Abstract
We recently developed a therapeutic biopolymer composed of an elastin-like polypeptide (ELP) fused to vascular endothelial growth factor (VEGF)121 and showed long-term renoprotective effects in experimental renovascular disease after a single intra-renal administration. Here, we sought to determine the specificity, safety, efficacy, and mechanisms of renoprotection of ELP-VEGF after systemic therapy in renovascular disease. We tested whether kidney selectivity of the ELP carrier would reduce off-target binding of VEGF in other organs. In vivo bio-distribution after systemic administration of ELP-VEGF in swine was determined in kidneys, liver, spleen, and heart. Stenotic-kidney renal blood flow and glomerular filtration rate were quantified in vivo using multi-detector computed tomography (CT) after six weeks of renovascular disease, then treated with a single intravenous dose of ELP-VEGF or placebo and observed for four weeks. CT studies were then repeated and the pigs euthanized. Ex vivo studies quantified renal microvascular density (micro-CT) and fibrosis. Kidneys, liver, spleen, and heart were excised to quantify the expression of angiogenic mediators and markers of progenitor cells. ELP-VEGF accumulated predominantly in the kidney and stimulated renal blood flow, glomerular filtration rate, improved cortical microvascular density, and renal fibrosis, and accompanied by enhanced renal expression of VEGF, downstream mediators of VEGF signaling, and markers of progenitor cells compared to placebo. Expression of angiogenic factors in liver, spleen, and heart were not different compared to placebo-control. Thus, ELP efficiently directs VEGF to the kidney after systemic administration and induces long-term renoprotection without off-target effects, supporting the feasibility and safety of renal therapeutic angiogenesis via systemic administration of a novel kidney-specific bioengineered compound.
Keywords: elastin-like polypeptide, vascular endothelial growth factor, renovascular disease, renal hemodynamics, imaging, drug delivery, renal injury
Introduction
The outcomes of renovascular disease (RVD) are still poor, and there is a noticeable lack of consensus regarding the best therapeutic strategy for these patients, which adds a burden of uncertainty to the treatment selection and course. Regardless of the chosen therapy (medical, interventional, or combined therapy), patients with RVD improve in about 30% of the cases1, 2. Furthermore, the results of the CORAL study support the notion that pharmacological or interventional (e.g. renal angioplasty to resolve the obstruction) strategies do not show significant differences in renal recovery to support one treatment over the other3, although secondary evaluations of the ASTRAL study suggest that interventional strategies may still be beneficial in selected populations4. These controversies feed a pressing need for novel and more effective therapeutic strategies for the growing population of patients suffering from RVD that are at higher cardiovascular risk and at risk for development of chronic kidney disease (CKD).
It is possible that the vascular obstruction in RVD may be a major instigator of renal injury, and it may also exacerbate pre-existing renal damage5, 6. However, the dynamic and progressive nature of RVD may be a driving force for evolving renal injury distal to the vascular obstruction and may determine the chances of renal recovery after therapeutic interventions. Thus, it is possible that the poor recovery in RVD results from a combination of doing too little or acting too late with the possibility of neglecting the stenotic renal parenchyma.
Renal microvascular (MV) dysfunction, remodeling, and even loss are hallmarks of CKD irrespective of the etiology7, 8. We have shown that damage of the small vessels in the kidney correlates with a significant deterioration of renal hemodynamics, filtration, and tubular function in a swine model of chronic RVD9–11. We also demonstrated that MV disease in the stenotic kidney is associated with blunted renal angiogenesis, which is driven by a progressive decrease in renal vascular endothelial growth factor (VEGF)10, 12. The pivotal role of this pro-angiogenic cytokine in the kidney is supported by proof of concept studies showing that intra-renal replenishment of VEGF ameliorated renal MV rarefaction and attenuated renal dysfunction and damage11, 13, 14.
We recently extended and refined renal VEGF therapy. We developed a novel fusion of a bioengineered protein for drug delivery with VEGF121. We used elastin-like polypeptides (ELP), which are genetically encoded drug-delivery vectors with long plasma half-life, low immunogenicity, and adaptability to be fused to nearly any therapeutic. Furthermore, ELPs naturally accumulate in kidney15–18. The ELP-VEGF construct displayed a prolonged circulation and tissue residence time, improved stenotic kidney targeting and long-term efficacy of VEGF therapy in the RVD model (compared to unconjugated VEGF) after a single intra-renal administration18. However, whether systemic administration of ELP-VEGF may target and protect the kidney is unknown and is important to determine from a clinical-translational perspective. Thus, we first seek to establish the renal specificity of the ELP-VEGF construct after systemic administration, as we also aim to determine the safety and efficacy in the RVD model through this route. We hypothesize that the fusing of VEGF to the ELP biopolymer carrier will lead to renal tissue specificity and kidney accumulation without decreasing therapeutic efficacy even after systemic administration. Finally, we intend to determine potential off-target effects (a concern from a clinical-translational perspective) and underlying mechanisms of long-term renoprotection after systemic ELP-VEGF therapy.
Results
Characterization and labeling of the ELP-VEGF construct with fluorescent probes
Labeling was performed on primary amine residues, including the protein’s N-terminus, its one lysine residue near the N-terminus, and its eight lysine residues on the surface of VEGF121 as highlighted in Supplementary Figure 1. Labeling did not alter VEGF potency. For more details, please see Supplementary File. The stability of the ELP-VEGF biopolymer was determined in vitro, as described in the Supplementary File. As shown in Supplementary Figure 2A, ELP-VEGF was present as a single band migrating at 74 kDa, and the free rhodamine label migrated below the 10 kDa marker. When incubated in PBS, very little ELP-VEGF degradation was observed for the first 24 hours (quantified in Supplementary Figure 2B). Degradation of the protein began between 24 and 48 hours of incubation and proceeded to near complete loss of the full-length band after 4 days. A similar slow degradation was observed when ELP-VEGF was incubated in plasma. The kinetics of the degradation in plasma were different from the PBS incubation, beginning more quickly. However, after 5 days in plasma, more full-length protein remained intact than in the PBS incubation, possibly reflecting a stabilizing decoy effect of other plasma proteins occupying proteases. Detection of free dye by using trichloroacetic acid to precipitate the protein component of each sample mirrored the gel electrophoresis data in the PBS incubation, with free dye slowly being released over the period between 20 and 96 hours. However, the amount of free dye peaked at only about 25%, indicating that most of the dye was still bound to a protein component and consistent with the presence of a band of approximately 10 kDa in Supplementary Figure 2A. After incubation in plasma, almost no free dye was detectable, though it is possible that some free dye bound to albumin or other plasma proteins and was thus precipitated by trichloroacetic acid. These analyses reveal that ELP-VEGF does degrade under physiological conditions, but the rate of degradation is quite slow relative to the rate of clearance from the body observed here and in other studies18, 19.
