Abstract
Purpose of Review
Changes in the bone marrow microenvironment, which accompany aging and obesity, including increased marrow adiposity, can compromise hematopoiesis. Here we review deleterious shifts in molecular, cellular and tissue activity, and consider the potential of exercise to slow degenerative changes associated with aging and obesity.
Recent Findings
While bone marrow hematopoietic stem cells (HSC) are increased in frequency and myeloid-biased with age, the effect of obesity on HSC proliferation and differentiation remains controversial. HSC from both aged and obese environment have reduced hematopoietic reconstitution capacity following bone marrow transplant. Increased marrow adiposity affects HSC function, causing up-regulation of myelopoiesis and down-regulation of lymphopoiesis. Exercise, in contrast, can reduce marrow adiposity and restore hematopoiesis.
Summary
The impact of marrow adiposity on hematopoiesis is determined mainly through correlations. Mechanistic studies are needed to determine a causative relationship between marrow adiposity and declines in hematopoiesis, which could aid in developing treatments for conditions that arise from disruptions in the marrow microenvironment.
Keywords: Bone marrow microenvironment, lymphopoiesis, myelopoiesis, exercise, whole body vibration
Bone Marrow Microenvironment
Bone marrow is a multicellular tissue located in the cavity of bones, encased by trabecular and cortical bone. Vasculature in the bone marrow not only delivers nutrients to the marrow, but also carries blood cells born within the marrow out into systemic circulation [1]. The complex and dynamic microenvironment of the marrow supports formation and function of many different types of cells, including undifferentiated multipotent stem cells, cells at different developmental stages, and terminally differentiated cells. Two principal types of multipotent stem cells that reside in adult marrow are mesenchymal stem cells (MSC) and hematopoietic stem cells (HSC), each critical to the regenerative and inflammatory response of the organism.
MSC are capable of differentiating into bone forming cells (osteoblasts), adipocytes, chondrocytes, myocytes, fibroblasts and epithelial cells, in addition to self-renewal [2]. MSC have been shown to make up about 0.6–1% of the bone marrow in mice [3], a fraction which is much smaller in humans, ranging between 0.001–0.02% of total cells [4]. Although bone marrow has been considered the primary source of MSC, recent studies have found multipotent stem cells in other tissues as well, such as adipose tissue, liver, and pancreas [5–7]. While locally these resident MSC can aide in tissue repair and regeneration by differentiating into local tissue cell types, they have the potential to differentiate into any cell type from the mesenchymal lineage in-vitro, given the proper differentiation conditions [7, 8]. MSC fate selection in the body is susceptible to environmental cues and systemic insults, such that an obese environment can prompt MSC to differentiate into adipocytes and away from other cell types [9, 3, 10]. Biasing MSC differentiation towards adipogenesis in the obese state might have a negative impact systemically on bone health and marrow architecture [11, 12]. Due to close proximity in the marrow space, a disrupted MSC environment may ultimately influence HSC fate.
HSC are the primary source of all the blood and immune cells in the body. HSC can differentiate into red blood cells, platelets, and leukocytes [13]. A single multipotent HSC has the ability of reconstituting the whole adult bone marrow under systemic stress such as irradiation [14]. Multipotent HSC in the bone marrow exist as dormant cells, in a state of long-term quiescence, and only divide five times over their lifetime [15]. These long-term HSC comprise approximately 0.01–0.03% and 0.04% of the marrow cellularity in mice [16] and in humans [17], respectively. While HSC are continuously differentiating to maintain the body’s demand for various blood and immune cells, HSC differentiation is also impacted by systemic insults, such as severe blood loss, irradiation, infection, and chronic inflammation.
Hematopoiesis
The process by which long-term HSC replenish the blood and immune cells in the body is known as hematopoiesis. In healthy adults, there is a high turnover of blood and immune cells daily leading to continuous hematopoiesis in the marrow. Average lifespan of a red blood cell, erythrocyte, in the circulation after being released from the bone marrow is ~115 days in healthy adults [18]. Once these cells reach senescence, they are removed via phagocytosis, prompting the release of erythropoietin, which signals the bone marrow to produce close to 200 billion new erythrocytes daily [19]. Platelets have a shorter lifespan of ~8–10 days and there are close to one trillion platelets in circulation at all times, creating a high level of daily need for platelet production in the bone marrow [20, 21]. Lifespan for lymphocytes is more complex. The naïve B and T cells have a short lifespan in circulation of only a few days to a few weeks [22]. However, if these naïve cells come in contact with a foreign antigen, they get converted into effector or memory cells that can survive indefinitely [22]. Neutrophils have an average lifespan of a few hours to a few days, leading to ~50–100 billion neutrophils produced by the bone marrow daily [23, 24]. Eosinophils are primarily found in much larger quantities in tissues, such as gut, rather than in circulation [25]. Their average lifespan ranges from 2–5 days in tissue, however, it can increase to 14 days in the presence of increased levels of cytokines [25]. Basophils only make up about 0.5% of the cells in circulation under homeostatic conditions and their average lifespan is ~1–2 days [26]. Intravascular monocytes have a lifespan of ~4 days, whereas monocytes with an ability to extravasate in response to an inflammatory response have a lifespan of ~11 days [27]. Thus, bone marrow hematopoiesis is a dynamic process, producing billions of cells daily to meet the body’s demand for sustaining a healthy immune and circulatory system.
Traditionally, hematopoiesis is thought to be a tree-like hierarchical process, such that long-term HSC give rise to short-term multipotent progenitor cells (MPP), which then further segregate into either common lymphoid progenitor (CLP) or common myeloid progenitor (CMP) [19]. CLP gives rise to B cells, T cells and natural killer cells [19]. CMP further segregates into either megakaryocyte-erythroid progenitor (MEP), which can produce erythrocytes and platelets, or granulocyte-macrophage progenitor (GMP), which can produce macrophages, eosinophils, basophils, neutrophils and mast cells [19]. A recent study led by Velten, et al. challenges this notion [28]. Using flow cytometry, transcriptomic and functional data acquisition at single-cell resolution, this study shows that HSC and MPP are initially part of a continuum of low-primed undifferentiated hematopoietic stem and progenitor cells (CLOUD-HSPC), which is characterized as Lin−CD34+CD38−. Lineage restriction is only shown to begin with CD38 upregulation, when the stem cell modules and certain early priming modules are turned off and specific lineage modules are activated [28]. Thus, HSC differentiation in young adults is shown to be a continuous process, where cells in CLOUD-HSPC have the propensity to convert into any lineage (lymphoid, myeloid, megakaryocytic, or erythroid) without any binary branch points.
