Abstract
Age-related declines in motor function may be due, in part, to an increase in oxidative stress in the aging brain leading to dopamine (DA) neuronal cell death. In this study, we examined the neuroprotective effects of natural antioxidants resveratrol and pinostilbene against age-related DAergic cell death and motor dysfunction using SH-SY5Y neuroblastoma cells and young, middle-aged, and old male C57BL/6 mice. Resveratrol and pinostilbene protected SH-SY5Y cells from a DA-induced decrease in cell viability. Dietary supplementation with resveratrol and pinostilbene inhibited the decline of motor function observed with age. While DA and its metabolites (DOPAC and HVA), dopamine transporter, and tyrosine hydroxylase levels remain unchanged during aging or treatment, resveratrol and pinostilbene increased ERK1/2 activation in vitro and in vivo in an age-dependent manner. Inhibition of ERK1/2 in SH-SY5Y cells decreased the protective effects of both compounds. These data suggest that resveratrol and pinostilbene alleviate age-related motor decline via the promotion of DA neuronal survival and activation of the ERK1/2 pathways.
Keywords: Resveratrol, pinostilbene, Striatum, Substantia nigra, aging, ERK1/2, SH-SY5Y, MAP kinases
1. Introduction
Physical performance contributes to the overall quality of life and declines with age. For example, balance and walking speed decline with aging, leading to a decrease in daily activities and a higher incidence of institutionalization and mortality. [1–4] The number of injuries due to falls also increases as a person ages, most likely from deficits in motor coordination. [5] However, there are few treatments for motor impairments that occur with non-diseased aging.
One hypothesis for the pathology that underlies age-related motor deficits is that there is an increase in oxidative stress with age that leads to an increase in neuronal cell death. [6–11] Dopamine (DA) neurotransmission plays an integral role in the coordination of voluntary movements and DA neurons are particularly sensitive to oxidative stress. [12–14] Therefore, we hypothesize that administration of antioxidants will protect DA neurons from oxidative stress-induced cell death and thereby improve motor function and physical activity with age.
Resveratrol, an antioxidant found in many foods, including grapes and blueberries, has been shown to increase the latency to fall on the rotarod. [15, 16] Additionally, a report in non-human primates showed that resveratrol treatment increased spontaneous activity. [17] One of the limitations to the use of resveratrol is that it has limited bioavailability. [18–20] Therefore, methylated resveratrol analogs with greater bioavailability have gained interest as potential antioxidant treatments. One such analog, pinostilbene, has been shown to protect neurons against oxidative stress in vitro and in vivo. [21] While there are multiple proposed mechanisms for resveratrol-mediated cell survival, the mechanisms behind the protective effects of pinostilbene are not well defined.
Signaling pathways that resveratrol has been shown to modulate are the extracellular signal-regulated kinases 1 and 2 (ERK1/2) pathways. ERK1/2 are members of the mitogen-activated protein kinase (MAPK) family that have been shown to play a critical role in cell survival in response to oxidative stress, both in PC12 cells and primary cortical neurons. [22, 23] Moreover, activation of ERK1/2 protect against hypoxia-induced cell death in primary cortical cultures [24] and H2O2-mediated cell death in primary striatal cell cultures. [23] While the mechanism for pinostilbene-induced neuroprotection remains unclear, resveratrol has been shown to both activate [25–27] and inhibit [28–30] the ERK1/2 pathways to provide neuroprotection. Until now, the effect of pinostilbene on ERK1/2 activation has not been reported.
In the current study, we investigated the effects of resveratrol and pinostilbene on dopamine induced cell death of SH-SY5Y cells and age-related motor deficits. Furthermore, we examined the role of the ERK1/2 signaling cascades in resveratrol and pinostilbene-mediated protection. Finally, we measured DA content and markers of DA neurons in the striatum and substantia nigra (SN) of C57BL/6 male mice. Because the DAergic neuronal systems play an integral role in voluntary motor functions and the paucity of therapies for motor deficits noted with aging, we aimed to investigate the potential mechanism of compounds that may alleviate these deficits. This study suggests that natural products that protect DA neurons from oxidative stress may be novel interventions for age-related motor decline.
2. Materials and methods
2.1 Experimental design and treatments
Resveratrol was purchased from ChemPacific, Baltimore, MD. Pinostilbene was synthesized by Dr. Cassia Mizuno in Dr. Agnes Rimando’s laboratory following published procedures. [31] Stock solutions of resveratrol and pinostilbene were made in DMSO (Sigma Aldrich) and further diluted in serum-free media. SH-SY5Y cells were treated with 1 or 5 µM of resveratrol, pinostilbene or DMSO (vehicle for resveratrol and pinostilbene). DA (Sigma Aldrich) was dissolved in sterile water to an initial concentration of 100 mM. Following treatment with resveratrol, pinostilbene, or DMSO (30 min), SH-SY5Y cells were treated with DA (50, 100, or 200 µM) or H2O (vehicle for DA) for 24 hrs. U0126 (Cell Signaling) was dissolved in DMSO to an initial concentration of 10 mM. Cells were treated with U0126 (10 µM) or DMSO (vehicle) 1 hr prior to resveratrol treatment/90 min prior to DA treatment.
2.2 Cell Culture
SH-SY5Y cells (ATCC, Manassas, VA) were grown on 10cm cell culture plates (Sarstedt; Newton, NC) in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, Carlsbad, CA) with 10% heat-inactivated fetal bovine serum (FBS; Atlanta Biological, Lawrenceville, GA), 0.1% Uridine (Sigma Aldrich; St. Louis, MO), 0.1% Pyruvate (Sigma Aldrich), and 1% penicillin/streptomycin (Gibco). Cells were maintained at 37°C with 5% CO2. Cells were plated at a density of 1.5 × 104 cells per well for 96-well plates and 6.0 × 106 cells per 35 mm plate.
2.3 Animals
Young (2 m.o.), middle-aged (10 m.o.), and old (22 m.o.) male C57BL/6 mice obtained from the NIA aging colony (Charles River, New York, NY) were maintained in temperature-controlled rooms in the barrier facility maintained by Duquesne University. Animals were housed in accordance with Guidelines for the Care and Use of Animals, at 23°C with lights on between 7 am and 7 pm. Animals were fed and watered ad libitum, and the woodchips in their cages changed every other day. Full-time trained animal care personnel performed all animal handling and maintenance. The directors of the Duquesne University animal facilities and veterinarians supervised animal care. All animal care was overseen by the Institutional Animal Care and Use Committee (IACUC).
2.4 Dietary supplementation
Mice were given 6 g of resveratrol- or pinostilbene-supplemented diet (120 mg compound/kg of diet; 0.72 mg/day/mouse) per day for eight weeks. The diets were prepared at Harlan Teklad (Madison, WI) by adding crystalline resveratrol (TCI America, Waltham, MA) or pinostilbene (synthesized by Dr. Cassia Mizuno, ARS USDA facility, University, MS) to the control diet of Purina Rodent Chow (~0.54 mg resveratrol or pinostilbene/day/mouse). Food was placed on the floor of the cage so there was adequate access for all age groups. Body weight (in grams) and food intake (in grams) were measured each morning when a new supply of food was given.
2.5 Cell Death Assays
SH-SY5Y cells were analyzed using the Cell Titer Glo Luminescent Cell Viability Assay (Promega, Madison, WI) as per manufacturer’s protocol. Luminescence was recorded using a microplate reader (Perkin Elmer, Waltham, MA).
2.6 Behavioral analyses
Cylinder test for spontaneous activity
Animals were tested for spontaneous activity using a cylinder test as previously described. [32, 33] For these experiments, a small, transparent, Plexiglas cylinder (height 15.5 cm, diameter 12.7 cm) and were videotaped for 3 min. Videos were rated for forelimb steps, hind-limb steps, rears, and time spent grooming by an investigator blinded to the treatments.