In vivo biodistribution of fluorescently labeled ELP-VEGF following single intravenous administration
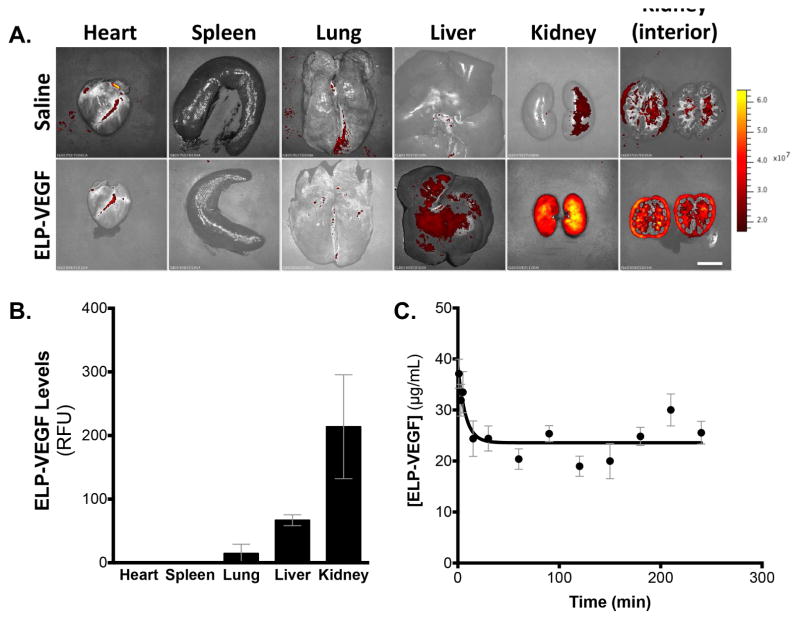
To determine the pharmacokinetics and biodistribution of ELP-VEGF, the protein was fluorescently labeled, and plasma levels and organ biodistribution were determined 4 hours after a single intra-venous administration (ear vein catheter) in the swine. The fluorescently labeled protein was administered intravenously in swine at a bolus dose of 1 mg/kg, plasma was sampled intermittently, and organ fluorescence was determined at sacrifice four hours after injection. Whole organ imaging revealed that ELP-VEGF predominantly accumulated in the kidney (Figure 1A). When the kidneys were cut in cross section, fluorescence imaging revealed ELP-VEGF localized at high levels in the renal cortex, with additional focal medullary localization in what are likely the large vascular branches. Retention of ELP-VEGF in the kidney was 3.2-fold higher than in the next most abundant organ, the liver. Additionally, ELP-VEGF levels in the kidney were 14.7-fold higher than in the lung, and the protein was undetectable in the heart and spleen at this dose (Figure 1B). Direct measurement of plasma fluorescence revealed a biphasic clearance of ELP-VEGF from the blood. A rapid distribution phase was evident within 30 minutes of injection, followed by a very slow clearance phase (Figure 1C). The slow clearance of ELP-VEGF is consistent with our observations of this protein after intra-renal administration, where we observed a half-life of approximately 13.5 hours18. However, the short duration of this experiment did not provide enough clearance time to achieve an accurate fit of the terminal half-life following intravenous administration.
Figure 1. Organ biodistribution and plasma clearance of ELP-VEGF in the pig.
A. Three pigs (average weight 30 kg) were given fluorescently labeled ELP-VEGF by direct intra-venous administration (ear vein). Organ biodistribution was determined 4 hours after injection by ex vivo whole organ fluorescence imaging imaging (A, scale bar = 5 cm) and quantification (B). Plasma was sampled intermittently during the 4-hour experiment, and ELP-VEGF levels were determined by direct fluorescence measurement (C).
Overall, these results demonstrate that ELP-VEGF is sufficiently stable under physiological conditions, clears slowly, and most of the injected protein is retained in the kidney even after systemic injection using an ear vein route, suggesting that ELP-VEGF is tissue specific and that systemic administration is a viable route for delivery of ELP-VEGF for renal therapy in RVD.
Renal and liver toxicity of systemic ELP-VEGF therapy
In addition to determining the pharmacokinetics and biodistribution of ELP-VEGF, blood from the pigs before injection and four hours after injection was assessed to determine if ELP-VEGF caused any acute effects. Quantification of blood urea nitrogen (BUN), creatinine, BUN/Creatinine ratio, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), and lactate dehydrogenase (LDH) showed that a single administration of ELP-VEGF did not induce any acute changes on parameters of renal or liver function, suggesting lack of acute toxicity and underscoring the safety of the construct (Table 1).
Table 1.
Parameters of renal and liver function (mean ± SEM) at time 0 and 4 hours after administration of ELP-VEGF intravenous injection (single dose, 1 mg/kg) for pharmacokinetics and biodistribution studies.
| Parameter | Plasma - Baseline | Plasma - 4 hours | Range | P value |
|---|---|---|---|---|
| BUN (mg/dL) | 5.3±0.6 | 5.33±0.3 | 8–24 mg/dL | 0.9 |
| Creatinine (mg/dL) | 1.2±0.1 | 1.3±0.1 | 1–3 mg/dL | 0.69 |
| BUN/Creatinine ratio | 4.56±0.5 | 4.39±0.6 | 0.85 | |
| ALT (U/L) | 38.0±7.7 | 34.5±4.6 | 31–58 U/L | 0.73 |
| AST (U/L) | 29.3±6.7 | 34.7±9.9 | 32–84 U/L | 0.68 |
| GGT (U/L) | 42.3±13.3 | 32.0±10.8 | 10–60 U/L | 0.57 |
| LDH (U/L) | 484.3±159.8 | 513.0±121.5 | 380–630 U/L | 0.89 |
p<0.05 4 hours vs. Baseline
BUN: blood urea nitrogen
ALT: alanine aminotransferase
AST: aspartate aminotransferase
GGT: gamma-glutamyl transferase
LDH: lactate dehydrogenase
In vivo efficacy of systemic ELP-VEGF therapy
We then sought to determine whether a single intravenous dose of ELP-VEGF was safe and efficacious for improving renal function and decreasing renal injury, and whether off-target effects were observed.
General characteristics
Body weight was similar among controls, RVD, and RVD+ELP-VEGF pigs 6 weeks after sham or induction of RVD and prior to treatment (Table 2). Pigs randomized to RVD and RVD+ELP-VEGF groups had elevated pre-treatment blood pressure relative to non-RVD controls, but the RVD+placebo (saline) and RVD+ELP-VEGF pre-treatment blood pressures were not significantly different from one another (Table 2). All animals were monitored during ELP-VEGF administration to determine the potential impact on heart rate and blood pressure, which were unchanged during injection. However, RVD+ELP-VEGF treated pigs showed a non-significant transient and asymptomatic decrease in blood pressure during the 24–48 hours following IV administration of the construct (from 142.5±10.3 to 126.2±9.7 mm/Hg, p=NS, Supplementary Figure 3) that then returned to hypertension pre-treatment values. Hypertension was not significantly different between RVD and RVD+ELP-VEGF pigs at 10 weeks (Table 2, Supplementary Figure 3).
Table 2.
Body weight, degree of stenosis, mean arterial pressure, renal vascular resistance, and renal cortical and medullary volumes (mean ± SEM) in normal, RVD, and RVD pigs before treatment (6 weeks after induction of RVD or sham operation) and four weeks after saline or ELP-VEGF treatment (10 weeks) (n=6–7 per group).
| Parameter | Normal | RVD | RVD+ELP-VEGF |
|---|---|---|---|
| Pre-treatment values (6 weeks) | |||
|
|
|||
| Body weight (kg) | 43.2±3.8 | 45.1±2.1 | 46.6±2.8 |
|
| |||
| Degree of stenosis (%) | 0.0±0.0 | 75.1±7.2* | 73.7±9.3* |
|
| |||
| MAP (mm/Hg) | 96.0±2.4 | 139.1±9.3* | 142.5±10.3* |
|
| |||
| RVR (mmHg/mL/min) | 0.21±0.02 | 0.58±0.04* | 0.64±0.05* |
|
| |||
| Cortical volume (cc) | 117.8±7.0 | 60.4±6.3* | 61.8±7.2* |
|
| |||
| Medullary volume (cc) | 34.0±5.3 | 17.4±2.0* | 18.1±1.9* |
|
| |||
| Post-Treatment values (10 weeks) | |||
|
|
|||
| Body weight (kg) | 51.3±5.2 | 54.2±2.9 | 54.5±2.8 |
|
| |||
| Degree of stenosis (%) | 0.0±0.0 | 74.9±6.5* | 75.2±8.7* |
|
| |||
| MAP (mm/Hg) | 100.1±1.9 | 150.4±10.4* | 142.4±3.8* |
|
| |||
| RVR (mmHg/mL/min) | 0.19±0.01 | 0.56±0.07* | 0.47±0.06* † |
|
| |||
| Cortical volume (cc) | 96.3±6.3 | 64.3±8.7* | 64.2±5.9* |
|
| |||
| Medullary volume (cc) | 31.1±6.5 | 18.4±2.1* | 21.2±7.0* |
p<0.05 vs. Normal;
p<0.05 vs. RVD.
