Abstract
High-age patients have higher rates of comorbidity that are associated with a poor prognosis. It is important to correctly evaluate their preoperative status to avoid mortality. The aim of this study was to clarify whether the Charlson comorbidity index (CCI) was useful for predicting postoperative outcomes. This retrospective study collected data from 250 consecutive patients over 75 years of age. The CCI takes into account 19 comorbid conditions. Inflammation-based scores, including the Glasgow prognostic score (GPS) and the platelet to lymphocyte ratio (PLR), are other preoperative scoring systems. The relationships among these scores and postoperative outcomes were evaluated. The patients were classified according to their vital status (dead, n = 30 or alive, n = 220). Comorbidities, the presence of double cancer, and lymph node metastases were significantly different between the groups (p < 0.01, p = 0.01, and p < 0.01). In regard to the scoring systems, the CCI, GPS, and PLR were significantly different (p = 0.02, p = 0.03, and p = 0.05). Multivariate analysis identified CCI ≥ 2 (hazard ratio (HR) = 5.24, 95 % confidence interval (CI) = 1.30–12.1, p = 0.01) as a significant determinant of postoperative outcome (p < 0.01). The overall survival tended to be lower in patients with high CCI scores group (p = 0.03). The CCI was useful to predict postoperative outcomes in high-age colorectal cancer patients.
Electronic supplementary material
The online version of this article (doi:10.1007/s12262-016-1544-4) contains supplementary material, which is available to authorized users.
Keywords: Charlson comorbidity index, Colorectal cancer, Older patients, Overall survival
Introduction
Colorectal cancer is the third leading cause of cancer-related death in Japan. As the population ages, the number of elderly colorectal cancer patients is likely to increase. In general, high-age patients are said to have higher rates of comorbidity than younger patients [1]. In some reports, a number of past illnesses tended to be associated with increased postoperative complications and a poor prognosis [2]. It is important to correctly evaluate high-age patients’ preoperative status to avoid poor outcomes. Until now, several scoring systems using preoperative information have been reported to predict postoperative outcomes. The Charlson comorbidity index (CCI) was reported to classify comorbid conditions that might alter the risk of mortality for use in longitudinal studies [3]. The index takes into account 19 comorbid conditions, including cardiovascular disease, diabetes mellitus, liver disease, and pulmonary disease.
On the other hand, there is a strong relationship between malignant disease and inflammation [4]. In colorectal cancer, inflammatory bowel diseases such as ulcerative colitis and Crohn’s disease are known as premalignant disease [5]. Today, several inflammatory-based scores using preoperative data have been reported. C-reactive protein (CRP) is an acute phase protein produced in the liver that is recognized as a systemic inflammation marker [6]. Albumin has been shown to be a marker of nutrition, and hypoalbuminemia could show a declining health status or malnutrition [7]. The Glasgow prognostic score (GPS) is one of the inflammatory-based scores that can be calculated from the CRP and the serum albumin level, and it has been reported to be effective for predicting postoperative mortality [8].
The platelet to lymphocyte ratio (PLR) is another inflammation score that consists of the platelet count and the lymphocyte count [9]. Platelets play an important role in the coagulation system and the inflammatory response, and they are known to correlate with tumor growth, invasion, and angiogenesis [10]. Lymphocytes play an important role in anti-tumor immunity and are important factors for the host immune system [11]. Therefore, a low lymphocyte level is considered to be associated with recurrence [12].
Several reports have shown the effectiveness of these preoperative scoring systems, including the CCI, GPS, and PLR, for predicting postoperative outcomes in various cancers [13–15]. However, few reports have examined their use in high-age patients.
The aim of this study was to evaluate whether preoperative scoring systems, including the CCI, GPS, and PLR, are useful prognostic factors in older colorectal cancer patients.
Patients and Methods
Patients
A total of 506 colorectal cancer patients who underwent colorectal resection of primary cancer at the Department of Surgical Oncology, Nagasaki University Graduate School of Biological Sciences from January 2009 to December 2014 were evaluated. This retrospective study collected data from 250 consecutive patients over 75 years of age. The median follow-up time was 24.0 months (range, 0.2–69.2 months).
