2002;40:794–811. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Jack K, McLean SM, Moffett JK, Gardiner E. Barriers to treatment adherence in physiotherapy outpatient clinics: a systematic review. Manual therapy. 2010;15:220–228. doi: 10.1016/j.math.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Archives of internal medicine. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 7.Bartlett EE, Grayson M, Barker R, Levine DM, Golden A, Libber S. The effects of physician communications skills on patient satisfaction; recall, and adherence. Journal of chronic diseases. 1984;37:755–764. doi: 10.1016/0021-9681(84)90044-4. [DOI] [PubMed] [Google Scholar]
- 8.Egolf RJ, BeGole EA, Upshaw HS. Factors associated with orthodontic patient compliance with intraoral elastic and headgear wear. American Journal of Orthodontics and Dentofacial Orthopedics. 1990;97:336–348. doi: 10.1016/0889-5406(90)70106-M. [DOI] [PubMed] [Google Scholar]
- 9.Chapman K, Walker L, Cluley S, Fabbri L. Improving patient compliance with asthma therapy. Respiratory medicine. 2000;94:2–9. doi: 10.1053/rmed.1999.0667. [DOI] [PubMed] [Google Scholar]
- 10.Berg JS, Dischler J, Wagner DJ, Raia JJ, Palmer-Shevlin N. Medication compliance: a healthcare problem. Ann Pharmacother. 1993;27:S1–S24. [PubMed] [Google Scholar]
- 11.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clinical therapeutics. 2001;23:1296–1310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 12.Morris LS, Schulz R. Patient compliance—an overview. Journal of clinical pharmacy and therapeutics. 1992;17:283–295. doi: 10.1111/j.1365-2710.1992.tb01306.x. [DOI] [PubMed] [Google Scholar]
- 13.Martin LR, Williams SL, Haskard KB, DiMatteo MR. The challenge of patient adherence. Ther Clin Risk Manag. 2005;1:189–199. [PMC free article] [PubMed] [Google Scholar]
- 14.Lancaster J. Dysphagia: its nature, assessment and management. British journal of community nursing. 2015 doi: 10.12968/bjcn.2015.20.Sup6a.S28. [DOI] [PubMed] [Google Scholar]
- 15.Sura L, Madhavan A, Carnaby G, Crary MA. Dysphagia in the elderly: management and nutritional considerations. Clin Interv Aging. 2012;7:98. doi: 10.2147/CIA.S23404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serra-Prat M, Palomera M, Gomez C, Sar-Shalom D, Saiz A, Montoya JG, Navajas M, Palomera E, Clavé P. Oropharyngeal dysphagia as a risk factor for malnutrition and lower respiratory tract infection in independently living older persons: a population-based prospective study. Age and ageing. 2012;41:376–381. doi: 10.1093/ageing/afs006. [DOI] [PubMed] [Google Scholar]
- 17.Low J, Wyles C, Wilkinson T, Sainsbury R. The effect of compliance on clinical outcomes for patients with dysphagia on videofluoroscopy. Dysphagia. 2001;16:123–127. doi: 10.1007/s004550011002. [DOI] [PubMed] [Google Scholar]
- 18.Baijens LW, Clavé P, Cras P, Ekberg O, Forster A, Kolb GF, Leners J-C, Masiero S, Mateos-Nozal J, Ortega O. European Society for Swallowing Disorders–European Union Geriatric Medicine Society white paper: oropharyngeal dysphagia as a geriatric syndrome. Clinical interventions in aging. 2016;11:1403. doi: 10.2147/CIA.S107750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zolnierek KBH, DiMatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Medical care. 2009;47:826. doi: 10.1097/MLR.0b013e31819a5acc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Namasivayam AM, Steele CM, Keller H. The effect of tongue strength on meal consumption in long term care. Clinical Nutrition. 2016;35:1078–1083. doi: 10.1016/j.clnu.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Wright RW, Brand RA, Dunn W, Spindler KP. How to write a systematic review. Clinical orthopaedics and related research. 2007;455:23–29. doi: 10.1097/BLO.0b013e31802c9098. [DOI] [PubMed] [Google Scholar]
