Abstract
Background
Even with the increased roll out of combination antiretroviral therapy (cART), paediatric HIV infection is associated with neurodevelopmental delays and neurocognitive deficits that may be accompanied by alterations in brain structure. Few neuroimaging studies have been done in children initiating ART before 2 years of age, and even fewer in children within the critical stage of brain development between 5-11 years. We hypothesized that early ART would limit HIV-related brain morphometric deficits at age 7.
Methods
Study participants were 7-year old HIV-infected (HIV+) children from the Children with HIV Early Antiretroviral Therapy (CHER) trial whose viral loads were supressed at a young age, and age-matched uninfected controls. We used structural magnetic resonance imaging (MRI) and FreeSurfer (http://www.freesurfer.net/) software to investigate effects of HIV and age at ART initiation on cortical thickness, gyrification and regional brain volumes.
Results
HIV+ children showed reduced gyrification compared to controls in bilateral medial parietal regions, as well as reduced volumes of the right putamen, left hippocampus, and global white and gray matter and thicker cortex in small lateral occipital region. Earlier ART initiation was associated with lower gyrification and thicker cortex in medial frontal regions.
Conclusions
Although early ART appears to preserve cortical thickness and volumes of certain brain structures, HIV infection is nevertheless associated with reduced gyrification in the parietal cortex, and lower putamen and hippocampus volumes. Our results indicate that in early childhood gyrification is more sensitive than cortical thickness to timing of ART initiation. Future work will clarify the implications of these morphometric effects for neuropsychological function.
Keywords: gyrification, morphometry, CHER, neurodevelopment, cortical thickness, paediatric HIV
1. Introduction
With increased antiretroviral therapy (ART) roll-out, the mortality rate of perinatally HIV-infected (HIV+) children has decreased substantially (Freeman 2016; Cotton et al. 2013; Violari et al. 2008). Hence, pediatric HIV is now a chronic infection and research efforts have shifted to understanding the effects of HIV and various combination ART regimens during childhood development (Freeman 2016; Blokhuis et al. 2016).
Even in the ART era, pediatric HIV infection is associated with neurodevelopmental delay and neurocognitive deficits such as language impairment, psychomotor slowing, and processing speed delay (Donald et al. 2014; Wilmhurst et al. 2014; Foster et al. 2006; Koekkoek et al. 2008; Martin et al. 2006; Van Arnhem et al. 2013). Behavioural abnormalities include lack of concentration and attentiveness, hyperactivity, anxiety and conduct disorders (Musielak and Fine 2015; Govender et al. 2011). Neurodevelopmental delays and deficits may be exacerbated by environmental and physical early life stressors, such as prenatal exposure to alcohol or drugs, and trauma, all of which are prevalent in South Africa (Kader et al. 2012).
Neuroimaging studies show that even when ART is initiated in childhood, there are alterations in brain structure due to HIV and/or ART (for reviews see Musielak and Fine 2015; Hoare et al. 2014). Findings have included lower cortical and total gray matter (GM) volumes (Lewis-de los Angeles et al. 2017; Cohen et al. 2016); regional cortical (Lewis-de los Angeles et al. 2017; Sarma et al. 2014) and subcortical (Yadav et al. 2017; Lewis-de los Angeles et al. 2016) volume and shape alterations; ventricular enlargement and sulcal widening (Van Arnhem et al. 2013); regional cortical thickness increases and decreases (Yadav et al. 2017); global (Cohen et al. 2016) and local white matter (WM) atrophy (Sarma et al. 2014); WM lesions/abnormalities (Hoare et al. 2015; Ackermann et al. 2016, 2014; Uban et al. 2015; Van Arnhem et al. 2013), and basal ganglia calcification (Govender et al. 2011).
The Children with HIV Early Antiretroviral Therapy (CHER) clinical trial (Violari et al. 2008; Cotton et al. 2013) showed that early HIV diagnosis and ART initiation (6-12 weeks of age) reduced infant mortality and HIV progression compared to standard 2006 guidelines which advised initiating ART when the percentage of CD4+ T lymphocytes (CD4 percentage) - an indicator of immune system health -declined below 25% or for severe clinical disease (WHO, 2006). Since 2008, most guidelines recommend initiating ART as soon as possible for all HIV+ infants regardless of CD4 measures, even if asymptomatic (WHO, 2008; PENTA 2009; ART Guideline, 2008).
Although early ART initiation improves clinical outcomes for HIV+ children (Phongsamart et al. 2014; Crowell et al. 2015; Laughton et al. 2013; Brahmbhatt et al. 2014), ART alone cannot completely reverse the neurological effects of HIV (Whitehead et al. 2014; Lowenthal et al. 2014; Van Arnhem et al. 2013; Laughton et al. 2013; Mbugua et al. 2016; Ackermann et al. 2016). In addition, there is concern about potential adverse effects of ART including metabolic abnormalities (Vigano et al. 2010) and neurotoxicity (Robertson et al. 2012).
Few neuroimaging studies have been done in children initiating ART before 2 years of age, and even fewer in children within the critical stage of brain development between 5-11 years. Most studies of HIV+ children in this age range focused on neurobehavioral and cognitive parameters (Govender et al. 2011; Shanbhag et al. 2005; Martin et al. 2006), while the few existing neuroimaging studies included children over a wide age range, with later ART initiation and/or without details of viral suppression (Van Arnhem et al. 2013; Van Dalen et al. 2016; Yadav et al. 2017). These studies may not be relevant to the growing population of children on early ART in whom viral loads are suppressed from a young age.
Despite early ART initiation and viral load suppression, HIV+ children from the CHER cohort demonstrate basal ganglia metabolite level and white matter alterations at age 5 years (Mbugua et al. 2016; Ackermann et al. 2016). Morphometric brain development of these children is unknown. Here, we used FreeSurfer's automated cortical and subcortical analysis methods to investigate the effects of HIV and timing of ART initiation on brain morphometry in a neuroimaging follow-up study of the Cape Town CHER cohort at age 7 years. To our knowledge, this is the first study of brain morphometry in a homogeneous sample of children in a narrow age range (7.00 – 7.83 years) all of whom initiated ART before 76 weeks and 93% of whom had viral load suppression at time of scanning.
We hypothesized that early ART would prevent much of the neurological damage associated with perinatal HIV infection and would lead to no or small differences from the uninfected control group with respect to gyrification, cortical thickness and gray and white matter volumes. Further, since cortical gyrification is positively related to cognitive performance and cortical volume, but negatively related to cortical thickness in many regions of the cortex (Gautam et al. 2015), we hypothesized that younger age of ART initiation would be associated with greater gyrification, thinner cortex, and larger brain volumes.
2. Methods
Study participants
The study population comprised 103 Xhosa children (sixty-one HIV+, forty-two uninfected controls, age 7.21 ± 0.14 years) from a follow-up study of CHER trial participants, conducted at the Family Clinical Research Unit at Tygerberg Children's Hospital, Cape Town. On the CHER trial, HIV+ infants with CD4 percentage ≥ 25% were randomized to limited ART between 6 and 12 weeks of age for either 40 or 96 weeks and restarted when clinical and/or immunological criteria were met, or started ART only when they became symptomatic or CD4 percentage dropped below 20% (25% in the first year), as per guidelines at the time (WHO, 2006). Of the HIV+ children, forty-six started ART at or before 12 weeks of age (Before-12wk) and fifteen after 12 weeks of age (After-12wk). All HIV+ children had started ART by 76 weeks of age and received comprehensive immunological and clinical follow-up thereafter. First line ART regimen consisted of Zidovudine (ZDV) + Lamivudine (3TC) + Lopinavir-Ritonavir (LPV/r, Kaletra®) (CHER, 2010; Violari et al. 2008; Cotton et al. 2013). Viral loads were suppressed (<400 copies/mL) in 93% of HIV+ children at the time of scanning. The uninfected control group was recruited from an interlinking vaccine trial (Madhi et al., 2010) and included both children born to HIV+ mothers (HIV-exposed, uninfected; HEU; n=20) and to uninfected mothers (HIV-unexposed; HU; n=22).
Study participants' cognitive abilities were assessed on the Kaufman Assessment Battery for Children second edition (KABC-II) at 7 years as part of a longitudinal neurocognitive study (Kaufman & Kaufman, 2004). The Luria model of the KABC-II is considered culture fair and appropriate for use within the South African context (Boivin and Giordani, 2009; Jansen and Greenop, 2008; Skuy et al., 2000; Van Wyhe et al., 2017). Here we provide two summary measures from the KABC-II, namely the Mental Processing Index (MPI), which is a composite score of intellectual functioning from four subscales (Learning, Planning, Sequential processing and Simultaneous processing), and the Non-verbal Index (NVI), a composite score obtained from subtests requiring non-verbal reasoning. Test scores are interpreted relative to standardized norms with mean 100 and SD 15; MPI and NVI standard scores between 85 and 115 are considered average.
