Abstract
Background
Early intervention for adolescent substance use and mental health problems may mitigate potential harm. We examined patient outcomes from a pragmatic trial of two modalities of delivering Screening, Brief Intervention and Referral to Treatment (SBIRT) and Usual Care in pediatric primary care.
Methods
All clinic pediatricians (n=52) were randomized to three arms: 1) pediatrician-only, in which pediatricians were trained to deliver SBIRT; 2) embedded behavioral clinician (BC), in which pediatricians were trained to refer eligible adolescents to a BC who administered SBIRT; and 3) Usual Care (UC). Using electronic health record data, changes in past year substance use and depression symptoms between index visit and next screening visit were examined across treatment arms.
Results
Among patients who endorsed substance use and/or depression symptoms or were eligible for further assessments, brief interventions and referrals based on clinician assessment at index visit, 648 patients (mean age=15.2[SD=1.2]) were rescreened at a follow-up visit between 6 months and 2 years later. Among all patients, self-reported substance use rates did not differ over time or across arms, and depression symptoms increased over time. The embedded-BC arm had lower odds of having depression symptoms at follow-up than the physician-only arm, and lower odds than the UC arm though not significant; we found no differences between the pediatrician-only and UC arms.
Conclusions
The increase in depression symptoms over time highlights this population’s vulnerability and the importance of developing appropriate interventions. An embedded BC in pediatric primary care trained in SBIRT may benefit patients with depression symptoms.
Keywords: Adolescent, SBIRT, Screening, Substance, Alcohol, Drug, Depression, Integrated, Primary Care
INTRODUCTION
Substance use and depression are among the most common pediatric health conditions in the U.S.[1], and they frequently co-occur. Early intervention may mitigate their harm, and primary care provides excellent opportunities for detection and treatment. Support for the integration of substance use and mental health interventions into primary care has grown [2, 3]. Still lacking is a thorough understanding of effective implementation methods, as well as population-based evidence on integrating interventions into pediatric workflows.
Screening, brief intervention and referral to treatment (SBIRT) refers to systematic screening for substance use risk using evidence-based instruments, a brief patient-centered intervention, and referral to specialty treatment if needed. Numerous health and medical organizations have endorsed SBIRT [4, 5], and the literature on brief, integrated behavioral healthcare for adolescents, including SBIRT, includes evidence of effectiveness [6–8].
A recent meta-analysis found that integrated behavioral health/medical care produced better behavioral health outcomes for children and adolescents than usual care [9]. Early studies of SBIRT in pediatric primary care had inconsistent results [10, 11], but newer studies suggest potential benefits. A study in pediatric clinics in the U.S. and the Czech Republic found reduced alcohol use in the U.S. and reduced cannabis use in the Czech Republic [12]. In both samples, those using substances increased cessation, and those not yet using had lower rates of initiation. Trials of brief interventions (BI) in a Federally Qualified Health Center found that receiving a BI was related to lower rates of cannabis initiation and other drug use among cannabis-naïve adolescents [13] and to reduced cannabis-related consequences and driving while intoxicated [14].
Studies of integrated pediatric depression care are also promising [15]. A trial in private and public primary care found that patients receiving integrated care reported fewer symptoms, and better quality of life and greater treatment satisfaction with integrated care compared to usual care, with improvements persisting at 12 and 18 months [16]. In another trial, adolescents receiving collaborative care had lower depression scores and higher remission rates at 12 months than those in usual care [17].
This study presents outcomes from a cluster-randomized, hybrid implementation and effectiveness trial of SBIRT in a general pediatrics clinic at Kaiser Permanente Northern California (KPNC). All adolescents who screened positive were included as potential participants. Due to the use of electronic health record (EHR) data and the integration of SBIRT into the normal workflow, consent was not required. Pilot studies of well-visit screening found that many adolescents initially endorsed emotional distress, and only in later assessments disclosed substance use [18]. The frequent comorbidity of substance use and mental health problems[19] and the relationship between these outcomes [20] supported including both self-reported substance use and depression symptoms as sufficient risk factors to warrant an SBIRT intervention. Therefore, we examined both substance use and depression symptoms as outcomes in this study. This intervention approach was innovative, since adolescent SBIRT studies generally have examined substance use alone.
The interventions tested were also innovative and included two modalities of SBIRT delivery: one using SBIRT-trained pediatricians, the other using pediatricians trained to assess and refer at-risk patients to an embedded behavioral clinician (BC), and usual care (UC). Prior studies have found that both pediatricians [21] and embedded BCs [22] can effectively provide behavioral health interventions. We hypothesized that patients in both intervention arms would have lower odds of substance use and depression symptoms at follow-up than in UC due to the pediatricians’ SBIRT training and the BC’s additional time and clinical expertise. We also hypothesized that patients in the embedded-BC arm would have lower odds than those in the pediatrician-only arm, because BCs have professional behavioral health training and generally longer appointment times.