MAP: mean arterial pressure, RVR: renal vascular resistance
Systemic ELP-VEGF therapy stimulated renal hemodynamics and function compared to pre-treatment/placebo values
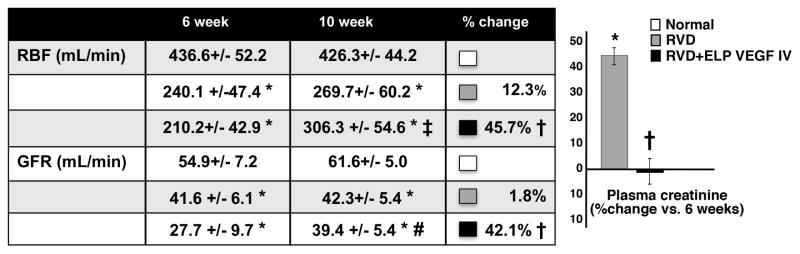
CT-derived stenotic kidney RBF, GFR, cortical and medullary perfusion were similarly decreased in all pigs with RVD after 6 weeks of observation, which correlated with a significant increase in renal vascular resistance (RVR) of the stenotic kidney (Table 2) and were accompanied by increased plasma creatinine (not shown). Blunted RBF, GFR (Figure 2), and regional perfusion (cortex: 3.3±0.5 mL/min/cc; medulla: 1.7±0.4 mL/min/cc) remained unchanged in RVD at 10 weeks (p=NS vs. 6 weeks) but RBF, GFR (Figure 2), and cortical perfusion (4.1±0.2 mL/min/cc, p<0.05 vs. 6 weeks) were stimulated after ELP-VEGF therapy whereas GFR showed a trend for a larger increase compared to pre-treatment values at 6 weeks (Figure 2). Improvements in RVR (Table 2) and a plateau in plasma creatinine (which continued to increase in untreated RVD, Figure 2) accompanied the improvements in renal function, suggesting slower or halted progression of renal dysfunction after ELP-VEGF therapy.
Figure 2. Systemic administration of ELP-VEGF improved renal hemodynamics and function in the stenotic kidney compared to pretreatment-/placebo values.
Effect of systemic ELP-VEGF on RBF, GFR, (table, absolute pre- and post-treatment values, and percent change after treatment/placebo) and plasma creatinine (bar graph, percent change after treatment) in normal, renovascular disease (RVD), and RVD+ELP-VEGF treated kidneys. * p<0.05 vs. Normal; † p<0.05 vs. RVD; ‡ p<0.05 vs. 6 weeks; # p=0.07 vs. 6 weeks.
RBF: renal blood flow; GFR: glomerular filtration rate.
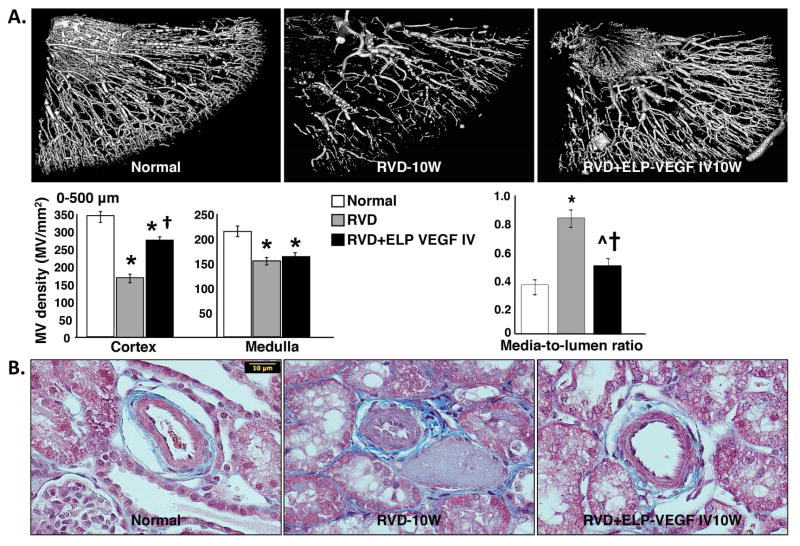
Systemic ELP-VEGF therapy improved cortical MV density and remodeling
The stenotic kidney showed a significant reduction in cortical and medullary MV density (quantified by micro-CT) accompanied by substantial MV remodeling compared to normal controls. Notably, systemically administered ELP-VEGF significantly improved cortical (but not medullary) MV density and remodeling of small and large microvessels (0–500 μm in diameter), which was evident throughout the renal cortical parenchyma (Figure 3A). This was accompanied by improved stenotic kidney media-to-lumen ratio, suggesting protection of the pre-existing microvasculature (Figure 3B).
Figure 3. Systemic administration of ELP-VEGF improved renal cortical microvascular density and remodeling in the stenotic kidney.
A. Representative pictures of renal microvascular (MV) density (stenotic kidney, 3D micro-CT reconstruction, MV diameter ≤ 500 μm, A) and MV remodeling (stenotic kidney, media to lumen ratio, B) in normal, renovascular disease (RVD), and RVD+ELP-VEGF treated kidneys. * p<0.05 vs. Normal; † p<0.05 vs. RVD; ^p=0.06 vs. Normal.
ELP-VEGF did not cause long-term deleterious effects
Plasma collected before treatment and four weeks after treatment in the RVD pigs was assessed with standard kidney and liver function assays to determine if treatment caused any long-term effects. Blood urea nitrogen (BUN) was reduced at the 10-week time point relative to pre-treatment values (Table 3). Creatinine levels were stable after treatment, consistent with ELISA data shown in Figure 2, and the BUN/creatinine ratio was significantly reduced by ELP-VEGF therapy. All measures of liver function were unchanged after ELP-VEGF treatment, and values were within normal ranges after treatment. Finally, no tumors were observed after ELP-VEGF therapy in any major organ.
Table 3.
Kidney and liver parameters (mean ± SEM) after 6 weeks of RVD (before treatment) and then 4 weeks after administration of ELP-VEGF intravenous injection (efficacy studies, single dose, 1 mg/kg).
| Parameter | Plasma-6 weeks | Plasma-10 weeks | Normal Range | P value |
|---|---|---|---|---|
| BUN (mg/dL) | 8.3±0.7 | 5.0±0.5 | 8–24 mg/dL | 0.03* |
| Creatinine (mg/dL) | 1.4±0.1 | 1.5±0.08 | 1–3 mg/dL | 0.64 |
| BUN/Creatinine ratio | 6.1±0.5 | 3.4±0.1 | 0.04* | |
| ALT (U/L) | 43.7±6.5 | 50.7±3.7 | 31–58 U/L | 0.91 |
| AST (U/L) | 38.0±2.2 | 32.3±3.3 | 32–84 U/L | 0.16 |
| GGT (U/L) | 32.3±4.3 | 29.0±2.3 | 10–60 U/L | 0.70 |
| LDH (U/L) | 488.7±19.6 | 487.3±63.9 | 380–630 U/L | 0.91 |
p<0.05 10 vs. 6 weeks (pre-treatment)
BUN: blood urea nitrogen
ALT: alanine aminotransferase
AST: aspartate aminotransferase
GGT: gamma-glutamyl transferase
LDH: lactate dehydrogenase
Renal angiogenic signaling, progenitor cell activation, and histology in the stenotic kidney
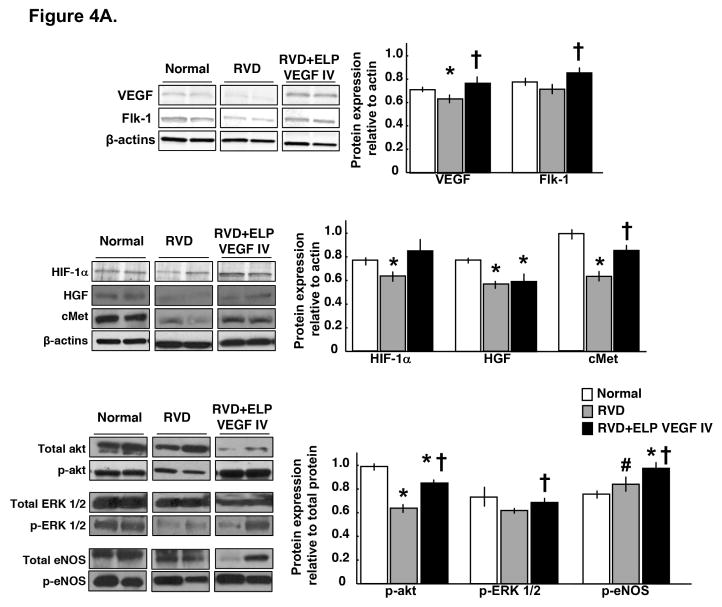
Systemic ELP-VEGF therapy improved renal expression of VEGF and downstream signaling
Protein extracts were made from the stenotic kidney harvested after sacrifice at the 10-week time point and examined by Western blotting to determine effects of ELP-VEGF treatment on VEGF downstream signaling. a single systemic administration of ELP-VEGF improved the expression of pro-angiogenic HIF-1α (upstream mediator of VEGF and HGF), VEGF and its receptor Flk-1, cMet (HGF was unchanged), and the expression of downstream mediators of VEGF signaling such as p-akt, p-ERK 1/2 and p-eNOS (Figure 4A).