Image acquisition and analysis
All participants received a T1-weighted high-resolution structural MRI scan on a 3T Allegra scanner (Siemens, Erlangen, Germany) at the Cape Universities Brain Imaging Centre (CUBIC). Children were scanned in sagittal orientation using a 3D echo planar imaging (EPI) navigated (Tisdall et al. 2012) multiecho magnetisation prepared rapid gradient echo (MEMPRAGE; van der Kouwe et al. 2008) sequence (FOV 224 × 224 mm2, TR 2530 ms, TI 1160 ms, TE's = 1.53/3.19/4.86/6.53 ms, bandwidth 650Hz/px, 144 slices, 1.3 × 1.0 × 1.0 mm3) that prospectively corrects for motion during the scan. To limit motion due to restlessness, children watched a movie via a mirror and rear projection screen during scanning. Scans were performed without sedation according to protocols approved by the Faculty of Health Sciences Human Research Ethics Committees of both the Universities of Cape Town and Stellenbosch. Parents/guardians provided written informed consent and the children gave oral assent.
FreeSurfer version 6.0 (https://surfer.nmr.mgh.harvard.edu) was used for automated cortical reconstruction and to measure cortical thickness and local gyrification index (LGI) across the cortical surface, as well as regional and total brain volumes. FreeSurfer outputs were manually checked for errors in cortical and sub-cortical segmentations. Minor pial edits (skull strip corrections) were required for some of the outputs, but no white matter correction. There were no failures in the LGI computation. Subjects were excluded from particular analyses if their mean values on these measures were extreme outliers (removed from the median of the sample by more than 3 times the interquartile range (IQR)).
Volumetric Analysis
Volumes of regions previously implicated in HIV infection, including the bilateral caudate, thalamus, hippocampus, putamen and lateral ventricles, as well as the corpus callosum, global white matter (WM), global gray matter (GM), and total intracranial volume (TIV), were analysed using the statistical analysis programming language, R (https://www.r-project.org/).
Linear regression was used to compare volumes between HIV+ and uninfected children. Since sex-related differences in cortical and sub-cortical structures have been reported in brain development (Fisher et al. 2016; Luder et al. 2010), sex was controlled for in all analyses. Analyses were repeated adjusting for TIV to examine whether regional volume changes were over and above the effect of HIV on TIV. Among HIV+ children, relationships of age at ART initiation and cumulative ART duration to total and regional brain volumes were also investigated adjusting for the effect of duration of ART interruption. Since treatment interruption could expose children's brains to HIV, this may have negated some of the benefits of earlier ART. Duration of interruption was set to zero for children on continuous treatment. We report p-values both uncorrected and after adjustment for multiple comparisons using the false discovery rate (FDR) method (Benjamini et al. 1995).
Vertex-wise analysis of cortical thickness and LGI
Cortical thickness and LGI were compared between HIV+ and uninfected children vertex-wise over the whole brain using the QDEC tool in FreeSurfer. The interaction of sex and HIV infection status was also investigated. To investigate the possible effect of earlier ART initiation, we explored among HIV+ children the relationship between age at ART initiation and morphometric measures, controlling for duration of ART interruption, as well as differences between the Before-12wk and After-12wk groups. To examine whether reduced gyrification in the Before-12wk group was attributed to treatment interruption in some children, we also compared the After-12wk group separately to children in the Before-12wk group who were interrupted and those who were not. Further, vertex-wise comparisons were performed between Before-12wk and After-12wk children and uninfected controls separately. The smoothing kernel for cortical thickness analyses was 10mm FWHM and no smoothing was used for LGI. Results of vertex-wise analyses controlling for sex were thresholded at an initial uncorrected threshold of p<0.05. Because of the spatial smoothness of the data, uncorrected p-values at neighbouring vertices can be assumed to covary to some degree, forming clusters. To adjust for multiple comparisons across vertices, cluster size limits for a two-tailed test, threshold of p<0.05, and for data of similar smoothness, were generated using Monte Carlo simulation (Forman et al., 1995). We report clusters surviving a cluster-size corrected threshold of p<0.05, with no adjustment for the two hemispheres.
3. Results
Sample characteristics
Only one child, a girl in the Before-12wk group, was excluded due to a poor quality MRI. Her data on a number of measures were also extreme outliers (> 3 IQRs below the 25th percentile). We therefore present data for 102 children. Population characteristics for all subjects are presented in Table 1. Groups did not differ in age, sex, TIV and MPI and NVI on the KABC-II (administered at age 7 years). Table 2 presents sample characteristics and clinical measures for HIV+ children in the Before-12wk and After-12wk groups. Since children were randomised to treatment arms, groups do not differ on any demographic or disease-related variables, except for CD4 count at age 7, which we controlled for in all analyses involving comparisons between the Before-12wk and After-12wk groups. Notably, no child starting ART after 12 weeks had treatment interrupted whereas the Before-12wk group included 28 children (62%) whose treatment was interrupted. All except 5 children had undetectable viral loads (<400 copies/mL) by 2 years of age, and all except 4 at the time of scanning.
Table 1. Sample characteristics.
| HIV+ | Uninfected | t or χ2 | p-value | |
|---|---|---|---|---|
|
| ||||
| Sample size (N) | 60 | 42 | ||
| Age at scan (years) | 7.20 (0.13) | 7.23 (0.16) | -1.12 | 0.26 |
| Number of males (% males) | 28 (47%) | 25 (58%) | 1.47 | 0.25 |
| Total intracranial volume (cm3) | 1427.99 (104.99) | 1477.77 (130.77) | -1.43 | 0.16 |
| Birth weight (g) | 3079.91 (443.02) | 3100.86 (466.99) | -0.23 | 0.82 |
| Kaufman Assessment Battery for Children (KABC) Second edition composite scores: | ||||
| Mental processing index | 72.18 (7.89) | 73.13 (9.48) | -0.53 | 0.59 |
| Non-verbal index | 73.12 (10.85) | 72.54 (11.21) | 0.26 | 0.80 |
Values are N (% of total) or mean (standard deviation)
Table 2. Sample characteristics and clinical profiles of HIV+ children initiating ART before and after 12 weeks.
| HIV+children | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| After-12wk (> 12 weeks) | Before-12wk (≤ 12 weeks) | t or χ2 | p-value | ||||
| Demographics | |||||||
| Sample size (N) | 15 | 45 | |||||
| Age at scan (years) | 7.22 (0.10) | 7.19 (0.14) | 0.64 | 0.53 | |||
| Males (% males) | 5 (33%) | 22 (49%) | 0.68 | 0.41 | |||
| Total intracranial volume (cm3) | 1295 (91.42) | 1259 (108.77) | 1.17 | 0.24 | |||
| Birth weight (g) | 3019.67 (439.05) | 3087.39 (466.34) | -0.50 | 0.62 | |||
| Clinical data at baseline (6-8 weeks) | |||||||
| CD4 count (cell/mm3)a | 2010.60 (682.69) | 1862.16 (992.67) | 0.54 | 0.59 | |||
| CD4% (cells/mm3) | 35.45 (7.78) | 32.76 (10.04) | 0.95 | 0.35 | |||
| CD4/CD8a | 1.32 (0.70) | 1.30 (0.80) | 0.09 | 0.93 | |||
| CD8 count (cells/mm3) | 1888.50 (974.78) | 1682.78 (949.77) | 0.70 | 0.48 | |||
| Viral load at enrolment | |||||||
| High (>750,000 copies/mL) | 9 (60%) | 24 (53.3%) | |||||
| Low (400-750,000 copies/mL) | 6 (40%) | 21 (46.7%) | |||||
| Suppressed (<400 copies/mL) | 0 | 0 | |||||
| Clinical data at scan (7 years) | |||||||
| CD4 count (cells/mm3) | 1446.27 (487.79) | 1172.81 (451.39) | 1.98 | 0.05 | |||
| CD4% (cells/mm3) | 37.94 (6.20) | 36.74 (6.64) | 0.61 | 0.54 | |||
| Viral load at scan (Age 7 years) | |||||||
| High (>750,000 copies/mL) | 0 | 0 | |||||
| Low (400-750,000 copies/mL) | 0 | 4 (8.9%) | |||||
| Suppressed (<400 copies/mL) | 15 (100%) | 41 (91.1%) | |||||
| Other | |||||||
| Age at ART initiation (weeks) | 34.14(17.56) | 8.41(1.59) | 9.99 | <0.0001 | |||
| Age at ART interruption (weeks) | - | 63.77 (29.32)b | - | - | |||
| Age of first viral load suppression (weeks)c | 47.57 (36.71) | 33.50 (16.32) | 1.06 | 0.29 | |||
| Duration of ART interruption (weeks) | - | 62.62 (76.08)b | - | - | |||
| Cumulative duration on ART (weeks) | 342.70 (18.87) | 318.70 (78.08) | 1.17 | 0.25 | |||
| CDC classification | |||||||
| A | 1 (6.7%) | 8 (17.8%) | |||||
| B | 2 (13.3%) | 11 (24.4%) | |||||
| Severe B | 4 (26.7%) | 2 (4.4%) | |||||
| C | 8 (53.3%) | 24 (53.3%) | |||||
| HIV encephalopathy diagnosis | 2 (13.3%) | 7 (15.6%) | |||||
| Kaufman Assessment Battery for Children (KABC) Second edition composite scores | |||||||
| Mental processing index | 74.07 (5.08) | 71.50 (8.63) | 1.08 | 0.28 | |||
| Non-verbal index | 75.33 (9.93) | 72.38 (11.15) | 0.91 | 0.37 | |||
Values are N (% of total) or mean (standard deviation) except for age of first viral load suppression
CD4 count at enrolment data were not available for 2 children.
