Abstract
A plethora of solid substrates, cultivation conditions and enzyme assay methods have been used for efficient production and estimation of polygalacturonase and pectin methylesterase enzymes. Recent developments in industrial biotechnology offer several opportunities for the utilization of low cost agro-industrial waste in Solid State Fermentation (SSF) for the pectinolytic enzyme production using fungi. Fruit waste mainly citrus fruit waste alone and along with other agro-industrial waste has been explored in SSF for enzyme production. Agro-industrial waste, due to the economic advantage of low procuring cost has been employed in SSF bioreactors for pectinolytic enzyme production. Acidic pectinases produced by fungi are utilized especially in food industries for clarification of fruit juices. This review focuses on the recent developments in SSF processes utilizing agro-industrial residues for polygalacturonase and pectin methylesterase production, their various assay methods and applications in fruit juice industries.
Keywords: Agro-industrial waste, Enzyme assay, Juice clarification, Pectinolytic enzymes, Solid state fermentation
Introduction
Pectinolytic enzymes are a group of related enzymes that hydrolyse pectic substances. Pectin is a complex polysaccharide present in the middle lamella of plant cell walls. It is composed of multiple units of D-Galacturonic acid linked by α (1, 4) glycosidic linkage. Pectinolytic enzymes have been reported in higher plants (Nighojkar et al. 1994; Jolie et al. 2010) and microorganisms including bacteria and fungi (Uzuner and Cekmecelioglu 2015; Patidar et al. 2016; Rebello et al. 2017). Pectin is completely digested by three major enzymes: pectin methylesterase (pectinesterase; EC: 3.1.1.11), pectinase (polygalacturonase; EC: 3.1.1.15) and pectin lyase (EC: 4.2.2.10) to release galacturonic acids and its oligomers (Combo et al. 2012). In nature, microorganisms have been endowed with vast potential. They produce a range of enzymes, which have been exploited commercially over the years. It has been reported that microbial enzymes account for 25% of total global enzyme sales (Jayani et al. 2005). The pectic substances can be converted by means of microorganisms or their enzymes into constituent monosaccharides or specific oligosaccharides without the production of undesirable by-products (Zykwinska et al. 2008; Martínez et al. 2009).
Pectinases are known for their tremendous potential in various industries. Pectin methylesterase and endo-polygalacturonase have important role in softening of fruits, extraction and clarification of juices, preparing gel, food manufacturing, retting of textile fibers, extraction of olive oil, protoplast isolation, etc. (Kashyap et al. 2001; Jayani et al. 2005; Kohli and Gupta 2015). Galacturonic acid, produced by action of pectinolytic enzymes, has various applications in industries mainly in pharmaceutical industries. It is used for the production of vitamin C as acidic agent in food industries and as washing powder agent in chemical industries (Molnar et al. 2009; Burana-Osot et al. 2010). Almost all the commercial preparations of pectinases are produced from fungal sources (Kertesz 1951). Filamentous fungi especially Aspergillus niger is the major producer of acidic pectinase used mainly in fruit juice and wine industries (Kashyap et al. 2001).
The utilization of SSF processes is interesting for pectinase production by fungi and has the advantages of low water requirement, high productivity, lesser chance of contamination, cost effectiveness and simpler fermentation technology (Pandey et al. 2000; Viniegra-González et al. 2003). Research on the selection of suitable solid substrates for SSF has mainly been centered on agricultural and industrial residues due to their potential advantages for filamentous fungi, which are capable of penetrating into the hardest of these solid substrates. In addition to the utilization of this agro-industrial waste, it provides alternative substrates and helps in solving the pollution problems (Pandey 2003).
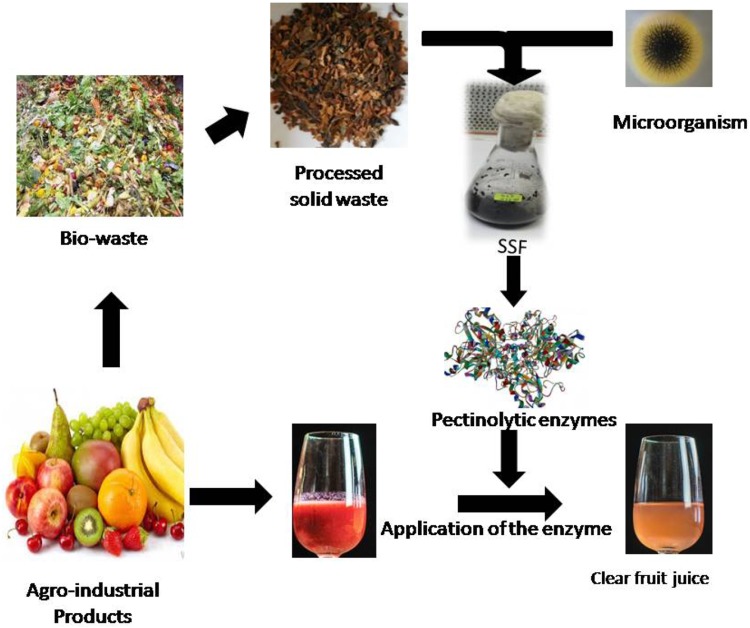
Due to the wide applications of polygalacturonase and pectin methylesterase, there is a need to highlight recent developments on several aspects related to their production in SSF. Microbial sources, production, characterization and application of pectinases have been reviewed (Kashyap et al. 2001; Jayani et al. 2005; Favela-Torres et al. 2006; Sharma et al. 2013). However, the most common sources of pectinolytic microorganisms, recent development in SSF process for polygalacturonase and pectin methylesterase production, and their assay methods have not been considered until now. Therefore, these aspects have been focused in the present review and described in Fig. 1. The applications of these enzymes in fruit juice clarification have also been discussed in the present review.
Fig. 1.
An overview of the pectinolytic enzyme production and their application for fruit juice clarification
Pectinolytic enzymes
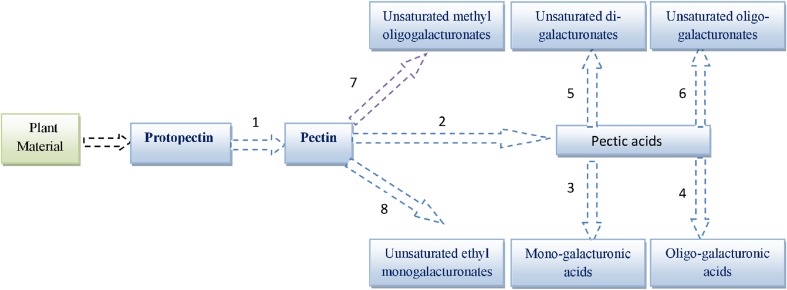
Pectinolytic enzymes are of great interest in industrial applications owing to their significant role in fruit juice industries. The pectinolytic enzymes can be divided into three main groups on the basis of their action on substrate as follows (Fig. 2):
Fig. 2.
Enzymatic reactions catalyzed by pectinolytic enzymes. 1. Protopectinase 2. Pectin methylesterase 3. Exo-polygalacturonase 4. Endo-polygalacturonase 5. Exo-polygalacturonate lyase 6. Endo-polygalacturonate lyase 7. Endo-polymethylgalacturonate lyase 8. Exo-polymethylgalacturonate lyase
Protopectinases
Protopectinases (3.2.1.99) degrade the insoluble protopectin which is present in unripe fruits and give rise to highly polymerized soluble pectin (Tapre and Jain 2014). The protopectinase name was originally given by Briton et al. (1927). Pectinosinase is also synonymous with protopectinase.
Pectin methylesterases
Pectin methylesterases (3.1.1.11) are responsible for the removal of methoxyl esters, resulting in acid pectins and methanol (Parmar and Rupasinghe 2013). Pectin methylesterase, often referred to as pectin pectylhydrolase, pectinesterase, pectase, pectin demethoxylase and pectolipase, is a carboxylic acid esterase and belongs to the hydrolase group of enzymes (Whitaker 1984). Fungal pectin methylesterase acts by a multi-chain mechanism, removing the methyl groups at random. In contrast, plant pectin methylesterases tend to act either at the non-reducing end or next to a free carboxyl group, and proceed along the molecule by a single chain mechanism.
Polygalacturonases
Polygalacturonases (3.2.1.15) are the pectinolytic enzymes that catalyze the hydrolytic cleavage of the polygalacturonic acid chain in the presence of water (Kant et al. 2013; Rebello et al. 2017). They are most extensively studied among the family of pectinolytic enzymes. The polygalacturonases are classified into two classes; endo-polygalacturonase (E.C. 3.2.1.15) and exo-polygalacturonase (E.C. 3.2.1.67). Endo-polygalacturonase hydrolyses polygalacturonic acids and liberates oligogalacturonic acids. Exo-polygalacturonase hydrolyzes pectic acids and liberates mono-galacturonate.
Pectin lyases
Lyases (or transeliminases) perform non-hydrolytic breakdown of pectates or pectinates, characterized by a trans-eliminative split of the pectic polymer (Jayani et al. 2005). The lyases break the glycosidic linkages at fourth carbon and simultaneously eliminate hydrogen from fifth carbon, producing a unsaturated product. Polygalacturonate lyases (Pectate lyases) are produced by many bacteria and some pathogenic fungi with endo- polygalacturonate lyases being more abundant than exo-polygalacturonate lyases. Polygalacturonate lyases have been isolated from bacteria and fungi associated with food spoilage and soft rot.
Lyases can be classified into four types on the basis of the pattern of action namely Endo-polygalacturonate lyase (Endo PGL; E.C. 4.2.2.2), Exo-polygalacturonate lyase (ExoPGL, E.C. 4.2.2.9), Endo-polymethylgalacturonate lyase (Endo PMGL; E.C. 4.2.2.10) and Exo-polymethylgalacturonate lyase (Exo PMGL).
Production system
SSF is defined as the fermentation process which is carried out in absence or near absence of free water. SSF and Submerged fermentation (SmF) processes have been widely used for pectinolytic enzyme production by different types of microorganisms. SSF is considered more suitable for fungi than for bacteria (Pandey 2003; Favela-Torres et al. 2006; Kumar et al. 2012).
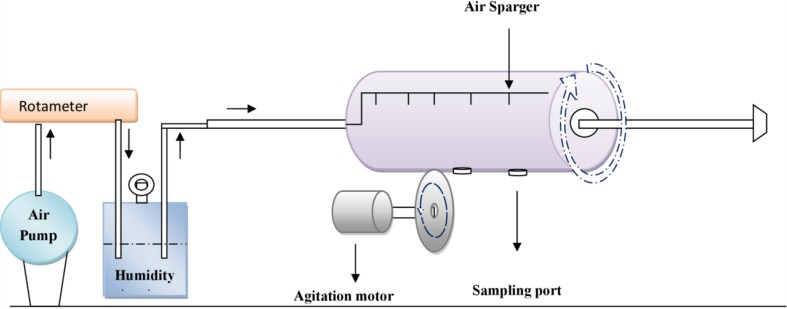
Agro-industrial waste is utilized in SSF as medium for metabolite production mainly by fungi. The capability of fungi to grow and utilize agro waste as its natural habitat makes them more interesting for their use in SSF processes. Fungi have been considered to be the organisms most adapted to SSF because their hypha can grow on particle surfaces and penetrate into the inter particle spaces thereby colonizing the solid substrates (Fig. 3). In contrast, in SmF the nutrients and microorganisms are both submerged in water and, therefore, SmF is suitable for bacterial isolates (Graminha et al. 2008). However, availability of several research articles proves that bacterial cultures can also be well manipulated and managed for SSF processes even for scarcely produced pectinolytic enzymes (Gupta et al. 2008; Kashyap et al. 2003).
Fig. 3.
Growth of fungi (A. tubingensis MP 30) on solid medium for production of pectinolytic enzymes (Patidar et al. 2016)
Comparative analysis of SSF and SmF process for fungal pectinase production reported higher production by A. niger in the former (Patil and Dayanand 2006). Similarly, the production of pectin methylesterase and Endo-polgalacturonase by A. niger has been studied in SmF and SSF systems by Maldonado and Strasser de Saad (1998). They reported four times higher pectin methylesterase production in SSF than in SmF system. Similarly polygalacturonase production was six times higher in SSF than in SmF system and it required a shorter time for enzyme production. Advantages of SSF over SmF are summarized in Table 1.
Table 1.
Advantages and disadvantages of SSF over SmF (Raimbault 1998; Hölker et al. 2004; Couto and Sanroma 2006)
| Advantages | Disadvantages |
|---|---|
| Low-cost media | Difficulties on scale-up |
| Higher productivity | Mixing of nutrients not uniform |
| Better oxygen circulation | Problems with heat build-up |
| Catabolite repression is negligible | Higher impurity product |
| Absence of foam formation so no requirement of antifoaming agent | Recovery cost is high |
| It resembles the natural habitat for several microorganisms | Difficult control of process parameters |
| Less effort in downstream processing | |
| Simple technology | |
| Less energy demand for heating | |
| Fermentation of water in-soluble material | |
| Requires smaller reactor volume | |
| Less chance of contamination |
Pectinolytic microorganisms and SSF conditions
Pectinolytic microorganisms
Pectinolytic enzymes are widely distributed among fungi and bacteria. They are also found in higher plants, parasitic plants and some plant parasitic nematodes (Shrivastava et al. 1994; Jayani et al. 2005). Sources of pectin degrading microorganisms have been listed in Table 2. Many workers have used several fungal strains in SSF for industrial production of pectinolytic enzymes. Most widely used Aspergillus sps. for polygalacturonase production are A. sojae (Heerd et al. 2012; Demir and Tari 2014), A. niger (Dhillon et al. 2007; Ruiz et al. 2012; Darah et al. 2013; Trentini et al. 2015; Anand et al. 2017; Mahmoodi et al. 2017; Patidar et al. 2017), A. fumigatus (Sandri et al. 2015; Wong et al. 2017), A. sydowii (Singh and Mandal 2012), A. tubingensis (Tai et al. 2014; Patidar et al. 2016), A. carbonarius (Nakkeeran et al. 2011), A. niveus (Maller et al. 2011), A. awamori (Botella et al. 2005; Diaz et al. 2012) and A. oryzae (Meneghel et al. 2014; Biz et al. 2016). In recent reports, bacterial isolates Bacillus subtilis SAV-21 (Kaur and Gupta 2017) and Bacillus licheniformisusin (Pervez et al. 2017) have been reported to produce pectinase in SSF. Pereira et al. (2017) reported edible mushroom Pleurotussajor-caju for the production of pectinase in SSF. A yeast Saccharomyces cerevisiae, isolated from fruit and industrial waste, has been applied for pectinase production using SSF (Poondla et al. 2016).
Table 2.