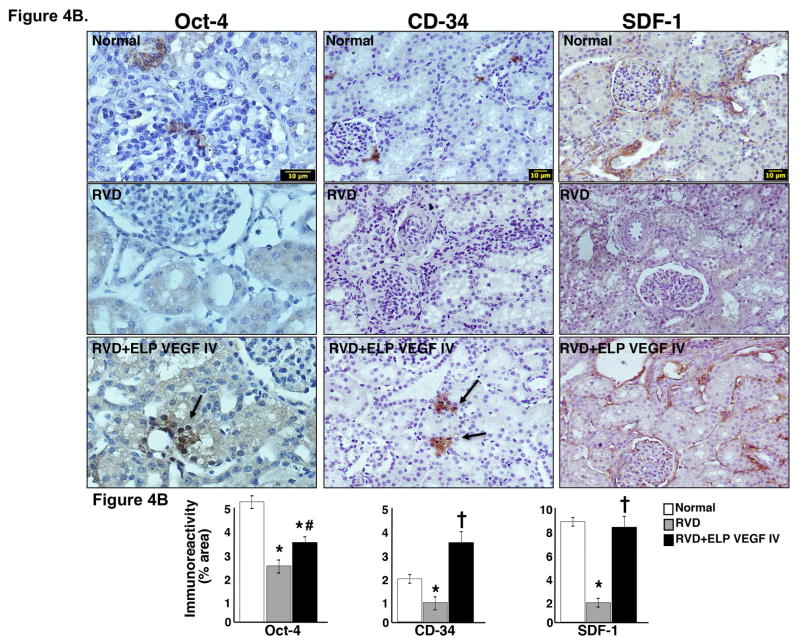
Figure 4. Systemic administration of ELP-VEGF improved angiogenic signaling and stimulated progenitor cells in the stenotic kidney.
4A) Representative renal protein expression (2 bands per group) and quantification of vascular endothelial growth factor (VEGF) and its receptor Flk-1 (top), hypoxia induced factor (HIF)-1α, hepatocyte growth factor (HGF), and HGF receptor cMet (middle), and total and phosphorylated (p)-akt, ERK ½, and endothelial nitric oxide synthase (eNOS, bottom) in normal, RVD and RVD+ELP-VEGF stenotic kidneys. 4B) Representative pictures of immunoreactivity against Oct-4 (40x), stromal-derived factor (SDF)-1 (20x), and CD-34 (20x) and quantification in normal, RVD and RVD+ELP-VEGF stenotic kidneys. * p<0.05 vs. Normal; † p<0.05 vs. RVD; #p>0.1 vs. RVD.
Systemic ELP-VEGF stimulated circulating and renal progenitor cells
Blocks of renal cortex were collected from the stenotic kidney after sacrifice and fixed and stained for markers of progenitor cells. The improvements in VEGF signaling in these kidneys were also accompanied by enhanced renal immunoreactivity against CD-34, stromal-derived factor (SDF)-1, and Oct-4 (mainly observed in the tubule-interstitial compartments and in MV proximity), suggesting potential stimulation, mobilization, and homing of systemic progenitor cells to the stenotic kidney and/or activation of resident cell progenitors in the stenotic kidney (Figure 4B).
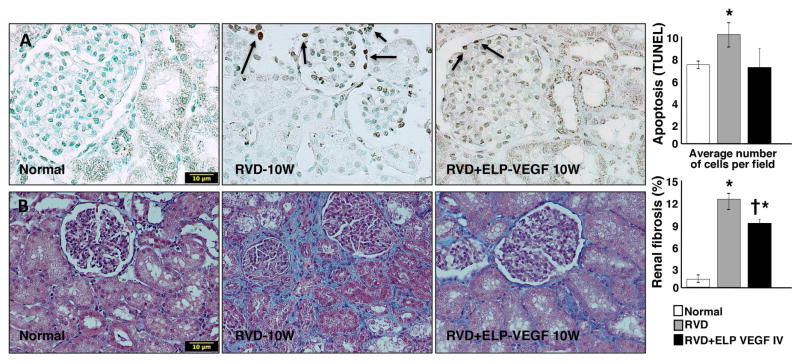
ELP-VEGF therapy attenuated apoptosis and fibrosis
Concurrent with the improved renal function and MV remodeling, the stenotic kidney showed a decrease in the fraction of TUNEL+ cells and attenuated glomerulosclerosis and cortical tubule-interstitial fibrosis compared to untreated animals, suggesting a reduction in apoptosis and fibrosis (Figure 5B–C).
Figure 5. Systemic administration of ELP-VEGF attenuated renal apoptosis and fibrosis in the stenotic kidney.
Representative stenotic kidney pictures (x40), showed as examples to illustrate and quantify the fraction of apoptotic cells (TUNEL, A) and fibrosis (trichrome, B) in the stenotic kidney after ELP-VEGF therapy or placebo. * p<0.05 vs. Normal; † p<0.05 vs. RVD.
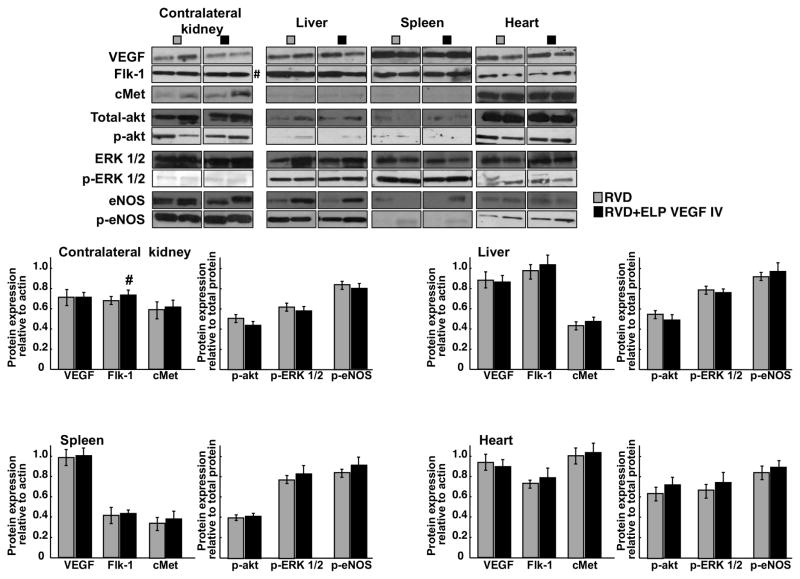
Systemic therapy did not induce off-target expression of angiogenic or apoptotic factors
Systemic administration may result in off-target accumulation and binding of the construct to other tissues than the stenotic kidney and stimulate vascular growth. Therefore, protein expression of angiogenic mediators were determined by western blot in the contralateral kidney, liver, spleen, and heart of the untreated and treated RVD pigs. We observed that 4 weeks after a single systemic administration of the ELP-VEGF construct, there were no changes in the expression of angiogenic mediators (except for a non-significant trend of Flk-1 in the contralateral kidney of the ELP-VEGF treated animals), which suggest minimum off-target activity of the construct (Figure 6).