Before surgery, the appropriateness of resection was determined by abdominal CT and colonoscopy. Data for age, sex, body mass index, performance status, tumor markers including CEA and CA19-9, International Union Against Cancer tumor staging, operation time, amount of blood loss, and postoperative data including pathology, depth of tumor invasion, and hospital stay were retrospectively collected. Postoperative complications were graded according to the Clavien-Dindo classification, categorizing surgical complications from grades 1 to 5 based on the invasiveness of the treatment required. In the present study, complications were defined as conditions that required treatment (Clavien-Dindo classification grades 2–5).
Colectomy, anterior resection, and abdominoperineal resection plus lymph node resection were performed according to the guideline of the Japanese Society for Cancer of the Colon and Rectum (JSCCR). A hand-sewn or an end-to-end anastomosis using double-stapling technique was performed according to tumor location. Mortality and morbidity data were collected from the database of our department and collaborating hospitals.
Scoring Systems Evaluated
The components of the CCI are shown in Supplementary Table 1. Weights were assigned for each condition the patients had, and their total was the score.
The GPS was established in the following manner. Patients with both hypoalbuminemia (<3.5 g/dL) and an elevated CRP level (>1.0 mg/dL) were allocated a score of 2; patients with only one of the two abnormalities were allocated a score of 1; and patients with neither of the above-mentioned two abnormalities were allocated a score of 0. The PLR was defined as the absolute platelet count divided by the absolute lymphocyte count.
Statistical Analysis
Data of different groups were compared using Student’s t test. Continuous data are expressed as means ± standard deviation (SD). On univariate analysis, comparisons of categorical variables were performed using the chi-square test or Fisher’s exact test. p < 0.05 was considered significant. Overall survival and disease-free survival were calculated according to Kaplan-Meier methods, and the differences between groups were tested for significance using the log-rank test. Statistical analysis was performed using SPSS ver. 22 (Chicago, IL, USA). Cox proportional hazard model was used to determine significant factors affecting the mortality.
Results
Baseline Patient Demographic Data
Table 1 shows the patient demographic data. The median age was 79.8 years (75–92 years), and 51.2 % of the patients were male. Patients’ performance status levels (0/1/2/3) were 164, 64, 20, and 0. Overall, 89 patients (35.6 %) had comorbidities, 63 had double cancer, 61 patients had rectal cancer, and the others had colon cancer. Average tumor size was 40.0 mm (3–105 mm). Lymph node metastases were present in 88 patients. TMN stages (0/1/2/3) were 21, 80, 68, and 81. Histological types were as follows: well differentiated, 126; moderately differentiated, 103; and poorly differentiated, 21. The average operation time was 240.2 min (63–860 min), and the average blood loss was 122.3 g (5–1550 g). A total of 155 patients underwent laparoscopic surgery, and 44 patients received perioperative chemotherapy. Postoperative complications occurred in 88 patients (35.2 %).
Table 1.
Clinicopathological characteristics of 250 colorectal cancer patients
| Number | 250 |
| Age (year) | 79 (75–92) |
| Sex (male/female) | 128:122 |
| Comorbidity (no/yes) | 161:89 |
| Performance status (0/1/2/3) | 164:64:20:0 |
| Double cancer (no/yes) | 187:63 |
| Location (C/A/T/D/S/R) | 27:56:27:11:55:74 |
| Tumor size (mm) | 40 (3–105) |
| Tumor depth (Tis/T1/T2/T3/T4) | 21:47:40:99:19 |
| Lymph node metastasis (N0/N1/N2) | 169:51:30 |
| Histological type (well/mod/poor) | 126:103:21 |
| TNM stage (0/I/II/III) | 21:80:68:81 |
| Operation time (min) | 240 (63–860) |
| Blood loss (g) | 122 (5–1550) |
| Laparoscopic surgery (no/yes) | 95:155 |
| Perioperative chemotherapy (no/yes) | 206:44 |
| Postoperative complication (no/yes) | 162:88 |
| Hospital stay (days) | 25.5 (12–112) |
Relationships between Patient Outcomes and Clinicopathological Features
Of the 250 patients, 30 patients died during the observation period. The patients were subdivided into two groups (dead, n = 30 or alive, n = 220). Table 2 shows the characteristics of each group. Age, sex, body weight, body mass index, performance status, tumor location, tumor size, tumor depth, and tumor markers including CEA and CA19-9 were not significantly different between the two groups. Comorbidities, the presence of double cancer, and lymph node metastases were significantly different between the groups (p < 0.01, p = 0.01, and p < 0.01).
