Abstract
Renal recovery after dialysis-requiring acute kidney injury (AKI-D) is an important clinical and patient-centered outcome. Here we examined whether the pre-admission proteinuria level independently influences risk for non-recovery after AKI-D in a community-based population. All adult members of Kaiser Permanente Northern California who experienced AKI-D between January 1, 2009 and September 30, 2015 were included. Pre-admission proteinuria levels were determined by dipstick up to four years before the AKI-D hospitalization and the outcome was renal recovery (survival and dialysis-independence four weeks and more) at 90 days after initiation of renal replacement therapy. We used multivariable logistic regression to adjust for baseline estimated glomerular filtration rate (eGFR), age, sex, ethnicity, short-term predicted risk of death, comorbidities, and medication use. Among 5,347 adults with AKI-D the mean age was 66 years, 59% were men, and 50% were white. Compared with negative/trace proteinuria, the adjusted odds ratios for non-recovery (continued dialysis-dependence or death) were 1.47 (95% confidence interval 1.19–1.82) for 1+ proteinuria and 1.92 (1.54–2.38) for 2+ or more proteinuria. Among survivors, the crude probability of recovery ranged from 83% for negative/trace proteinuria with baseline eGFR over 60 mL/min/1.73m2 to 25% for 2+ or more proteinuria with eGFR 15–29 mL/min/1.73m2. Thus, the pre-AKI-D level of proteinuria is a graded, independent risk factor for non-recovery and helps to improve short-term risk stratification for patients with AKI-D.
Keywords: dialysis-requiring acute kidney injury, renal recovery, proteinuria, risk factor
Introduction
Dialysis-requiring acute kidney injury (AKI-D) is one of the most severe acute medical conditions affecting hospitalized patients1,2 and is independently associated with increased short-term mortality and long-term adverse outcomes.2–5 Some reports indicate that the incidence of AKI-D has recently increased as much as 10% per year nationally.6–10 Meanwhile, in-hospital mortality rates among patients with AKI-D have declined,6,11 suggesting that the overall number of AKI-D survivors is increasing and that outcomes in this vulnerable population deserve more attention. Existing studies among AKI-D survivors have reported that up to one-third remain dialysis-dependent at the time of hospital discharge, of whom 40–80% remain dialysis-dependent at 90 days after initiation of renal replacement therapy (RRT) and are typically deemed to have end-stage renal disease (ESRD).1,12–14
Renal recovery after AKI-D—defined here as return of sufficient native kidney function to come off dialysis—is a critically important clinical and patient-oriented outcome. Although a majority of patients with normal baseline renal function eventually recover enough to come off dialysis if they survive the AKI-D hospitalization,15 many cases of AKI-D represent acute kidney injury (AKI) superimposed on chronic kidney disease (CKD), and these patients may transition directly to ESRD.3,15,16
Predicting the probability of recovery after AKI-D is a common dilemma faced by physicians (including nephrologists, intensivists, and hospitalists), patients, and patients’ families. The ability to predict recovery accurately would help guide decisions regarding dialysis access placement (e.g., temporary versus tunneled catheters during the AKI-D hospitalization, earlier fistula or graft placement after hospital discharge). Improved prognostic abilities would also influence the timing of outpatient dialysis-chair placement (which could affect initial hospital length of stay) and guide appropriate counseling for patients and their families.
However, we currently have limited ability to predict renal recovery in an individual AKI-D patient.12,17 The identification of independent risk factors for non-recovery is the first step toward improving risk stratification after AKI-D. Reduced baseline estimated glomerular filtration rate (eGFR) before an AKI-D episode has been consistently shown to predict non-recovery after AKI-D.3,16 Other selected risk factors that have been implicated include older age, diabetes mellitus, heart failure, and greater comorbidity burden.12,13,18,19
Recently, there has been increasing recognition of the importance of proteinuria – independent of eGFR – as a risk factor for kidney-related adverse outcomes,20–23 and these associations are consistent even with proteinuria determined semi-quantitatively using urine-dipstick assessment.24–28 Whether pre-admission proteinuria level is an independent risk factor for and helps predict non-recovery after AKI-D is not known. We examined these questions in a large, diverse, community-based population to fill this key prognostic knowledge gap.
Results
Cohort assembly and baseline characteristics
We initially identified 13,213 patients who received RRT in the hospital between January 1, 2009 and September 30, 2015. After excluding patients who were on chronic dialysis before the index hospitalization, age <18 years, unknown gender, had <12 consecutive months of membership or drug coverage before the index hospitalization, or had baseline eGFR >150 mL/min/1.73m2, we had a final analytic cohort of 5,347 AKI-D patients (Figure 1).
Figure 1.
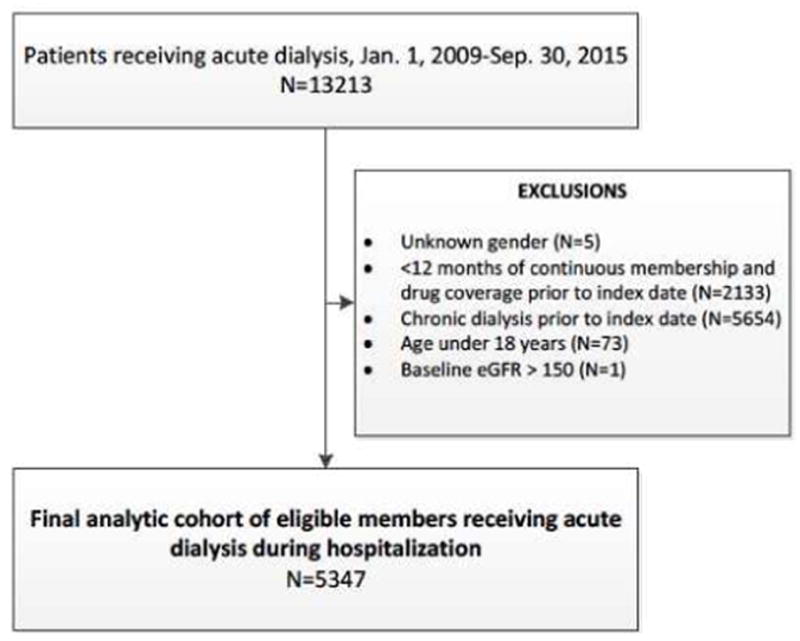
Identification of study cohort containing adults experiencing dialysis-requiring acute kidney injury.