Sources of pectinolytic microorganisms
| Source | Microorganism | References |
|---|---|---|
| Agricultural waste |
Aspergillus sp. Penicillium sp. |
Zeni et al. (2011) |
| Decomposing passion fruit peels | Aspergillus oryzae | Biz et al. (2016) |
| Decaying orange peel | Asergillus fumigatus | Phutela et al. (2005) |
| Soil of plum orchid waste site | Aspergillus sp. | Sunnotel and Nigam (2002) |
| Rotten orange | Aspergillus niger | Darah et al. (2013) and Mahmoodi et al. (2017) |
| Mango industrial waste | Fusarium sp. | Reddy and Saritha (2015) |
| Rotten mango | Aspergillus niveus | Maller et al. (2011) |
| Citrus fruit peels | Aspergillus niger | Martos et al. (2009) |
| Agro waste dumping pit soil | Aspergillus niger | Patil and Dayanand (2006) |
| Oak (Quercus spp) | Penicillium pinophilum | Ruiz et al. (2012) |
| Olive paste and olives |
Aspergillus niger
Aspergillus fumigatus |
Sanchez et al. (2015) |
| Soil of fruit processing site |
Aspergillus niger
Aspergillus tubingensis |
Patidar et al. (2016, 2017) |
| Fruit waste disposal site | Pseudozyma sp. | Sharma et al. (2014) |
| Pulp and paper mill, paper mulberry bark, vegetables and fruits |
Erwinia carotovora
Erwinia chrysanthemi Bacillus sp. |
Sittidilokratna et al. (2007) |
| Agricultural and vegetable waste | Bacillus sp. | Soares et al. (1999) |
| Rotten vegetable | Bacillus licheniformis | Rehman et al. (2014) |
| Soil sample | Bacillus pumilus | Sharma and Satyanarayana (2006) |
| Soil, water, rotten fruit and vegetables | Bacillus sp. | Tariq and Latif (2012) |
| Soil contaminated with effluents of paper and pulp industry | Bacillus subtilis | Kaur et al. (2011) |
| Decomposing kitchen waste | Bacillus subtilis RCK | Gupta et al. (2008) |
| Glass fibre microfilters | Geotrichum klebahnii | Zapata and Voget (2012) |
In microorganisms, pectin methylesterase is mainly found in fungi, such as A. tubingensis (Patidar et al. 2016), A. aculeatus (Duvetter et al. 2005), A. niger (Van Alebeek et al. 2003; Hasunuma et al. 2003; Joshi et al. 2006; Jiang et al. 2013), Fusarium asiaticum (Glinka and Liao 2011), Penicillium notatum (Gayen and Ghosh 2011), Fusarium oxysporum (Miller and Macmillan 1971) and Botrytis cinerea (Valette-Collet et al. 2003). It is also reported in bacteria such as Xanthomonas sp., Bacillus sp. and in very few yeast such as Saccharomyces cerevisiae and Candida boidinii (Nakagawa et al. 2000; Jayani et al. 2005; Rehman et al. 2014; Kohli et al. 2015).
However, there are very few reports available which show the use of microorganisms in SSF for pectin methylesterase production (Gayen and Ghosh 2011; Patidar et al. 2016). Aspergillus sp. and Penicillium sp. have been used in SSF for pectin methylesterase production (Maldonado and Strasser de Saad 1998; Taragano and Pilosof 1999; Joshi et al. 2006; Gayen and Ghosh 2011). Pectin methylesterase and polygalacturonase production has been recently reported in SSF by A. tubingensis MP30 and A. niger AN07, respectively (Patidar et al. 2016, 2017).
SSF process conditions
Solid medium
The choice of medium for the production of pectinolytic enzymes can highly affect the yield, as well as the cost of the final product. The most economical and environmental friendly choice as solid medium for high enzyme production is agro-industrial waste.
Agricultural waste products and waste from bio-refineries provide abundant possibilities for novel low-cost production media. In general, agricultural and food processing waste consists of large amounts of organic matter with high nutrient value (Table 3) and, therefore, have been used in SSF for the production of polygalacturonase and pectin methylesterase enzymes. Agro wastes utilized as solid medium in SSF are listed in Table 4. In present review, agro-industrial waste has been classified into two categories namely crop waste and fruit waste.
Table 3.
Nutritional value of important agro-industrial waste
| Components | Lemon pulp | Orange peel | Wheat bran | Grape skin | Sugarcane bagasse | Orange bagasse | Apple pomace | Rice bran | Olive pomace |
|---|---|---|---|---|---|---|---|---|---|
| Crude fiber | 25.78 | NA | 66.12 | NA | NA | 41.5 | 51.1% | 10.10% | NA |
| Crude protein | 3.89 | 7.7 | 15.73 | NA | NA | 4.0 | 4.0% | 12.26% | NA |
| Total sugars | 37.59 | 43.1 | 2.34 | NA | NA | NA | 9.5–22.0% | 43.29 | NA |
| Pectin | NA | 14.7 | NA | 1.3 | NA | NA | 7.2% | NA | 5.8 |
| Cellulose | NA | 16.7 | NA | 25.9 | 35.2 | NA | 6.8% | NA | 22.9 |
| Hemicellulose | NA | 11.8 | NA | 3.6 | 24.5 | NA | NA | NA | 5.9 |
| Starch | NA | NA | NA | NA | NA | 7.1 | NA | NA | NA |
| Lignin | NA | 1.2 | NA | 22.5 | 22.2 | NA | NA | NA | 22.6 |
| Ash | 3.1–4.0 | 1.2 | 3.24 | 6.5 | 20.9 | 2.6 | 0.5% | 10.63 | 3.3 |
| Moisture | 7.00 | 2.31 | 9.36 | 5.6 | NA | 9.9 | 10.8% | 13% | 3.4 |
| Fat | 3.46 | 3.6 | – | 9.8 | NA | 0.6 | NA | 24.60% | 15.9 |
| Reference | De Gregorio et al. (2002) and Ruiz et al. (2012) | Zhou et al. (2011) | Demir and Tari (2014) | Sanchez et al. (2015) | Rezende et al. (2011) | Romero-Lopez et al. (2011) | Shalini and Gupta (2009) and Sudha et al. (2007) | Satter et al. (2014) | Sanchez et al. (2015) |
Table 4.
Agro-industrial waste and their fermentation conditions used in SSF for pectinolytic enzyme production
| Solid substrate | Organism | Temp | Inoculum size | Moisture content | Particle size | Type of fermenter | Fermentation time | Agitation per day | Enzyme activity | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Wheat bran | Aspergillus sojae | 37 °C | 107 spore/g substrate | 62% | 100–250 µm | Flask type | 4 days | 3 | 535.4 U/g | Demir and Tarı (2014) |
| Wheat bran | Aspergillus sojae | 37 °C | 107 spore/g substrate | 62% | 100–250 µm | Tray type | 4 days | Static | 298 U/g | Demir and Tarı (2016) |
| Wheat bran | Aspergillus niger | 30 °C | 107 spores per g | 29.6% (v/w) | Unsieved | Double surface bioreactor | 96 h | Static | 45 U/g | Hendges et al. (2011) |
| Wheat bran | Bacillus subtilis | 35 °C | 2.7a | 1:7 | Unsieved | Flask reactor | 48 h | Shaking | 5769.2 IU/g | Gupta et al. (2008) |
| Wheat bran and soy bran | Aspergillus niger | 30 °C | 1 × 108 spores | 40% | NA | Flask type | 22 h | Static | Castilho et al. (2000) | |
| Wheat bran and orange peel | Aspergillus sps. | RT | 2 × 107 spores | 120% | Fine particle | Flask reactor | 5 days | Static | 23 U/g | Heerd et al. (2012) |
| Wheat bran and sugar beet pulp | Aspergillus sojae | 30 °C | 2 × 107 spores/10 g | 160% | 90% < 630 μm) | Flask reactor | 8 days | Static | 909.5 U/g | Heerd et al. (2014) |
| Wheat bran, rice bran and apple pomace | Bacillus sp. DT7 | 37 °C | NA | 75% | Unsieved | Flask reactor | 36 h | Static | 8050 U/g | Kashyap et al. (2003) |
| Orange bagasse, sugar cane bagasse and wheat bran | Thermoascus aurantiacus | 65 °C | 5 mg dry mycelial mass/g | 1:2 (v/w) | NA | Flask reactor | 6 days | Static | 43 U/g | Martins et al. (2002) |
| Orange bagasse and wheat bran | Penicillium viridicatum | 28 °C | 107 spores/g | 70%/80% | Unsieved | Flask/polypropylene packs | 96 h | Static | 0.70 U/ml | Silva et al. (2005) |
| Apple pomace | Aspergillus niger | 35 °C | 5 × 108/ml | <50% | – | Stirred tank reactor | 70 h | Static | – | Berovic’and Ostrovers’nik (1997) |
| Strawberry pomace | Lentinus edodes | 25 °C | – | 1:2 (v/w) | – | Flask reactor | 40 days | static | 29.4 U | Zheng and Shetty (2000) |
| Grape pomace | Aspergillus awamori | 30 °C | 5 × 105 spores/g | 60% | Flask reactor | 7 day | Static | 0.113 IU/gds | Botella et al. (2005) | |
| Pomelo citrus grandis | Aspergillus niger | 30 °C | 1 × 107 spores/ml | 1:1 (v/w) | 0.75 mm | Flask reactor | 5 days | Static | 8.90 U/g | Darah et al. (2013) |
| Citrus peel | Aspergillus niger | 30 °C | 10% | 1:10 (v/w) | Unsieved | Flask type | 120 h | Static | 0.940 U/ml | Dhillon et al. (2007) |
| Grape pomace and orange peel | Aspergillus awamori | 30 °C | 4.5 × 108 spores/g | 70% | Unsieved | Flask | 4 days | Static | 16.9 IU/g | Diaz et al. (2012) |
| Grape pomace and orange peel | Aspergillus awamori | 30 °C | 4.5 × 107 spores | 70% | 1 mm (OP) | Packed bed and tray-type | 5 days | Static | 4 IU/g | Diaz et al. (2013) |
| Lemon peel | Aspergillus niveus | 40 °C | 5 × 106 conidia/ml | 1:2 (v/w) | Unsieved | Flask reactor | 5 days | Static/agitated | 125U/140U | Maller et al. (2011) |
| Sunflower head | Aspergillus niger | 34 °C | 1 × 105 spores/ml | 65% | 500 µm | Flask reactor | 96 h | Static | 19.8U/g | Patil and Dayanand (2006) |
| Lemon peel pomace | Aspergillus niger | 30 °C | 2 × 107 spores/ml | 70% | 2–0.7 mm | Column tray bioreactor | 96 h | Static | 2181 U/L. | Ruiz et al. (2012) |
| Rice husk | Aspergillus tubingensis | 35 °C | 5 × 106 spores | 1:5 (v/w) | Unsieved | Flask type | 3 days | Static | 15.9 U/mL | Tai et al. (2014) |
| Papaya peel | Aspergillus tubingensis | 30 °C | 1 × 107 | 86% | 2 mm | Flask type | 120 h | Static | 246.83 U/g | Patidar et al. (2016) |
| Sugarcane bagasse | Aspergillus niger | 35 °C | 0.3 g/cm3 | 70% | NA | Flask type | 72 h | Static | NA | Acuna-Arguelles et al. (1994) |
| Sugar beet pulp | Bacillus gibsonii | 35 °C | NA | 1:3 (v/w) | NA | Flask type | 48 h | Shaking | 3600 U/g | Li et al. (2005) |
| Mango peel | Aspergillus foetidus | 35 °C | 2 × 107 spores/g | 1:1 (v/w) | Unsieved | Flask type | 120 h | Static | NA | Kumar et al. (2012) |
| Cassava bagasse | Bacillus subtilis | 50 °C | 1 × 108 CFU/ml | 70% | Unsieved | Flask type | 6 days | Static | 228.5 U/g | Swain et al. (2009) |
| Bingtang sweet oranges | Eupenicillium javanicum | 30 °C | 106–107/ml | 80% | Unsieved | Flask type | 96 h | Static | 51.87 U/g | Tao et al. (2011) |
RT room temperature
aOD at 600 nm
Crop waste
Wheat bran Wheat bran is one of the most popular agro-industrial residue preferred by many researchers to produce enzymes from various microorganisms in SSF (Balkan and Ertan 2010; Freitas et al. 2006; Demir and Tari 2014). According to a report of United States Department of Agriculture (USDA 2013), a total of 655,270,000 tons of wheat were produced in the period 2012–2013 in the world. Since about 15–20% of wheat bran was reported to be discarded during wheat flour production process (Dobrev et al. 2007, Demir and Tari 2014), it has been accepted as a sustainable by-product for the microbial production of industrially important enzymes in SSF. In recent reports, wheat bran along with tea extract has been used for the production of polygalacturonase under SSF condition (Anand et al. 2017). Jahan et al. (2017) reported high yield of polygalacturonase in fermentation broth having 1.0% wheat bran as a substrate supplemented with NaNO3 and yeast extract as source of nitrogen and KH2PO4 was selected as suitable micronutrient. Wheat bran along with orange peel and lemon peels has been reported as best substrate combination for pectinase production by Aspergillus giganteus NRRL10 (Ortiz et al. 2017). Demir and Tari (2016) have reported the effect of moisture content on wheat bran thickness, which affects polygalacturonase production in SSF. In earlier reports, Demir and Tari (2014) have used wheat bran as most suitable solid substrate without the addition of any nutritive supplement for the production of polygalacturonase in SSF by mutant strain of A. sojae. Heerd et al. (2014) reported enhanced pectinase production by A. sojae in SSF using wheat bran supplemented with 30% sugar beet pulp as inducer. Various other microorganisms such as A. niger (Hendges et al. 2011; Heerd et al. 2012), Bacillus sp. (Kashyap et al. 2003; Gupta et al. 2008) have been used in SSF for polygalacturonase production using wheat bran as solid substrate. Wheat bran has also been mixed with other agro-industrial waste such as orange bagasse (1:1w/w), orange peel (7:3w/w) and soy bran (1:1w/w) for increased production of pectinolytic enzyme in SSF (Silva et al. 2005; Heerd et al. 2012; Castilho et al. 2000).
Rice husk and rice bran Rice husk and rice bran, the main by-products of rice processing industries, are widely used agricultural wastes employed in various metabolite productions, thus reducing the environmental impact associated with improper disposal of it. Rice husk has been used in SSF for production of polygalacturonase and feruloyl esterase (Tai et al. 2014). Rice husk, supplemented with (g/l) pectin, 10; sucrose, 20; K2HPO4, 1.0; NaNO3, 30; MgSO4·7H2O, 5.0; KCl, 10; FeSO4·7H2O, 0.01 and pH 4.0 has been used as solid carrier for the production of polygalacturonase (Tai et al. 2014). Wong et al. (2017) has reported utilization of rice bran as solid substrate for polygalacturonase production by newly isolated A. fumigatus R6.
Sugarcane bagasse Sugarcane bagasse serves as an ideal substrate in microbial processes for the production of value-added products, due to its abundant availability (Pandey et al. 2000). It has been impregnated with high glucose concentration solution for pectinase production using SSF in a packed-bed column fermenter (Solis-Pereyra et al. 1996). The effect of water activity on pectinase production has been reported using bagasse impregnated with a medium containing pectin and sucrose (Acuna-Arguelles et al. 1994).
Maldonado and Strasser de Saad (1998) used sugarcane bagasse as solid substrate for the production of polygalacturonase and pectin methylesterase. The fermentation medium was composed of (g/l): urea, 0.3; K2HPO4, 0.65; (NH4)2SO4, 1.26; MgSO4, 0.02; FeSO4, 0.029; pectin, 1.5; sugar cane bagasse as support, 23.1. The pH of the medium was adjusted to 4.5.
Sunflower head The northern part of India is a semi arid tropical region, well known for the production of oil seed sun flower, which is cultivated in about 30,000 hectare area. Deseeded dried sun flower head is burnt to ash after harvesting. Patil and Dayanand (2006) reported pectinase production in SSF and SmF using deseeded sunflower head. Higher productions of polygalacturonase were observed when the medium was supplemented with 6% sucrose as carbon source and 0.3% ammonium sulphate as nitrogen source in SSF.
Onion waste India is the second largest producer of vegetables with a global production share of 14%. According to the Ministry of Agriculture, Govt. of India, India alone produces 16813 metric tones of onion per year in area of 1052 hectare (IHD 2014). According to the FAO (Food and Agriculture Organization of the United Nations), Europe produces about 600 000 tons of onion waste per year (FAO 2013). The onion waste cannot be discarded in landfills due to enhanced growth of phyto-pathogens (Ng et al. 1998). Dried onion waste has been characterized and % of pectin was found to be 3.25. Utilization of onion waste has been reported for the production of pectinase in SSF by edible mushroom Pleurotus sajor-caju (Pereira et al. 2017).
