Abstract
INTRODUCTION
It is unclear whether abnormalities in brain glucose homeostasis are associated with AD pathogenesis.
METHODS
Within the autopsy cohort of the Baltimore Longitudinal Study of Aging, we measured brain glucose concentration and assessed the ratios of the glycolytic amino acids, serine, glycine and alanine to glucose. We also quantified protein levels of the neuronal (GLUT3) and astrocytic (GLUT1) glucose transporters. Finally, we assessed the relationships between plasma glucose measured prior to death and brain tissue glucose.
RESULTS
Higher brain tissue glucose concentration, reduced glycolytic flux, and lower GLUT3 are related to severity of AD pathology and the expression of AD symptoms. Longitudinal increases in fasting plasma glucose levels are associated with higher brain tissue glucose concentrations.
DISCUSSION
Impaired glucose metabolism due to reduced glycolytic flux may be intrinsic to AD pathogenesis. Abnormalities in brain glucose homeostasis may begin several years prior to the onset of clinical symptoms.
Keywords: glucose, insulin resistance, Alzheimer’s disease, GLUT3, GLUT1, neuritic plaque, neurofibrillary tangles, mass spectrometry, glycolysis
1. Introduction
While numerous epidemiological studies indicate that peripheral insulin resistance (IR) and diabetes are risk factors for Alzheimer’s disease (AD) [1–3], it is not known whether brain glucose dysregulation is a key feature of AD and is related to severity of AD pathology or symptom expression [4, 5]. Previous studies have shown that several components of the insulin signaling pathway are abnormal in AD brains relative to controls, including genes encoding insulin, IGF-1, and IGF-2 peptides and their receptors [6–10]. Since these abnormalities appear to be a common feature of both type-1 and type-2 diabetes, the term “type-3 diabetes” was proposed to describe brain-specific abnormalities in insulin signaling associated with AD [11, 12]. Taken together, the large body of evidence implicating abnormal insulin signaling in AD has led to clinical trials targeting these abnormalities in patients with mild cognitive impairment (MCI) and AD [13–15]. However, it is well recognized that glucose transport from the peripheral circulation across the blood brain barrier (BBB) and capillary endothelial cells into the interstitial fluid and brain tissue are largely insulin-independent processes [16, 17]. Similarly, the transport of glucose across the cell membrane into neurons is largely independent of insulin [18]. While 18Fdeoxyglucose positron emission tomography (18FDG-PET) studies have shown reduced brain glucose uptake in regions vulnerable to AD pathology [19–22], it is unclear whether an overall failure of regulation of brain glucose metabolism is a key etiopathogenic factor in AD and whether abnormalities of brain glucose homeostasis in AD are related to peripheral glucose concentration. Answering these questions is critical to establishing whether central glucose homeostasis is a potential target for disease-modifying treatments in AD.
In this study we asked the following main questions:
Is brain tissue glucose concentration altered in AD?
What is the relationship between brain tissue glucose concentration and severity of AD pathology?
What are plausible molecular mechanisms underlying abnormalities of brain glucose homeostasis in AD?
What is the relationship between trajectories of blood glucose concentration during life and brain tissue glucose levels measured at death?
Our results provide the first evidence for brain glucose dysregulation as a critical event in AD pathogenesis that closely reflects both severity of AD pathology and the expression of symptoms.
2. Methods
2.1 Participants
The Baltimore Longitudinal Study of Aging (BLSA) is a prospective, ongoing cohort study of community-dwelling volunteer participants in Baltimore that began in 1958 and has been described in detail previously [23, 24]. Historically, participants underwent extensive biomedical examination and neuropsychological testing every two years. From 2003, participants under age 60 are assessed every 4 years; those aged 60 to 79 years every 2 years, and participants aged 80 and older are assessed annually. Written informed consent was obtained at each visit, and the study was approved by the local Institutional Review Board and the National Institute on Aging. The participants in this report were from the autopsy program of the BLSA that was initiated in 1986 and has been described previously [25]) They provided data on concentrations of brain tissue glucose and the glycolytic amino acids, serine, glycine and alanine (N = 43; from the middle frontal gyrus; MFG, inferior temporal gyrus; ITG and cerebellum), as well as proteomic data from the MFG (N=47). The mean age at death in the sample was 86.6 ± 9.5 years (range 62.9–99.2). As reported previously, the autopsy subsample is not significantly different from the BLSA cohort as a whole in terms of the rates of dementia and clinical stroke [26].
2.2 Cognitive status
At each assessment, participants underwent a battery of neuropsychological testing. Clinical and neuropsychological data were reviewed at consensus case conferences if they made four or more errors on the Blessed Information Memory Concentration (BIMC) test, if their Clinical Dementia Rating (CDR) score was equal to or greater than 0.5, or if concerns were raised about their cognitive status. In addition, all participants were evaluated by case conference on death or withdrawal. The diagnoses of dementia and AD were based on the Diagnostic and Statistical Manual (DSM)-III-R [27] and the National Institute of Neurological and Communication Disorders and Stroke — Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria [28], respectively.
2.3 Neuropathological studies
Postmortem brain examinations were performed by an experienced neuropathologist (J.C.T.). Assessment of neuritic plaques and neurofibrillary tangles using Consortium to Establish a Registry for Alzheimer's Disease (CERAD) [29] and Braak criteria [30] respectively have been described previously [31]. We have previously described the clinico-pathological features of BLSA participants categorized as “asymptomatic Alzheimer’s disease (ASYMAD)” after neuropathological assessment at death [32]. Briefly, these individuals have significant AD neuropathology at autopsy, but without evidence for cognitive impairment during life, as assessed by longitudinal cognitive evaluations during their BLSA research visits.
2.4 Plasma glucose measurement
Plasma glucose measurements were obtained from venous blood samples after an overnight fast by the glucose-oxidase method as described previously [33]. We used all available longitudinal plasma glucose data (445 observations, mean follow up interval. 19.1 years). We excluded 10 data points where fasting plasma glucose values were beyond three standard deviations from the mean value.
2.5 Quantitative metabolomics assays of brain tissue glucose and glycolytic amino acids (serine, glycine and alanine)
Glucose concentration was measured in frozen brain tissue samples from 43 BLSA participants (N=14 AD; N=14 control and N=15 ‘ASYMAD’) on the BIOCRATES P180 platform. Brain tissue regions for glucose assays were selected a priori in the MFG and ITG to represent brain regions vulnerable to amyloid and tau deposition respectively. The cerebellum was sampled to represent an additional brain region resistant to classical AD pathology [34].
A sterile 4 mm diameter tissue punch was extracted from the cortical surface of the three brain regions i.e. MFG, IRG and the cerebellum from brain tissue samples stored at −80°C. To extract metabolites from brain tissue, samples were homogenized using Precellys® with ethanol phosphate buffer. Samples were centrifuged and the supernatant was used for analysis. The BIOCRATES P180 platform was used for the quantification of glucose and the three glycolytic amino acids, serine, glycine and alanine. The fully automated assay was based on PITC (phenylisothiocyanate) derivatization in the presence of internal standards followed by flow injection analysis-mass spectrometry (FIA-MS/MS; for glucose) and liquid chromatography-tandem mass spectrometry (LC-MS/MS; for glycolytic amino acids) using a SCIEX 4000 QTrap® mass spectrometer (SCIEX, Darmstadt, Germany) with electrospray ionization. Brain tissue glucose concentration was derived as the sum of hexoses detected and absolute concentration expressed as nmol/mg tissue.
