Abstract
Inflammatory bowel diseases (IBD) are caused by the convergence of microbial, environmental, and genetic factors. Diet significantly alters these interactions by affecting both the host and microbiome. Using a mucosal inflammatory model that resembles the human condition of ileal pouchitis, we investigated the effects of Control (CONT) or Antioxidant (AOX) diet, containing pharmacologically relevant levels of 4 micronutrients, on disease risk in wild-type and IL-10−/− animals following surgical self-filling (SF) ileal blind loop placement. Although no differences were found in body weight change or survival, IL-10−/− CONT animals had significantly larger lymphoid organs compared with IL-10−/− AOX or with WT. SF loops from IL-10−/− CONT loop mucosa demonstrated histological inflammation, characterized by goblet cell depletion, increased mucosal myeloperoxidase (MPO), and elevated IFNγ, TNFα, and IL-17α gene expression, which AOX attenuated. AOX elevated luminal IgA in IL-10−/− animals, but not significantly in WT. In IL-10−/− animals, AOX significantly decreased the percentage of CD4+T-bet and CD4+RORγ T-cells compared with CONT, with no changes in CD4+Foxp3+ Treg cells. 16S rRNA gene sequencing demonstrated AOX increased microbial alpha diversity compared with CONT in both genotypes. Notably, colonizing germ-free IL-10−/− hosts with CONT bacterial communities, but not AOX, recapitulated the inflammatory phenotype. Collectively, these findings highlight that common dietary antioxidant micronutrients reshape the gut microbial community to mitigate intestinal inflammatory profiles in genetically susceptible hosts. Insights into the dietary-immune-microbial nexus may improve understanding for recurrent inflammatory episodes in susceptible patient populations and opportunities for practical therapeutics to restore immune and microbial homeostasis.
Keywords: Self-filling Loops, Pouchitis, Inflammatory Bowel Disease, Nutrition Therapy, T Regulatory Lymphocytes, Antioxidants, Retinoic Acid, Ascorbic Acid, Ulcerative Colitis
Introduction
The incidence of inflammatory bowel diseases (IBD) has increased in recent decades. [1] While over 200 genetic variants are now associated with increased disease risk, genetic drift alone cannot account for these emerging trends. [2] Other factors associated with the ‘western’ lifestyle, such as diet, environment, and lifestyle (improved sanitation and antibiotics), which are often associated with microbial disturbances, are more plausible triggers for disease. [3–5] Ulcerative colitis is one clinical subset of IBD that occurs exclusively in the colon and never involves the small intestine. Ultimately, the final treatment in medically refractory UC is removal of the colon followed by the surgical creation of an ileal pouch with anal anastomosis (IPAA). Unfortunately, about half of IBD patients who receive IPAA develop pouch inflammation, termed ‘pouchitis’, which is in contrast to patients with colon cancer (familial adenomatous polyposis) who are treated with IPAA and exhibit much lower risk. [6]
We recently reported a surgical model of ileal self-filling loops (SFLs), which exhibits immune, microbiome, and histologic features similar to human pouchitis. [7] Specifically, our work found stasis in the SFL, but not self-emptying loops (SELs), promote changes in mucosal morphology, transcriptome profiles, and microbial communities that subsequently resemble the colon. These changes were driven in part by innate toll-like receptor (TLR) signaling, as adaptations were dramatically attenuated in TLR4-deficient animals. In wild type animals, SFL results in adaptations that mimic colonic morphology, but are otherwise devoid of inflammatory disease. However, in mice susceptible to colonic inflammation – IL-10 deficient animals – we observe the SFL displays severe inflammation, resembling pouchitis. SELs in IL-10 animals remain similar to native ileum in those animals. Furthermore, it was demonstrated the microbial community in SFLs, but not SELs, induce colonic disease when transferred to germ-free recipient IL-10 deficient mice, highlighting a causal role for gut dysbiosis in triggering mucosal inflammation in susceptible hosts. [7]
Building upon that work, we investigated whether dietary factors influence disease pathogenesis in this model. David et al previously showed dietary alterations rapidly and predictably changes (within 24 hours) gut microbial communities. [8] Persson et al reported dietary sugars may be a risk factor for IBD while Chassaing et al reported food additives, such as emulsifiers, induced dysbiosis and promoted intestinal inflammation. [9,10] However, in the setting of the IBD, particularly in UC IPAA where disease risk is high, few clinical studies and no experimental data are available for the impact of diet and/or dietary supplements. In the context of micronutrients, Lanco et al reported a prospective food frequency survey in human patients with endoscopic and histological pouch examination in 160 patients, identifying increased pouchitis incidence in patients with decreased intake of several micronutrients (including vitamin A, vitamin C, cryptoxanthin, and lycopene). Since little research has been performed on the role of micronutrients in the context of IBD, we focused on potential protective vitamins or minerals in the context of ulcerative colitis and pouchitis. From an immunological standpoint, retinoic acid (vitamin A) suppresses T cell differentiation (Th1 and Th17) responses, mediated in part through CD103+ tolerizing dendritic cells (DCs) [11,12], and modulates plasma cell immunoglobulin-A class switch and production. [13] CD103+ DCs induce expression of the CC-chemokine receptor 9, which serves as an immune cell ligand to traffic lymphocytes to the lamina propria. Since the Th1 and Th17 T cell populations are hallmarks of ulcerative colitis response, this evidence provided a cogent mechanism of possible protection in pouchitis. In addition, ascorbic acid and Vitamin E were studied empirically in models of chemical injury to the mucosa, specifically in DSS and acetic acid induced colitis, respectively [14,15]. These micronutrients appeared to provide antioxidant functions to the mucosa and attenuated tissue damage. Finally, low selenium levels have been found in patients with Ulcerative Colitis and Crohn’s disease [16]. Collectively, these observations suggested that certain dietary micronutrients may interact with disease disk in the setting of IBD, although it remained unclear to what extent under pouchitis genetic susceptibility.
Therefore, while focus has been placed on macronutrient intake in other chronic disease settings, such as fiber and dietary lipids, greater understanding of micronutrients role in recurrent IBDs is largely unexplored. Finally, since UC related pouchitis also responds to antibiotics evidence clearly suggests strong etiological roles for the microbiota in pouchitis. Here, we employed our established pouchitis model of SFL disease in genetically susceptible hosts to address the hypothesis that the addition of specific micronutrients (retinoic acid, Vitamin C, Vitamin E, and selenium) at pharmacologically relevant levels would alter the inflammatory profiles and associated microbial structure and function in genetically susceptible hosts.