Fruit waste
Papaya peel Papaya (Carica papaya L.) family Caricaceae is grown in Australia, Hawaii, Philippines, Sri Lanka, South Africa, India, Bangladesh, Malaysia and a number of other countries in tropical America (OECD 2005). South Mexico and Costa Rica are its origins. According to the Indian Horticulture Board, Ministry of Agriculture, Govt. of India, India alone produces a total of 56,39,000 metric tons of papaya every year (IHD 2014). Papaya due to its high vitamin content (vitamin A and C) and high fibre content is extensively utilized worldwide for fruit juice preparation, salad preparation and also in cosmetics and medications (Ittimongkol et al. 2002; Almora et al. 2004; Silva et al. 2007). The by-product of papaya is its peel, approximately 20–25% of the fruit weight, which can be used as animal feed but is generally discarded into the environment causing organic pollution (Koubala et al. 2014). The papaya processing units often dispose off these papaya peels.
Papaya peel has huge amounts of monosaccharides, pectin and protein which facilitate the growth of fungus (Chaiwut et al. 2010; Maran and Prakash 2015). The preferred substrate of A. niger for the production of pectin methylesterase is partially esterified pectin (Van Alebeek et al. 2003). Papaya peel contains partially esterified (45–51%) pectin (Boonrod et al. 2006). Utilization of this by-product has been reported to produce the enzymes pectin methylesterase and polygalacturonase in SSF, which will also help to solve the pollution problem of papaya processing units. Papaya peel has been used along with 10% (w/v) orange peel for the production of pectin methylesterase (Patidar et al. 2016). It has also been used for polygalacturonase production in SSF using dried papaya peel and orange peel in ratio of 2:1 (Patidar et al. 2017).
Mango peel Mango is an important tropical fruit, cultivated in many tropical regions and distributed worldwide. India is largest producer of mango amongst 90 countries in the world, with its production of 18,643,000 metric tons on 2,209,000 hectare area which is 35.8% of total fruit area (DACFW 2015; Reddy and Saritha 2015). Mango peel, one of the major by-products from the mango pulp industry, constitutes about 20–25% of the mango fruit processing waste and is discarded during the process. It is a rich source of pectin, which comprises 12.2–21.2% pectin with degrees of esterification from 56.3 to 65.6% (Cheok et al. 2016). Mango peel has been used in SSF for pectinase production by A. foetidus (Kumar et al. 2012). The substrate mango peel was supplemented with salt solution consisting of (g/l) (NH4)2SO4, 25; MgSO4, 0.6; FeSO4, 0.4; urea, 3; peptone, 5 and KH2PO4, 6 at pH 7.0 for use in SSF. Reddy and Saritha (2016) also used mango fruit processing waste (0.5%) for production of pectinase by Enterobacter sp. PSTB-1in SmF.
Citrus fruits waste The family of citrus fruits consists of oranges, Kinnow, Khatta, Lemon, Grapefruit, Malta, Mausami, Sweet orange, etc. The cell wall of these fruits is known to contain high amounts of pectin ingredients (Alexander and Sulebele 1980; Dhillon et al. 2007). Spain is the largest producer of citrus fruit in Europe with an output of 6 million tons/yr. Approximately 800,000–900,000 tons of peel and pulp waste are generated every year only in Spain. Its disposal is the biggest problem for the environment. Lemon peel, orange peel, pomelo peel, orange pomace, etc. have been used extensively in SSF for pectinolytic enzymes production (Table 4). Orange peel has been reported as an inducer for pectinolytic enzyme production in SSF and SmF due to high % of pectin (14.7%) Nighojkar et al. 2006; Zhou et al. 2011; Ahmed et al. 2016). In a recent report, mixture of citrus pulp (51.6%) and sugarcane bagasse (48.4%) has been used in SSF for pectinase production by A. oryzae (Biz et al.2016). Utilization of orange peel along with coconut fibre in ratio of 4:1 has been reported in SSF for the production of pectinase by Bacterial isolate Bacillus subtilis SAV-21 (Kaur and Gupta 2017). Poondla et al. (2016) reported orange peel and ground nut cake as significant solid substrates for pectinase production by Saccharomyces cerevisiae using Response Surface Methodology. Sharma et al. (2014) reported utilization of citrus peel for hyper production of pectinase in SSF by a yeast isolate Pseudozyma sp. SPJ. Citrus fruit waste has been exploited with wheat bran in SSF for polygalacturonase enzyme production (Heerd et al. 2012). Ruiz et al. (2012) reported enhanced pectinase production when SSF process is operated in a column-tray bioreactor at 30 °C and 70% moisture for 96 h. Biz et al. (2016) used packed bed reactor for the production of pectinase enzyme in SSF using citrus peel as solid substrate. Silva et al. (2005) reported utilization of orange bagasse and wheat bran (1:1w/w) for pectinase production in SSF by Penicillium viridicatu. Another citrus waste, orange pomace has been used for pectinase production by A. niger in SSF (Mahmoodi et al. 2017).
Gayen and Ghosh (2011) used orange peel and wheat bran in ratio of 1:1 (w/w) for pectin methylesterase production in SSF.
Apple pomace Apple pomace is a waste from the apple processing industry which presents considerable disposal problems. The production of apple in India is about 2,521,000 metric tons on 277,000 hectare area (DACFW 2015). It has a pH range of 3.1–3.8 (Hang et al. 1982). When dried, it is rich in carbohydrates, pectin and proteins. Apple pomace is the residue left after juice extraction and constitutes about 25–35% of the weight of fresh fruit. It contains 85% carbohydrate and 15% protein (Reid et al. 1999) with 12.3% fermentable sugar (Hang and Woodams 1986). Water soluble components of apple pomace are composed of monosaccharide, oligosaccharide, water soluble polysaccharide and water insoluble components including pectic substances. Apple pomace has been used in SSF for the production of pectinolytic enzyme using A. niger (Berovic’ and Ostrovers’nik 1997). Solid medium was consisted of 1500 g of apple pomace, 750 g of soya flour, 450 g of wheat bran, 1200 g of wheat corn, 90 g of dry whey, 15 g (NH4)2SO4 and 60 g NH4NO3 in 2000 ml water at pH 7.
Joshi et al. (2006) also used dried apple pomace as solid substrate for pectin methylesterase production by A. niger in SSF.
Strawberry pomace Another fruit processing waste, strawberry pomace, obtained from the strawberry processing industry, has similar disposal problem. This waste has been targeted as raw materials for fungal polygalacturonase production by Lentinus edodes, a Chinese mushroom (Zheng and Shetty 2000). Maximum polygalacturonase production in SSF (29.4 U/g pomace) occurred at 40 days incubation using strawberry pomace as solid substrate.
Grape pomace According to a report of DACFW, Govt. of India, Grape production in India is about 2,590,000 metric tons per year in area of 122,000 hectares (DACFW 2015). Grape pomace is the residue left after juice extraction from grapes in the food processing and wine making industry. Diaz et al. (2012, 2013) reported synthesis of polygalacturonase, xylanase and cellulase by SSF on a mixture of washed grape pomace supplemented with orange peel in ratio of 1:1(w/w) and nutrient solution composed of (g/l) urea, 2.4; (NH4)2SO4, 9.8; KH2PO4, 5.0; FeSO4·7H2O, 0.001; ZnSO4·7H2O, 0.0008; MgSO4·7H2O, 0.004; CuSO4·5H2O, 0.001 and pectin, 11.5. Diaz et al. (2012) obtained increase in enzyme production as compared to whole grape pomace used alone.
Banana peel According to 2015 report of Department of Agriculture, Cooperation and Farmers Welfare, Govt. of India (DACFW), banana production of 29,135,000 metric tons over an area of 841,000 hectares is maximum amongst all fruits produced in India (DACFW 2015). Sethi et al. (2016) has reported utilization of banana peel as solid substrate for the production of pectinase by A. terreus NCFT4269.10 in SSF. Sethi et al. (2016) also reported combination of banana peel and apple pomace in ratio of 9:1 for enhanced production of pectinase. Riboflavin in concentration of 10 mg % and gibberellic acid 0.025% supported pectinase production when these were mixed with banana peel in SSF. Barman et al. (2014) also used banana peel as solid substrate in SSF for pectinase production by A. niger strain. The pectinase enzyme activity is significantly affected by concentration of the banana peel in the initial concentration.
Algal biomass Algal biomass is an economically important renewable source for the production of valuable industrial products due to its nutritional value. Dried algal biomass has been used in SSF and SmF for pectinase enzyme production by Bacillus licheniformis KIBGE-IB4 (Pervez et al. 2017). The solid medium containing 10.0 g of dried algal biomass moistened with 10.0 ml mineral salt medium of K2HPO4: 2.0 g L−1 and MgSO4: 2.0 g L−1 was used for the production of pectinase. Among different dried algal biomass used, green algae Ulva lactuca has been reported as most suitable substrate for pectinase enzyme production (Pervez et al. 2017).
Production time
The time course of pectinolytic enzyme production varied with nature of solid substrate and microorganism used. Maximum production of pectic enzyme from different microorganisms varies mainly from 1 to 7 days (Table 4). Wong et al. (2017) reported 129 h of incubation period for pectinase production in SSF by A. fumigatus. Demir and Tari (2014) showed increase in polygalacturonase production sharply by A. sojae after the second day of incubation period and maximum polygalacturonase production (136.9 U/gds) could be achieved on the 4th day of incubation using wheat bran as solid substrate. The production of polygalacturonase begins between the 3rd and 4th day utilizing the other carbon sources present in the media. However, Botella et al. (2005) have reported the maximum polygalacturonase activity on the 25th hour of fermentation by Aspergillus awamori strain cultivated on grape pomace. Barman et al. (2014) reported 65.82 h of fermentation period for pectinase production by A. niger in SSF using banana peel as solid substrate. Zheng and Shetty (2000) reported 40 days of production period using strawberry pomace as solid medium. The addition of orange peels reduced the time needed for fermentation with increase in the production of the pectinolytic enzymes with grape pomace (Diaz et al. 2012) and papaya peel (Patidar et al. 2016) as solid substrate.
Gayen and Ghosh (2011) and Patidar et al. (2016) reported 120 h as optimum time for the production of pectin methylesterase enzyme in SSF. Joshi et al. (2006) reported 96 h production time for maximum pectin methylesterase production in SSF using apple pomace powder as solid substrate and A. niger as microbial source.
Production temperature
Patil and Dayanand (2006), Hendges et al. (2011), Demir and Tari (2014), Barman et al. (2014) and Wong et al. (2017) obtained the maximum polygalacturonase production by Aspergillus strains at 34, 30, 37, 32.37 and 33 °C, respectively. Jahan et al. (2017) reported decrease in production by 29 and 32% when fermentation was carried out at 40 and 45 °C, respectively. The influence of temperature is associated with the growth of the organism (Uzuner and Cekmecelioglu 2015). Darah et al. (2013) reported highest fungal growth rate at 30 °C production temperature. However, the production temperature and growth temperature can be different as reported by Demir and Tari (2014).
Joshi et al. (2006) reported 25 °C production temperature for maximum pectin methylesterase production by A. niger. Similarly, Maldonado and Strasser de Saad (1998) and Patidar et al. (2016) used 30 °C production temperature for maximum pectin methylesterase production by Aspergillus sp.
Moisture content and moistening agent
Moisture content is an important aspect in SSF for industrial enzyme production. The reported initial moisture content value varies from 40 to 80% for the production of pectinases from various solid substrates using different fungal strains. However, Heerd et al. (2012) used 120% moisture content maintained by 0.2 N HCl in SSF containing wheat bran and orange peel (70:30) as solid substrate. Heerd et al. (2014) also used 0.2 N HCl for maintaining the 160% moisture content of solid medium wheat bran and sugar beet pulp. Wong et al. (2017) reported 49.6% moisture content maintained with mineral solution. Mahmoodi et al. (2017) reported maximum polygalacturonase activity at 70% (w/w) of the initial moisture content. Ortiz et al. (2017) optimized 130% (w/w) moisture content using statistical approach. Diaz et al. (2012) reported enhanced production of xylanase, polygalacturonase and carboxymethyl cellulase in grape pomace using 70% moisture content maintained with nutrient solution. Demir and Tari (2014) optimized 62% moisture content in SSF using wheat bran as solid substrate. They compared buffer solution pH 6 and distilled water for higher polygalacturonase production and reported 47% higher polygalacturonase production (212 U/gds) in SSF using distilled water. Ruiz et al. (2012) used 70% moisture content maintained with Czapek-Dox medium. Patidar et al. (2017) reported 90% moisture content maintained with distilled water for maximum polygalacturonase production.
Maldonado and Strasser de Saad (1998) used 70% moisture content maintained with distilled water for pectin methylesterase production in SSF. Gayen and Ghosh (2011) used distilled water (1:1v/w) to moist citrus peel and what bran in SSF. However, Patidar et al. (2016) reported 86% (v/w) moisture content maintained with 10% orange peel suspension for maximum pectin methylesterase production in SSF using papaya peel as solid substrate.
Particle size
The particle size is important for any SSF process due to its influence on aeration, specific area and porosity of substrate. The particle size range of 150–250 µm has been reported by Demir and Tari (2014) for maximum polygalacturonase production. The sized particles gave 92% more of enzyme activity than the un-sized wheat bran. Darah et al. (2013) reported 0.75 mm particle size of pomelo peel for maximum polygalacturonase production. Ruiz et al. (2012) reported optimum particle size range 0.7–2 mm (mesh size 25) of lemon peel pomace for maximum polygalacturonase production.
Patidar et al. (2016) used scanning electron microscope images to show effects of particle size on fungal growth and reported optimum particle size 2 mm for maximum pectin methylesterase production.
Inoculum of microorganisms
Fungal inoculum can be prepared using a vegetative seed culture or spore suspension prepared on slant. The size of inoculum is important in the SSF process. Taragano and Pilosof (1999), Silva et al. (2007), Tai et al. (2014) used spore suspension as inoculum for fungal enzyme production. However, Demir and Tari (2014, 2016) used seed culture inoculum for production of polygalacturonase in SSF. Different type of solutions viz. distilled water, 0.1% Tween 80 and 0.2% Tween 80, have been used for polygalacturonase production in SSF by Taragano and Pilosof (1999), Silva et al. (2007) and Darah et al. (2013), respectively. Mahmoodi et al. (2017) used spore suspension prepared by washing a 5-day incubated plate agar media with isotonic saline solution. Pereira et al. (2017) used boiled wheat grains for preparation of edible mushroom Pleurotussajor-caju spawn. The spawn was prepared by inoculating fungal mycelium agar disks into sterile wheat grain and it was incubated at 30 °C, in the absence of light, until their complete colonization. Most of the literature shows inoculum size ranging from 105 to 107 spores/g of substrate (Silva et al. 2005; Botella et al. 2005; Hendges et al. 2011, Demir and Tari 2016). Uzuner and Cekmecelioglu (2015) used overnight grown Bacillus subtilis inoculum containing 106 CFU/ml which was incubated under agitation at 130 rpm. Joshi et al. (2006) prepared spore suspension of A. niger in distilled water for production of pectin methylesterase enzyme in SSF. Patidar et al. (2016) reported 1 × 107 spores per ml prepared in 0.1% Tween 80 for maximum pectin methylesterase production.
Agitation frequency
In SSF, agitation is required for the removal of heat generated during metabolic process of fermentation and to mix the oxygen transferred to the solid medium. In SSF, the speed of agitation is very slow. It is reported from once to thrice a day. Demir and Tari (2014) reported that agitation speed of 3 times per day enhances the polygalacturonase production in SSF by 17.2% as compared to that in static medium. However, Darah et al. (2013) reported that no agitation is required for maximum polygalacturonase production. According to Darah et al. (2013) agitation of medium may affect the nature of particle and may damage fungal mycelia. Agitation is a major challenge for successful implementation of SSF (Favela-Torres et al. 2006).