Age and duration of ART interruption mean and standard deviation based only on children in whom treatment was interrupted (N=28)
where values are median (interquartile range (IQR)).
Volumetrics
After controlling for TIV, volumes of global gray and white matter, bilateral hippocampus and putamen, and right thalamus were smaller (p≤0.05) in HIV+ children compared to controls (Table 3). On adjusting for multiple comparisons, global gray and white matter, left hippocampus and right putamen volumes remained significantly smaller, while right hippocampus, left putamen and right thalamus showed a trend for being smaller in HIV+ children than uninfected controls. Age at ART initiation and cumulative duration on ART showed no relationship with both global and regional brain volumes after multiple comparison correction.
Table 3.
Regression of regional brain volumes at age 7 years with HIV infection status.
| Brain region | Volume (mm3)a | Regression controlling for sex | Regression controlling for sex and total intracranial volume | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| HIV+ | Uninfected | Effect size (standard error) | p | FDR Adjusted p-value | Effect size (standard error) | p | FDR Adjusted p-value | |
| Total intracranial volume | 1427991 (104991) | 1477778 (130776) | 19199 (22288) | 0.02* | 0.09† | - | - | - |
| Global gray matter | 713353 (52485) | 731931 (63920) | 24521 (14204) | 0.07† | 0.14 | 18518 (12489) | 0.01* | 0.04* |
| Global white matter | 380733 (41701) | 401041 (52381) | 19685 (10213) | 0.01* | 0.09† | 14388 (8229) | <0.001* | 0.01* |
| Corpus callosum | 3349 (450) | 3462 (470)) | 76(89) | 0.21 | 0.27 | 56 (86) | 0.16 | 0.20 |
| L caudate | 3701 (501) | 3807 (458) | 98 (100) | 0.26 | 0.30 | 50 (84) | 0.19 | 0.22 |
| R caudate | 3868 (499) | 3992 (451) | 121 (104) | 0.18 | 0.27 | 71 (87) | 0.11 | 0.15 |
| L hippocampus1 | 3443 (321) | 3583 (362) | 114 (89) | 0.03* | 0.11 | 97 (87) | 0.02* | 0.05* |
| R hippocampus2 | 3554 (358) | 3696 (391) | 184 (89) | 0.05* | 0.14 | 159 (85) | 0.03* | 0.06† |
| L putamen | 5006 (526) | 5205 (612) | 371 (133) | 0.06† | 0.14 | 324 (123) | 0.03* | 0.06† |
| R putamen | 5069 (538) | 5313 (585) | 360 (120) | 0.02* | 0.09† | 326 (114) | 0.01* | 0.04* |
| L thalamus | 7205 (647) | 7365 (694) | -12 (125) | 0.19 | 0.27 | -81 (97) | 0.09† | 0.14 |
| R thalamus | 7151 (672) | 7349 (731) | 76 (134) | 0.11 | 0.19 | 9 (110) | 0.05* | 0.08† |
| L lateral ventricle | 6214 (2320) | 6517 (2754) | -49 (498) | 0.54 | 0.58 | -291 (415) | 0.51 | 0.54 |
| R lateral ventricle | 5731 (1937) | 5870 (2458) | -127 (441) | 0.75 | 0.75 | -357 (354) | 0.72 | 0.72 |
Values are Mean (Standard deviation)
Left and right hippocampus each had an extreme outlier, which was removed before analysis (N=101).
p ≤ 0.05 (denoted in bold)
p < 0.1
Vertex-wise analysis of cortical thickness and LGIs
HIV+ children demonstrated thicker cortex compared to uninfected controls in a small left inferior lateral occipital region (MNI co-ordinates at peak: -32.9, -83.4, 1.6, size: 865.57 mm2) (Figure 1), and lower local gyrification in bilateral paracentral (Left: MNI co-ordinates at peak: -6.4, -35.8, 56.5, 1966.90 mm2; Right: MNI co-ordinates at peak: 11.2, 1.4, 46.1, 1109.49 mm2) and right temporal (MNI co-ordinates at peak: 54.6, -12.3, -17.3, 802.37 mm2) regions (Figure 2).
Fig. 1.
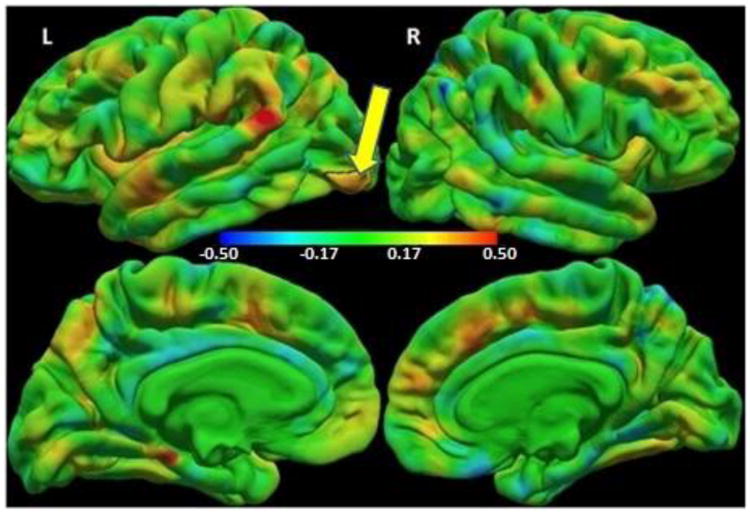
Colour map of regression coefficients for cortical thickness (mm) against group (HIV+ children/controls) controlling for sex. Positive regression coefficients (red/yellow) indicate HIV+ > controls, and negative coefficients (cyan/blue) indicate HIV+ < controls. The color bar scale applies to both lateral (top) and medial (bottom) views.
The small left lateral occipital region outlined in black shows where HIV+ children have thicker cortex compared to uninfected controls at a threshold of p<0.05, with a cluster size corrected threshold of p<0.05 (Peak MNI co-ordinates 32.9, -83.4, 1.6, size 865.57 mm2).
Fig.2.
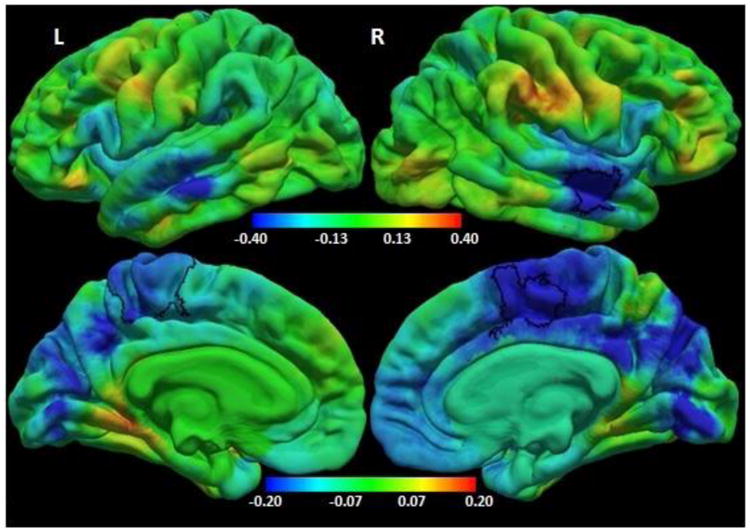
Colour map of regression coefficients for local gyrification index (LGI) against group (HIV+ children /controls) controlling for sex. Positive regression coefficients (red/yellow) indicate HIV+ > controls, and negative coefficients (cyan/blue) indicate HIV+ < controls. Note different color bar scales for lateral (top) and medial (bottom) views.