Materials and Methods
Animals
All animal protocols were approved by IACUC at the University of Chicago. Animals were either wild-type (WT) or IL-10−/− gene-deficient mice on the C57Bl/6 background bred and housed under standard 12:12 light/dark conditions at the University of Chicago. Female mice, 6–8 weeks old, were fed ad libitum gel diet 76A (Cat# 72-07-5022, Clear H20, Portland, ME) for 5-days prior to surgery to avoid obstruction at the surgical anastomotic sight. Females were used because of their better survival rate post-operatively. Animals were anesthetized with ketamine/xylazine. Aseptic surgery was performed to resect 2.5 cm of ileum 3 cm proximal to the ileal-cecal value with anastomosis to the ileum using 8-0 suture (Figure 1). In contrast to human surgery, colons are not removed in our mouse model, since this procedure dramatically complicates the surgical procedure, prolongs surgical recovery, and decreases animal survival. The abdominal wall was closed with interrupted 4-0 silk sutures and skin with staples. Analgesics (buprenorphine 20 mg/kg BW) were provided post-operatively. Animals remained on gel diet 76A for 12 days during surgical recovery and then were fed a purified Control diet (CONT) or Antioxidant diet (AOX) (Table 1) for 16 days. The antioxidant diet was identical to CONT but with pharmacologically relevant doses of 4 micronutrients: retinoic acid, vitamin C, vitamin E, and selenium. Animals were weighed daily. Mice were humanely euthanized and intestinal loop mucosal scrapings were collected. Mesenteric lymph nodes were weighed and normalized to body weight. Loop contents were collected and snap frozen at −80°C for microbiota analysis, Biolog assay, and IgA/IgG measurements.
Figure 1.
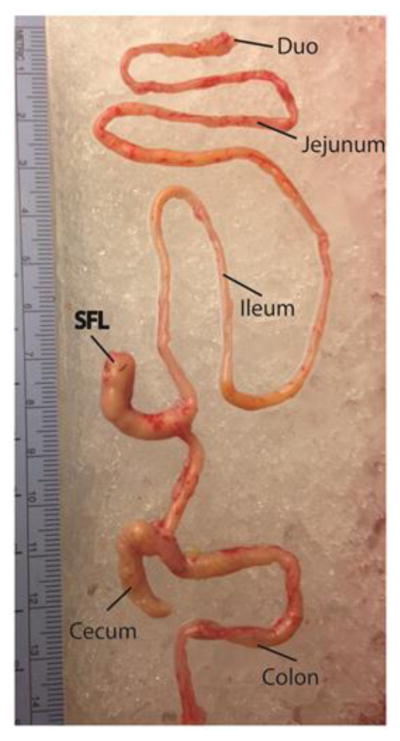
Gross Anatomy of the Self-Filling Loop (SFL) in relation to other regions of the small and large intestine. Ruler in centimeters.
Table 1.
Experimental diets.
| Ingredient, g/kg | Control Diet | AOX Diet |
|---|---|---|
| Casein | 180 | 180 |
| L-Methionine | 2.5 | 2.5 |
| Sucrose | 100 | 100 |
| Corn Starch | 240 | 240 |
| Maltodextrin | 120 | 120 |
| Cellulose | 220.18 | 202.143 |
| Soybean Oil | 80 | 80 |
| Choline Bitartrate | 2.5 | 2.5 |
| Mineral Mix, AIN-93G-MX (94046) | 38.5 | 38.5 |
| Calcium Phosphate, monobasic, monohydrate | 3.4 | 3.4 |
| Calcium Carbonate | 1 | 1 |
| Ferric Citrate | 0.4 | 0.4 |
| TBHQ, antioxidant | 0.02 | 0.02 |
| Vitamin Mix, AIN-93-VX w/A, D, E (94047) | 11.5 | 11.5 |
| Vitamin Mix, AIN-93 w/o A, D, E (120379) | 0 | 0 |
| Vitamin A Palmitate (500,000 IU/g) | 0 | 0.037 |
| Vitamin C, L-ascorbyl-2-polyphosphate (35%) | 0 | 16 |
| Vitamin E, DL-alpha tocopheryl acetate (500 IU/g) | 0 | 0.35 |
| Vitamin D3, cholecalciferol (500,000 IU/g) | 0 | 0 |
| Sodium Selenite (0.0445% in sucrose) | 0 | 1.65 |
|
| ||
| Calculated Nutrient Profile | ||
|
| ||
| Kcal/g | 3.2 | 3.2 |
|
| ||
| Macronutrient, % by weight | ||
|
| ||
| Protein | 16 | 16 |
| Carbohydrate | 45 | 45 |
| Fat | 8 | 8 |
| Vitamin A, IU/kg | 4600 | 23100 |
| Vitamin E, IU/kg | 86 | 261 |
| Vitamin C, g/kg | 0 | 5.6 |
| Se, mg/kg | 0.16 | 0.5 |
RNA extraction, cDNA synthesis, and quantitative real-time PCR
Total RNA isolation was performed on mucosal scrapings with the Trizol (Ambion) and chloroform method. RNA concentration and quality was determined by UV spectrophotometry. RNA was reverse transcribed using anchored-oligo (dT) and random hexamer primers. Following RT (Transcriptor Reverse Transcriptase Reaction buffer 5X; Protector RNase Inhibitor 40U/μl; Deoxynucleotide Mix, 10mM; Transcriptor Reverse Transcriptase 20U/μl), RT-PCR was performed on resulting cDNA in triplicate, using the manufacturer’s protocol (Roche Applied Science), in LightCycler® capillary. Gene-specific primers from murine GAPDH, TNFα, IFNγ, IL-17α, IL-10, CD103, and CCR9 are shown in reference [17] Table 1. The optimal concentration of cDNA and primers, as well as the maximum efficiency of amplification, were obtained through five-point, two-fold dilution curve analysis for each gene. RT-PCR amplification consisted of an initial denaturation step (95°C for 10 min), 45 cycles of denaturation (95°C for 10s), annealing (55°C for 20s) and extension (60°C for 30s), followed by a final incubation at 55°C for 30s and cooling at 40°C for 30s. All measurements were normalized by the expression of GAPDH gene, considered as a stable housekeeping gene. Gene expression was determined using the delta-delta Ct method: 2−▲▲CT(▲▲CT=[Ct(target gene) – Ct(GAPDH)]patient – [Ct(target gene) – Ct(GAPDH)]control). Real-time data were analyzed using the Roche LightCycle® (Roche Applied Science).
Immunofluorescence (IF), & Histopathological Scoring
Mucosal tissues were fixed in 4% formalin/PBS overnight. Five-micron sections were cut, deparaffinized, and processed for H&E, periodic acid Schiff-base (PAS), alcian-blue staining, or immunofluorescence (IF) with Anti-MPO and Anti-Syndecan. For IF, antigen retrieval was performed in sodium citrate (pH 6.0), blocked in 10% BSA, and primary antibody (1:200 rabbit anti-MPO; 1:1000 rabbit anti-syndecan) overnight at 4°C. Samples were washed and Alexa Fluor conjugated secondary antibody (555 goat anti-rabbit, Invitrogen life science) was applied. Slides were counterstained with DAPI and visualized with a Leica DM2500 microscope (Leica Microsystems, Wetzlar, Germany) through a 20X lens objective using Image Pro-Plus software (Media Cybernetics, Silver Springs, MD, USA) for image capture. Goblet cell depletion scoring was blindly assessed by two investigators with the following system (0 = 0% loss, 1 = 15%, 2 = 35%, 3 = 50%, 4 = 65%, 5 = 85%, 6 = 100%) and displayed as GC depletion score.
Loop Content IgA and IgG Enzyme-Linked Immunosorbent Assay
Loop contents were weighed and placed in PBS + protease inhibitor, vortexed for 20 minutes, and spun down for 2 minutes at 1000 x g. Samples were diluted 1:100 and the IgA and IgG ELISAs were performed per manufacturer instructions (Cat# 88-50450, Cat# 88-50400; eBiosciences, San Diego, CA) and normalized to content wet weight.