Gayen and Ghosh (2011) tried different agitation speed for pectin methylesterase production in SSF and reported maximum production of pectin methylesterase in stationary state.
Determination of pectinolytic activity
Several different assays have been used for the quantification of pectinolytic enzyme activity. The various methods extensively used for assay of polygalacturonase and pectin methylesterase have been reported in literature.
Assay methods for polygalacturonase
Di-nitro salicylic acid method (Miller’s method)
Di-nitro salicylic acid (DNS) method is commonly used for determination of polygalacturonase activity. It is used to determine the amount of reducing sugars (galacturonic acid) released from the polygalacturonic acid substrate (Miller 1959; Li et al. 2015). In this method, after incubation, DNS solution is added in the reaction mixture to stop the reaction and tubes are kept in boiling water for 15 min (Jayani et al. 2005). Both polygalacturonic acid and low methoxyl pectin have been reported as substrate for pectinase assay (Joshi et al. 2013; Ibrahim et al. 2014). Biz et al. (2016) used 75% methylated citrus pectin in the pectinase assay. Zhang et al. (2017) used DNS method for acidic pectinase where substrate of the enzyme was prepared in buffer at pH 3.5. Amid et al. (2014) reported use of DNS method for alkaline pectinase at pH 8.0. Anand et al. (2017) added 1 ml of distilled water in the reaction mixture after the addition of 3 ml DNS to dilute the colour density. One unit (U) of Polygalacturonase activity is calculated as the amount of enzyme required to release one µmol equivalent of galacturonic acid per min under the assay condition and it is expressed in U/ml (Dey et al. 2014).
Enzyme activity can be calculated using following formula (Li et al. 2015)
where “v” is the enzyme volume used in the assay, “194.1” is the molecular weight of galacturonic acid, and t is the reaction time in min.
Arsenomolybdate–copper reagent method (Nelson and Somogyi Method)
The amount of galacturonic acid liberated by polygalacturonase enzyme can also be measured using Nelson (1944) and Somogyi (1952) method. This is colorimetric method in which arsenomolybdate and copper reagent are used for the development of colour (Esawy et al. 2013). Gadre et al. (2003) used 0.24% (w/v) polygalacturonic acid prepared in 60 mM sodium acetate buffer, pH 5.4 as substrate. Pectin and polygalacturonic acid both can be used as substrate for pectinase activity in Nelson-Somogyi method (Nakkeeran et al. 2011; Esawy et al. 2013; Babbar et al. 2016).
The density of colour generated by reaction of arsenomolybdate and copper sulphate reagents is proportional to the amount of reducing sugar. One unit of pectinase activity is defined as the amount of enzyme that releases one micromole of reducing sugar per minute under standard assay conditions (Babbar et al. 2016). Enzyme activity can be calculated using the same formula mentioned for DNS method.
Viscometric method
In this method, viscosity of the solution containing pectin as substrate was measured using viscometer (White and Fabian 1953; Jayani et al. 2005; Esawy et al. 2013; Mahmoodi et al. 2017). The amount of enzyme is directly proportional to the decrease in viscosity of the solution. One unit of enzyme activity is defined as the amount of enzyme required for attaining a certain decrease of viscosity per unit time (Esawy et al. 2013; Ramírez-Tapias et al. 2014). Poletto et al. (2017) defined total enzyme unit as the quantity of enzyme that causes a 50% decrease in the viscosity of the pectin solution, under standard conditions. The percentage of decrease in relative viscosity of pectin solution (w/v) is calculated according to the following formula (White and Fabian 1953).
where “A” is time of flow (seconds) of a given volume of inactivated reaction mixture (blank); “B” is time of flow (seconds) of the same volume of active reaction mixture. However, this method has met with limited success. There is no direct correlation between viscosity reduction and number of glycosidic bonds hydrolyzed (Jayani et al. 2005).
Ruthenium-red microplate assay
As an alternative to viscometric and reducing sugar estimation method, Torres et al. (2011) presented a quantitative method based on the ruthenium red precipitation of polygalacturonic acid. The ruthenium red microplate assay method was described by Ortiz et al. (2014). The microplate assay is carried out in 96-well polymerase chain reaction (PCR) plates. The enzyme solution is mixed with substrate in the microplate well and incubated at 40 °C for 20 min. The enzyme reaction was stopped by placing plate in ice and ruthenium red is added. The absorbance at 535 nm was recorded with a microplate reader. Alternatively, the measurements could be performed at 492 nm in the spectrophotometer. The calibration curve is prepared using polygalacturonic acid as standard.
The enzyme unit is defined as the amount of enzyme required to hydrolyze 1 µg of polygalacturonic acid into smaller fragments unable to precipitate with the ruthenium red dye per minute under the assay conditions. Activity of enzyme is calculated according to the following equation:
where SB is the absorbance of the enzyme sample blank obtained from a mixture of enzyme dilution and polygalacturonic acid solution without incubation, SA is the polygalacturonic acid-enzyme reaction absorbance, and SLP is the slope of the least-squares adjusted calibration curve obtained from A535 nm versus micrograms (µg) of polygalacturonic acid, t is the time (in min) taken for the reaction (typically 20 min), and v is the volume (in ml) of enzyme dilution used in the reaction.
Assay methods for pectin methylesterase
Microbial pectin methylesterase methods are based on the estimation of carboxyl groups by either pH meter or spectrophotometer and estimation of methanol spectrophotometrically.
Spectrophotometric assay
Spectrophotometric assay method is based on continuous monitoring of change in absorbance due to carboxyl groups liberated by pectin methylesterase activity. Patidar et al. (2016) used continuous spectrophotometric method of Vilarino et al. (1993) for A. tubingensis pectin methylesterase using bromocresol green (BCG) dye as pH indicator. The change in BCG color due to action of pectin methylesterase activity was recorded spectrophotometrically at 617 nm. BCG works in acidic conditions. However, for alkaline pectin methylesterase, bromothymol blue has been used (Hagerman and Austin 1986; Nighojkar et al. 1994; Zocca et al. 2007). One unit of enzyme activity is taken as the amount of enzyme that catalyses the release of 1 µmol of galacturonic acid equivalent per minute under the conditions of enzyme assay.
where V = Total volume of Assay (ml); v = Volume (ml) of enzyme solution; DF = Dilution Factor.
Titrimetric method
Titrimetric method is commonly used for determination of pectin methylesterase activity (Christgau et al. 1996; Arotupin et al. 2008). Nowadays, an automated titrator is used for enzyme activity assay (Plaza et al. 2008). The incubations are done in a 15 ml reactor cell connected to a magnetic stirrer and a thermostat is used to maintain temperature. In this method, the reactor is filled with 15 ml of a 0.2% solution of pectin (DE > 75%) in water. The required pH of pectin is adjusted with NaOH or HCl, and the optimum temperature for the reaction is maintained. The enzyme sample is injected and the reagents stirred for 1 min. The reaction mixture is incubated for 1 h in the reactor cell. The constant pH is maintained in the reactor cell by injecting 0.01 M NaOH. The number of hydrolyzed methyl ester linkages (hmel) is determined according to the following equation:
The pKa of galacturonic acid is approx. 3.5 at 30 °C in aqueous solution, and this value has been used for polygalacturonic acid.
Wilinska et al. (2008) used titration method in which pectin methylesterase activity was measured by determining the carboxyl groups released, using titration with 0.02 M NaOH. One unit of pectin methylesterase is defined as the amount of enzyme releasing one carboxyl group per ml per min. Jiang et al. (2013) gave another definition of pectin methylesterase activity in which the rate of NaOH consumption is monitored and the activity of pectin methylesterase is calculated from the slope of the curve. One unit of pectin methylesterase activity is defined as the consumption of 1 µmol of NaOH/min under optimal conditions.
Gel diffusion assay
This method is independent of pH and insensitive to other pectinolytic enzymes viz. pectin lyase and pectinase. The concept of increased binding of ruthenium red to pectin with difference in the number of methyl esters attached to the pectin forms the basis for gel diffusion assay for pectin methylesterase activity (Downie et al. 1998). The stained zone diameters decrease with increasing percentage of substrate esterification. The substrate required in this method is pectin which must have high degree of esterification usually more than 90%.
Gel diffusion is performed in 2% (w/v) agarose gel containing citrus pectin (90% esterified) in citrate phosphate buffer. The enzyme sample is added in wells prepared in gel plate. The plate is incubated at 30 °C for 16 h and the presence of enzyme is detected by incubating it in 0.05% (w/v) ruthenium red for 45 min (Jiang et al. 2013). Lionetti (2015) reported pectoplate assay method which can discriminate the enzyme activity between plant and fungal pathogen. Recently, Lionetti et al. (2017) also used pectoplate assay for plant pectin methylesterase activity determination. The samples were loaded in the wells and incubated for 16 h at 30 °C. The activity of enzyme was calculated using 0.05% ruthenium red for 30 min. The amount of activity in nano- or picokatals is calculated based on the standard curve generated from the commercial enzyme activity versus stained zone diameter. The commercial enzyme activity is expressed as the production of acid equivalents which is interpreted as units for conversion to nanokatals.
Methanol estimation method
Methanol is one of the products of pectin methylesterase reaction. Pectin methylesterase can be assayed directly by quantifying methanol using gas liquid chromatography (Salas-Tovar et al. 2017). Pectin methylesterase can be quantified by methanol oxidation through potassium permanganate (Wood and Siddiqui 1971). In 1998, a close correlation has been reported between pectin methylesterase activity and levels of methanol in fruit tissues, indicating that pectin methylesterase is on the primary biosynthetic pathway for methanol production in plant tissue and fruits (Frenkel et al. 1998; Micheli 2001). The enzyme–substrate reaction, after incubation is stopped by placing the tube at 100 °C for 10 min. The alcohol oxidase enzyme is added to the reaction mixture to oxidize methanol produced by pectin methylesterase activity. The 2–4 pentanodione (20 mM) in 2 M ammonium phosphate was added in the reaction mixture and placed in a water bath at 60 °C for 15 min. The absorbance is measured at 412 nm against a blank made with the same components but with heat denatured enzyme extract. A calibration curve using 0–250 nmol/ml methanol is prepared as a standard. The amount of methanol released allows the determination of pectin methylesterase activity expressed in nkat (Deytieux-Belleau et al. 2008).
It is currently impossible to compare different pectinolytic enzyme assay methods reported in the literature due to different assay conditions. Therefore, it is important to develop a standardized assay for evaluating the pectinase complexes (Biz et al. 2014).
Bioreactor employed for pectinolytic enzyme production using SSF
The various important factors to be considered for the development of a SSF process include selection of microorganism and substrate, optimum process parameters and purification of the end product. Development of any fermentation process requires proper relationships between the physiology of the microorganisms and the fermentation parameters such as temperature, pH, aeration, moisture content, nature of solid substrate employed, etc.
Scale up of SSF process and biomass estimation has become the major challenge that led the researchers to thrive hard to find the solutions (Singhania et al. 2009). A number of bioreactors have been designed which could overcome the problems of scale up and to an extent also the on-line monitoring of several parameters. These bioreactors also regulate the heat and mass transfer which are difficult to manage in a basic system of fermentation. Several types of bioreactors have been designed for small scale as well as large scale applications of SSF, including the tray bioreactors, packed bed bioreactors and drum bioreactors.
Tray bioreactor
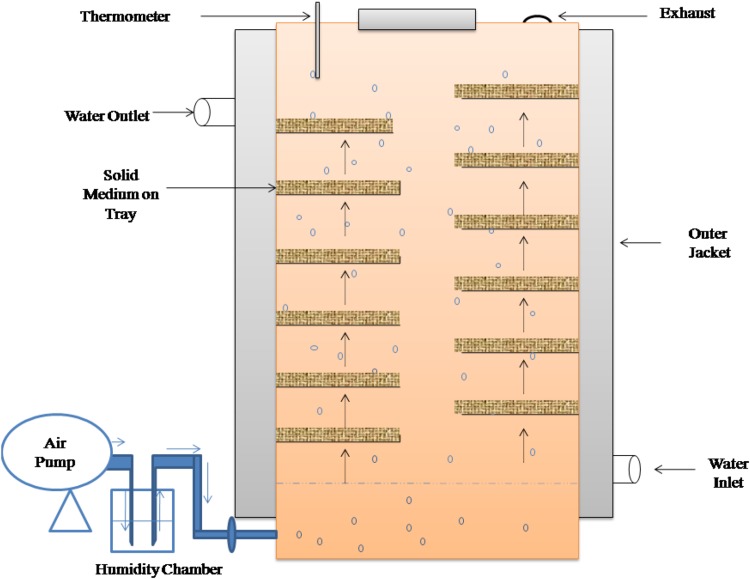
Tray bioreactor is static type of bioreactor in which wood or stainless steel trays are used to place the solid substrate. It has provision of air circulation in the reactor through perforated trays (Fig. 4). Trays are arranged in a chamber or room with controlled temperature and humidity. Ortiz et al. (2017) reported scaling up of the pectinase production using a tray bioreactor under optimum fermentation conditions. In this study, solid media consisted of 0.1 kg of wheat bran, orange and lemon peels in proportion of 66:17:17, respectively, have been used. Diaz et al. (2013) reported column tray bioreactor for the production of polygalacturonase, xylanase and carboxy methyl cellulase (CMCase) in SSF. In this reactor, ten sterile 250 ml interconnected roux flasks were used which had provision of water-saturated aeration system. The sterile air enters the first plate and leaves the reactor at the tenth plate after passing through the whole reactor. Ruiz et al. (2012) also used tray bioreactor for the production of pectinase by Aspergillus niger Aa-20 using lemon peel pomace as support and carbon source. The advantage of using tray reactors is that the labour intensity is very low if it is run as a continuous system and also it can be easily scaled to larger operations. However, in this type of reactor, the control of temperature and oxygen transfer during SSF is a big challenge. Roussos et al. (2014) patented a novel designs of the tray reactor in which temperature can be easily controlled. The limit of oxygen is affected by formation of the mycelium and release of CO2 and heat, especially for higher substrate layers (Soccol et al. 2017).
Fig. 4.
An overview of Tray Bioreactor used for enzyme production in SSF.
Modified from Ruiz et al. (2012)
Packed bed bioreactor
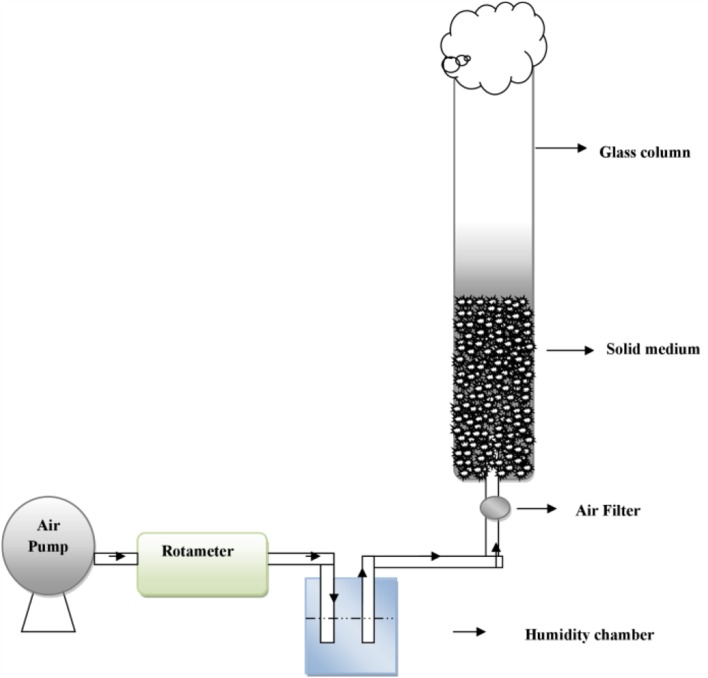
Packed bed bioreactor has been used widely for production of pectinolytic enzymes (Fig. 5). It is difficult to control the temperature within bioreactor at pilot or large scale due to the limitation of heat and mass transfer. Therefore, reliable scale-up strategies are essential which can be fulfilled by packed bed bioreactor (Pitol et al. 2016). Packed Bed Reactor provides larger substrate bed and easier product recovery (Singhania et al. 2009). Biz et al. (2016) reported production of pectinase by A. oryzae in pilot-scale packed bed reactor. In this bioreactor, 15 kg of substrate containing citrus pulp (51.6%) and sugarcane bagasse (48.4%) are used for enzyme production. It was observed that the combination of citrus pulp and sugarcane bagasse avoided the problems of formation of agglomerates and bed shrinkage that were reported in the earlier work of Pitol et al. (2016). Pitol et al. (2016) scaled up the production of pectinases in packed-bed bioreactors, from 12 g to 30 kg of dry substrate. In this reactor, 40 cm high bed containing 27 kg of wheat bran and 3 kg of sugarcane bagasse has been used. This is the biggest scale yet reported for pectinase production in SSF. Packed bed reactor has been used for the production of polygalacturonase, xylanase and carboxy methyl cellulase (CMCase) in SSF (Diaz et al. 2013). A novel design of the packed bed reactor has been made in which aeration in the reactor brings about mixing of the solids (Singhania et al. 2009).
