Abstract
Cognitive control of attention in conflict situations is a basic skill that is vital for goal-oriented behaviors. Behavioral evidence shows that conflict control occurs over successive trials as well as longer time scales of trial blocks, but the relation among time scales as well as their neural mechanisms are unclear. This study used measures of behavior, EEG, and a simple quantitative model to test the hypothesis that conflict control at the block level is not exclusively driven by the control adjustments over successive trials. Young adults performed an auditory Simon task, and the base rate of compatible vs. incompatible trials was manipulated in separate blocks (25, 50, 75% compatible). EEG data were analyzed using independent component analysis (ICA) to define cortical mechanisms of any base rate and trial-by-trial sequence effects. Reaction time measures had both sequence and base rate effects. Two fronto-medial ICA components indexed sequence and base rate effects, with specific profiles for evoked potentials and oscillations in the theta and alpha frequency bands. Predictive modeling showed that sequence effects accounted for a minority of the variance on behavioral and ICA measures (all <36%). The results strongly suggest that the base rate manipulation affected behavior and many neural measures in addition to the influences of sequence effects.
Keywords: cognitive control, EEG, theta, prefrontal cortex, attention
1. Introduction
The ability to pursue and achieve goals is vital for intelligent behavior. Setting attentional biases to promote goal attainment is a basic process in cognitive control (Miller & Cohen, 2001). Nonetheless, task-irrelevant information still influences performance, and when performance suffers this is termed a “conflict” between relevant and irrelevant information (Botvinick, Braver, Barch, Carter, & Cohen, 2001). The Stroop, Flanker, and Simon tasks are commonly used to study cognitive conflict (Eriksen & Eriksen, 1974; Simon & Rudell, 1967; Stroop, 1935). The specific nature of the conflict varies in the three tasks, but all are thought to generally involve conflict that arises during the response selection stage (Egner, 2017).
The Simon task indexes the degree that irrelevant spatial information captures attention (Simon & Rudell, 1967). Subjects respond using the left or right hand on the basis of a non-spatial stimulus feature, such as pitch. Performance in the Simon task is better when the hand and sound location are on the same side (compatible trials) relative to having different locations (incompatible trials). The degree of conflict is quantified by differences in reaction time and accuracy among compatible versus incompatible trials (termed the “Simon effect”). Spatial hearing has particular ecological importance as an early warning system (Hartmann, 1999; Scharf, 1998). Potent attention capture in spatial hearing may be why, as compared to vision, the auditory Simon effect is usually larger (Vu, Proctor, & Urcuioli, 2003) and evident across a wider range of reaction times (Wascher, Schatz, Kuder, & Verleger, 2001).
The degree of conflict on the time scale of minutes can be manipulated by varying the base rates of compatible and incompatible trials within an entire block of trials (Stürmer, Leuthold, Soetens, Schröter, & Sommer, 2002); also termed “list-wide proportion congruence” (Logan & Zbrodoff, 1979). For simplicity, here we use the term base rate. The basic result is that the conflict effect increases with the base rate of compatible trials (reviewed in Bugg & Crump, 2012). Base rate effects are often characterized as an indication of cognitive control strategy (e.g. Risko, Blais, Stolz, & Besner, 2008). Participants may decrease cognitive control bias if compatible trials are frequent because it is redundant with location information. Thus, in the Simon task compatible trials may reinforce the tendency to respond with the hand that matches stimulus location, and incompatible trials may serve as a reminder to try to ignore spatial information. Therefore, trial blocks with mostly compatible trials have fewer reminders and larger Simon effects. This explanation fits in with a Dual Mechanisms Framework for cognitive control in which participants activate “proactive control” to set attentional biases in anticipation of conflict for the next stimulus (Braver, 2012). Other researchers have also explained the base rate effect as being caused by a strengthening of correct stimulus-response associations with decreasing compatible trial base rate (Cao, Cao, Yue, & Wang, 2017).
Cognitive control has also been examined on the shorter time frame of successive trials (Egner, 2007; Gratton, Coles, & Donchin, 1992; Larson, Kaufman, & Perlstein, 2009; Wühr & Ansorge, 2005). Results show that the Simon Effect is smaller on the trial after an incompatible trial, relative to having a previous compatible trial (Gratton et al., 1992). These “sequence effects” may be due to a system that monitors for conflict in response selection, and when conflict exceeds a threshold cognitive control then enhances task-related biases on the next trial (Botvinick et al., 2001). These trial-by-trial adjustments may map onto the idea of “reactive control” in which participants respond to stimuli across shorter timescales rather than proactively adjusting control across longer periods of time (Braver, 2012). Proactive and reactive control are not necessarily exclusive of each other, and some researchers have suggested that anticipatory proactive control can adjust on shorter timescales as well (van Driel, Swart, Egner, Ridderinkhof, and Cohen, 2015). It is therefore possible that with sequence effects participants are proactively adjusting attention on a trial-by-trial basis dependent on the previous trial type.
The base rate and sequence effects are interrelated because the proportion of each type of sequence covaries with base rate. The higher the proportion of compatible trials, the greater the chance that for any given trial the previous trial was compatible. Since there is a larger Simon effect following compatible trials, it must also be the case that blocks with higher compatible base rates (and thus more trials preceded by compatible trials) will have larger Simon effects. It is even possible that sequence effects could entirely drive base rate effects. However, researchers often study base rate effects without considering sequence effects. An exception is Risko et al. (2008), who found that controlling for the specific sequences of stimulus and response locations across pairs of trials reduced the base rate effect by 47%. In another study Torres-Quesada, Milliken, Lupiáñez, & Funes (2014) found that base rate effects were present when compatibility sequence effects were absent. Together these results suggest that base rate effects on longer time scales are only partially driven by sequence effects on short time scales.
The issue of how sequence effects relate to base rate effects can also be approached by including measures of neural activity (e.g. Cieslik, Mueller, Eickhoff, Langner, & Eickhoff, 2015; Larson & Lee, 2014). The anterior cingulate cortex (ACC) has been implicated in cognitive control in fMRI (Kerns, 2006), EEG (van Veen & Carter, 2002), and lesion studies (di Pellegrino, Ciaramelli, & Làdavas, 2007). Kerns (2006) found that the ACC was involved in detecting and responding to Simon task conflict, and that ACC activity then predicted prefrontal cortex activity on the next trial. This finding supports a conflict-monitoring hypothesis in which the ACC monitors conflict and then signals to other brain areas, such as the dorsolateral prefrontal cortex, to help deal with conflict on upcoming trials (Botvinick, Cohen, & Carter, 2004; Kerns, 2006). These findings highlight the importance of frontal midline areas in modulating cognitive conflict, and provide a foundation to test how well neural mechanisms of sequence effects can account for base rate effects. On the other hand, some researchers have suggested that the ACC does not monitor conflict (Burle, Roger, Allain, Vidal, & Hasbroucq, 2008). The current study aimed to clarify by looking at neural activity during conflict across multiple timescales.
Frontal event-related potentials (ERPs) and EEG oscillations that index conflict likely reflect activity in the same medial frontal brain areas previously implicated in cognitive control and found to be sources of frontal theta (Gevins, Smith, McEvoy, & Yu, 1997; Kerns, 2006). For example, Hanslmayr et al. (2008) used source localization to find that a conflict-sensitive ERP and frontal theta during a Stroop task both originated in the ACC. ERP components known as the “N2” in Flanker tasks (Clayson & Larson, 2011) or as the “N450” or “medial frontal negativity” (MFN) in Stroop or other conflict tasks are larger (more negative) for incompatible vs. compatible trials and often thought to originate in the ACC (Larson et al., 2009; West & Bailey, 2012). The MFN/N450 peaks at ~450 ms in visual Stroop tasks (Tillman & Wiens, 2011; West & Bailey, 2012). It shows both base rate and sequence effects and is thought to reflect conflict monitoring (Cespón, Galdo-Álvarez, & Díaz, 2016; Larson et al., 2009; West & Bailey, 2012). Also in Stroop tasks, the amplitude of a subsequent component called the “frontal slow wave” (latency ~600-800 ms or later) is also sensitive to conflict and base rate (Bailey, West, & Anderson, 2010; West & Bailey, 2012). The medial frontal negativity occurs before a response is made, and is likely related to conflict detection and processing. Because the frontal slow wave occurs after the average response time, it may reflect across-trial adaptations such as increases in proactive conflict monitoring or between-trial responses such as working memory capacity updates (West, Bailey, Tiernan, Boonsuk, & Gilbert, 2012).
Neural oscillations have also implicated frontal and midline brain areas such as the ACC in cognitive control (Buzsaki & Draguhn, 2004; Cohen & Ridderinkhof, 2013; Cohen, Ridderinkhof, Haupt, Elger, & Fell, 2008). Increases in power following a stimulus indicate synchronized cortical cell activity in response to the stimulus, and decreases indicate desynchronization (Pfurtscheller & Lopes da Silva, 1999). Theta power (4-8 Hz) in prefrontal brain areas increases during conflict (Cavanagh & Frank, 2014; Cohen & Cavanagh, 2011; Gulbinaite, van Rijn, & Cohen, 2014). In an auditory Stroop task Oehrn, et al. (2014) found higher theta power in the dorsomedial prefrontal cortex during conflict, as measured using electrocorticography. Frontal and parietal theta power in the visual Simon task exhibits sequence effects, with a larger Simon effect for the trials after a compatible trial (Gulbinaite et al., 2014; Tang, Hu, & Chen, 2013; Töllner et al., 2017). Thus, both behavioral and neural measures of cognitive control adjust on long and short timescales in the Simon task, but to date no study has defined similarities and differences in conflict processing on these two time scales.