The bilateral paracentral (left: precentral/ postcentral/ paracentral/ posterior cingulate/ precuneus/ superior parietal gyri, MNI co-ordinates -6.4, -35.8, 56.5, size 1966.90mm2; right: superior frontal/ paracentral/ posterior cingulate gyri, MNI co-ordinates 11.2, 1.4, 46.1, size 1109.49 mm2) and right temporal (superior / middle temporal gyri, MNI co-ordinates 54.6, -12.3, -17.3, size 802.37mm2) regions outlined in black show where HIV+ children have smaller local gyrification indices compared to uninfected controls at a threshold of p<0.05, with a cluster size corrected threshold of p<0.05.
Although there was no interaction effect of sex on the relationship between HIV status and cortical thickness significant at p<0.05, there was a significant interaction effect of sex on the relationship between HIV status and LGI in bilateral parietal/middle temporal regions (left: MNI co-ordinates at peak: -47.2, -60.0, 8.8, 2538.16 mm2; Right: MNI co-ordinates at peak: 45.6, -52.0, 19.7, 2484.04 mm2) (Figure 3a). The plots in Figure 3b show that the mean LGIs in the bilateral clusters were similar or higher in HIV+ girls compared to uninfected girls (Left p=0.09, Right: p=0.004), while LGIs of HIV+ boys were lower than in uninfected boys (Left p=0.01, Right p=0.02).
Fig. 3.
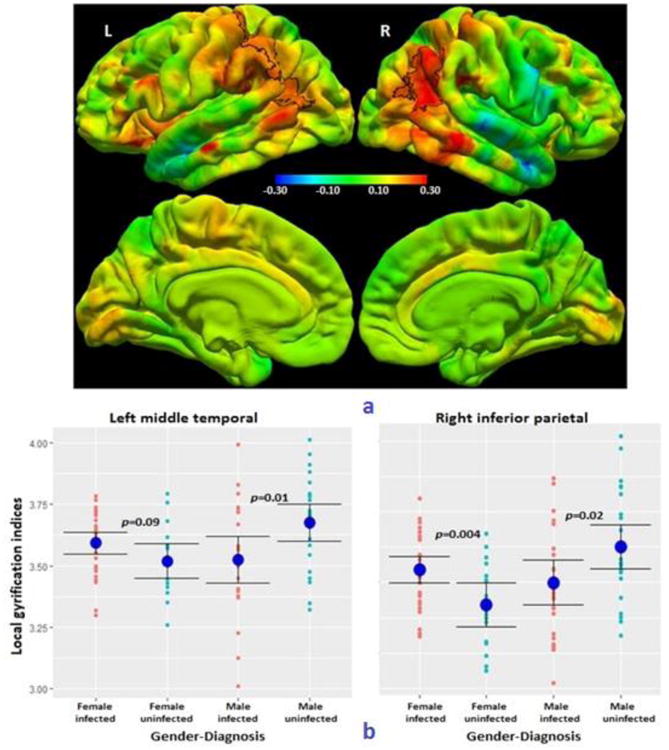
a) Colour map of regression coefficients for the interaction effect of sex on the relationship between HIV infection status and local gyrification index (LGI). The same color bar scale applies to all views.
The bilateral parieto-temporal regions outlined in black show a significant sex-HIV interaction effect at a threshold of p<0.05, with a cluster size threshold of p<0.05. (Left postcentral/ superior parietal/ supramarginal/ inferior parietal/ middle temporal gyri, MNI co-ordinates -47.2, -60.0, 8.8, size 2538.16 mm2; right inferior parietal/ middle temporal/ supramarginal/ superior parietal gyri, MNI co-ordinates 45.6, -52.0, 19.7, size 2484.04 mm2)
b) Plots of peak LGI (and group means ± SD) in the left and right parieto-temporal clusters, showing how the effect of HIV infection on LGIs differs in girls and boys. On the left, the peak LGI was located in the middle temporal gyrus, and on the right in the inferior parietal gyrus.
Linear regression that adjusted for sex and duration of interruption showed that right medio-frontal (MNI co-ordinates at peak: 10.3, 51.2, 3.3, cluster size: 882.87 mm2) cortex was thinner with later ART initiation (Figure 4a), while LGI was greater with later ART initiation in symmetric bilateral medial frontal regions (Left: MNI co-ordinates at peak: -5.7 29.6 13.6, cluster size: 3609.06 mm2; Right: MNI co-ordinates at peak: 14.7, 39.0, 9.2, cluster size: 5285.53 mm2), as well as a right lateral fronto-temporal region (MNI co-ordinates at peak: 43.2, -0.8, -18.7, cluster size: 838.81 mm2) (Figure 4b). Group analyses adjusted for sex confirmed that After-12wk children had significantly greater LGIs than both interrupted and continuous Before-12wk children in the same bilateral medial frontal regions. Comparison with the uninfected children showed that whereas the After-12wk children had greater LGIs than the uninfected children in these bilateral frontal regions, the Before-12wk children had smaller LGIs in similar regions.
Fig. 4.
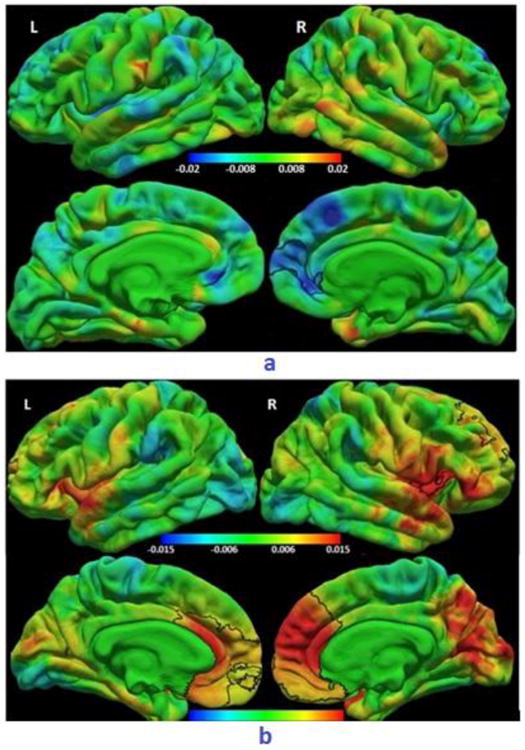
a) Colour map of regression coefficients for the regression of cortical thickness (mm) on age at ART initiation (weeks). The cluster outlined in black shows a right medial frontal region where older age at ART initiation was associated with thinner cortex at a threshold of p<0.05, with a cluster size threshold of p<0.05, after controlling for sex and duration of interruption. (Right rostral anterior cingulate/ medial orbitofrontal/ superior frontal/ frontal pole/ rostral middle frontal gyri, MNI co-ordinates 10.3, 51.2, 3.3, size 882.87 mm2). A similar region showed thinner cortex in After-12wk children compared to Before-12wk children. The same color bar scale applies to all views.
b) Colour map of regression coefficients for LGI on age at ART initiation (weeks). Clusters outlined in black show (top) right lateral fronto-temporal (right pars orbitalis/ pars triangularis/ lateral orbitofrontal/ insula/ superior temporal, MNI co-ordinates 43.2, -0.8, -18.7, size 838.81 mm2) and (bottom) bilateral medial frontal (left: medial orbitofrontal/ rostral anterior cingulate/ caudal anterior cingulate/ superior frontal/ frontal pole/ rostral middle frontal, MNI co-ordinates -5.7, 29.6, 13.6, size 3609.06 mm2; right: caudal anterior cingulate/ rostral anterior cingulate/ medial orbitofrontal/ superior frontal/ rostral middle frontal/ frontal pole, MNI co-ordinates 14.7, 39.0, 9.2, size 5285.53 mm2) regions where older age at ART initiation was associated with greater LGIs at a threshold of p<0.05, with a cluster size threshold of p<0.05, after controlling for sex and duration of interruption. Similar regions showed greater gyrification in After-12wk children compared to Before-12wk children. Note different color bar scales for lateral (top) and medial (bottom) views.