Overall, 3,633 (68%) of eligible patients had pre-admission dipstick proteinuria data available within four years before the AKI-D hospitalization (median 128 days, interquartile range 35 to 390) (Table 1): 876 (24.1%) had negative or trace proteinuria, 930 (25.6%) had 1+ proteinuria, and 1,827 (50.3%) had ≥2+ proteinuria. Compared to patients with negative/trace proteinuria, those with greater proteinuria were more likely to be persons of color, to be current smokers, and to have lower baseline kidney function, hypertension, diabetes, and anemia. Those with greater proteinuria were less likely to have documented mitral/aortic valvular disease, atrial flutter/fibrillation, and cirrhosis.
Table 1.
Baseline characteristics of adults with dialysis-requiring acute kidney injury, stratified by pre-admission proteinuria level. P-values compare results across the four proteinuria groups.
| Characteristic | Overall (N=5347) | Negative/Trace (N=876) | 1+ (N=930) | ≥2+ (N=1827) | Unknown (N=1714) | P-Value |
|---|---|---|---|---|---|---|
| Age, years, Mean (±SD) | 66.2 (±14.0) | 67.9 (±13.6) | 69.0 (±12.9) | 66.1 (±13.3) | 63.8 (±15.1) | <0.001 |
| Male gender, N (%) | 3143 (58.8) | 476 (54.3) | 543 (58.4) | 1079 (59.1) | 1045 (61.0) | <0.05 |
| Self-reported race, N (%) | <0.001 | |||||
| White | 2676 (50.0) | 541 (61.8) | 478 (51.4) | 682 (37.3) | 975 (56.9) | |
| Black/African American | 752 (14.1) | 95 (10.8) | 126 (13.5) | 331 (18.1) | 200 (11.7) | |
| Asian/Pacific Islander | 762 (14.3) | 75 (8.6) | 107 (11.5) | 367 (20.1) | 213 (12.4) | |
| Other/Unknown | 1157 (21.6) | 165 (18.8) | 219 (23.5) | 447 (24.5) | 326 (19.0) | |
| Hispanic ethnicity, N (%) | 962 (18.0) | 140 (16.0) | 179 (19.2) | 374 (20.5) | 269 (15.7) | <0.001 |
| Medical history, N (%) | ||||||
| Acute myocardial infarction | 332 (6.2) | 47 (5.4) | 75 (8.1) | 147 (8.0) | 63 (3.7) | <0.001 |
| Heart failure | 1803 (33.7) | 344 (39.3) | 376 (40.4) | 754 (41.3) | 329 (19.2) | <0.001 |
| Ischemic stroke or TIA | 242 (4.5) | 33 (3.8) | 47 (5.1) | 113 (6.2) | 49 (2.9) | <0.001 |
| Mitral and/or aortic valvular disease | 760 (14.2) | 191 (21.8) | 170 (18.3) | 217 (11.9) | 182 (10.6) | <0.001 |
| Atrial flutter or fibrillation | 1050 (19.6) | 259 (29.6) | 236 (25.4) | 319 (17.5) | 236 (13.8) | <0.001 |
| Coronary artery bypass graft surgery | 99 (1.9) | 22 (2.5) | 16 (1.7) | 39 (2.1) | 22 (1.3) | 0.11 |
| Percutaneous coronary intervention | 243 (4.5) | 39 (4.5) | 49 (5.3) | 99 (5.4) | 56 (3.3) | <0.05 |
| Diabetes mellitus | 3031 (56.7) | 459 (52.4) | 566 (60.9) | 1392 (76.2) | 614 (35.8) | <0.001 |
| Hypertension | 4398 (82.3) | 716 (81.7) | 818 (88.0) | 1732 (94.8) | 1132 (66.0) | <0.001 |
| Cirrhosis | 389 (7.3) | 106 (12.1) | 78 (8.4) | 49 (2.7) | 156 (9.1) | <0.001 |
| Chronic lung disease | 1629 (30.5) | 335 (38.2) | 296 (31.8) | 559 (30.6) | 439 (25.6) | <0.001 |
| Smoking status, N (%) | <0.001 | |||||
| Current Smoker | 490 (9.2) | 48 (5.5) | 70 (7.5) | 876 (47.9) | 215 (12.5) | |
| Former smoker | 2294 (42.9) | 423 (48.3) | 436 (46.9) | 794 (43.5) | 641 (37.4) | |
| Nonsmoker | 2563 (47.9) | 405 (46.2) | 424 (45.6) | 157 (8.6) | 858 (50.1) | |
| Medication use, N (%) | ||||||
| ACE inhibitor | 1545 (28.9) | 240 (27.4) | 299 (32.2) | 458 (25.1) | 548 (32.0) | <0.001 |
| Angiotensin II receptor blocker | 830 (15.5) | 156 (17.8) | 170 (18.3) | 319 (17.5) | 185 (10.8) | <0.001 |
| Beta blocker | 2957 (55.3) | 495 (56.5) | 574 (61.7) | 1203 (65.8) | 685 (40.0) | <0.001 |
| Calcium channel blocker | 1957 (36.6) | 218 (24.9) | 356 (38.3) | 1059 (58.0) | 324 (18.9) | <0.001 |
| Diuretic | 3294 (61.6) | 601 (68.6) | 616 (66.2) | 1273 (69.7) | 804 (46.9) | <0.001 |
| Alpha blocker | 1028 (19.2) | 145 (16.6) | 188 (20.2) | 540 (29.6) | 155 (9.0) | <0.001 |
| Aldosterone receptor antagonist | 442 (8.3) | 135 (15.4) | 83 (8.9) | 70 (3.8) | 154 (9.0) | <0.001 |
| Nitrate | 832 (15.6) | 147 (16.8) | 166 (17.8) | 380 (20.8) | 139 (8.1) | <0.001 |
| Statin | 3049 (57.0) | 487 (55.6) | 566 (60.9) | 1236 (67.7) | 760 (44.3) | <0.001 |
| Non-steroidal anti-inflammatory drug | 360 (6.7) | 61 (7.0) | 59 (6.3) | 60 (3.3) | 180 (10.5) | <0.001 |
| Short Term Mortality Score50, N (%) | <0.001 | |||||
| <0.001 | 20 (0.4) | 2 (0.2) | 1 (0.1) | 1 (0.1) | 16 (0.9) | |
| 0.001–0.005 | 178 (3.3) | 23 (2.6) | 28 (3.0) | 50 (2.7) | 77 (4.5) | |