Challenging beam test for motor coordination
Animals were tested on the challenging beam to assess motor coordination and balance as previously described. [34–37]
2.7 Tissue processing
Mice were sacrificed 24 hr following the last behavioral test, and the striatum and hippocampus were rapidly dissected, frozen on dry ice, and stored at −80°C until assayed. The hindbrain was stored in fix solution (4% formaldehyde/4% NaF in PBS) for one week and then changed to a 30% sucrose solution in 1XPBS. Samples were sonicated in 0.1N perchloric acid (20 µl/mg wet tissue weight) for analysis of DA levels or in ice-cold lysis buffer (20 mM Tris, pH 6.8, 137 mM NaCl, 25 mM β-glycerophosphate, pH 7.14, 2 mM NaPPi, 2 mM EDTA, 1 mM Na3VO4, 1% Triton X-100, 10% glycerol, 5 µg/ml leupeptin, 5 µg/ml aprotinin, 2 mM benzamidine, 0.5 mM DTT and 1 mM PMSF) for Western blot analysis.
2.8 High Performance Liquid Cromatography
Striatal tissue was analyzed for DA, DOPAC, and HVA content as previously described. [38, 39] Briefly, a 20 µl aliquot of each sample was injected onto an ESA C18 column (2.1 × 150 mm, ESA, Inc., Chelmsford, MA). The mobile phase consisting of 75 mM H2NaPO4, 1.7 mM octanesulfonic acid, 25 mM Na2EDTA 0.00001% triethylamine (v/v), and 10% acetonitrile (v/v), pH 3.0 was pumped through the system at a rate of 0.3 ml/min using an ESA LC-10AD pump (ESA Inc., Chelmsford, MA). Samples were detected coulometrically using an ESA Coulochem Model 4100A detector, an ESA Model 5010 conditioning cell, and an ESA Model 5014B microdialysis cell (ESA Inc., Chelmsford, MA). The settings for detection were E1 = −0.26 V, E2 = +0.28 V, guard cell = +0.4 V. The limits of detection were in the femtomole range. Quantification was done using standard curves for DA, DOPAC and HVA.
2.9 Western Blot Analysis
For in vitro studies, SH-SY5Y cells were plated in 60 mm plates and treated with resveratrol (Resv; 5 µM) or pinostilbene (Pino; 5 µM) over a time-course (15 min – 48 hrs). For striatal tissue, one half of the striatum from each animal was sonicated in ice-cold lysis buffer. Proteins (60 µg for striatal tissue and 30 µg for SH-SY5Y cells) were separated on 8% SDS-PAGE minigels. The gels were then transferred to nitrocellulose membranes (LI-COR Biosciences; Lincoln, NE) and membranes were blocked in casein blocking buffer (LI-COR Biosciences) for 1 hr at room temperature and incubated overnight at 4°C in the following primary antibodies: mouse anti-ERK1/2 (1:1000, catalog # 9107; Cell Signaling Technology; Danvers, MA), rabbit anti-phospho-ERK1/2 (1:1000, #9101; Cell Signaling Technology), rabbit monoclonal anti-dopamine transporter (DAT) (1:3000, #AB2231; Millipore), mouse anti- tyrosine hydroxylase (TH) (1:2000; #MAB318, Millipore), or GAPDH (1:1000; Millipore) in casein blocking buffer (LI-COR Biosciences). Membranes were then incubated with IR680-labeled goat anti-rabbit or IR800-labeled goat anti-mouse secondary antibody (1:10,000, LI-COR Biosciences). Blots were scanned on an Odyssey Imager and quantified with Odyssey software, using background subtraction above and below each band. Grayscale images were used for quantification. Images were pseudo-colored red (700 nm, goat anti-rabbit) and green (800 nm goat anti-mouse) to delineate using distinct fluorescent wavelengths.
2.10 Immunohistochemistry
Approximately six separate series of 30 µm coronal brain sections were obtained from each hindbrain using a sliding microtome (Microm HM 450; Thermo Scientific; Asheville, NC). The slices were then subjected to unbiased stereological cell counting to determine the number of TH+ neurons using the novel motorized stage approach. [40, 41] Coronal SN sections were rinsed 6 times in PBS and blocked in 10% serum solution in PBS containing 0.3% Triton X-100 for 30 min at RT. Then, brain sections were incubated in a solution containing a mouse monoclonal anti-MAP2 (1:2000; #MAB378, Millipore) and polyclonal anti-TH (1:2000; #AB1542, Millipore) primary antibodies in PBS. Following 3 rinses in PBS, sections were then incubated with secondary antibodies: Cy3-conjugated anti-sheep (1:500; #713-165-003, Jackson-ImmunoResearch) and Alexa Fluor-conjugated 647 anti-mouse (1:500; #A31571, Invitrogen) for 2 hr at RT. Next, tissue sections were washed in 3 changes of PBS for 10 min, mounted onto plus-coated slides and coverslipped using gelvatol mounting media. Images were acquired using an automated Nikon 90i upright fluorescence microscope equipped with five fluorescent channels (blue, green, red, far red and near IR), and high N.A. plan fluor/apochromat objectives and a linear encoded motorized stage (Prior Electronics). The studies described here were all performed using 40× objective (0.75 N.A.). Images were collected using the Nikon NIS-Elements software and a Q-imaging Retiga cooled CCD camera. Neuron counting was performed by an investigator blinded to treatment and age. All slides were scanned under the same conditions for magnification, exposure time, lamp intensity, and camera gain. Quantitative analysis was performed on fluorescent images generated in 3 fluorescent colors (stained for MAP2, TH+, and H 33342). Images were stitched with NIS-Elements, following background subtraction and thresholding for each individual channel. Once stitched together, colocalization and subsequent exclusion was performed on the images.
2.10 Analysis of stilbenes by gas chromatography-mass spectrometry (GC-MS)
Tissue samples were kept in −80°C freezer until used for analysis. Tissues were thawed on ice, then homogenized in 150µl of sodium phosphate buffer (0.2M NaHPO4:0.2M Na2HPO4; 80:20), pH 7.4, and centrifuged for 15 mins at 7000g, 4°C. The pellet was homogenized a second time with 150µl of phosphate buffer. The supernatants were combined; half-volume was treated with s-glucuronidase (5000 U/ml potassium phosphate buffer, 75mM, pH 6.8; 50 µl/125µl extract) and incubated at 37°C with shaking at 750 rpm for 20 hrs. Potassium phosphate buffer was added to the other half volume as control. The mixture was partitioned with ethyl acetate (200µl × 3). The combined ethyl acetate extracts was dried under a stream of nitrogen, and derivatized with 30µL of a 1:1 mixture N,O-bis[trimethylsilyl]trifluoroacetamide and dimethylformamide (Pierce Biotechnology, Inc., Rockford, IL, USA), heated at 70°C for 40 min, and used for analysis of pinostilbene and resveratrol.
Analysis of pinostilbene and resveratrol in the HPC tissues by GC-MS (JEOL GCMate II Instrument; JEOL USA Inc., Peabody, MA, USA) was performed using a J&W DB-5 capillary column (0.25 mm internal diameter, 0.25µm film thickness, and 30m length; Agilent Technologies, Foster City, CA, USA). The GC temperature program was: initial 190°C, increased to 242°C at 30°C/min rate, increased to 248°C at the rate of 0.4°C/min, then finally increased to 300°C at the rate of 30°C/min and held at this temperature for 0.5 min. The carrier gas was ultrahigh purity helium (1 mL/min flowrate). The injection port, GC-MS interface, and ionization chamber were kept at 250, 230, and 230°C, respectively. The volume of injection was 2µL (splitless injection). The mass spectrum was acquired in the positive, selected ion-monitoring mode; electron 48 impact 70eV. The retention time for pinostilbene was 10.4 min (monitored with m/z 388, 373, and 356). The retention time for resveratrol was 11.2 min (monitored with m/z 446, 431, and 373). GC-MS analyses were done in duplicates. Quantitation of pinostilbene and resveratrol was done using external standards. These analyses were done in the laboratory of our collaborator, Dr. Agnes Rimando.
2.11 Statistical Analysis
All statistical tests were performed using Graph Pad Prism 6 (Graphpad Prism, Inc., La Jolla, CA). The data were analyzed using either one- or two-way ANOVA (p < 0.05) followed by post hoc comparison using an uncorrected Fisher’s LSD test.