16S DNA isolation
Intestinal contents were homogenized in 1 mL extraction buffer [50mM Tris (pH 7.4), 100mM EDTA (pH 8.0), 400mM NaCl, 0.5% SDS] containing 20uL proteinase K (20mg/ml). 0.1-mm-diameter zirconia/silica beads (BioSpec Products, Bartlesville, OK, USA) were added to the extraction tubes and a Mini-Beadbeater-8 cell disrupter (BioSpec Products) for 2 × 1 min to lyse cells. After overnight incubation at 55°C with agitation, extraction with phenol:chloroform:isoamyl alcohol, and precipitation with ethanol were performed. Isolated DNA was dissolved in nuclease-free water and stored at −80°C.
16S rRNA-based Polymerase Chain Reaction, llumina Library Preparation, and Data Analysis
Polymerase chain reaction was performed as follows: 5μL of 10x Ex Taq buffer containing 20mM MgCl2 (Takara, Tokyo, Japan), 4μL of 2.5mM dNTP Mixture (Takara), 1μL each of forward (27F, 5′-AGA GTT TGA TCC TGG CTC AG-3′) and reverse (1492R, GGT TAC CTT GTT ACG ACT-3′) primer (10mM each), 0.25μL of Taq polymerase (Takara), 36.75μL nuclease-free water, and 2μL of DNA template. The PCR conditions were: 94°C for 5 min followed by 30 cycles of amplification consisting of denaturation at 94°C for 30 sec, annealing at 58°C for 1 min, and extension at 72°C for 1.5 min.
PCR primers used were specific for the 338–806 bp region of the 16S rRNA encoding gene and contained Illumina 3′ adapter sequences as well as a 12-bp barcode as previously described. [18] This barcode-based primer approach allowed sequencing of multiple samples in a single sequencing run without the need for physical partitioning. Sequencing was performed using an Illumina MiSeq DNA sequencer at Argonne National Laboratory’s Next Generation Sequencing Core. Forward sequences were then trimmed and classified with the QIIME toolkit (version 1.8.0). Using the QIIME wrappers, OTUs were picked at 97% sequence identity using uclust and a representative sequence (centroid) was then chosen for each OTU by selecting the most abundant sequence in that OTU. These representative sequences were aligned using PyNAST and taxonomy was assigned to them using the uclust consensus taxonomy assigner. The PyNAST-aligned sequences were also used to build a phylogenetic tree with FastTree and weighted UniFrac distances then computed between all samples for additional ecological analyses, including principal coordinates analysis (PCoA).
Biolog assay
To examine the substrate utilization of loop microbial communities, biolog assays were performed. Under anaerobic conditions, equal amounts of loop contents (~10 mg) were resuspended in 1 mL Biolog Inoculum Fluid-A (IF-A; Cat #72401, Biolog, Heyward, CA) containing tetrazolium indicator dye to measure metabolic redox. After thorough vortex homogenization, particulate was pelleted at low speed for 10s on a bench top mini-centrifuge and 10 μL supernatant were transferred and thoroughly mixed in a new tube of IF-A (~12 mL). Then, microbial samples were transferred using a multi-channel pipette reservoir at 100 μL per well to a GenIII plate (Cat #2030, Biolog). After inoculation in the anaerobic chamber, plates were incubated overnight at 37°C. The following day, optical densities at 590 nm were recorded for each plate-well and Bray-Curtis dissimilarity was used to compare experimental groups Two-way cluster dendrograms and Indicator analysis. [19]
Flow Cytometry
To examine cellular immune changes in response to dietary treatments, an additional experiment in IL-10−/− animals was included for flow cytometry analysis. The following conjugated antibodies were purchased from eBioscience: T-bet (4B10), Foxp3 (FJK-16s), Rorgt (AFKJS-9), Mouse IgG1, Rat IgG2a. The following antibodies were purchased from BD Biosciences: CD45 (30-F11), Fc Block™ (2.4G2). The following antibodies were purchased from Biolegend: CD4 (GK1.5), TCRb (H57-597). Aqua LIVE/DEAD® Fixable Aqua Dead Cell Stain Kit was purchased from Life Technologies. Cells were isolated from lamina propria as previously described [20] to assess intra-nuclear levels of Foxp3, T-bet, and RORγt in live CD45+ TCRb+ CD4+ T cells. Cells were incubated in Fc Block™ for 5 min following 10 min of Aqua LIVE/DEAD® and 20 min surface antibody incubation. Subsequently, cells were permeabilized with the Foxp3 fixation/permeabilization kit (eBioscience) for 45 minutes at 4°C following transcription factor staining for 30 minutes at 4 °C. Flow cytometry analysis was performed with a 9-color BD FACSCanto (BD Biosciences) using FlowJo software (FlowJo, LLC).
Conventionalization of germ-free IL-10 mice
Germ-free animals were individually housed and maintained in plastic isolators. Gnotobiotic diets were sterilized by autoclave and sterility was screened weekly. To conventionalize germ-free IL-10 animals, 1 g of luminal contents was collected from wild-type CONT and AOX loops, placed in 5 mL sterile PBS, and vigorously resuspended. Particulate matter was gently centrifuged in a bench-top centrifuge briefly (1000 x g for 10 seconds) and 200 μL of microbial supernatant was administered in each mouse via oral gavage. This process was repeated 7 days later to reinforce conventionalization. Donor contents and the resulting colonic communities in conventionalized animals were collected for 16S rRNA gene sequencing 3 weeks later. Colonic tissue was collected for histology and mucosal gene expression as described above.
Statistics
Data from the in vivo studies are presented as mean ± SEM; statistical significance was analyzed by the Analysis of Variance test (ANOVA) for multiple groups followed by analysis between two groups (Tukey-Frame’s multiple comparisons test) or T-test. P<0.05 was considered statistically significant, unless otherwise stated.
Results
Mucosal Cytokines
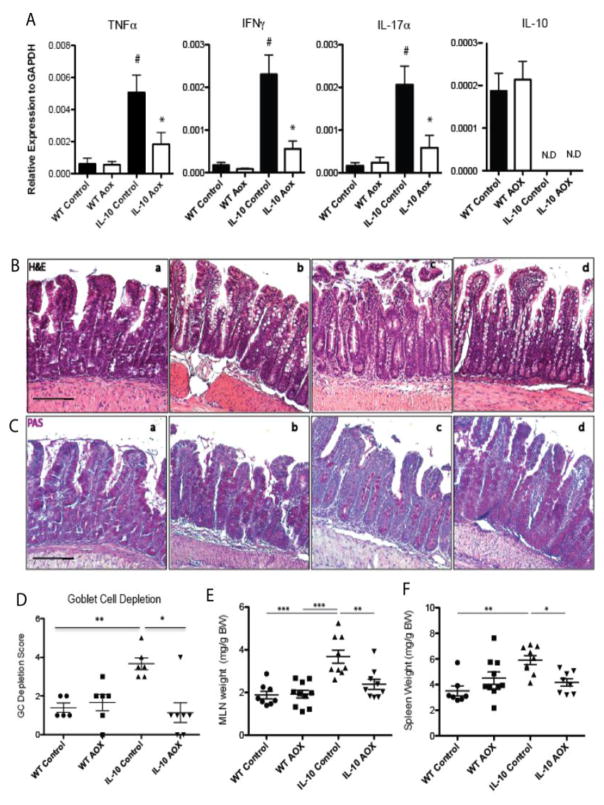
The gene expression of the mucosal cytokines, TNFα, IFNγ, IL-17α, and IL-10, were measured in mucosal samples (Figure 2A). There were no significant changes in the relative expression of mucosal cytokines in WT mice between diets, however, compared with WT CONT, TNFα, IFNγ, and IL-17α were significantly elevated in IL-10−/− CONT samples (P < 0.01 each). Compared with IL-10−/− CONT, the expression of TNFα, IFNγ, IL-17α was significantly decreased in IL-10−/− AOX (P < 0.05; P< 0.01; P< 0.05, respectively). Despite lower cytokine levels found in IL-10−/− AOX compared with IL-10−/− CONT, cytokines levels did not reach levels observed in WT suggesting an underlying inflammatory state.