| 0.005–0.02 | 982 (18.4) | 127 (14.5) | 148 (15.9) | 434 (23.8) | 273 (15.9) | |
| 0.02–0.05 | 1190 (22.3) | 189 (21.6) | 192 (20.6) | 503 (27.5) | 306 (17.9) | |
| 0.05–0.10 | 748 (14.0) | 160 (18.3) | 143 (15.4) | 247 (13.5) | 198 (11.6) | |
| 0.10–0.15 | 349 (6.5) | 65 (7.4) | 68 (7.3) | 105 (5.7) | 111 (6.5) | |
| 0.15–0.30 | 417 (7.8) | 65 (7.4) | 83 (8.9) | 118 (6.5) | 151 (8.8) | |
| >=0.30 | 454 (8.5) | 75 (8.6) | 85 (9.1) | 98 (5.4) | 196 (11.4) | |
| Body mass index, kg/m2, Mean (±SD) | 30.8 (±8.3) | 30.6 (±8.3) | 30.4 (±8.2) | 31.2 (±8.3) | 30.6 (±8.3) | 0.05 |
| Systolic blood pressure, mmHg, Mean (±SD) | 128.5 (±22.7) | 120.9 (±21.1) | 124.2 (±20.7) | 138.0 (±23.7) | 124.2 (±19.8) | <0.001 |
| Estimated glomerular filtration rate, mL/min/1.73m2 | ||||||
| Median (IQR) | 30.3 (14.3–60.0) | 42.6 (24.6–70.5) | 31.1 (15.6–53.6) | 14.9 (9.9–25.3) | 58.8 (35.8–83.2) | <0.001 |
| Missing or prior renal transplant, N (%) | 611 (11.4) | 52 (5.9) | 47 (5.1) | 89 (4.9) | 423 (24.7) | |
| Serum creatinine, mg/dL, Mean (±SD) | 2.8 (±2.4) | 1.9 (±1.4) | 2.6 (±2.1) | 4.1 (±2.7) | 1.8 (±2.0) | <0.001 |
| Hemoglobin, g/L, Mean (±SD) | 11.2 (±2.1) | 11.4 (±2.0) | 11.1 (±2.0) | 10.6 (±1.9) | 12.1 (±2.3) | <0.001 |
Outcomes after AKI-D by proteinuria level
In the overall cohort, 3,601 (67.3%) were classified as non-recovery at 90 days after initiation of acute RRT. Among those who did not recover, 1,542 died while on dialysis or within 28 days of stopping dialysis.
The crude risk for non-recovery was higher with greater pre-admission proteinuria level: 57.8% (95% CI: 54.5–61.0%) for negative/trace, 68.8% (95% CI: 65.8–71.8%) for 1+, and 81.0% (95% CI: 79.2–82.8%) for ≥2+ (Table 2 shows results further stratified by eGFR). In multivariable analyses, compared with negative/trace proteinuria, higher level of pre-admission proteinuria was associated with a graded, increased adjusted odds of non-recovery, even after accounting for potential differences in demographic characteristics, baseline eGFR, comorbidities, short-term predicted mortality,29 and pre-admission medication use (Figure 2, Supplementary Table 1). The addition of proteinuria to our multivariable model significantly improved the discrimination of non-recovery after AKI-D, as demonstrated by changes in the C-statistic, net reclassification improvement, and integrated discrimination improvement (Table 3).
Table 2.
Crude probabilities (% risk [95% CI]) of non-recovery, stratified by pre-admission proteinuria level and baseline estimated glomerular filtration rate (eGFR).
| eGFR (mL/min/1.73m2) | Pre-admission Proteinuria Level | ||
|---|---|---|---|
|
| |||
| Negative/Trace | 1+ | ≥2+ | |
| 60–150 | 46.6 (40.6 – 52.6) | 55.2 (47.8 – 62.6) | 53.1 (43.9 – 62.3) |
| 45–59 | 48.0 (39.2 – 56.8) | 64.1 (55.8 – 72.4) | 48.7 (37.6 – 59.8) |
| 30–44 | 55.8 (48.0 – 63.6) | 63.6 (56.0 – 71.2) | 55.4 (47.7 – 63.1) |
| 15–29 | 68.7 (62.2 – 75.2) | 75.8 (70.1 – 81.5) | 80.1 (76.6 – 83.6) |
Figure 2.
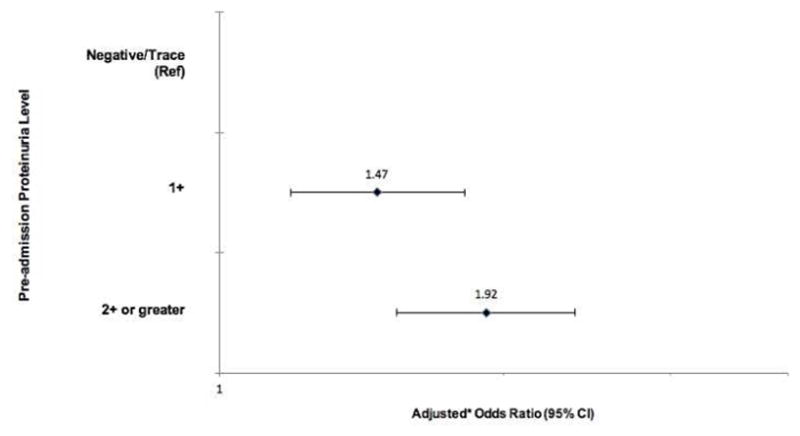
Multivariable-adjusted* association of pre-admission proteinuria level and non-recovery after dialysis-requiring acute kidney injury.
Table 3.
Discrimination metrics for proteinuria in multivariable logistic prediction model for 90-day non-recovery after dialysis-requiring acute kidney injury.
| Statistic | Improvement (95% CI) | P-value | |
|---|---|---|---|
| C-statistic with proteinuria | 0.769 | 0.005 (0.002, 0.008) | 0.0035 |
| C-statistic without proteinuria | 0.764 | ||
| Net reclassification improvement (NRI) | 0.32 (0.26, 0.37) | <0.0001 | |
| Integrated discrimination improvement (IDI) | 0.008 (0.005, 0.01) | <0.0001 |
In a sensitivity analysis that excluded patients with the highest 20% predicted risk of short-term death, similar findings were observed: adjusted odds ratios were 1.42 (95% CI: 1.12–1.79) for 1+ proteinuria and 2.06 (95% CI: 1.62–2.61) for ≥2+ proteinuria as compared with negative/trace proteinuria. We also performed another sensitivity analysis including only patients who had dipstick proteinuria measured within one year of the index hospitalization and obtained similar results: compared to negative/trace proteinuria, adjusted odds ratios were 1.33 (95% CI: 1.12–1.57) and 1.74 (95% CI: 1.48–2.06) for the 1+ and ≥2+ proteinuria groups, respectively.