The current study had two main purposes. First, we defined the impact of manipulating the base rate of compatible trials on auditory cortical potentials (ERPs and event-related spectral perturbations/ERSPs) identified using independent component analysis (ICA). We hypothesized that a subset of the independent components would be strongly modulated by base rate, and would likely reflect frontal midline areas (anterior cingulate cortex, medial prefrontal cortex) that are associated with cognitive control. Most EEG studies on cognitive conflict have analyzed ERP data at the channel level. The current study used independent component analysis to further examine functionally distinct and/or independent components of electrical activity at the scalp. The second, and main, objective was to determine if behavior and the components modulated by base rate reflect a genuine effect of base rate. The null hypothesis was that base rate effects on behavior and independent component activity are accounted for by sequence effects, which have been shown by a lesion study to also involve the anterior cingulate cortex and to be reduced in patients with lesions in this area (di Pellegrino et al., 2007). We developed a quantitative model to predict what base rate effects would be for each participant if they were driven solely by that participant’s observed sequence effects. We then compared the predicted base rate effects to the observed base rate effects. Rejection of the null model predicted only from two-trial sequence effects would imply a separate process for adjustment of cognitive control across longer timescales.
2. Method
2.1. Subjects
Fifty-seven subjects were recruited from a University community in exchange for extra credit (mean age = 20.1 ± 2.5 years, 19 males, 2 left-handed). None reported a history of major neurological or psychiatric disorders. Normal hearing thresholds were verified for all subjects with audiometric testing (0.5-8.0 kHz). A subset of subjects (n=35) completed additional cognitive tests and surveys not presented here, including two working memory capacity tests and a questionnaire about musical experience. All subjects were given a handedness questionnaire (Oldfield, 1971). Ten subjects were excluded from analysis due to technical issues, hairstyles that prevented EEG recording, or excessive EEG artifacts.
2.2. Design
Subjects performed the Simon task under three base rate conditions (25, 50, and 75% compatible trials) tested in separate blocks. Factors of current trial type (compatible vs. incompatible), base rate (25%, 50%, 75% compatible), and previous trial type were used to examine current trial type × base rate and current trial type × previous trial type (sequence effect) interactions. We expected increases in trial type effects as the base rate of compatible trials increased, as well as compatibility sequence effects. The 50% base rate had equal proportions of each trial type and of each possible two-trial sequence. Thus, participants could gather no information on which trial type was most likely to occur during this condition. By making predictive models based on sequence effects in the 50% base rate, we tested whether short-timescale sequence effects fully predicted changes in the Simon effect across base rates or cognitive control adjusted across longer timescales as well.
2.3. Simon Task
Participants were familiarized with two monaural white noise sounds (100-10,000 Hz, 200 ms duration, ~65 dB nHL, 5 ms rise/fall) that had one of two amplitude modulation (AM) rates (25 or 75 Hz, 90% depth). Subjects pressed a left or right button with their left or right hand, respectively, to indicate AM rate (counterbalanced across subjects). The sounds were presented every 2.0 seconds to the left or right ear with insert headphones. For “compatible” trials, the sound’s location matched the side of its button assignment (e.g. right ear stimulus/right hand response). For “incompatible” trials, the AM rate specified a response by the hand opposite the ear receiving the sound (e.g. right ear stimulus/left hand response). Each participant received three blocks of each compatible base rate (25%, 50%, and 75%) with 161 trials in each block. The first trial in each block was not analyzed because the analysis focused on sequence effects. The order of base rates was randomized across subjects. A practice block with 40 trials was given before testing.
2.4. Electrophysiological Recordings
Electroencephalography (EEG) was recorded using a 64-channel Ag/AgCl electrode cap positioned using the 10/20 system (Compumedics Neuroscan, Charlotte, NC). The cap had a reference electrode between Cz and CPz and four electrodes placed above and below the left eye and near the lateral corners of each eye to track eye movements. Recording during the Simon Task took place inside a sound attenuating, electrically shielded booth (IAC Acoustics, Bronx, New York). EEG data was digitized at 500Hz with a DC-100Hz band-pass filter and recorded using Curry 7 Neuroimaging Suite Software (Compumedics Neuroscan, Charlotte, NC).
2.5. Data Analysis
2.5.1. EEG Processing
EEG data was processed offline using the EEGLAB (Delorme & Makeig, 2004) Matlab plugin (The Mathworks, Inc., Natick, MA). Continuous EEG data was high-pass filtered at 1Hz, resampled at 250 Hz, epoched from −800 to 1200 ms around stimulus onset, and re-referenced to the average. Channels and epochs containing lots of noise in the recording, EKG, or excessive non-stereotyped artifacts such as muscle activity were removed. Epochs were coded for factors of current Simon Task trial type (compatible vs. incompatible), previous trial type (compatible vs. incompatible), and base rate (25%, 50%, or 75% compatible). ERPs were baselined from −100 to 0 ms and low-pass filtered at 30Hz.
2.5.2. Independent Component Analysis
Following the EEG processing steps described above, extended infomax independent component analysis (ICA) was used on the epoched data to identify physiologically or functionally separate sources of electrical activity recorded at the scalp, referred to as independent components (ICs) (Bell & Sejnowski, 1995; Delorme & Makeig, 2004). ICs are statistically categorized as independent; therefore, they must reflect physiologically different brain responses in order to have been found to be statistically independent (Jung et al., 2001). ICs with scalp map distributions that were smooth across channels, log-function shaped spectral power curves with peaks at frequency bands that reflect brain activity (θ, α, β), and/or consistent ERP peaks across task trials were deemed consistent with brain activity. ICs made up of eye movements, muscle activity, EKG, or noise in the recording (Delorme, Sejnowski, & Makeig, 2007) were excluded from analyses. Dipoles for each IC were modeled with the DIPFIT2 plug-in from the FIELDTRIP toolbox for MATLAB for the purpose of helping with cluster analysis (Oostenveld, Fries, Maris, & Schoffelen, 2011; http://www.ru.nl/neuroimaging/fieldtrip). Time-frequency analysis was calculated for each IC to show average changes in event-related spectral power across time and frequency. Results were plotted as color heat maps representing power in decibels (dB) along time and frequency axes. To run ANOVA statistics, results were quantified as average power over time-frequency windows of interest. Our ICA and time-frequency analysis procedures are described further by Mock, Seay, Charney, Holmes, & Golob (2015).
2.5.3. Cluster Analysis
Principal component cluster analysis using EEGLAB’s k-means algorithm was used to group physiologically and functionally similar ICs from different subjects. To cluster biologically similar ICs together, IC dipole location was weighted three times as heavily as the scalp map, ERP, and ERSP. Clustering was performed several times using k=8 to k=14 k-means and an outlier cluster with IC centroids > 3 standard deviations from cluster centroids. Eight clusters had relative stability and contained most subjects across increasing values of k. In some cases, a subject contributed more than one IC to a cluster. Because we wanted individual subjects to be weighted equally in statistical analyses, one IC per subject per cluster was selected based on quality of the component measures (ERP, ERSP, scalp map, dipole location). To verify that this pruning did not influence our results, we confirmed that clusters showed the same statistically significant ERP and ERSP effects before and after pruning.
Statistical analyses used time windows based on previous studies of the N450 and the later frontal slow wave (e.g. West & Bailey, 2012). In addition, non-parametric statistics that corrected for multiple comparisons between 0-1000 ms were also used to identify time periods of significant effects.
2.6. Statistical Analysis
Only trials with correct behavioral responses were analyzed. Median trial reaction times for each condition and each subject were used to reduce the influence of outliers. Greenhouse-Geisser corrected p-values are reported when follow-up ANOVAs violated the sphericity assumption.
2.6.1. Preliminary Analysis
One concern was whether the ERSP sequence effects may have been due in part to inter-trial adjustments that affected the current trial’s ERSP baseline. In order to rule out this possibility, we tested the effect of previous trial type on current trial baseline theta and alpha power for midfrontal and central clusters. We found that although previous trial type affected the current trial ERSP, it was not related to the current trial baseline. Therefore, inter-trial effects that could have affected the ERSP baseline were not analyzed further.
2.6.2. Base Rate and Sequence Effects
The base rate and sequence effects on cognitive control were analyzed for Simon Task reaction time, accuracy, and electrophysiological measures (ERPs and ERSPs). For ERPs and ERSPs, interaction statistics for clusters were first graphed in EEGLAB using a false discovery rate statistical correction for multiple comparisons. The plots were used to select windows with significant predicted effects. Window averages were computed and then analyzed using repeated-measures ANOVAs conducted in R (R Foundation for Statistical Computing, 2015). A 2 × 3 repeated-measures ANOVA with factors of Simon Task trial type (compatible vs. incompatible) and Simon Task base rate (25%, 50%, 75% compatible) tested the trial type × base rate interaction. A 2 × 2 repeated measures ANOVA with factors of current trial type (compatible vs. incompatible) and previous trial type (compatible vs. incompatible) tested sequence effects. Sequence effects were only examined in the 50% base rate because the 25% and 75% base rates had too few trials of the least frequent sequence to analyze the EEG data with an adequate signal-to-noise ratio. Follow-up comparisons across base rates were made within each trial type following a significant trial type × base rate interaction, with a Bonferroni corrected alpha level of 0.025. Compatible vs. incompatible comparisons within each level of previous trial type were made when sequence effects were significant, with a Bonferroni corrected alpha level of 0.025.