3. Results
3.1 Resveratrol and pinostilbene protect against DA-induced cell death via activation of ERK1/2
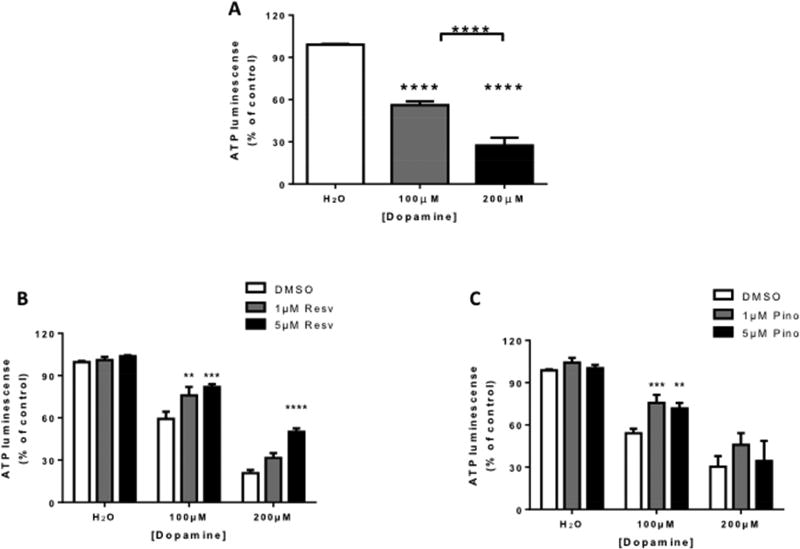
To determine whether resveratrol or pinostilbene protect against DA-induced cell death, SH-SY5Y cells were pretreated with varying concentrations of resveratrol (Resv) or pinostilbene (Pino) followed by DA treatment (Fig. 1). Treatment with DA significantly decreased SH-SY5Y cell viability as measured by ATP luminescence (Fig. 1A). Pretreatment with resveratrol and pinostilbene protected against the loss of cell viability (Fig. 1B and 1C).
Figure 1. Effect of resveratrol and pinostilbene on dopamine induced loss of ATP in SH-SY5Y cells.
(A) DA increased the loss of ATP in SH-SY5Y cells in a dose-dependent manner. (B) Pretreatment with resveratrol inhibited the loss of ATP induced by DA treatment. (C) Pretreatment with pinostilbene inhibited the loss of ATP induced by 100 µM, but not 200 µM, DA treatment. n=3–4 experiments done in triplicate for each group. Data are expressed as mean ± SEM. **** p < 0.0001 compared to the H2O treated group (A), between DA treated groups (A), and compared to the DMSO treated group (B). ***p < 0.001 and **p < 0.01 compared to the DMSO treated group.
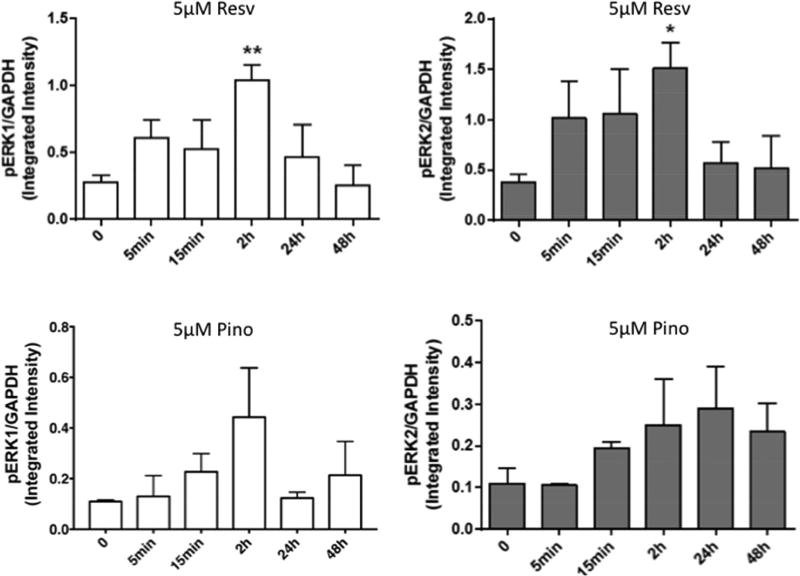
To examine the effect resveratrol or pinostilbene treatment on phosphorylated (activated) ERK1/2, SH-SY5Y cells were treated with resveratrol (Resv; 5 µM) or pinostilbene (Pino; 5 µM). Resveratrol transiently increased ERK1 and 2 activation in SH-SY5Y cells, such that activation was returned to basal levels by 24 hr (Fig. 2A and 2B). Pinostilbene increased ERK1 phosphorylation at 2 hr post-treatment with a return to baseline by 24hrs (Fig. 2C). In contrast, pinostilbene increased activation of ERK2 by 15 min and this activation was sustained up to 48 hrs (Fig. 2D).
Figure 2. Expression of phosphorylated ERK1/2 in SH-SY5Y cells following resveratrol or pinostilbene treatment.
SH-SY5Y cells were treated with 5 µM resveratrol (A, B) or 5 µM pinostilbene (C, D) for the different time points. Cell lysates were collected, resolved on SDS-PAGE gels and probed for phospho and total ERK1 and ERK2. GAPDH was used as a loading control. (A, B) Immunoblot analysis indicating that resveratrol increases ERK1 and ERK2 phosphorylation in a time dependent manner. (C, D) Immunoblot analysis indicating that pinostilbene increases ERK1 and ERK2 phosphorylation in a time dependent manner distinct from resveratrol. The values are calculated using the integrated intensity. Protein expression was standardized to the intensity of GAPDH. n=3–4 for each time point. Data are expressed as mean ± SEM. ** p < 0.01 and * p < 0.05 compared to the 0 time point.
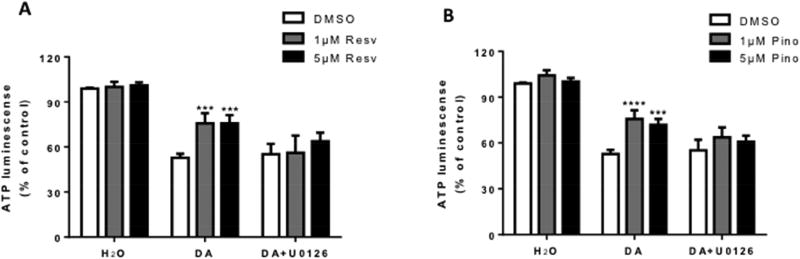
Further, to investigate the role of ERK1/2 activation in resveratrol and pinostilbene-mediated protection from DA-induced cell death, SH-SY5Y cells were pretreated with an inhibitor of the ERK1/2 pathway, U0126 (10 µM), prior to resveratrol or pinostilbene (1 or 5 µM) and DA (100 µM) treatment. Pretreatment with U0126 inhibited the resveratrol- and pinostilbene mediated protection, as indicated by the loss of ATP (Fig. 3A and 3B, respectively).
Figure 3. Effect of pharmacological ERK1/2 inhibition on resveratrol- and pinostilbene-mediated protection from DA-induced ATP loss in SH-SY5Y cells.
U0126 (10 µM) inhibited the (B) resveratrol- and (C) pinostilbene-mediated protection from 100 µM DA-induced ATP loss. n=3–4 experiments done in triplicate for each group. Data are expressed as mean ± SEM. **** p < 0.0001 and *** p < 0.001 compared to DMSO treatment.
3.2 Dietary supplementation with resveratrol or pinostilbene decreases age-related motor deficits
Table 1 shows the levels of resveratrol and pinostilbene in the brain as measured by GC-MS. The levels of pinostilbene in the brain are greater than that of resveratrol at all ages. Resveratrol was undetectable in the brains of the young animals. Interestingly, trace amounts of resveratrol were noted in the brains of the middle-aged and old mice.
Table 1.