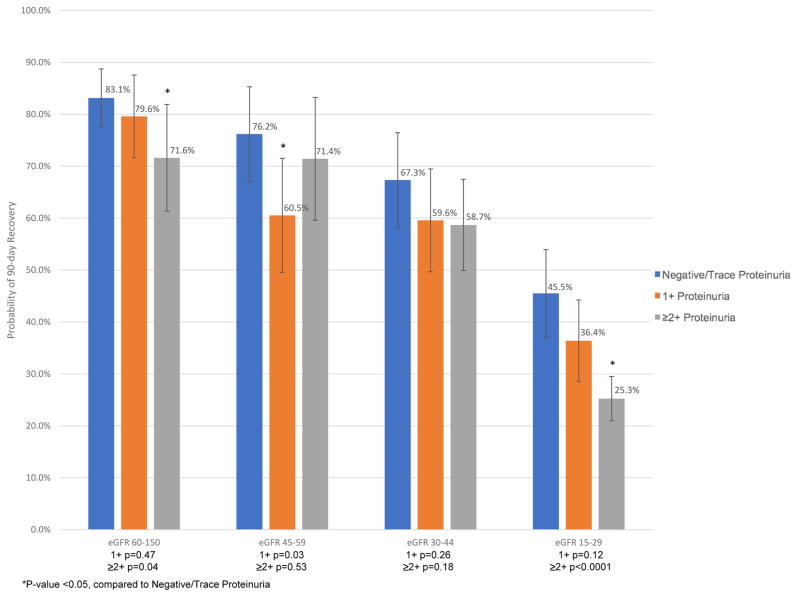
Finally, crude probabilities for recovery at 90 days among survivors showed an overall trend toward decreasing probability of recovery with lower baseline eGFR and more baseline proteinuria (Figure 3, Supplementary Table 2). For example, among AKI-D survivors, the probability of recovery for those with negative/trace proteinuria was 83.1% among patients with eGFR >60 mL/min/1.73m2 but only 45.5% among those with eGFR 15–29 mL/min/1.73m2 (Figure 3). Furthermore, for patients with eGFR 15–29 mL/min/1.73m2, probability of recovery was 45.5% for those with negative/trace proteinuria, 36.4% for 1+ proteinuria, and 25.3% for ≥2+ proteinuria (Figure 3). Overall, across all eGFR categories, the adjusted odds ratio for survival with non-recovery (i.e., alive but remained dialysis-dependent) in those with ≥2+ proteinuria compared to negative/trace proteinuria was 2.22 [95% CI 1.72–2.88] (Table 4).
Figure 3.
Crude probability of recovery conditional on survival, by baseline estimated glomerular filtration rate (eGFR, mL/min/1.73m2) and pre-admission proteinuria level. P-values for pair-wise comparisons to the negative/trace proteinuria group within each eGFR category are shown.
Table 4.
Multivariable-adjusted* association of pre-admission proteinuria level and non-recovery after dialysis-requiring acute kidney injury, by vital status at 90 days after initiation of acute renal replacement therapy.
| Pre-admission Proteinuria | Adjusted* Odds Ratio (95% Confidence Interval) | |
|---|---|---|
| Non-recovery at 90 days, Dead | Non-recovery at 90 days, Alive | |
| Negative/Trace | Reference | Reference |
| 1+ | 1.38 (1.05–1.81) | 1.50 (1.14–1.96) |
| 2+ or greater | 1.37 (1.03–1.83) | 2.22 (1.72–2.88) |
Adjusted for baseline: estimated glomerular filtration rate, age, gender, race, ethnicity, smoking status, pre-existing co-morbidities (heart failure, coronary heart disease, prior ischemic stroke or transient ischemic attack, peripheral arterial disease, atrial flutter or fibrillation, mitral or aortic valvular disease, venous thromboembolism, hypertension, diabetes mellitus, dyslipidemia, prior gastrointestinal bleed requiring hospitalization, thyroid disease, cirrhosis, chronic lung disease, dementia, depression), body mass index, short-term mortality score, pre-admission systolic blood pressure, pre-admission high-density and low-density lipoprotein levels, pre-admission hemoglobin level, pre-admission medication use (angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, β-blockers, calcium channel blockers, diuretics, aldosterone receptor antagonists, alpha blockers, antiarrhythmic agents, nitrates, other vasodilators, non-aspirin antiplatelet agents, low-molecular-weight heparin, statins, other lipid-lowering agents, anti-diabetic agents, non-steroidal anti-inflammatory drugs), most recent inpatient hemoglobin before acute RRT initiation, and most recent inpatient platelet count before acute RRT initiation.
Discussion
To our knowledge, our study is the first to demonstrate that severity of proteinuria before an episode of AKI-D is a graded, independent predictor of subsequent non-recovery, even after adjustment for other potential explanatory factors, including baseline eGFR, age, gender, race, and other comorbidities as well as short-term predicted risk of death. We also found that proteinuria significantly improves the discrimination of non-recovery after AKI-D.
We found that more than two thirds of patients did not recover after AKI-D, which is consistent with previously reported estimates.12,13,30,31 One recent single-center study of critically ill septic patients with AKI (n=136 for AKI-D) found that dipstick albuminuria measured within 72 hours after intensive care unit admission was independently associated with lower rates of recovery (defined as return of serum creatinine to <1.5 times baseline) at 30 days post-discharge.32 However, since proteinuria was measured during the index AKI-D hospitalization, the albuminuria detected may be a result of acute medical conditions such as trauma, febrile illness, and/or sepsis,33–36 and may not represent baseline kidney function status. Interpretation of these results is further clouded by the fact that AKI itself often results in increased proteinuria as a manifestation of acute parenchymal damage.37–39
To further aid physicians in counseling AKI-D patients and their families about the chances for coming off dialysis, we present observed probabilities of recovery conditional on surviving at 90 days. Reliable prognostic information is important to allow shared decisions about what care to provide, and the clinical relevance of our findings is more pronounced when considering only survivors (Figure 3). In our study, crude recovery probabilities for those with baseline eGFR >45 mL/min/1.73m2, eGFR 30–45 mL/min/1.73m2, and eGFR 15–29 mL/min/1.73m2 were generally consistent with estimates reported in prior studies,3,15,16 although there are important differences in the study populations analyzed and methods: prior publications were based on data more than a decade older than that in our study and the timeline for assessment of recovery differed for previous studies, which used the end of hospitalization15 or 30 days after hospital discharge3,16. We note that taking into account baseline proteinuria allowed further refinement of risk stratification, particularly for those with eGFR 15–29 mL/min/1.73m2 (the largest eGFR subgroup). The chances of recovery among survivors in this eGFR stratum was previously reported to be 37%.16 Here, we note that chances of recovery among survivors actually ranged from 45.5% for those with negative/trace proteinuria to 25.3% for those with ≥2+ proteinuria.