2.6.3. Base Rate vs. Sequence Effects (Tables 1 & 2)
Table 1.
Trial type percentages in each base rate.
| 25% Compatible | 75% Compatible | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Compatible (C) | Incompatible (IC) | Compatible (C) | Incompatible (IC) | |||||
| Sequence Type | C-C | IC-C | C-IC | IC-IC | C-C | IC-C | C-IC | IC-IC |
| % of Sequence Type within Condition | 6.25 | 18.75 | 18.75 | 56.25 | 56.25 | 18.75 | 18.75 | 6.25 |
Table 2.
Predictive model calculations
| 25% Compatible | 75% Compatible |
|---|---|
| .25(M50%C-C) + .75(M50%IC-C) = PC | .75(M50%C-C) + .25(M50%IC-C) = PC |
| .25(M50%C-IC) + .75(M50%IC-IC) = PIC | .75(M50%C-IC) + .25(M50%IC-IC) = PIC |
| PSimonEffect = PIC - PC | PSimonEffect = PIC - PC |
Abbreviations are as follows. M = Mean, P = Predicted Response, C-C = Compatible following compatible, IC-C = Compatible following incompatible, IC-IC = Incompatible following incompatible, C-IC = Incompatible following compatible
Quantitative models were constructed to predict the Simon effect in each base rate condition from sequence effects in the 50% compatible base rate. For each subject a given ICA measure for each sequence type (compatible-compatible, compatible-incompatible, incompatible-compatible, incompatible-incompatible) was weighted based on the percentage of each sequence type in the base rate and applied to each base rate. The weights are given in Table 1 and predictive calculations are given in Table 2. An ordinary least squares regression slope fitting procedure was then used to get each subject’s observed and predicted Simon effect slope across base rates. We tested the predicted versus observed slopes using paired-sample t-tests to see whether base rate attenuated the Simon effect on the timescale of minutes, beyond trial-to-trial adjustments on the timescale of seconds. The null hypothesis was that the observed slopes across base rates would not differ from the predicted slopes calculated from the model.
3. Results
3.1. Behavior
Behavioral results are shown in Figure 1, with reaction time and accuracy as a function of base rate on the left and sequence effects on the right. Overall, Simon effects for reaction time and accuracy increased with compatible base rate, and were most evident after a compatible trial (See Table 3 for a summary of results).
Figure 1.
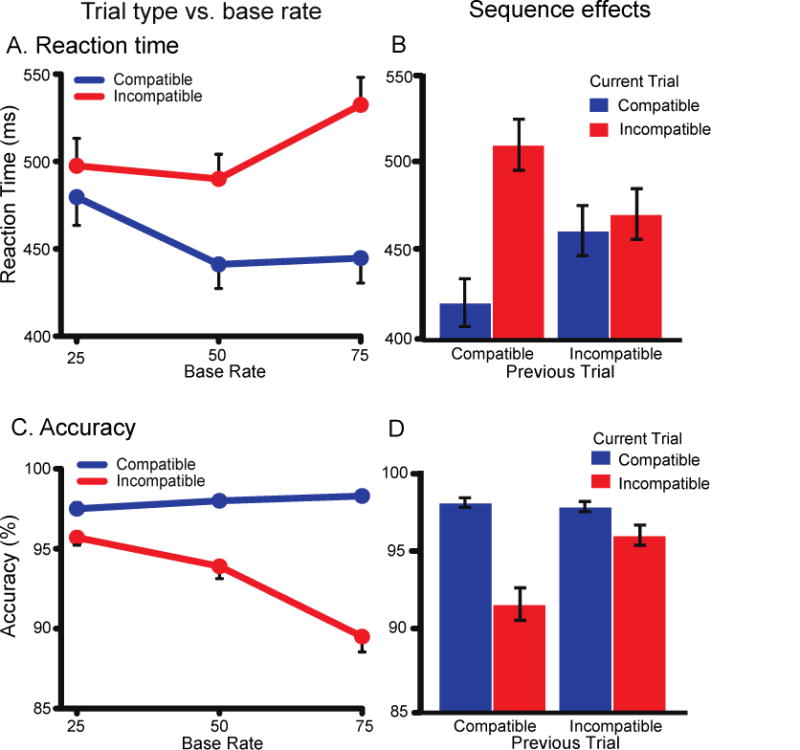
A) The Trial type × Base Rate interaction for reaction time during the Simon task. B) Sequence effects (Current Trial type × Previous Trial Type) for reaction time during the Simon task. B) Trial type × Base Rate interaction for accuracy. C) Sequence effects for accuracy.
Table 3.
Summary of significant effects.
| Measure | Window | Base Rate × Trial Type | Sequence Effect | Observed Simon effect slope > Model | |
|---|---|---|---|---|---|
| Behavior | Reaction Time | + | + | + | |
| Accuracy | + | + | + | ||
|
| |||||
| Frontal | ERP | 400–500 ms | − | + | − |
| Midline Cluster | ERSP | 4–12 Hz, 300–500 ms | + | + | + |
|
| |||||
| Central Cluster | ERP | 400-700 ms | + | − | + |
| ERSP | 4–8 Hz, 300–600 ms | + | + | + | |
| 8–12 Hz, 300–500 ms | + | + | + | ||
indicates a significant effect.
Indicates no effect
3.1.1. Base rate analysis
Reaction time and accuracy were examined separately using 2 (trial type) × 3 (base rate) ANOVA tests. Reaction time showed a Simon effect, with slower reaction times on incompatible vs. compatible trials (F(1,46) = 164.6, p<.001). Reaction time slowed with increases in the compatible base rate; indicated by a main effect of base rate (F(2,92) = 4.54, p<.05; Figure 1A). Importantly, a significant trial type × base rate interaction showed that the Simon Effect increased in tandem with compatible trial base rate (F(2,92) = 69.5, p<.001)1.
For accuracy there was also a significant Simon effect, as subjects were more accurate on compatible vs. incompatible trials (F(1,46) = 81.12, p<.001). Accuracy also decreased as compatible base rate increased (F(2,92) = 32.06, p<.001; Figure 1C). These main effects were qualified by a significant trial type × base rate interaction. As with reaction time, the Simon effect became greater with increases in the compatible trial base rate (F(2,92) = 48.47, p<.001)1.
3.1.2. Sequence effects in 50% base rate condition
Potential carryover effects from the previous trial were examined in the 50% base rate condition using a 2 (previous trial type) × 2 (current trial type) ANOVA (Figure 1B, D). For reaction time there was a significant previous × current trial interaction (F(1,46) = 171.24, p<.001; Figure 1B). The interaction showed that the Simon effect was large after a compatible trial (t(46) = 16.39, p<.001), but was not evident after an incompatible trial with a Bonferroni correction (p > .03). Analysis of accuracy also had significant sequence effects (F(1,46) = 26.84, p<.001; Figure 1D), with a larger difference between trial types after a compatible trial.
3.2. Independent Component Analysis
Analyses focused on the frontal and central midline clusters because the ERPs and ERSPs had conflict effects and the topography was consistent with generators in frontal areas important for cognitive control (Figure 2). Most subjects (42 and 45 out of 47, respectively) contributed ICs to these clusters (See supplemental materials for additional analysis of lateral clusters). As with the behavioral data, we first analyzed the base rate effects and then examined sequence effects in the 50% base rate condition.
Figure 2.
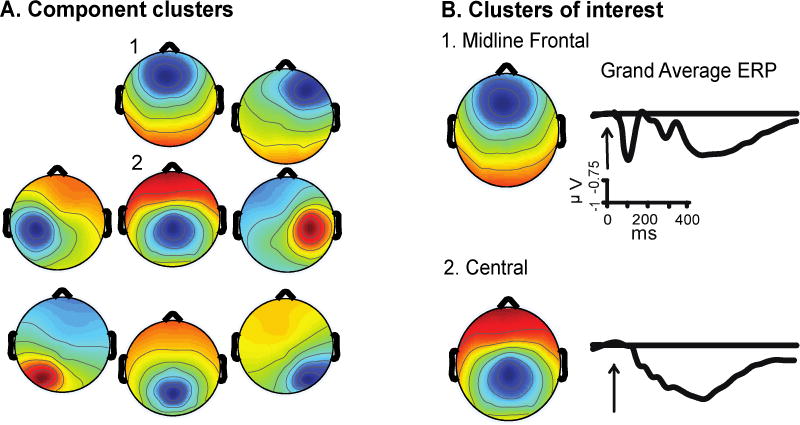
A) Independent component analysis cluster scalp maps. To cluster ICs together, k-means cluster analysis used the IC dipole location, scalp map, ERP, and ERSP. To ensure that biologically similar ICs were grouped together dipole location was weighted three times more heavily than the scalp map, ERP, and ERSP. B) Scalp maps and grand average ERPs for clusters examined in this paper.
3.3. Midline frontal component cluster
3.3.1. Base rate analysis
Event-related potential amplitudes were analyzed using 2 (trial type) × 3 (base rate) ANOVA tests (Figure 3A, C). The time window from 400-500 ms had greater negativity for compatible vs incompatible trials (F(1,41) = 10.41, p<.01) and no main effect or interaction involving base rate. The time period of the medial frontal negativity (250-350 ms) and later slow wave (400-700 ms) did not have significant effects, and are not further analyzed for this cluster.
Figure 3.