Pinostilbene and resveratrol penetrate the blood-brain barrier.
| Sample description | Pinostilbene (ng/mg tissue) |
Resveratrol (ng/mg tissue) |
|---|---|---|
| Young-Pinostilbene | 0.0365 | NA |
| Middle-Pinostilbene | 0.0772 | NA |
| Old-Pinostilbene | 0.0776 | NA |
| Young-Resveratrol | NA | nd |
| Middle -Resveratrol | NA | trace |
| Old-Resveratrol | NA | trace |
Values are presented as ng/mg fresh tissue; GC-MS analysis in duplicate.
Pinostilbene and resveratrol were detected in the hippocampus of middle and old-aged animals.
Pinostilbene was found in the hippocampus of young mice.
NA = not applicable; pinostilbene or resveratrol was not analyzed
nd = not detected
trace = peaks are below limit of quantitation.
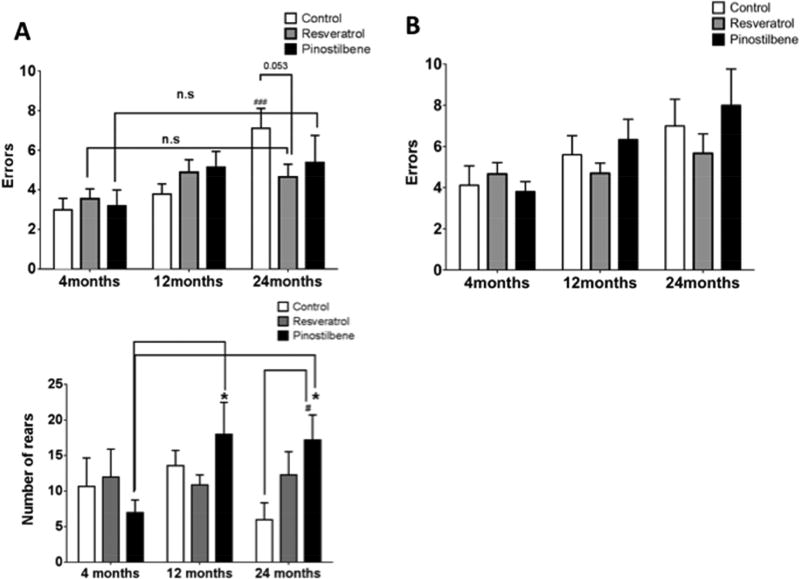
We have previously reported that motor coordination decreases in C57Bl/6 male mice with age. [37, 39] Similarly, in the current study, aged animals fed the control diet made significantly more errors on the challenge beam than young animals (Fig. 4A, control group). However, there was no significant difference between the errors made by old mice on resveratrol and pinostilbene diet compared to their diet-matched young counterparts as well as young control mice. Old mice fed a resveratrol-supplemented diet for four weeks showed reduced errors on the challenge beam compared to their age-matched controls (p=0.053). Surprisingly, at eight weeks, mice on pinostilbene made more errors than old control mice (Figure 4B). However in the cylinder test for spontaneous exploratory behavior, pinostilbene, but not resveratrol, supplementation increased the number of rears made by both middle-aged as well as old animals (Fig. 4C).
Figure 4. Effects of 4 and 8-week dietary supplementation with resveratrol or pinostilbene on motor coordination and spontaneous activity as measured by the challenging beam and cylinder test.
(A) Dietary supplementation for 4 weeks with resveratrol decreased the number of errors made by 24 month old animals. (B) Dietary supplementation for 8 weeks with resveratrol or pinostilbene did not improve motor function of old animals. (C) Dietary supplementation with pinostilbene but not resveratrol increased the number of rears made by middle-aged and old animals.
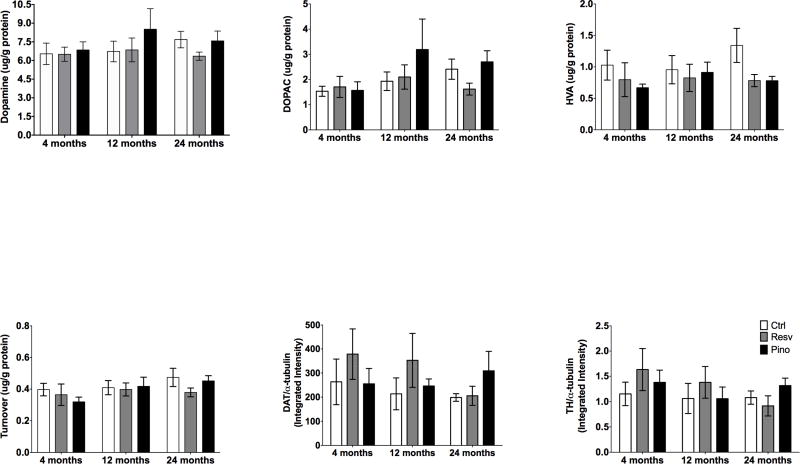
3.5 Dietary supplementation with resveratrol or pinostilbene does not alter the levels of dopamine, its metabolites, or dopamine transporter in the striatum with age
To determine if there were changes in the nigrostriatal DA system associated with age and/or dietary supplementation, high performance liquid chromatography (HPLC) was employed to measure the content of DA and its metabolites, including DOPAC and HVA, in the striata of young, middle-aged, and old animals. DA, DOPAC, and HVA levels did not change with age or dietary supplementation in the striatum (Fig. 5A–D). Similarly, TH and DAT expression in the striatum was unchanged with age or dietary supplementation (Fig. 5E–F).
Figure 5. Effect of dietary supplementation with resveratrol or pinostilbene on markers of dopaminergic neurons and function in the striatum with age.
(A–F) Dietary supplementation with resveratrol or pinostilbene had no effect on striatal DA, DOPAC, HVA, DAT or TH levels or expression. n=5–7 per group.
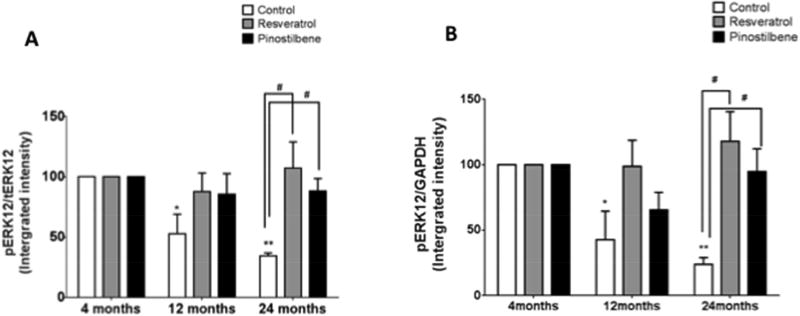
3.6 Dietary supplementation with resveratrol or pinostilbene increases ERK1/2 phosphorylation in the striatum of old mice
Striata isolated from young, middle-aged, and old animals were subject to Western blot analysis for phosphorylated (activated) ERK1/2 after eight weeks of dietary supplementation with resveratrol or pinostilbene. The ERK1/2 activation for young mice was set at 100%. Both diets lead to increases in ERK1/2 phosphorylation in the striata from the old animals as compared to age-matched control (Fig. 6A and 6B).
Figure 6. Effect of dietary supplementation with resveratrol or pinostilbene on the ERK1/2 phosphorylation in striatal tissue with age.
(A and B) Resveratrol and pinostilbene diet increased ERK1/2 phosphorylation in the striatum of old animals. ERK1/2 phosphorylation in young animals was set at 100%. Data are expressed as mean ± SEM. ** p < 0.01, * p < 0.05 compared to 4 month young control group. #p<0.05 compared to age-matched controls. n=3–4 for each treatment and age.