METHODS
Study Participants
KPNC is an integrated healthcare delivery system of 4 million members. The study was conducted from 11/1/2011 – 10/31/2013 in KPNC’s Oakland Pediatrics Department, which treats a racially and socio-economically diverse population. All clinic pediatricians (n=52) were randomized to one of three study arms: 1) Pediatrician only: Pediatricians trained to assess substance use and depression symptoms using evidence-based screening tools, to deliver BIs, and to refer patients to specialty substance use or mental health treatment; 2) Embedded BC: Pediatricians trained to assess and refer patients to an embedded BC for further assessment, BI, and referral to treatment; and 3) Usual Care (UC): care as usual (no SBIRT training or access to the embedded BC, but with access to EHR screening tools and patient screening information). Patients aged 12–18 were eligible. The study had no exclusion criteria. Provider assignment to study arm was not blinded. Consistent with other comparative effectiveness studies, we used EHR measures to examine outcomes, and patients were not recruited to the study or informed of which study arm included their pediatrician. We were not required by the Institutional Review Boards (IRB) to consent pediatricians, and the study was approved by the IRBs of KPNC and the University of California, San Francisco.
Pediatricians in both intervention arms were offered on-site trainings (three 60-minute sessions in the pediatrician-only arm, and one 60-minute session in the embedded-BC arm) for which they received lunch and continuing education credit. In the pediatrician-only arm, 64% of pediatricians attended at least two trainings; in the embedded-BC arm, 75% of pediatricians attended the training. Trainings in the pediatrician-only arm covered adolescent substance use and mental health prevalence, comorbidity and consequences; assessment; BI strategies for substance use (e.g., motivational interviewing (MI) skills [23], decisional balance exercises, and goal-setting) and depression (e.g., empathic listening, psychoeducation, problem-solving, behavioral activation, stress reduction, and exploration of challenges in interpersonal relationships)[24]; and protocols for referring patients to specialty substance use and psychiatric treatment. Pediatricians in the pediatrician-only and UC arms incorporated the SBIRT elements into their normal clinical workflow during the well-visit appointment times (typically 15–30 minutes). Training in the embedded-BC arm covered similar elements, but focused less on intervention delivery and more on how to assess severity and refer patients to the BC. This training approach has been used in prior studies and has been associated with sufficient skill acquisition [25]. The BC (N=1) was a licensed clinical psychologist who received 10 hours of MI-based SBIRT training, and had depression and substance use treatment experience. She provided brief cognitive behavioral therapy-based treatment and crisis management for substance and mood problems, spending from 30 to 60 minutes on SBIRT activities, and received weekly clinical supervision with the study intervention trainer, an experienced clinical psychologist. Pediatricians in the treatment arms received supplemental recordings and slides, and research staff was available for technical assistance. Performance feedback and discussion of SBIRT techniques were provided at quarterly meetings.
As in prior SBIRT implementation studies, [26–28] feedback on SBIRT rates (in the pediatrician-only arm) and referral to BHC rates (in the embedded-BHC arm) were discussed quarterly with pediatricians at feedback meetings, including a review of SBIRT skills, to enhance fidelity.[29] Also, emails and staff meetings informed providers equally across all arms regarding the screening and assessment tools in the EHR, and reminded them of the requirement to document clinical activities.
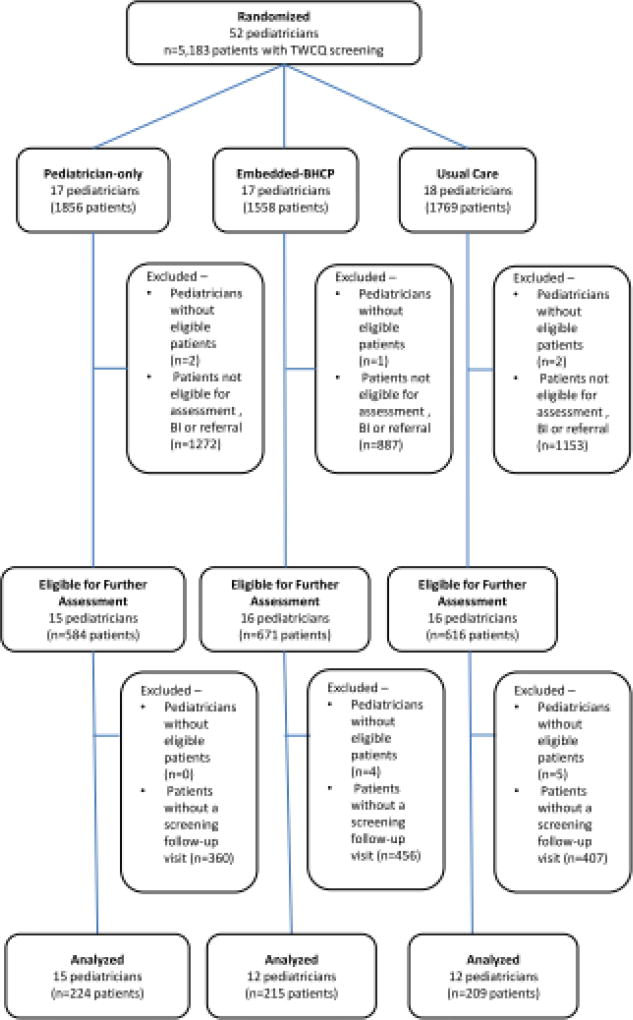
Per established workflow, all patients presenting for well visits completed a paper and pencil comprehensive health screener, the “Teen Well Check Questionnaire” (TWCQ), a comprehensive health screening instrument developed by health system leaders, based on the Bright Futures [30] screening guidance. TWCQ responses are entered into the patient’s EHR. The TWCQ includes items (Yes/No) on past-year alcohol, marijuana and other drug use, and recent depression symptoms (“During the past few weeks, have you OFTEN felt sad, down or hopeless?” and “Have you seriously thought about killing yourself, made a plan, or tried to kill yourself?”) which served as the initial substance use and mental health risk screening questions. Medical assistants enter patient