Various cells in the bone marrow niche can regulate hematopoiesis. MSC have been shown to support HSC growth, viability and hematopoiesis by secreting growth factors and cytokines such as stromal cell derived factor 1, stem cell factor, leukemia inhibitory factor, macrophage colony stimulating factor, osteopontin, interleukin 6, and interleukin 11 [29–31]. MSC also regulate immune cell behavior outside of the bone marrow by reducing proliferation of lymphocytes in-vivo and in-vitro, by prompting a change in macrophage polarity to secrete anti-inflammatory cytokine interleukin 10, and by modulating secretion of pro-inflammatory cytokines via TNF-α stimulated gene/protein 6 [32–34]. MSC-derived osteoblasts also regulate and support proliferation, maturation and activation of cells in the hematopoietic lineage by secreting granulocyte colony stimulating factor, macrophage colony stimulating factor, interleukin 1 and interleukin 6 [35]. In addition, osteoblasts play a major role in osteoclast proliferation and activation, and in attracting them to the mineralized surfaces to initiate bone resorption [36, 37]. Conversely, as shown in-vitro, osteoclasts can control osteoblast chemotaxis by platelet derived growth factor bb/platelet derived growth factor receptor β signaling [38]. Coupling between osteoblasts and osteoclasts mediates bone remodeling and aids in maintaining healthy bone phenotype. The osteoblasts embedded in the bone matrix, known as osteocytes, play a role in maintaining the osteoblast-osteoclast coupling [39]. Osteocytes also influence myelopoiesis in the bone marrow, potentially by Gsα-dependent signaling, which controls the production of granulocyte colony stimulating factor [40]. Hence, multiple cells in the bone marrow microenvironment closely regulate hematopoiesis to maintain a delicate balance between many cell types in the bone marrow, and ultimately, in circulation, at all times.
Marrow Adipose Tissue
Adipose tissue is functionally divided into brown adipose tissue (BAT) and white adipose tissue (WAT). BAT aids in heat production during early development and gradually gets replaced by WAT after birth [41]. WAT plays vital roles in temperature regulation and mechanical cushion, but its primary role is energy storage [42]. WAT located just underneath the skin is known as subcutaneous adipose tissue (SAT), whereas the WAT deposited in and around internal organs is known as visceral adipose tissue (VAT). In healthy individuals, a majority of WAT consists of SAT, which can promote adipogenesis to facilitate excess energy storage [43]. With high calorie intake, more energy gets stored in the form of VAT, which can impede normal functionality of internal organs such as liver, pancreas, kidneys, testes, intestines, and heart, and lead to metabolic syndrome [11]. Besides the abdominal and the thoracic cavities, adipose tissue can also be found in the bone marrow.
Marrow adipose tissue (MAT) has recently been of interest due to its proximity to stem and immune cells in the bone marrow. Under normal physiological conditions, while almost all of the bone marrow at birth consists of hematopoietic red bone marrow [44], by adulthood, 50% or more of the bone marrow gets occupied by the fatty yellow bone marrow [45]. MAT development starts in distal regions of the bone and moves proximally with time [46]. This has repeatedly been shown in rodents. In long bones, distal marrow is mostly yellow, while proximal marrow is mostly red; similarly, the caudal vertebrae have mainly yellow marrow, while the lumbar vertebrae mainly has red marrow [47–49].
A recent study by Scheller, et al. highlights region-specific differences in MAT using a murine model [49]. Distal MAT, which consists of densely packed adipocytes that resembles WAT and contains high levels of unsaturated lipids, is called constitutive MAT (cMAT). While proximal MAT, which consists of loosely dispersed adipocytes in the red marrow surrounded by cells in the hematopoietic lineage and contains high levels of saturated lipids, is called regulated MAT (rMAT). cMAT and rMAT have functional differences as well; for instance, cold exposure leads to 56–71% reduction in rMAT in tibial epiphysis and proximal tibia, and no loss of cMAT in distal tibia in mice, suggesting that rMAT might be more responsive to environmental cues [49].
The presence of MAT is not necessarily pathological and has been shown to have physiological functions. Cawthorn, et al. demonstrate that MAT increases in the initial stage of anorexia nervosa and secretes adiponectin, a protein shown to play a role in mitigating inflammation, atherosclerosis, and metabolic syndrome [50]. However, in the late stage of anorexia nervosa, MAT decreases, suggesting that MAT is utilized to account for calorie deficit [51]. Hence, in the presence of inadequate amount of WAT, MAT can serve as an energy depot. In addition, MAT might be playing a role in delaying metabolic syndrome as evident through congenital vs. acquired lipodystrophy. Congenital lipodystrophy is often paralleled by insulin resistance and type 2 diabetes, whereas the onset of these comorbidities is delayed in acquired lipodystrophy. Interestingly, patients with congenital lipodystrophy have reduced proportions of MAT, whereas MAT proportions are preserved in patients with acquired lipodystrophy [52]. MAT might also be aiding in preventing disuse-induced bone loss, such that genetic deficiency to produce MAT led to increased bone loss when the mice were subjected to hindlimb unloading, compared to wild-type mice [53]. The function of MAT is still not very well defined. The studies that show beneficial effects of MAT are comparing genetic deficiency to produce MAT with the presence of physiological levels of MAT. This redirects our attention to differences in cMAT vs rMAT as outlined by Scheller, et al. We hypothesize that the presence of cMAT might be necessary for normal physiological functions, however, increased accumulation of rMAT might still have pathological consequences since rMAT is located within the hematopoietic red marrow, which might disrupt function of surrounding cells in the marrow.
Effect of Aging and Obesity on Marrow Architecture and Hematopoiesis
Aging
Aging can adversely affect bone marrow microenvironment. In humans, bone marrow cellularity has been shown to decrease immensely between ages 80 and 100, partly due to increased apoptosis, and decreased lymphocytes and macrophage populations [54]. On the other hand, the number of HSC in the marrow increases with age in both mice and humans, partially due to reduced quiescence in the HSC population, which promotes cell proliferation [17, 55]. However, old HSC are functionally inferior to young HSC due to accumulation of oxidative stress, which reduces their capacity of self-renewal and of reconstituting the hematopoietic system [56]. Aging also leads to altered hematopoiesis. Aging leads to myeloid bias in the marrow, with increased proportion of myeloid progenitor cells and decreased proportion of lymphoid progenitor cells [17, 57]. To the contrary, the repopulation capacity of human HSC to generate myeloid population decreases with age, as confirmed by in-vivo transplantation in a murine model and in-vitro colony-forming assay [57]. Certainly, more studies are needed to determine the degree to which aging alters or compromises HSC function and potential.
Aging also leads to altered bone marrow microarchitecture with increased marrow adiposity [58–60]. There are gender-associated differences in MAT accumulation with aging. In females, MAT increases dramatically between the ages of 55 and 65, whereas in males, it increases gradually throughout the life [59]. Females over 60 years old have 10% higher MAT than males of the same age group [59]. Excessive fat content in the marrow with aging can limit space for other cells to grow and can also alter functionality of surrounding cells, such that the MSC in the fatty marrow might have reduced potential to support hematopoiesis [61].
Obesity
Obesity leads to bone marrow hyperplasia with ~20–30% increased marrow cellularity [62, 63]. Effect of obesity on long-term HSC and progenitor cells varies in diet-induced obesity (DIO) murine models. Eighteen weeks of 45% kcal from fat diet led to decreased HSC and HSPC population in the bone marrow, by reducing proliferation and promoting differentiation [64]. Six weeks of 60% kcal from fat diet did not alter bone marrow HSPC population as compared to regular diet controls [12]. Conversely, 12 weeks of 60% kcal from fat diet had increased HSC population in the bone marrow compared to controls [65]. The differences in the HSC and HSPC populations in DIO murine models might be due to different percentage of fat in the diet or due to the varying duration of high fat diet feeding. Further studies are needed to confirm the effect of obesity on HSC and HSPC populations in the bone marrow.