Figure 2.
Evidence of inflammation in the Self-Filling Loop (SFL) mucosa and immune organs. (A) Cytokine expression for TNFα, IFNγ, IL-17α, and IL-10 in mucosal scrapings of WT and IL-10−/− animals on Control and AOX diet. (B) Histology of the SFL mucosa stained by H&E in (a) WT Control, (b) WT AOX, (c) IL-10−/− Control, and (d) IL-10−/− AOX: IL-10−/− Controls demonstrate evidence of epithelial erosion. (C) Periodic Acid-Schiff (PAS) staining of goblet cells in the SFL mucosa of (a) WT Control, (b) WT AOX, (c) IL-10−/− Control, and (d) IL-10−/− AOX demonstrates goblet cell loss in IL-10−/− Control and goblet cell hyperplasia in AOX fed animals. (D) Goblet cell depletion as PAS stained sections. (E) Normalized mesenteric lymph node organ (MLN) weight. (F) Normalized spleen weight. * P < 0.05, ** P < 0.01, *** P < 0.005. Scale bars indicate 100um. Results are n = 7–10/group for all measurements, except goblet cell depletion, n = 5–6/group.
Loop Mucosal Histology and Immunofluorescence (IF)
Consistent with our previous report [7], the SFL mucosa were characterized by short villi lengths and an elongated crypt compartment upon H&E assessment (Figure 2B), while IL-10−/− loops were characterized by epithelial erosion, cellular infiltrates, and loss of goblet cells (PAS stain; Figure 2C). Compared with WT CONT, the AOX diet enlarged goblet cells, but did not alter total goblet cell numbers or gene expression of the mucin glycoprotein, MUC2 (data not shown). In contrast to the marked histological inflammation visible in IL-10−/− CONT, IL-10−/− AOX prevented goblet cell depletion and epithelial erosion.
To investigate inflammatory contribution from lamina propria neutrophils, IF was performed for myeloperoxidase staining (see reference [17], Fig 2), which was found to be significantly elevated in IL-10−/− CONT compared with WT CONT (P <0.05) and WT AOX (P < 0.01), but mitigated in IL-10−/− AOX samples (P < 0.05) compared with IL-10−/− CONT. There was no difference between WT AOX and WT CONT.
Body and Immune Organ Weight
There were no differences in weight loss between genotypes during surgical recovery (−7±1 vs −8±1 %, p=0.45) (see reference [17], Fig 1). Following introduction of experimental diets, no differences in body weight were observed between diets in either genotype (WT: 0.2±3 vs. 0.2±3 %, p=0.99; IL-10−/−: −3.5±3 vs. 0.5±2, p=0.27). Mesenteric lymph nodes (MLNs) were collected and weighed from all animals. There were no MLN weight (mg/gram body weight) differences in WT animals (Figure 2E). However, the MLN weights in IL-10−/− CONT were significantly greater than WT CONT (P < 0.01) and IL-10−/− AOX MLN weight were significantly decreased compared with IL-10−/− CONT (P < 0.05). At baseline, IL-10−/− CONT animals had larger spleen weight (Figure 2F) compared with WT CONT (P<0.01), and AOX mitigated this in IL-10−/− animals (P<0.05).
Mucosal T Lymphocyte Populations
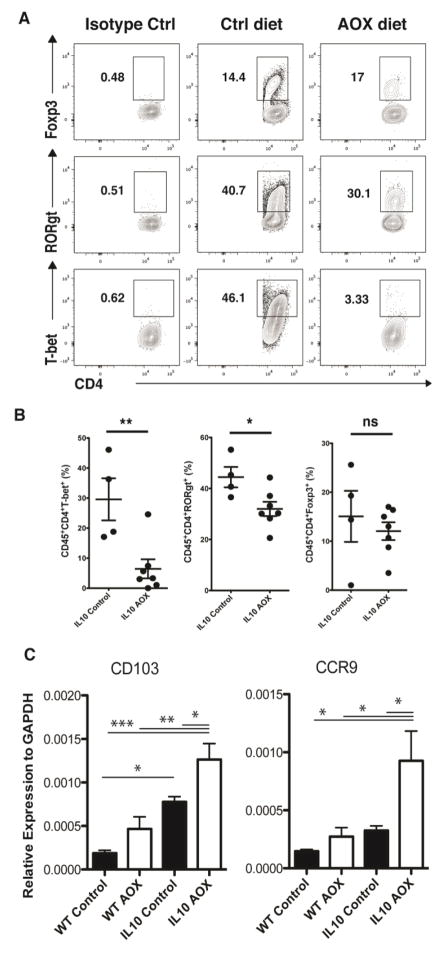
To characterize changes in cytokines at the cellular level, additional experiments were performed for flow cytometry analysis of lamina propria T lymphocytes in IL-10−/− animals (Figure 3A). Compared with IL-10−/− CONT animals, IL-10−/− AOX displayed fewer relative percentages of Th17 (CD45+CD4+RORgT+) and Th1 (CD45+CD4+T-bet+) cells, but unchanged levels of Treg (CD45+CD4+Foxp3+) (Figure 3B). Because T lymphocyte trafficking to the lamina propria is influenced by the lymphocyte integrin CCR9 under the regulation of CD103+ dendritic cells, we further measured gene expression of these markers in the loop mucosa. Compared with WT CONT animals, IL-10−/− CONT animals expressed greater levels of CD103 expression (P < 0.05) at baseline, while CCR9 did not reach significance. Increased CD103 expression in IL-10−/− animals is potentially a compensatory response to the underlying inflammation in this model (Figure 3C). Following AOX, the level of CD103 and CCR9 gene expression was increased in both genotypes and reached significance in IL-10−/− animals (P <0.05), suggesting the diet potentially stimulated tolerizing dendritic cell expansion and altered immune cell trafficking.
Figure 3.