While many previous studies of AKI-D have focused on what happens during the index hospitalization, including identification of inpatient risk factors for developing AKI-D and subsequent complications,12,13,18,19,31,40 few studies have examined the association between pre-admission patient characteristics and important outcomes among AKI-D patients. One of our study’s strengths was that it included comprehensive information on non-recovery based on a regional health system ESRD Treatment Registry3,16,24,41,42 that allowed for integration of both inpatient and outpatient (both pre- and post-hospital admission) data. Our study had other strengths. We tracked a large, contemporary sample of >5,000 adults with AKI-D with broad demographic diversity, and the majority had pre-admission dipstick proteinuria levels. In addition, in contrast to prior studies that only studied critically ill patients, our cohort also included hospitalized patients not in intensive care units who experienced AKI-D in 21 medical centers in Northern California, which increases the generalizability of our findings, especially given that KPNC members are highly representative of the Northern California and statewide population.43 Our approach of anchoring outcomes from time of RRT initiation enhances our results’ generalizability, since timing of hospital discharge may be affected by social and systems-based factors unrelated to the natural history of AKI-D.
Limitations of our study included that we did not have uniform capture of pre-admission proteinuria or eGFR on all study participants, but we believe this situation is typical for “real-world” clinical practice and thus does not detract from external validity. Although all patients were included in all analyses, parameter estimates for those with missing proteinuria data were not shown, as it is not clear how best to interpret outcomes in those patients. While we relied on electronic medical records for key data elements, the KPNC data sources used are much more comprehensive than administrative databases and allow for thorough characterization of individual patients.42,44–54 Although use of urine protein-to-creatinine ratio (or albumin-to-creatinine ratio) would be a more accurate quantitative measure for proteinuria than dipstick assessment, these measurements are performed much less often than dipstick measurements.21 Our findings extend the literature linking dipstick proteinuria to a broad range of kidney-related adverse outcomes.21,55,56 Another limitation was that we did not have all the clinical details surrounding the AKI-D admissions, such as etiology of baseline CKD and indication(s) for RRT initiation (e.g., hyperkalemia, volume overload). While prior studies suggest that sepsis, surgery, vasopressor use, and mechanical ventilation may influence recovery after AKI,57,58 these clinical features are not well-established risk factors for non-recovery after AKI-D as compared with other characteristics like baseline eGFR, for example. Etiology of AKI-D was also not available in our dataset, but prior evaluation of KPNC medical records showed that almost all AKI-D cases were due to causes consistent with a diagnosis of acute tubular necrosis (such as sepsis, hypotension, and nephrotoxins); only rarely were cases due to other causes such as glomerulonephritis.3,16,59 Our results regarding outcomes among those with eGFR <15 ml/min/1.73m2 should be interpreted with caution, since at such low levels of kidney function, it is difficult to distinguish between true superimposed AKI-D versus progression of underlying CKD to the point of needing RRT. Finally, our results should be validated in other comparable datasets.
In sum, leading AKI opinion leaders and guidelines have identified renal recovery after AKI-D as a research area of “serious unmet need, with great potential to benefit patients.”12,60 Identification of risk factors for non-recovery is crucial for refining our abilities to predict recovery, appropriately counsel patients, and improve outcomes for this vulnerable population. Determining which patients have a reasonable chance of recovery is important so that their medical treatment can be appropriately tailored to maximize chances of recovery. For example, there is general consensus that hemodynamic instability associated with excessive fluid removal on hemodialysis delays or prevents renal recovery.60 It is therefore particularly important that intra-dialytic hypotension be avoided in AKI-D patients, who will be increasingly discharged to chronic hemodialysis units in the U.S. as a result of recent Medicare reimbursement policy changes. Before 2017, Medicare did not reimburse free-standing chronic hemodialysis facilities for dialysis services provided to AKI-D patients, which limited the available dialysis options for AKI-D patients otherwise ready for hospital discharge.61 The Trade Preferences Extension Act of 2015 recently extended Medicare reimbursement to chronic hemodialysis facilities that dialyze AKI-D patients beginning January 1, 2017. This timely policy change will allow more AKI-D patients to receive outpatient dialysis more easily. However, personnel in chronic hemodialysis units may not be used to caring for AKI-D patients, and several treatment approaches designed for ESRD patients (such as aggressive stepwise increases in fluid removal as a method to control blood pressure)62 may not be appropriate for AKI-D patients who may recover. Finally, identifying AKI-D patients with a reasonable chance of recovery would be important to facilitate targeted enrollment into future clinical trials testing interventions to promote faster and more complete recovery of kidney function.
Methods
Source population
The source population was based within Kaiser Permanente Northern California (KPNC), a large, integrated health care delivery system that currently provides comprehensive care for >4.1 million members. The KPNC membership is highly representative of the local surrounding and statewide population.43 Nearly all aspects of care – including complete laboratory and RRT data – are captured through KPNC’s electronic medical record system.
This study was approved by institutional review boards at KPNC and the University of California, San Francisco. A waiver of informed consent was obtained given the nature of the study.
Study sample
We conducted a retrospective cohort study of all adult (age ≥18 years) KPNC members who developed AKI-D between January 1, 2009 and September 30, 2015 and who had ≥12 consecutive months of health plan membership and pharmacy benefits before the index hospitalization in order to ensure adequate capture of relevant comorbidities, laboratory tests, and prescription medication use. We identified cases of AKI-D as having receipt of acute RRT during hospitalization in the absence of any pre-admission chronic RRT, which was ascertained through a comprehensive KPNC ESRD Treatment Registry that tracks initiation and cessation of RRT treatments and date(s) of renal transplantation.3,16,24,41,42 RRT included receipt of acute peritoneal dialysis, hemodialysis, and hemofiltration that were identified by International Classification of Diseases, Ninth Revision (ICD-9) procedure codes (54.98, 39.95) and Current Procedural Terminology codes (90935, 90937, 90945, 90947, 90999). We previously demonstrated the accuracy of these codes for AKI-D across the spectrum of pre-admission eGFR based on adjudication of medical records by a board-certified KPNC nephrologist in a random sample of 100 patients (positive predictive value 94%).10
Assessment of pre-admission proteinuria level and baseline estimated glomerular filtration rate
The primary predictor was pre-admission proteinuria level, as reflected by the most recent outpatient dipstick urinalysis result within four years before the AKI-D hospitalization. The “negative” (laboratory result 0 mg/dL) and “trace” (laboratory result <30 mg/dL) proteinuria groups were combined as a reference group and compared to the 1+ and ≥2+ proteinuria groups. Patients for whom pre-admission proteinuria level was not available were grouped and included in analysis as “unknown proteinuria.” Baseline eGFR was calculated using the CKD-EPI equation63 based on the most recent outpatient, non-emergency-department serum creatinine measurement within one year before the AKI-D hospitalization.24 Patients who had a previous renal transplant were included and analyzed as a “transplant” group.