Figure 6. Systemic administration of ELP-VEGF did not result in significant changes in off-target expression of angiogenic factors.
Comparative protein expression and quantification (2 representative bands per group) of vascular endothelial growth factor (VEGF), its receptor Flk-1, HGF-receptor cMet, total and phosphorylated (p)-akt, ERK ½, and endothelial nitric oxide synthase (eNOS) in the contralateral kidney, liver, spleen, and heart of untreated and ELP-VEGF IV treated pigs. #p=0.06 vs. RVD.
Discussion
The purpose of the current study was multifold. We first aimed to determine the feasibility and efficacy of targeting the kidney through systemic administration of ELP-VEGF therapy based on the higher preference of ELP for the kidney compared to other organs18. Determining a potential targeted renal therapy via a systemic administration could have implications towards clinical application. We observed that a single administration of ELP-VEGF using a peripheral vessel was able to target the kidney and accumulate in the renal parenchyma, which was accompanied by improved renal function, improved angiogenic and reduced apoptotic signaling (which may precede renal fibrosis20), attenuated renal MV rarefaction, and reduced fibrosis, indicating that effects of ELP-VEGF therapy in the stenotic kidney were not limited to vascular proliferation and repair. However, VEGF is a highly ubiquitous cytokine with autocrine, paracrine, and receptor-driven endocrine effects21, 22 that was administered via a systemic route, hence possible off-target effects are a concern from a (potential) clinical perspective. Thus, our secondary goal was to determine the safety of systemic administration. Notably, renal targeting and effects of ELP-VEGF were not associated with significant accumulation or changes in the off-target expression of angiogenic factors in liver, spleen or heart. Therefore, the current study extends our previous work18 and supports the kidney-selectivity of the ELP-VEGF construct. Our biodistribution data demonstrate that ELP-VEGF does accumulate in the liver, though at levels 3.2-fold lower than in the kidney. However, markers of VEGF signaling were not changed in the livers of treated pigs. This suggests that at the dose used (1 mg/kg, determined by a pilot study using escalating doses), the liver levels of ELP-VEGF are too low or the retention of the protein is too short to result in sustained VEGF signaling 4 weeks after treatment. Our study suggests that off-target effects, if any, have not developed 4 weeks after single administration whereas renoprotection persists. The current work may set a milestone for a novel kidney-targeting strategy using a non-invasive administration of ELP-VEGF.
Major pathological features of RVD are secondary hypertension and a degree of renal compromise that is often hard to predict and may range from non-significant changes to a significant and progressive deterioration of renal function. It is unquestionable that patients with RVD are at higher risk of developing CKD, which is largely driven by a chronic reduction of blood flow into the kidneys and subsequent development of tissue ischemia, renal dysfunction, and injury. Resolution of the stenosis was historically a major treatment goal in RVD, but conclusions from the ASTRAL23 and CORAL3 studies supported the view that targeting the stenotic renal artery should not be applied as the first line of therapy or in all patients with RVD based on the lack of significant advantages of renal angioplasty compared to medical therapy. Such conclusions may help to decrease the risks of an unnecessary exposure of patients with RVD to the potential risks of this intervention, although renal angioplasty and stenting may still be beneficial in selected patients with RVD4. Furthermore, a puzzling fact is that regardless of the chosen therapeutic strategy (medical or interventional), meaningful recovery of renal function is observed in a relatively small fraction of the patients1, 2. Therefore, if the vascular obstruction is not the main problem, targeting the renal parenchyma could likely be a major determinant on outcomes and recovery.
The current study extends our previous observations from both the feasibility and therapeutic angles. We showed that the ELP-VEGF construct is active in vitro and displays a prolonged half-life in vivo compared to unconjugated VEGF after a single intra-renal administration in rodent and swine models18, 19. For the current study, we first performed organ biodistribution studies in the swine model after a single systemic administration through an ear vein. As shown in Figure 1, we observed a significantly greater accumulation in the renal cortex over medulla, and a higher renal accumulation compared to liver and other organs. The greater cortical accumulation may be related to the higher vascularity of the renal cortex (it handles about 70% of the RBF) combined with the large size of the construct (74kDa) that may slow down its filtration. Thus, our data suggest that the construct may be greater retained in the stenotic renal cortex partly because of a combination of size of ELP-VEGF and that the lack of renal VEGF (but with relatively preserved VEGF receptor Flk-111, 18) may help to direct the construct to the ischemic environment. Another possibility is that VEGF and VEGF receptor levels increase (and remain elevated) in the stenotic kidney after treatment simply because of more vascularization and thus binding (e.g. the more endothelial cells in a given sample, the more VEGF/Flk-1 signal) in a context of a persistent reduction of blood flow, since stenosis remains unaltered and serves as a persistent stimulus.
Therefore, a first conclusion is that a systemic, non-invasive administration may be a feasible approach to target the kidney. The greater accumulation in renal cortex compared to the medulla was consequential since it was followed by a greater relative increase in RBF and GFR compared to pre-treatment values (Figure 2), cortical perfusion, cortical MV density, and decreased RVR. However, the recovery of renal hemodynamics was not as impressive as that observed following intra-renal therapy18 and shows a limited comparative efficacy of single intra-venous administration. Dose optimization studies are warranted to determine if systemic ELP-VEGF can achieve equivalent renal protection/recovery to intra-renal administration, whether repeated doses are needed, and whether the optimal dose for renal protection (single or eventually, multiple) is still safe from inducing effects in off-target organs. Some accumulation was observed in the liver, and although it was a significantly lower organ retention compared to the kidney, it may have played a role in limiting renal targeting and therapeutic availability of ELP-VEGF. Virtually no traces were observed in other organs such as heart or spleen. It is important to emphasize that systemic administration was not accompanied by acute effects on markers of renal or liver function, (Table 1) nor chronic effects on liver function (Table 3) or changes in angiogenic signaling, as suggested by Figure 6. Thus, our data support the notion that a systemic administration is safe and is able to target renal tissue, but also suggest that ELP-VEGF may suffer from some off-target accumulation in route to the kidneys, thus reducing some of the therapeutic efficacy compared to intra-renal administration.
Our study shows that systemic administration of ELP-VEGF therapy can reach the kidney and resulted in a greater relative increase in stenotic kidney RBF and GFR compared to pre-treatment values and placebo-treated RVD (Figure 1 and 2). Interestingly, despite the similar renal accumulation of ELP-VEGF in both kidneys observed in the biodistribution studies (done in healthy pigs), the contralateral kidney did not show significant changes in renal hemodynamics or function. One explanation may be that accumulation of ELP-VEGF may differ in the stenotic versus contralateral kidney in the context of unilateral RVD, and future studies in pigs with RVD are warranted to determine whether the ischemic environment in the stenotic kidney may play a role in directing organ biodistribution. The contralateral kidney in the RVD model shows relatively preserved hemodynamics and MV architecture, although we showed that it develops MV endothelial dysfunction, mild MV remodeling, and mild tubule-interstitial fibrosis11. The comparatively minor damage of the contralateral kidney at this stage is likely driven by hypertension24 and not ischemia as in the stenotic kidney25, and thus the severity of the insult differs as does the extent of renal injury. Unlike the stenotic kidney, we observed that contralateral kidney expression of VEGF and downstream mediators such as the Flk-1 receptor, and pro-angiogenic cMet, p-akt, p-ERK1/2 and p-eNOS were not attenuated and remained unmodified after systemic administration of ELP-VEGF. The distinct effects in the stenotic kidney may reflect comparatively more accentuated defects on mechanisms of tissue repair26 that may have been driven by blunted VEGF availability and signaling in an ischemic milieu, and thus improved by VEGF therapy. Such differences in turn underscore the importance of VEGF for a kidney facing ischemia.