Fig. 5.
Packed bed bioreactor used for enzyme production in SSF.
Modified from Diaz et al. (2013)
Drum bioreactor
Another type of bioreactor in which rotating drum is employed for the production of enzymes in SSF (Fig. 6). In drum bioreactor continuous or intermittent agitation of solid medium occurs which provides homogeneity of the solid medium and better circulation of oxygen (Soccol et al. 2017). Poletto et al. (2017) reported production of pectinase by A. niger LB-02-SF in a bench-scale rotating drum bioreactor. Poletto et al. (2017) reported the effect of drum bioreactor volume occupied by substrate on growth rate and enzyme production. It has been found that with 30% of the bioreactor volume occupied, agitation promoted increasing fungal growth and decreasing enzyme production, whereas with 50% occupation, an opposite trend occurred. Rodríguez-Fernández et al. (2011) also carried out pectinase production in horizontal drum bioreactor. In this reactor, CO2 production is measured by an infrared sensor while consumption of O2 by the microorganism is measured by electrochemical sensor. To accurately measure the O2 balance, one sensor is fitted in the inlet and the other is located in the outlet of the bioreactor. Drum bioreactors can perform constant mixing thereby ensuring thermal equilibrium, or intermittent stirring whereby stationary phase is maintained similar to the tray fermenter. A disadvantage of the continuously stirred drum bioreactor is that agglomerates may form and shear stress is increased (Singhania et al. 2009).
Fig. 6.
An overview of drum bioreactor used in SSF for pectinolytic enzyme production.
Modified from Rodríguez-Fernández et al. (2011)
Biomass estimation
Separation of biomass, which is essential for the kinetic studies, is a big challenge in SSF. Glucosamine estimation, ergosterol estimation, protein (kjeldahl) estimation, DNA estimation, dry weight changes and CO2 evolution are indirect biomass estimation methods used in SSF (Rodríguez-Fernández et al. 2011; Ruiz et al. 2012). Recently, digital image processing has been developed for biomass estimation in SSF. In recent times, estimation of oxygen intake and carbon dioxide evolution rate are considered to be most accurate for the determination of the growth of microorganism (Singhania et al. 2009).
Application of enzyme in fruit juice clarification
Pectinolytic enzymes are one of the upcoming enzymes of fruit industries. Fruit juices are naturally cloudy mainly due to the presence of pectin polysaccharides (Sharma et al. 2017). The high concentration of pectin leads to colloid formation in the juice, which leads to create problem in the processing of clear fruit juices. The role of acidic pectinases in bringing down the cloudiness and bitterness of fruit juices is well established (Kashyap et al. 2001; Jayani et al. 2005). In an unripe fruit, pectin is bound to cellulose micro fibrils in the cell wall. Such pectin is insoluble and hence confers rigidity to cell walls. However, during ripening the structure of pectin is altered by naturally occurring enzymes in the fruits. As a result of this, the pectin becomes more soluble and softens the plant tissues (Caffall and Mohnen 2009). Therefore, pectin degrading enzymes have been used to clarify following important fruit juices:
Apple juice Pectinolytic enzymes produced by Aspergillus sp. have been widely used for purification of apple juice. Amin et al. (2016) reported 73.78% reduction in turbidity of apple juice by exo-polygalacturonase produced by P. notatum. Yuan et al. (2011) also used polygalacturonase of Penicillium sp. and reported 4.5% reduction in viscosity and 71.8% increased the light transmission. Apple juice clarification by polygalacturonase produced by Aspergillus sp. has been reported in recent literature (Mahmoodi et al. 2017; Dey et al. 2014; Kant et al. 2013; Sandri et al. 2015). Mahmoodi et al. (2017) reported 7.2% reduction in apple juice viscosity by A. niger pectinase. Dey et al. (2014) treated apple juice with polygalacturonase produced by A. awamori Nakazawa and reported 38% reduction in viscosity and 93% increase in transmission at 660 nm when it was incubated at 50 °C for 2 h. Apple juice treated with polygalacturonase produced by A. niger showed the maximum clarification by increase in % transmission from 2.5 to 20.4 upon overnight incubation (Kant et al. 2013). A 90% decrease in apple juice turbidity has been reported by Sandri et al. (2015) using A. niger pectinase enzyme extract. Yang et al. (2011) also treated apple juice with Bispora sp. pectinase and reported 84% increase in transmittance and reduction in viscosity by 7.7%. Baron et al. (2006) studied effect of high pressure on apple juice clarification by pectin methylesterase. Rajdeo et al. (2016) reported apple juice clarification with immobilized pectinase. The properties of apple juice treated with immobilized enzyme were similar to those of that treated with free pectinase.
Orange and mosambi juice Citrus fruits viz. oranges, lemon, and grapefruit contain the highest reported concentrations of pectin in their tissues (Galant et al. 2014). In commercially prepared citrus juices, pectin accounts for approximately one-third of the insoluble material that is found within the juice cloud (Baker and Bruemmer 1969). Pectin from orange is only partially methylated because of the large amounts of pectin methylesterase which remove methoxyl group from pectin (Kashyap et al. 2001; Maran et al. 2013). In the presence of calcium ions, insoluble calcium pectate is formed in orange juice leading to the undesirable precipitation of haze particles. Tounsi et al. (2016) reported citrus fruit juice clarification using polygalacturonase of Penicillium occitanis. Anand et al. (2017) reported orange juice clarification using exo-polygalacturonase produced by A. niger. Diaz et al. (2013) reported 95% clarification in orange juice on addition of mixture of pectinase, xylanase and CMCase produced in tray reactor by A. awamori. Rai et al. (2004) have obtained 89% clarified mosambi juice by a pectinase of Aspergillus niger using enzyme protein concentration 0.004 g/l for 99 min, at temperature of 42 °C.
Passion Fruit Juice This juice has commercial importance due to its pleasant unique aroma and flavor and high nutritional values (Cheok et al. 2016). Brazil is the largest producer of passion fruit in the world and about 50% of the production is used in the juice processing industry (Canteri et al. 2012). Jiraratananon and Chanachai (1996) observed a viscosity reduction of 18% with enzymatic treatment of passion fruit juice with pectinase. Domingues et al. (2012) reported passion fruit juice clarification using chitosan treatment. The juice is centrifuged at 4000 rpm followed by coagulation/flocculation process with chitosan at 300 ppm and at pH 6.
Banana Juice The turbidity of banana juice is caused mainly by the polysaccharides in the juice such as pectin and starch. It is a major challenge to process banana after its harvesting (Mohapatra et al. (2011). Pectin makes the clarification process difficult because of its fibre such as molecular structure. Cheng et al. (2017) used acidic endo-polygalacturonase produced by Penicillium oxalicum CZ1028 for banana juice clarification at 45 °C for 1 h. Cheng et al. (2017) reported 31.76% reduction in viscosity and 6% increase in transmission at 660 nm. Barman et al. (2014) reported banana juice clarification by pectinase produced by A. niger. The optimum results (0.10 OD at 660 nm) were obtained when raw banana juice was incubated for 60 min with 2% concentration of partially purified pectinase. Sagu et al. (2013) optimized conditions for banana juice clarification using commercial pectinase and reported 33 °C and 108 min for best results. Lee et al. (2006) employed response surface methodology to clarify banana juice in which optimum conditions for clarification are found to be 0.084% enzyme concentration, incubation temperature of 43.2 °C and incubation time of 80 min.
Lemon juice Maktouf et al. (2014) have reported lemon juice clarification using Penicillium occitanis pectinase. The optimum treatment conditions reported were 600 U/l enzyme concentration, 45 min and 30 °C. Under optimized conditions, 77% reduction of viscosity and 47% reduction in turbidity is reported.
Mango juice Pectinase from A. foetidus has been reported for mango juice clarification (Kumar et al. 2012). Mango juice was treated with 20 ml of crude enzyme preparation (specific activity 228 IU/ml). The maximum mango juice clarification (92.5%) was obtained at temperature of 40 °C and 150 min incubation time. Cheng et al. (2017) used acidic endo-polygalacturonase produced by Penicillium oxalicum CZ1028 for mango juice clarification at 45 °C for 1 h and reported 5% increase in transmission at 660 nm.
Pineapple juice Pectin methylesterase produced by A. tubingensis in SSF has been reported for pineapple juice clarification (Patidar et al. 2016). It was found that increase in amount of enzyme from 10 to 100 U increased the juice clarification from 3.1 to 19.5% and decreased the pH from 4.3 to 3.0 at 30 °C. Tochi et al. (2009) reported pineapple juice clarification by commercially available A. niger pectinase (Sigma- Aldrich) which showed reduction in turbidity from 1.5 to 0.8 at 35 °C. de Carvalho et al. (2008) reported 5% change in pineapple juice sugar content due to the addition of pectinase and cellulase followed by cross flow micro- and ultra-filtration to maintain the nutritional quality of pineapple juice.
Blue berry juice Taking into consideration the high nutritional potential, blueberry juice has been clarified by Sandri et al. (2015). Pectinase of A. niger produced in SSF has been used for the clarification of blueberry juices.
Guava juice South Africa, India and Hawaii are major producers of Guava (Kashyap et al. 2001). Kant et al. (2013) reported guava juice clarification using A. niger polygalacturonase. In this study, addition of enzyme increased the sugar content and % transmission at 650 nm, whereas the pH of the juice decreased. The % transmission at 650 nm and mg % sugar content increased from 1.7 to 20.4 and 1.9–4.8, respectively.
Date syrup Dates are important products in the hot desert regions of the world and are marketed globally as a high-value fruit. It always plays an important part in the economic and social lives of the people of these regions (Abbès et al. 2011). The commercial quality of date syrup increases on addition of pectinase. The use of pectinase and cellulase enzyme (50U/5U) gave the highest recovery of total soluble solids and the lowest turbidity compared with control sample.
Papaya juice Cheng et al. (2017) used acidic endo-polygalacturonase produced by Penicillium oxalicum CZ1028 for banana juice clarification at 45 °C for 1 h. Cheng et al. (2017) reported 78.36% reduction in viscosity and 43.3% increase in transmission at 660 nm. Tu et al. (2013) reported reduction in viscosity of papaya juice by 17.6%, and increased its transmittance by 59.1% when papaya juice was treated with Achaetomium sp. polygalacturonase. Tu et al. (2013) also reported higher efficiency with a synergy degree of more than 1.25, when juice was treated with commercial pectin methylesterase and polygalacturonase produced by Achaetomium sp.
Grape juice Li et al. (2017) used combination of two polygalacturonase (exo- and endo-) produced by Talaromyces leycettanus in ratio of 1:4 for grape juice clarification and reported 140% pectin-degrading efficiency with light transmittance improvement from 14 to 82%. Sreenath and Santhanam (1992) reported 25% reduction in viscosity when white grape juice was treated with commercial A. niger pectinase.
Bitter gourd juice (Karela Juice) Bitter gourd exhibits significant anti-diabetic activity. Bitter gourd is tropical medicinal plant which belongs to the Cucurbitaceae family. Its fruit bitter melon or karela has a distinct bitter taste (Deshaware et al. 2017). The optimum conditions, i.e., pectinase concentration (10.2 ml/kg), incubation time (140 min) and temperature (48.8 °C) resulted in juice yield of 82%, α–amylase and α-glucosidase inhibition activity of 23 and 59%, respectively.
Challenges and future aspects in fruit juice clarification
The main parameters to be considered in any enzymatic reaction for clarification are pH, temperature, amount of enzyme, treatment time, and presence of inhibitors. The enzymatic treatment conditions depend heavily on the type of raw material employed and optimum conditions for each fruit juice which have to be determined experimentally (Sharma et al. 2017; Garcıa 2018). Apart from process conditions, there are some other factors that pose challenges in the production of fruit juices and affect the global juice market. One of the main challenges is associated with the constant supply of fruits, as most of the fruits are seasonal and this affects continuous supply of fruits (Rajauria and Tiwari 2018).
Few more aspects can be considered for future in fruit juice industry. The eradication of food borne pathogens and spoilage microorganisms may be searched for. The prevention of fruit juice contamination and newer presentation methods may be studied. The use of ozone as microbial agent against bacteria, fungi, parasites and viruses growing over fruits may also be tried in future to enhance the shelf life of fruits. The effects of SCCD (Supercritical Carbondioxide Technology) may also be investigated for enhancing fruit juice characteristics such as pH, acid content, total soluble solids and bioactive compounds.
Conclusion
Pectinolytic enzymes have been extensively used in various fruit processing industries. The enzymes are mostly produced by SSF and to some extent SmF using numerous microorganisms isolated from decaying fruit waste and soil. The different techniques of SSF, assay methods and different applications of these enzymes particularly in fruit juice have been discussed in the review. Several bioreactors such as tray, packed bed and drum bioreactor have been described for the efficient production of polygalacturonase and pectin methylesrase, their scale up and control of production parameters have been discussed. A lot of scope exists in the upgradation of the process parameters in production as well as its application in fruit juice clarification. Many authors have mentioned the use of enzymes obtained from different microbial sources, and also used different solid substrate for production. However, recent studies depict that the most widely used production media uses wheat bran which can be easily replaced by fruits wastes such as papaya peel for more efficient production.
Acknowledgements
The authors acknowledge the facilities provided by the Department of Biotechnology, Ministry of Science and Technology, Government of India, New Delhi (DBT) in the School of Biotechnology, Devi Ahilya University, Indore under M.Sc. Biotechnology Program and Bioinformatics sub center. The authors also acknowledge facilities of Department of Biosciences, Maharaja Ranjit Singh College of Professional Sciences, Indore.
Compliance with ethical standards
Conflict of interest
All the authors declare no conflict of interests publishing this article in 3 Biotech.