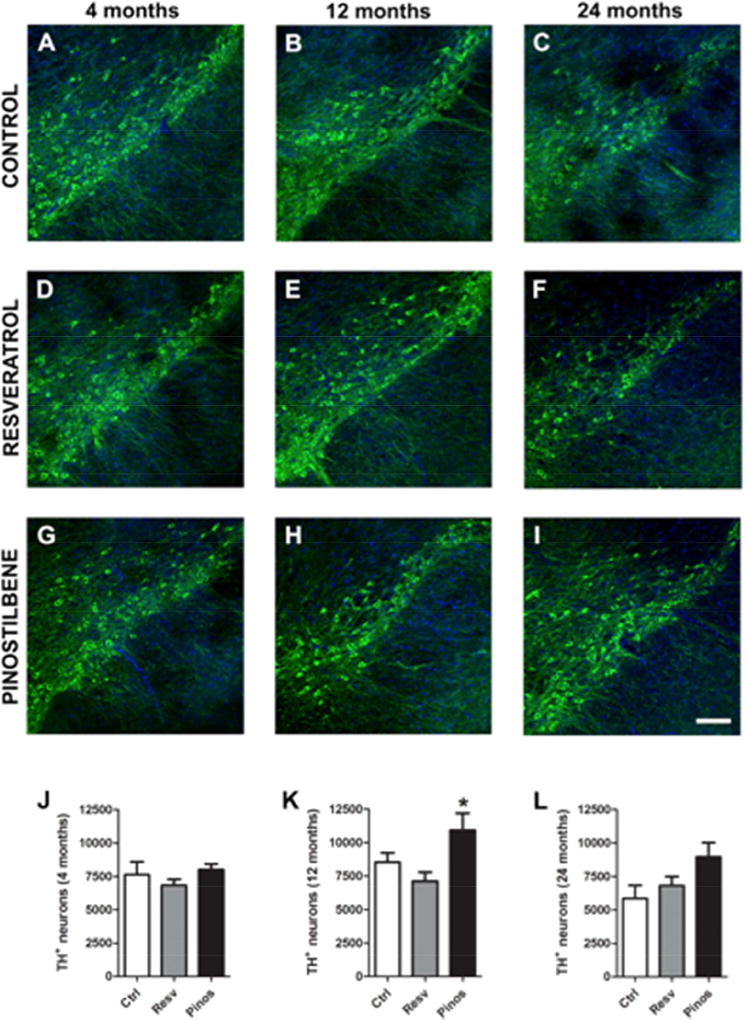
S1.1 Dietary supplementation with pinostilbene, but not resveratrol, increases the number of of TH+ neurons in the SN with age
Hindbrain slices were stained for TH after eight weeks of dietary supplementation with resveratrol or pinostilbene. Quantification of the images revealed that although there was a decline in the number of TH+ neurons with age, it was not a significant decrease (p= 0.13; Fig. 1S J–L control bars). Dietary supplementation with resveratrol did not alter the number of TH+ neurons in the SN in any age group. In contrast, dietary supplementation with pinostilbene significantly increased TH+ neurons in both middle-aged and old mice as compared to age-matched controls (Fig. 1S K and L, p<0.05).
4. Discussion
DA neurons in the ventral midbrain are important for motor coordination and voluntary movement. Therefore, a loss and/or decrease in the function of these DA neurons may lead to deficits in motor performance. For example, it has been reported that there is a decline in DA neurons [42, 43] and their nerve terminals [44–46] as well as decreases in motor performance with normal aging. [37, 47–50] Consequently, there is great interest in exogenous compounds that may combat DA neuronal loss and improve motor coordination associated with aging. In this study, we used SH-SY5Y cells as a model for DA neurons and a mouse model of aging to investigate the effect of resveratrol and pinostilbene, natural products that affect cell viability, cell signaling pathways, and age-related alterations in motor coordination and the nigrostriatal DA system [21, 51].
4.1 Natural products in vitro
Resveratrol, an isolated compound found in grapes, blueberries, and walnuts, is a well-studied natural antioxidant. [51–53] However, resveratrol has low bioavailability due to rapid metabolism into glucuronides and sulfate conjugates. [19, 54, 55] Even so, resveratrol has been shown to decrease 6-OHDA-induced cell death in SH-SY5Y cells. [21, 56] Analogs of resveratrol, in which the hydroxyl groups are methylated, possess prolonged bioavailability and increased ability to permeate cell membranes. [21, 57, 58] In this study, we compared the effects of resveratrol and pinostilbene, a methylated analog of resveratrol, on DA-induced cell death in SH-SY5Y cells. In agreement with a previous report [59], resveratrol protected SH-SY5Y cells from DA-induced cell death. However, ours is the first study to show that pinostilbene inhibits DA-induced cell death in these cells.
4.2 Natural products in vivo
In animal models of normal aging, others and we have reported declines in spontaneous motor activity and motor coordination. [37, 47–49] Motor coordination is largely controlled by the DA system. [60–63] Loss of DA neurons in the SN and projections to the striatum occur with age and in Parkinson’s disease. [43, 64, 65] Our in vitro data in DAergic cells suggest that resveratrol and pinostilbene may alleviate the loss of motor function seen with age. As a result, we sought to assess the effect of natural product supplementation on age-related motor deficits in C57BL/6 male mice using the challenging beam and cylinder behavioral tests to measure motor coordination and spontaneous activity, respectively. [34, 36] Ours is the first study to examine the effect of pinostilbene on age-related motor function and our data (Table 1) suggest that pinostilbene is more abundant in the brain compared to resveratrol. At four weeks, aged mice exhibited significantly higher number of errors on the challenging beam than young mice. After four weeks, there was no significant difference between errors made by old mice on the resveratrol and pinostilbene diet compared to their diet-matched young counterparts, indicating that dietary supplementation with the natural compounds improved motor coordination. Old mice on resveratrol-diet made less errors compared to their age-matched counterparts (p=0.053). This is in agreement with a previous report in which resveratrol-supplemented diet improved performance on the accelerating rotarod in 18–21 month old C57Bl/6 mice. [66] There was also a trend toward an increase in rearing activity in old animals fed resveratrol. These behavioral data are also consistent with the increase in the amounts of resveratrol, albeit trace amounts, detected in the brains of the middle-aged and old animals (Table 1). At four weeks, although not statistically significant, pinostilbene fed mice made less errors made on the challenging beam test compared to old control mice. Surprisingly, motor function of pinostilbene-fed mice appears to worsen at eight weeks. It is possible that resveratrol affects not only the brain regions responsible for motor control, but also improves skeletal muscle strength and function. Resveratrol is known to possess beneficial effects in attenuating damage and loss of muscle fibers, both in vitro and in vivo, likely through a reduction in oxidative damage [15, 67]. Alternatively, there may be differences in the pharmacokinetic profiles of resveratrol and pinostilbene with increasing age that contribute to the disparities seen in their in vivo effects. Middle-aged and old mice fed a pinostilbene-supplemented diet exhibited a significant increase in the number of rears as compared to diet-matched young mice indicating that resveratrol and pinostilbene may affect different components of motor function and, therefore, may provide neuroprotection in distinct brain regions.
To investigate any underlying changes in the DAergic system with age that may cause the decline in motor function, we examined the levels of DA, its metabolites, TH, and DAT present in the striatum. Striatal DA, TH, DAT, and the number of DA neurons in the SN have been reported to decrease with age. [42, 44–46, 48, 49, 68–71] However, as others and we have previously reported [37, 47] the age-related decline in motor function was not accompanied by age-related alterations in striatal DA, DOPAC, HVA, TH, or DAT in the groups of animals used in this study. Therefore, these DA neuronal markers may decrease with age in a species and/or strain specific manner. We also found no change in any of these striatal proteins following dietary supplementation with resveratrol or pinostilbene. Our current resveratrol data are in contrast to previous results from our lab in rat pups exposed to resveratrol in utero [72] and results from other labs following i.p. administration of resveratrol to female mice and male rats. [73, 74] These data suggest that the route of administration of resveratrol, species tested, and/or the sex and age of the animals are important considerations.