Table. 2.
Relationship between patient outcomes and clinicopathological features
| Dead | Alive | p value | |
|---|---|---|---|
| Number | 30 | 220 | |
| Age (year) | 79.6 | 77.9 | 0.96 |
| Sex (male/female) | 11:19 | 117:113 | 0.08 |
| Body weight (kg) | 50.5 | 52.6 | 0.76 |
| Body mass index (kg/m2) | 23.3 | 23.5 | 0.91 |
| Comorbidity (no/yes) | 9:21 | 123:97 | <0.01 |
| Performance status (0/1, 2, 3) | 14:16 | 80:140 | 0.27 |
| Double cancer (no/yes) | 17:13 | 170:50 | 0.01 |
| Location (C/A/T/D/S/R) | 6:12:4:0:4:6 | 21:44:23:11:51:68 | 0.58 |
| Tumor size (mm) | 41.5 | 39.7 | 0.63 |
| Tumor depth (Tis/T1/T2/T3/T4a/T4b) | 3:3:3:17:2:2 | 20:50:42:90:13:5 | 0.35 |
| Lymph node metastasis (N0/1/2a/2b) | 8:8:14:1 | 161:43:14:1 | <0.01 |
| CEA | 8.1 | 9.1 | 0.69 |
| CA19-9 | 26.1 | 21.4 | 0.97 |
| Histological type (well/mod/poor) | 11:15:4 | 117:92:11 | 0.08 |
| Lymphatic invasion (no/yes) | 4:26 | 64:156 | 0.07 |
| Vessel invasion (no/yes) | 6:24 | 75:145 | 0.12 |
| Dukes classification (A/B/C) | 8:8:14 | 98:61:61 | 0.07 |
| Operation time (min) | 200.3 | 246.3 | 0.06 |
| Blood loss (g) | 127 | 81.3 | 0.04 |
| Laparoscopic surgery (no/yes) | 15:15 | 92:128 | 0.39 |
| Complication ablation (no/yes) | 27:3 | 196:24 | 0.88 |
| Perioperative chemotherapy (no/yes) | 24:6 | 182:38 | 0.71 |
| Postoperative complication (no/yes) | 16:14 | 144:76 | 0.19 |
| Hospital stay (days) | 25.7 | 25.5 | 0.93 |
| Prognostic score | |||
| GPS (0/1, 2) | 13:17 | 55:165 | 0.03 |
| PLR | 185.6 | 157.2 | 0.05 |
| CCI (0, 1/≥2 ) | 14:16 | 60:160 | 0.02 |
Surgical Features, Outcomes, and Scoring Systems
There were no significant differences in operation time, blood loss, the performance of laparoscopic surgery, perioperative chemotherapy, and postoperative complications. The pathological features including histological type, lymphatic invasion, and vessel invasion were not significantly different between the groups. In regard to the scoring systems, GPS, PLR, and CCI were significantly different (p = 0.03, p = 0.05, and p = 0.02).
Baseline Characteristics of Colorectal Cancer Patients Stratified by GPS, PLR, and CCI
As shown in Table 3, tumor depth, vessel invasion, and combined resection were significantly different according to the GPS score. Tumor depth, Dukes classification, lymphatic invasion, vessel invasion, operation time, and laparoscopic surgery were significantly different according to the PLR score. With the CCI score, only sex was significantly different.