References
- Abbès F, Bouaziz MA, Blecker C, Masmoudi M, Attia H, Besbes S. Date syrup: effect of hydrolytic enzymes (pectinase/cellulase) on physicochemical characteristics, sensory and functional properties. LWT Food Sci Technol. 2011;44:1827–1834. [Google Scholar]
- Acuna-Arguelles M, Gutierrez-Rojas M, Viniegra-Gonzalez G, Favela-Torres E. Effect of water activity on exo-pectinase production by Aspergillus niger CH4 on solid state fermentation. Biotechnol Lett. 1994;16:23–28. [Google Scholar]
- Ahmed I, Zia MA, Hussain MA, Akram Z, Naveed MT, Nowrouzi A. Bioprocessing of citrus waste peel for induced pectinase production by Aspergillus niger; its purification and characterization. J Radiat Res Appl Sci. 2016;9:148–154. [Google Scholar]
- Alexander MM, Sulebele GA. Characteristics of pectins from Indian citrus peels. J Food Sci Technol. 1980;17:180–182. [Google Scholar]
- Almora K, Pino JA, Fernandez M, Duarte C, Gonzalez J, Roncal E. Evaluation of volatiles from ripening papaya (Carica papaya L., var. Maradol roja) Food Chem. 2004;86:127–130. [Google Scholar]
- Amid M, Manap Y, Zohdi K. Purification and characterisation of thermo-alkaline pectinase enzyme from Hylocereus polyrhizus. Eur Food Res Technol. 2014 [Google Scholar]
- Amin F, Bhatti HN, Bilal M, Asgher M. Improvement of activity, thermo-stability and fruit juice clarification characteristics of fungal exo-polygalacturonase. Int J Biol Macromol. 2016 doi: 10.1016/j.ijbiomac.2016.10.086. [DOI] [PubMed] [Google Scholar]
- Anand G, Yadav S, Yadav D. Production, purification and biochemical characterization of an exo-polygalacturonase from Aspergillus niger MTCC 478 suitable for clarification of orange juice. 3 Biotech. 2017;7:122. doi: 10.1007/s13205-017-0760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arotupin DJ, Akinyosoye FA, Onifade AK. Purification and characterization of pectinmethylesterase from Aspergillus repens isolated from cultivated soil. Afr J Biotechnol. 2008;7:1991–1998. [Google Scholar]
- Babbar N, Baldassarre S, Maesen M, Prandi B, Dejonghea W, Sforzab S, Elst K. Enzymatic production of pectic oligosaccharides from onion skins. Carbohydr Poly. 2016;146:245–252. doi: 10.1016/j.carbpol.2016.03.011. [DOI] [PubMed] [Google Scholar]
- Baker RA, Bruemmer JH. Cloud stability in the absence of various orange juice soluble components. Proc Fla State Hortic Soc. 1969;82:215–220. [Google Scholar]
- Balkan B, Ertan F. The production of a new fungal alpha-amylase degraded the raw starch by means of solid-state fermentation. Prep Biochem Biotechnol. 2010;40:213–228. doi: 10.1080/10826068.2010.488549. [DOI] [PubMed] [Google Scholar]
- Barman S, Sit N, Badwaik LS, Deka SC. Pectinase production by Aspergillus niger using banana (Musa balbisiana) peel as substrate and its effect on clarification of banana juice. J Food Sci Technol. 2014 doi: 10.1007/s13197-014-1413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron A, Denes J, Durier C. High-pressure treatment of cloudy apple juice. LWT Food Sci Technol. 2006;39:1005–1013. [Google Scholar]
- Berovic’ M, Ostrovers’nik H. Production of Aspergillus niger pectolytic enzymes by solid state bioprocessing of apple pomace. J Biotechnol. 1997;53:47–53. doi: 10.1016/s0168-1656(96)01661-6. [DOI] [PubMed] [Google Scholar]
- Biz A, Farias FC, Motter FA, de Paula DH, Richard P, Krieger N, Mitchell DA. Pectinase Activity Determination: an Early Deceleration in the Release of Reducing Sugars Throws a Spanner in the Works! PLoS ONE. 2014;9(10):e109529. doi: 10.1371/journal.pone.0109529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biz A, Finkler ATJ, Pitol LO, Medina BS, Krieger N, Mitchell DA. Production of pectinases by solid-state fermentation of a mixture of citrus waste and sugarcane bagasse in a pilot-scale packed-bed bioreactor. Biochem Eng J. 2016;111:54–62. [Google Scholar]
- Boonrod D, Reanma K, Niamsup H. Extraction and physicochemical characteristics of acid-soluble pectin from raw papaya (Carica papaya) peel. Chiang Mai J Sci. 2006;33:129–135. [Google Scholar]
- Botella C, de Ory I, Webb C, Cantero D, Blandino A. Hydrolytic enzyme production by Aspergillus awamori on grape pomace. Biochem Eng J. 2005;26:100–106. [Google Scholar]
- Brinton CS, Dore WH, Wichmann HJ, Willaman JJ, Wilson CP. Definitions written by the committee on nomenclature of pectin of the agriculture-food division. J Am Chem Soc. 1927;49:38–40. [Google Scholar]
- Burana-Osot J, Soonthornchareonnonb N, Chaidedgumjorna A, Hosoyamac S, Toidac T. Determination of galacturonic acid from Pomelo pectin in term of galactose by HPAEC with fluorescence detection. Carbohydr Pol. 2010;81:461–465. [Google Scholar]
- Caffall KH, Mohnen D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr Res. 2009;344:1879–1900. doi: 10.1016/j.carres.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Canteri MHG, Scheer AP, Ginies C, Reich M, Renard CMCG, Wosiacki G. Rheological and macromolecular quality of pectin extracted with nitric acid from passion fruit rind. J Food Process Eng. 2012;35:800–809. [Google Scholar]
- Castilho LR, Medronho RA, Alves TLM. Production and extraction of pectinases obtained by solid-state fermentation of agroindustrial residues with Aspergillus niger. Bioresour Technol. 2000;71:45–50. [Google Scholar]
- Chaiwut P, Nitsawang S, Shank L, Kanasawud P. A comparative study on proteolytic components of papaya peel and latex proteases. Chiang Mai J Sci. 2010;34:109–118. [Google Scholar]
- Cheng Z, Chen D, Wang Q, Xian L, Lu B, Wei Y, Tang H, Lu Z, Zhu Q, Chen Y, Huang R. Identification of an acidic endo-polygalacturonase from Penicillium oxalicum CZ1028 and its broad use in major tropical and subtropical fruit juices production. J Biosci Bioeng. 2017;123(6):665–672. doi: 10.1016/j.jbiosc.2017.01.013. [DOI] [PubMed] [Google Scholar]
- Cheok CY, Adzahan NM, Rahman RA, Abedin NHZ, Hussain N, Sulaiman R, Chong GH. Current trends of tropical fruit waste utilization. Cri Rev Food Sci Nutr. 2016 doi: 10.1080/10408398.2016.1176009. [DOI] [PubMed] [Google Scholar]
- Christgau S, Kofod LV, Halkier T, Andersen LN, Hockauf M, Dorreich K, Dalboge H, Kauppinen S. Pectin methyl esterase from Aspergillus aculeatus: expression cloning in yeast and characterization of the recombinant enzyme. Biochem J. 1996;319:705–712. doi: 10.1042/bj3190705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combo AMM, Aguedo M, Goffin D, Wathelet B, Paquot M. Enzymatic production of pectic oligosaccharides from polygalacturonic acid with commercial pectinase preparations. Food Bioprod Process. 2012;90:588–596. [Google Scholar]
- Couto SR, Sanroma MA. Application of solid-state fermentation to food industry—A review. J Food Eng. 2006;76:291–302. [Google Scholar]
- DACFW (2015) Department of Agriculture Cooperation, Farmers and Welfare, Ministry of Agriculture, Government of India
- Darah I, Taufiq MMJ, Lim SH. Pomelo citrus grandis (l.) osbeck peel as an economical alternative substrate for fungal pectinase production. Food Sci Biotechnol. 2013 [Google Scholar]
- de Carvalho LMJ, de Castro IM, da Silva CAB. A study of retention of sugars in the process of clarification of pineapple juice (Ananas comosus, L. Merril) by micro- and ultra-filtration. J Food Eng. 2008;87:447–454. [Google Scholar]
- De Gregorio A, Mandalari G, Arena N, Nucita F, Tripodo MM, Lo Curto RB. SCP and crude pectinase production by slurry-state fermentation of lemon pulps. Bioresour Technol. 2002;83:89–94. doi: 10.1016/s0960-8524(01)00209-7. [DOI] [PubMed] [Google Scholar]
- Demir H, Tari C. Valorization of wheat bran for the production of polygalacturonase in SSF of Aspergillus sojae. Ind Crops Prod. 2014;54:302–309. [Google Scholar]
- Demir H, Tari C. Bioconversion of wheat bran for polygalacturonase production by Aspergillus sojae in tray type solid-state fermentation. Int Biodeterio Biodegrad. 2016;106:60–66. [Google Scholar]
- Deshaware S, Gupta S, Singhal RS, Variyar PS. Enhancing anti-diabetic potential of bitter gourd juice using pectinase: a response surface methodology approach. LWT Food Sci Technol. 2017 [Google Scholar]
- Dey TB, Adak S, Bhattacharya P, Banerjee R. Purification of polygalacturonase from Aspergillus awamori Nakazawa MTCC 6652 and its application in apple juice clarification. LWT Food Sci Technol. 2014;59:591–595. [Google Scholar]
- Deytieux-Belleau C, Vallet A, Doneche B, Geny Pectin methylesterase and polygalacturonase in the developing grape skin. Plant Physiol Biochem. 2008;46:638–646. doi: 10.1016/j.plaphy.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Dhillon SS, Gill RK, Gill SS, Singh M. Studies on the utilization of citrus peel for pectinase production using fungus Aspergillus niger. Int J Environ Stud. 2007;61:199–210. [Google Scholar]
- Diaz AB, Alvarado O, de Ory I, Caro I, Blandino A. Enhance hydrolytic enzymes production by Aspergillus awamori on supplemented grape pomace. Food Bioprod Process. 2012;90:72–78. [Google Scholar]
- Diaz AB, Alvarado O, de Ory I, Caro I, Blandino A. Valorization of grape pomace and orange peels: improved production of hydrolytic enzymes for the clarification of orange juice. Food Bioprod Process. 2013;91:580–586. [Google Scholar]
- Dobrev GT, Pishtiyski IG, Stanchev VS, Mircheva R. Optimization of nutrient medium containing agricultural wastes for xylanase production by Aspergillus niger B03 using optimal composite experimental design. Bioresour Technol. 2007;98:2671–2678. doi: 10.1016/j.biortech.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Domingues RCC, Faria Junior SB, Silva RB, Cardoso VL, Miranda Reis MH. Clarification of passion fruit juice with chitosan: effects of coagulation process variables and comparison with centrifugation and enzymatic treatments. Process Biochem. 2012;47:467–471. [Google Scholar]
- Downie B, Dirk LM, Hadfield KA, Wilkins TA, Bebbett AB, Bradford KJ. A gel diffusion assay for quantification of pectin methylesterase activity. Anal Biochem. 1998;15:149–157. doi: 10.1006/abio.1998.2847. [DOI] [PubMed] [Google Scholar]
- Duvetter T, Van Loey A, Smout C, Verlent I, Ly Nguyen B, Hendrickx M. Aspergillus aculeatus pectin methylesterase: study of the inactivation by temperature and pressure and the inhibition by pectin methylesterase inhibitor. Enzym Microb Technol. 2005;36:385–390. [Google Scholar]
- Esawy MA, Gamal AA, Kamel Z, Ismail AS, Abdel-Fattah AF. Evaluation of free and immobilized Aspergillus niger NRC1ami pectinase applicable in industrial processes. Carbohydr Poly. 2013;92:1463–1469. doi: 10.1016/j.carbpol.2012.10.061. [DOI] [PubMed] [Google Scholar]
- FAO (2013) Food and Agriculture Organization of the United Nations and World Health Organization (FAO/WHO). Faostat. Geneva, Switzerland. http://faostat.fao.org/site/567/desktopdefault.aspx?pageid=567#ancor.Freixo
- Favela-Torres E, Volke-Sepúlveda T, Viniegra-González G. Production of hydrolytic depolymerising pectinases. Food Technol Biotechnol. 2006;44:221–227. [Google Scholar]
- Freitas P, Martin N, Silva D, Silva R, Gomes E. Production and partial characterization of polygalacturonases produced by thermophilic Monascus sp. N8 and by thermotolerant Aspergillus sp. N12 on solid-state fermentation. Braz J Microbiol. 2006;37:302–306. [Google Scholar]
- Frenkel C, Peters JS, Tieman DM, Tiznado ME, Handa AK. Pectin methylesterase regulates methanol and ethanol accumulation in ripening tomato (Lycopersicon esculentum) fruit. J Biol Chem. 1998;273:4293–4295. doi: 10.1074/jbc.273.8.4293. [DOI] [PubMed] [Google Scholar]
- Gadre RV, Driessche GV, Beeumen JV, Bhat MK. Purification, characterisation and mode of action of an endo-polygalacturonase from the psychrophilic fungus Mucor flavus. Enzym Microb Technol. 2003;32:321–330. [Google Scholar]
- Galant AL, Luzio ZA, Widmer WW, Cameron RG. Compositional and structural characterization of pectic material from frozen concentrated orange juice. Food Hydrocolloid. 2014;35:661–669. [Google Scholar]
- Garcıa CA. Application of enzymes for fruit juice processing. Cambridge: Academic Press; 2018. pp. 201–216. [Google Scholar]
- Gayen S, Ghosh U. Pectinmethylesterase Production from mixed agro- wastes by Penicillium notatum NCIM. 923 in Solid-State fermentation. Bioremed Biodegrad. 2011;2:1–4. [Google Scholar]
- Glinka EM, Liao YC. Purification and partial characterisation of pectin methylesterase produced by Fusarium asiaticum. Fungal Biol. 2011;15:1112–1121. doi: 10.1016/j.funbio.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Graminha EBN, Goncalves AZL, Pirota RDPB, Balsalobre MAA, de Silva R, Gomes E. Enzyme production by solid-state fermentation: application to animal nutrition. Animal Feed Sci Technol. 2008;144:1–22. [Google Scholar]
- Gupta S, Kapoor M, Sharma KK, Nair LM, Kuhad RC. Production and recovery of an alkaline exo-polygalacturonase from Bacillus subtilis RCK under solid-state fermentation using statistical approach. Bioresour Technol. 2008;99:937–945. doi: 10.1016/j.biortech.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Hagerman AE, Austin PJ. Continuous spectrophotometric assay for plant pectin methyl esterase. J Agri Food Chem. 1986;34:440–444. [Google Scholar]
- Hang YD, Woodams EE. Solid state fermentation of apple pomace for citric acid production. MIRCEN J. 1986;2:283–287. [Google Scholar]
- Hang YD, Lee CY, Woodams EE. A solid state fermentation system for production of ethanol from apple pomace. J Food Sci. 1982;47:1851–1852. [Google Scholar]
- Hasunuma T, Fukusaki E, Kobayash A. Methanol production is enhanced by expression of an Aspergillus niger pectin methylesterase in tobacco cells. J Biotechnol. 2003;106:45–52. doi: 10.1016/j.jbiotec.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Heerd D, Yegin S, Tari C, Fernandez-Lahore M. Pectinase enzyme-complex production by Aspergillus spp. in solid-state fermentation: a comparative study. Food Bioprod Process. 2012;90:102–110. [Google Scholar]
- Heerd D, Diercks-Horn S, Fernández-Lahore M. Efficient polygalacturonase production from agricultural and agro-industrial residues by solid-state culture of Aspergillus sojae under optimized conditions. Springer Plus. 2014 doi: 10.1186/2193-1801-3-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendges DH, Montanari Q, Malvessi E, da Silveira MM. Production and characterization of endo-polygalacturonase from Aspergillus niger in solid-state fermentation in double-surface bioreactor. Brazilian Arch Biol Technol. 2011;54:253–258. [Google Scholar]