4. Discussion
This is the first study reporting possible effects of HIV and timing of ART initiation on cortical thickness and gyrification in children. Specifically, HIV+ children demonstrated thicker cortex in a small left inferior lateral occipital region and lower gyrification in bilateral paracentral and right temporal regions than uninfected controls, despite early ART and viral load suppression. Additional HIV-related gyrification reductions were evident in lateral parieto-temporal regions in boys, but not in girls. Contrary to our hypothesis, ART initiation at a younger age was associated with less gyrification in bilateral medial frontal and right fronto-temporal regions, but thicker cortex in an overlapping right medial frontal region. Separate analyses demonstrated greater gyrification in similar bilateral medial frontal regions in After-12wk children compared to both uninfected controls and Before-12wk children, who had the least gyrification in these regions. WM and GM volumes, right putamen and left hippocampus were relatively smaller in HIV+ children after adjustment for reductions in total brain volume and multiple comparisons, while timing of ART initiation did not affect global or regional brain volumes.
Volumetrics
Our finding of lower global white and gray matter volumes is consistent with other studies in youths on ART with viral load suppression (Cohen et al. 2016; Lewis-de los Angeles et al. 2017). However, findings in subcortical regions have been less consistent. While Lewis-de los Angeles et al. (2016) found subcortical shape deformations in the absence of HIV-associated volumetric differences, Yadav and colleagues (2017) reported hippocampal volume decreases and nucleus accumbens increases in slightly older HIV+ children, and Blokhuis et al. (2017) reported trend-level but not significant putamen increases.
Before the roll-out of early ART, common structural neuroimaging findings in HIV+ children included ventricular enlargement, white matter signal abnormalities, cerebral and subcortical atrophy, corpus callosum damage and basal ganglia calcification (George et al. 2009; Hoare et al. 2014, Martin et al. 2006). The subcortical neuronal atrophy observed in HIV infection was thought to be due to the proximity to the ventricle, which acts as a channel for cerebrospinal fluid and a carrier of HIV. This proximity may facilitate easier penetration by HIV-infected mononuclear cells and also HIV toxic products, such as gp120 and Tat, leading to neuronal atrophy (Ances et al. 2012). Early ART has both clinical and immunological benefits by suppressing HIV replication, in the CNS and elsewhere, and restoring immune function, thereby protecting brain morphology and neurodevelopment (Laughton et al. 2012; Cotton et al. 2013; Lindsey et al. 2007; Violari et al. 2008). Notably, we did not find volume reduction in the caudate and a trend in right thalamus – the regions closest to the ventricles.
The decreased volume observed here in the putamen and hippocampus, despite early ART initiation and viral load suppression, suggests either ongoing neurodevelopmental delay or damage from early neuro-infiltration that persists into childhood. The latter ties in with recent spectroscopy findings in the same cohort where immunocompromise at baseline was associated with lower basal ganglia metabolite levels (choline, NAA) five years later (Mbugua et al. 2016).
Interestingly, we did not find corpus callosum (CC) reductions or ventricular enlargement. Although CC atrophy was found in youths with suppressed viral loads some of whom started ART early (Sarma et al. 2014; Yadav et al. 2017), most children included in studies reporting ventricular enlargement did not have suppressed viral loads (Hoare et al. 2014; van Arnhem et al. 2013; Martin et al. 2006), whereas viral loads were suppressed in 93% of children in this study. It is possible that CC atrophy is not evident at age 7 years, as children in the two studies cited above were older and initiated ART over a wider age range, whereas in our study all children initiated ART before 76 weeks of age. These data suggest that earlier ART may be neuroprotective to the CC, consistent with results of recent DTI findings in our cohort where no microstructural differences were found between HIV+ and control children in the CC at age 5 years (Ackermann et al. 2016). However, manual segmentation in these children at 5 years showed corpus callosum reductions in HIV+ children, even though automated segmentation showed little difference between groups (Randall et al., 2017). CC volume alterations may be so small that automated segmentation measures used here are not sensitive enough to detect differences, due to imperfect co-registration of pediatric brains at this age to adult templates (Narayanan et al. 2016; Yoon et al. 2009). A study by Andronikou et al. (2015) including HIV+ children from our cohort (age range 7 to 49 months) and using semi-automated segmentation methods, also did not find CC volume and thickness differences compared to controls.
LGI and cortical thickness
Contrary to our hypothesis that early ART would minimise morphometric alterations, HIV+ children had lower gyrification than uninfected controls in large bilateral medial parietal and right temporal regions, with affected regions being more extensive in boys than in girls. Although sulcal widening, characterised by reduced gyrification, curvature and steepness (White et al. 2010), was reported in HIV+ children on ART (Van Arnhem et al. 2013), only 24% of children in that study had suppressed viral loads compared to 93% here. Unfortunately, that study reported only the incidence of sulcal widening and not the locations, making direct comparison of affected regions impossible. Sex differences are not surprising as both gyrification and cortical thickness differ between sexes (Fisher et al. 2016; Luder et al. 2010; Im et al. 2006).
The fact that HIV+ children had thicker cortex than controls only in one small left lateral occipital region suggests that early ART may have reversed or prevented some of the previously reported effects of HIV on the cortex. This is consistent with reports of improved neurodevelopmental function in HIV+ children started on ART in early infancy (Brahmbhatt et al. 2014; Cotton et al. 2013; Crowell et al. 2015; Laughton et al. 2012; Lindsey et al. 2007; Shanbhag et al. 2005). However, a recent study by Yadav and colleagues (2017) in 10 to 11-year old HIV+ children on ART found regional cortical thickness decreases in bilateral postcentral and right superior temporal regions, but increases in bilateral medial frontal regions, which the authors suggest could be related to stress-induced hypertrophy of medium spiny neurons. It is noteworthy that the regions showing greater cortical thickness in that study overlap with those where we found lower gyrification and thicker cortex in children who started ART before 12 weeks of age.
Effects of timing of ART initiation
Previous studies have shown a relationship between earlier ART initiation and improved neurodevelopmental (IQ, memory, psychomotor, and behavior) outcomes, at least in the short term (Brahmbhatt et al. 2014; Crowell et al. 2015; Laughton et al. 2012), as well as metabolic advantages in the basal ganglia at age 5 years (Mbugua et al. 2016). Although we found no effect of age of ART initiation on brain volumes, the relationships observed in the medial frontal regions for both cortica thickness and gyrification, and for gyrification in fronto-temporal regions, suggest that time of ART initiation affects cortical development in these regions. The fact that more extensive regions show greater gyrification with later ART initiation than thinner cortex, suggests that gyral formation in early childhood may be more sensitive to earlier ART than cortical thickness. It is known that gyrification is influenced primarily by early events, in contrast to cortical thickness, which is most affected by maturational changes during childhood, adolescence and early adulthood (Schaer et al. 2012).
The findings of positive association between bilateral frontal LGI and age at ART initiation, and reduced frontal LGI in Before-12wk children compared to both controls and After-12wk children, suggest that earlier ART rather than HIV infection reduces gyrification in this region. In the region showing an effect of ART initiation age on cortical thickness, Before-12-wk children, who had lowest gyrification, also had the thickest cortex (2.88 ± 0.19 mm); thicker than both controls (2.78 ± 0.19 mm, p=0.005) and After-12wk children (2.70 ± 0.15 mm, p<0.001). ZDV, used in first line treatment, has been associated with mitochondrial dysfunction (Robertson et al. 2012; Musielak & Fine, 2015) and neurological abnormalities (Tardieu et al. 2005). A DTI study in children from the same cohort at age 5 years similarly found poorer regional white matter (WM) integrity in children starting ART before 12 weeks compared to those starting after 12 weeks (Ackermann et al. 2016). In that study, the poorer WM integrity was attributable to treatment interruption. In contrast, the relations of LGI and cortical thickness with age at ART initiation in our study survived adjustment for treatment interruption. Also, gyrification increases in the After-12wk children were evident compared to both interrupted and continuous Before-12wk children separately, confirming that the lower gyrification observed here in the Before-12wk group is not a consequence of treatment interruption.