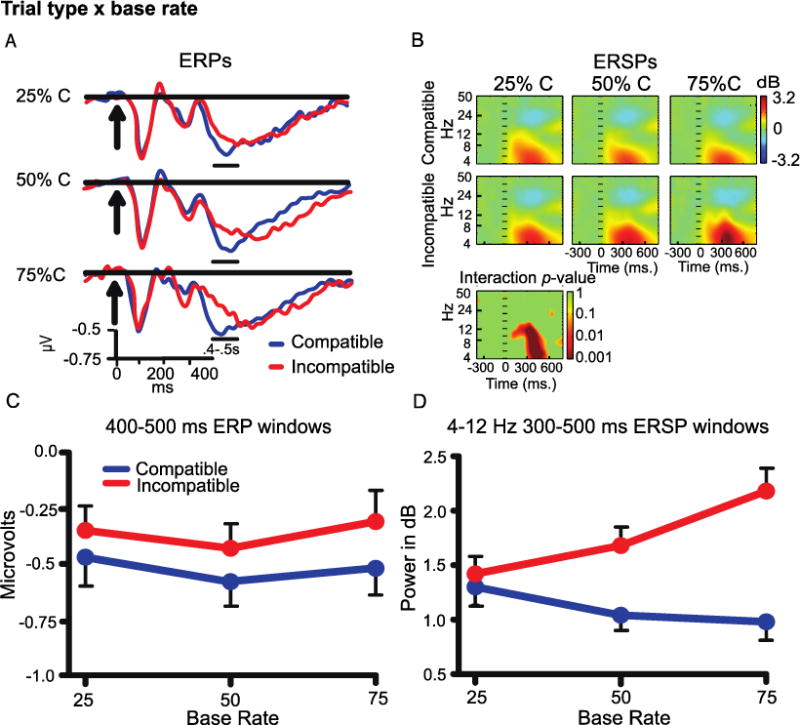
A) Midfrontal cluster ERP waveforms in each base rate. B) ERSP color heat maps including a plot of non-parametric permutation statistics for the interaction with a false discovery correction. C) ERP window averages Trial Type × Base Rate effect. D) ERSP window averages Trial Type × Base Rate effects.
A plot of ERSP power over time showed large increases in the theta and alpha bands (4-12 Hz) between ~300-500 ms after stimulus onset (Figure 3B, D)2. A 2 (trial type) × 3 (base rate) ANOVA had a significant effect of trial type (incompatible > compatible) (F(1,41) = 53.07, p<.001)3. This effect was consistent with previous work implicating frontal theta in cognitive control (e.g. Cavanagh & Frank, 2014). The main effect of trial type was qualified by a significant 2 (trial type) × 3 (base rate) interaction (F(2,82) = 20.76, p<.001). The interaction showed that the Simon effect increased with increases in compatible base rate (See Figure 3D). Follow-up ANOVAs on each trial type showed that power across base rates was comparable for compatible trials but increased for incompatible trials (F(2,82) = 21.04, p<.001).
3.3.2. Sequence effects in 50% base rate condition
A 2 (previous trial type) × 2 (current trial type) ANOVA on ERP amplitudes in the 400-500 ms time window had a significant interaction (F(1,41) = 6.91, p=.012), indicating sequence effects (Figure 4A, C). Similar to the reaction time results, the Simon effect for this window was present after compatible trials (t(41) = 3.07, p<.01), but not incompatible trials.
Figure 4.
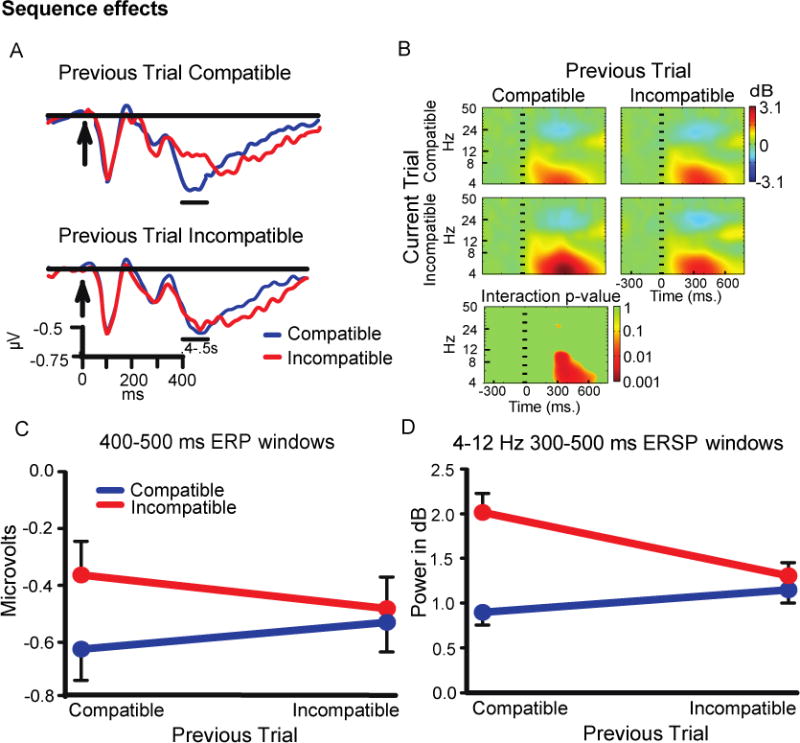
A) Midfrontal cluster ERP waveforms. B) ERSP color heat maps, including a plot of non-parametric permutation statistics for the interaction with a false discovery correction. C) ERP window average sequence effects. D) ERSP window average sequence effects.
Analysis of 4-12 Hz power between 300-500 ms also had a significant interaction (F(1,41) = 25.93, p<.001; Figure B, D), and post-hoc ANOVAs found a significant Simon effect after compatible trials (t(41) = 6.92, p<.001) but not after incompatible trials (p>.08).
3.4. Central component cluster
3.4.1. Base rate analysis
For the central cluster there was a prominent negative-going ERP between 400-700 ms (Figure 5A). Analysis revealed a main effect of trial type (incompatible > compatible trials)(F(1,44) = 16.12, p<.001), and a trial type × base rate interaction (F(2, 88) = 16.41, p<.001; Figure 5C). As with the findings above, the interaction reflected increases in the Simon effect as the percentage of compatible trials increased. Post-hoc ANOVAs showed that compatible trial amplitudes did not differ across base rates, but amplitude for incompatible trials increased (i.e. became more negative) with increases in compatible base rate (F(2, 88) = 7.08, p<.01).
Figure 5.
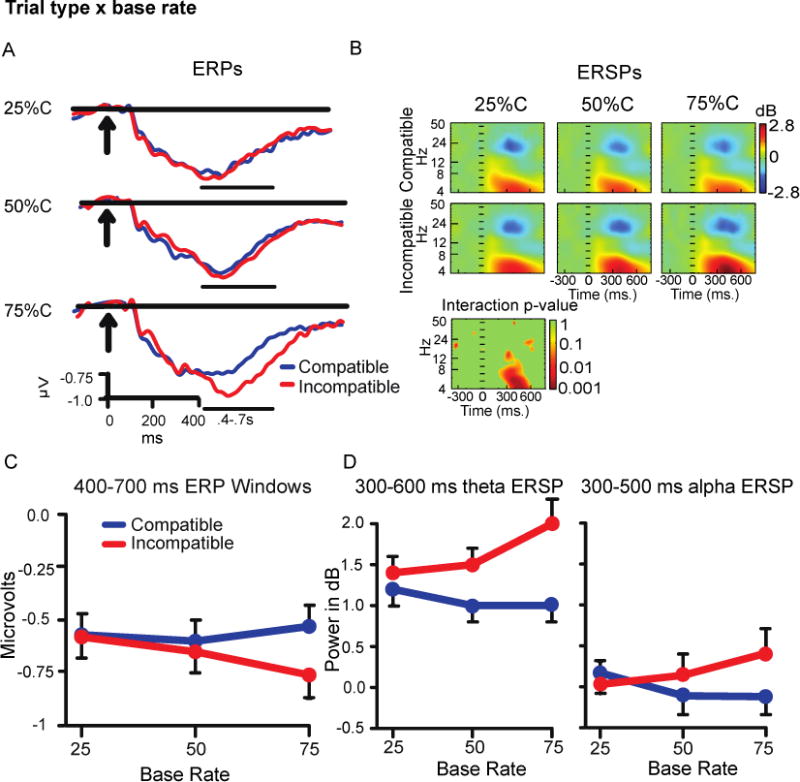
A) Central cluster ERP waveforms. B) ERSP color heat maps including a plot of non-parametric permutation statistics for the interaction with a false discovery correction. C) ERP window average Trial Type × Base Rate effects. D) ERSP window average Trial Type × Base Rate effects.
Power in the theta and alpha bands increased at ~300-600 ms and ~300-500 ms, respectively (Figure 5B). For the theta power measure (300-600 ms) there were main effects of trial type (F(1,44) = 37.42, p<.001) and base rate (F(2,88) = 3.57, p<.05), and a significant 2 (trial type) × 3 (base rate) interaction (F(2,88) = 13.7, p<.001; Figure 5D). The interaction showed that the size of the Simon effect increased with the percentage of compatible trials. Post-hoc ANOVAs showed no difference in compatible trials across base rate, while incompatible trial power increased as base rate increased, (F(2,88) = 12.33, p<.001). Note that power reductions were also observed in the beta band (~16-26 Hz) between ~300-600 ms (blue regions of Figure 5B), but they were not affected by base rate or trial type. Analysis of alpha ERSP power (300-500 ms) showed a significant trial type × base rate interaction (F(2,88) = 6.65, p<.01; Figure 5B, D). There was also a main effect of trial type (incompatible > compatible trials; F(1,44) = 4.42, p<.05). The interaction was due to increases in the Simon effect as compatible trials became more common, but power did not significantly vary across base rates within either trial type.