Table 3.
Baseline characteristics of patients stratified by GPS, PLR, and CCI
| GPS | PLR | CCI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0, 1 | ≥2 | p | <136.4 | >136.4 | p | 0, 1 | ≥2 | p | ||
| Sex | Male | 113 | 15 | 67 | 61 | 82 | 46 | |||
| Female | 109 | 13 | 0.68 | 59 | 64 | 0.42 | 96 | 28 | 0.01 | |
| Location | Colon | 158 | 18 | 86 | 90 | 121 | 55 | |||
| Rectum | 64 | 10 | 0.89 | 39 | 35 | 0.55 | 55 | 19 | 0.18 | |
| Tumor | Tis-T2 | 113 | 7 | 75 | 45 | 83 | 37 | |||
| T3-T4 | 109 | 21 | <0.01 | 50 | 80 | <0.01 | 93 | 37 | 0.68 | |
| Dukes | A | 98 | 8 | 66 | 40 | 73 | 33 | |||
| B | 61 | 14 | 32 | 43 | 49 | 26 | ||||
| C | 63 | 6 | 0.07 | 28 | 41 | <0.01 | 52 | 17 | 0.65 | |
| CEA | <5 | 165 | 15 | 91 | 81 | 122 | 50 | |||
| ≥5 | 57 | 13 | 0.08 | 36 | 44 | 0.14 | 54 | 24 | 0.97 | |
| CA19-9 | <37 | 197 | 19 | 111 | 105 | 156 | 60 | |||
| ≥37 | 25 | 9 | 0.2 | 14 | 20 | 0.17 | 20 | 14 | 0.32 | |
| Histology | Well | 115 | 11 | 70 | 56 | 87 | 39 | |||
| Mod | 95 | 16 | 50 | 61 | 80 | 31 | ||||
| Por | 12 | 1 | 0.26 | 5 | 8 | 0.16 | 9 | 4 | 0.8 | |
| Lymph nodes | N0 | 153 | 16 | 91 | 78 | 118 | 51 | |||
| N1–3 | 69 | 12 | 0.53 | 34 | 47 | 0.06 | 58 | 23 | 0.53 | |
| ly | 0–1 | 153 | 17 | 92 | 78 | 122 | 48 | |||
| 2–3 | 69 | 11 | 0.91 | 33 | 47 | 0.04 | 54 | 26 | 0.66 | |
| v | 0–1 | 163 | 14 | 96 | 81 | 126 | 51 | |||
| 2–3 | 59 | 14 | 0.03 | 29 | 44 | 0.02 | 50 | 23 | 0.92 | |
| Stage | I-II | 153 | 16 | 91 | 78 | 118 | 51 | |||
| III | 69 | 12 | 0.53 | 34 | 47 | 0.06 | 58 | 23 | 0.53 | |
| Chemotherapy | yes | 41 | 3 | 23 | 31 | 31 | 13 | |||
| no | 186 | 22 | 0.09 | 102 | 104 | 0.7 | 145 | 61 | 0.44 | |
| Operation time (min) | 243 | 203 | 0.09 | 260 | 221 | 0.01 | 246 | 225 | 0.23 | |
| Bleeding (g) | 117 | 181 | 0.3 | 122 | 131 | 0.41 | 126 | 110 | 0.49 | |
| Laparoscopic surgery (no/yes) | no | 84 | 11 | 34 | 61 | 70 | 25 | |||
| yes | 138 | 17 | 0.63 | 91 | 64 | 0.02 | 106 | 49 | 0.24 | |
| Combined resection (no/yes) | no | 210 | 20 | 119 | 111 | 163 | 67 | |||
| yes | 12 | 8 | <0.01 | 7 | 14 | 0.2 | 13 | 7 | 0.92 | |
GPS Glasgow prognostic score, PLR platelet to lymphocyte ratio, CCI Charlson comorbidity index
Predictors of Mortality by Logistic Regression Analysis
Multivariate analysis using the clinicopathological factors that were selected according to the backward elimination method identified CCI ≥ 2 (hazard ratio [HR] = 5.24, 95 % confidence interval [CI] = 1.30–12.1, p = 0.01) as a significant determinant of postoperative complications (p < 0.01) (Table 4).