Flow Cytometry Analysis of Self-Filling Loop (SFL) lamina propria T cells in IL-10−/− animals fed Control or AOX diet. (A) Representative gating for Live, CD45+ CD4+ lymphocytes stained with T-bet+, RORgt+, and FOXp3+. (B) Percentage change in T lymphocytes between IL-10−/− Control and AOX, where AOX decreased relative proportions of T-Bet+ (Th1) and RORgT+ (Th17), but did not significantly affect FOXp3+ (Treg) cell percentages. (C) Quantitative gene expression for CCR9 and CD103 in WT and IL-10−/− animals on Control and AOX diet. * P < 0.05, ** P < 0.01, *** P < 0.005. Flow cytometry data are representative of 3 independent experiments, each containing n=4–6/group.
Loop Luminal Immunoglobulin Levels
To investigate loop immunoglobulins following dietary intake, luminal IgA and IgG were quantified in loop contents. Mucosal plasma cells were also imaged with histology. No trends were observed in lamina propria plasma cell numbers (see reference [17], Fig 3A); however, the luminal level of IgA was significantly altered (see reference [17], Fig 3B). Compared with WT CONT, IgA levels were higher but not significant in IL-10−/− CONT at baseline. Following AOX diet, the level of IgA increased in both diets, but this reached significance only in IL-10 animals (P < 0.01). The luminal level of IgG decreased in both genotypes following AOX, but did not reach significance (see reference [17], Fig 3C). Since IgA has been shown to specifically target species belonging to Bacteriodietes, we next focused on the microbial taxonomy in the SFL.
Loop Microbial Taxonomy and Function
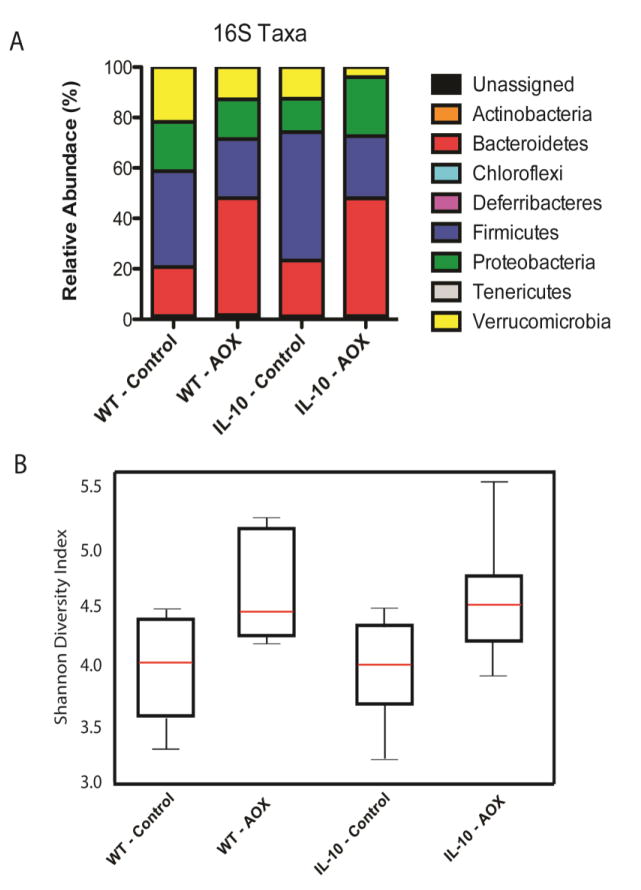
At the phylum level, similar patterns were observed in community composition between diets in both genotypes (Figure 4A). Following AOX, the relative percent of Bacteroidetes increased while Firmicutes decreased in both genotypes. Proteobacteria levels were largely unchanged, while the level of Verrucomicrobia decreased after AOX in both genotypes. Genera levels are displayed in reference [17] Table 2. Interestingly, the alpha diversity (Shannon diversity index) was increased after AOX diet in both genotypes (Figure 4B), indicating the AOX diet cultivated greater community membership, either directly or through changes in host immune function, compared with the CONT diet.
Figure 4.
Bacterial Taxonomy assessed by 16S sequencing. (A) Relative abundance of phylum in WT and IL-10−/− animals fed Control and AOX, where diet led to similar compositions regardless of genotype. (B) Shannon Diversity index between WT and IL-10−/− animals fed Control and AOX, where AOX led to similar increases in alpha diversity compared to Control diet in both Phenotypes. Taxonomy of Genera level changes are displayed in reference [17] Table 2. Results are n=7–9/group.
To explore functional characteristics of these microbial communities, the substrate utilization assay – Biolog - was performed (see reference [17] Fig 4). In this experiment, loop microbiota are diluted and grown anaerobically in the presence of 72 carbon substrates (i.e., sugars, carboxylic acids, amino acids) and 24 environmental sensitivities (i.e., low pH, salt concentrations, antibiotics), providing a signature of substrate utilization. This assay demonstrates that the dietary intake, but not host genotype, drives microbiota function, where WT or IL-10−/− animals on AOX diet cluster together and WT and IL-10−/− on CONT diet cluster more similarly. This finding is consistent with the taxonomic characterization where diet drives similar changes in either genotype.
Conventionalization of Germ-Free IL-10−/− mice with Loop Microbiota
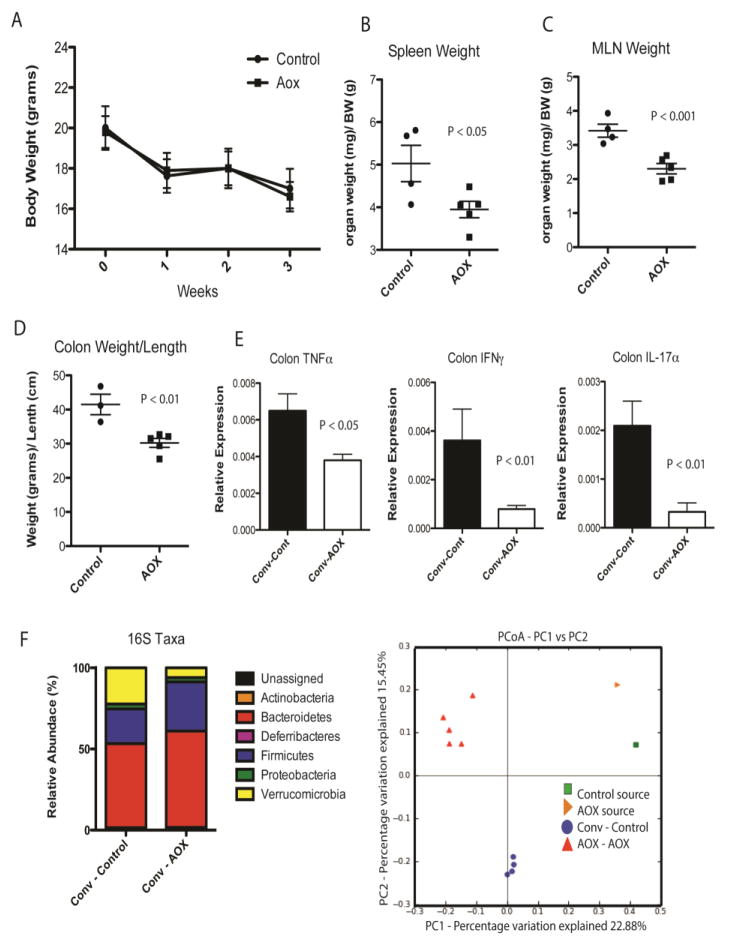
Finally, to test the role of the microbiome in driving mucosal inflammation, the loop microbiota from WT animals fed CONT or AOX were used to colonize germ-free IL-10−/− animals (Figure 5). There were no differences in body weight between groups after 3 weeks, however, all animals lost weight consistent with the response to microbial colonization (Figure 5A). The spleen and MLN weights (normalized to body weight) were greater in CONT compared with AOX conventionalized animals. Similarly, colon weight/length ratio, and mucosal cytokines were elevated in CONT compared with AOX (Figure 5D, E). Consistent with the donor microbiota, GF animals colonized with AOX had greater relative levels of the phylum Bacteriodetes and less Verrucomicrobia than CONT animals (Figure 5F). Principal component analysis demonstrates the composition of CONT and AOX colonized animals clustered differentially (Figure 5F). Taxonomic changes at the genera level are displayed in reference [17] Table 3.