In addition to changes in the striatal DA system, alterations at the level of the SN have been also reported with age, including a loss of DA neurons. [43, 64, 75] We examined the number of DA neurons in the SN across ages and treatment groups. In the present study, quantitative analysis using a novel fluorescence unbiased stereology approach [40, 41] indicated that there was a trend toward fewer TH+ neurons with age. Dietary supplementation with pinostilbene for eight weeks, but not resveratrol, significantly increased the number of TH+ neurons in the middle-aged and old mice compared to the age-matched controls. Our behavioral data suggest that pinostilbene is more effective than resveratrol at restoring spontaneous activity with age whereas resveratrol is more effective in improving motor coordination with age as measured by the cylinder test and challenging beam test, respectively. These data support the hypothesis mentioned above that resveratrol and pinostilbene provide protection in distinct areas of the brain related to motor function. In seeming contrast to our current data, others have noted an increase in TH immunoreactivity in the SN following resveratrol exposure. [76, 77] The distinction between these previous reports and our current observation is that these groups measured the intensity of the immunoreactivity, whereas we counted the number of TH+ neurons using a novel stereological approach. Moreover, in the previous studies, diabetic mice, rats, and young animals were administered resveratrol via gavage. Together with the striatal DAergic markers, these data suggest that the effects of natural antioxidants may be dependent upon the route of administration, species, and/or age of the animals.
Next, we proceeded to examine the effects of resveratrol and pinostilbene on the ERK1/2 signaling pathways in the striatum. In both the resveratrol and pinostilbene supplemented groups, striatal ERK1/2 activation increased as compared to that of animals fed the standard diet. While others have noted increases and decreases in ERK1/2 activation following resveratrol administration in the hippocampus [12, 28], these are the first studies to examine the effects of resveratrol or pinostilbene on ERK1/2 activity in the striatum. This data complements work published by Parmar and colleagues demonstrating that ERK1/2 is important for DA cell survival [78] and possible DAergic region development [79]; therefore, the increase in the ERK1/2 by these phytochemicals could be contributing to the healthy state or functioning of DAergic neurons.
Overall, our data suggest that some of the motor deficits associated with aging may be alleviated by diets supplemented with resveratrol or pinostilbene. As these natural products are already widely used, it is important to understand the mechanism by which they may provide neuroprotection. In addition, as the elderly population increases and the incidence of slip, falls, and fractures increase, it is important to explore options for the treatment of age-related motor decline. Natural supplements provide an attractive option as they produce less unwanted side effects in a vulnerable population.
Supplementary Material
Figure 7. Effect of dietary supplementation with resveratrol or pinostilbene on the number of TH+ neurons in the substantia nigra with age.
(A–C; J–L) The number of TH+ neurons decreased with age in the SN. (D–F; J–L) Resveratrol diet did not alter the number of TH+ neurons in an age-dependent manner. (G–I; J–L) Pinostilbene diet increased the number of TH+ neurons in 12 and 24 month old animals. Data are expressed as mean ± SEM. * p < 0.05 compared to age matched control group.
Acknowledgments
These studies were funded, in part, by the National Institute of Aging (AG25848; JEC) and the Faculty Development Fund at Duquesne University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statements
The authors have no actual or potential conflicts of interest.
Bibliography
- 1.Onder G, Pedone C, Gambassi G. Risk of hip fracture in women: not only a smoking issue. Arch Intern Med. 2002;162:101–2. doi: 10.1001/archinte.162.1.101-a. [DOI] [PubMed] [Google Scholar]
- 2.Onder G, Penninx BW, Lapuerta P, Fried LP, Ostir GV, Guralnik JM, et al. Change in physical performance over time in older women: the Women's Health and Aging Study. J Gerontol A Biol Sci Med Sci. 2002;57:M289–93. doi: 10.1093/gerona/57.5.m289. [DOI] [PubMed] [Google Scholar]
- 3.Guralnik JM, Seeman TE, Tinetti ME, Nevitt MC, Berkman LF. Validation and use of performance measures of functioning in a non-disabled older population: MacArthur studies of successful aging. Aging (Milano) 1994;6:410–9. doi: 10.1007/BF03324272. [DOI] [PubMed] [Google Scholar]
- 4.Guralnik JM, Winograd CH. Physical performance measures in the assessment of older persons. Aging (Milano) 1994;6:303–5. doi: 10.1007/BF03324256. [DOI] [PubMed] [Google Scholar]
- 5.Allum JH, Carpenter MG, Honegger F, Adkin AL, Bloem BR. Age-dependent variations in the directional sensitivity of balance corrections and compensatory arm movements in man. J Physiol. 2002;542:643–63. doi: 10.1113/jphysiol.2001.015644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harman D. Role of free radicals in aging and disease. Ann N Y Acad Sci. 1992;673:126–41. doi: 10.1111/j.1749-6632.1992.tb27444.x. [DOI] [PubMed] [Google Scholar]
- 7.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915–22. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stadtman ER. Protein oxidation and aging. Science. 1992;257:1220–4. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- 9.Joseph JA, Denisova N, Villalobos-Molina R, Erat S, Strain J. Oxidative stress and age-related neuronal deficits. Mol Chem Neuropathol. 1996;28:35–40. doi: 10.1007/BF02815202. [DOI] [PubMed] [Google Scholar]
- 10.Floyd RA, Hensley K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol Aging. 2002;23:795–807. doi: 10.1016/s0197-4580(02)00019-2. [DOI] [PubMed] [Google Scholar]
- 11.Mariani E, Polidori MC, Cherubini A, Mecocci P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: an overview. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;827:65–75. doi: 10.1016/j.jchromb.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, Gu J, Wang X, Xie K, Luan Q, Wan N, et al. Antidepressant-like activity of resveratrol treatment in the forced swim test and tail suspension test in mice: the HPA axis, BDNF expression and phosphorylation of ERK. Pharmacology, biochemistry, and behavior. 2013;112:104–10. doi: 10.1016/j.pbb.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Roeper J. Dissecting the diversity of midbrain dopamine neurons. Trends Neurosci. 2013;36:336–42. doi: 10.1016/j.tins.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Michaelis EK. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. 2010;2:12. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon BS, Delgado-Diaz DC, Carson J, Fayad R, Wilson LB, Kostek MC. Resveratrol improves muscle function but not oxidative capacity in young mdx mice. Can J Physiol Pharmacol. 2014;92:243–51. doi: 10.1139/cjpp-2013-0350. [DOI] [PubMed] [Google Scholar]
- 16.Mancuso R, del Valle J, Modol L, Martinez A, Granado-Serrano AB, Ramirez-Nunez O, et al. Resveratrol improves motoneuron function and extends survival in SOD1(G93A) ALS mice. Neurotherapeutics. 2014;11:419–32. doi: 10.1007/s13311-013-0253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dal-Pan A, Terrien J, Pifferi F, Botalla R, Hardy I, Marchal J, et al. Caloric restriction or resveratrol supplementation and ageing in a non-human primate: first-year outcome of the RESTRIKAL study in Microcebus murinus. Age (Dordr) 2011;33:15–31. doi: 10.1007/s11357-010-9156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andlauer W, Kolb J, Siebert K, Furst P. Assessment of resveratrol bioavailability in the perfused small intestine of the rat. Drugs Exp Clin Res. 2000;26:47–55. [PubMed] [Google Scholar]