TWCQ responses into the EHR. Endorsement of TWCQ substance use or depression symptoms or pediatrician clinical judgment made patients eligible for further assessment with the CRAFFT substance use assessment tool [31] and additional substance use quantity/frequency questions. These tools were available in the EHR to all providers. Assessment was followed by BI and/or referral to psychiatric or substance use treatment, as needed in the treatment arms and care as usual in the UC arm. Across the 52 randomized pediatricians, 5183 patients were screened and 1871 were eligible for further assessment. The analytical sample consisted of the 648 patients eligible for assessment at index visit and rescreened between 6 months to 2 years later. No pediatricians in the UC arm used the CRAFFT though it was available to all pediatricians. Quantity/frequency items in the EHR were also available to all physicians, however, only 65 patients were administered the questions by pediatricians in the intervention arms at both index and follow-up; therefore, these outcomes could not be examined.
Measures and Data Sources
Patient age, sex and race/ethnicity, and provider age, gender and years of experience were extracted from the EHR.
Outcomes
Substance use and depression measures were dichotomous variables from the TWCQ indicating any substance use in the past year (alcohol, marijuana and/or other drug use) and any depression symptoms in the prior few weeks (“During the past few weeks, have you OFTEN felt sad, down or hopeless?” and “Have you seriously thought about killing yourself, made a plan, or tried to kill yourself?”), respectively, which were extracted from the EHR.
KPNC adolescents have a well visit and complete the TWCQ roughly every one to two years, but visit frequency fluctuates. To exclude visits which might be part of the index encounter episode, we designated the follow-up visit as the first visit between 6 months and 2 years after the index visit. Patients must have responded to the outcome questions at both time points to be included in the analyses.
Statistical Methods
We used ANOVA and chi-square tests to examine bivariate differences between continuous and categorical patient and provider characteristics, respectively, across arms. As patients are nested within providers, and observations within clusters may be correlated, we used generalized estimation equation (GEE) techniques assuming an exchangeable working correlation structure to fit multivariable logistic regression models. Models examined differences in outcomes (any substance use or depression symptoms) across the three arms (ref = UC), and between the intervention arms (ref = pediatrician-only), between the two time points, with time treated as a continuous measure. Initial models adjusted for patient and provider characteristics, but because provider characteristics did not differ and were not significant in the main models, only patient characteristics were included in the final models. Analyses were performed using SAS statistical software, version 9.3 (SAS Institute Inc.); significance was defined at p<.05.
Power calculations accounted for intra-class correlation (ICC) among patients clustered within providers (unit of randomization), which reduced effective sample size by a factor of 1+(n-1)*ICC, where n is the average cluster size [32]. For our primary outcome of change in symptom endorsement, our sample size is 648 patients across 39 pediatricians, with an ICC estimate of 0.02, with adequate power (.80) to detect a small-medium effect size of 18% in changes across arms.
RESULTS
Participation in Follow-up Visits
Among the 1871 patients eligible for assessment, 35.0% (n=648) had a follow-up visit between 6 months to 2 years after the index visit; there were no differences across arms (33.4% embedded BC, 36.8% pediatrician only, 33.9% UC; p=0.401). Fewer patients with a follow-up visit were Black (30.9% with a follow-up visit vs. 34.9% without a follow-up visit) and Hispanic (20.8% vs. 25.4%), and more were White (30.7% vs. 22.3%; p=0.001). Younger patients were more likely to have a follow-up visit (mean age[SD]= 15.2[1.2] vs. 16.2[1.5]; p<.001); there were no differences by gender.
Among patients with follow-up visits, the average length of time between visits was 434 (SD=122) days, and did not differ between arms (mean[SD]=436[122] embedded BC; 439[128] pediatrician-only; 428[115] UC; p=0.619). There were more males in the UC arm (52.6%, 43.8% embedded-BC, 38.1% pediatrician-only; p=0.010). The UC arm also had more Whites (38.8%, 28.1% embedded-BC, 25.6% pediatrician-only), and fewer Blacks (29.2%, 30.4% embedded-BC, 33.0% pediatrician-only; p=0.015); age did not differ across the arms (Table 1).