- 22.Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, Porter AC, Tugwell P, Moher D, Bouter LM. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC medical research methodology. 2007;7:10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benzies KM, Premji S, Hayden KA, Serrett K. State-of-the-evidence reviews: advantages and challenges of including grey literature. Worldviews on Evidence-Based Nursing. 2006;3:55–61. doi: 10.1111/j.1741-6787.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- 24.Shinn EH, Basen-Engquist K, Baum G, Steen S, Bauman RF, Morrison W, Garden AS, Sheil C, Kilgore K, Hutcheson KA. Adherence to preventive exercises and self-reported swallowing outcomes in post-radiation head and neck cancer patients. Head & neck. 2013;35:1707–1712. doi: 10.1002/hed.23255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutcheson KA, Bhayani MK, Beadle BM, Gold KA, Shinn EH, Lai SY, Lewin J. Eat and exercise during radiotherapy or chemoradiotherapy for pharyngeal cancers: use it or lose it. JAMA Otolaryngology–Head & Neck Surgery. 2013;139:1127–1134. doi: 10.1001/jamaoto.2013.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duarte VM, Chhetri DK, Liu YF, Erman AA, Wang MB. Swallow preservation exercises during chemoradiation therapy maintains swallow function. Otolaryngology--Head and Neck Surgery. 2013 doi: 10.1177/0194599813502310. 0194599813502310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Starmer H, Sanguineti G, Marur S, Gourin CG. Multidisciplinary head and neck cancer clinic and adherence with speech pathology. The Laryngoscope. 2011;121:2131–2135. doi: 10.1002/lary.21746. [DOI] [PubMed] [Google Scholar]
- 28.Cnossen IC, van Uden-Kraan CF, Rinkel RN, Aalders IJ, de Goede CJ, de Bree R, Doornaert P, Rietveld DH, Langendijk JA, Witte BI. Multimodal guided self-help exercise program to prevent speech, swallowing, and shoulder problems among head and neck cancer patients: a feasibility study. Journal of medical Internet research. 2014;16:e74. doi: 10.2196/jmir.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cnossen IC, van Uden-Kraan CF, Witte BI, Aalders YJ, de Goede CJ, de Bree R, Doornaert P, Rietveld DH, Buter J, Langendijk JA. Prophylactic exercises among head and neck cancer patients during and after swallowing sparing intensity modulated radiation: adherence and exercise performance levels of a 12-week guided home-based program. European Archives of Oto-Rhino-Laryngology. 2016:1–10. doi: 10.1007/s00405-016-4367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hajdú SF, Wessel I, Johansen C, Kristensen CA, Kadkhoda ZT, Plaschke CC, Dalton SO. Swallowing therapy and progressive resistance training in head and neck cancer patients undergoing radiotherapy treatment: randomized control trial protocol and preliminary data. Acta Oncologica. 2017;56:354–359. doi: 10.1080/0284186X.2016.1269193. [DOI] [PubMed] [Google Scholar]
- 31.Krisciunas GP, Castellano K, McCulloch TM, Lazarus CL, Pauloski BR, Meyer TK, Graner D, Van Daele DJ, Silbergleit AK, Crujido LR. Impact of compliance on dysphagia rehabilitation in head and neck cancer patients: results from a multi-center clinical trial. Dysphagia. 2016:1–10. doi: 10.1007/s00455-016-9760-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wall LR, Ward EC, Cartmill B, Hill AJ, Porceddu SV. Adherence to a Prophylactic Swallowing Therapy Program During (Chemo) Radiotherapy: Impact of Service-Delivery Model and Patient Factors. Dysphagia. 2016:1–14. doi: 10.1007/s00455-016-9757-z. [DOI] [PubMed] [Google Scholar]
- 33.Leiter A, Windsor J. Compliance of geriatric dysphagic patients with safe-swallowing instructions. JOURNAL OF MEDICAL SPEECH LANGUAGE PATHOLOGY. 1996;4:289–299. [Google Scholar]
- 34.Shim JS, Oh B-M, Han TR. Factors associated with compliance with viscosity-modified diet among dysphagic patients. Annals of rehabilitation medicine. 2013;37:628–632. doi: 10.5535/arm.2013.37.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Medical care. 2004;42:200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 36.DiMatteo MR. Evidence-based strategies to foster adherence and improve patient outcomes: the author’s recent meta-analysis indicates that patients do not follow treatment recommendations unless they know what to do, are committed to doing it, and have the resources to be able to adhere. JAAPA-Journal of the American Academy of Physicians Assistants. 2004;17:18–22. [PubMed] [Google Scholar]