3.4.2. Sequence effects in 50% base rate condition
A 2 (current trial type) × 2 (previous trial type) repeated-measures ANOVA testing sequence effects on ERP amplitude in the 400-700 ms time window was not statistically significant (p =.084; Figure 6C). The trend was toward a larger Simon effect following compatible than incompatible trials.
Figure 6.
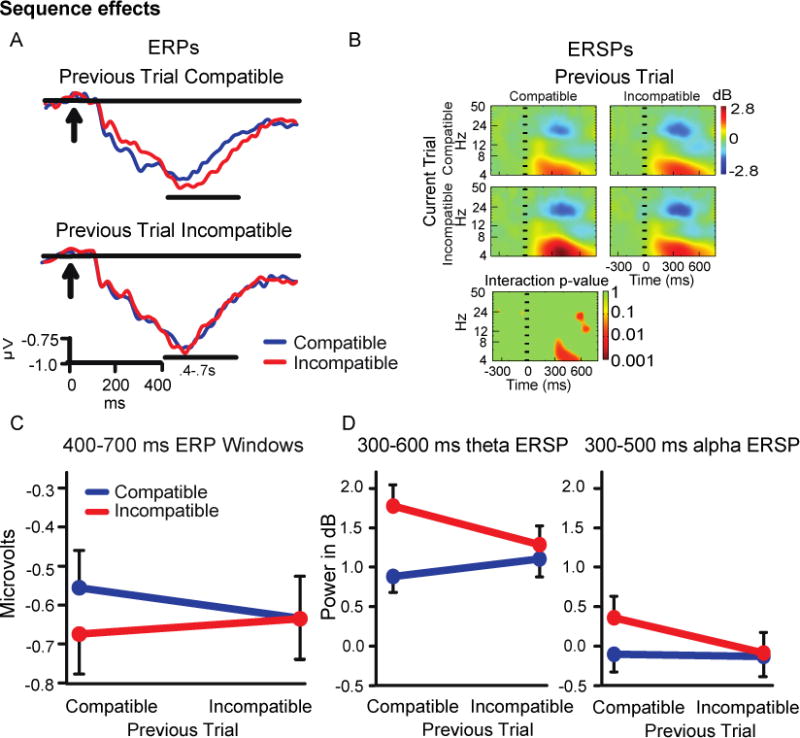
A) Central cluster ERP waveforms. B) ERSP color heat maps including a plot of non-parametric permutation statistics for the interaction with a false discovery correction. C) ERP sequence effects. D) ERSP sequence effects.
For ERSP power in the 300-600 ms theta band window there was a significant current × previous trial type interaction, indicating a compatibility sequence effect (F(1,44) = 13.38, p<.001; Figure 6D). As with behavioral reaction time and some of our other neural measures, the difference between compatible and incompatible trials was significant following compatible trials (t(44) = 5.21, p<.001) but not following incompatible trials.
Alpha ERSPs (300-500ms window) showed a comparable significant 2 (previous trial type) × 2 (current trial type) repeated-measures ANOVA (F(1,44) =4.25, p=.043; Figure 6D). Once again post-hoc tests revealed that the Simon effect was present following compatible trials (t(44) = 2.58, p<.025) but not incompatible trials.
In summary, with only two exceptions the Simon effects defined by behavioral and ERP/ERSP measures increased as compatible trials became more common, and were also larger after a compatible vs. incompatible trial. This pattern of results is consistent with the idea that base rate effects are a byproduct of sequence effects, which covary with base rate. The next section employed quantitative modeling to test this hypothesis.
3.5. Predictive Models
We next compared the predicted versus observed slopes for behavioral and ICA measures of the Simon effect as a function of base rate. Predicted slopes were derived from the sequence effects in the 50% base rate condition. The 50% base rate served as an unbiased estimate of sequence effects because the four combinations of compatible/incompatible trial sequences were equally likely. To quantify how much sequence effects account for base rate effects, r2 values were computed on the correlation between slope values predicted from sequence effects in the 50% condition and the observed slope values. Large r2 values would indicate that sequence effects drive most of the base rate effect, while low r2 values suggest that other factors also contribute to the base rate effects.
3.5.1. Behavior
The direction of the Simon effect slope as a function of base rate was very consistent across subjects. For example, reaction time measures in 46/47 subjects showed increases in the Simon Effect across base rates. For reaction time the slope was significantly steeper for observed vs. predicted values (t(46) = 4.65, p<.001; Figure 7A). The r2 values for the correlation between predicted and observed was rather small (16.6% of the variance).
Figure 7.
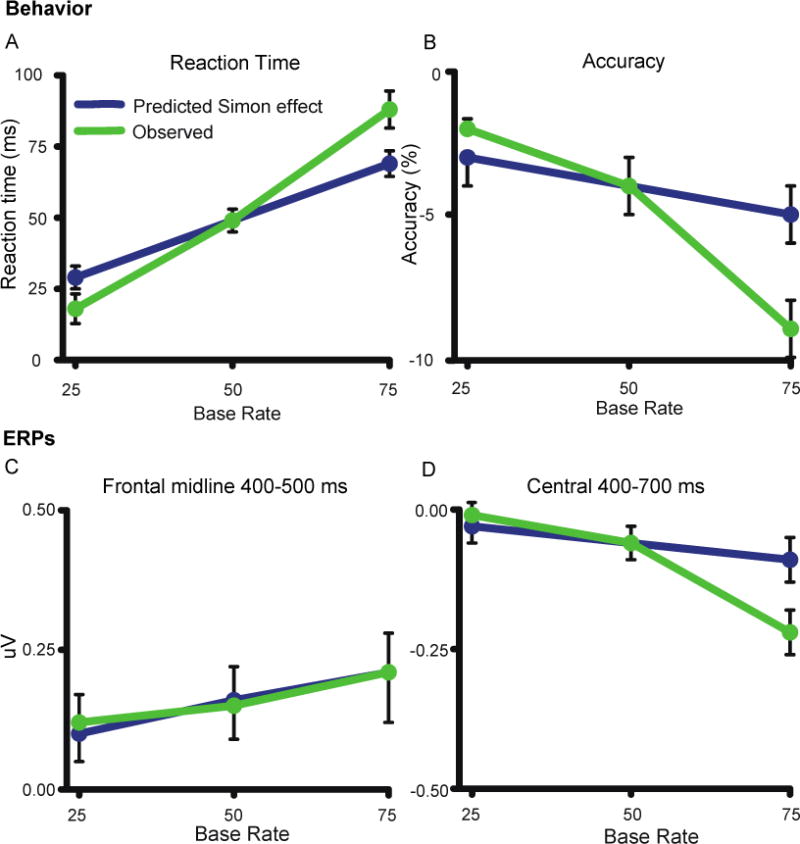
Quantitative modeling results for predicted versus observed Simon effect. Predictions were based on sequence effects in the 50% base rate condition, and applied to 25% and 75% base rate conditions. A) Predicted vs. observed Simon effects (incompatible-compatible) for reaction time. B) Predicted vs. observed Simon effects for accuracy. C) Predicted vs. observed Simon effects for frontal midline ERP. D) Predicted vs. observed Simon effects for central ERP.
The same results were seen for accuracy; the observed Simon effect slope was steeper than predicted (t(46) = −6.88, p<.001; Figure 7B), and sequence effects accounted for 35.6% of the variance in observed slope across base rates. These findings show that sequence effects accounted for a relatively small percent of the variance in base rate effects.
3.5.2. Midline frontal cluster ERPs and ERSPs
For the ERP measure (400-500 ms) there was no significant difference between the slopes of observed and predicted Simon effects (Figure 7C). The slope was modest, and consistent with the lack of a significant trial type × base rate interaction.
Results from the midline frontal ERSP measure (300-500 ms, 4-12 Hz) were consistent with the behavioral results above. The observed Simon effect slope was larger than the predicted slope (t(41) = 3.15, p<.01; Figure 8, left panel). Sequence effects accounted for only 8.0% of the observed variance.
Figure 8.
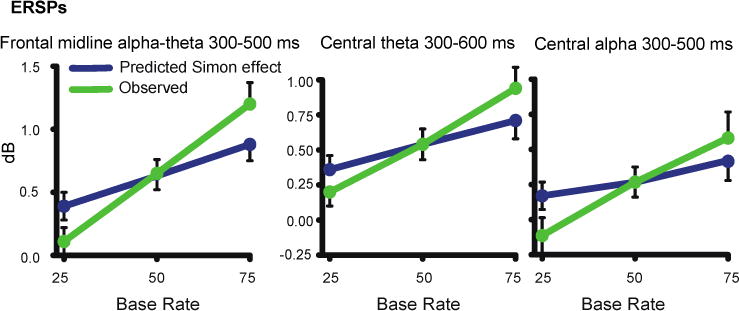
Predicted versus observed Simon effect across base rate for ERSPs.
3.5.3. Central cluster ERPs and ERSPs
The Simon effect slope for the central cluster ERP (400-700 ms) was steeper than predicted from the model (t(44) = −3.94, p<.001; Figure 7D). Sequence effects accounted for 22.1% of the observed slope. The same results were seen with the theta (300-600 ms, 4-8 Hz)(t(44) = 2.5, p = 0.02) and alpha (300-500 ms, 8-12 Hz)(t(44) = 2.15, p <.05) ERSP power measures. Sequence effects accounted for 13.7% of the observed theta variance and 1.9% of the observed alpha variance.