Obesity also promotes secretion of granulocyte colony stimulating factor and granulocyte macrophage colony stimulating factor in the bone marrow [63]. Consequently, most studies have shown increased myelopoiesis, and suppressed lymphopoiesis in bone marrow HSC during obesity [12, 65, 63, 11]. However, one study has shown increased lymphopoiesis and no significant change in myelopoiesis with 162 days of 45% kcal from fat diet [62]. In competitive bone marrow transplantation, HSC from obese mice have deteriorated multi-lineage reconstitution capacity [64]. Outside of the marrow, obesity creates a systemic chronic inflammatory state, leading to increased immune cell populations in circulation both in mice and in humans [66–68], immune cell influx to tissues in the visceral cavity, and pro-inflammatory cytokine release [5, 69, 70].
Obesity also leads to an aging-like shift in bone marrow microarchitecture, by increasing the proportion of marrow adiposity as shown in multiple murine models [71, 58, 12, 72, 73]. Six weeks of 60% kcal from fat diet led to 363% increase in MAT compared to regular diet control [12]. MAT proportion increased by 5-fold with a longer high fat diet feeding (12 weeks of 60% kcal from fat diet) [71]. This trend in MAT expansion with continued high-fat diet feeding is shown by Scheller, et al., where 12 week, 16 week, and 20 week of 60% kcal from fat diet feeding leads to approximately 3-fold, 5-fold, and 12-fold increase in MAT, respectively, in the proximal tibial metaphysis [72]. A less severe fat diet (45% kcal from fat) fed for 6 weeks led to 2.6-fold increase in MAT compared to controls [73]. Interestingly, MAT proportion reduces to the similar level as control mice following weight loss [72].
Effect of MAT on Hematopoiesis and Bone Remodeling
Increase in MAT, which parallels aging or obesity, adversely impacts hematopoiesis, due to HSC’s close interactions with other cells and tissues in the marrow space as summarized in Figure 1. Increased MAT has been shown to negatively correlate with plasma insulin-like growth factor 1 (IGF-1) and plasma stromal-derived factor 1 (SDF-1), as demonstrated in the bone marrow derived from the aged [60]. A similar trend is shown in obese women, where MAT is negatively correlated with IGF-1 and positively correlated with visceral adiposity [74]. IGF-1 has been shown to increase HSC engraftment following bone marrow transplantation in murine models, while also protecting HSC and HSPC from apoptosis and enhancing proliferation and differentiation of surviving cells [75, 76]. SDF-1 has been shown to promote hematopoietic reconstitution following lethal radiation and bone marrow transplantation [77, 78]. Thus, reduced level of IGF-1 and SDF-1, paralleled by increased MAT, can adversely affect HSC engraftment and hematopoiesis.
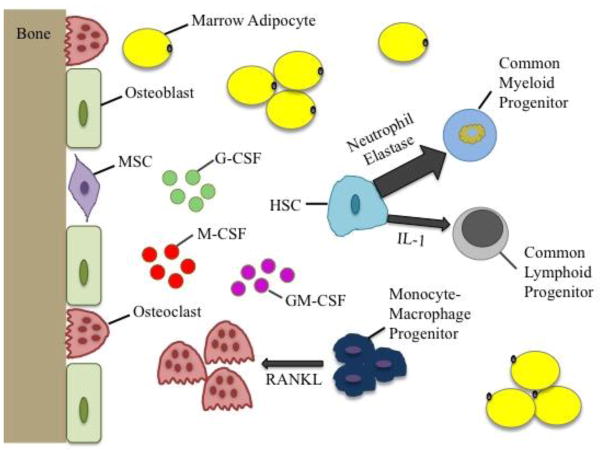
Figure 1.
Schematic summarizing the effect of marrow adiposity on hematopoiesis. Aging or obesity associated increase in marrow adiposity leads to elevated secretion of G-CSF, M-CSF, and GM-CSF, which subsequently results in elevated myelopoiesis (mediated by neutrophil elastase) and suppressed lymphopoiesis (mediated by IL-1). Marrow adipocytes can also secrete RANKL, which can prompt monocyte-macrophage progenitors towards osteoclasts. Osteoblasts then attract osteoclasts towards mineralized bone surfaces to initiate bone resorption, which can impair bone remodeling and bone health. [G-CSF – granulocyte colony stimulating factor, M-CSF – macrophage colony stimulating factor, GM-CSF – granulocyte macrophage colony stimulating factor, RANKL – receptor activator of nuclear factor kappa-B ligand, IL-1 – interleukin 1, HSC – hematopoietic stem cell, MSC – mesenchymal stem cell]
MAT has been shown to increase expression of receptor activator of nuclear factor kappa-B ligand (RANKL), osteoprotegerin, and macrophage colony stimulating factor, leading to increased osteoclast production, potentially suppressing other myeloid populations [79, 80]. In competitive bone marrow transplantation, animals with increased MAT showed reduced marrow cellularity and impaired hematopoietic reconstitution potential [58]. MAT is paralleled by reduced B lymphopoiesis, and increased myelopoiesis, as a precursor to systemic inflammation [12, 81]. MAT-induced depletion in B lymphopoiesis seems to be mediated by IL-1 since adding anti-IL-1 in bone marrow cultures in-vitro restores B lymphopoiesis [81]. Obesity-induced increased myelopoiesis seems to be mediated by secretion of neutrophil elastase, which gets up-regulated in response to high fat diet feeding [82]. Mice with neutrophil elastase deficiency had significantly reduced neutrophils and monocytes and significantly increased B-lymphocytes compared to wild type mice during obesity, not just in the bone marrow, but also in the blood and spleen [82].
A recent study by Walji, et al., studied the effect of MAT accumulation on hematopoiesis in microfilbril-associated glycoprotein-1 deficient (Mfap2−/−) mice, that display excess adiposity, without increased calorie intake, impaired glucose metabolism or aging [83]. By 10 weeks of age, these mice had 5-fold expansion of MAT compared with wild-type (WT) controls. Interestingly, despite drastic increases in MAT, Mfap2−/− mice did not have altered bone marrow cellularity compared to WT. However, increased MAT was paralleled by increased proportion of macrophages, myeloid-derived dendritic cells, and B cells and reduced proportion of neutrophils [83]. Data from this study is not consistent with previous studies that show reduced B lymphopoiesis and increased myelopoiesis in response to increased MAT. Further studies are needed to determine whether knocking out microfibril-associated glycoprotein-1 triggers other physiological responses beyond increased adiposity that might be interfering with hematopoiesis.