- 19.Soleas GJ, Angelini M, Grass L, Diamandis EP, Goldberg DM. Absorption of trans-resveratrol in rats. Methods Enzymol. 2001;335:145–54. doi: 10.1016/s0076-6879(01)35239-4. [DOI] [PubMed] [Google Scholar]
- 20.Soleas GJ, Yan J, Goldberg DM. Measurement of trans-resveratrol, (+)-catechin, and quercetin in rat and human blood and urine by gas chromatography with mass selective detection. Methods Enzymol. 2001;335:130–45. doi: 10.1016/s0076-6879(01)35238-2. [DOI] [PubMed] [Google Scholar]
- 21.Chao J, Li H, Cheng KW, Yu MS, Chang RC, Wang M. Protective effects of pinostilbene, a resveratrol methylated derivative, against 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells. J Nutr Biochem. 2010;21:482–9. doi: 10.1016/j.jnutbio.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Guyton KZ, Liu Y, Gorospe M, Xu Q, Holbrook NJ. Activation of mitogen-activated protein kinase by H2O2. Role in cell survival following oxidant injury. J Biol Chem. 1996;271:4138–42. doi: 10.1074/jbc.271.8.4138. [DOI] [PubMed] [Google Scholar]
- 23.Crossthwaite AJ, Hasan S, Williams RJ. Hydrogen peroxide-mediated phosphorylation of ERK1/2, Akt/PKB and JNK in cortical neurones: dependence on Ca(2+) and PI3-kinase. J Neurochem. 2002;80:24–35. doi: 10.1046/j.0022-3042.2001.00637.x. [DOI] [PubMed] [Google Scholar]
- 24.Jin K, Mao XO, Zhu Y, Greenberg DA. MEK and ERK protect hypoxic cortical neurons via phosphorylation of Bad. J Neurochem. 2002;80:119–25. doi: 10.1046/j.0022-3042.2001.00678.x. [DOI] [PubMed] [Google Scholar]
- 25.Bobermin LD, Quincozes-Santos A, Guerra MC, Leite MC, Souza DO, Goncalves CA, et al. Resveratrol prevents ammonia toxicity in astroglial cells. PLoS One. 2012;7:e52164. doi: 10.1371/journal.pone.0052164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maher P, Dargusch R, Bodai L, Gerard PE, Purcell JM, Marsh JL. ERK activation by the polyphenols fisetin and resveratrol provides neuroprotection in multiple models of Huntington's disease. Hum Mol Genet. 2011;20:261–70. doi: 10.1093/hmg/ddq460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simao F, Pagnussat AS, Seo JH, Navaratna D, Leung W, Lok J, et al. Pro-angiogenic effects of resveratrol in brain endothelial cells: nitric oxide-mediated regulation of vascular endothelial growth factor and metalloproteinases. J Cereb Blood Flow Metab. 2012;32:884–95. doi: 10.1038/jcbfm.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Almeida LM, Leite MC, Thomazi AP, Battu C, Nardin P, Tortorelli LS, et al. Resveratrol protects against oxidative injury induced by H2O2 in acute hippocampal slice preparations from Wistar rats. Archives of biochemistry and biophysics. 2008;480:27–32. doi: 10.1016/j.abb.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Lee EO, Park HJ, Kang JL, Kim HS, Chong YH. Resveratrol reduces glutamate-mediated monocyte chemotactic protein-1 expression via inhibition of extracellular signal-regulated kinase 1/2 pathway in rat hippocampal slice cultures. J Neurochem. 2010;112:1477–87. doi: 10.1111/j.1471-4159.2009.06564.x. [DOI] [PubMed] [Google Scholar]
- 30.Lee SJ, Kim MM. Resveratrol with antioxidant activity inhibits matrix metalloproteinase via modulation of SIRT1 in human fibrosarcoma cells. Life Sci. 2011;88:465–72. doi: 10.1016/j.lfs.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Polunin KE, Schmalz H-G. Application of Chromium-Arene Complexes in the Organic Synthesis. Efficient Synthesis of Stilbene Phytoalexins. Russian Journal of Coordination Chemistry. 2004;30:252–61. [Google Scholar]
- 32.Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–87. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 33.Glajch KE, Fleming SM, Surmeier DJ, Osten P. Sensorimotor assessment of the unilateral 6-hydroxydopamine mouse model of Parkinson's disease. Behav Brain Res. 2012;230:309–16. doi: 10.1016/j.bbr.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fleming SM, Salcedo J, Fernagut PO, Rockenstein E, Masliah E, Levine MS, et al. Early and progressive sensorimotor anomalies in mice overexpressing wild-type human alpha-synuclein. J Neurosci. 2004;24:9434–40. doi: 10.1523/JNEUROSCI.3080-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fleming SM, Fernagut PO, Chesselet MF. Genetic mouse models of parkinsonism: strengths and limitations. NeuroRx. 2005;2:495–503. doi: 10.1602/neurorx.2.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hwang DY, Fleming SM, Ardayfio P, Moran-Gates T, Kim H, Tarazi FI, et al. 3,4-dihydroxyphenylalanine reverses the motor deficits in Pitx3-deficient aphakia mice: behavioral characterization of a novel genetic model of Parkinson's disease. J Neurosci. 2005;25:2132–7. doi: 10.1523/JNEUROSCI.3718-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allen EN, Cavanaugh JE. Loss of motor coordination in an aging mouse model. Behav Brain Res. 2014;267:119–25. doi: 10.1016/j.bbr.2014.03.032. [DOI] [PubMed] [Google Scholar]
- 38.Smith AD, Kozlowski DA, Bohn MC, Zigmond MJ. Effect of AdGDNF on dopaminergic neurotransmission in the striatum of 6-OHDA-treated rats. Exp Neurol. 2005;193:420–6. doi: 10.1016/j.expneurol.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 39.Allen E, Carlson KM, Zigmond MJ, Cavanaugh JE. L-DOPA reverses motor deficits associated with normal aging in mice. Neurosci Lett. 2011;489:1–4. doi: 10.1016/j.neulet.2010.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tapias V, Greenamyre JT. A rapid and sensitive automated image-based approach for in vitro and in vivo characterization of cell morphology and quantification of cell number and neurite architecture. Curr Protoc Cytom. 2014;68:12–33. 1–22. doi: 10.1002/0471142956.cy1233s68. [DOI] [PubMed] [Google Scholar]
- 41.Tapias V, Greenamyre JT, Watkins SC. Automated imaging system for fast quantitation of neurons, cell morphology and neurite morphometry in vivo and in vitro. Neurobiol Dis. 2013;54:158–68. doi: 10.1016/j.nbd.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Emborg ME, Ma SY, Mufson EJ, Levey AI, Taylor MD, Brown WD, et al. Age-related declines in nigral neuronal function correlate with motor impairments in rhesus monkeys. The Journal of comparative neurology. 1998;401:253–65. [PubMed] [Google Scholar]
- 43.Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 44.Salvatore MF, Apparsundaram S, Gerhardt GA. Decreased plasma membrane expression of striatal dopamine transporter in aging. Neurobiol Aging. 2003;24:1147–54. doi: 10.1016/s0197-4580(03)00129-5. [DOI] [PubMed] [Google Scholar]
- 45.Yue F, Zeng S, Wu D, Yi D, Alex Zhang Y, Chan P. Age-related decline in motor behavior and striatal dopamine transporter in cynomolgus monkeys. Journal of neural transmission (Vienna, Austria : 1996) 2012;119:943–52. doi: 10.1007/s00702-012-0770-6. [DOI] [PubMed] [Google Scholar]
- 46.Troiano AR, Schulzer M, de la Fuente-Fernandez R, Mak E, McKenzie J, Sossi V, et al. Dopamine transporter PET in normal aging: dopamine transporter decline and its possible role in preservation of motor function. Synapse. 2010;64:146–51. doi: 10.1002/syn.20708. [DOI] [PubMed] [Google Scholar]
- 47.Cantuti-Castelvetri I, Shukitt-Hale B, Joseph JA. Dopamine neurotoxicity: age-dependent behavioral and histological effects. Neurobiol Aging. 2003;24:697–706. doi: 10.1016/s0197-4580(02)00186-0. [DOI] [PubMed] [Google Scholar]
- 48.Boger HA, Middaugh LD, Huang P, Zaman V, Smith AC, Hoffer BJ, et al. A partial GDNF depletion leads to earlier age-related deterioration of motor function and tyrosine hydroxylase expression in the substantia nigra. Exp Neurol. 2006;202:336–47. doi: 10.1016/j.expneurol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 49.Colebrooke RE, Humby T, Lynch PJ, McGowan DP, Xia J, Emson PC. Age-related decline in striatal dopamine content and motor performance occurs in the absence of nigral cell loss in a genetic mouse model of Parkinson's disease. Eur J Neurosci. 2006;24:2622–30. doi: 10.1111/j.1460-9568.2006.05143.x. [DOI] [PubMed] [Google Scholar]