Table 1.
Patient Demographics by Treatment Arm (n=648)
| Embedded-BC (n=224) |
Pediatrician- only (n=215) |
UC (n=209) |
p- value |
||
|---|---|---|---|---|---|
| Male, n (%) | 98 (43.8) | 82 (38.1) | 110 (52.6) | 0.010 | |
| Race/ethnicity, n (%) | |||||
| Asian | 21 (9.4) | 31 (14.4) | 19 (9.1) | 0.015 | |
| Black | 68 (30.4) | 71 (33.0) | 61(29.2) | ||
| Hispanic | 60 (26.8) | 39 (18.1) | 36 (17.2) | ||
| White | 63 (28.1) | 55 (25.6) | 81 (38.8) | ||
| Other/Unknown | 12 (5.4) | 19 (8.8) | 12 (5.7) | ||
| Age, mean(SD) | 15.1 (1.2) | 15.2 (1.2) | 15.3 (1.2) | 0.497 | |
Note: BC=behavioral; UC= usual care
Substance Use Endorsement
Among all patients, 11% endorsed substance use symptoms at the index visit only, 15% endorsed substance use symptoms at the follow-up visit only, 48% endorsed substance use symptoms at both visits and 26% did not endorse substance use symptoms at either visit. More adolescents in the embedded-BC arm endorsed symptoms at only the initial visit compared with the other arms. The UC arm had more patients who endorsed substance use symptoms at both visits (p<.05, Table 2).
Table 2.
Symptom endorsement by treatment arm
| Substance Use* | Mood | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| embedded- BC |
physician- only |
UC | embedded- BC |
physician- only |
UC | |
|
|
||||||
| Initial visit only | 16.1 | 8.4 | 9.1 | 6.7 | 3.3 | 5.3 |
| Follow-up visit only | 16.1 | 13.5 | 14.4 | 27.2 | 31.6 | 27.8 |
| Both visits | 42.4 | 47.0 | 54.1 | 13.8 | 20.5 | 16.8 |
| No symptoms | 25.5 | 31.2 | 22.5 | 52.2 | 44.7 | 50.2 |
p<.05
The odds of substance use did not differ over time (adjusted odds ratio [AOR]=1.19, 95% CI=0.97–1.46), and did not differ across treatment arms. Asians, Blacks and Hispanics were less likely to endorse substance use than Whites, while older adolescents and males were more likely (Table 3).
Table 3.
Substance Use Symptom Endorsement Over Time
| 3a. Substance Use Symptom Endorsement between Index and Follow-up Visits Across All Arms (n=648) | ||||
|---|---|---|---|---|
|
|
||||
| AOR | 95% CI | p-value | ||
| Time | 1.19 | 0.97 | 1.46 | 0.089 |
| Treatment arms (reference: UC) | ||||
| Embedded-BC | 0.87 | 0.61 | 1.23 | 0.425 |
| Physician-only | 0.82 | 0.57 | 1.27 | 0.274 |
| Male (reference: female) | 1.52 | 1.13 | 2.03 | 0.005 |
| Age | 2.04 | 1.79 | 2.31 | <.001 |
| Race/ethnicity (reference: white) | ||||
| Asian | 0.31 | 0.19 | 0.51 | <.001 |
| Black | 0.39 | 0.27 | 0.56 | <.001 |
| Hispanic | 0.43 | 0.29 | 0.65 | <.001 |
| Other/Unknown | 0.53 | 0.28 | 1.02 | 0.057 |
| 3b. Substance Use Symptom Endorsement between Index and Follow-up Visits Across Intervention Arms Only (n= 439) | ||||
|---|---|---|---|---|
|
|
||||
| AOR | 95% CI | p-value | ||
| Time | 1.13 | 0.89 | 1.45 | 0.313 |
| Embedded-BC (reference: physician-only) | 1.07 | 0.76 | 1.51 | 0.691 |
| Male (reference: female) | 1.58 | 1.12 | 2.24 | 0.010 |
| Age | 2.09 | 1.78 | 2.45 | <.001 |
| Race/ethnicity (reference: white) | ||||
| Asian | 0.40 | 0.22 | 0.73 | 0.003 |
| Black | 0.49 | 0.31 | 0.76 | 0.002 |
| Hispanic | 0.44 | 0.27 | 0.71 | 0.001 |
| Other/Unknown | 0.47 | 0.22 | 1.01 | 0.052 |
Note: Patients were required to have responses at both the index and follow-up visits; AOR=adjusted odds ratio; UC= usual care; BC=behavioral clinician
Note: Patients were required to have responses at both the index and follow-up visits; AOR=adjusted odds ratio; BC=behavioral clinician
Depression Symptom Endorsement
Only 5% of patients endorsed mood symptoms only at the index visit, 29% endorsed at the follow-up visit only, 17% endorsed mood symptoms at both visits and 49% did not endorsed mood symptoms at either time point. The embedded-BC arm had slightly more patients with endorsed mood symptoms at only the initial visit, and fewer patients who endorsed at both visits compared to the other arms, though the differences were not statistically different (Table 2).