The effect of increased MAT on osteoblasts and osteocytes remains unclear. However, a recent study by Fairfield, et al. demonstrates in-vitro and in-vivo that sclerostin, a Wnt-inhibitory molecule secreted by osteocytes, may be mediating marrow adipogenesis [84]. Circulating sclerostin levels have been shown to correlate with increased vertebral MAT in older men, indicating a potential relationship between osteocytes and MAT [85]. Further studies need to be conducted to determine whether increase in marrow adiposity affects osteocyte and osteoblast quantity and/or function. On the other hand, MAT-associated increase in RANKL can promote osteoclastogenesis and differentiation of CFU macrophages into osteoclast lineage [79, 86]. The imbalance in osteoblast-osteoclast coupling with increased marrow adiposity can result in impaired bone remodeling, leading to reduced bone formation and increased bone resorption. Indeed, MAT expansion in humans has been associated with bone loss, reduced bone mineral density and increased risk of fracture, the characteristics of osteopenia and osteoporosis, despite correcting for multiple confounding variables such as age, sex, race, ethnicity, menopausal status, and fat composition [87, 88].
Exercise as a Countermeasure
Exercise and MAT
Exercise has been shown to suppress MAT expansion in both mice and humans. In a study led by Styner, et al., six weeks of voluntary running wheel exercise led to 50% reduction in MAT in a model of diet-induced obesity [89]. Similarly, in young female athletes, exercise increased bone marrow density, as a predictor of marrow adiposity, by 0.5% [90]. Reduction in MAT, following exercise, may partially be mediated by increased level of perilipin 3, which has been shown to play a role in β-oxidation of lipids and basal lipolysis [89, 91, 92]. MAT expansion following 60 days of bed rest in young men was prevented when subjects underwent resistance training during the bed rest [93]. As a surrogate for exercise, whole body vibration (WBV), delivered with accelerations of approximately 0.7g (g = Earth’s gravitational force, 9.81 m/s2), strengthened resistance training’s ability to suppress MAT expansion [93]. WBV could also be effective as a stand-alone treatment in preventing MAT expansion as evident by 55% reduction in MAT with 6 weeks of vibration treatment (0.3g acceleration, 90 Hz frequency) in an ovariectomy murine model [94]. MAT can be used as a predictor of bone strength since it correlates with strength strain index, cortical area, and bone mineral density [90]. Reduction in MAT is paralleled by increase in bone quality as evident by 19% increase in trabecular bone volume fraction, following 48% reduction in MAT [89].
Exercise and Hematopoiesis
Although not many studies have evaluated the effect of exercise on HSC and hematopoiesis, exercise has been shown to promote HSC differentiation and increase HSC progenitors in both the bone marrow and in circulation [95–97]. Baker, et al., showed 78% reduction in MAT, paralleled by 49% and 229% increase in colony forming cells in the bone marrow and blood, respectively, following 10 weeks of exercise in mice [95]. Eight weeks of exercise in mice has been shown to increase HSC population in the vascular bone marrow by 20%, paralleled by 48% increased cellularity in the spleen colonies [96]. Interestingly, HSPC show an adaptive response to exercise, such that runners have 3–4 fold higher level of circulating HSPC compared to sedentary people at rest [97]. Although exercise affects HSC proliferation and differentiation, it does not alter HSC engraftment, homing, and self-renewal potential after bone marrow transplantation [96]. Similar to exercise, WBV has also been shown to restore lymphopoiesis, as demonstrated by 32% and 57% increase in B cells in the bone marrow and in circulation, respectively, following 4 months of WBV treatment (0.2g acceleration, 90 Hz frequency), in a murine model of diet-induced obesity [11]. Outside of the bone marrow, WBV can also regulate inflammatory response in the adipose tissue as evident by reduction in B cell, T cell, and macrophage populations (16%, 22%, and 13%, respectively) during obesity (Figure 2) [5]. A positive correlation has been demonstrated between circulating blood and immune cells (white blood cells, red blood cells, and platelets) and bone mineral density in post-menopausal women, suggesting a relationship between hematopoiesis and bone health [98].
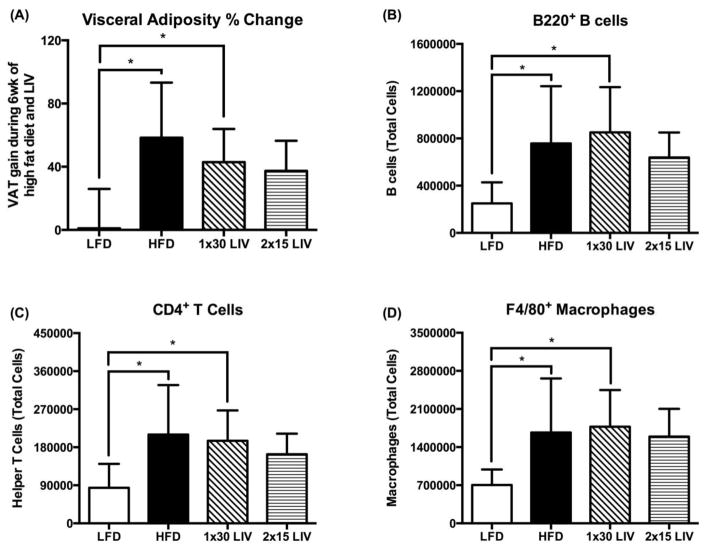
Figure 2.
Visceral adiposity and infiltration of immune cells in gonadal adipose tissue in a murine model of diet-induced obesity (45% kcal from fat diet) in adult male C57BL/6J mice. All mice were fed their respective diets for 2wk, followed by 6wk of low intensity vibration (LIV; 90Hz, 0.2g peak acceleration (g = Earth’s gravitational force), 5d/wk) treatment, while continuing on high fat diet. Continued gain in visceral adiposity with high fat diet feeding in HFD and 1×30 LIV group, which was prevented with 2×15 LIV (A). Increased infiltration of B cells (B), T cells (C), and macrophages (D) in gonadal adipose tissue with high fat diet, which was mitigated by 2×15 LIV, but not 1×30 LIV. *p<0.05. All data are presented as mean±SD. (Figure modified from [5]). Used with permission from Nature Publishing.
Effectiveness of exercise, or specifically of mechanical element of exercise, reduces with aging, partly due to reduced cell mechanosensitivity with aging [99]. One potential solution to avoid plateauing of the response to exercise, could be insertion of rest periods between bouts of physical activity [100]. Insertion of a 3-hour rest period between bouts of WBV has been shown to reduce adipogenesis in MSC in-vitro, as evident by 70% reduction in adiponectin after 7 days of treatment (0.7g acceleration, 90Hz frequency) [101]. A recent study from our lab shows that this effect translates in-vivo as well in an adult murine model of diet-induced obesity. WBV was only effective in adult mice when 30 minutes of treatment (0.2g acceleration, 90Hz frequency) was separated in two bouts of 15 minutes with 5-hour rest period per day [5]. Indeed, inclusion of a rest period resulted in reduced visceral adiposity (Figure 2A) and restored B cell (Figure 2B), T cell (Figure 2C), and macrophage (Figure 2D) populations in the adipose tissue [5]. Further studies are needed to optimize the treatment duration and the rest period between bouts to achieve maximum response to physical activity.
Thus, exercise has been shown to simultaneously be beneficial for both MAT prevention and restoring hematopoiesis in the bone marrow. However, for patients who might not be capable of strenuous physical exertion, low magnitude WBV (<1.0g) can serve somewhat as surrogate to exercise.