Table 4.
Predictors of mortality on logistic regression analysis
| HR | 95 % CI, lowest-highest CI | p value | |
|---|---|---|---|
| Sex male vs. female | 3.24 | 0.76–13.7 | 0.11 |
| Dukes b/c vs. a | 7.32 | 0.90–59.2 | 0.06 |
| CCI ≥2 vs. 0,1 | 5.24 | 1.30–12.1 | 0.01 |
HR hazard ratio, CI confidence interval, CCI Charlson comorbidity index
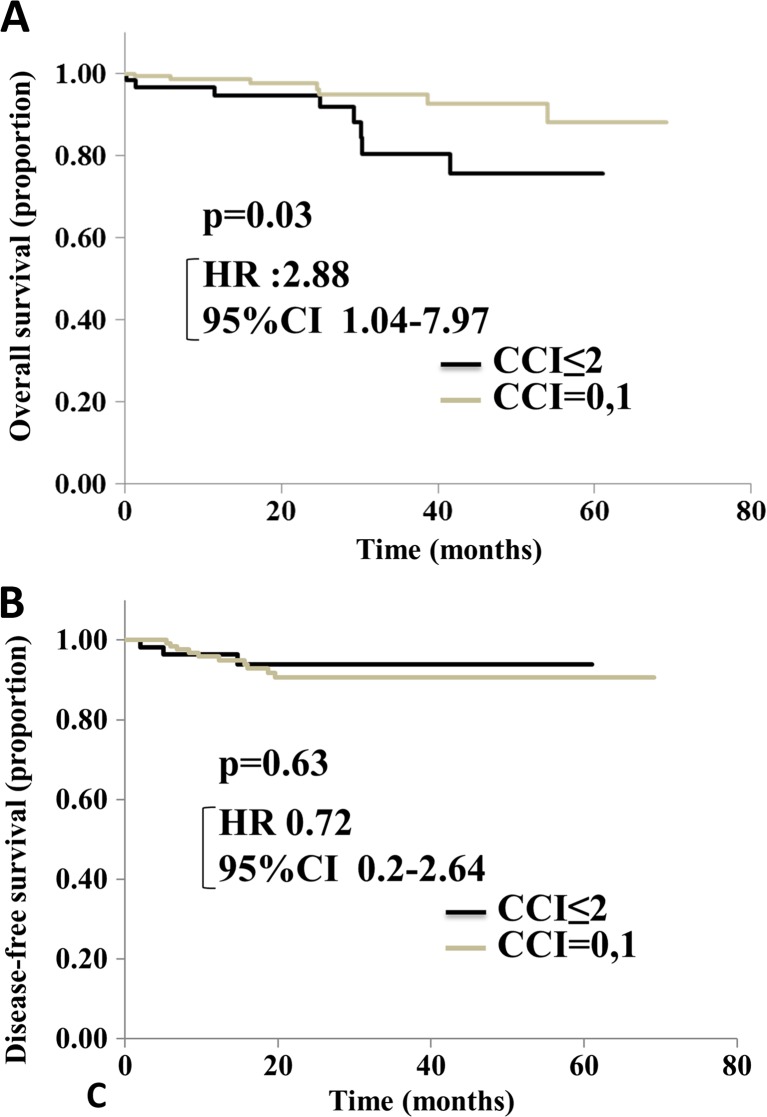
Kaplan-Meier Curves of DFS, OS, and CSS for CCI
The Kaplan-Meier method and log-rank tests showed that overall survival tended to be lower in patients with a high CCI score than in those with a low CCI score (Fig. 1a; p = 0.03). Disease-free survival was not significantly different between the groups (Fig. 1b; p = 0.63).