The odds of endorsing depression symptoms increased between visits for all patients (AOR=3.53, 95% CI=2.78–4.40). Patients in the embedded-BC arm had lower odds of endorsing depression symptoms than those in the pediatrician-only arm (AOR=0.71, 95% CI=0.50–0.99); the pediatrician-only arm did not differ from UC (AOR=1.02, 95% CI=0.72–1.43). Blacks and those of other/unknown ethnicity had higher odds of endorsing depression symptoms over time than Whites, while older adolescents and males had lower odds (Table 4).
Table 4.
Depression Symptom Endorsement Over Time
| 4a. Depression Symptom Endorsement between Index and Follow-up Visits Across All Arms (n=648) | ||||
|---|---|---|---|---|
|
|
||||
| AOR | 95% CI | p-value | ||
| Time | 3.53 | 2.78 | 4.40 | <.001 |
| Treatment arms (reference: UC) | ||||
| Embedded-BC | 0.72 | 0.51 | 1.02 | 0.063 |
| Physician-only | 1.02 | 0.72 | 1.43 | 0.928 |
| Male (reference: female) | 0.42 | 0.31 | 0.55 | <.001 |
| Age | 0.63 | 0.56 | 0.71 | <.001 |
| Race/ethnicity (reference: white) | ||||
| Asian | 1.47 | 0.89 | 2.42 | 0.133 |
| Black | 2.15 | 1.49 | 3.10 | <.001 |
| Hispanic | 1.48 | 0.98 | 2.24 | 0.061 |
| Other/Unknown | 2.18 | 1.15 | 4.17 | 0.018 |
| 4b. Depression Symptom Endorsement between Index and Follow-up Visits Across Intervention Arms Only (n= 439) | ||||
|---|---|---|---|---|
|
|
||||
| AOR | 95% CI | p-value | ||
| Time | 3.59 | 2.71 | 4.77 | <.001 |
| Embedded-BC (reference: physician-only) | 0.71 | 0.50 | 0.99 | 0.047 |
| Male (reference: female) | 0.41 | 0.29 | 0.58 | <.001 |
| Age | 0.64 | 0.55 | 0.74 | <.001 |
| Race/ethnicity (reference: white) | ||||
| Asian | 1.43 | 0.76 | 2.67 | 0.266 |
| Black | 2.12 | 1.35 | 3.34 | 0.001 |
| Hispanic | 1.46 | 0.89 | 2.41 | 0.133 |
| Other/Unknown | 2.21 | 1.00 | 4.89 | 0.049 |
Note: Patients were required to have responses at both the index and follow-up visits; AOR=adjusted odds ratio; UC= usual care; BC=behavioral clinician
Note: Patients were required to have responses at both the index and follow-up visits; AOR=adjusted odds ratio; BC=behavioral clinician
DISCUSSION
Evidence suggests that primary care-based behavioral health may be preferable to non-integrated approaches, but barriers to integration – such as competing priorities and full schedules – are well documented. It is unclear whether it is feasible to expect pediatricians to provide behavioral interventions, or whether they are more effectively delivered by other clinicians. The literature has not directly examined whether non-physician clinicians can deliver SBIRT as effectively as pediatricians. To help answer this important question, we compared clinical outcomes of pediatrician-delivered versus BC-delivered SBIRT in pediatric primary care.
We found no differences in substance use at follow-up visits, either between the intervention arms or between them and usual care. However, patients in the embedded-BC arm were less likely to report depression symptoms at follow-up. These patients may have benefitted from meeting with the BC, who had more experience treating, and more time to discuss, emotional concerns than their pediatrician. Other factors potentially contribute to the effects we observed, such as demographic differences in patient characteristics or variability in intervention fidelity by type of provider. Yet our results suggest that having a BC readily available to assist patients experiencing distress may be more effective than training pediatricians to address depression, a significant health condition, and an important risk factor and precursor of adolescent substance use problems [33]. Finding a significant impact on depression symptoms from a BI is notable given the small effect sizes and modest evidence base on the efficacy and effectiveness of adolescent depression interventions [34].
Not surprisingly, older adolescents were more likely to endorse substance use, as they had more time to be exposed to substances or to have experienced emotional distress. Depression symptoms increased over time across arms, but especially among younger adolescents, consistent with research suggesting an increase in depression prevalence during early adolescence [35]. Healthcare providers should consider screening and early intervention efforts during this vulnerable developmental phase. Adolescents of color were less likely than Whites to endorse substance use, but more likely to endorse depression symptoms, suggesting an unmet need for mental health interventions. Given documented racial disparities in mental health treatment utilization [36], healthcare providers should consider delivery models which integrate behavioral health clinicians into primary care, which may reduce these disparities [37].