Conclusion
Bone marrow contains a complex niche that maintains the balance between many cell types, including those from the mesenchymal and hematopoietic lineage. This specialized bone marrow microenvironment is sensitive to systemic stressors such as aging and obesity, which can increase the proportion of adipose tissue within the bone marrow, and lead to reduced engraftment, homing, and self-renewal capacity of HSC. Increased marrow adiposity affects HSC function by disrupting HSC differentiation pathways, biasing lineage selection towards myelopoiesis and away from lymphopoiesis. Marrow adiposity adversely affects bone health by reducing bone volume fraction and bone mineral density, and compromising the regenerative potential of the bone cell precursor pool, including osteoblasts (from MSC) and osteoclasts (from HSC). Exercise has been shown to prevent marrow adipose tissue expansion, protect the marrow phenotype, and to restore MAT-induced changes in hematopoiesis. There is early preclinical and clinical evidence that suggests that - for patients that are unable to perform strenuous exercise - low magnitude whole body vibration can serve as a simultaneous treatment for reducing marrow adiposity and restoring hematopoiesis.
Footnotes
Compliance with Ethical Guidelines
Conflict of Interest
Janet Rubin, Meilin Chan, Vihitaben Patel, Ete Chan declare no conflict of interest. Clinton Rubin has authored patents related to use of mechanical signals to bias stem cell fate and mechanical regulation of metabolic diseases. He also serves as the Chief Scientific Officer at Marodyne Medical. Other authors have nothing to disclose.
Jay Cao declares no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Travlos GS. Normal structure, function, and histology of the bone marrow. Toxicol Pathol. 2006;34(5):548–65. doi: 10.1080/01926230600939856. [DOI] [PubMed] [Google Scholar]
- 2.Augello A, De Bari C. The regulation of differentiation in mesenchymal stem cells. Hum Gene Ther. 2010;21(10):1226–38. doi: 10.1089/hum.2010.173. [DOI] [PubMed] [Google Scholar]
- 3.Luu YK, Capilla E, Rosen CJ, Gilsanz V, Pessin JE, Judex S, et al. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J Bone Miner Res. 2009;24(1):50–61. doi: 10.1359/jbmr.080817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez-Viejo M, Menendez-Menendez Y, Blanco-Gelaz MA, Ferrero-Gutierrez A, Fernandez-Rodriguez MA, Gala J, et al. Quantifying mesenchymal stem cells in the mononuclear cell fraction of bone marrow samples obtained for cell therapy. Transplant Proc. 2013;45(1):434–9. doi: 10.1016/j.transproceed.2012.05.091. [DOI] [PubMed] [Google Scholar]
- ••5.Patel VS, Chan ME, Pagnotti GM, Frechette DM, Rubin J, Rubin CT. Incorporating Refractory Period in Mechanical Stimulation Mitigates Obesity-Induced Adipose Tissue Dysfunction in Adult Mice. Obesity (Silver Spring) 2017;25(10):1745–53. doi: 10.1002/oby.21958. This study demonstrates that obesity leads to systemic chronic inflammatory state by inducing increased infiltration of immune cells in the adipose tissue, which is restored via whole body vibration. In addition, this study emphasizes that in adults, whole body vibration treatment is only effective when it is separated with a rest period. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Yu X, Chen E, Li L. Liver-derived human mesenchymal stem cells: a novel therapeutic source for liver diseases. Stem Cell Res Ther. 2016;7(1):71. doi: 10.1186/s13287-016-0330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seeberger KL, Dufour JM, Shapiro AM, Lakey JR, Rajotte RV, Korbutt GS. Expansion of mesenchymal stem cells from human pancreatic ductal epithelium. Lab Invest. 2006;86(2):141–53. doi: 10.1038/labinvest.3700377. [DOI] [PubMed] [Google Scholar]
- 8.Yin L, Zhu Y, Yang J, Ni Y, Zhou Z, Chen Y, et al. Adipose tissue-derived mesenchymal stem cells differentiated into hepatocyte-like cells in vivo and in vitro. Mol Med Rep. 2015;11(3):1722–32. doi: 10.3892/mmr.2014.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubin CT, Capilla E, Luu YK, Busa B, Crawford H, Nolan DJ, et al. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc Natl Acad Sci U S A. 2007;104(45):17879–84. doi: 10.1073/pnas.0708467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.da Silva SV, Renovato-Martins M, Ribeiro-Pereira C, Citelli M, Barja-Fidalgo C. Obesity modifies bone marrow microenvironment and directs bone marrow mesenchymal cells to adipogenesis. Obesity (Silver Spring) 2016;24(12):2522–32. doi: 10.1002/oby.21660. [DOI] [PubMed] [Google Scholar]
- 11.Chan ME, Adler BJ, Green DE, Rubin CT. Bone structure and B-cell populations, crippled by obesity, are partially rescued by brief daily exposure to low-magnitude mechanical signals. Faseb j. 2012;26(12):4855–63. doi: 10.1096/fj.12-209841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••12.Adler BJ, Green DE, Pagnotti GM, Chan ME, Rubin CT. High fat diet rapidly suppresses B lymphopoiesis by disrupting the supportive capacity of the bone marrow niche. PLoS One. 2014;9(3):e90639. doi: 10.1371/journal.pone.0090639. This paper demonstrates that high fat diet leads to increase in marrow adiposity, paralleled by reduced B lymphopoiesis and increased myelopoiesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132(4):631–44. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Notta F, Doulatov S, Laurenti E, Poeppl A, Jurisica I, Dick JE. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333(6039):218–21. doi: 10.1126/science.1201219. [DOI] [PubMed] [Google Scholar]
- 15.Tesio M, Tang Y, Mudder K, Saini M, von Paleske L, Macintyre E, et al. Hematopoietic stem cell quiescence and function are controlled by the CYLD-TRAF2-p38MAPK pathway. J Exp Med. 2015;212(4):525–38. doi: 10.1084/jem.20141438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Challen GA, Boles N, Lin KK, Goodell MA. Mouse hematopoietic stem cell identification and analysis. Cytometry A. 2009;75(1):14–24. doi: 10.1002/cyto.a.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pang WW, Price EA, Sahoo D, Beerman I, Maloney WJ, Rossi DJ, et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci U S A. 2011;108(50):20012–7. doi: 10.1073/pnas.1116110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franco RS. Measurement of red cell lifespan and aging. Transfus Med Hemother. 2012;39(5):302–7. doi: 10.1159/000342232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dzierzak E, Philipsen S. Erythropoiesis: development and differentiation. Cold Spring Harb Perspect Med. 2013;3(4):a011601. doi: 10.1101/cshperspect.a011601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen JA, Leeksma CH. Determination of the life span of human blood platelets using labelled diisopropylfluorophosphonate. J Clin Invest. 1956;35(9):964–9. doi: 10.1172/jci103356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thon JN, Italiano JE. Platelet formation. Semin Hematol. 2010;47(3):220–6. doi: 10.1053/j.seminhematol.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sprent J, Tough DF. Lymphocyte life-span and memory. Science. 1994;265(5177):1395–400. doi: 10.1126/science.8073282. [DOI] [PubMed] [Google Scholar]