Fig. 1.
Overall survival (OS) (a) and disease-free survival (DFS) (b) after colorectal resection, according to the CCI score. There are significant differences in OS (p = 0.03). DFS shows no significant difference between the two groups (p = 0.63). HR hazard ratio; CI confidence interval
Discussion
This study verified that preoperative scoring systems could predict postoperative outcomes in high-age colorectal cancer patients and showed that CCI was the independent prognostic factor. This result supports the previous reports that showed the usefulness of the CCI score for predicting mortality in cancer of the digestive system. Dias-Santos and colleagues examined 497 pancreas cancer patients who underwent curative resection and they calculated the CCI scores. A high CCI score was a predictor of postoperative complications, longer hospital stay, and mortality within 1 year of pancreas resection [16]. In colorectal cancer, the CCI score was reported to correlate with long-term outcomes [17]. Colonic cancer patients with high CCI scores had a 28 % greater risk of death within 5 years. Rectal cancer patients with high CCI scores also had 14 times greater risk of death within 5 years. As previously described, high-age patients had several comorbidities prior to surgery. The CCI score could be useful for predicting patient prognosis in the older patient group. In the present study, however, the CCI score was not significantly correlated with postoperative complications (p = 0.72). With respect to postoperative complications, several reports showed the importance of operative factors, including operation time, severity, and amount of blood loss [18, 19]. The scoring of this study included only the preoperative factors, which is why the CCI was not significantly correlated with postoperative complications.
The GPS score that is calculated from the CRP and albumin levels has been demonstrated to be of prognostic value in several solid cancers [20]. In colorectal cancer, Choi and colleagues reported that patients with a high GPS score (GPS = 2) had poorer cancer-specific survival than those with a low GPS score (GPS = 0, 1) [21]. On multivariate analysis, GPS was identified as an independent prognostic factor in colorectal cancer (GPS 2; GPS 0 or 1; HR 5.168; p = 0.003). Contrary to the previous report, in the present study, the GPS score was not correlated with patient prognosis. There were relatively younger patients (72.3 % of patients were under 70 years) in Choi’s report. However, all patients in the present study were over 75 years. It has been reported that the older the individual, the higher the CRP level [22]. Cytokines such as IL-6 stimulate the production of CRP in hepatocytes [23]. High-age patients usually have more hypercytokinemia than younger patients because of comorbidities, including lung disease, heart disease, and malignancy [24]. It has been reported that serum albumin levels change with age, with a progressive reduction of the serum albumin concentration of between 0.08 and 0.17 g/L/year [25–27]. The same as the CRP level, hypercytokinemia also affects hypoalbuminemia [28]. In addition to high cytokine levels, several factors including low oral intake, loss of muscle mass, and many comorbidities, which were strongly correlated with high age, were related to a low level of albumin. This is because the GPS score, which consists of the CRP and albumin levels, could have some bias in high-age patients.
In fact, a high PLR level is reported to be associated with reduced OS and decreased time to recurrence [29]. However, the present result showed no significant correlation between the PLR level and mortality. The platelet count was not affected by age. However, the lymphocyte count has been reported to decrease with age because of weakness of the immune system [30]. For these reasons, inflammatory-based scores including the GPS and PLR could be less effective as prognostic markers in elderly patients than in younger patients.
There were some limitations in this study. First, the number of patients was relatively small, and the follow-up period was limited. Second, this was a retrospective, single-institution study. Third, prognosis was evaluated using only some inflammatory-based scores. There are other inflammatory-based scores such as the neutrophil to lymphocyte ratio, the derived neutrophil to lymphocyte ratio, and the lymphocyte to monocyte ratio [15]. Further large-scale studies and analyses are needed to clarify which score is most relevant for old individuals.
The CCI was useful to predict postoperative outcomes in high-age colorectal cancer patients. Inflammatory-based scores could be biased in older patients because of abnormalities in the immune, coagulation, and inflammatory systems.