Figure 5.
Conventionalization of germ-free IL-10−/− mice with the microbiota of Control and AOX loops. (A) Conventionalization of both microbiota communities led to similar changes in body weight over 21 days. (B) Normalized Spleen and (C) Mesenteric Lymph Node weight. (D) Ratio of colon weight per length. (E) Colonic mucosal cytokines, TNFα, IFNγ, and IL-17α. (F) Bacterial community taxonomy at the phylum level and beta diversity in mice conventionalized with Control and AOX derived microbiota. Resulting community structure resembled the donor groups in composition following 21 days of conventionalization and remained distinct in beta diversity between groups. Principal component analysis (PCoA) of conventionalized microbiota beta diversity demonstrate clear separation between Control and AOX communities. Taxonomy of Genera level changes are displayed in reference [17] Table 3. P values displayed in figures. Results are n = 4–5/group.
Discussion
Inflammatory bowel diseases (IBD) are believed to result from host genetic susceptibility in response to dietary, environmental, and microbial triggers that lead to chronic inflammatory responses. Ulcerative colitis is one form of IBD that involves only the colon, and in medically refractory disease, many patients require total removal of the colon. In these patients, the terminal small bowel is often used to surgically create a reservoir - termed an ileal pouch - that allows continence. Unfortunately, up to 50% of IBD patients who receive a pouch subsequently develop an inflammatory condition called pouchitis [21,22] In contrast, patients with familial adenomatous polyposis who undergo IPAA develop this condition far less frequency. Our previous experimental work demonstrated the inflammation in genetically susceptible animals is related to the microbial stasis, which mimics colonic microbiota, and drive toll-like receptor (TLR) mediated mucosal adaptations that histologically and transcriptionally resemble colon. [7] Elevated TLR signaling is observed in human pouches, [23] but the presence of endoscopic and histological mucosal metaplasia is variable in humans, likely reflecting a degree of heterogeneity of patient populations and surgical techniques in pouch size and style between surgeons. Stasis itself is also a trigger for enterocolitis symptoms. [7,24] While treatments for pouchitis include antibiotics, steroids, and immunologics, suggesting a critical role for microbiota-immune cross talk, little attention has been placed on potential dietary triggers or risk factors that lead to disease. It is increasingly recognized that diet can rapidly and dramatically alter microbial community structure and function, and therefore dietary factors likely provide underappreciated etiologies in pouchitis. Limited numbers of studies have focused on fiber and probiotics (e.g. VSL#3) in pouch patients, [25–27] with the aim of directly altering the microbial communities, but little focus has been placed on other dietary factors that influence the microbial compartment or host immune compartment directly.
Here we employed our established self-filling loop model, mimicking pouchitis, to explore the role of dietary intervention in genetically susceptible hosts. We specially focused on the dietary micronutrients, retinoic acid, ascorbic acid, vitamin E, and selenium (AOX diet) as a cocktail to screen for differential responses to diet in the IL-10−/− host. We selected retinoic acid because of the described influence of this micronutrient on T cell differentiation, including Th1 and Th17 subsets, through interactions with CD103+ Dendritic cells, as well as plasma cell differentaiton and function. [28–31] Furthermore, recent experimental modeling with chemically induced colitis demonstrated the potential protective effects of ascorbic acid and Vitamin E upon mucosal tissue damage. [14,15] However, since our model results from genetic susceptibility to inflammation induced by bacterial triggers, it remained unclear in molecules protective under chemical injury would be protective. Finally, we selected selenium since recent reports suggest human IBD patients with Ulcerative colitis and Crohn’s disease have reduced levels. The absence of IL-10−/− provides a background of inflammatory responsiveness, and decreased immune tolerance, to screen for environmental and dietary effects on disease processes. Therefore, our rational was to determine of elevating the dietary concentration of these micronutrients had any influence on the genetic susceptibility to pouchitis-like inflammation observed in our established model. The current study was also influenced in part by human clinical study observations that identified an association between lower intake of certain dietary micronutrients and elevated pouchitis incidence. [32] Because active disease or symptoms might alter dietary preferences in humans, providing alternative explanations for this reported association, our expectations were conservative. Accordingly, our initial hypothesis was that these specific micronutrients, widely considered antioxidants, would alter circulating reactive oxygen species or levels of antioxidant enzymes, such as superoxide dismutase (SOD), and may have a protective role in preventing free radical formation. Altered SOD expression has been reported in IBD. [33] Indeed, reduced oxidative stress has even been reported in the chemically induced models of colitis following Vitamin E supplementation. [15] However, our initial investigations failed to identify differences in serum free radicals or mucosal expression of SOD1/2 following AOX (data not shown), despite marked improvements found in histology assessment (Figure 2B). We therefore next focused on the immune compartment and microbiota structure to explore the mechanism behind improved mucosal injury.
In addition to improved histology with AOX in IL-10−/− animals, we observed altered mucosal cytokines compared with CONT diet. Specifically, TNFα, IFNγ, IL-17α are associated with pouchitis in humans and drive the pathophysiology of disease. Histologically, the mucosa displayed less goblet cell depletion following AOX, possibly due to decreased TNFα levels, a known stimulator of mucus release. [34] Despite these visual changes, there were no differences in mucosal MUC2 gene expression, the most abundantly produced mucin in the small intestine (data not shown), supporting the notion that goblet cell differences were due to decreased mucin secretion instead of increased mucin production. We then performed flow cytometry analyses of the loop to quantify immune cells, which indicated AOX leads to decreased levels of Th17 and Th1 lymphocytes in IL-10−/− animals, without altering Treg subsets, consistent with the observed cytokine expression data. These results were also consistent with the known influence of retinoic acid upon these cell subsets. [35,28] T lymphocyte trafficking is in part regulated by lymphocyte intergrins, including CC-chemokine receptor 9 (CCR9), which is influenced by CD103+ dendritic cells. [30] Interestingly, in both genotypes AOX elevated the gene expression of CCR9 and CD103, compared with CONT diet. The role of CCR9 could be seen as beneficial or detrimental, depending upon cellular population recruitment to the intestinal effector sites. Globally, however, animals lacking CCR9 display exacerbated IBD in response to chemical challenge, [36] suggesting a role in mediating protective mechanisms.