- 23.Pillay J, den Braber I, Vrisekoop N, Kwast LM, de Boer RJ, Borghans JA, et al. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood. 2010;116(4):625–7. doi: 10.1182/blood-2010-01-259028. [DOI] [PubMed] [Google Scholar]
- 24.Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31(8):318–24. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park YM, Bochner BS. Eosinophil survival and apoptosis in health and disease. Allergy Asthma Immunol Res. 2010;2(2):87–101. doi: 10.4168/aair.2010.2.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Min B, Brown MA, Legros G. Understanding the roles of basophils: breaking dawn. Immunology. 2012;135(3):192–7. doi: 10.1111/j.1365-2567.2011.03530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janssen WJ, Bratton DL, Jakubzick CV, Henson PM. Myeloid Cell Turnover and Clearance. Microbiol Spectr. 2016;4(6) doi: 10.1128/microbiolspec.MCHD-0005-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Velten L, Haas SF, Raffel S, Blaszkiewicz S, Islam S, Hennig BP, et al. Human haematopoietic stem cell lineage commitment is a continuous process. Nat Cell Biol. 2017;19(4):271–81. doi: 10.1038/ncb3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP, Gerson SL. Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res. 2000;9(6):841–8. doi: 10.1089/152581600750062264. [DOI] [PubMed] [Google Scholar]
- 30.Mishima S, Nagai A, Abdullah S, Matsuda C, Taketani T, Kumakura S, et al. Effective ex vivo expansion of hematopoietic stem cells using osteoblast-differentiated mesenchymal stem cells is CXCL12 dependent. Eur J Haematol. 2010;84(6):538–46. doi: 10.1111/j.1600-0609.2010.01419.x. [DOI] [PubMed] [Google Scholar]
- 31.Carrancio S, Blanco B, Romo C, Muntion S, Lopez-Holgado N, Blanco JF, et al. Bone marrow mesenchymal stem cells for improving hematopoietic function: an in vitro and in vivo model. Part 2: Effect on bone marrow microenvironment. PLoS One. 2011;6(10):e26241. doi: 10.1371/journal.pone.0026241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30(1):42–8. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 33.Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42–9. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roddy GW, Oh JY, Lee RH, Bartosh TJ, Ylostalo J, Coble K, et al. Action at a distance: systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TNF-alpha stimulated gene/protein 6. Stem Cells. 2011;29(10):1572–9. doi: 10.1002/stem.708. [DOI] [PubMed] [Google Scholar]
- 35.Taichman RS, Emerson SG. The role of osteoblasts in the hematopoietic microenvironment. Stem Cells. 1998;16(1):7–15. doi: 10.1002/stem.160007. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi N, Udagawa N, Akatsu T, Tanaka H, Shionome M, Suda T. Role of colony-stimulating factors in osteoclast development. J Bone Miner Res. 1991;6(9):977–85. doi: 10.1002/jbmr.5650060912. [DOI] [PubMed] [Google Scholar]
- 37.Marks SC, Jr, Mackay CA, Jackson ME, Larson EK, Cielinski MJ, Stanley ER, et al. The skeletal effects of colony-stimulating factor-1 in toothless (osteopetrotic) rats: persistent metaphyseal sclerosis and the failure to restore subepiphyseal osteoclasts. Bone. 1993;14(4):675–80. doi: 10.1016/8756-3282(93)90091-n. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez-Fernandez MA, Gallois A, Riedl T, Jurdic P, Hoflack B. Osteoclasts control osteoblast chemotaxis via PDGF-BB/PDGF receptor beta signaling. PLoS One. 2008;3(10):e3537. doi: 10.1371/journal.pone.0003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26(2):229–38. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fulzele K, Krause DS, Panaroni C, Saini V, Barry KJ, Liu X, et al. Myelopoiesis is regulated by osteocytes through Gsalpha-dependent signaling. Blood. 2013;121(6):930–9. doi: 10.1182/blood-2012-06-437160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoneshiro T, Aita S, Matsushita M, Okamatsu-Ogura Y, Kameya T, Kawai Y, et al. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity (Silver Spring) 2011;19(9):1755–60. doi: 10.1038/oby.2011.125. [DOI] [PubMed] [Google Scholar]
- 42.Trayhurn P, Beattie JH. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc. 2001;60(3):329–39. doi: 10.1079/pns200194. [DOI] [PubMed] [Google Scholar]
- 43.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453(7196):783–7. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 44.Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res. 1971;80:147–54. doi: 10.1097/00003086-197110000-00021. [DOI] [PubMed] [Google Scholar]
- 45.Moore SG, Dawson KL. Red and yellow marrow in the femur: age-related changes in appearance at MR imaging. Radiology. 1990;175(1):219–23. doi: 10.1148/radiology.175.1.2315484. [DOI] [PubMed] [Google Scholar]
- 46.Scheller EL, Rosen CJ. What’s the matter with MAT? Marrow adipose tissue, metabolism, and skeletal health. Ann N Y Acad Sci. 2014;1311:14–30. doi: 10.1111/nyas.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huggins C, Blocksom BH. CHANGES IN OUTLYING BONE MARROW ACCOMPANYING A LOCAL INCREASE OF TEMPERATURE WITHIN PHYSIOLOGICAL LIMITS. J Exp Med. 1936;64(2):253–74. doi: 10.1084/jem.64.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460(7252):259–63. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scheller EL, Doucette CR, Learman BS, Cawthorn WP, Khandaker S, Schell B, et al. Corrigendum: Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat Commun. 2016;7:13775. doi: 10.1038/ncomms13775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lihn AS, Pedersen SB, Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obes Rev. 2005;6(1):13–21. doi: 10.1111/j.1467-789X.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 51.Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014;20(2):368–75. doi: 10.1016/j.cmet.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suchacki KJ, Cawthorn WP, Rosen CJ. Bone marrow adipose tissue: formation, function and regulation. Curr Opin Pharmacol. 2016;28:50–6. doi: 10.1016/j.coph.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keune JA, Wong CP, Branscum AJ, Iwaniec UT, Turner RT. Bone Marrow Adipose Tissue Deficiency Increases Disuse-Induced Bone Loss in Male Mice. Sci Rep. 2017;7:46325. doi: 10.1038/srep46325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ogawa T, Kitagawa M, Hirokawa K. Age-related changes of human bone marrow: a histometric estimation of proliferative cells, apoptotic cells, T cells, B cells and macrophages. Mech Ageing Dev. 2000;117(1–3):57–68. doi: 10.1016/s0047-6374(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 55.Pearce DJ, Anjos-Afonso F, Ridler CM, Eddaoudi A, Bonnet D. Age-dependent increase in side population distribution within hematopoiesis: implications for our understanding of the mechanism of aging. Stem Cells. 2007;25(4):828–35. doi: 10.1634/stemcells.2006-0405. [DOI] [PubMed] [Google Scholar]