This study has several limitations. It was conducted in an integrated system with an insured population, and may not be generalizable to uninsured populations, and clinician practices here may differ from other settings. However, it is feasible that many pediatrician practices could employ a BC, and some are doing so [38]. Use of a single clinic may have led to spillover effects between study arms, and uptake of training in the pediatrician-only arm was limited. Differences in fidelity among providers in the two intervention arms may also have influenced the study results.
We can assume that follow-up visits were not missing at random; thus we included only patients with responses at each time point and examined differences between those with and without a follow-up visit during the specified time period. We found no differences in attrition rates across treatment arms.
This was a pragmatic trial that did not recruit patients and used EHR-based clinical information to examine outcomes. This strengthened the study by allowing us to examine the population base of adolescents with pediatric visits in the clinic. However, we relied on data which depended on patients returning for another well visit during the study period and completing the TWCQ. Patients may have returned for other reasons, e.g., for acute medical symptoms, and not received the TWCQ. Care data were reliant on information recorded in the EHR by providers. Additionally, no UC arm pediatricians and few in either intervention arm collected CRAFFT or substance use quantity/frequency data at follow-up visits (although both were part of follow-up protocol). As a result, we had to rely on the any past-year substance use items in the TWCQ administered at subsequent well visits to examine outcomes, rather than the more detailed measures often collected in traditional randomized trials. In so doing, we were unable to examine changes in quantity or frequency of use, which we might have detected with more sensitive instruments. Relatively few patients received a brief intervention in either intervention arm (27% in the BHC arm and 16.5% in the pediatrician-only arm).
Provider skill and intervention fidelity likely had an impact on effectiveness. However, our only gauge of intervention fidelity was providers’ own descriptions of their practices during feedback meetings, as we could not directly observe physician-patient interactions.
Most importantly, the length of time between index and follow up averaged over one year, while most studies have six month follow-ups. This longer follow-up period may have contributed to decay in intervention impact (or increased impact of confounders) over time. We also note that rescreening could have occurred 6 months later which would overlap with the measure of use in the prior 12 months. This, and the limitation of only measuring any use rather than amount of use, may help explain the lack of substance use findings. We also relied on two brief screening items to measure depression severity, but nevertheless found treatment group differences in depression symptoms.
CONCLUSION
The U.S. Preventive Services Task Force has found the evidence for screening and interventions for adolescents in pediatric primary care insufficient to recommend employing them for alcohol or drug use, but does recommend screening and intervention for depression [39]. Given the growing prevalence of depression, treatment needs, and costs of specialty psychiatric care for adolescents [40], integrated behavioral health approaches, including SBIRT, may be beneficial. Adolescents with lower-severity problems of either kind are unlikely to seek specialty mental health treatment, but may benefit from early intervention in primary care [6, 15]. Although our null findings regarding substance use are disappointing given the positive findings in some recent SBIRT trials [12, 14], this paper adds to the literature suggesting that integrated behavioral health may be effective for reducing depression symptoms, thus potentially beneficial for reducing substance use risk as well. The study is important because it examined the effectiveness of an evidence-based intervention implemented in a real-world clinical setting, in which outcomes measurement and fidelity are difficult to assess and control. More SBIRT research is needed in pediatric primary care to broaden the evidence base for its effectiveness among adolescents.
Figure 1.
CONSORT Diagram
IMPLICATIONS AND CONTRIBUTION.
Although substance use outcomes did not differ across modalities in this trial of SBIRT for adolescents in primary care, depression symptoms were lower for those receiving SBIRT from a behavioral health clinician, suggesting that they may be more effective than paediatricians in addressing depression symptoms in primary care.
Acknowledgments
We thank Agatha Hinman for editorial assistance with the manuscript. We thank the KPNC Adolescent Chemical Dependency Coordinating Committee, the KPNC Adolescent Medicine Specialists Committee, and Thekla Brumder Ross, PsyD, and Jennifer Mertens, PhD, for their guidance. We also thank Anna Wong, PhD, David Bacchus, MD, Patricia Castaneda-Davis, MD, and all the physicians, medical assistants, nurses, receptionists, managers, and especially the patients and parents, of KPNC’s Oakland Pediatrics clinic for their participation in the activities related to this study. All contributing authors have been included above.
Funding: This work was supported by the National Institute on Alcohol Abuse and Alcoholism (grant R01 AA016204)
Abbreviations
- SBIRT
Screening, Brief Intervention and Referral to Treatment
- EHR
Electronic Health Record
- KPNC
Kaiser Permanente Northern California
- UC
Usual Care
- BC
Behavioral Clinician
- AOR
Adjusted Odds Ratio
- MI
Motivational Interviewing
- CI
Confidence Interval
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have no conflicts of interest to disclose.