4. Discussion
The main finding of this study is that quantitative modeling showed that two fronto-medial components, defined by ICA, indexed conflict at both longer (minutes) and shorter (seconds) timescales. The base rate effects, on the time scale of minutes, were only weakly predicted from sequence effects occurring on successive trials. Oscillations in the theta and alpha bands were sensitive to conflict, base rate, and sequence effects. Event-related potentials were modulated by conflict and either base rate or sequence, but not both. Our main conclusion is straightforward: behavioral and neurophysiological data converge to show that sequence effects do not account for base rate effects. This leaves other factors such as how well findings map onto proactive and reactive control. Below we consider the theoretical implications of the findings, and relations of the ICs to frontal networks implicated in cognitive control and conflict.
4.1. Behavioral Results and Implications for Contextual Effects
As with previous studies, the impact of conflict on performance (Simon effect) increased in tandem with the base rate of compatible trials (Stürmer et al., 2002; West & Bailey, 2012). In principle, sequence effects could account for the influence of base rate on the Simon effect, as the two variables covary. Instead, our modeling based on sequence effects in the 50% base rate condition found that sequence effects accounted for only 16.6 % of the variance in reaction time and 35.6% of the variance in accuracy across the three compatible base rates (Figure 7A, B).
Sequence effects are one of many “low-level” influences that could be manifest as base rate effects. For example, the degree of conflict in the Stroop task can be influenced by varying the probability of compatible trials at the level of specific color words (Jacoby, Lindsay, and Hessels, 2003; Schmidt & Besner, 2008; Bugg & Crump, 2012). Item-level compatibility can also covary with base rate, although research has shown distinct item-specific and base rate effects (Bugg & Chanani, 2011). One limitation of the current study is that it did not look at item effects. Future work using a Simon task with more than two locations is needed to examine these possibilities. Likewise, conflict effects can vary depending on the specific combination of stimulus and response locations in a sequence (Hommel, Proctor, & Vu, 2004). Risko et al. (2008) found that stimulus-response location sequences accounted for 47% of the base rate effect. However, another study found that base rate and congruency sequence information accounted for conflict task performance without specific stimulus and response information (Jiang, Heller, & Egner, 2014). The current approach using behavior, EEG, and quantitative modeling contributes to the research agenda of examining detailed cognitive and neural mechanisms of cognitive control. We next turn to the quantitative modeling results, which were designed to better understand relations among base rate and sequence effects.
4.2. Quantitative Modeling
All of the behavioral measures, and most of the neural measures, had the same basic result: in terms of Simon conflict, sequence effects were not good predictors of base rate effects. Differences among measures were quantitative, with sequence effects accounting between 1.9%-35.6% of the variance due to base rate. Thus, adjustments in cognitive control at the timescale of minutes went above and beyond what would be expected from trial-by-trial adjustments. The modeling results support a Dual Mechanisms of Control model in which proactive control before trials and stimulus-induced reactive control both contribute to cognitive control (Braver, 2012). Results also support a Bayesian model of cognitive control in which both sequence effects on shorter timescales and the proportion of congruent effects across longer timescales contribute to cognitive control performance (Jiang et al., 2014).
The two independent component clusters that are the focus of this paper have fronto-central topography that is symmetrical about the midline, with conflict-related ERP and ERSP activity before and shortly after the time of behavioral responses. Taken together, these components add to convergent evidence for the importance of dorsolateral prefrontal and anterior cingulate cortex areas in mediating conflict between stimulus dimensions (Oehrn et al., 2014), including during the Simon effect (Kerns, 2006). Previous evidence supports two differentiable control networks which operate at rapid (trial-to-trial or slightly longer) and longer (multiple-trial) time scales and involve the dorsolateral prefrontal cortex or the dorsolateral anterior cingulate cortex (Dosenbach, Fair, Cohen, Schlaggar, & Peterson, 2008). Although localization precision of ICA components is constrained by EEG spatial resolution, we found some differentiable effects that point to separate control networks due to adjustments on different timescales for some measures. When considered with the scalp map topography and previous research above, it seems that these components could possibly reflect the ACC and/or dorsolateral prefrontal cortex.
Most of the IC measures (ERP, ERSP) had both base rate and sequence effects, but the midfrontal ERP measure (400-500 ms) had sequence effects in the absence of significant base rate effects. The opposite dissociation was also observed, where the central slow wave ERP (400-700 ms) had a base rate × trial type interaction but no sequence effects. Given the mathematics of the model we used, in the absence of a counteracting influence sequence effects should, with an adequate number of subjects, be reflected in a significant base rate effect. Taken together, the ERP dissociations between the two timescales suggest they may be elements of distinct proactive (central ERP) and reactive (frontal ERP) control mechanisms, as specified in models such as the dual processing framework.
Base rate effects map well onto proactive control in which participants detect a frequent or infrequent need for cognitive control and proactively adjust control intensity (Braver, 2012). Sequence effects adjust on a shorter timescale, more similar to reactive control. However, unlike tasks that are designed to assess proactive and reactive control (Gonthier, Macnamara, Chow, Conway, & Braver, 2016) our task does not cleanly separate proactive and reactive control. For example, sequence effects could potentially be proactive adjustments on short timescales immediately preceding each trial. To test the possibility of ERSP sequence effects mapping onto shorter-term proactive control versus reactive control, we tested the effect of previous ERSP trial type on the current trial baseline and found no effect. Therefore, these ERSP sequence effects are more likely to reflect reactive than proactive control. The 400-500 ms ERP effect was in the opposite direction of our ERSP effect for this cluster. Therefore, although conflict ERPs such as the N200/N450 may partially drive ERSP effects in some cases, evoked and induced activity showed a dissociation here which makes it unlikely that our 400-500 ms ERP drove the frontal theta effect in this cluster.
There has been some debate in the field regarding whether the ACC monitors conflict. Conflict activity has been observed in the ACC by numerous studies (e.g. Kern, 2006; Botvinick, Nystrom, Fissell, Carter, & Cohen, 1999; Roberts & Hall, 2008). At the same time, some research has suggested that the ACC does not monitor conflict because amplitude of the “error negativity” ERP generated by the ACC does not increase with greater conflict (Burle et al., 2008). In contrast, Amiez, Joseph, and Procyk (2006) found that the ACC in monkeys was involved in detecting probabilities of higher reward values and adapting task choices accordingly on a trial-by-trial basis. Our data suggest that some midline ERPs and ERSPs detect conflict across longer timescales and also monitor conflict on a trial-by-trial basis. Due to the limited spatial resolution of EEG, we cannot be sure that these ICs originate in the ACC, but it is possible that one or both reflect conflict monitoring or detection of conflict probability in the ACC and/or dorsomedial PFC.
For reaction time, both compatible and incompatible trial types showed performance improvements as they became the most common trial type in a block. In contrast, for most neural measures with a base rate × trial type interaction (midline frontal cluster 4-12 Hz ERSPs, central cluster ERPs, central cluster theta ERSPs, left cluster 300-500 ms ERPs) base rate effects were only evident for incompatible trials. Thus, changes in the neural measures were not a direct indicator of performance, which rules-out the possibility that the components index activity that directly generates the motor response. Instead these mechanisms seem to largely reflect inhibitory processing or other features associated with incompatible trials, and are not associated with performance on compatible trials. One limitation of the current study is that it did not identify a neural mechanism specific to compatible trial response patterns, although such a mechanism may be more reflective of a motor response than conflict processing.
4.3. Independent Component Oscillations (ERSPs)
The midline theta power Simon effect increased with the base rate of compatible trials, which is consistent with studies implicating frontal theta in cognitive control (Cavanagh & Frank, 2014; Cohen & Cavanagh, 2011; Tang et al., 2013) and executive functioning (Clayton, Yeung, & Cohen Kadosh, 2015; Miller & Cohen, 2001; von Stein & Sarnthein, 2000). The average increase in power appears before and during our average response time, suggesting that theta plays a role in conflict detection and response. Increased frontal theta power when incompatible trials are less common suggests a mechanism where theta is enhanced when handling rare conflict, and together with the modeling results suggests it is largely due to proactive control. We found this effect for the midline frontal and central clusters. Similarly, Töllner et al. (2017) also found two midline frontal clusters with frontal theta during conflict in a Simon task. However, only one of their two clusters exhibited a Simon effect.
Examining shorter timescales, van Driel et al. (2015) found that when participants were cued that conflict was likely on the next trial, frontal theta increased and response time slowed on that upcoming trial. In contrast, the likelihood of conflict in our task varied in blocks lasting minutes. Under these conditions, we found less of an increase in frontal theta for conflict when conflict was more likely compared to when it was less likely. Thus, the current study suggests that proactive control responds differently to conflict probability across longer timescales than to cues immediately preceding a trial. Researchers have also examined frontal theta on shorter timescales in a cued task switching experiment and found that proactive control, and the associated frontal theta, had adjustments over longer periods of time as well as across trials (Cooper, Wong, McKewen, Michie, Karayanidis, 2017). When considered along with these lines of research using trial-by-trial conflict cues, our findings provide new information about proactive control. Future work could further examine why adjustments of likelihood across longer timescales had such different effects than the short-term cues indicating conflict likelihood used by van Driel et al. (2015).
The basic pattern of theta power findings extended into the alpha band (8-12 Hz) at midline frontal and central components, and comports with work showing that EEG oscillations associated with cognitive control are broadly tuned and can include alpha (Hacker, Snyder, Pahwa, Corbetta, & Leuthardt, 2017). Alpha oscillations are also related to attentional control, and alpha synchronization tends to increase with attentional inhibition (for a review see Klimesch, 2012).