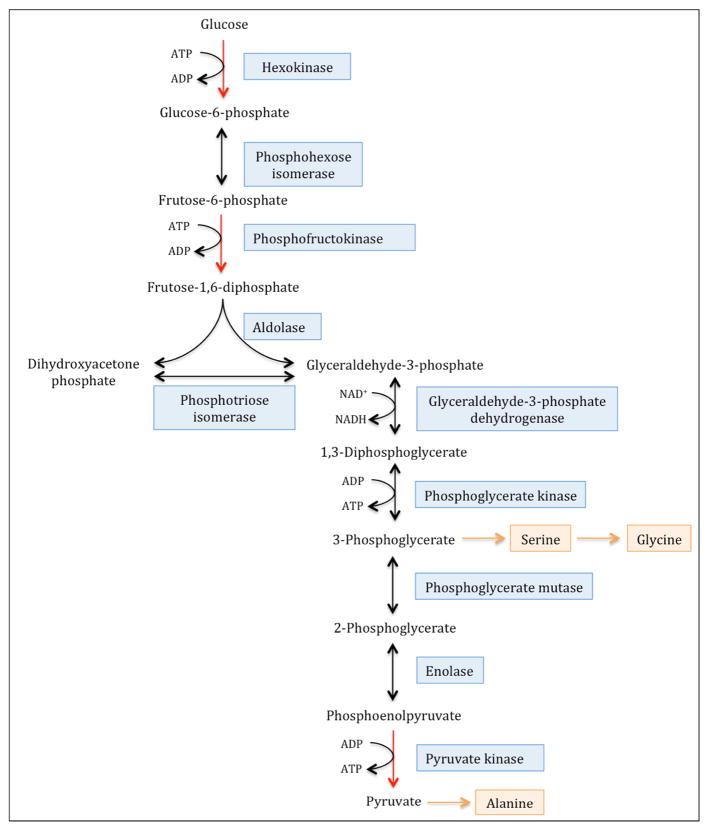
To assess activities of the three rate-controlling enzymes of glycolysis (i.e. hexokinase; HK, phosphofructokinase; PFK and pyruvate kinase; PK), we calculated the ratios of the concentrations of the glycolytic amino acids serine, glycine and alanine to glucose. These amino acids are biosynthetic derivatives of intermediate metabolites in glycolysis and thus their concentrations relative to glucose, are an indirect measure of the net enzymatic activities catalyzing the three sequential, rate-controlling, irreversible steps of glycolysis [35]. As shown in Figure 1, the amino acid serine is synthesized from the glycolytic intermediate 3-phosphoglycerate and can be converted to glycine following transfer of a methyl group from its side chain. The ratio of the concentrations of serine and glycine to glucose (serine+glycine:glucose) therefore reflects the net activities of the two irreversible enzymatic reactions in glycolysis that are upstream of 3-phosphoglycerate:
Figure 1. Glycolytic intermediates are biosynthetic precursors of the non-essential amino acids, serine, glycine and alanine.
The three irreversible and rate-controlling steps of glycolysis are catalyzed by the enzymes, hexokinase, phosphofructokinase and pyruvate kinase, yielding intermediate metabolites that can undergo conversion to serine, glycine and alanine. The ratio of the concentrations of serine and glycine to glucose (serine+glycine:glucose) therefore reflects the net activities of the two irreversible enzymatic reactions in glycolysis that are upstream of 3-phosphoglycerate, catalyzed by hexokinase and phosphofructokinase. Similarly, the ratio of serine+glycine+alanine to glucose provides an indirect assessment of the net activity of pyruvate kinase, which catalyzes the conversion of phosphoenolpyruvate (PEP) to pyruvate.
the first reaction in glycolysis, i.e. the phosphorylation of glucose to glucose 6-phosphate, catalyzed by the inducible enzyme, hexokinase (abbreviated here as HK) and
the phosphorylation of fructose 6-phosphate to fructose 1, 6-bisphosphate in the presence of phosphofructokinase (abbreviated here as PFK)
The conversion of phosphoenolpyruvate (PEP) to pyruvate is the last step of glycolysis and is an irreversible reaction catalyzed by the enzyme pyruvate kinase (abbreviated here as PK) (fig-1). The amino acid alanine is synthesized from pyruvate by a transamination reaction catalyzed by alanine amino transferase (ALT). Therefore the ratio of serine+glycine+alanine to glucose provides an indirect assessment of the net activity of pyruvate kinase.
2.6 Quantification of brain tissue glucose transporter proteins
We examined protein levels of the two principal glucose transporters in the brain i.e. neuronal glucose transporter-3 (GLUT3) and glucose transporter-1 (GLUT1); the main glucose transporter in astrocytes and within vascular endothelial cells of the blood brain barrier (BBB) [36].
Label free quantification (LFQ) of the brain proteome by LC-MS/MS was performed in the MFG using a ThermoFisher Scientific (San Jose, CA) Q-Exactive Plus mass spectrometer. Analysis of raw data was performed using MaxQuant 1.5.3.28 software (Max Planck Institute of Biochemistry, Martinsried, Germany) and bootstrap regressed against age and postmortem interval (PMI) of each individual. In addition to protein levels of GLUT3 and GLUT1, we also quantified levels of nesprin1, a neuronal nuclear protein. Additional details on collection of proteomic data, raw data availability, and analyses parameters are provided through the Synapse AMP-AD portal (https://www.synapse.org/#!Synapse:syn3606086) and have been reported previously [37]. Additional details on the specificity of the LFQ workflow to identify GLUT3 and GLUT1 proteins are provided in supplementary materials (table-S1).
The proteomic analyses were performed on the MFG in 47 BLSA participants (N=20 AD; N=13 control and N=14 ‘ASYMAD’) and included 40 individuals in whom brain tissue glucose and glycolytic amino acids concentrations were also estimated by quantitative metabolomics as described in the previous section.
2.7 Statistical analyses
Differences in severity of AD pathology between groups were examined using the Mantel-Haenszel Chi-Square Test of correlation. Proportional odds ordinal logistic models [38] are a generalization of the Wilcoxon and Kruskal-Wallis tests that allow for covariates adjustment. We used these tests to compare group differences (i.e. AD, control and ASYMAD) in brain tissue glucose concentrations as well as ratios of glycolytic amino acids to glucose within each of the three brain regions (i.e ITG, MFG and cerebellum). Sensitivity analyses were conducted using sex and age at death as covariates.