A recent study showed that renal bioavailability of VEGF is critical for maintenance of the peritubular microvasculature via tubular-vascular crosstalk with the Flk-1 receptors27, which are abundant in renal endothelial cells and pivotal for VEGF-induced angiogenic effects. It is possible that renal up-regulation of VEGF receptors11 after ELP-VEGF therapy may have attracted circulating VEGF to the stenotic kidney, enhancing angiogenic activity and VEGF-driven renoprotection. As shown in Figure 4A, the increase in Flk-1 expression appears to be small but it was statistically significant and succeeded in inducing angiogenesis in the stenotic kidney. Furthermore, we observed that ELP-VEGF administration led to an increase in HIF-1α expression, which is a potent upstream mediator for VEGF stimulation and can be activated by the HGF pathway28. Moreover, ELP-VEGF therapy also resulted in augmented expression of the HGF receptor cMet, which could have contributed to the upstream increase in HIF-1α and downstream increased expression of VEGF/HGF mediators28–30. Therefore, it is possible that ELP-VEGF therapy restored and boosted the interaction of the HIF-1α/VEGF/HGF pathways that in turn led to the improvements in downstream expression of p-ERK ½, p-akt, and p-eNOS. Notably, such changes were followed by improved stenotic kidney cortical MV density and decreased MV media-to-lumen ratio, which may support the notion of a combined effect on vascular proliferation and protection of the pre-existing vasculature of the stenotic kidney (Figure 3). The distinct increase in CD 34, SDF-1, and Oct-4 positive cells in the stenotic kidneys of treated animals (Figure 4B), which were mainly observed in the tubule-interstitial compartments and in MV proximity (less in the glomerulus), supports this speculation and suggests that ELP-VEGF therapy may have stimulated mobilization and homing of endogenous circulating progenitor cells to the kidney31–34 and, possibly, also resident progenitors as suggested by Oct-435, 36. Recent studies have challenged the role of extra-renal cell progenitors in renal tissue repair37 and suggested a prominent contribution of renal resident pluripotent stem cells in healing the kidney when facing ischemia38, 39. Altogether, the improved signaling may have promoted and sustained renal MV proliferation, repair, and functional recovery, which may also be supported by the decrease in RVR after ELP-VEGF therapy. It is thus possible that VEGF-mediated stimulation of circulating and renal resident pluripotent cells play an important role in renal recovery and may explain the long term effects we have consistently observed after single-dose VEGF therapy11, 14, 18. In turn, a healthier renal parenchyma may have resulted in greater protection of VEGF sources (e.g.: glomeruli, tubules27), which likely contributed to improve the expression of VEGF and downstream mediators after ELP-VEGF therapy.
Limitations
Our results attest the kidney-targeting capabilities of the ELP-VEGF construct, which is paired with a sustained stimulation of VEGF signaling and consequent recovered and/or amplified VEGF-driven long-term renoprotection compared to unconjugated VEGF18. However, the current study is not free of limitations. The swine model is clinically relevant due to size, human-like anatomy and cardio-renal physiology, and because it shows almost all the renal pathological features observed in human RVD. However, we are aware that this model may better represent an early stage of the disease and that we are reporting results of a chronic study but with a single time-point of observation. Thus, future studies in a model with more severe compromise of renal function and damage, inclusion of additional time-points, and increased length of observation are necessary to support the capability of ELP-VEGF to target the kidney and enhance the potential for clinical translation of this strategy. In turn, such studies may contribute to define whether repeated administration is necessary and may help to determine the optimal therapeutic window for ELP-VEGF to rescue the kidney in RVD without side effects. On a similar note, multiple time points are also warranted in the search for potential off-target effects if higher or repeated doses are considered, as a single time-point as in the current study cannot rule out potential later effects in other organs.
The current study shows that the ELP-VEGF fusion protein has a distinct renal preference that supports potential for therapeutic application to protect the kidney in chronic RVD regardless of the route of administration18. The characteristics and efficacy of the ELP-VEGF construct make it an attractive tool for future studies on other forms of progressive renal damage with significant MV abnormalities, such as hypertensive and diabetic nephropathy, which are the major causes of CKD. The possibility of targeting the kidney via a non-invasive therapy may have significant implications towards clinical application of this unique technology.
Methods
In vitro studies
Generation of constructs, purification of polypeptides, and labeling ELP-VEGF with fluorescent probes
The coding sequence for human VEGF121 was fused in frame with the ELP coding sequence, and the chimeric protein was recombinantly expressed, purified, and characterized in vitro, as recently described18, 19. ELP-VEGF was labeled with 5/6-carboxy-tetramethyl-rhodamine succinimidyl ester (Life Technologies), as recently described18, 19. Stability and activity of the ELP-VEGF construct was assessed in vitro. For details, please see Supplementary File and Figures.
Determination of optimal dose for in vivo studies
The Institutional Animal Care and Use Committee at the University of Mississippi Medical Center approved all the studies. pilot study was first performed to determine the optimal dose to perform organ biodistribution and long-term efficacy studies. For details, please Supplementary File.
In vivo organ biodistribution
Three pigs were anesthetized and injected via an ear vein cannula with rhodamine-labeled ELP-VEGF to achieve a dose of 1 mg/kg body weight. Plasma was sampled intermittently from an indwelling carotid catheter. Four hours after IV injection of ELP-VEGF, the pigs were euthanized by an overdose injection of sodium pentobarbital (100mg/kg), and the organs were removed for analysis. Whole-organ ex vivo fluorescence imaging was conducted on kidneys, liver, spleen, lungs, and heart using an IVIS Spectrum (Caliper Life Sciences, Perkin Elmer) with 535 nm excitation, 580 nm emission, and auto exposure. Mean fluorescence radiant efficiency was determined for each organ using Living Image Software (Caliper), as described18. The mean fluorescence radiant efficiency was corrected for autofluorescence (by subtracting the autofluorescence values for each organ determined from an untreated pig) and fit to a standard curve of the fluorescently labeled protein. Data represent the mean±SEM. Plasma levels were determined by direct fluorescence detection in undiluted plasma using a fluorescence plate reader and Nanoquant® plate (Tecan). Plasma fluorescence was fit to a standard curve made from serial dilutions of known quantities of the same batch of fluorescently labeled ELP-VEGF.
Renal and liver toxicity of systemic ELP-VEGF therapy
Plasma samples obtained during biodistribution studies at 0 hour and 4 hours after injection, and plasma samples obtained pre-treatment after 6 weeks of RVD and then 4 weeks after single IV administration were used to assess acute and chronic effects on renal (blood urea nitrogen –BUN-, creatinine, and BUN/Creatinine) and liver (alanine aminotransferase -ALT-, aspartate aminotransferase –AST-, gamma-glutamyl transferase –GGT-, and lactate dehydrogenase –LDH-) function.
In vivo renal functional studies
Twenty juvenile domestic pigs (sus scrofa domestica) were used for the study, which lasted a total of 10 weeks. In 13 pigs, unilateral RVD was induced (by renal artery stenosis) and blood pressure continuously measured by telemetry, as described9, 13, 14, 18. Six weeks after induction of RVD, the degree of renal artery stenosis was quantified in all pigs by renal angiography, as shown9, 13, 14, 18. In vivo helical MDCT flow studies were then performed for quantification of basal single-kidney blood flow (RBF), regional perfusion, and glomerular filtration rate (GFR), as described9, 13, 14, 18. Immediately after completion of the MDCT studies, and while still under anesthesia, all RVD animals were treated with a single intravenous infusion of vehicle (RVD, n=7) or ELP-VEGF (1mg/kg, RVD+ELP-VEGF, n=6) through a catheter placed in the ear vein. All animals were monitored during ELP-VEGF administration to determine the potential impact on heart rate and blood pressure during injection. Additional animals were used as normal controls (Normal, n=7). Blood was collected (at 6 and 10 weeks) to measure plasma creatinine following vendors’ instructions. Pigs were then observed for 4 additional weeks and then MDCT in vivo studies repeated. After completion of all the in vivo studies, the pigs were euthanized and kidneys, liver, spleen, and hearts removed. All organs were carefully examined at necropsy to determine whether ELP-VEGF therapy induced development of tumors or aberrant vascularization. No gross tumor formation was observed in any of the organs. After inspection, tissues were processed and ex vivo studies performed, as shown9, 13, 14, 18.