- 50.Barreto G, Huang TT, Giffard RG. Age-related defects in sensorimotor activity, spatial learning, and memory in C57BL/6 mice. Journal of neurosurgical anesthesiology. 2010;22:214–9. doi: 10.1097/ANA.0b013e3181d56c98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pangeni R, Sahni JK, Ali J, Sharma S, Baboota S. Resveratrol: review on therapeutic potential and recent advances in drug delivery. Expert Opin Drug Deliv. 2014;11:1285–98. doi: 10.1517/17425247.2014.919253. [DOI] [PubMed] [Google Scholar]
- 52.Pangeni R, Sharma S, Mustafa G, Ali J, Baboota S. Vitamin E loaded resveratrol nanoemulsion for brain targeting for the treatment of Parkinson's disease by reducing oxidative stress. Nanotechnology. 2014;25:485102. doi: 10.1088/0957-4484/25/48/485102. [DOI] [PubMed] [Google Scholar]
- 53.Bastianetto S, Menard C, Quirion R. Neuroprotective action of resveratrol. Biochim Biophys Acta. 2015;1852:1195–201. doi: 10.1016/j.bbadis.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 54.Marier JF, Vachon P, Gritsas A, Zhang J, Moreau JP, Ducharme MP. Metabolism and disposition of resveratrol in rats: extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model. J Pharmacol Exp Ther. 2002;302:369–73. doi: 10.1124/jpet.102.033340. [DOI] [PubMed] [Google Scholar]
- 55.Goldberg DM, Yan J, Soleas GJ. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin Biochem. 2003;36:79–87. doi: 10.1016/s0009-9120(02)00397-1. [DOI] [PubMed] [Google Scholar]
- 56.Chao J, Yu MS, Ho YS, Wang M, Chang RC. Dietary oxyresveratrol prevents parkinsonian mimetic 6-hydroxydopamine neurotoxicity. Free Radic Biol Med. 2008;45:1019–26. doi: 10.1016/j.freeradbiomed.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 57.Wen X, Walle T. Methylated flavonoids have greatly improved intestinal absorption and metabolic stability. Drug Metab Dispos. 2006;34:1786–92. doi: 10.1124/dmd.106.011122. [DOI] [PubMed] [Google Scholar]
- 58.Wen X, Walle T. Methylation protects dietary flavonoids from rapid hepatic metabolism. Xenobiotica. 2006;36:387–97. doi: 10.1080/00498250600630636. [DOI] [PubMed] [Google Scholar]
- 59.Lee MK, Kang SJ, Poncz M, Song KJ, Park KS. Resveratrol protects SH-SY5Y neuroblastoma cells from apoptosis induced by dopamine. Exp Mol Med. 2007;39:376–84. doi: 10.1038/emm.2007.42. [DOI] [PubMed] [Google Scholar]
- 60.Pijnenburg AJ, van Rossum JM. Letter: Stimulation of locomotor activity following injection of dopamine into the nucleus accumbens. The Journal of pharmacy and pharmacology. 1973;25:1003–5. doi: 10.1111/j.2042-7158.1973.tb09995.x. [DOI] [PubMed] [Google Scholar]
- 61.Jackson DM, Anden NE, Dahlstrom A. A functional effect of dopamine in the nucleus accumbens and in some other dopamine-rich parts of the rat brain. Psychopharmacologia. 1975;45:139–49. doi: 10.1007/BF00429052. [DOI] [PubMed] [Google Scholar]
- 62.Costall B, Marsden CD, Naylor RJ, Pycock CJ. The relationship between striatal and mesolimbic dopamine dysfunction and the nature of circling responses following 6-hydroxydopamine and electrolytic lesions of the ascending dopamine systems of rat brain. Brain Res. 1976;118:87–113. doi: 10.1016/0006-8993(76)90843-x. [DOI] [PubMed] [Google Scholar]
- 63.Costall B, Naylor RJ. Actions of dopaminergic agonists on motor function. Adv Neurol. 1975;9:285–97. [PubMed] [Google Scholar]
- 64.McGeer PL, McGeer EG, Suzuki JS. Aging and extrapyramidal function. Arch Neurol. 1977;34:33–5. doi: 10.1001/archneur.1977.00500130053010. [DOI] [PubMed] [Google Scholar]
- 65.Fearnley JM, Revesz T, Brooks DJ, Frackowiak RS, Lees AJ. Diffuse Lewy body disease presenting with a supranuclear gaze palsy. J Neurol Neurosurg Psychiatry. 1991;54:159–61. doi: 10.1136/jnnp.54.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–68. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ryan MJ, Jackson JR, Hao Y, Williamson CL, Dabkowski ER, Hollander JM, et al. Suppression of oxidative stress by resveratrol after isometric contractions in gastrocnemius muscles of aged mice. J Gerontol A Biol Sci Med Sci. 2010;65:815–31. doi: 10.1093/gerona/glq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cruz-Muros I, Afonso-Oramas D, Abreu P, Barroso-Chinea P, Rodriguez M, Gonzalez MC, et al. Aging of the rat mesostriatal system: differences between the nigrostriatal and the mesolimbic compartments. Exp Neurol. 2007;204:147–61. doi: 10.1016/j.expneurol.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 69.McNeill TH, Koek LL. Differential effects of advancing age on neurotransmitter cell loss in the substantia nigra and striatum of C57BL/6N mice. Brain Res. 1990;521:107–17. doi: 10.1016/0006-8993(90)91530-t. [DOI] [PubMed] [Google Scholar]
- 70.Meng SZ, Ozawa Y, Itoh M, Takashima S. Developmental and age-related changes of dopamine transporter, and dopamine D1 and D2 receptors in human basal ganglia. Brain Res. 1999;843:136–44. doi: 10.1016/s0006-8993(99)01933-2. [DOI] [PubMed] [Google Scholar]
- 71.Morgan DG. The dopamine and serotonin systems during aging in human and rodent brain. A brief review. Prog Neuropsychopharmacol Biol Psychiatry. 1987;11:153–7. doi: 10.1016/0278-5846(87)90053-4. [DOI] [PubMed] [Google Scholar]
- 72.Rose KM, Parmar MS, Cavanaugh JE. Dietary supplementation with resveratrol protects against striatal dopaminergic deficits produced by in utero LPS exposure. Brain Res. 2014;1573:37–43. doi: 10.1016/j.brainres.2014.05.028. [DOI] [PubMed] [Google Scholar]
- 73.Di Liberto V, Makela J, Korhonen L, Olivieri M, Tselykh T, Malkia A, et al. Involvement of estrogen receptors in the resveratrol-mediated increase in dopamine transporter in human dopaminergic neurons and in striatum of female mice. Neuropharmacology. 2012;62:1011–8. doi: 10.1016/j.neuropharm.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 74.Sarubbo F, Ramis MR, Aparicio S, Ruiz L, Esteban S, Miralles A, et al. Improving effect of chronic resveratrol treatment on central monoamine synthesis and cognition in aged rats. Age (Dordr) 2015;37:9777. doi: 10.1007/s11357-015-9777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fleming SM, Chesselet MF. Behavioral phenotypes and pharmacology in genetic mouse models of Parkinsonism. Behav Pharmacol. 2006;17:383–91. doi: 10.1097/00008877-200609000-00004. [DOI] [PubMed] [Google Scholar]
- 76.Brambilla Bagatini P, Xavier LL, Neves L, Saur L, Barbosa S, Baptista PP, et al. Resveratrol prevents akinesia and restores neuronal tyrosine hydroxylase immunoreactivity in the substantia nigra pars compacta of diabetic rats. Brain Res. 2014;1592:101–12. doi: 10.1016/j.brainres.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 77.Lofrumento DD, Nicolardi G, Cianciulli A, De Nuccio F, La Pesa V, Carofiglio V, et al. Neuroprotective effects of resveratrol in an MPTP mouse model of Parkinson's-like disease: possible role of SOCS-1 in reducing pro-inflammatory responses. Innate Immun. 2014;20:249–60. doi: 10.1177/1753425913488429. [DOI] [PubMed] [Google Scholar]
- 78.Parmar MS, Jaumotte JD, Wyrostek SL, Zigmond MJ, Cavanaugh JE. Role of ERK1, 2, and 5 in dopamine neuron survival during aging. Neurobiol Aging. 2014;35:669–79. doi: 10.1016/j.neurobiolaging.2013.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parmar MS, Jaumotte JD, Zigmond MJ, Cavanaugh JE. ERK1, 2, and 5 expression and activation in dopaminergic brain regions during postnatal development. Int J Dev Neurosci. 2015;46:44–50. doi: 10.1016/j.ijdevneu.2015.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.