A similar approach was taken for analyses of group differences in protein levels of the glucose transporters, GLUT3 and GLUT1. In order to confirm that observed changes in levels of the neuronal glucose transporter, GLUT3 were not driven primarily by neuronal loss, LFQ-derived protein levels of the neuronal nuclear protein, nesprin-1 [39], was used as an additional covariate in addition to sex and age of death in sensitivity analyses comparing GLUT3 protein levels between groups.
Associations between brain tissue glucose concentration, ratios of the glycolytic amino acids to glucose and AD pathology were examined using Spearman's rank correlation before and after adjusting for sex and age at death. Associations between protein levels of the glucose transporters and AD pathology were examined similarly with protein levels of nesprin-1 as an additional covariate in analyses involving GLUT3.
To investigate the associations between brain tissue glucose concentrations and AD pathology with longitudinal fasting plasma glucose concentrations measured during the BLSA research visits, separate linear mixed-effects models (LME) were fit with longitudinal glucose measures as the outcome and each pathology variable (i.e. CERAD and Braak score) and brain glucose concentration as the main predictors. The time of follow up was anchored at the last measurement of fasting plasma glucose (using time of last measurement as the time origin, time = 0), with all previous longitudinal observations considered negative relative to the last measurement. This re-centering of the time variable allows us to test the effects of the last plasma fasting glucose levels as well as the rates of change in fasting plasma glucose concentrations on pathology and brain tissue glucose concentration simultaneously in a single model. Other covariates included age at measurement of the last plasma fasting glucose concentration and sex. Brain tissue glucose concentration was natural log transformed and z-scored, age was mean centered and sex coded as −0.5 for female and 0.5 as male.
All the analyses were conducted in SAS 9.4 (Cary, NC)
3. Results
3.1 Brain tissue glucose concentration in AD
The demographic characteristics of BLSA participants who contributed data to the measurements of brain tissue glucose concentration are shown in Table 1A. The three groups i.e. AD, controls and ‘ASYMAD’ did not differ significantly in age-at-death or the postmortem interval to autopsy. There were also no group differences in sex or APOE ε4 carrier status.
Table 1A.
Sample characteristics of participants who provided data on brain glucose concentrations and ratios of glycolytic amino acids:glucose
| Whole sample | CN | AYSMAD | AD | P-value | |
|---|---|---|---|---|---|
| N | 43 | 14 | 15 | 14 | |
| Sex (f/m) | 16/27 | 4/10 | 5/10 | 7/7 | 0.53 |
| Age death | 86.6 (9.5) 62.9 – 99.2 |
82.6 (11.0) 64.2 – 99.2 |
89.2 (7.9) 71.9 – 96.4 |
87.9 (8.9) 62.9 – 98.7 |
0.15 |
| PMI (Hours) | 15.3 (6.8) 2.0–33.0 |
16.9 (6.4) 7.0–28.0 |
14.8 (8.1) 2.0–33.0 |
14.7 (6.0) 3.0–23.0 |
0.70 |
| APOE (ε4−/ε4+) | 33/10 | 12/2 | 11/4 | 10/4 | 0.73 |
AD = Alzheimer’s disease; CN = control; ASYMAD = asymptomatic Alzheimer’s disease Sex, and APOE genotype variables were compared using Fisher’s exact test, age of death and PMI were compared using the Kruskal-Wallis Test
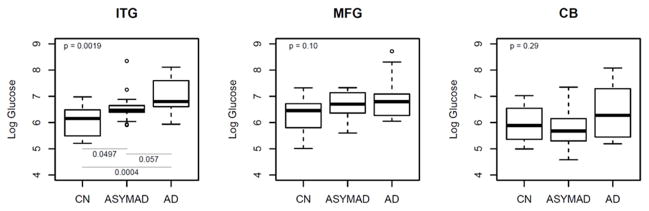
Figure 2A summarizes results of analyses comparing brain tissue glucose concentrations between the three groups. In unadjusted models, brain tissue glucose concentrations were significantly different in the ITG (global p value for significance across groups=0.0019) with tissue glucose concentration following the pattern AD>ASYMAD>control (Figure 2A). Pair-wise comparisons between the groups in the ITG showed significantly higher concentration of glucose in the AD (P=0.0004) and ASYMAD groups (P=0.0497) relative to controls and a trend towards higher concentration in AD relative to ASYMADs (P=0.057). Results were similar after adjusting for sex and age of death. In the MFG, group differences from unadjusted models did not reach significance (global p=0.10) (Figure 2A), but after additionally adjusting for sex and age at death, results approached significance (global p value for significance across groups=0.051). Pair-wise comparisons between the groups in the MFG showed significantly higher concentration of glucose in the AD (P=0.032) and ASYMAD groups (P=0.026) relative to controls. There were no group differences in tissue glucose concentrations within the cerebellum in either unadjusted (global p value for significance across groups =0.29; Figure 2A) or adjusted models.
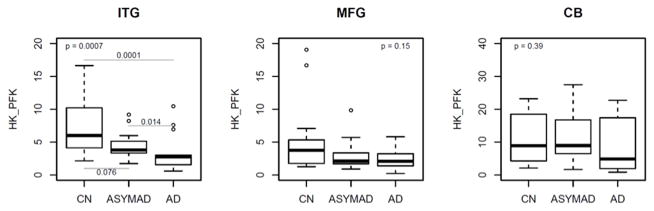
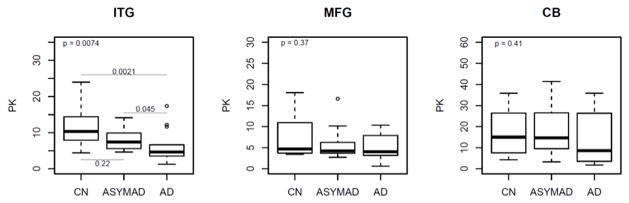
Figure 2. Box plots showing differences in brain tissue glucose concentrations (2A) and, brain tissue ratios of glycolytic amino acids: glucose (2B and 2C) between groups (unadjusted models).
ITG = inferior temporal gyrus; MFG = middle frontal gyrus; CB = cerebellum; AD = Alzheimer’s disease; CN = control; ASYMAD = asymptomatic Alzheimer’s disease; HK_PFK = hexokinase and phosphofructokinase; PK = pyruvate kinase
Upper corner shows the overall p-values comparing all 3 groups. Post-hoc pair-wise comparisons are only conducted for regions with overall p-value <= 0.05 and are highlighted by lines showing the relevant pair-wise comparisons (2A–2C). ‘HK_PFK’ (2B) indicates activities of hexokinase and phosphofructokinase enzymes assessed by ratios of the glycolytic amino acids, serine and glycine to glucose (serine+glycine:glucose).
‘PK’ (2C) indicates activity of the pyruvate kinase enzyme assessed by ratios of the glycolytic amino acids, serine, glycine and alanine to glucose (serine+glycine+alanine:glucose)
3.2. Brain tissue glucose concentration and AD pathology
The three groups differed significantly in the severity of both neuritic plaque and neurofibrillary tangle pathology following a linear trend (Mantel-Haenszel Chi-Square Test of correlation between group and Braak scores: p<.0001; CERAD scores: p<.0001) with the AD group showing the highest, ASYMAD intermediate, and controls lowest levels of pathology.