4.4. Independent Component Event-related Potentials (ERPs)
The relationship between the midline frontal component’s ERP and the MFN/N450, which was not defined here using ICA, is unclear (Bailey et al., 2010; Tillman & Wiens, 2011; West & Bailey, 2012). The waveforms in our study are similar in topography and waveform shape to conflict studies in the visual modality (e.g. Bailey et al., 2010; West & Bailey, 2012). Analysis of the scalp channel data before ICA (data not shown) showed that there was a significant MFN effect that was consistent with previous visual and auditory (Donohue, Liotti, Perez, & Woldorff, 2012) studies that did not use ICA. The frontal cluster ERP had a significant effect of trial type between 400-500 ms, which is later than the usual MFN window. The effect was in the opposite direction, with greater negativity for compatible than incompatible trials. The N100, which is a very large and reliable ERP to auditory stimuli, was present and negative in polarity. Therefore, this opposite effect is not due to inverted polarity of ICA scalp projections (Hyvärinen, Karhunen, & Oja, 2004). Our ICA data have grand average differences consistent with a base rate × trial type interaction in the 250-350 ms time window, but visual inspection revealed inconsistency in this peak across subjects.
The central cluster had slow wave ERP effects (400-700 ms), with a significant trial type × base rate effect. This window may have been earlier in our task than for previous studies using the visual Stroop task (Bailey et al., 2010; West & Bailey, 2012) due to faster processing in the auditory versus visual modality. Post-hoc tests revealed an increase in slow wave negativity for incompatible trials as compatible trial base rate increased but no change across base rates for compatible trials. Because this window occurs after the average response time, it may reflect post-response, across-trial adaptations in conflict processing rather than pre-response cognitive control of conflict.
Supplementary Material
Highlights.
Cognitive control dynamically adjusts across shorter and longer timescales.
Sequence effects account for minimal variance in base rate (% compatible) effects.
Frontal theta adjusts with compatible trial base rate and sequence effects.
Acknowledgments
This study was supported by NSF CAREER award BCS-0844961, NIH R01DC014736, and Tulane Flowerree Summer Research Fellowships. We are thankful to Mike Seay for assistance with data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Dr. Leslie Fellows
Section Editor, Neuropsychologia
Dr. Stephan Hamann
Editor-in-Chief, Neuropsychologia
Post-hoc ANOVAs showed decreasing compatible trial reaction time as base rate increased (F(2,92) = 10.14, p<001). For incompatible trials, reaction time slowed as compatible trial base rate increased (F(2, 92) = 12.26, p<.001). For compatible trial accuracy, there was a slight increase as base rate increased (F(2, 92) = 4.75, p < .025). Accuracy decreased for incompatible trials as base rate increased (F(2, 92) = 46.80, p<.001).
Because theta and alpha were analyzed separately for the central cluster, we also ran the analyses here for the midline frontal cluster looking at theta and alpha separately. The trial Type × Base rate and sequence effects interactions were still significant and had the same patterns of means when theta and alpha were separated as when they were analyzed together.
Note the difference in direction relative to the 400-500 ms ERP effect for this cluster. The non-phase-locked ERSP was larger for incompatible trials, while the phase-locked ERP effect had the opposite pattern. This indicates different cortical mechanisms for these two findings.
References
- Amiez C, Joseph JP, Procyk E. Reward Encoding in the Monkey Anterior Cingulate Cortex. Cerebral Cortex. 2006;16(7):1040–1055. doi: 10.1093/cercor/bhj046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey K, West R, Anderson CA. A negative association between video game experience and proactive cognitive control. Psychophysiology. 2010;47(1):34–42. doi: 10.1111/j.1469-8986.2009.00925.x. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen J, Carter C. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004 doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402(6758):179–81. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Braver TS. The variable nature of cognitive control: a dual mechanisms framework. Trends in Cognitive Sciences. 2012;16(2):106–113. doi: 10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugg JM, Chanani S. List-wide control is not entirely elusive: Evidence from picture–word Stroop. Psychonomic Bulletin & Review. 2011;18(5):930–936. doi: 10.3758/s13423-011-0112-y. [DOI] [PubMed] [Google Scholar]
- Bugg JM, Crump MJC. In Support of a Distinction between Voluntary and Stimulus-Driven Control: A Review of the Literature on Proportion Congruent Effects. Frontiers in Psychology. 2012;3:367. doi: 10.3389/fpsyg.2012.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burle B, Roger C, Allain S, Vidal F, Hasbroucq T. Error Negativity Does Not Reflect Conflict: A Reappraisal of Conflict Monitoring and Anterior Cingulate Cortex Activity. Journal of Cognitive Neuroscience. 2008;20(9):1637–1655. doi: 10.1162/jocn.2008.20110. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal Oscillations in Cortical Networks. Science. 2004;304(5679):1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Cao Y, Cao X, Yue Z, Wang L. Temporal and spectral dynamics underlying cognitive control modulated by task-irrelevant stimulus-response learning. Cognitive, Affective, & Behavioral Neuroscience. 2017;17(1):158–173. doi: 10.3758/s13415-016-0469-5. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends in Cognitive Sciences. 2014:1–8. doi: 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cespón J, Galdo-Álvarez S, Díaz F. Cognitive control activity is modulated by the magnitude of interference and pre-activation of monitoring mechanisms. Scientific Reports. 2016;6 doi: 10.1038/srep39595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik EC, Mueller VI, Eickhoff CR, Langner R, Eickhoff SB. Three key regions for supervisory attentional control: evidence from neuroimaging meta-analyses. Neuroscience and Biobehavioral Reviews. 2015;48:22–34. doi: 10.1016/j.neubiorev.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayson PE, Larson MJ. Conflict adaptation and sequential trial effects: Support for the conflict monitoring theory. Neuropsychologia. 2011;49(7):1953–1961. doi: 10.1016/j.neuropsychologia.2011.03.023. [DOI] [PubMed] [Google Scholar]
- Clayton MS, Yeung N, Cohen Kadosh R. The roles of cortical oscillations in sustained attention. Trends in Cognitive Sciences. 2015;19(4):188–95. doi: 10.1016/j.tics.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Cavanagh JF. Single-trial regression elucidates the role of prefrontal theta oscillations in response conflict. Frontiers in Psychology. 2011;2:30. doi: 10.3389/fpsyg.2011.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Ridderinkhof KR. EEG source reconstruction reveals frontal-parietal dynamics of spatial conflict processing. PloS One. 2013;8(2):e57293. doi: 10.1371/journal.pone.0057293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Ridderinkhof KR, Haupt S, Elger CE, Fell J. Medial frontal cortex and response conflict: Evidence from human intracranial EEG and medial frontal cortex lesion. Brain Research. 2008;1238:127–142. doi: 10.1016/j.brainres.2008.07.114. [DOI] [PubMed] [Google Scholar]
- Cooper PS, Wong ASW, McKewen M, Michie PT, Karayanidis F. Frontoparietal theta oscillations during proactive control are associated with goal-updating and reduced behavioral variability. Biological Psychology. 2017;129:253–264. doi: 10.1016/j.biopsycho.2017.09.008. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Delorme A, Sejnowski T, Makeig S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. NeuroImage. 2007;34(4):1443–9. doi: 10.1016/j.neuroimage.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Pellegrino G, Ciaramelli E, Làdavas E. The Regulation of Cognitive Control following Rostral Anterior Cingulate Cortex Lesion in Humans. Journal of Cognitive Neuroscience. 2007;19(2):275–286. doi: 10.1162/jocn.2007.19.2.275. [DOI] [PubMed] [Google Scholar]
- Donohue SE, Liotti M, Perez R, Woldorff MG. Is conflict monitoring supramodal? Spatiotemporal dynamics of cognitive control processes in an auditory Stroop task. Cognitive, Affective & Behavioral Neuroscience. 2012;12(1):1–15. doi: 10.3758/s13415-011-0060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends in Cognitive Sciences. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T. The Wiley Handbook of Cognitive Control. Chichester, UK: John Wiley & Sons, Ltd; 2017. Conflict Adaptation; pp. 64–78. [Google Scholar]
- Egner T. Congruency sequence effects and cognitive control. Cognitive, Affective, & Behavioral Neuroscience. 2007;7(4):380–390. doi: 10.3758/cabn.7.4.380. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics. 1974;16(1):143–149. [Google Scholar]
- Gevins A, Smith ME, McEvoy L, Yu D. High-resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cerebral Cortex. 1997;7(4):374–85. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- Gonthier C, Macnamara BN, Chow M, Conway ARA, Braver TS. Inducing Proactive Control Shifts in the AX-CPT. Frontiers in Psychology. 2016;7:1822. doi: 10.3389/fpsyg.2016.01822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E. Optimizing the use of information: Strategic control of activation of responses. Journal of Experimental Psychology. 1992;121(4):480–506. doi: 10.1037//0096-3445.121.4.480. [DOI] [PubMed] [Google Scholar]