It is possible that higher gyrification in After-12wk children is a consequence of abnormally increased sulcal depth in children due to untreated HIV infection. Abnormal increases in gyrification relative to healthy controls are observed in children and adults, for example, in autism (Wallace et al. 2013), Williams syndrome (Gaser et al. 2006), schizophrenia (Schultz et al. 2010) and preterm birth (Kesler et al. 2006), and together with thinner cortex in children with developmental dyslexia (Williams et al. 2017). In this scenario, unless earlier ART and untreated HIV have opposite effects on early gyrification, it is difficult to explain why children receiving ART before 12 weeks should have lower frontal LGIs than controls while children receiving ART after 12 weeks have higher LGIs than controls in similar regions. It should be noted, however, that the control group includes HEU children, exposed in utero to HIV and antiretrovirals which could have influenced brain development, and HU children (Robertson et al. 2012; Le Doare et al. 2012). Even though HEU and HU children did not differ on any demographic variables, when comparing the After-12wk children to HEU and HU children separately, the After-12wk children demonstrated greater LGIs in the same bilateral frontal regions than HEU but not HU children. These results suggest that gyrification increases in the After-12wk children may be attributable to lower gyrification in HEU children. Notably, cortical thickness in the region showing association with age at ART initiation did not differ between After-12wk and uninfected children (p=0.16) Neuropsychological test data are needed to understand the implication of increased gyrification in these regions in After-12wk children and to reveal whether earlier ART affects cognitive function in the longer term.
Our study includes a relatively large sample size, at a specific age, with participants on homogenous ART regimens and clinical follow-up from birth until age 7 years. Although the narrow age range is a strength of the study, it limits our ability to draw any conclusions about longitudinal brain development.
In this study, we could not disentangle the contributions of HIV and ART to the current findings, due to all children being on ART, although associations with ART initiation age may provide some insights. This is, however, complicated by the fact that a subset of the children receiving early ART had protocol-defined treatment interruption while those starting later remained on continuous ART. Although we controlled for duration of treatment interruption, it is possible that there are critical times in which exposure to HIV may impair gyrification or cortical thickness. In addition, environmental variables associated with HIV may also profoundly affect neurodevelopment. Even though the uninfected control children were from a similar disadvantaged socioeconomic environment, HIV+ children are more likely to have experienced stressors such as poor health, nutrition or the loss of a parent, which may influence brain development. The limited socioeconomic and environmental data obtained for these children makes it difficult to explore these factors fully in the current study. Future studies obtaining more descriptive sociodemographic and environmental data may be able to untangle the effects of these variables from the effects of HIV/ART itself on brain development. The co-registration of children's brains to an adult brain template is also a limitation and may have obscured some morphometric outcomes.
5. Conclusions
In this study regional differences in cortical thickness, gyrification, global white and gray matter volume, left hippocampus and right putamen suggest persisting and residual structural alterations at age 7 years despite early ART initiation and viral load suppression. However, no differences in volumes of many subcortical regions, corpus callosum, and lateral ventricles were found between HIV+ children and controls. There was also no effect of earlier ART on brain volumes at this age, but decreased medial frontal gyrification and increased cortical thickness in children starting ART before 12 weeks relative to both controls and children initiating ART after 12 weeks. Neuropsychological scores may clarify whether these frontal cortical changes are associated with better or worse cognitive outcomes.
Acknowledgments
Support for this study was provided by
National Research Foundation (NRF) / Department of Science and Technology (DST) South African Research Chairs Initiative.
US National Institute of Allergy and Infectious Diseases (NIAID) through the Comprehensive International Program of Research on AIDS (CIPRA) network, Grant U19 AI53217.
National Institutes of Health (NIH) grants R01HD071664 and R21MH096559.
NRF grant CPR20110614000019421, and the Medical Research Council (MRC).
The Departments of Health of the Western Cape and Gauteng, South Africa and ViiV Healthcare/GlaxoSmithKline plc provided additional support for the CHER.
Postgraduate Funding Office (PFO), University of Cape Town, South Africa.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national Human Research Ethics Committees (HREC) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Ackermann C, Andronikou S, Laughton B, Kidd M, Dobbels E, Innes S, van Toorn R, Cotton M. White matter signal abnormalities in children with suspected HIV-related neurologic disease on early combination antiretroviral therapy. The Pediatric infectious disease journal. 2014;33(8):e207–12. doi: 10.1097/INF.0000000000000288. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann C, Andronikou S, Saleh MG, Laughton B, Alhamud AA, van der Kouwe A, Meintjes EM. Early Antiretroviral Therapy in HIV-Infected Children Is Associated with Diffuse White Matter Structural Abnormality and Corpus Callosum Sparing. American Journal of Neuroradiology. 2016;37(12):2363–2369. doi: 10.3174/ajnr.A4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Ortega M, Vaida F, Heaps J, Paul R. Independent effects of HIV, aging, and HAART on brain volumetric measures. Journal of acquired immune deficiency syndromes (1999) 2012;59(5):469. doi: 10.1097/QAI.0b013e318249db17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andronikou S, Ackermann C, Laughton B, Cotton M, Tomazos N, Spottiswoode B, Mauff K, Pettifor JM. Corpus callosum thickness on mid-sagittal MRI as a marker of brain volume: a pilot study in children with HIV-related brain disease and controls. Pediatric radiology. 2015;45(7):1016–1025. doi: 10.1007/s00247-014-3255-y. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the royal statistical society Series B (Methodological) 1995:289–300. [Google Scholar]
- Blokhuis C, Kootstra NA, Caan MWA, Pajkrt D. Neurodevelopmental delay in pediatric HIV/AIDS: Current perspectives. Neurobehavioral HIV Medicine. 2016;7(1):1–13. doi: 10.2147/NBHIV.S68954. [DOI] [Google Scholar]
- Blokhuis C, Mutsaerts HJ, Cohen S, Scherpbier HJ, Caan MW, Majoie CB, Kuijpers TW, Reiss P, Wit FW, Pajkrt D. Higher subcortical and white matter cerebral blood flow in perinatally HIV-infected children. Medicine. 2017;96(7) doi: 10.1097/MD.0000000000005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin MJ, Giordani B. Neuropsychological assessment of African children: evidence for a universal brain/behavior omnibus within a coconstructivist paradigm. Prog Brain Res. 2009;178:113–35. doi: 10.1016/S0079-6123(09)17808-1. [DOI] [PubMed] [Google Scholar]
- Brahmbhatt H, Boivin M, Ssempijja V, Kigozi G, Kagaayi J, Serwadda D, Gray RH. Neurodevelopmental benefits of Anti-Retroviral Therapy in Ugandan children 0–6 Years of age with HIV. Journal of acquired immune deficiency syndromes (1999) 2014;67(3):316. doi: 10.1097/QAI.0000000000000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (CHER 2010) A phase III randomised, open label trial to evaluate strategies for providing Antiretroviral Therapy to infants shortly after primary infection in a resource poor setting. The National Institute of Allergy and Infectious Diseases, Division of AIDS (DAIDS); Children with HIV Early Antiretroviral Therapy (CHER) Trial; pp. 22–23. [Google Scholar]
- Cohen S, Caan MW, Mutsaerts H, Scherpbier HJ, Kuijpers TW, Reiss P, Majoie CB, Pajkrt D. Cerebral injury in perinatally HIV-infected children compared to matched healthy controls. Neurology. 2016;86(1):19–27. doi: 10.1212/WNL.0000000000002209. [DOI] [PubMed] [Google Scholar]