- 56.Yahata T, Takanashi T, Muguruma Y, Ibrahim AA, Matsuzawa H, Uno T, et al. Accumulation of oxidative DNA damage restricts the self-renewal capacity of human hematopoietic stem cells. Blood. 2011;118(11):2941–50. doi: 10.1182/blood-2011-01-330050. [DOI] [PubMed] [Google Scholar]
- 57.Kuranda K, Vargaftig J, de la Rochere P, Dosquet C, Charron D, Bardin F, et al. Age-related changes in human hematopoietic stem/progenitor cells. Aging Cell. 2011;10(3):542–6. doi: 10.1111/j.1474-9726.2011.00675.x. [DOI] [PubMed] [Google Scholar]
- 58.Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank AM, Bocian C, et al. Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration. Cell Stem Cell. 2017;20(6):771–84e6. doi: 10.1016/j.stem.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Griffith JF, Yeung DK, Ma HT, Leung JC, Kwok TC, Leung PC. Bone marrow fat content in the elderly: a reversal of sex difference seen in younger subjects. J Magn Reson Imaging. 2012;36(1):225–30. doi: 10.1002/jmri.23619. [DOI] [PubMed] [Google Scholar]
- 60.Tuljapurkar SR, McGuire TR, Brusnahan SK, Jackson JD, Garvin KL, Kessinger MA, et al. Changes in human bone marrow fat content associated with changes in hematopoietic stem cell numbers and cytokine levels with aging. J Anat. 2011;219(5):574–81. doi: 10.1111/j.1469-7580.2011.01423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reinisch A, Etchart N, Thomas D, Hofmann NA, Fruehwirth M, Sinha S, et al. Epigenetic and in vivo comparison of diverse MSC sources reveals an endochondral signature for human hematopoietic niche formation. Blood. 2015;125(2):249–60. doi: 10.1182/blood-2014-04-572255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trottier MD, Naaz A, Li Y, Fraker PJ. Enhancement of hematopoiesis and lymphopoiesis in diet-induced obese mice. Proc Natl Acad Sci U S A. 2012;109(20):7622–9. doi: 10.1073/pnas.1205129109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.do Carmo LS, Rogero MM, Paredes-Gamero EJ, Nogueira-Pedro A, Xavier JG, Cortez M, et al. A high-fat diet increases interleukin-3 and granulocyte colony-stimulating factor production by bone marrow cells and triggers bone marrow hyperplasia and neutrophilia in Wistar rats. Exp Biol Med (Maywood) 2013;238(4):375–84. doi: 10.1177/1535370213477976. [DOI] [PubMed] [Google Scholar]
- 64.van den Berg SM, Seijkens TT, Kusters PJ, Beckers L, den Toom M, Smeets E, et al. Diet-induced obesity in mice diminishes hematopoietic stem and progenitor cells in the bone marrow. Faseb j. 2016;30(5):1779–88. doi: 10.1096/fj.201500175. [DOI] [PubMed] [Google Scholar]
- 65.Singer K, DelProposto J, Morris DL, Zamarron B, Mergian T, Maley N, et al. Diet-induced obesity promotes myelopoiesis in hematopoietic stem cells. Mol Metab. 2014;3(6):664–75. doi: 10.1016/j.molmet.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takahashi K, Mizuarai S, Araki H, Mashiko S, Ishihara A, Kanatani A, et al. Adiposity elevates plasma MCP-1 levels leading to the increased CD11b-positive monocytes in mice. J Biol Chem. 2003;278(47):46654–60. doi: 10.1074/jbc.M309895200. [DOI] [PubMed] [Google Scholar]
- 67.Nanji AA, Freeman JB. Relationship between body weight and total leukocyte count in morbid obesity. Am J Clin Pathol. 1985;84(3):346–7. doi: 10.1093/ajcp/84.3.346. [DOI] [PubMed] [Google Scholar]
- 68.Womack J, Tien PC, Feldman J, Shin JH, Fennie K, Anastos K, et al. Obesity and immune cell counts in women. Metabolism. 2007;56(7):998–1004. doi: 10.1016/j.metabol.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nteeba J, Ortinau LC, Perfield JW, 2nd, Keating AF. Diet-induced obesity alters immune cell infiltration and expression of inflammatory cytokine genes in mouse ovarian and peri-ovarian adipose depot tissues. Mol Reprod Dev. 2013;80(11):948–58. doi: 10.1002/mrd.22231. [DOI] [PubMed] [Google Scholar]
- 70.Caer C, Rouault C, Le Roy T, Poitou C, Aron-Wisnewsky J, Torcivia A, et al. Immune cell-derived cytokines contribute to obesity-related inflammation, fibrogenesis and metabolic deregulation in human adipose tissue. Sci Rep. 2017;7(1):3000. doi: 10.1038/s41598-017-02660-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Doucette CR, Horowitz MC, Berry R, MacDougald OA, Anunciado-Koza R, Koza RA, et al. A High Fat Diet Increases Bone Marrow Adipose Tissue (MAT) But Does Not Alter Trabecular or Cortical Bone Mass in C57BL/6J Mice. J Cell Physiol. 2015;230(9):2032–7. doi: 10.1002/jcp.24954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scheller EL, Khoury B, Moller KL, Wee NK, Khandaker S, Kozloff KM, et al. Changes in Skeletal Integrity and Marrow Adiposity during High-Fat Diet and after Weight Loss. Front Endocrinol (Lausanne) 2016;7:102. doi: 10.3389/fendo.2016.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••73.Styner M, Thompson WR, Galior K, Uzer G, Wu X, Kadari S, et al. Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise. Bone. 2014;64:39–46. doi: 10.1016/j.bone.2014.03.044. This paper demonstrates that high fat diet leads to increased marrow adiposity, which gets suppressed by exercise. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV, et al. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity (Silver Spring) 2011;19(1):49–53. doi: 10.1038/oby.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caselli A, Olson TS, Otsuru S, Chen X, Hofmann TJ, Nah HD, et al. IGF-1-mediated osteoblastic niche expansion enhances long-term hematopoietic stem cell engraftment after murine bone marrow transplantation. Stem Cells. 2013;31(10):2193–204. doi: 10.1002/stem.1463. [DOI] [PubMed] [Google Scholar]
- 76.Zhou D, Deoliveira D, Kang Y, Choi SS, Li Z, Chao NJ, et al. Insulin-like growth factor 1 mitigates hematopoietic toxicity after lethal total body irradiation. Int J Radiat Oncol Biol Phys. 2013;85(4):1141–8. doi: 10.1016/j.ijrobp.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liang X, Su YP, Kong PY, Zeng DF, Chen XH, Peng XG, et al. Human bone marrow mesenchymal stem cells expressing SDF-1 promote hematopoietic stem cell function of human mobilised peripheral blood CD34+ cells in vivo and in vitro. Int J Radiat Biol. 2010;86(3):230–7. doi: 10.3109/09553000903422555. [DOI] [PubMed] [Google Scholar]