Non-recovery after AKI-D
The primary outcome (chosen a priori) was non-recovery of kidney function after an episode of AKI-D. Recovery was defined as being alive and no longer needing RRT for ≥4 consecutive weeks at 90 days after initiation of acute RRT. We used status at 90 days because conventionally, patients are considered as having ESRD if they remain dialysis-dependent for at least 90 days.12 We chose to define recovery such that it could occur during the initial AKI-D hospitalization or in the outpatient setting after discharge to capture relevant outcomes comprehensively. We anchored our analysis based on the date of acute RRT initiation (rather than hospital discharge or some other date) to link it more closely to the natural history of the AKI episode rather than other extraneous factors. We required that patients be alive and off dialysis for ≥4 weeks to reduce misclassification of people who stopped dialysis due to withdrawal of care.
Covariates
Demographic characteristics (age, gender, self-reported race/ethnicity) were obtained from health plan databases.64–66 Relevant comorbidities were defined by diagnostic or procedure ICD-9 codes and supplemented with laboratory test results (e.g., blood glucose), outpatient vital signs, and/or prescribed medications using electronic health record-based data that was cleaned and linked at the individual-patient level into the Kaiser Permanente Virtual Data Warehouse as previously described and validated.42,44–54,67 Patient vital status was based on comprehensive information from health plan administrative and clinical databases, member proxy reporting, Social Security Administration vital status files, and California state death certificate information.68,69
Statistical approach
All analyses were conducted using SAS, version 9.3 (Cary, N.C.). Baseline characteristics were compared across pre-admission proteinuria levels using ANOVA for continuous variables and χ2 tests for categorical variables. We calculated the crude risk (and associated 95% confidence limits) for non-recovery after RRT initiation by pre-admission proteinuria level.
We next conducted a multivariable logistic regression model evaluating pre-admission proteinuria level and non-recovery, with adjustment for baseline eGFR, age, gender, race, ethnicity, smoking status, pre-existing comorbidities (heart failure, coronary heart disease, prior ischemic stroke, peripheral arterial disease, atrial fibrillation, mitral or aortic valvular disease, venous thromboembolism, hypertension, diabetes mellitus, dyslipidemia, prior gastrointestinal bleed requiring hospitalization, thyroid disease, cirrhosis, chronic lung disease, dementia, and depression), body mass index, short-term mortality score,29 pre-admission systolic blood pressure, pre-admission high-density and low-density lipoprotein levels, pre-admission hemoglobin level, pre-admission medication use (angiotensin converting enzyme [ACE] inhibitors, angiotensin II receptor blockers [ARBs], β-blockers, calcium channel blockers, diuretics, aldosterone receptor antagonists, alpha blockers, antiarrhythmic agents, nitrates, other vasodilators, non-aspirin antiplatelet agents, low-molecular-weight heparin, statins, other lipid-lowering agents, anti-diabetic agents, and non-steroidal anti-inflammatory drugs), and most recent inpatient hemoglobin level and platelet count before acute RRT initiation.
We assessed the incremental prognostic value of incorporating proteinuria into our logistic regression model by calculating change in C-statistic, net reclassification improvement, and integrated discrimination improvement metrics.
We conducted a sensitivity analysis by excluding the patients with the highest 20% predicted short-term mortality risk (calculated using a validated inpatient mortality scoring system derived and validated within the KPNC population29) to ensure that the association between pre-admission proteinuria level and non-recovery after AKI-D was not primarily driven by excess mortality. We also conducted a separate sensitivity analysis that included only patients who had dipstick proteinuria assessed within one year of the index hospitalization.
Finally, we ascertained probabilities for renal recovery among those who survived until ≥90 days after AKI-D, stratified by baseline eGFR and pre-admission proteinuria level. We also conducted multivariable polytomous logistic regression for a multi-level composite outcome of survival and non-recovery.
Supplementary Material
Acknowledgments
Sources of Support: This study was supported by the UCSF-Kaiser Permanente Northern California Grants Program for Fellows (BJL, ASG, CYH) and NIH-NIDDK Grants T32DK007219 (BJL), F32DK115030 (BJL), K24DK92291 (CYH), K23DK100468 (RKH), K24113381 (KDL) and R01DK101507 (KDL, ASG, CYH).
We thank Charles E. McCulloch, PhD, for helpful statistical discussions.
Footnotes
Disclosure
The authors of this manuscript have no relevant disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 2.Metnitz PG, Krenn CG, Steltzer H, et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002;30(9):2051–2058. doi: 10.1097/00003246-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Lo LJ, Go AS, Chertow GM, et al. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney international. 2009;76(8):893–899. doi: 10.1038/ki.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu VC, Shiao CC, Chang CH, et al. Long-term outcomes after dialysis-requiring acute kidney injury. Biomed Res Int. 2014;2014:365186. doi: 10.1155/2014/365186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu VC, Wu PC, Wu CH, et al. The impact of acute kidney injury on the long-term risk of stroke. J Am Heart Assoc. 2014;3(4) doi: 10.1161/JAHA.114.000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu CY. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol. 2013;24(1):37–42. doi: 10.1681/ASN.2012080800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wald R, McArthur E, Adhikari NK, et al. Changing incidence and outcomes following dialysis-requiring acute kidney injury among critically ill adults: a population-based cohort study. Am J Kidney Dis. 2015;65(6):870–877. doi: 10.1053/j.ajkd.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 8.Kolhe NV, Muirhead AW, Wilkes SR, Fluck RJ, Taal MW. National trends in acute kidney injury requiring dialysis in England between 1998 and 2013. Kidney international. 2015;88(5):1161–1169. doi: 10.1038/ki.2015.234. [DOI] [PubMed] [Google Scholar]