Table 2 shows correlations between brain tissue glucose concentration and measures of AD pathology. Brain tissue glucose concentration in the ITG was significantly associated with both Braak (Spearman’s rho=0.37; p=0.014) and CERAD scores (Spearman’s rho =0.47; p=0.0014). Tissue glucose concentrations in the MFG, were significantly associated with CERAD scores (Spearman’s rho =0.39; p=0.0096). No significant association was observed between glucose concentration in the cerebellum and AD pathology. These results remained similar after adjusting for sex and age at death.
Table 2.
Correlations between brain tissue glucose concentration, brain tissue ratios of glycolytic amino acids:glucose, protein levels of the neuronal (GLUT3) and astrocytic (GLUT1) glucose transporters and AD pathology (Braak and CERAD scores)
| rho P value | Brain tissue glucose concentration | Serine+glycine:glucose (Hexokinase_and Phosphofructokinase activities) | Serine+glycine+alanine :glucose (Pyruvate kinase activity) | protein levels of glucose transporters (MFG) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ITG | MFG | CB | ITG | MFG | CB | ITG | MFG | CB | GLUT3 | GLUT1 | |
| Braak | 0.37 0.014 |
0.12 0.44 |
0.21 0.24 |
−0.37 0.016 |
−0.13 0.41 |
−0.22 0.23 |
−0.46 0.0093 |
−0.14 0.46 |
−0.22 0.22 |
−0.40 0.0051 |
0.038 0.80 |
| CERAD | 0.47 0.0014 |
0.39 0.0096 |
0.25 0.16 |
−0.52 0.0004 |
−0.38 0.013 |
−0.25 0.17 |
−0.55 0.0010 |
−0.31 0.087 |
−0.24 0.18 |
−0.42 0.0033 |
−0.21 0.16 |
ITG = inferior temporal gyrus; MFG = middle frontal gyrus; CB = cerebellum The numbers in each cell show the unadjusted Spearman’s rank correlation rho and corresponding p values.
3.3 Brain tissue ratios of glycolytic amino acids to glucose in AD
Figures 2B and 2C summarize results of analyses comparing brain tissue ratios of the glycolytic amino acids, serine+glycine to glucose; assessing hexokinase and phosphofructokinase activities (‘HK_PFK’) and serine+glycine+alanine to glucose; assessing pyruvate kinase activity ( ‘PK’) between the three groups respectively. In unadjusted models, brain tissue HK_PFK activities were significantly different in the inferior temporal gyrus (ITG; global p value for significance across groups=0.0007) following the pattern AD<ASYMAD<control (Figure 2B). Pair-wise comparisons between the groups in the ITG showed significantly lower HK_PFK activity in the AD group relative to controls (P=0.0001) as well as lower activity in AD relative to ASYMADs (P=0.014). Similarly, in unadjusted models within the ITG, we found that brain tissue PK activity was also significantly different between the groups (ITG; global p value for significance across groups=0.0074) with the pattern, AD<ASYMAD<control (Figure 2C). Pair-wise comparisons between the groups in the ITG showed significantly lower PK activity in the AD group relative to controls (P=0.0021) as well as lower activity in AD relative to ASYMADs (P=0.045). These results were similar in models additionally adjusted for sex and age at death. In the MFG, group difference in brain tissue HK_PFK activities did not reach statistical significance in the unadjusted model (2B), but showed a trend towards significance in adjusted models (HK_PFK; global p value for significance across groups =0.058). There were no significant group differences in PK activity in the MFG in either unadjusted (PK; global p value for significance across groups =0.368; Figure 2C) or adjusted models. There were no group differences in brain tissue activities of either HK_PFK or PK within the cerebellum in either unadjusted (HK_PFK; global p value for significance across groups =0.394; PK; global p value for significance across groups =0.410, Figures 2B and 2C) or adjusted models.
3.4 Brain tissue ratios of glycolytic amino acid to glucose and AD pathology
Table 2 shows correlations between ratios of the glycolytic amino acids, serine+glycine to glucose; assessing hexokinase and phosphofructokinase activities i.e. ‘HK_PFK’) and serine+glycine+alanine to glucose; assessing pyruvate kinase activity i.e. ‘PK’) against measures of AD pathology. Brain tissue HK_PFK activities in the ITG were significantly associated with both Braak (Spearman’s rho=−0.37; p=0.016) and CERAD (Spearman’s rho =−0.52; p=0.0004) scores. Similarly PK activity in the ITG was also significantly associated with both Braak (Spearman’s rho-0.46; p=0.0093) and CERAD (Spearman’s rho −0.55; p=0.0010) scores. In the MFG, HK_PFK activities were significantly associated with the CERAD (Spearman’s rho −0.38; p=0.013) score. These results remained similar after adjusting for sex and age of death.
3.5 Brain tissue protein levels of glucose transporters (GLUT3 and GLUT1)
The demographic characteristics of BLSA participants who contributed data to the proteomic analyses of brain tissue glucose transporter levels are shown in Table 1B. The three groups i.e. AD, controls and ‘ASYMAD’ did not differ significantly in age-at-death or the postmortem interval to autopsy. There were also no group differences in sex or APOE ε4 carrier status.
Table 1B.
Sample characteristics of participants who provided proteomic data from the MFG. These participants include 40 individuals in whom tissue glucose concentrations were also estimated by flow injection analysis-mass spectrometry (FIA-MS)
| Whole sample | CN | AYSMAD | AD | P-value | |
|---|---|---|---|---|---|
| N | 47 | 13 | 14 | 20 | |
| Sex (f/m) | 17/30 | 3/10 | 4/10 | 10/10 | 0.25 |
| Age death | 86.1 (9.4) 62.9 – 99.2 |
81.8 (10.9) 64.2 – 99.2 |
88.9 (8.0) 71.9 – 96.4 |
87.0 (8.7) 62.9 – 98.7 |
0.18 |
| PMI (Hours) | 14.5 (6.1) 2.0–28.0 |
17.4 (5.9) 7.0–28.0 |
12.1 (7.1) 2.0–24.0 |
14.3 (4.9) 6.0–23.0 |
0.12 |
| Nesprin-1 × e6 (protein levels) | 55.6 (16.3) 19.1 – 94.4 |
60.4 (14.1) 35.9 – 85.5 |
47.9(16.8) 19.1 – 70.0 |
58.0 (16.1) 21.6 −94.4 |
0.22 |
| APOE (ε4−/ε4+) | 36/11 | 11/2 | 11/3 | 14/6 | 0.69 |
AD = Alzheimer’s disease; CN = control; ASYMAD = asymptomatic Alzheimer’s disease Sex and APOE genotype variables were compared using Fisher’s exact test, age at death, post-mortem interval (PMI) and Nesprin-1 levels were compared using the Kruskal-Wallis Test. Nesprin-1 (SYNE1 gene product) levels represent protein LFQ intensity values from LC-MS/MS measurements as described previously [37].