- Gulbinaite R, van Rijn H, Cohen MX. Fronto-parietal network oscillations reveal relationship between working memory capacity and cognitive control. Frontiers in Human Neuroscience. 2014;8:761. doi: 10.3389/fnhum.2014.00761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker CD, Snyder AZ, Pahwa M, Corbetta M, Leuthardt EC. Frequency-specific electrophysiologic correlates of resting state fMRI networks. NeuroImage. 2017;149:446–457. doi: 10.1016/j.neuroimage.2017.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S, Pastötter B, Bäuml KH, Gruber S, Wimber M, Klimesch W. The Electrophysiological Dynamics of Interference during the Stroop Task. Journal of Cognitive Neuroscience. 2008;20(2):215–225. doi: 10.1162/jocn.2008.20020. [DOI] [PubMed] [Google Scholar]
- Hartmann WM. How We Localize Sound. Physics Today. 1999;52(11):24. [Google Scholar]
- Henkin Y, Yaar-Soffer Y, Gilat S, Muchnik C. Auditory conflict processing: behavioral and electrophysiologic manifestations of the stroop effect. Journal of the American Academy of Audiology. 2010;21(7):474–86. doi: 10.3766/jaaa.21.7.6. [DOI] [PubMed] [Google Scholar]
- Hommel B, Proctor RW, Vu KPL. A feature-integration account of sequential effects in the Simon task. Psychological Research. 2004;68(1):1–17. doi: 10.1007/s00426-003-0132-y. [DOI] [PubMed] [Google Scholar]
- Hyvärinen A, Karhunen J, Oja E. Independent Component Analysis. John Wiley & Sons; 2004. [Google Scholar]
- Jacoby LL, Lindsay DS, Hessels S. Item-specific control of automatic processes: stroop process dissociations. Psychonomic Bulletin & Review. 2003;10(3):638–44. doi: 10.3758/bf03196526. [DOI] [PubMed] [Google Scholar]
- Jiang J, Heller K, Egner T. Bayesian modeling of flexible cognitive control. Neuroscience and Biobehavioral Reviews. 2014;46(Pt 1):30–43. doi: 10.1016/j.neubiorev.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung TP, Makeig S, Mckeown MJ, Bell AJ, Lee TW, Sejnowski TJ. Imaging Brain Dynamics Using Independent Component Analysis. IEEE Proceedings. 2001;88(7):1107–22. doi: 10.1109/5.939827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanske P, Kotz SA. Positive emotion speeds up conflict processing: ERP responses in an auditory Simon task. Biological Psychology. 2011;87(1):122–127. doi: 10.1016/j.biopsycho.2011.02.018. [DOI] [PubMed] [Google Scholar]
- Kerns JG. Anterior cingulate and prefrontal cortex activity in an FMRI study of trial-to-trial adjustments on the Simon task. NeuroImage. 2006;33(1):399–405. doi: 10.1016/j.neuroimage.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303(5660):1023–6. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Klimesch W. A-Band Oscillations, Attention, and Controlled Access To Stored Information. Trends in Cognitive Sciences. 2012;16(12):606–617. doi: 10.1016/j.tics.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause CM, Lang HA, Laine M, Helle SI, Kuusisto MJ, Pörn B. Event-related desynchronization evoked by auditory stimuli. Brain Topography. 1994;7(2):107–12. doi: 10.1007/BF01186768. [DOI] [PubMed] [Google Scholar]
- Larson MJ, Kaufman DAS, Perlstein WM. Neural time course of conflict adaptation effects on the Stroop task. Neuropsychologia. 2009;47(3):663–670. doi: 10.1016/j.neuropsychologia.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Larson E, Lee AKC. Switching auditory attention using spatial and non-spatial features recruits different cortical networks. NeuroImage. 2014;84:681–687. doi: 10.1016/j.neuroimage.2013.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuthold H, Schroter H. Electrophysiological evidence for response priming and conflict regulation in the auditory Simon task. Brain Research. 2006;1097(1):167–180. doi: 10.1016/j.brainres.2006.04.055. [DOI] [PubMed] [Google Scholar]
- Logan GD, Zbrodoff NJ. When it helps to be misled: Facilitative effects of increasing the frequency of conflicting stimuli in a Stroop-like task. Memory & Cognition. 1979;7(3):166–174. [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mock JR, Seay MJ, Charney DR, Holmes JL, Golob EJ. Rapid cortical dynamics associated with auditory spatial attention gradients. Frontiers in Neuroscience. 2015;9:179. doi: 10.3389/fnins.2015.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naatanen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24(4):375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Oehrn CR, Hanslmayr S, Fell J, Deuker L, Kremers NA, Do Lam AT, Elger CE, Axmacher N. Neural communication patterns underlying conflict detection, resolution, and adaptation. The Journal of Neuroscience. 2014;34(31):10438–52. doi: 10.1523/JNEUROSCI.3099-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clinican Neurophysiology. 1999;110(11):1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Risko EF, Blais C, Stolz JA, Besner D. Nonstrategic contributions to putatively strategic effects in selective attention tasks. Journal of Experimental Psychology. Human Perception and Performance. 2008;34(4):1044–52. doi: 10.1037/0096-1523.34.4.1044. [DOI] [PubMed] [Google Scholar]
- Roberts KL, Hall DA. Examining a supramodal network for conflict processing: a systematic review and novel functional magnetic resonance imaging data for related visual and auditory stroop tasks. Journal of Cognitive Neuroscience. 2008;20(6):1063–1078. doi: 10.1162/jocn.2008.20074. [DOI] [PubMed] [Google Scholar]
- Sacchet MD, LaPlante RA, Wan Q, Pritchett DL, Lee AKC, Hämäläinen M, Moore CI, Kerr CE, Jones SR. Attention Drives Synchronization of Alpha and Beta Rhythms between Right Inferior Frontal and Primary Sensory Neocortex. Journal of Neuroscience. 2015;35(5) doi: 10.1523/JNEUROSCI.1292-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf B. Auditory attention: The psychoacoustical approach. In: Pashler H, editor. Attention. East Sussex, UK: Psychology Press; 1998. pp. 75–117. [Google Scholar]
- Schmidt JR, Besner D. The Stroop Effect: Why Proportion Congruent Has Nothing to Do with Congruency and Everything to Do with Contingency. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34(3):514–523. doi: 10.1037/0278-7393.34.3.514. [DOI] [PubMed] [Google Scholar]
- Simon JR, Rudell AP. Auditory S-R compatibility: The effect of an irrelevant cue on information processing. Journal of Applied Psychology. 1967;51(3):300–4. doi: 10.1037/h0020586. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Stürmer B, Leuthold H, Soetens E, Schröter H, Sommer W. Control over location-based response activation in the Simon task: behavioral and electrophysiological evidence. Journal of Experimental Psychology. 2002;28(6):1345–63. doi: 10.1037//0096-1523.28.6.1345. [DOI] [PubMed] [Google Scholar]
- Tang D, Hu L, Chen A. The neural oscillations of conflict adaptation in the human frontal region. Biological Psychology. 2013;93(3):364–72. doi: 10.1016/j.biopsycho.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Core Team R. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. URL http://www.R-project.org/ [Google Scholar]
- Tillman CM, Wiens S. Behavioral and ERP indices of response conflict in Stroop and flanker tasks. Psychophysiology. 2011;48(10):1405–11. doi: 10.1111/j.1469-8986.2011.01203.x. [DOI] [PubMed] [Google Scholar]
- Töllner T, Wang Y, Makeig S, Müller HJ, Jung TP, Gramann K. Two Independent Frontal Midline Theta Oscillations During Conflict Detection and Adaptation in a Simon-type Manual Reaching Task. Journal of Neuroscience. 1523;10:1752–16. doi: 10.1523/JNEUROSCI.1752-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Quesada M, Milliken B, Lupiáñez J, Funes MJ. Proportion Congruent effects in the absence of Sequential Congruent effects. Psicológica. 2014;35:101–115. doi: 10.1016/j.actpsy.2014.03.006. [DOI] [PubMed] [Google Scholar]
- van Driel J, Swart JC, Egner T, Ridderinkhof KR, Cohen MX. (No) time for control: Frontal theta dynamics reveal the cost of temporally guided conflict anticipation. Cognitive, Affective, & Behavioral Neuroscience. 2015;15(4):787–807. doi: 10.3758/s13415-015-0367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiology & Behavior. 2002;77(4–5):477–82. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- von Stein A, Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. International Journal of Psychophysiology. 2000;38(3):301–313. doi: 10.1016/s0167-8760(00)00172-0. [DOI] [PubMed] [Google Scholar]
- Vu KPL, Proctor RW, Urcuioli P. Transfer effects of incompatible location-relevant mappings on a subsequent visual or auditory Simon task. Memory & Cognition. 2003;31(7):1146–1152. doi: 10.3758/bf03196135. [DOI] [PubMed] [Google Scholar]
- Wascher E, Schatz U, Kuder T, Verleger R. Validity and boundary conditions of automatic response activation in the Simon task. Journal of Experimental Psychology: Human Perception and Performance. 2001;27(3):731–751. doi: 10.1037//0096-1523.27.3.731. [DOI] [PubMed] [Google Scholar]
- West R, Bailey K. ERP correlates of dual mechanisms of control in the counting Stroop task. Psychophysiology. 2012;49(10):1309–18. doi: 10.1111/j.1469-8986.2012.01464.x. [DOI] [PubMed] [Google Scholar]
- West R, Bailey K, Tiernan BN, Boonsuk W, Gilbert S. The temporal dynamics of medial and lateral frontal neural activity related to proactive cognitive control. Neuropsychologia. 2012;50(14):3450–3460. doi: 10.1016/j.neuropsychologia.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Wühr P, Ansorge U. Exploring trial-by-trial modulations of the Simon effect. The Quarterly Journal of Experimental Psychology. 2005;58(4):705–31. doi: 10.1080/02724980443000269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