- 9.Siew ED, Davenport A. The growth of acute kidney injury: a rising tide or just closer attention to detail? Kidney international. 2015;87(1):46–61. doi: 10.1038/ki.2014.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu CY, McCulloch CE, Fan D, Ordonez JD, Chertow GM, Go AS. Community-based incidence of acute renal failure. Kidney international. 2007;72(2):208–212. doi: 10.1038/sj.ki.5002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM. Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol. 2006;17(4):1143–1150. doi: 10.1681/ASN.2005091017. [DOI] [PubMed] [Google Scholar]
- 12.Cerda J, Liu KD, Cruz DN, et al. Promoting kidney function recovery in patients with AKI requiring RRT. Clinical journal of the American Society of Nephrology: CJASN. 2015;10(10):1859–1867. doi: 10.2215/CJN.01170215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hickson LJ, Chaudhary S, Williams AW, et al. Predictors of outpatient kidney function recovery among patients who initiate hemodialysis in the hospital. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2015;65(4):592–602. doi: 10.1053/j.ajkd.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chertow GM, Christiansen CL, Cleary PD, Munro C, Lazarus JM. Prognostic stratification in critically ill patients with acute renal failure requiring dialysis. Arch Intern Med. 1995;155(14):1505–1511. [PubMed] [Google Scholar]
- 15.Schiffl H. Renal recovery from acute tubular necrosis requiring renal replacement therapy: a prospective study in critically ill patients. Nephrol Dial Transplant. 2006;21(5):1248–1252. doi: 10.1093/ndt/gfk069. [DOI] [PubMed] [Google Scholar]
- 16.Hsu CY, Chertow GM, McCulloch CE, Fan D, Ordonez JD, Go AS. Nonrecovery of kidney function and death after acute on chronic renal failure. Clinical journal of the American Society of Nephrology: CJASN. 2009;4(5):891–898. doi: 10.2215/CJN.05571008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P Acute Dialysis Quality Initiative w. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bagshaw SM, Laupland KB, Doig CJ, et al. Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: a population-based study. Crit Care. 2005;9(6):R700–709. doi: 10.1186/cc3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craven AM, Hawley CM, McDonald SP, Rosman JB, Brown FG, Johnson DW. Predictors of renal recovery in Australian and New Zealand end-stage renal failure patients treated with peritoneal dialysis. Perit Dial Int. 2007;27(2):184–191. [PubMed] [Google Scholar]
- 20.Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79(12):1331–1340. doi: 10.1038/ki.2010.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303(5):423–429. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 22.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu RK, Hsu CY. Proteinuria and reduced glomerular filtration rate as risk factors for acute kidney injury. Curr Opin Nephrol Hypertens. 2011;20(3):211–217. doi: 10.1097/MNH.0b013e3283454f8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu CY, Ordonez JD, Chertow GM, Fan D, McCulloch CE, Go AS. The risk of acute renal failure in patients with chronic kidney disease. Kidney international. 2008;74(1):101–107. doi: 10.1038/ki.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grams ME, Astor BC, Bash LD, Matsushita K, Wang Y, Coresh J. Albuminuria and estimated glomerular filtration rate independently associate with acute kidney injury. Journal of the American Society of Nephrology. 2010;21:1757–1764. doi: 10.1681/ASN.2010010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang TM, Wu VC, Young GH, et al. Preoperative proteinuria predicts adverse renal outcomes after coronary artery bypass grafting. Journal of the American Society of Nephrology. 2010;22:156–163. doi: 10.1681/ASN.2010050553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James MT, Grams ME, Woodward M, et al. A Meta-analysis of the association of estimated GFR, albuminuria, diabetes mellitus, and hypertension with acute kidney injury. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2015;66(4):602–612. doi: 10.1053/j.ajkd.2015.02.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grams ME, Sang Y, Ballew SH, et al. A Meta-analysis of the association of estimated GFR, albuminuria, age, race, and sex with acute kidney injury. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2015;66(4):591–601. doi: 10.1053/j.ajkd.2015.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Escobar GJ, Gardner MN, Greene JD, Draper D, Kipnis P. Risk-adjusting hospital mortality using a comprehensive electronic record in an integrated health care delivery system. Med Care. 2013;51(5):446–453. doi: 10.1097/MLR.0b013e3182881c8e. [DOI] [PubMed] [Google Scholar]
- 30.Gautam SC, Brooks CH, Balogun RA, Xin W, Ma JZ, Abdel-Rahman EM. Predictors and Outcomes of Post-Hospitalization Dialysis Dependent Acute Kidney Injury. Nephron. 2015;131(3):185–190. doi: 10.1159/000441607. [DOI] [PubMed] [Google Scholar]
- 31.Heung M, Wolfgram DF, Kommareddi M, Hu Y, Song PX, Ojo AO. Fluid overload at initiation of renal replacement therapy is associated with lack of renal recovery in patients with acute kidney injury. Nephrol Dial Transplant. 2012;27(3):956–961. doi: 10.1093/ndt/gfr470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neyra JA, Li X, Yessayan L, et al. Dipstick albuminuria and acute kidney injury recovery in critically ill septic patients. Nephrology (Carlton) 2016;21(6):512–518. doi: 10.1111/nep.12637. [DOI] [PubMed] [Google Scholar]
- 33.Marks MI, McLaine PN, Drummond KN. Proteinuria in children with febrile illnesses. Arch Dis Child. 1970;45(240):250–253. doi: 10.1136/adc.45.240.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alpert HC, Lohavichan C, Presser JI, Papper S. “Febrile” proteinuria. South Med J. 1974;67(5):552–554. doi: 10.1097/00007611-197405000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Richmond JM, Sibbald WJ, Linton AM, Linton AL. Patterns of urinary protein excretion in patients with sepsis. Nephron. 1982;31(3):219–223. doi: 10.1159/000182650. [DOI] [PubMed] [Google Scholar]
- 36.Gosling P, Sutcliffe AJ. Proteinuria following trauma. Ann Clin Biochem. 1986;23(Pt 6):681–685. doi: 10.1177/000456328602300610. [DOI] [PubMed] [Google Scholar]
- 37.Zappitelli M, Coca SG, Garg AX, et al. The association of albumin/creatinine ratio with postoperative AKI in children undergoing cardiac surgery. Clinical journal of the American Society of Nephrology: CJASN. 2012;7(11):1761–1769. doi: 10.2215/CJN.12751211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsu RK, Hsu CY. We can diagnose AKI “early”. Clinical journal of the American Society of Nephrology: CJASN. 2012;7(11):1741–1742. doi: 10.2215/CJN.09740912. [DOI] [PubMed] [Google Scholar]