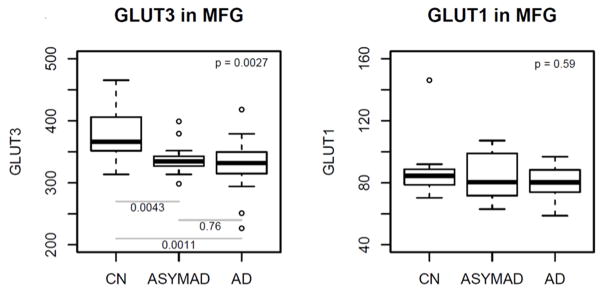
Figure 3 summarizes results of analyses comparing brain tissue glucose transporter protein levels between the three groups in the MFG. In unadjusted models, brain tissue protein levels of the neuronal glucose transporter GLUT3 were significantly different across the groups (global p value for significance=0.0027) (Figure 3). Pair-wise comparisons showed significantly lower protein levels of GLUT3 in the AD (P=0.0011) and ASYMAD (P=0.0043) groups relative to controls. These results remained similar in models adjusted for sex, age at death and levels of the neuronal nuclear protein, nesprin-1. No significant differences were observed in protein levels of the astrocytic glucose transporter GLUT1.
Figure 3. Box plots showing differences in protein levels of the glucose transporters GLUT3 and GLUT1 in the middle frontal gyrus (unadjusted models).
MFG = middle frontal gyrus; GLUT3 = glucose transporter-3; GLUT1 = glucose transporter-1
Upper corner shows the overall p-values comparing all 3 groups and post-hoc pairwise p-values are shown at the bottom
3.6 Brain tissue protein levels of glucose transporters and AD pathology
Protein levels of GLUT3 were significantly associated with both Braak (Spearman’s rho = −0.40; p=0.0051) and CERAD (Spearman’s rho =−0.42; p=0.0033) scores in the MFG. There were no significant associations between protein levels of GLUT1 and Braak (Spearman’s rho = 0.038; p=0.80) or CERAD (Spearman’s rho = −0.21; p=0.16) scores. These results remained unchanged in models adjusted for sex, age at death and protein levels of the neuronal nuclear protein nesprin-1 (Table 2).
3.7 Associations between plasma fasting glucose and brain tissue glucose concentrations
Table 1C summarizes the plasma fasting glucose measurements that were used in the analyses examining their relationship with brain tissue glucose concentrations. A total of 445 plasma fasting glucose measures were available in the 43 BLSA participants who also contributed measures of brain tissue glucose concentration described above. The average follow up interval for the plasma glucose measurements was 19.1 years and the average interval between the last available fasting plasma glucose assessment and death was five years.
Table 1C.
Sample characteristics of BLSA participants who contributed data for longitudinal analyses of fasting plasma glucose concentrations and their relationship to brain tissue glucose levels.
| Whole sample | CN | AYSMAD | AD | P-value | |
|---|---|---|---|---|---|
| N subjects | 43 | 14 | 15 | 14 | |
| Total # of glucose measurements | 445 | 150 | 156 | 139 | |
| Total # of fasting plasma glucose measurements in the diabetic range (>=126 mg/dl) | 26 | 11 | 15 | 0 | |
| Age at first plasma glucose measurement | 62.6 (16.3) 27.4–89.3 |
57.4 (17.8) 27.4–89.3 |
68.7 (15.2) 32.1–83.3 |
61.2 (14.8) 33.0–81.9 |
0.14 |
| Follow up time (yrs) | 19.1 (11.7) 0–44.4 |
21.5 (11.2) 1.6–36.2 |
17.8 (13.3) 0–44.4 |
17.9 (10.7) 0–39.7 |
0.54 |
| # of visits per subject | 10.3 (6.1) 1–24 |
10.7 (5.8) 2–23 |
10.4 (6.8) 1–24 |
9.9 (6.0) 1–24 |
0.86 |
| Average plasma glucose concentration (mg/dl) over follow up | 100.1 (13.3) 83.7–142.5 |
98.7 (10.0) 89.2–131.8 |
107.0 (18.2) 83.7–142.5 |
94.3 (4.8) 86.5–101.5 |
0.047 |
| Time between last plasma glucose and death | 5.0 (4.0) 0.5–17.8 |
3.7 (2.0) 1.1–7.4 |
2.7 (2.0) 0.5–6.5 |
8.8 (4.3) 2.9–17.8 |
<0.0001 |
| Age at last plasma glucose measurement | 81.6 (10.0) 56.9–95.0 |
78.9 (11.6) 56.9–94.1 |
86.5 (7.7) 69.3–95.0 |
79.1 (9.0) 56.9–93.3 |
0.053 |
AD = Alzheimer’s disease; CN = control; ASYMAD = asymptomatic Alzheimer’s disease P-values are from the Kruskal-Wallis Test comparing all 3 groups (CN; controls, ASYMAD; asymptomatic Alzheimer’s disease, AD; Alzheimer’s disease).
In linear mixed models adjusted for age at the last available plasma glucose measurement and sex, we found that both the last plasma fasting glucose concentration as well as longitudinal trajectories of change in plasma fasting glucose were associated with brain tissue glucose concentrations in all three regions examined i.e. ITG, MFG and cerebellum (Table 3). The direction of this association indicates that higher levels (i.e. last measured fasting plasma glucose concentration) as well as greater increases over time in plasma fasting glucose are associated with higher brain tissue glucose concentrations. These results were similar in models that were further adjusted for clinical status i.e. AD/control/ASYMAD.
Table 3.
Associations between plasma fasting glucose and brain tissue glucose concentrations
| Effect of last plasma fasting glucose concentration | Effect of rates of change in fasting plasma glucose concentrations | |
|---|---|---|
| CB | 5.52 (2.35) p=0.025 |
0.18 (0.093) p=0.069 |
| ITG | 4.24 (2.30) p=0.073 |
0.25(0.10) p=0.018 |
| MFG | 4.54 (2.46) p=0.073 |
0.24 (0.093) p=0.017 |
CB = cerebellum; ITG = inferior temporal gyrus; MFG = middle frontal gyrus The numbers in the table are estimated regression beta coefficients, standard error in parentheses and the corresponding p values.
In sensitivity analyses, we excluded 26 plasma fasting glucose values in the diabetic range i.e. >=126 mg/dl; longitudinal associations remained unchanged in the MFG and ITG, however cross-sectional associations only remained significant in the ITG. When we examined the relationship between plasma fasting glucose concentrations and AD pathology, there were no significant associations with either Braak or CERAD scores.
4. Discussion
In this study we have demonstrated that abnormalities in brain glucose homeostasis are intrinsic to AD pathogenesis and may begin several years prior to the onset of clinical symptoms. We first showed that brain regions vulnerable to amyloid deposition and neurofibrillary pathology show significantly higher tissue glucose concentrations in AD. Moreover, higher concentrations of brain tissue glucose are associated with greater severity of both amyloid plaque deposition and neurofibrillary pathology.