- Hölker U, Höfer M, Lenz J. Biotechnological advantages of laboratory-scale solid- state fermentation with fungi. Appl Microbiol Biotechnol. 2004;64:175–186. doi: 10.1007/s00253-003-1504-3. [DOI] [PubMed] [Google Scholar]
- Ibrahim D, Weloosamy H, Sheh-Hong L. Potential use of nylon scouring pad cubes attachment method for pectinase production by Aspergillus niger HFD5A-1. Process Biochem. 2014;49:660–667. [Google Scholar]
- IHD (Indian horticulture Database) Ministry of Agriculture Government of India (2014) Indian Horticulture Production at a Glance. pp 100–105
- Ittimongkol B, Srisukh V, Tungrugsasut W, Sukkhow B, Chinganjanarod P, Jaicharoen P. Instant dietary fiber supplemented beverage mixes from papaya. Thai J Phytopharma. 2002;9:13–21. [Google Scholar]
- Jahan N, Shahid F, Aman A, Mujahid TY, Qader SAU. Utilization of agro waste pectin for the production of industrially Important polygalacturonase. Heliyon 3. 2017 doi: 10.1016/j.heliyon.2017.e00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayani RS, Saxena S, Gupta R. Microbial pectinolytic enzymes: a review. Process Biochem. 2005;40:2931–2944. [Google Scholar]
- Jiang X, Chen P, Yin M, Yang Q. Constitutive expression, purification and characterization of pectin methylesterase from Aspergillus niger in Pichia pastoris for potential application in the fruit juice industry. J Sci Food Agric. 2013;93:375–381. doi: 10.1002/jsfa.5771. [DOI] [PubMed] [Google Scholar]
- Jiraratananon R, Chanachai A. A study of fouling in the ultra filtration of passion fruit juice. J Membr Sci. 1996;111:39–48. [Google Scholar]
- Jolie RP, Duvetter T, Van Loey AM, Hendrickx ME. Pectin methylesterase and its proteinaceous inhibitor: a review. Carbohydr Res. 2010;345:2583–2595. doi: 10.1016/j.carres.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Joshi VK, Parmar M, Rana NS. Pectin Esterase Production from Apple Pomace in Solid-State and Submerged Fermentations. Food Technol Biotechnol. 2006;44:253–256. [Google Scholar]
- Joshi M, Nerurkar M, Badhe P, Adivarekar R. Scouring of cotton using marine pectinase. J Mol Cataly B Enzym. 2013;98:106–113. [Google Scholar]
- Kant S, Vohra A, Gupta R. Purification and physicochemical properties of polygalacturonase from Aspergillus niger MTCC 3323. Protein Exp Pur. 2013;87:11–16. doi: 10.1016/j.pep.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Kashyap DR, Vohra PK, Chopra S, Tewari R. Applications of pectinases in the commercial sector: a review. Bioresour Technol. 2001;77:215–227. doi: 10.1016/s0960-8524(00)00118-8. [DOI] [PubMed] [Google Scholar]
- Kashyap DR, Soni SK, Tewari R. Enhanced production of polygalacturonase by Bacillus sp. DT7 using solid state fermentation. Bioresour Technol. 2003;88:251–254. doi: 10.1016/s0960-8524(02)00206-7. [DOI] [PubMed] [Google Scholar]
- Kaur SJ, Gupta VK. Production of pectinolytic enzymes pectinase and pectin lyase by Bacillus subtilis SAV-21 in solid state fermentation. Ann Microbiol. 2017 [Google Scholar]
- Kaur A, Mahajan R, Singh A, Garg G, Sharma J. A novel and cost effective methodology for qualitative screening of alkalo-thermophilic cellulase free xylano-pectinolytic microorganisms using agricultural wastes. World J Microbiol Biotechnol. 2011;27:459–463. [Google Scholar]
- Kertesz ZI. The pectic substances. New York and London: Interscience Publisher; 1951. p. 628. [Google Scholar]
- Kohli P, Gupta R. Alkaline pectinase: a review. Biocataly Agri Biotech. 2015;4:279–285. [Google Scholar]
- Kohli P, Kalia M, Gupta R. Pectin Methylesterases: a Review. J Bioproc Biotech. 2015;5:1–7. [Google Scholar]
- Koubala BB, Christiaens G, Kansci S, Loey AMV, Hendrickx ME. Isolation and structural characterization of papaya peel pectin. Food Res Int. 2014;55:215–221. [Google Scholar]
- Kumar YS, Kumar PV, Reddy OVS. Pectinase production from mango peel using Aspergillus foetidus and its application in processing of mango juice. Food Biotechnol. 2012;26:107–123. [Google Scholar]
- Lee WC, Yusof S, Hamid NSA, Baharin BS. Optimizing conditions for enzymatic clarification of banana juice using response surface methodology (RSM) J Food Eng. 2006;73:55–63. [Google Scholar]
- Li Z, Bai Z, Zhang B, Xie H, Hu Q, Hao C, Xue W, Zhang H. Newly isolated Bacillus gibsonii S-2 capable of using sugar beet pulp for alkaline pectinase production. World J Microbiol Biotechnol. 2005;21:1483–1486. [Google Scholar]
- Li K, Meng K, Pan X, Ma R, Yang P, Huang H, Yao B, Su X. Two thermophilic fungal pectinases from Neosartorya fischeri P1: gene cloning, expression, and biochemical characterization. J Mol Cataly B: Enzym. 2015;118:70–78. [Google Scholar]
- Li Y, Wang Y, Tu T, Zhang D, Ma R, You S, Wang X, Yao B, Luo H, Xu B. Two acidic, thermophilic GH28 polygalacturonases from Talaromyces leycettanus JCM 12802 with application potentials for grape juice clarification. Food Chem. 2017;237:997–1003. doi: 10.1016/j.foodchem.2017.06.037. [DOI] [PubMed] [Google Scholar]
- Lionetti V. PECTOPLATE: the simultaneous phenotyping of pectin methylesterases, pectinases, and oligogalacturonides in plants during biotic stresses. Front Plant. 2015;331:1–8. doi: 10.3389/fpls.2015.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionetti V, Fabri E, Caroli MD, Hansen AR, Willats WGT, Piro G, Bellincampi D. Three pectin methyl esterase inhibitors protect cell wall integrity for immunity to Botrytis. 2017 doi: 10.1104/pp.16.01185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoodi M, Najafpour GD, Mohammadi M. Production of pectinases for quality apple juice through fermentation of orange pomace. J Food Sci Technol. 2017 doi: 10.1007/s13197-017-2829-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maktouf S, Neifar M, Drira SJ, Baklouti S, Fendri M, Châabouni SE. Lemon juice clarification using fungal pectinolytic enzymes coupled to membrane ultra filtration. Food Bioprod Process. 2014;92:14–19. [Google Scholar]
- Maldonado MC, Strasser de Saad AM. Production of pectinesterase and polygalacturonase by Aspergillus niger in submerged and solid state systems. J Ind Microb Biotechnol. 1998;20:34–38. doi: 10.1038/sj.jim.2900470. [DOI] [PubMed] [Google Scholar]
- Maller A, Damasio ARL, da Silva TM, Jorge JA, Terenzi HF, de Moraes Polizeli MLT. Biotechnological potential of agro-industrial wastes as a carbon source to thermostable polygalacturonase production in Aspergillus niveus. Enzym Res. 2011 doi: 10.4061/2011/289206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maran JP, Prakash KA. Process variables influence on microwave assisted extraction of pectin from waste Carcia papaya L. peel. Int J Biol Macromol. 2015;73:202–206. doi: 10.1016/j.ijbiomac.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Maran JP, Shivkumar V, Thirugnanasambandham K, Shridhar R. Optimization of microwave assisted extraction of pectin from orange peel. Carbohydr Poly. 2013;97:703–709. doi: 10.1016/j.carbpol.2013.05.052. [DOI] [PubMed] [Google Scholar]
- Martínez JMG, Gomez EV, Peman J, Canton E, García MG, del Castillo Agudo L. Identification of pathogenic yeast species by polymerase chain reaction amplification of the RPS0 gene intron fragment. J Appl Microbiol. 2009 doi: 10.1111/j.1365-2672.2009.04595.x. [DOI] [PubMed] [Google Scholar]
- Martins ES, Silva D, Da Silva R, Gomes E. Solid state production of thermostable pectinases from thermophilic Thermoascus aurantiacus. Process Biochem. 2002;37:949–954. [Google Scholar]
- Martos MA, Vazquez FM, Benassi FO, Hours RA. Production of pectinases by A. niger: influence of fermentation conditions. Braz Arch Biol Technol. 2009;52:567–572. [Google Scholar]
- Meneghel L, Pellenz Reis G, Reginatto C, Malvessi E, Moura da Silveira M. Assessment of pectinase production by Aspergillus oryzae ingrowth-limiting liquid medium under limited and non-limited oxygen supply. Process Biochem. 2014;49:1800–1807. [Google Scholar]
- Micheli F. Pectin methylesterase: cell wall enzymes with important roles in plant physiology. Trends Plant Sci. 2001;6:414–419. doi: 10.1016/s1360-1385(01)02045-3. [DOI] [PubMed] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analy Chem. 1959;31:426–428. [Google Scholar]
- Miller L, Macmillan JD. Purification and pattern of action of pectinesterase from Fusarium oxysporum f. sp. vasinfectum. Biochem. 1971;10:570–576. doi: 10.1021/bi00780a005. [DOI] [PubMed] [Google Scholar]
- Mohapatra D, Mishra S, Singh CB, Jayas DS. Post-harvest processing of banana: opportunities and challenges. Food Bioprocess Technol. 2011;4:327–339. [Google Scholar]
- Molnar E, Eszterle M, Kiss K, Nemestóthy N, Fekete J, Bélafi-Bako K. Utilization of electrodialysis for galacturonic acid recovery. Desalinat. 2009;241:81–85. [Google Scholar]
- Nakagawa T, Miyaji T, Yurimoto H, Sakai Y, Kato N, Tomizuka N. A methylotrophic pathway participates in pectin utilization of Candida boidinii. Appl Environ Microbiol. 2000;66:4253–4257. doi: 10.1128/aem.66.10.4253-4257.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakkeeran E, Umesh-Kumar S, Subramanian R. Aspergillus carbonarius polygalacturonases purified by integrated membrane process and affinity precipitation for apple juice production. Bioresour Technol. 2011;102:3293–3297. doi: 10.1016/j.biortech.2010.10.048. [DOI] [PubMed] [Google Scholar]
- Nelson N. A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem. 1944;153:375–380. [Google Scholar]
- Ng A, Smith AC, Waldron KW. Effect of tissue type and variety on cell wall chemistry of onion (Allium cepa L.) Food Chem. 1998;63:17–24. [Google Scholar]
- Nighojkar A, Srivastava S, Kumar A. Pectin methylesterase from germinating Vigna sinesis seeds. Plant Sci. 1994;103:115–120. [Google Scholar]
- Nighojkar S, Phanse Y, Sinha D, Nighojkar A, Kumar A. Production of polygalacturonase by immobilized cells of Aspergillus niger using orange peel as inducer. Process Biochem. 2006;41:1136–1140. [Google Scholar]
- OECD (Environment Directorate Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides and Biotechnology) (2005) Consensus document on the biology of papaya (Carica papaya) series on harmonisation of regulatory oversight in biotechnology No. 33
- Ortiz GE, Guitart ME, Albertó E, Fernández Lahore HM, Blasco M. Microplate assay for endo-polygalacturonase activity determination based on ruthenium red method. Anal Biochem. 2014;454:33–35. doi: 10.1016/j.ab.2014.02.028. [DOI] [PubMed] [Google Scholar]
- Ortiz GE, Ponce-Mora MC, Noseda DG, Cazabat G, Saravalli C, López MC, Gil GP, Blasco M, Albertó EO. Pectinase production by Aspergillus giganteus in solid-state fermentation: optimization, scale-up, biochemical characterization and its application in olive-oil extraction. J Ind Microbiol Biotechnol. 2017 doi: 10.1007/s10295-016-1873-0. [DOI] [PubMed] [Google Scholar]
- Pandey A. Solid-state fermentation. Biochem Eng J. 2003;13:81–84. doi: 10.1016/s1369-703x(00)00065-6. [DOI] [PubMed] [Google Scholar]
- Pandey A, Soccol CR, Nigam P, Soccol VT. Biotechnological potential of agro-industrial residues. I: sugar cane bagasse. Bioresour Technol. 2000;74:69–80. [Google Scholar]
- Parmar I, Rupasinghe HPV. Bio-conversion of apple pomace into ethanol and acetic acid: enzymatic hydrolysis and fermentation. Bioresour Technol. 2013;130:613–620. doi: 10.1016/j.biortech.2012.12.084. [DOI] [PubMed] [Google Scholar]
- Patidar MK, Nighojkar S, Kumar A, Nighojkar A. Papaya peel valorization for production of acidic pectin methylesterase by Aspergillus tubingensis and its application for fruit juice clarification. Biocatal Agri Biotechnol. 2016;6:58–67. [Google Scholar]
- Patidar MK, Nighojkar S, Nighojkar A, Kumar A. Purification and characterization of polygalacturonase produced by Aspergillus niger AN07 in Solid State Fermentation. Can J Biotech. 2017;1:11–18. [Google Scholar]
- Patil SR, Dayanand A. Production of polygalacturonase from deseeded sunflower head by Aspergillus niger in submerged and solid-state conditions. Bioresour Technol. 2006;97:2054–2058. doi: 10.1016/j.biortech.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Pereira GS, Cipriani M, Wisbeck E, Souza O, Strapazzon JO, Gern RMM. Onion juice waste for production of Pleurotussajor-caju and pectinases. Food Bioprod Process. 2017;106:11–18. [Google Scholar]
- Pervez S, Shahid F, Aman A, Qader SAU. Algal biomass: a sustainable, economical and renewable approach for microbial production of pectinolytic enzymes using submerged and solid state fermentation techniques. Biocataly Biotransform. 2017 [Google Scholar]
- Phutela U, Dhuna V, Sandhu S, Chadha BS. Pectinase and polygalacturonase production by a thermophilic Aspergillus fumigatus isolated from decomposting orange peels. Braz J Microbiol. 2005;36:63–69. [Google Scholar]
- Pitol LO, Biz A, Mallmann E, Krieger N, Mitchell DA. Production of pectinases by solid-state fermentation in a pilot-scale packed-bed bioreactor. Chem Eng J. 2016;283:1009–1018. [Google Scholar]
- Plaza L, Duvetter T, der Plancken IV, Meersman F, Loey AV, Hendrickx M. Influence of environmental conditions on thermal stability of recombinant Aspergillus aculeatus pectinmethylesterase. Food Chem. 2008;111:912–920. [Google Scholar]
- Poletto P, Polidoro TA, Zeni M, da Silveira MM. Evaluation of the operating conditions for the solid-state production of pectinases by Aspergillus niger in a bench-scale, intermittently agitated rotating drum bioreactor. LWT - Food Sci Technol. 2017 [Google Scholar]
- Poondla V, Yannam SK, Gummadi SN, Subramanyam R, Obulam VSR. Enhanced production of pectinase by Saccharomyces cerevisiae isolate using fruit and agro-industrial wastes: its application in fruit and fiber processing. Biocataly Agri Biotechnol. 2016 [Google Scholar]
- Rai P, Majumdar GC, Das Gupta S, De S. Optimizing pectinase usage in pretreatment of mosambi juice for clarification by response surface methodology. J Food Eng. 2004;64:397–403. [Google Scholar]
- Raimbault M. General and microbiological aspects of solid substrate fermentation. Elec J Biotech. 1998;1:1–15. [Google Scholar]
- Rajauria G, Tiwari BK. Fruit juices: an overview. Cambridge: Academic Press; 2018. pp. 3–13. [Google Scholar]
- Rajdeo K, Harini T, Lavanya K, Fadnavis NW. Immobilization of pectinase on reusable polymer support for clarification of apple juice. Food Bioprod Process. 2016;99:12–19. [Google Scholar]