High-resolution CT imaging
MDCT analysis was used to calculate single-kidney RBF (mL/min), GFR (mL/min), cortical and medullary perfusion (mL/minute/cc tissue), using previously validated methods18, 40, 41.
Micro-CT reconstruction and quantification of renal MV density was performed as extensively described10, 12, 18, 42.
Ex vivo studies
Expression and renal morphology were assessed in Normal, RVD and RVD+ELP-VEGF pigs.
Western blotting
Standard blotting protocols were followed, as described9, 18, 43, to determine renal expression of pro-angiogenic hypoxia-induced factor (HIF)-1α, VEGF (Santa Cruz, CA, 1:200), hepatocyte growth factor (HGF; Abcam, Cambridge, UK, 1:200), the specific VEGF and HGF receptors Flk-1 (Santa Cruz, CA, 1:200 for all) and cMet (Abbiotec, San Diego, CA, 1:200), total and phosphorylated (p)-eNOS (Cell Signaling, 1:500, Danvers, MA, 1:500), ERK ½ and akt (Santa Cruz, CA, 1:200).
Immunostaining and renal morphology
Mid-hilar 5 μm cross sections of each kidney (1 per animal) stained with trichrome were examined to quantify (using Image J 1.45s, National Institutes of Health, USA) tubule-interstitial fibrosis, glomerulosclerosis, and media-to-lumen ratio, as shown9, 18, 43. Fraction of apoptotic cells was quantified by TUNEL, as previously shown43. Finally, immunoreactivity against progenitor cells markers Oct-4 (Abcam, Cambridge, UK, 1:100), CD 34 (Aviva Systems Biology, CA, 1:100), and SDF-1 (Santa Cruz, CA, 1:100) was performed in paraffin-embedded renal sections following vendor’s instructions and quantified using Image J 1.45s, National Institutes of Health, USA.
Statistical Analysis
Results are expressed as mean ± SD or SEM as indicated. Comparisons within groups were performed using paired student’s t-test, and among groups using one-way ANOVA, with Bonferroni correction for multiple comparisons. Statistical significance was accepted for p≤0.05.
Supplementary Material
Acknowledgments
Sources of support
This work was supported by grant HL095638, HL51971, and GM104357 (ARC); and HL121527 (GLB) from the National Institutes of Health, and by grant 18490005 from the American Heart Association (ARC).
Footnotes
Disclosures: GLB is owner of Leflore Technologies LLC, a private company working to commercialize ELP-based technologies in several disease areas.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Choi SS. Atherosclerotic renal artery stenosis and revascularization. Expert review of cardiovascular therapy. 2014;12:1419–25. doi: 10.1586/14779072.2014.977258. [DOI] [PubMed] [Google Scholar]
- 2.Yu MS, Folt DA, Drummond CA, Haller ST, Cooper EL, Brewster P, Evans KL, Cooper CJ. Endovascular versus medical therapy for atherosclerotic renovascular disease. Current atherosclerosis reports. 2014;16:459. doi: 10.1007/s11883-014-0459-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, Cohen DJ, Matsumoto AH, Steffes M, Jaff MR, Prince MR, Lewis EF, Tuttle KR, Shapiro JI, Rundback JH, Massaro JM, D’Agostino RB, Sr, Dworkin LD. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med. 2014;370:13–22. doi: 10.1056/NEJMoa1310753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ritchie J, Green D, Chrysochou C, Chalmers N, Foley RN, Kalra PA. High-risk clinical presentations in atherosclerotic renovascular disease: prognosis and response to renal artery revascularization. Am J Kidney Dis. 2014;63:186–97. doi: 10.1053/j.ajkd.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Textor SC, Lerman LO. Reality and renovascular disease: when does renal artery stenosis warrant revascularization? Am J Kidney Dis. 2014;63:175–7. doi: 10.1053/j.ajkd.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Textor SC, Misra S, Oderich GS. Percutaneous revascularization for ischemic nephropathy: the past, present, and future. Kidney Int. 2013;83:28–40. doi: 10.1038/ki.2012.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mack M, Yanagita M. Origin of myofibroblasts and cellular events triggering fibrosis. Kidney Int. 2015;87:297–307. doi: 10.1038/ki.2014.287. [DOI] [PubMed] [Google Scholar]
- 8.Xavier S, Vasko R, Matsumoto K, Zullo JA, Chen R, Maizel J, Chander PN, Goligorsky MS. Curtailing endothelial TGF-beta signaling is sufficient to reduce endothelial-mesenchymal transition and fibrosis in CKD. Journal of the American Society of Nephrology: JASN. 2015;26:817–29. doi: 10.1681/ASN.2013101137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chade AR, Rodriguez-Porcel M, Grande JP, Krier JD, Lerman A, Romero JC, Napoli C, Lerman LO. Distinct renal injury in early atherosclerosis and renovascular disease. Circulation. 2002;106:1165–71. doi: 10.1161/01.cir.0000027105.02327.48. [DOI] [PubMed] [Google Scholar]
- 10.Chade AR, Zhu X, Mushin OP, Napoli C, Lerman A, Lerman LO. Simvastatin promotes angiogenesis and prevents microvascular remodeling in chronic renal ischemia. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2006;20:1706–8. doi: 10.1096/fj.05-5680fje. [DOI] [PubMed] [Google Scholar]
- 11.Iliescu R, Fernandez SR, Kelsen S, Maric C, Chade AR. Role of renal microcirculation in experimental renovascular disease. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2010;25:1079–87. doi: 10.1093/ndt/gfp605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu XY, Chade AR, Rodriguez-Porcel M, Bentley MD, Ritman EL, Lerman A, Lerman LO. Cortical microvascular remodeling in the stenotic kidney: role of increased oxidative stress. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:1854–9. doi: 10.1161/01.ATV.0000142443.52606.81. [DOI] [PubMed] [Google Scholar]
- 13.Chade AR, Kelsen S. Renal microvascular disease determines the responses to revascularization in experimental renovascular disease. Circulation Cardiovascular interventions. 2010;3:376–83. doi: 10.1161/CIRCINTERVENTIONS.110.951277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chade AR, Kelsen S. Reversal of renal dysfunction by targeted administration of VEGF into the stenotic kidney: a novel potential therapeutic approach. American journal of physiology Renal physiology. 2012;302:F1342–50. doi: 10.1152/ajprenal.00674.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bidwell GL, 3rd, Mahdi F, Shao Q, Logue OC, Waller JP, Reese C, Chade AR. A kidney-selective biopolymer for targeted drug delivery. American journal of physiology Renal physiology. 2017;312:F54–F64. doi: 10.1152/ajprenal.00143.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bidwell GL, 3rd, Perkins E, Hughes J, Khan M, James JR, Raucher D. Thermally targeted delivery of a c-Myc inhibitory polypeptide inhibits tumor progression and extends survival in a rat glioma model. PloS one. 2013;8:e55104. doi: 10.1371/journal.pone.0055104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bidwell GL, 3rd, Perkins E, Raucher D. A thermally targeted c-Myc inhibitory polypeptide inhibits breast tumor growth. Cancer letters. 2012;319:136–43. doi: 10.1016/j.canlet.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chade AR, Tullos NA, Harvey TW, Mahdi F, Bidwell GL., 3rd Renal Therapeutic Angiogenesis Using a Bioengineered Polymer-Stabilized Vascular Endothelial Growth Factor Construct. Journal of the American Society of Nephrology: JASN. 2016;27:1741–52. doi: 10.1681/ASN.2015040346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.George EM, Liu H, Robinson GG, Mahdi F, Perkins E, Bidwell GL., 3rd Growth factor purification and delivery systems (PADS) for therapeutic angiogenesis. Vasc Cell. 2015;7:1. doi: 10.1186/s13221-014-0026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitamura H, Shimizu A, Masuda Y, Ishizaki M, Sugisaki Y, Yamanaka N. Apoptosis in glomerular endothelial cells during the development of glomerulosclerosis in the remnant-kidney model. Exp Nephrol. 1998;6:328–36. doi: 10.1159/000020540. [DOI] [PubMed] [Google Scholar]