- 37.Mekhail TM, Adelstein DJ, Rybicki LA, Larto MA, Saxton JP, Lavertu P. Enteral nutrition during the treatment of head and neck carcinoma. Cancer. 2001;91:1785–1790. [PubMed] [Google Scholar]
- 38.Pezner RD, Archambeau JO, Lipsett JA, Kokal WA, Thayer W, Robert L. Tube feeding enteral nutritional support in patients receiving radiation therapy for advanced head and neck cancer. International Journal of Radiation Oncology* Biology* Physics. 1987;13:935–939. doi: 10.1016/0360-3016(87)90110-6. [DOI] [PubMed] [Google Scholar]
- 39.Eisbruch A, Schwartz M, Rasch C, Vineberg K, Damen E, Van As CJ, Marsh R, Pameijer FA, Balm AJ. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? International Journal of Radiation Oncology* Biology* Physics. 2004;60:1425–1439. doi: 10.1016/j.ijrobp.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 40.Logemann JA, Pauloski BR, Rademaker AW, Colangelo LA. Speech and swallowing rehabilitation for head and neck cancer patients. Oncology (Williston Park, NY) 1997;11:651–656. 659. discussion 659: 663–654. [PubMed] [Google Scholar]
- 41.Frowen JJ, Perry AR. Swallowing outcomes after radiotherapy for head and neck cancer: a systematic review. Head & neck. 2006;28:932–944. doi: 10.1002/hed.20438. [DOI] [PubMed] [Google Scholar]
- 42.Frowen J, Cotton S, Corry J, Perry A. Impact of demographics, tumor characteristics, and treatment factors on swallowing after (chemo) radiotherapy for head and neck cancer. Head & neck. 2010;32:513–528. doi: 10.1002/hed.21218. [DOI] [PubMed] [Google Scholar]
- 43.Stenson KM, MacCracken E, List M, Haraf DJ, Brockstein B, Weichselbaum R, Vokes EE. Swallowing function in patients with head and neck cancer prior to treatment. Archives of Otolaryngology–Head & Neck Surgery. 2000;126:371–377. doi: 10.1001/archotol.126.3.371. [DOI] [PubMed] [Google Scholar]
- 44.Kotz T, Federman AD, Kao J, Milman L, Packer S, Lopez-Prieto C, Forsythe K, Genden EM. Prophylactic swallowing exercises in patients with head and neck cancer undergoing chemoradiation: a randomized trial. Archives of Otolaryngology–Head & Neck Surgery. 2012;138:376–382. doi: 10.1001/archoto.2012.187. [DOI] [PubMed] [Google Scholar]
- 45.Mittal BB, Pauloski BR, Haraf DJ, Pelzer HJ, Argiris A, Vokes EE, Rademaker A, Logemann JA. Swallowing dysfunction—preventative and rehabilitation strategies in patients with head-and-neck cancers treated with surgery, radiotherapy, and chemotherapy: a critical review. International Journal of Radiation Oncology* Biology* Physics. 2003;57:1219–1230. doi: 10.1016/s0360-3016(03)01454-8. [DOI] [PubMed] [Google Scholar]
- 46.Adams AS, Soumerai SB, Lomas J, Ross-Degnan D. Evidence of self-report bias in assessing adherence to guidelines. International Journal for Quality in Health Care. 1999;11:187–192. doi: 10.1093/intqhc/11.3.187. [DOI] [PubMed] [Google Scholar]
- 47.Garber MC, Nau DP, Erickson SR, Aikens JE, Lawrence JB. The concordance of self-report with other measures of medication adherence: a summary of the literature. Medical care. 2004;42:649–652. doi: 10.1097/01.mlr.0000129496.05898.02. [DOI] [PubMed] [Google Scholar]
- 48.Bollen JC, Dean SG, Siegert RJ, Howe TE, Goodwin VA. A systematic review of measures of self-reported adherence to unsupervised home-based rehabilitation exercise programmes, and their psychometric properties. BMJ open. 2014;4:e005044. doi: 10.1136/bmjopen-2014-005044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robbins J, Gangnon RE, Theis SM, Kays SA, Hewitt AL, Hind JA. The effects of lingual exercise on swallowing in older adults. Journal of the American Geriatrics Society. 2005;53:1483–1489. doi: 10.1111/j.1532-5415.2005.53467.x. [DOI] [PubMed] [Google Scholar]