To further investigate abnormalities in glucose metabolism associated with higher glucose concentrations in brain regions vulnerable to AD pathology, we utilized concentrations of the glycolytic amino acids, serine, glycine and alanine to assess the three rate controlling, irreversible steps of glycolysis. Besides the generation of energy through ATP from the breakdown of glucose, intermediates of glycolysis serve as biosynthetic precursors of several metabolites including the non-essential amino acids, serine, glycine and alanine (fig-1) [35]. The ratios of these glycolytic amino acids to that of glucose, the primary substrate of glycolysis, are hence useful as indirect measures of the enzymatic activities of hexokinase, phosphofructokinase and pyruvate kinase. Our results indicate that the activities of these principal rate controlling enzymes of glycolysis are significantly reduced in the ITG and MFG, but not cerebellum, of individuals with AD. Moreover, lower activities of these enzymes are associated with greater severity of both neurofibrillary and amlyoid pathology.
Next, using mass spectrometry-based proteomics in brain tissue samples from the same participants, we showed that protein levels of the neuronal glucose transporter, GLUT3 are significantly reduced in AD and that lower levels of GLUT3 protein reflect greater severity of both amyloid plaque and neurofibrillary tangle pathology. Finally, we demonstrated that longitudinal increases in fasting plasma glucose levels, measured up to four decades prior to death, are associated with higher brain tissue glucose concentrations globally.
To the best of our knowledge, this report is the first to measure brain tissue glucose concentrations and assess glycolytic flux to demonstrate their relationships with both severity of AD pathology and the expression of AD symptoms. Including brain tissue samples from ‘ASYMAD’ individuals who represent an intermediate group in the gradation of neuropathology from controls to AD patients in the absence of cognitive impairment during life, allowed us to relate measures of brain glucose concentration and glycolytic flux to incremental levels of AD pathology and symptom expression. Equally importantly, by measuring glucose concentrations in brain regions both vulnerable to distinct pathological features of AD i.e. MFG (amyloid deposition) and ITG (tau accumulation) as well as in a region relatively resistant to AD pathology i.e. cerebellum [34], we were able to determine whether the observed alterations in brain glucose concentrations and abnormalities in glycolysis were related to AD-defining pathological processes.
Our observation that in the ITG, brain glucose concentrations are highest in AD, lowest in controls and intermediate in ASYMADs suggests that there may be regionally-specific threshold levels of tissue glucose beyond which the accumulation of AD pathology triggers the expression of clinical symptoms. Complementing these observations on brain glucose concentrations in the ITG, we also observed that activities of the three rate-controlling enzymes of glycolysis, i.e. hexokinase, phosphofructokinase and pyruvate kinase, are lowest in AD, highest in controls and intermediate in ASYMADs, suggesting that regionally-specific abnormalities in glycolytic flux may lead to the build-up of brain glucose levels, evolution of AD pathology and ultimately, the development of AD symptoms. Furthermore, the absence of significant group differences in either brain tissue glucose concentration or glycolytic flux in the cerebellum, a region that is relatively spared of classical AD pathology, suggests that these abnormalities are specifically linked to the defining pathological hallmarks of AD. This interpretation is further strengthened by the significant associations between concentrations of brain tissue glucose and measures of glycolytic flux with severity of AD pathology both in the ITG and MFG.
Our proteomic analysis of brain tissue from the same groups of participants in the BLSA autopsy sample allowed us to further explore potential mechanisms underlying alterations in brain glucose concentration in AD. We found that protein levels of the neuronal glucose transporter, GLUT3 are significantly lower in AD and ASYMAD brains relative to controls. Furthermore, lower protein levels of GLUT3 are associated with greater severity of both neuritic plaque and neurofibrillary tangle pathology. Equally importantly, we find that both the group differences in GLUT3 protein levels as well as their association with AD pathology are independent of neuronal loss as they remain significant even after adjustment for levels of the neuronal nuclear protein nesprin-1 [39]. This suggests that lower GLUT3 protein levels in AD are likely to reflect early changes in the evolution of AD pathophysiology, rather than downstream consequences of neurodegeneration. Together with the observation that protein levels of the astrocytic glucose transporter GLUT1 are unchanged, our results implicate a specific loss of the neuronal glucose transporter GLUT3 as an integral feature of AD pathogenesis.
Our findings also complement and extend previous functional neuroimaging studies using 18FDG-PET showing that decreases in neuronal glucose uptake may be an early feature of AD pathogenesis [19–22, 40]. These studies show a characteristic pattern of hypometabolism affecting primarily the parieto-temporal, posterior cingulate and frontal cortices with relative sparing of the cerebellum, thalamus and basal ganglia [22]. This pattern of lower cerebral glucose uptake on FDG-PET imaging therefore appears consistent with our current observation of higher brain tissue glucose levels in the frontal and temporal cortices in AD, but sparing the cerebellum. Previous studies have shown that the density of the neuronal GLUT3 transporter in the brain is coupled to local cerebral glucose utilization [41]. Our current results indicate that lower cerebral glucose utilization, as reflected in lower rates of glycolysis and higher brain tissue glucose concentrations, may thus down-regulate GLUT3 protein levels, especially in brain regions vulnerable to AD pathology.
Previous studies of GLUT3 and GLUT1 protein levels in the AD brain have relied upon small sample sizes and important methodological differences with our current report merit consideration. Simpson and colleagues measured GLUT3 and GLUT1 protein levels using immunoblotting and autoradiography from a sample of 12 AD and 12 control brains [42]. The mean age of the control group (56±22 years) in their report was considerably lower than the AD group (76±5 years). While they observed overall reductions in GLUT1 and GLUT3 protein levels averaged across several regions, considerable inter-individual variability made comparisons of regional differences in glucose transporter levels difficult. Using cytochalasin B binding assays, Kalaria and colleagues demonstrated lower glucose transporter levels in AD brains compared to controls [43]. However, as cytochalasin B is a non-specific blocker of both GLUT1 and GLUT3 transporters [44], a reliable assessment of differences in neuronal versus astrocytic glucose transporter levels was not feasible. Moreover, in both these studies, the absence of ‘high-pathology controls’ or ‘ASYMADs’ as in our current report, precludes assessment of changes in glucose transporter levels in relation to severity of AD pathology and expression of AD symptoms. A definitive assessment of regional differences in GLUT3 and GLUT1 levels in AD may require a larger sample size and proteomic profiling across several brain regions.
It is important to note that our measure of brain tissue glucose is based on mass spectrometric quantification of total hexose levels transported from the circulation. Glucose is the predominant hexose in blood and is present in far greater concentrations (5450.0±1200 μM) than other hexoses such as fructose (46.26 ± 25.22 μM), galactose (88.3±34.7 μM) and mannose (64.0 ±12.0 μM 12.0 uM) [45] [46, 47]. Therefore the total concentration of brain hexoses measured in our samples is likely to comprise predominantly of glucose.