- 21.Eichmann A, Simons M. VEGF signaling inside vascular endothelial cells and beyond. Curr Opin Cell Biol. 2012;24:188–93. doi: 10.1016/j.ceb.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 23.Investigators A. Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, Baigent C, Carr S, Chalmers N, Eadington D, Hamilton G, Lipkin G, Nicholson A, Scoble J. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med. 2009;361:1953–62. doi: 10.1056/NEJMoa0905368. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Porcel M, Krier JD, Lerman A, Sheedy PF, 2nd, Romero JC, Napoli C, Lerman LO. Combination of hypercholesterolemia and hypertension augments renal function abnormalities. Hypertension. 2001;37:774–80. doi: 10.1161/01.hyp.37.2.774. [DOI] [PubMed] [Google Scholar]
- 25.Gomez SI, Warner L, Haas JA, Bolterman RJ, Textor SC, Lerman LO, Romero JC. Increased hypoxia and reduced renal tubular response to furosemide detected by BOLD magnetic resonance imaging in swine renovascular hypertension. American journal of physiology Renal physiology. 2009;297:F981–6. doi: 10.1152/ajprenal.90757.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chade AR, Zhu XY, Krier JD, Jordan KL, Textor SC, Grande JP, Lerman A, Lerman LO. Endothelial progenitor cells homing and renal repair in experimental renovascular disease. Stem cells. 2010;28:1039–47. doi: 10.1002/stem.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dimke H, Sparks MA, Thomson BR, Frische S, Coffman TM, Quaggin SE. Tubulovascular cross-talk by vascular endothelial growth factor a maintains peritubular microvasculature in kidney. Journal of the American Society of Nephrology: JASN. 2015;26:1027–38. doi: 10.1681/ASN.2014010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumura A, Kubota T, Taiyoh H, Fujiwara H, Okamoto K, Ichikawa D, Shiozaki A, Komatsu S, Nakanishi M, Kuriu Y, Murayama Y, Ikoma H, Ochiai T, Kokuba Y, Nakamura T, Matsumoto K, Otsuji E. HGF regulates VEGF expression via the c-Met receptor downstream pathways, PI3K/Akt, MAPK and STAT3, in CT26 murine cells. Int J Oncol. 2013;42:535–42. doi: 10.3892/ijo.2012.1728. [DOI] [PubMed] [Google Scholar]
- 29.Kubota T, Matsumura A, Taiyoh H, Izumiya Y, Fujiwara H, Okamoto K, Ichikawa D, Shiozaki A, Komatsu S, Nakanishi M, Kuriu Y, Murayama Y, Ikoma H, Ochiai T, Nakamura T, Matsumoto K, Nakamura T, Otsuji E. Interruption of the HGF paracrine loop by NK4, an HGF antagonist, reduces VEGF expression of CT26 cells. Oncol Rep. 2013;30:567–72. doi: 10.3892/or.2013.2509. [DOI] [PubMed] [Google Scholar]
- 30.Sulpice E, Ding S, Muscatelli-Groux B, Berge M, Han ZC, Plouet J, Tobelem G, Merkulova-Rainon T. Cross-talk between the VEGF-A and HGF signalling pathways in endothelial cells. Biol Cell. 2009;101:525–39. doi: 10.1042/BC20080221. [DOI] [PubMed] [Google Scholar]
- 31.Cencioni C, Capogrossi MC, Napolitano M. The SDF-1/CXCR4 axis in stem cell preconditioning. Cardiovascular research. 2012;94:400–7. doi: 10.1093/cvr/cvs132. [DOI] [PubMed] [Google Scholar]
- 32.Chen LH, Advani SL, Thai K, Kabir MG, Sood MM, Gibson IW, Yuen DA, Connelly KA, Marsden PA, Kelly DJ, Gilbert RE, Advani A. SDF-1/CXCR4 signaling preserves microvascular integrity and renal function in chronic kidney disease. PloS one. 2014;9:e92227. doi: 10.1371/journal.pone.0092227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Togel F, Isaac J, Hu Z, Weiss K, Westenfelder C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int. 2005;67:1772–84. doi: 10.1111/j.1523-1755.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 34.Yoder MC. Human endothelial progenitor cells. Cold Spring Harb Perspect Med. 2012;2:a006692. doi: 10.1101/cshperspect.a006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chade AR, Zhu X, Lavi R, Krier JD, Pislaru S, Simari RD, Napoli C, Lerman A, Lerman LO. Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation. 2009;119:547–57. doi: 10.1161/CIRCULATIONAHA.108.788653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta S, Verfaillie C, Chmielewski D, Kren S, Eidman K, Connaire J, Heremans Y, Lund T, Blackstad M, Jiang Y, Luttun A, Rosenberg ME. Isolation and characterization of kidney-derived stem cells. Journal of the American Society of Nephrology: JASN. 2006;17:3028–40. doi: 10.1681/ASN.2006030275. [DOI] [PubMed] [Google Scholar]
- 37.Sradnick J, Rong S, Luedemann A, Parmentier SP, Bartaun C, Todorov VT, Gueler F, Hugo CP, Hohenstein B. Extrarenal Progenitor Cells Do Not Contribute to Renal Endothelial Repair. Journal of the American Society of Nephrology: JASN. 2016;27:1714–26. doi: 10.1681/ASN.2015030321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q. The renal papilla is a niche for adult kidney stem cells. J Clin Invest. 2004;114:795–804. doi: 10.1172/JCI20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park HC, Yasuda K, Kuo MC, Ni J, Ratliff B, Chander P, Goligorsky MS. Renal capsule as a stem cell niche. American journal of physiology Renal physiology. 2010;298:F1254–62. doi: 10.1152/ajprenal.00406.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daghini E, Primak AN, Chade AR, Krier JD, Zhu XY, Ritman EL, McCollough CH, Lerman LO. Assessment of renal hemodynamics and function in pigs with 64-section multidetector CT: comparison with electron-beam CT. Radiology. 2007;243:405–12. doi: 10.1148/radiol.2432060655. [DOI] [PubMed] [Google Scholar]
- 41.Krier JD, Ritman EL, Bajzer Z, Romero JC, Lerman A, Lerman LO. Noninvasive measurement of concurrent single-kidney perfusion, glomerular filtration, and tubular function. American journal of physiology Renal physiology. 2001;281:F630–8. doi: 10.1152/ajprenal.2001.281.4.F630. [DOI] [PubMed] [Google Scholar]
- 42.Chade AR, Krier JD, Textor SC, Lerman A, Lerman LO. Endothelin-a receptor blockade improves renal microvascular architecture and function in experimental hypercholesterolemia. Journal of the American Society of Nephrology: JASN. 2006;17:3394–403. doi: 10.1681/ASN.2006060635. [DOI] [PubMed] [Google Scholar]
- 43.Chade AR, Rodriguez-Porcel M, Grande JP, Zhu X, Sica V, Napoli C, Sawamura T, Textor SC, Lerman A, Lerman LO. Mechanisms of renal structural alterations in combined hypercholesterolemia and renal artery stenosis. Arteriosclerosis, thrombosis, and vascular biology. 2003;23:1295–301. doi: 10.1161/01.ATV.0000077477.40824.52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.