To complement lymphocyte analysis, we next investigated immunoglobulins in the loop lumen and found IgA was elevated following AOX diet in IL-10−/− animals, indicating either enhanced plasma cell IgA class switching, elevated plasma cell IgA production, or increased epithelial IgA transport and release. IgA is thought to be protective in the lumen, where IgA-transport protein, pIgR, deficient animals or IgA deficient human populations are at increased risk of colitis or IBD, respectively. [37,38] Elevated IgA is consistent with the known effects of retinoic acid upon IgA production. [13] Interestingly, it has been recently reported that while IgA is constitutively expressed in the intestine, substantial amounts of IgA are released that are specific to species of the phylum Bacteriodetes. [39] In addition to histological and immunological changes, AOX led to an altered microbial community structure via 16S sequencing that included increased microbial diversity – in both genotypes - and altered microbial function assessed through an in vitro assay of substrate utilization. It should be noted that phylum level analysis provides limited insight, but we noted increased Bacteriodetes and decreased Firmicutes. The level of the phylum Verrucomicrobia was decreased in both genotypes following AOX diet, which was interesting because this phylum is sometimes associated with protective effects. However, since Verrucomicrobiota is comprised of a single species, Akkermansia Muciniphila, which feeds on mucus release by the host, it may have decreased in relative abundance as a result of lower goblet cell mucus release as indicated by histology and gene expression analysis.
The changes observed following AOX in IL-10−/− animals lead to multiple interesting questions that are fundamental to the nature of diet, microbe, and host interactions. Does AOX directly or indirectly impact the loop microbiome and the mucosal immune compartments? How do these compartments influence one another? While the former question remains difficult to tease apart, we addressed the later question by performing microbial conventionalization studies. Conventionalization of germ-free animals remains the best technique to study the direct effect of defined microbial communities upon host parameters without confounding variables, including antibiotics or reemergence of microbial species present before introduction of test communities. To test this, ileal loop microbiota were collected from WT animals fed CONT or AOX diet and subsequently administered to germ-free IL-10−/− animals. This experiment demonstrated that CONT microbiota led to more pronounced inflammatory responses, including morphometric analysis of the MLN and colon and elevated mucosal cytokines, in the IL-10−/− hosts compared with AOX microbiota, supporting the notion that following AOX diet the microbiota directly alters the host immune response. To address the role of the immune compartment upon the microbiota following AOX diet, cell transfer studies would be needed from the loop mucosa into lymphocyte deficient animals. Furthermore, feeding sterile AOX diet to germ-free animals may allow investigation of the dietary micronutrients upon the immune system alone; however, these animals have fewer gut immune cells at baseline. The primary limitation of the current study is the inability to elucidate the contribution of each dietary component - retinoic acid, ascorbic acid, vitamin E, and selenium - since they were provided as a cocktail in AOX diet. Further studies with individual compounds are needed, such as a focused examination of retinoic acid, although it is possible the results observed in this study resulted from the synergistic contribution of each compound. Additionally, when considering the potential benefit of supplementing with individual micronutrients, one must be acutely aware of the risk of toxicity, especially in regards to retinoic acid and hepatic dysfunction. Well the dietary doses used here remain the only 2 to 4 times higher than concentrations found in maintenance diets, future work is needed to determine safe levels of intake in both mice and humans.
In conclusion, pouchitis remains a chronic problem for many IBD patients who have an ileal pouch reservoir. The current treatments target the microbial communities (through non-specific antibiotics) and inhibit immune responses (through the use of steroids and immunologics). Recent advancements in studying the microbiome demonstrate microbial communities are highly responsive to diet, both in their composition and function. Likewise, much attention is currently placed on the use of probiotics to directly manipulate the microbiota community in pouch patients and other forms of IBD. However, given the importance of diet in shaping the community, we empirically modeled this disease to examine if dietary micronutrients influenced disease in genetically susceptible animals. Our results demonstrate that dietary micronutirents, in addition to macronutrients, can influence mucosal immune responses and mucosal pathophysiology. These outcomes are related to shifts in the microbiome, which has a causal role, since the transfer of microbial communities to naïve germ-free hosts can differentially drive disease pathogenesis. Globally the observed protective effect of our dietary treatment were consistent with the influence of retinoic acid upon mucosal and immune compartment function. Deeper understanding of the immune-microbiome cross talk under different dietary settings may enable us to predict and prevent key risk factors for inflammatory disease in pouch patient populations.
Acknowledgments
Funding: This work was supported by NIH grants DK097268, DK47722, P30DK42086; GI Research Foundation, Chicago, IL; Leona M. and Harry B. Helmsley Charitable Trust (Sinai-Helmsley Alliance for Research Excellence).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ng SC, Bernstein CN, Vatn MH, Lakatos PL, Loftus EV, Tysk C, et al. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut. 2013;62:630–49. doi: 10.1136/gutjnl-2012-303661. [DOI] [PubMed] [Google Scholar]
- 2.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–17. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–24. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuoka K, Kanai T. The gut microbiota and inflammatory bowel disease. Semin Immunopathol. 2015;37:47–55. doi: 10.1007/s00281-014-0454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shreiner AB, Kao JY, Young VB. The gut microbiome in health and in disease. Curr Opin Gastroenterol. 2015;31:69–75. doi: 10.1097/MOG.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angriman I. Relationship between pouch microbiota and pouchitis following restorative proctocolectomy for ulcerative colitis. World J Gastroenterol. 2014;20:9665. doi: 10.3748/wjg.v20.i29.9665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward MA, Pierre JF, Leal RF, Huang Y, Shogan BD, Dalal SR, et al. Insights into the pathogenesis of ulcerative colitis from a murine model of stasis-induced dysbiosis, colonic metaplasia, and genetic susceptibility. Am J Physiol Gastrointest Liver Physiol. 2016 doi: 10.1152/ajpgi.00017.2016. ajpgi.00017.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–63. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Persson P-G, Ahlbom A, Hellers G. Diet and Inflammatory Bowel Disease: A Case-Control Study. Epidemiology. n.d;3:47–52. doi: 10.2307/3702936. [DOI] [PubMed] [Google Scholar]