Electronic supplementary material
(DOCX 14 kb)
Compliance with Ethical Standards
The work presented herein is original, has not been previously published in whole or in part, and is not under consideration for publication at any other journal.
Conflicts of Interest
No financial or other potential conflicts of interest exist for any of the authors.
Financial Disclosure
None.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12262-016-1544-4) contains supplementary material, which is available to authorized users.
References
- 1.Teriokhin AT, Budilova EV, Thomas F, et al. World wide variation in life-span sexual dimorphism and sex-specific environmental mortality rates. Hum Biol. 2004;76:623–641. doi: 10.1353/hub.2004.0061. [DOI] [PubMed] [Google Scholar]
- 2.Alves A, Panis Y, Mathieu P, et al. Postoperative mortality and morbidity in French patients undergoing colorectal surgery: result of a prospective multicenter study. Arch Surg. 2005;140:278–283. doi: 10.1001/archsurg.140.3.278. [DOI] [PubMed] [Google Scholar]
- 3.Mary EC, Peter P, Kathy LA, et al. A new method of classifying prognostic comorbity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 4.Trinchieri G. Cancer and inflammation: an old intuition with rapidly evolving new concept. Annu Rev Immunol. 2012;30:677–706. doi: 10.1146/annurev-immunol-020711-075008. [DOI] [PubMed] [Google Scholar]
- 5.Triantafillidis JK, Nasioulas G, Kosmidis PA. Colorectal cancer and inflammatory bowel disease: epidemiology,risk factors, mechanism of carcinogenesis and prevention strategies. Anticancer Res. 2009;29:2727–2737. [PubMed] [Google Scholar]
- 6.Marnell L, Mold C, Du Cos TW. C-reactive protein: ligands, receptors and role in inflammation. Clin Immunol. 2005;117:104–111. doi: 10.1016/j.clim.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 7.McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12:223–226. doi: 10.1097/MCO.0b013e32832a7902. [DOI] [PubMed] [Google Scholar]
- 8.Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6:146–163. doi: 10.2217/fon.09.136. [DOI] [PubMed] [Google Scholar]
- 9.Xie X, Luo KJ, Hu Y, et al. Prognostic value of preoperative platelet-lymphocyte and neutrophil-lymphocyte ratio in patients undergoing surgery for esophageal squamous cell carcinoma. Di Esophagus. 2014;19:10. doi: 10.1111/dote.12296. [DOI] [PubMed] [Google Scholar]
- 10.Trousseau A, Phlegmasia AD. Lectures on clinical medicine, delivered at the Holte-Dieu. Paris. 1865;5:281–332. [Google Scholar]
- 11.Katayama J, Yasuda K, Kawai K, et al. Circulating lymphocyte is an important determinant of the effectiveness of preoperative radiotherapy in advanced rectal cancer. BMC Cancer. 2011;11:16. doi: 10.1186/1471-2407-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ceze N, Thibault G, Goujoin G, et al. Pretreatment lymphopenia as a prognostic biomarker in colorectal cancer patients receiving chemotherapy. Cancer Chemother Pharmacol. 2011;68:1305–1313. doi: 10.1007/s00280-011-1610-3. [DOI] [PubMed] [Google Scholar]
- 13.Birim O, Kappetein AP, Bogers AJ. Charlson cormorbidity index as a predictor of long term outcome after surgery for non-small cell lung cancer. Eur J Cardiothoracic Surg. 2005;28:759–762. doi: 10.1016/j.ejcts.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 14.Smith RA, Bosonnet L, Raraty M, et al. Preoperative platelet lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg. 2009;197:466–472. doi: 10.1016/j.amjsurg.2007.12.057. [DOI] [PubMed] [Google Scholar]
- 15.McMillan DC, Crozier JE, Canna K, et al. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectum cancer. Int J Color Dis. 2007;22:881–886. doi: 10.1007/s00384-006-0259-6. [DOI] [PubMed] [Google Scholar]