Clinical Trials Registration: ClinicalTrials.gov #NCT02408952
References
- 1.Perou R, Bitsko RH, Blumberg SJ, et al. Mental health surveillance among children--United States, 2005–2011. Morbidity and mortality weekly report Surveillance summaries. 2013;62(Suppl 2):1–35. [PubMed] [Google Scholar]
- 2.Gabel S. The integration of mental health into pediatric practice: pediatricians and child and adolescent psychiatrists working together in new models of care. J Pediatr. 2010;157:848–851. doi: 10.1016/j.jpeds.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 3.SAMHSA-HRSA Center for Integrated Health Solutions. Integrating behavioral health and primary care for children and youth: Concepts and strategies. Available at: http://www.integration.samhsa.gov/integrated-care-models/Overview_CIHS_Integrated_Care_Systems_for_Children.pdf.
- 4.Subramaniam GA, Volkow ND. Substance misuse among adolescents: to screen or not to screen? JAMA Pediatr. 2014;168:798–799. doi: 10.1001/jamapediatrics.2014.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Institute on Alcohol Abuse and Alcoholism. Alcohol screening and brief intervention for youth: A practitioner's guide. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2011. Oct, 2015. Report No.: NIH Publication No. 11-7805. [Google Scholar]
- 6.Tanner-Smith EE, Lipsey MW. Brief alcohol interventions for adolescents and young adults: a systematic review and meta-analysis. J Subst Abuse Treat. 2015;51:1–18. doi: 10.1016/j.jsat.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown RA, Abrantes AM, Minami H, et al. Motivational interviewing to reduce substance use in adolescents with psychiatric comorbidity. J Subst Abuse Treat. 2015;59:20–29. doi: 10.1016/j.jsat.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babowitch JD, Antshel KM. Adolescent treatment outcomes for comorbid depression and substance misuse: A systematic review and synthesis of the literature. J Affect Disord. 2016;201:25–33. doi: 10.1016/j.jad.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 9.Asarnow JR, Rozenman M, Wiblin J, et al. Integrated Medical-Behavioral Care Compared With Usual Primary Care for Child and Adolescent Behavioral Health: A Meta-analysis. JAMA pediatrics. 2015;169:929–937. doi: 10.1001/jamapediatrics.2015.1141. [DOI] [PubMed] [Google Scholar]
- 10.Boekeloo BO, Jerry J, Lee-Ougo WI, et al. Randomized trial of brief office-based interventions to reduce adolescent alcohol use. Archives of pediatrics & adolescent medicine. 2004;158:635–642. doi: 10.1001/archpedi.158.7.635. [DOI] [PubMed] [Google Scholar]
- 11.D'Amico EJ, Miles JN, Stern SA, et al. Brief motivational interviewing for teens at risk of substance use consequences: a randomized pilot study in a primary care clinic. J Subst Abuse Treat. 2008;35:53–61. doi: 10.1016/j.jsat.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Harris SK, Csemy L, Sherritt L, et al. Computer-facilitated substance use screening and brief advice for teens in primary care: an international trial. Pediatrics. 2012;129:1072–1082. doi: 10.1542/peds.2011-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walton MA, Resko S, Barry KL, et al. A randomized controlled trial testing the efficacy of a brief cannabis universal prevention program among adolescents in primary care. Addiction. 2013 doi: 10.1111/add.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walton MA, Bohnert K, Resko S, et al. Computer and therapist based brief interventions among cannabis-using adolescents presenting to primary care: one year outcomes. Drug and alcohol dependence. 2013;132:646–653. doi: 10.1016/j.drugalcdep.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asarnow JR, Rozenman M. Integrating depression treatment within primary care improves outcomes in adolescents. Evid Based Ment Health. 2015;18:94. doi: 10.1136/eb-2014-101994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asarnow JR, Jaycox LH, Tang L, et al. Long-term benefits of short-term quality improvement interventions for depressed youths in primary care. Am J Psychiatry. 2009;166:1002–1010. doi: 10.1176/appi.ajp.2009.08121909. [DOI] [PubMed] [Google Scholar]
- 17.Richardson LP, Ludman E, McCauley E, et al. Collaborative care for adolescents with depression in primary care: a randomized clinical trial. Jama. 2014;312:809–816. doi: 10.1001/jama.2014.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sterling SA, Kline-Simon AH, Wibbelsman CJ, et al. Screening for adolescent alcohol and drug use in pediatric health-care settings: predictors and implications for practice and policy. Addiction Science & Clinical Practice. 2012;7:13. doi: 10.1186/1940-0640-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kline-Simon AH, Weisner C, Sterling S. Point prevalence of co-occurring behavioral health conditions and associated chronic disease burden among adolescents. J Am Acad Child Adolesc Psychiatry. 2016;55:408–414. doi: 10.1016/j.jaac.