- 10.Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519:92–6. doi: 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai A, Lu N, Guo Y, Liu Z, Chen J, Peng Z. All-trans retinoic acid down-regulates inflammatory responses by shifting the Treg/Th17 profile in human ulcerative and murine colitis. J Leukoc Biol. 2009;86:959–69. doi: 10.1189/jlb.0109006. [DOI] [PubMed] [Google Scholar]
- 12.Magnusson MK, Brynjólfsson SF, Dige A, Uronen-Hansson H, Börjesson LG, Bengtsson JL, et al. Macrophage and dendritic cell subsets in IBD: ALDH+ cells are reduced in colon tissue of patients with ulcerative colitis regardless of inflammation. Mucosal Immunol. 2016;9:171–82. doi: 10.1038/mi.2015.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seo G-Y, Jang Y-S, Kim H-A, Lee M-R, Park M-H, Park S-R, et al. Retinoic acid, acting as a highly specific IgA isotype switch factor, cooperates with TGF-β1 to enhance the overall IgA response. J Leukoc Biol. 2013;94:325–35. doi: 10.1189/jlb.0313128. [DOI] [PubMed] [Google Scholar]
- 14.Yan H, Wang H, Zhang X, Li X, Yu J. Ascorbic acid ameliorates oxidative stress and inflammation in dextran sulfate sodium-induced ulcerative colitis in mice. Int J Clin Exp Med. 2015 [PMC free article] [PubMed] [Google Scholar]
- 15.Tahan G, Aytac E, Aytekin H, Gunduz F, Dogusoy G, Aydin S, et al. Vitamin E has a dual effect of anti-inflammatory and antioxidant activities in acetic acid-induced ulcerative colitis in rats. Can J Surg. 2011 doi: 10.1503/cjs.013610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castro Aguilar-Tablada T, Navarro-Alarcón M, Quesada Granados J, Samaniego Sánchez C, Ángel Rufián-Henares J, Nogueras-Lopez F. Ulcerative Colitis and Crohn’s Disease Are Associated with Decreased Serum Selenium Concentrations and Increased Cardiovascular Risk. Nutrients. 2016 doi: 10.3390/nu8120780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pierre JF, Hinterleitner R, Bouziat R, Hubert N, Leone V, Miyoshi J, Jabri BCE. Data on Changes to Mucosal Inflammation and the Intestinal Microbiota following Dietary Micronutrients in Genetically Susceptible Hosts. Data Br. 2017 doi: 10.1016/j.dib.2018.08.026. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierre JF, Martinez KB, Ye H, Nadimpalli A, Morton TC, Yang J, et al. Activation of bile acid signaling improves metabolic phenotypes in high-fat diet-induced obese mice. Am J Physiol - Gastrointest Liver Physiol. 2016;311:G286–304. doi: 10.1152/ajpgi.00202.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCune B, Mefford MJ. Softw Des Gleneden Beach Oregon USA. 1999. Multivariate Analysis of Ecological Data. [Google Scholar]
- 20.Bouziat R, Hinterleitner R, Brown JJ, Stencel-Baerenwald JE, Ikizler M, Mayassi T, et al. Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science. 2017;356:44–50. doi: 10.1126/science.aah5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuisma J, Mentula S, Luukkonen P, Jarvinen H, Kahri A, Farkkila M. Factors associated with ileal mucosal morphology and inflammation in patients with ileal pouch-anal anastomosis for ulcerative colitis. Dis Colon Rectum. 2003;46:1476–83. doi: 10.1097/01.DCR.0000093821.07912.96. [DOI] [PubMed] [Google Scholar]
- 22.Stocchi L, Pemberton JH. Pouch and pouchitis. Gastroenterol Clin North Am. 2001;30:223–41. doi: 10.1016/s0889-8553(05)70175-8. [DOI] [PubMed] [Google Scholar]
- 23.Scarpa M, Grillo A, Pozza A, Faggian D, Ruffolo C, Scarpa M, et al. TLR2 and TLR4 up-regulation and colonization of the ileal mucosa by Clostridiaceae spp. in chronic/relapsing pouchitis. J Surg Res. 2011;169:e145–54. doi: 10.1016/j.jss.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Pierre JFJF, Barlow-Anacker AJAJ, Erickson CSCS, Heneghan AFAF, Leverson GE, Dowd SESE, et al. Intestinal dysbiosis and bacterial enteroinvasion in a murine model of Hirschsprung’s disease. J Pediatr Surg. 2014;49:1242–51. doi: 10.1016/j.jpedsurg.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, et al. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53:108–14. doi: 10.1136/gut.53.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305–9. doi: 10.1053/gast.2000.9370. [DOI] [PubMed] [Google Scholar]
- 27.Shen J, Zuo Z-X, Mao A-P. Effect of Probiotics on Inducing Remission and Maintaining Therapy in Ulcerative Colitis, Crohn’s Disease, and Pouchitis. Inflamm Bowel Dis. 2014;20:21–35. doi: 10.1097/01.MIB.0000437495.30052.be. [DOI] [PubMed] [Google Scholar]
- 28.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, et al. Reciprocal TH17 and Regulatory T Cell Differentiation Mediated by Retinoic Acid. Science (80− ) 2007:317. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 29.Whelan JT, Chen J, Miller J, Morrow RL, Lingo JD, Merrell K, et al. 9-cis-Retinoic acid promotes cell adhesion through integrin dependent and independent mechanisms across immune lineages. J Nutr Biochem. 2013;24:832–41. doi: 10.1016/j.jnutbio.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakdash G, Vogelpoel LT, van Capel TM, Kapsenberg ML, de Jong EC. Retinoic acid primes human dendritic cells to induce gut-homing, IL-10-producing regulatory T cells. Mucosal Immunol. 2015;8:265–78. doi: 10.1038/mi.2014.64. [DOI] [PubMed] [Google Scholar]
- 31.Park M-H, Park S-R, Lee M-R, Kim Y-H, Kim P-H. Retinoic acid induces expression of Ig germ line α transcript, an IgA isotype switching indicative, through retinoic acid receptor. Genes Genomics. 2011;33:83–8. doi: 10.1007/s13258-010-0168-5. [DOI] [Google Scholar]
- 32.Ianco O, Tulchinsky H, Lusthaus M, Ofer A, Santo E, Vaisman N, et al. Diet of patients after pouch surgery may affect pouch inflammation. World J Gastroenterol. 2013;19:6458–64. doi: 10.3748/wjg.v19.i38.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kruidenier L, Kuiper I, van Duijn W, Marklund SL, van Hogezand RA, Lamers CBHW, et al. Differential mucosal expression of three superoxide dismutase isoforms in inflammatory bowel disease. J Pathol. 2003;201:7–16. doi: 10.1002/path.1407. [DOI] [PubMed] [Google Scholar]
- 34.Dharmani P, Leung P, Chadee K, Innes D, Ravdin J. Tumor Necrosis Factor-α and Muc2 Mucin Play Major Roles in Disease Onset and Progression in Dextran Sodium Sulphate-Induced Colitis. PLoS One. 2011;6:e25058. doi: 10.1371/journal.pone.0025058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elias KM, Laurence A, Davidson TS, Stephens G, Kanno Y, Shevach EM, et al. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 2008;111:1013–20. doi: 10.1182/blood-2007-06-096438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wurbel M-A, McIntire MG, Dwyer P, Fiebiger E, Thompson C. CCL25/CCR9 Interactions Regulate Large Intestinal Inflammation in a Murine Model of Acute Colitis. PLoS One. 2011;6:e16442. doi: 10.1371/journal.pone.0016442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reikvam DH, Derrien M, Islam R, Erofeev A, Grcic V, Sandvik A, et al. Epithelial-microbial crosstalk in polymeric Ig receptor deficient mice. Eur J Immunol. 2012;42:2959–70. doi: 10.1002/eji.201242543. [DOI] [PubMed] [Google Scholar]
- 38.Ludvigsson JF, Neovius M, Hammarström L. Association between IgA deficiency & other autoimmune conditions: a population-based matched cohort study. J Clin Immunol. 2014;34:444–51. doi: 10.1007/s10875-014-0009-4. [DOI] [PubMed] [Google Scholar]
- 39.Bunker JJ, Flynn TM, Koval JC, Jabri B, Antonopoulos DA, Correspondence AB, et al. Innate and Adaptive Humoral Responses Coat Distinct Commensal Bacteria with Immunoglobulin A. 2015 doi: 10.1016/j.immuni.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]