- Cotton MF, Violari A, Otwombe K, Panchia R, Dobbels E, Rabie H, van Rensburg AJ. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. The Lancet. 2013;382(9904):1555–1563. doi: 10.1016/S0140-6736(13)61409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell CS, Yanling HUO, Tassiopoulos K, Malee KM, Yogev R, Hazra R, Rutstein RM, Nichols SL, Smith RA, Williams PL, Oleske J, Muller WJ PACTG 219C and PHACS study teams. Early viral suppression improves neurocognitive outcomes in HIV-infected children. AIDS (London England) 2015;29(3):295. doi: 10.1097/QAD.0000000000000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald KA, Hoare J, Eley B, Wilmshurst JM. Neurologic complications of pediatric human immunodeficiency virus: Implications for clinical practice and management challenges in the African setting. Seminars in Paediatric Neurology. 2014;21(1):3–11. doi: 10.1016/j.spen.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Fisher AM, Cachia A, Fischer C, Mankiw C, Reardon PK, Clasen LS, Blumenthal JD, Greenstein D, Giedd JN, Mangin JF, Raznahan A. Influences of brain size, sex, and sex chromosome complement on the architecture of human cortical folding. Cerebral Cortex. 2016 doi: 10.1093/cercor/bhw323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster- size threshold. Magnetic Resonance in Medicine. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Foster CJ, Biggs RL, Melvin D, Walters MDS, Tudor- Williams G, Lyall EGH. Neurodevelopmental outcomes in children with HIV infection under 3 years of age. Developmental Medicine & Child Neurology. 2006;48(8):677–682. doi: 10.1017/S0012162206001423. [DOI] [PubMed] [Google Scholar]
- Freeman A. The role of neuropsychology in UK pediatric HIV care: Relevance to clinical practice and research. Child Neuropsychology. 2016:1–10. doi: 10.1080/09297049.2016.1207757. [DOI] [PubMed] [Google Scholar]
- Gaser C, Luders E, Thompson PM, Lee AD, Dutton RA, Geaga JA, Hayashi KM, Bellugi U, Galaburda AM, Korenberg JR, Mills DL, Toga AW, Reiss AL. Increased local gyrification mapped in Williams syndrome. NeuroImage. 2006;33(1):46–54. doi: 10.1016/j.neuroimage.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Gautam P, Anstey KJ, Wen W, Sachdev PS, Cherbuin N. Cortical gyrification and its relationships with cortical volume, cortical thickness, and cognitive performance in healthy mid-life adults. Behavioural Brain Research. 2015;287:331–9. doi: 10.1016/j.bbr.2015.03.018. [DOI] [PubMed] [Google Scholar]
- George R, Andronikou S, du Plessis J, du Plessis A, Van Toorn R, Maydell A. Central nervous system manifestations of HIV infection in children. Pediatric Radiology. 2009;39(6):575–585. doi: 10.1007/s00247-009-1170-4. [DOI] [PubMed] [Google Scholar]
- Govender R, Eley B, Walker K, Petersen R, Wilmshurst JM. Neurologic and neurobehavioral sequelae in children with human immunodeficiency virus (HIV-1) infection. Journal of child neurology. 2011:0883073811405203. doi: 10.1177/0883073811405203. [DOI] [PubMed] [Google Scholar]
- Hoare J, Ransford GL, Phillips N, Amos T, Donald K, Stein DJ. Systematic review of neuroimaging studies in vertically transmitted HIV positive children and adolescents. Metabolic Brain Disease. 2014;29(2):221–229. doi: 10.1007/s11011-013-9456-5. [DOI] [PubMed] [Google Scholar]
- Hoare J, Fouche JP, Phillips N, Joska JA, Donald KA, Thomas K, Stein DJ. Clinical associations of white matter damage in cART-treated HIV-positive children in South Africa. Journal of neurovirology. 2015;21(2):120–128. doi: 10.1007/s13365-014-0311-1. [DOI] [PubMed] [Google Scholar]
- Im K, Jong-Min L, Junki L, Yong-Wook S, In Young K, Jun Soo K, Sun IK. Gender difference analysis of cortical thickness in healthy young adults with surface-based methods. NeuroImage. 2006;31:31–38. doi: 10.1016/j.neuroimage.2005.11.042. [DOI] [PubMed] [Google Scholar]
- Jansen P, Greenop K. Factor analyses of the Kaufman assessment battery for children assessed longitudinally at 5 and 10 years. South African Journal of Psychology. 2008;38(2):355–365. [Google Scholar]
- Kader R, Seedat S, Koch JR, Parry CD. A preliminary investigation of the AUDIT and DUDIT in comparison to biomarkers for alcohol and drug use among HIV-infected clinic attendees in Cape Town, South Africa. African Journal of Psychiatry. 2012;15(5):346–351. doi: 10.4314/ajpsy.v15i5.43. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Assessment Battery for Children. 2nd. Minneapolis, MN: Pearson; 2004. [Google Scholar]
- Kaufman AS, Kaufman NL. Manual for the Kaufman Assessment Battery for Children. Second. Circle Pines, MN: American Guidance Service Publishing/Pearson Products Inc; 2004. [Google Scholar]
- Kesler SR, Vohr B, Schneider KC, Katz KH, Makuch RW, Reiss AL, Ment LR. Increased temporal lobe gyrification in preterm children. Neuropsychologia. 2006;44(3):445–453. doi: 10.1016/j.neuropsychologia.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Koekkoek S, de Sonneville LM, Wolfs TF, Licht R, Geelen SP. Neurocognitive function profile in HIV-infected school-age children. European Journal of Paediatric Neurology. 2008;12(4):290–297. doi: 10.1016/j.ejpn.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Laughton B, Cornell M, Grove D, Kidd M, Springer PE, Dobbels E, Janse van Rensburg A, Violari A, Babiker A, Madhi SA, Jean-Philippe P, Gibb DM, Cotton MF. Early antiretroviral therapy improves neurodevelopmental outcomes in infants. AIDS (London England) 2012;26(13):1685. doi: 10.1097/QAD.0b013e328355d0ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughton B, Cornell M, Boivin M, Van Rie A. Neurodevelopment in perinatally HIV-infected children: a concern for adolescence. Journal of the International AIDS Society. 2013;16(1) doi: 10.7448/IAS.16.1.18603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Doaré K, Bland R, Newell ML. Neurodevelopment in children born to HIV-infected mothers by infection and treatment status. Paediatrics peds-2012. 2012 doi: 10.1542/peds.2012-0405. [DOI] [PubMed] [Google Scholar]
- Lewis-de los Angeles CP, Alpert KI, Williams PL, Malee K, Huo Y, Csernansky JG, Yogev R, Van Dyke RB, Sowell ER, Wang L. Deformed Subcortical Structures Are Related to Past HIV Disease Severity in Youth With Perinatally Acquired HIV Infection. Journal of the Paediatric Infectious Diseases Society. 2016;5(suppl 1):S6–S14. doi: 10.1093/jpids/piw051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis-De Los Angeles CP, Williams PL, Huo Y, Wang SD, Uban KA, Herting MM, Malee K, Yogev R, Csernansky JG, Nichols S, Van Dyke RB, Sowell ER, Wang L. Lower Total and Regional Grey Matter Brain Volumes in Youth with Perinatally-Acquired HIV Infection: Associations with HIV Disease Severity, Substance Use, and Cognition. Brain, Behavior, and Immunity. 2017 doi: 10.1016/j.bbi.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey JC, Malee KM, Brouwers P, Hughes MD. Neurodevelopmental functioning in HIV-infected infants and young children before and after the introduction of protease inhibitor–based highly active antiretroviral therapy. Paediatrics. 2007;119(3):e681–e693. doi: 10.1542/peds.2006-1145. [DOI] [PubMed] [Google Scholar]
- Lowenthal ED, Bakeera-Kitaka S, Marukutira T, Chapman J, Goldrath K, Ferrand RA. Perinatally acquired HIV infection in adolescents from sub-saharan Africa: A review of emerging challenges. The Lancet Infectious Diseases. 2014;14(7):627–639. doi: 10.1016/S1473-3099(13)70363-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Toga AW. Sex Differences in the Human Brain, Their Underpinnings and Implications. Elsevier; Amsterdam: 2010. Sex differences in brain anatomy. [Google Scholar]
- Madhi SA, Adrian P, Cotton MF, McIntyre JA, Jean-Philippe P, Meadows S, Nachman S, Kayhty H, Klugman KP, Violari A. Effect of HIV infection status and anti-retroviral treatment on quantitative and qualitative antibody responses to pneumococcal conjugate vaccine in infants. The Journal of infectious diseases. 2010;202(3):355–361. doi: 10.1086/653704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SC, Wolters PL, Toledo-Tamula MA, Zeichner SL, Hazra R, Civitello L. Cognitive functioning in school-aged children with vertically acquired HIV infection being treated with highly active antiretroviral therapy (HAART) Developmental Neuropsychology. 2006;30(2):633–657. doi: 10.1207/s15326942dn3002_1. [DOI] [PubMed] [Google Scholar]