- Ramírez-Tapias YA, Rivero CW, Britos CN, Trelles JA. Alkaline and thermostable polygalacturonase from Streptomyces halstedii ATCC10897 with applications in waste waters. Biocatal Agri Biotechnol. 2014;4:221–228. [Google Scholar]
- Rebello S, Mohandas A, Mathachan Aneesh E, Sindhu R, Binod P, Pandey A. Recent advancements in the production and application of microbial pectinases: an overview. Rev Environ Sci Biotechnol. 2017 [Google Scholar]
- Reddy PM, Saritha VK. Bio-catalysis of mango industrial waste by newly isolated Fusarium sp. (PSTF1) for pectinase production. 3 Biotech. 2015;5:893–900. doi: 10.1007/s13205-015-0288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PM, Saritha VK. Effects of the culture media optimization on pectinase production by Enterobacter sp. PSTB-1. 3 Biotech. 2016;6:207. doi: 10.1007/s13205-016-0502-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman HU, Aman A, Zohra RR, Ul Qader SA. Immobilization of pectin degrading enzyme from Bacillus licheniformis KIBGE IB-21 using agar-agar as a support. Carbohy Poly. 2014;102:622–626. doi: 10.1016/j.carbpol.2013.11.073. [DOI] [PubMed] [Google Scholar]
- Reid S, Sims IM, Melton LD, Gane AM. Characterization of extracellular polysaccharides from suspension cultures of apple (Malus domestica) Carbohydr Polym. 1999;39:369–379. [Google Scholar]
- Rezende CA, de Lima MA, Maziero P, de Azevedo ER, Garcia W, Polikarpov I. Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnol Biofuels. 2011;4:54–72. doi: 10.1186/1754-6834-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Fernández DE, Odríguez-León JA, de Carvalho JC, Sturm W, Soccol CR. The behavior of kinetic parameters in production of pectinase and xylanase by solid-state fermentation. Bioresour Technol. 2011;102:10657–10662. doi: 10.1016/j.biortech.2011.08.106. [DOI] [PubMed] [Google Scholar]
- Romero-Lopez MR, Osorio-Diaz P, Bello-Perez LA, Tovar J, Bernardino-Nicanor A. Fiber concentrate from orange (Citrus sinensis L.) bagasse: characterization and application as bakery product ingredient. Int J Mol Sci. 2011;12:2174–2186. doi: 10.3390/ijms12042174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussos S, Jean-Charles Labrousse Y, Lakhtar H, Tranier MS (2014) Solid state fermentation device and products obtained. Fr Patent. WO2014/118757 A1
- Ruiz HA, Rodríguez-Jasso RM, Rodríguez R, Contreras-Esquivel JC, Aguilar CN. Pectinase production from lemon peel pomace as support and carbon source in solid-state fermentation column-tray bioreactor. Biochem Eng J. 2012;65:90–95. [Google Scholar]
- Sagu ST, Nso EJ, Karmakar S, De S. Optimization of low temperature extraction of banana juice using commercial pectinase. Food Chem. 2013;151:182–190. doi: 10.1016/j.foodchem.2013.11.031. [DOI] [PubMed] [Google Scholar]
- Salas-Tovar JA, Flores-Gallegos AC, Contreras-Esquivel JC, Escobedo-García S, Morlett-Chávez JA, Rodríguez-Herrera R. Analytical Methods for Pectin Methylesterase Activity Determination: a Review. Food Anal Method. 2017 [Google Scholar]
- Sanchez SR, Sanchez IG, Arevalo-Villena M, Briones Perez A. Production and immobilization of enzymes by solid-state fermentation of agroindustrial waste. Bioprocess Biosyst Eng. 2015;38:587–593. doi: 10.1007/s00449-014-1298-y. [DOI] [PubMed] [Google Scholar]
- Sandri IG, Fontana RC, da Silveira MM. Influence of pH and temperature on the production of polygalacturonases by Aspergillus fumigatus. LWT - Food SciTechnol. 2015;61:430–436. [Google Scholar]
- Satter MA, Ara H, Jabin SA, Abedin N, Azad AK, Hossain A, Ara U. Nutritional Composition and Stabilization of Local Variety Rice Bran BRRI-28. Int J Sci Technol. 2014;3:306–313. [Google Scholar]
- Sethi BK, Nanda PK, Sahoo S. Enhanced production of pectinase by Aspergillus terreus NCFT 4269.10 using banana peels as substrate. 3. Biotech. 2016;6:36. doi: 10.1007/s13205-015-0353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalini R, Gupta DK. Utilization of pomace from apple processing industries: a review. J Food Sci Technol. 2009;47:365–371. doi: 10.1007/s13197-010-0061-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma DC, Satyanarayana T. A marked enhancement in the production of a highly alkaline and thermostable pectinase by Bacillus pumilus dcsr1 in submerged fermentation by using statistical methods. Bioresour Technol. 2006;97:727–733. doi: 10.1016/j.biortech.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Sharma N, Rathore M, Sharma M. Microbial pectinase: sources, characterization and applications. Rev Environ Sci Biotechnol. 2013;12:45–60. [Google Scholar]
- Sharma S, Sharma J, Mandhan RP. Lucrative pectinase production by novel strain Pseudozyma sp. SPJ with statistical optimization techniques using agro-industrial residues. Front Biol. 2014;9:317–323. [Google Scholar]
- Sharma HP, Patel H, Sugandha Enzymatic added extraction and clarification of fruit juices–a review. Crit Rev Food Sci Nutr. 2017;57(6):1215–1227. doi: 10.1080/10408398.2014.977434. [DOI] [PubMed] [Google Scholar]
- Shrivastava S, Nighojkar A, Kumar A. Multiple forms of pectin methylesterase from Cuscuta reflexa filaments. Phytochem. 1994;37(5):1233–1236. [Google Scholar]
- Silva D, Tokuioshi K, Martin ES, Silva RD, Gomes E. Production of pectinase by solid state fermentation with Penicillium viridicatum RFC3. Process Biochem. 2005;40:2885–2889. [Google Scholar]
- Silva TD, Rashid JA, Tan Z, Sivakumar D, Gera DN, Teixeira AMS, et al. Papaya (Carica papaya L.) biology and biotechnology. Tree Forestry Sci Biotechonol. 2007;1(1):47–73. [Google Scholar]
- Singh S, Mandal SK. Optimization of processing parameters for production of pectinolytic enzymes from fermented pineapple residue of mixed Aspergillus species. Jordan J Biol Sci. 2012;5:307–314. [Google Scholar]
- Singhania RR, Patel AK, Soccol CR, Pandey A. Recent advances in solid-state fermentation. Biochem Eng J. 2009;44:13–18. [Google Scholar]
- Sittidilokratna C, Suthirawut S, Chitradon L, Punsuvon V, Vaithanomsat P, Siriacha P. Screening of pectinase producing bacteria and their efficiency in biopulping of paper mulberry bark. Sci Asia. 2007;33:131–135. [Google Scholar]
- Soares MMCN, da Silva R, Gomes E. Screening of bacterial strains for pectinolytic activity: characterization of the polygalacturonase produced by bacillus sp. Revista de Microbiol. 1999;30:299–303. [Google Scholar]
- Soccol CR, da Costa ESF, Letti LAJ, Karp SG, Woiciechowski AL, Vandenberghe LPS. Recent developments and innovations in solid state fermentation. Biotechnol Res Inno. 2017;1:52–71. [Google Scholar]
- Solis-Pereyra S, Favela-Torres E, Gutierrez-Rojas M, Roussos S, Saucedo-Castaneda G, Gunasekaran P, Viniegra-Gonzalez G. Effect of different carbon sources on synthesis of pectinase by Aspergillus niger in submerged and solid state fermentation. World J Microbiol Biotechnol. 1996;12:257–260. doi: 10.1007/BF00360924. [DOI] [PubMed] [Google Scholar]
- Somogyi M. Notes on sugar determination. J Biol Chem. 1952;195:19–25. [PubMed] [Google Scholar]
- Sreenath HK, Santhanam K. The use of commercial enzymes in white grape juice clarification. J Fer Bioeng. 1992;73:241–243. [Google Scholar]
- Sudha ML, Baskaran V, Leelavathi K. Apple pomace as a source of dietary fiber and polyphenols and its effect on the rheological characteristics and cake making. Food Chem. 2007;104:686–692. [Google Scholar]
- Sunnotel O, Nigam P. Pectinolytic activity of bacteria isolated from soil and two fungal strains during submerged fermentation. World J Microbiol Biotechnol. 2002;18:835–839. [Google Scholar]
- Swain MR, Kar S, Ray RC. Exo-polygalacturonase production by Bacillus subtilis CM5 in solid state fermentation using cassava bagasse. Braz J Microbiol. 2009;40:636–648. doi: 10.1590/S1517-838220090003000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai E, Hsieh P, Sheu S. Effect of polygalacturonase and feruloyl esterase from Aspergillus tubingensis on demucilage and quality of coffee beans. Process Biochem. 2014;49:1274–1280. [Google Scholar]
- Tao N, Shi W, Liu Y, Huang S. Production of feed enzymes from citrus processing waste by solid-state fermentation with Eupenicillium javanicum. Int J Food Sci Technol. 2011;46:1073–1079. [Google Scholar]
- Tapre AR, Jain RK. Pectinases; enzymes for fruit processing industry. Int Food Res J. 2014;21:447–453. [Google Scholar]
- Taragano VM, Pilosof AMR. Application of Doehlert designs for water activity, pH, and fermentation time optimization for Aspergillus niger pectinolytic activities production in solid-state and submerged fermentation. Enzym Microb Technol. 1999;25:411–419. [Google Scholar]
- Tariq A, Latif Z. Isolation and biochemical characterization of bacterial isolates producing different levels of polygalacturonases from various sources. African J Microbiol Res. 2012;6(45):7259–7264. [Google Scholar]
- Tochi BN, Wang Z, Xu SY, Zhang W. The influence of a pectinase and pectinase/hemicellulases enzyme preparations on percent pineapple juice recovery, particulates and sensory attributes. Pak J Nutr. 2009;8:1184–1189. [Google Scholar]
- Torres S, Sayago JE, Ordoñez RM, Isla MI. A colorimetric method to quantify endo-polygalacturonase activity. Enzym Microb Technol. 2011;48:123–128. doi: 10.1016/j.enzmictec.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Tounsi H, Sassi HD, Romdhane ZB, Lajnef M, Dupuy JW, Lapaillerie D, Lomenech AM, Bonneu M, Gargouri A, Hadj-Taieb N. Catalytic properties of a highly thermoactive polygalacturonase from the mesophilic fungus Penicillium occitanis and use in juiceclarification. J Mol Cataly B Enzy. 2016;127:56–66. [Google Scholar]
- Trentini MMS, Toniazzo G, Zeni J, Pili J, DiLuccio M, Valduga E. Purification of pectinases from Aspergillus niger ATCC9642 by ethanol precipitation. Biocatal Agri Biotechnol. 2015;4:315–320. [Google Scholar]
- Tu T, Meng K, Bai Y, Shi P, Luo H, Wang Y, Yang P, Zhang Y, Zhang W, Yao B. High-yield production of a low-temperature-active polygalacturonase for papaya juice clarification. Food Chem. 2013;141:2974–2981. doi: 10.1016/j.foodchem.2013.05.132. [DOI] [PubMed] [Google Scholar]
- USDA (2013) Grain: world markets and trade circular series FG 08-13: p 8 [Online], http://www.fas.usda.gov/. Accessed 28 Aug 2013
- Uzuner S, Cekmecelioglu D. Enhanced pectinase production by optimizing fermentation conditions of Bacillus subtilis growing on hazelnut shell hydrolyzate. J Mol Cataly B Enzym. 2015;113:62–67. [Google Scholar]
- Valette-Collet O, Cimerman A, Reignault P, Levis C, Boccara M. Disruption of Botrytis cinerea pectin methylesterase gene Bcpme1 reduces virulence on several host plants. Mol Plante Microb Interact. 2003;16:360–367. doi: 10.1094/MPMI.2003.16.4.360. [DOI] [PubMed] [Google Scholar]
- Van Alebeek GJ, Van Scherpenzeel K, Beldman G, Schols HA, Voragen AG. Partially esterified oligogalacturonides are the preferred substrates for pectin methylesterase of Aspergillus niger. Biochem J. 2003;372:211–218. doi: 10.1042/BJ20021471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilarino C, Del Giorgio JF, Hours RA, Cascone O. Spectrophotometric method for fungal pectinesterase activity determination. LWT - Food Sci Technol. 1993;26:107–110. [Google Scholar]
- Viniegra-González G, Favela-Torres E, Aguilar CN, Rómero-Gomez SJ, Dıaz-Godınez G, Augur C. Advantages of fungal enzyme production in solid state over liquid fermentation systems. Biochem Eng J. 2003;13:157–167. [Google Scholar]
- Whitaker JR. Pectic substances, pectic enzymes and haze formation in fruit juices. Enzym Microb Technol. 1984;6:341–347. [Google Scholar]
- White L, Fabian F. Notes on viscometric method. J Appl Microbiol. 1953;1:724. [Google Scholar]
- Wilinska A, Rodrigues ASF, Bryjak J, Polakovic M. Thermal inactivation of exogenous pectin methylesterase in apple and cloudberry juices. J Food Eng. 2008;85:459–465. [Google Scholar]
- Wong LY, Saad WZ, Mohamad R, Tahir PM. Optimization of cultural conditions for polygalacturonase production by a newly isolated Aspergillus fumigatus R6 capable of retting kenaf. Indust Crop Prod. 2017;97:175–183. [Google Scholar]
- Wood PJ, Siddiqui IR. Determination of methanol and its application to measurement of pectin ester content and pectin methyl esterase activity. Anal Biochem. 1971;39(2):418–428. doi: 10.1016/0003-2697(71)90432-5. [DOI] [PubMed] [Google Scholar]
- Yang J, Luo H, Li J, Wang K, Cheng H, Bai Y, Yuan T, Fan Y, Yao B. Cloning, expression and characterization of an acidic endo-polygalacturonase from Bispora sp. MEY-1 and its potential application in juice clarification. Process Biochem. 2011;46:272–277. [Google Scholar]
- Yuan P, Meng K, Huang H, Shi P, Luo H, Yang P, Yao B. A novel acidic and low-temperature-active endo-polygalacturonase from Penicillium sp. CGMCC 1669 with potential for application in apple juice clarification. Food Chem. 2011;129:1369–1375. [Google Scholar]
- Zapata ADZ, Voget CE. Primary isolation of Geotrichum klebahnii polygalacturonase by capturing with glass fiber microfilters. Process Biochem. 2012;47:1277–1281. [Google Scholar]
- Zeni J, Cence K, Grando CE, Tiggermann L, Colet R, Lerin LA, Cansian RL, Toniazzo G, de Oliveira D, Valduga E. Screening of pectinase-producing microorganisms with polygalacturonase activity. Appl Biochem Biotechnol. 2011;163:383–392. doi: 10.1007/s12010-010-9046-5. [DOI] [PubMed] [Google Scholar]
- Zhang F, Tian M, Du M, Fang T. Enhancing the activity of pectinase using pulsed electric field (PEF) treatment. J Food Eng. 2017;205:56–63. [Google Scholar]
- Zheng ZX, Shetty K. Solid-state production of polygalacturonase by Lentinus edodes using fruit processing wastes. Process Biochem. 2000;35:825–830. [Google Scholar]
- Zhou J, Ge X, Zhang W. Improvement of polygalacturonase production at high temperature by mixed culture of Aspergillus niger and Saccharomyces cerevisiae. Bioresour Technol. 2011;102:10085–10088. doi: 10.1016/j.biortech.2011.08.077. [DOI] [PubMed] [Google Scholar]
- Zocca F, Lomolino G, Curioni A, Spettoli P, Lante A. Detection of pectin methylesterase activity in presence of methanol during grape pomace storage. Food Chem. 2007;102:59–65. [Google Scholar]
- Zykwinska A, Boiffard M, Kontkanen H, Buchert J, Franc J, Thibault O, Bonnin E. Extraction of Green Labeled Pectins and Pectic Oligosaccharides from Plant Byproducts. J Agri Food Chem. 2008;56:926–935. doi: 10.1021/jf801705a. [DOI] [PubMed] [Google Scholar]