- 39.Molnar AO, Parikh CR, Sint K, et al. Association of postoperative proteinuria with AKI after cardiac surgery among patients at high risk. Clinical journal of the American Society of Nephrology: CJASN. 2012;7(11):1749–1760. doi: 10.2215/CJN.13421211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franzen D, Rupprecht C, Hauri D, Bleisch JA, Staubli M, Puhan MA. Predicting outcomes in critically ill patients with acute kidney injury undergoing intermittent hemodialysis--a retrospective cohort analysis. Int J Artif Organs. 2010;33(1):15–21. [PubMed] [Google Scholar]
- 41.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA: the journal of the American Medical Association. 2002;287(19):2519–2527. doi: 10.1001/jama.287.19.2519. [DOI] [PubMed] [Google Scholar]
- 42.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002;287(19):2519–2527. doi: 10.1001/jama.287.19.2519. [DOI] [PubMed] [Google Scholar]
- 43.Gordon NP. Characteristics of Adult Health Plan Members in the Northern California Region Membership, as Estimated from the 2011 Member Health Survey. Division of Research, Kaiser Permanente Medical Care Program; Oakland, CA: 2013. [Google Scholar]
- 44.Go AS, Hylek EM, Borowsky LH, Phillips KA, Selby JV, Singer DE. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Annals of internal medicine. 1999;131(12):927–934. doi: 10.7326/0003-4819-131-12-199912210-00004. [DOI] [PubMed] [Google Scholar]
- 45.Go AS, Lee WY, Yang J, Lo JC, Gurwitz JH. Statin therapy and risks for death and hospitalization in chronic heart failure. JAMA. 2006;296(17):2105–2111. doi: 10.1001/jama.296.17.2105. [DOI] [PubMed] [Google Scholar]
- 46.Go AS, Iribarren C, Chandra M, et al. Statin and beta-blocker therapy and the initial presentation of coronary heart disease. Annals of internal medicine. 2006;144(4):229–238. doi: 10.7326/0003-4819-144-4-200602210-00004. [DOI] [PubMed] [Google Scholar]
- 47.Go AS, Yang J, Ackerson LM, et al. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation. 2006;113(23):2713–2723. doi: 10.1161/CIRCULATIONAHA.105.577577. [DOI] [PubMed] [Google Scholar]
- 48.Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Annals of internal medicine. 2009;151(5):297–305. doi: 10.7326/0003-4819-151-5-200909010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silverberg MJ, Leyden W, Hurley L, et al. Response to newly prescribed lipid-lowering therapy in patients with and without HIV infection. Annals of internal medicine. 2009;150(5):301–313. doi: 10.7326/0003-4819-150-5-200903030-00006. [DOI] [PubMed] [Google Scholar]
- 50.Go AS, Fang MC, Udaltsova N, et al. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation. 2009;119(10):1363–1369. doi: 10.1161/CIRCULATIONAHA.108.816082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Go AS, Yang J, Gurwitz JH, Hsu J, Lane K, Platt R. Comparative effectiveness of different beta-adrenergic antagonists on mortality among adults with heart failure in clinical practice. Arch Intern Med. 2008;168(22):2415–2421. doi: 10.1001/archinternmed.2008.506. [DOI] [PubMed] [Google Scholar]
- 52.Lo JC, Go AS, Chandra M, Fan D, Kaysen GA. GFR, body mass index, and low high-density lipoprotein concentration in adults with and without CKD. Am J Kidney Dis. 2007;50(4):552–558. doi: 10.1053/j.ajkd.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 53.Iribarren C, Darbinian JA, Go AS, Fireman BH, Lee CD, Grey DP. Traditional and novel risk factors for clinically diagnosed abdominal aortic aneurysm: the Kaiser multiphasic health checkup cohort study. Annals of epidemiology. 2007;17(9):669–678. doi: 10.1016/j.annepidem.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 54.Go AS, Yang J, Gurwitz JH, Hsu J, Lane K, Platt R. Comparative effectiveness of beta-adrenergic antagonists (atenolol, metoprolol tartrate, carvedilol) on the risk of rehospitalization in adults with heart failure. Am J Cardiol. 2007;100(4):690–696. doi: 10.1016/j.amjcard.2007.03.084. [DOI] [PubMed] [Google Scholar]
- 55.James MT, Hemmelgarn BR, Wiebe N, et al. Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: a cohort study. Lancet. 2010;376(9758):2096–2103. doi: 10.1016/S0140-6736(10)61271-8. [DOI] [PubMed] [Google Scholar]
- 56.Turin TC, James M, Ravani P, et al. Proteinuria and rate of change in kidney function in a community-based population. J Am Soc Nephrol. 2013;24(10):1661–1667. doi: 10.1681/ASN.2012111118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bagshaw SM, Uchino S, Bellomo R, et al. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clinical journal of the American Society of Nephrology: CJASN. 2007;2(3):431–439. doi: 10.2215/CJN.03681106. [DOI] [PubMed] [Google Scholar]
- 58.Kellum JA, Sileanu FE, Bihorac A, Hoste EA, Chawla LS. Recovery after Acute Kidney Injury. Am J Respir Crit Care Med. 2017;195(6):784–791. doi: 10.1164/rccm.201604-0799OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930–936. doi: 10.1053/ajkd.2002.32766. [DOI] [PubMed] [Google Scholar]
- 60.Chawla LS, Bellomo R, Bihorac A, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017 doi: 10.1038/nrneph.2017.2. [DOI] [PubMed] [Google Scholar]
- 61.Heung M, Faubel S, Watnick S, et al. Outpatient Dialysis for Patients with AKI: A Policy Approach to Improving Care. Clinical journal of the American Society of Nephrology: CJASN. 2015;10(10):1868–1874. doi: 10.2215/CJN.02290215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Agarwal R, Alborzi P, Satyan S, Light RP. Dry-weight reduction in hypertensive hemodialysis patients (DRIP): a randomized, controlled trial. Hypertension. 2009;53(3):500–507. doi: 10.1161/HYPERTENSIONAHA.108.125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carretta HJ, Mick SS. Geocoding public health data. Am J Public Health. 2003;93(5):699. doi: 10.2105/ajph.93.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Swallen KC, Glaser SL, Stewart SL, West DW, Jenkins CN, McPhee SJ. Accuracy of racial classification of Vietnamese patients in a population-based cancer registry. Ethn Dis. 1998;8(2):218–227. [PubMed] [Google Scholar]
- 66.Stewart SL, Swallen KC, Glaser SL, Horn-Ross PL, West DW. Comparison of methods for classifying Hispanic ethnicity in a population-based cancer registry. Am J Epidemiol. 1999;149(11):1063–1071. doi: 10.1093/oxfordjournals.aje.a009752. [DOI] [PubMed] [Google Scholar]
- 67.Go AS, Magid DJ, Wells B, et al. The Cardiovascular Research Network: a new paradigm for cardiovascular quality and outcomes research. Circ Cardiovasc Qual Outcomes. 2008;1(2):138–147. doi: 10.1161/CIRCOUTCOMES.108.801654. [DOI] [PubMed] [Google Scholar]
- 68.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. The New England journal of medicine. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 69.Arellano MG, Petersen GR, Petitti DB, Smith RE. The California Automated Mortality Linkage System (CAMLIS) Am J Public Health. 1984;74(12):1324–1330. doi: 10.2105/ajph.74.12.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.