- 78.Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia. 2002;16(10):1992–2003. doi: 10.1038/sj.leu.2402684. [DOI] [PubMed] [Google Scholar]
- 79.Takeshita S, Fumoto T, Naoe Y, Ikeda K. Age-related marrow adipogenesis is linked to increased expression of RANKL. J Biol Chem. 2014;289(24):16699–710. doi: 10.1074/jbc.M114.547919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Holt V, Caplan AI, Haynesworth SE. Identification of a subpopulation of marrow MSC-derived medullary adipocytes that express osteoclast-regulating molecules: marrow adipocytes express osteoclast mediators. PLoS One. 2014;9(10):e108920. doi: 10.1371/journal.pone.0108920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •81.Kennedy DE, Knight KL. Inhibition of B Lymphopoiesis by Adipocytes and IL-1-Producing Myeloid-Derived Suppressor Cells. J Immunol. 2015;195(6):2666–74. doi: 10.4049/jimmunol.1500957. This study demonstrates that MAT-induced depletion in B lymphopoiesis is mediated by secretion of IL-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •82.Huang JY, Zhou QL, Huang CH, Song Y, Sharma AG, Liao Z, et al. Neutrophil Elastase Regulates Emergency Myelopoiesis Preceding Systemic Inflammation in Diet-induced Obesity. J Biol Chem. 2017;292(12):4770–76. doi: 10.1074/jbc.C116.758748. This study demonstrates that obesity-induced myeloid bias is mediated via secretion of neutrophil elastase. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Walji TA, Turecamo SE, Sanchez AC, Anthony BA, Abou-Ezzi G, Scheller EL, et al. Marrow Adipose Tissue Expansion Coincides with Insulin Resistance in MAGP1-Deficient Mice. Front Endocrinol (Lausanne) 2016;7:87. doi: 10.3389/fendo.2016.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fairfield H, Falank C, Harris E, Demambro V, McDonald M, Pettitt JA, et al. The skeletal cell-derived molecule sclerostin drives bone marrow adipogenesis. J Cell Physiol. 2018;233(2):1156–67. doi: 10.1002/jcp.25976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ma YH, Schwartz AV, Sigurdsson S, Hue TF, Lang TF, Harris TB, et al. Circulating sclerostin associated with vertebral bone marrow fat in older men but not women. J Clin Endocrinol Metab. 2014;99(12):E2584–90. doi: 10.1210/jc.2013-4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kohli SS, Kohli VS. Role of RANKL-RANK/osteoprotegerin molecular complex in bone remodeling and its immunopathologic implications. Indian J Endocrinol Metab. 2011;15(3):175–81. doi: 10.4103/2230-8210.83401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Styner M, Pagnotti GM, McGrath C, Wu X, Sen B, Uzer G, et al. Exercise Decreases Marrow Adipose Tissue Through ss-Oxidation in Obese Running Mice. J Bone Miner Res. 2017 doi: 10.1002/jbmr.3159. This study demonstrates that reduction in high-fat diet induced accumulation in marrow adiposity during exercise is mediated via basal lyposis and β-oxidation, which is partially mediated by perilipin 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shen W, Chen J, Gantz M, Punyanitya M, Heymsfield SB, Gallagher D, et al. MRI-measured pelvic bone marrow adipose tissue is inversely related to DXA-measured bone mineral in younger and older adults. Eur J Clin Nutr. 2012;66(9):983–8. doi: 10.1038/ejcn.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Styner M, Pagnotti GM, McGrath C, Wu X, Sen B, Uzer G, et al. Exercise Decreases Marrow Adipose Tissue Through ss-Oxidation in Obese Running Mice. J Bone Miner Res. 2017 doi: 10.1002/jbmr.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rantalainen T, Nikander R, Heinonen A, Cervinka T, Sievanen H, Daly RM. Differential effects of exercise on tibial shaft marrow density in young female athletes. J Clin Endocrinol Metab. 2013;98(5):2037–44. doi: 10.1210/jc.2012-3748. [DOI] [PubMed] [Google Scholar]
- 91.Covington JD, Noland RC, Hebert RC, Masinter BS, Smith SR, Rustan AC, et al. Perilipin 3 Differentially Regulates Skeletal Muscle Lipid Oxidation in Active, Sedentary, and Type 2 Diabetic Males. J Clin Endocrinol Metab. 2015;100(10):3683–92. doi: 10.1210/jc.2014-4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Patel S, Yang W, Kozusko K, Saudek V, Savage DB. Perilipins 2 and 3 lack a carboxy-terminal domain present in perilipin 1 involved in sequestering ABHD5 and suppressing basal lipolysis. Proc Natl Acad Sci U S A. 2014;111(25):9163–8. doi: 10.1073/pnas.1318791111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Trudel G, Coletta E, Cameron I, Belavy DL, Lecompte M, Armbrecht G, et al. Resistive exercises, with or without whole body vibration, prevent vertebral marrow fat accumulation during 60 days of head-down tilt bed rest in men. J Appl Physiol (1985) 2012;112(11):1824–31. doi: 10.1152/japplphysiol.00029.2012. [DOI] [PubMed] [Google Scholar]
- 94.Krishnamoorthy D, Frechette DM, Adler BJ, Green DE, Chan ME, Rubin CT. Marrow adipogenesis and bone loss that parallels estrogen deficiency is slowed by low-intensity mechanical signals. Osteoporos Int. 2016;27(2):747–56. doi: 10.1007/s00198-015-3289-5. [DOI] [PubMed] [Google Scholar]
- 95.Baker JM, De Lisio M, Parise G. Endurance exercise training promotes medullary hematopoiesis. Faseb j. 2011;25(12):4348–57. doi: 10.1096/fj.11-189043. [DOI] [PubMed] [Google Scholar]
- 96.De Lisio M, Parise G. Characterization of the effects of exercise training on hematopoietic stem cell quantity and function. J Appl Physiol (1985) 2012;113(10):1576–84. doi: 10.1152/japplphysiol.00717.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bonsignore MR, Morici G, Santoro A, Pagano M, Cascio L, Bonanno A, et al. Circulating hematopoietic progenitor cells in runners. J Appl Physiol (1985) 2002;93(5):1691–7. doi: 10.1152/japplphysiol.00376.2002. [DOI] [PubMed] [Google Scholar]
- 98.Kim HL, Cho HY, Park IY, Choi JM, Kim M, Jang HJ, et al. The positive association between peripheral blood cell counts and bone mineral density in postmenopausal women. Yonsei Med J. 2011;52(5):739–45. doi: 10.3349/ymj.2011.52.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Klein-Nulend J, Sterck JG, Semeins CM, Lips P, Joldersma M, Baart JA, et al. Donor age and mechanosensitivity of human bone cells. Osteoporos Int. 2002;13(2):137–46. doi: 10.1007/s001980200005. [DOI] [PubMed] [Google Scholar]
- 100.Robling AG, Burr DB, Turner CH. Recovery periods restore mechanosensitivity to dynamically loaded bone. J Exp Biol. 2001;204(Pt 19):3389–99. doi: 10.1242/jeb.204.19.3389. [DOI] [PubMed] [Google Scholar]
- 101.Sen B, Xie Z, Case N, Styner M, Rubin CT, Rubin J. Mechanical signal influence on mesenchymal stem cell fate is enhanced by incorporation of refractory periods into the loading regimen. J Biomech. 2011;44(4):593–9. doi: 10.1016/j.jbiomech.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]