Given previous epidemiological evidence that insulin resistance and diabetes are associated with increased risk of AD [1–3], we also examined whether peripheral glucose levels measured decades prior to death may influence brain tissue glucose concentration. We have previously shown that this relationship does not appear to be mediated by a direct effect of blood glucose concentrations on either brain amyloid deposition or neurofibrillary pathology [48]. In the current report, we found that higher concentrations of plasma fasting glucose measured prior to death as well as greater increases in fasting plasma glucose over time were associated with higher brain tissue glucose concentrations in all three regions examined. In additional sensitivity analyses, we further confirmed that the longitudinal associations between fasting plasma glucose and brain tissue glucose concentrations are not driven by individuals with diabetes. These findings open up new lines of investigation in appropriate animal models of AD where longitudinal analyses of brain tissue and blood glucose concentrations in relation to accumulating AD pathology may provide important insights into the role of glucose dysregulation in early stages of AD pathogenesis. Such experimental studies are essential to test both causal relationships and confirm the temporal sequence of molecular events related to glucose dysregulation and AD pathogenesis in humans. It would also be important to test whether abnormalities of brain glucose utilization are a specific feature of AD pathogenesis or may be shared by other neurodegenerative diseases. The lack of brain and blood tissue samples from well-characterized cohorts representing other non-AD neurological diseases is a limitation of our present report.
Considering our results together, we propose that failure of neuronal glucose utilization due to impaired glycolysis is a fundamental feature of AD. At regionally specific threshold levels of brain glucose and impaired glycolytic flux, accumulating pathology in vulnerable brain regions triggers the onset of AD symptoms. Our results have important translational implications and set the stage for future studies that may uncover therapeutic interventions targeting brain glucose dysregulation in AD.
Supplementary Material
Brain tissue glucose is associated with severity of AD pathology and symptom onset
Reduced brain glycolytic flux is associated with severity of AD pathology and symptom onset
Neuronal glucose transporter GLUT3 is lower in AD
Lower GLUT3 levels are associated with more severe AD pathology
Increase in plasma glucose decades before death is related to higher brain glucose
1. Systematic review
We reviewed (using PubMed) all publications describing abnormalities of glucose metabolism in Alzheimer’s disease (AD). It is unclear whether glucose dysregulation in the brain is related to severity of AD pathology and symptom expression. Similarly, the relationships between longitudinal changes in blood glucose concentration and brain tissue glucose levels are unclear. We measured brain tissue concentrations of glucose and the glycolytic amino acids, serine, glycine and alanine as well as protein levels of the neuronal (GLUT3) and astrocytic (GLUT1) glucose transporters in the autopsy sample of the Baltimore Longitudinal Study of Aging (BLSA). Higher brain tissue glucose concentration, reduced glycolytic flux and lower levels of GLUT3 are related to severity of AD pathology and the expression of AD symptoms. Increasing fasting plasma glucose levels are associated with higher brain tissue glucose concentrations.
2. Interpretation
Abnormalities in brain glucose homeostasis are intrinsic to AD pathogenesis and begin several years prior to clinical symptoms.
3. Future directions
These results set the stage for future studies testing brain glucose dysregulation as a target of disease-modifying interventions in AD.
Acknowledgments
We are grateful to participants in the Baltimore Longitudinal Study of Aging for their invaluable contribution.
Funding
This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging. Support was provided by the Accelerating Medicine Partnership AD grant [grant number: U01AG046161-02], the NINDS Emory Neuroscience Core [grant number: P30NS055077], and the Emory Alzheimer’s Disease Research Center [grant number: P50AG025688].
Role of the funding source
The Intramural Research Program of the National Institute on Aging (NIH) had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Conflicts of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leibson CL, Rocca WA, Hanson VA, Cha R, Kokmen E, O'Brien PC, et al. Risk of dementia among persons with diabetes mellitus: a population-based cohort study. American journal of epidemiology. 1997;145:301–8. doi: 10.1093/oxfordjournals.aje.a009106. [DOI] [PubMed] [Google Scholar]
- 2.Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R. Diabetes mellitus and risk of Alzheimer's disease and dementia with stroke in a multiethnic cohort. American journal of epidemiology. 2001;154:635–41. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- 3.Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–42. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, Zhong C. Decoding Alzheimer's disease from perturbed cerebral glucose metabolism: implications for diagnostic and therapeutic strategies. Progress in neurobiology. 2013;108:21–43. doi: 10.1016/j.pneurobio.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Hoyer S. Brain glucose and energy metabolism abnormalities in sporadic Alzheimer disease. Causes and consequences: an update. Experimental gerontology. 2000;35:1363–72. doi: 10.1016/s0531-5565(00)00156-x. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed S, Mahmood Z, Zahid S. Linking insulin with Alzheimer's disease: emergence as type III diabetes. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2015;36:1763–9. doi: 10.1007/s10072-015-2352-5. [DOI] [PubMed] [Google Scholar]
- 7.Chami B, Steel AJ, De La Monte SM, Sutherland GT. The rise and fall of insulin signaling in Alzheimer's disease. Metabolic brain disease. 2016;31:497–515. doi: 10.1007/s11011-016-9806-1. [DOI] [PubMed] [Google Scholar]
- 8.Freude S, Schilbach K, Schubert M. The role of IGF-1 receptor and insulin receptor signaling for the pathogenesis of Alzheimer's disease: from model organisms to human disease. Current Alzheimer research. 2009;6:213–23. doi: 10.2174/156720509788486527. [DOI] [PubMed] [Google Scholar]
- 9.Hokama M, Oka S, Leon J, Ninomiya T, Honda H, Sasaki K, et al. Altered expression of diabetes-related genes in Alzheimer's disease brains: the Hisayama study. Cerebral cortex (New York, NY : 1991) 2014;24:2476–88. doi: 10.1093/cercor/bht101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, et al. Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. The Journal of clinical investigation. 2012;122:1316–38. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de la Monte SM. Type 3 diabetes is sporadic Alzheimers disease: mini-review. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2014;24:1954–60. doi: 10.1016/j.euroneuro.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de la Monte SM, Wands JR. Alzheimer's disease is type 3 diabetes-evidence reviewed. Journal of diabetes science and technology. 2008;2:1101–13. doi: 10.1177/193229680800200619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Claxton A, Baker LD, Hanson A, Trittschuh EH, Cholerton B, Morgan A, et al. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer's disease dementia. Journal of Alzheimer's disease : JAD. 2015;44:897–906. doi: 10.3233/JAD-141791. [DOI] [PubMed] [Google Scholar]