- 50.Rogus-Pulia N, Rusche N, Hind JA, Zielinski J, Gangnon R, Safdar N, Robbins J. Effects of Device-Facilitated Isometric Progressive Resistance Oropharyngeal Therapy on Swallowing and Health-Related Outcomes in Older Adults with Dysphagia. Journal of the American Geriatrics Society. 2016;64:417–424. doi: 10.1111/jgs.13933. [DOI] [PubMed] [Google Scholar]
- 51.Oh J-C. Effects of tongue strength training and detraining on tongue pressures in healthy adults. Dysphagia. 2015;30:315–320. doi: 10.1007/s00455-015-9601-x. [DOI] [PubMed] [Google Scholar]
- 52.Colodny N. Dysphagic independent feeders’ justifications for noncompliance with recommendations by a speech-language pathologist. American Journal of Speech-Language Pathology. 2005;14:61–70. doi: 10.1044/1058-0360(2005/008). [DOI] [PubMed] [Google Scholar]
- 53.Sabaté E. Adherence to long-term therapies: evidence for action. World Health Organization; 2003. [PubMed] [Google Scholar]
- 54.Mills EJ, Nachega JB, Bangsberg DR, Singh S, Rachlis B, Wu P, Wilson K, Buchan I, Gill CJ, Cooper C. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006;3:e438. doi: 10.1371/journal.pmed.0030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodrigues IB, Armstrong JJ, Adachi JD, MacDermid JC. Facilitators and barriers to exercise adherence in patients with osteopenia and osteoporosis: a systematic review. Osteoporosis International. 2017;28:735–745. doi: 10.1007/s00198-016-3793-2. [DOI] [PubMed] [Google Scholar]
- 56.Warburton DE, Nicol CW, Bredin SS. Prescribing exercise as preventive therapy. Canadian Medical Association Journal. 2006;174:961–974. doi: 10.1503/cmaj.1040750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carayon P, Hundt AS, Karsh B, Gurses A, Alvarado C, Smith M, Brennan PF. Work system design for patient safety: the SEIPS model. Quality and Safety in Health Care. 2006;15:i50–i58. doi: 10.1136/qshc.2005.015842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith-Tamaray M, Wilson L, McAllister L. Factors affecting dysphagia management and compliance with recommendations in non-metropolitan healthcare settings. International journal of speech-language pathology. 2011;13:268–279. doi: 10.3109/17549507.2011.573575. [DOI] [PubMed] [Google Scholar]
- 59.Edmonds MF, McGuire DB. Treatment adherence in head and neck cancer patients undergoing radiation therapy: challenges for nursing. Journal of Radiology Nursing. 2007;26:87–92. [Google Scholar]
- 60.Rosenvinge SK, Starke ID. Improving care for patients with dysphagia. Age and ageing. 2005;34:587–593. doi: 10.1093/ageing/afi187. [DOI] [PubMed] [Google Scholar]
- 61.Rogus-Pulia N, Hind J. Patient-Centered Dysphagia Therapy-The Critical Impact of Self-Efficacy. SIG 13 Perspectives on Swallowing and Swallowing Disorders (Dysphagia) 2015;24:146–154. [Google Scholar]
- 62.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychological review. 1977;84:191. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 63.Zimmerman BJ. Self-efficacy: An essential motive to learn. Contemporary educational psychology. 2000;25:82–91. doi: 10.1006/ceps.1999.1016. [DOI] [PubMed] [Google Scholar]
- 64.van Leer E, Hapner ER, Connor NP. Transtheoretical model of health behavior change applied to voice therapy. Journal of Voice. 2008;22:688–698. doi: 10.1016/j.jvoice.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Desharnais R, Bouillon J, Godin G. Self-efficacy and outcome expectations as determinants of exercise adherence. Psychological Reports. 1986;59:1155–1159. [Google Scholar]
- 66.Marks R, Allegrante JP. A review and synthesis of research evidence for self-efficacy-enhancing interventions for reducing chronic disability: implications for health education practice (part II) Health promotion practice. 2005;6:148–156. doi: 10.1177/1524839904266792. [DOI] [PubMed] [Google Scholar]
- 67.van Dulmen S, Sluijs E, van Dijk L, de Ridder D, Heerdink R, Bensing J. Patient adherence to medical treatment: a review of reviews. BMC health services research. 2007;7:55. doi: 10.1186/1472-6963-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.