- 16.Santos DD, Ferrone CR, Sheng H, et al. The Charlson age comorbidity index predicts early mortality after surgery for pancreatic cancer. Sirgery. 2015;157:881–887. doi: 10.1016/j.surg.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Wu CC, Hsu TW, Chang CM, et al. Age-adjusted Charlson comorbidity index scores as predictor of survival in colorectal cancer patients who underwent surgical resection and chemoradiation. Medicine. 2015;94:431. doi: 10.1097/MD.0000000000000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haga Y, Ikei S, Ogawa M. Estimation of physiologic ability and surgical stress (E-PASS) as a new prediction scoring system for postoperative morbidity and mortality following elective gastrointestinal surgery. Surg Today. 1999;29:219–225. doi: 10.1007/BF02483010. [DOI] [PubMed] [Google Scholar]
- 19.Prytherch DR, Whiteley MS, Higgins B, et al. POSSUM and Portsmouth POSSUM for predicting mortality. Physiological and operative severity score for the enumeration of mortality and morbidity. Br J Surg. 1998;85:1217–1220. doi: 10.1046/j.1365-2168.1998.00840.x. [DOI] [PubMed] [Google Scholar]
- 20.Forrest LM, McMillan DC, McArdle CS, et al. Evaliiation of cumulative prognostic scores based on the systemic inflammatory response I patients with inoperable non-small-cell lung cancer. Br J Cancer. 2003;89:1028–1030. doi: 10.1038/sj.bjc.6601242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi KW, Hong SW, Chang YG, et al. Inflammation-based score (Glasgow prognostic score) as an independent prognostic factor in colorectal cancer patients. Ann Surg Treat Res. 2014;86:309–313. doi: 10.4174/astr.2014.86.6.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aparecida SC, Fattori A, Sousa AL, et al. Determining the C-reactive protein level in patients with different clinical forms of Chagas disease. Rev Esp Cardiol. 2010;63:1096–1099. doi: 10.1016/S0300-8932(10)70233-5. [DOI] [PubMed] [Google Scholar]
- 23.McMillan DC, Canna K, McArdle CS. Systemic inflammatory response predicts survival following curative resection of colorectal cancer. Br J Surg. 2003;90:215–219. doi: 10.1002/bjs.4038. [DOI] [PubMed] [Google Scholar]
- 24.Cheng CC, Sun CF, Pai Wang WK, et al. Preoperative serum C-reactive protein and gastric cancer ; clinical-pathological correlation and prognostic significace. Chang Gung Med J. 2010;33:301–311. [PubMed] [Google Scholar]
- 25.Cooper JK, Gardner C. Effect of aging on serum albumin. J Am Geriaty Soc. 1989;37:1039–1042. doi: 10.1111/j.1532-5415.1989.tb06917.x. [DOI] [PubMed] [Google Scholar]
- 26.Gom I, Fukushima H, Shiraki M, et al. Relationship between serum albumin level and aging in community-dwelling self-supported elderly populations. J Nutr Sci Vitaminol. 2007;53:37–42. doi: 10.3177/jnsv.53.37. [DOI] [PubMed] [Google Scholar]
- 27.Sahyoun NR, Jacques PF, Dallal G, et al. Use of albumin as a predictor of mortality in community dwelling and institutionalized elderly populations. J Clin Epidemiol. 1996;49:981–988. doi: 10.1016/0895-4356(96)00135-7. [DOI] [PubMed] [Google Scholar]
- 28.Cabrerizo S, Cuadras D, Gomez-Busto F, et al. Serum albumin and health in older people: review and meta analysis. Maturitas. 2015;81:17–27. doi: 10.1016/j.maturitas.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Kwon HC, Kim SH, Oh SY. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers. 2012;17:216–222. doi: 10.3109/1354750X.2012.656705. [DOI] [PubMed] [Google Scholar]
- 30.Jain S, Harris J, Ware J. Platelets: linking hemostasis and cancer. Arterioscler Thromb Vasc Biol. 2010;30:2362–2367. doi: 10.1161/ATVBAHA.110.207514. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 14 kb)