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Hulvershorn LA, Quinn PD, Scott EL. Treatment of Adolescent Substance Use Disorders and Co-Occurring Internalizing Disorders: A Critical Review and Proposed Model. Curr Drug Abuse Rev. 2015;8:41–49. doi: 10.2174/1874473708666150514102745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoek W, Marko M, Fogel J, et al. Randomized controlled trial of primary care physician motivational interviewing versus brief advice to engage adolescents with an Internet-based depression prevention intervention: 6-month outcomes and predictors of improvement. Transl Res. 2011;158:315–325. doi: 10.1016/j.trsl.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller-Matero LR, Dubaybo F, Ziadni MS, et al. Embedding a psychologist into primary care increases access to behavioral health services. J Prim Care Community Health. 2015;6:100–104. doi: 10.1177/2150131914550831. [DOI] [PubMed] [Google Scholar]
- 23.Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3. New York: Guilford Press; 2013. [Google Scholar]
- 24.Stein RE, Zitner LE, Jensen PS. Interventions for adolescent depression in primary care. Pediatrics. 2006;118:669–682. doi: 10.1542/peds.2005-2086. [DOI] [PubMed] [Google Scholar]
- 25.Fallucco EM, Seago RD, Cuffe SP, et al. Primary care provider training in screening, assessment, and treatment of adolescent depression. Acad Pediatr. 2015;15:326–332. doi: 10.1016/j.acap.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Babor TE, Higgins-Biddle J, Dauser D, et al. Alcohol screening and brief intervention in primary care settings: Implementation models and predictors. J Stud Alcohol. 2005;66:361–368. doi: 10.15288/jsa.2005.66.361. [DOI] [PubMed] [Google Scholar]
- 27.Mertens JR, Chi FW, Weisner CM, et al. Physician versus non-physician delivery of alcohol screening, brief intervention and referral to treatment in adult primary care: the ADVISe cluster randomized controlled implementation trial. Addiction Science & Clinical Practice. 2015;10:26. doi: 10.1186/s13722-015-0047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sterling S, Kline-Simon AH, Satre DD, et al. Implementation of screening, brief intervention, and referral to treatment for adolescents in pediatric primary care: a cluster randomized trial. JAMA Pediatrics. 2015;169:e153145. doi: 10.1001/jamapediatrics.2015.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellg AJ, Borrelli B, Resnick B, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol. 2004;23:443–451. doi: 10.1037/0278-6133.23.5.443. [DOI] [PubMed] [Google Scholar]
- 30.Simon GR, Baker C, Barden GA, 3rd, et al. 2014 Recommendations for Pediatric Preventive Health Care. Pediatrics. 2014;133:568–570. doi: 10.1542/peds.2013-4096. [DOI] [PubMed] [Google Scholar]
- 31.Knight JR, Sherritt L, Shrier LA, et al. Validity of the CRAFFT substance abuse screening test among adolescent clinic patients. Arch Pediatr Adolesc Med. 2002;156:607–614. doi: 10.1001/archpedi.156.6.607. [DOI] [PubMed] [Google Scholar]
- 32.Campbell MK, Mollison J, Steen N, et al. Analysis of cluster randomized trials in primary care: a practical approach. Fam Pract. 2000;17:192–196. doi: 10.1093/fampra/17.2.192. [DOI] [PubMed] [Google Scholar]
- 33.Parrish KH, Atherton OE, Quintana A, et al. Reciprocal relations between internalizing symptoms and frequency of alcohol use: Findings from a longitudinal study of Mexican-origin youth. Psychol Addict Behav. 2016;30:203–208. doi: 10.1037/adb0000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weersing VR, Jeffreys M, Do MT, et al. Evidence Base Update of Psychosocial Treatments for Child and Adolescent Depression. J Clin Child Adolesc Psychol. 2017;46:11–43. doi: 10.1080/15374416.2016.1220310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A) Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alegria M, Carson NJ, Goncalves M, et al. Disparities in treatment for substance use disorders and co-occurring disorders for ethnic/racial minority youth. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50:22–31. doi: 10.1016/j.jaac.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hodgkinson S, Godoy L, Beers LS, et al. Improving mental health access for low-income children and families in the primary care setting. Pediatrics. 2017:139. doi: 10.1542/peds.2015-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hine JF, Grennan AQ, Menousek KM, et al. Physician satisfaction with integrated behavioral health in pediatric primary care. J Prim Care Community Health. 2017;8:89–93. doi: 10.1177/2150131916668115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siu AL. Screening for Depression in Children and Adolescents: US Preventive Services Task Force Recommendation Statement. Pediatrics. 2016;137:e20154467. doi: 10.1542/peds.2015-4467. [DOI] [PubMed] [Google Scholar]
- 40.Mojtabai R, Olfson M, Han B. National trends in the prevalence and treatment of depression in adolescents and young adults. Pediatrics. 2016;138:e20161878. doi: 10.1542/peds.2016-1878. doi: 20161810.20161542/peds.20162016-20161878. [DOI] [PMC free article] [PubMed] [Google Scholar]