- Mbugua KK, Holmes MJ, Cotton MF, Ratai EM, Little F, Hess AT, Dobbels E, Van der Kouwe AJ, Laughton B, Meintjes EM. HIV-associated CD4/8 depletion in infancy is associated with neurometabolic reductions in the basal ganglia at age 5 years despite early antiretroviral therapy. AIDS (London, England) 2016;30(9):1353. doi: 10.1097/QAD.0000000000001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musielak KA, Fine JG. An Updated Systematic Review of Neuroimaging Studies of Children and Adolescents with Perinatally Acquired HIV. Journal of Pediatric Neuropsychology. 2015:1–16. [Google Scholar]
- Narayanan PL, Warton C, Boonzaier NR, Molteno CD, Joseph J, Jacobson JL, Jacobson SW, Zollei L, Meintjes EM. Improved segmentation of cerebellar structures in children. Journal of neuroscience methods. 2016;262:1–13. doi: 10.1016/j.jneumeth.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENTA Steering Committee: PENTA. guidelines for the use of antiretroviral therapy in paediatric HIV-1 infection (2009) HIV Med. 2009;10:591–613. doi: 10.1111/j.1468-1293.2009.00759.x. [DOI] [PubMed] [Google Scholar]
- Phongsamart W, Hansudewechakul R, Bunupuradah T, Klinbuayaem V, Teeraananchai S, Prasithsirikul W, Kerr SJ, Akarathum N, Denjunta S, Ananworanich J, Chokephaibulkit K. Long-term outcomes of HIV-infected children in Thailand: the Thailand Pediatric HIV Observational Database. International Journal of Infectious Diseases. 2014;22:19–24. doi: 10.1016/j.ijid.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Randall SR, Warton CM, Holmes MJ, Cotton MF, Laughton B, van der Kouwe AJ, Meintjes EM. Larger Subcortical Gray Matter Structures and Smaller Corpora Callosa at age 5 years in HIV Infected Children on early ART. Frontiers in Neuroanatomy. 2017 doi: 10.3389/fnana.2017.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson K, Liner J, Meeker RB. Antiretroviral neurotoxicity. Journal of Neurovirology. 2012;18(5):388–399. doi: 10.1007/s13365-012-0120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma MK, Nagarajan R, Keller MA, Kumar R, Nielsen-Saines K, Michalik DE, Thomas MA. Regional brain gray and white matter changes in perinatally HIV-infected adolescents. NeuroImage: Clinical. 2014;4:29–34. doi: 10.1016/j.nicl.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaer M, Cuadra MB, Schmansky N, Fischl B, Thiran JP, Eliez S. How to measure cortical folding from MR images: a step-by-step tutorial to compute local gyrification index. Journal of visualized experiments: JoVE. 2012;(59) doi: 10.3791/3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz CC, Koch K, Wagner G, Roebel M, Nenadic I, Gaser C, Schachtzabel C, Reichenbach JR, Sauer H, Schlösser RG. Increased parahippocampal and lingual gyrification in first-episode schizophrenia. Schizophrenia Research. 2010;123(no2):137–144. doi: 10.1016/j.schres.2010.08.033. [DOI] [PubMed] [Google Scholar]
- Shanbhag MC, Rutstein RM, Zaoutis T, Zhao H, Chao D, Radcliffe J. Neurocognitive functioning in paediatric human immunodeficiency virus infection: effects of combined therapy. Archives of paediatrics & adolescent medicine. 2005;159(7):651–656. doi: 10.1001/archpedi.159.7.651. [DOI] [PubMed] [Google Scholar]
- Skuy M, Taylor M, O'Carroll S, Fridjhon P, Rosenthal L. Performance of black and white South African children on the Wechsler Intelligence Scale for Children—revised and the Kaufman Assessment Battery. Psychological Reports. 2000;86(3):727–737. doi: 10.2466/pr0.2000.86.3.727. [DOI] [PubMed] [Google Scholar]
- Tardieu M, Brunelle F, Raybaud C, Ball W, Barret B, Pautard B, Lachassine E, Mayaaux M, Blanche S. Cerebral MR Imaging in uninfected children born to HIV-seropositive mothers and perinatally exposed to zidovudine. American Journal of Neuroradiology. 2005;26:695–701. [PMC free article] [PubMed] [Google Scholar]
- Tisdall MD, Hess AT, Reuter M, Meintjes EM, Fischl B, Van der Kouwe AJ. Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magnetic Resonance in Medicine. 2012;68(2):389–399. doi: 10.1002/mrm.23228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uban KA, Herting MM, Williams PL, Ajmera T, Gautam P, Yanling H, Malee KM, Yogev R, Csernansky JG, Wang L, Nichols SL, Sowell ER. White matter microstructure among youth with perinatally acquired HIV is associated with disease severity. AIDS (London, England) 2015;29(9):1035. doi: 10.1097/QAD.0000000000000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Arnhem LA, Bunders MJ, Scherpbier HJ, Majoie CBLM, Reneman L, Frinking O, Poll-The BT, Kuijpers TW, Pajkrt D. Neurologic abnormalities in HIV-1 infected children in the era of combination antiretroviral therapy. PLoS ONE. 2013;8(5) doi: 10.1371/journal.pone.0064398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dalen YW, Blokhuis C, Cohen S, Ter Stege JA, Teunissen CE, Kuhle J, Kootstra NA, Scherpbier HJ, Kuijpers TW, Reiss P, Majoie CB. Neurometabolite Alterations Associated With Cognitive Performance in Perinatally HIV-Infected Children. Medicine. 2016;95(12):e3093. doi: 10.1097/MD.0000000000003093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kouwe AJ, Benner T, Salat DH, Fischl B. Brain morphometry with multiecho MPRAGE. NeuroImage. 2008;40(2):559–569. doi: 10.1016/j.neuroimage.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wyhe KS, Water Tvd, Boivin MJ, Cotton MF, Thomas KGF. Cross- cultural assessment of HIV Associated Neurocognitive Impairment using the Kaufman Assessment Battery for Children: A Systematic review. Journal of the International AIDS Society. 2017 doi: 10.7448/IAS.20.1.21412. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigano A, Cerini C, Pattarino G, Fasan S, Zuccotti GV. Metabolic complications associated with antiretroviral therapy in HIV-infected and HIV-exposed uninfected paediatric patients. Expert Opinion on Drug Safety. 2010;9(3):431–445. doi: 10.1517/14740330903579991. [DOI] [PubMed] [Google Scholar]
- Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, Jean-Philippe P, McIntyre JA. Early antiretroviral therapy and mortality among HIV-infected infants. New England Journal of Medicine. 2008;359(21):2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace GL, Robustelli B, Dankner N, Kenworthy L, Giedd JN, Martin A. Increased gyrification, but comparable surface area in adolescents with autism spectrum disorders. Brain. 2013 doi: 10.1093/brain/awt106. awt106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T, Su S, Schmidt M, Kao CY, Sapiro G. The development of gyrification in childhood and adolescence. Brain and cognition. 2010;72(1):36–45. doi: 10.1016/j.bandc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead N, Potterton J, Coovadia A. The neurodevelopment of HIV-infected infants on HAART compared to HIV-exposed but uninfected infants. AIDS Care - Psychological and Socio-Medical Aspects of AIDS/HIV. 2014;26(4):497–504. doi: 10.1080/09540121.2013.841828. [DOI] [PubMed] [Google Scholar]
- Williams VJ, Juranek J, Cirino P, Fletcher JM. Cortical Thickness and Local Gyrification in Children with Developmental Dyslexia. Cerebral Cortex. 2017 doi: 10.1093/cercor/bhx001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmshurst JM, Donald KA, Eley B. Update on the key developments of the neurologic complications in children infected with HIV. Current Opinion in HIV and AIDS. 2014;9(6):533–538. doi: 10.1097/COH.0000000000000101. [DOI] [PubMed] [Google Scholar]
- Working Group on Antiretroviral Therapy and Medical Management of HIV-Infected Children. Guidelines for the Use of Antiretroviral Agents in Paediatric HIV Infection. ART Guideline. 2008:1–126. [Google Scholar]
- World Health Organization (WHO) Antiretroviral therapy of HIV infection in infants and children in resource-limited settings: towards universal access. Recommendations for a public health approach. [accessed 03.11.2016];2006 Retrieved from http://www.who.int/hiv/pub/guidelines/WHOpaediatric.pdf. [PubMed]
- World Health Organization (WHO) Paediatric HIV/ART Care Guideline Group Meeting In. WHO Headquarter; Geneva, Switzerland: 2008. Report of the WHO Technical Reference Group. [Google Scholar]
- Yadav SK, Gupta RK, Garg RK, Venkatesh V, Gupta PK, Singh AK, Hashem S, Al-Sulaiti A, Kaura D, Wang E, Marincola FM, Haris M. Altered structural brain changes and neurocognitive performance in pediatric HIV. NeuroImage: Clinical. 2017;14:316–322. doi: 10.1016/j.nicl.2017.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon U, Fonov VS, Perusse D, Evans AC Brain Development Cooperative Group. The effect of template choice on morphometric analysis of paediatric brain data. NeuroImage. 2009;45(3):769–777. doi: 10.1016/j.neuroimage.2008.12.046. [DOI] [PubMed] [Google Scholar]