- 14.Claxton A, Baker LD, Wilkinson CW, Trittschuh EH, Chapman D, Watson GS, et al. Sex and ApoE genotype differences in treatment response to two doses of intranasal insulin in adults with mild cognitive impairment or Alzheimer's disease. Journal of Alzheimer's disease : JAD. 2013;35:789–97. doi: 10.3233/JAD-122308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, et al. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008;70:440–8. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- 16.Benarroch EE. Brain glucose transporters: implications for neurologic disease. Neurology. 2014;82:1374–9. doi: 10.1212/WNL.0000000000000328. [DOI] [PubMed] [Google Scholar]
- 17.Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007;27:1766–91. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joost HG, Thorens B. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members (review) Molecular membrane biology. 2001;18:247–56. doi: 10.1080/09687680110090456. [DOI] [PubMed] [Google Scholar]
- 19.Herholz K. Cerebral glucose metabolism in preclinical and prodromal Alzheimer's disease. Expert review of neurotherapeutics. 2010;10:1667–73. doi: 10.1586/ern.10.136. [DOI] [PubMed] [Google Scholar]
- 20.Hunt A, Schonknecht P, Henze M, Seidl U, Haberkorn U, Schroder J. Reduced cerebral glucose metabolism in patients at risk for Alzheimer's disease. Psychiatry research. 2007;155:147–54. doi: 10.1016/j.pscychresns.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Jagust WJ, Haan MN, Eberling JL, Wolfe N, Reed BR. Functional imaging predicts cognitive decline in Alzheimer's disease. Journal of neuroimaging : official journal of the American Society of Neuroimaging. 1996;6:156–60. doi: 10.1111/jon199663156. [DOI] [PubMed] [Google Scholar]
- 22.Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer's disease. FDG-PET studies in MCI and AD. European journal of nuclear medicine and molecular imaging. 2005;32:486–510. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- 23.Ferrucci L. The Baltimore Longitudinal Study of Aging (BLSA): a 50-year-long journey and plans for the future. J Gerontol A Biol Sci Med Sci. 2008;63:1416–9. doi: 10.1093/gerona/63.12.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shock NW, Gruelich R, Andres R, Arenberg D, Costa PT, Lakatta E, et al. The Baltimore Longitudinal Study of Aging. Washington, DC: US Government Printing Office; 1984. Normal human aging. [Google Scholar]
- 25.O'Brien RJ, Resnick SM, Zonderman AB, Ferrucci L, Crain BJ, Pletnikova O, et al. Neuropathologic studies of the Baltimore Longitudinal Study of Aging (BLSA) Journal of Alzheimer's disease : JAD. 2009;18:665–75. doi: 10.3233/JAD-2009-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gamaldo A, Moghekar A, Kilada S, Resnick SM, Zonderman AB, O'Brien R. Effect of a clinical stroke on the risk of dementia in a prospective cohort. Neurology. 2006;67:1363–9. doi: 10.1212/01.wnl.0000240285.89067.3f. [DOI] [PubMed] [Google Scholar]
- 27.Diagnostic and statistical manual of mental disorders, DSM-III-R. Washington, D.C: American Psychiatric Association; [Google Scholar]
- 28.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 29.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD) Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 30.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta neuropathologica. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 31.Troncoso JC, Zonderman AB, Resnick SM, Crain B, Pletnikova O, O'Brien RJ. Effect of infarcts on dementia in the Baltimore longitudinal study of aging. Annals of neurology. 2008;64:168–76. doi: 10.1002/ana.21413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iacono D, Resnick SM, O'Brien R, Zonderman AB, An Y, Pletnikova O, et al. Mild cognitive impairment and asymptomatic Alzheimer disease subjects: equivalent beta-amyloid and tau loads with divergent cognitive outcomes. Journal of neuropathology and experimental neurology. 2014;73:295–304. doi: 10.1097/NEN.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Metter EJ, Windham BG, Maggio M, Simonsick EM, Ling SM, Egan JM, et al. Glucose and insulin measurements from the oral glucose tolerance test and mortality prediction. Diabetes care. 2008;31:1026–30. doi: 10.2337/dc07-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larner AJ. The cerebellum in Alzheimer's disease. Dementia and geriatric cognitive disorders. 1997;8:203–9. doi: 10.1159/000106632. [DOI] [PubMed] [Google Scholar]
- 35.Berg JM, Tymoczko JL, Stryer L, Stryer L. Biochemistry. 5. New York: W.H. Freeman; 2002. [Google Scholar]
- 36.Vannucci SJ, Maher F, Simpson IA. Glucose transporter proteins in brain: delivery of glucose to neurons and glia. Glia. 1997;21:2–21. doi: 10.1002/(sici)1098-1136(199709)21:1<2::aid-glia2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 37.Seyfried NT, Dammer EB, Swarup V, Nandakumar D, Duong DM, Yin L, et al. A Multi-network Approach Identifies Protein-Specific Co-expression in Asymptomatic and Symptomatic Alzheimer's Disease. Cell systems. 2017;4:60–72. e4. doi: 10.1016/j.cels.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harrell FE. Regression modeling strategies with applications to linear models, logistic and ordinal regression, and survival analysis. Cham: Springer; 2015. [Google Scholar]
- 39.Dammer EB, Duong DM, Diner I, Gearing M, Feng Y, Lah JJ, et al. Neuron enriched nuclear proteome isolated from human brain. Journal of proteome research. 2013;12:3193–206. doi: 10.1021/pr400246t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:284–9. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duelli R, Kuschinsky W. Brain glucose transporters: relationship to local energy demand. News in physiological sciences : an international journal of physiology produced jointly by the International Union of Physiological Sciences and the American Physiological Society. 2001;16:71–6. doi: 10.1152/physiologyonline.2001.16.2.71. [DOI] [PubMed] [Google Scholar]
- 42.Simpson IA, Chundu KR, Davies-Hill T, Honer WG, Davies P. Decreased concentrations of GLUT1 and GLUT3 glucose transporters in the brains of patients with Alzheimer's disease. Annals of neurology. 1994;35:546–51. doi: 10.1002/ana.410350507. [DOI] [PubMed] [Google Scholar]
- 43.Kalaria RN, Harik SI. Reduced glucose transporter at the blood-brain barrier and in cerebral cortex in Alzheimer disease. Journal of neurochemistry. 1989;53:1083–8. doi: 10.1111/j.1471-4159.1989.tb07399.x. [DOI] [PubMed] [Google Scholar]
- 44.Dick AP, Harik SI, Klip A, Walker DM. Identification and characterization of the glucose transporter of the blood-brain barrier by cytochalasin B binding and immunological reactivity. Proceedings of the National Academy of Sciences. 1984;81:7233–7. doi: 10.1073/pnas.81.22.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wahjudi PN, Patterson ME, Lim S, Yee JK, Mao CS, Lee WN. Measurement of glucose and fructose in clinical samples using gas chromatography/mass spectrometry. Clinical biochemistry. 2010;43:198–207. doi: 10.1016/j.clinbiochem.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lentner C. Geigy scientific tables. Vol. 1. Basle: Ciba-Geigy; 1981. [Google Scholar]
- 47.Wu AHB. Tietz clinical guide to laboratory tests. St. Louis: Saunders Elsevier; 2006. [Google Scholar]
- 48.Thambisetty M, Jeffrey Metter E, Yang A, Dolan H, Marano C, Zonderman AB, et al. Glucose intolerance, insulin resistance, and pathological features of Alzheimer disease in the Baltimore Longitudinal Study of Aging. JAMA Neurol. 2013;70:1167–72. doi: 10.1001/jamaneurol.2013.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.