Abstract
We introduce an approach to predict deterioration of reaction state for people having neurological movement disorders such as hand tremors and nonvoluntary movements. These involuntary motor features are closely related to the symptoms occurring in patients suffering from Huntington's disease (HD). We propose a hybrid (neurofuzzy) model that combines an artificial neural network (ANN) to predict the functional capacity level (FCL) of a person and a fuzzy logic system (FLS) to determine a stage of reaction. We analyzed our own dataset of 3032 records collected from 20 test subjects (both healthy and HD patients) using smart phones or tablets by asking a patient to locate circular objects on the device's screen. We describe the preparation and labelling of data for the neural network, selection of training algorithms, modelling of the fuzzy logic controller, and construction and implementation of the hybrid model. The feed-forward backpropagation (FFBP) neural network achieved the regression R value of 0.98 and mean squared error (MSE) values of 0.08, while the FLS provides a final evaluation of subject's reaction condition in terms of FCL.
1. Introduction
Huntington disease (HD) is a progressive genetic neurodegenerative disorder causing involuntary movement and cognitive problems that significantly affect daily life of HD patients. HD affects about 1 in 10,000 to 20,000 people of European (Caucasian) descent [1], though in some isolated populations it is much higher. HD reduces life expectancy due to heart disease, pneumonia, physical injury from falls, and suicide. The most visible symptom of HD is chorea, which consists of jerky, involuntary movements of the upper and lower extremities, face or body, and occurs in about 90% of patients at some stage of their illness [2]. Other symptoms include behavioural problems, cognitive impairment, psychiatric disorders, and dementia, which have a serious impact on daily living of a patient and often result in hospitalization. The societal and financial cost of HD on health and social care systems is significant and is estimated to be £195 million per year in the UK alone [3].
HD is currently incurable so most of the current research in this area focuses on identifying the deficits at the early stage of the disease, to benefit from future medical interventions that may help delaying the progress of the disease [4, 5]. This is also the case of the work presented in this paper. Traditional HD research often include magnetic resonance neuroimaging (MRI) measures of striatum and white matter volume, CAG repeat length in chromosome analysis, age, and striatal atrophy [6, 7]. Moreover, medical personnel and doctors who have experience in caring after HD patients and knowing that disease is cureless are not usually motivated to conduct scientific research themselves or to support multidiscipline (e.g., bioinformatics) investigations.
Any scientific result (device, technology, and theoretical model) that could contribute towards improvement of daily life of HD patient's and help to monitor or predict the progress of the disease can be useful for both doctors and HD patients.
The problematics of data prediction evolved with the rise of artificial intelligence (AI) and machine learning (ML) methods and algorithms. Artificial neural networks (ANN) such as multilayer perceptron (MLP) can be used for classification of accelerometer-based tremor signals invoked by Parkinson patient's involuntary movements [8]. Prediction of Parkinson disease onset by adapting radial basis function neural network (RBFNN) for tremor activity data recorded via stimulation electrodes using electromyography (EMG) signals is described in [9]. Dynamic neural network (DNN) is used to detect time-varying occurrences of tremor and dyskinesia from time series data acquired from EMG sensors and triaxial accelerometers worn by Parkinson patients [10]. Another approach of designing a prediction model for Parkinson's disease uses a decision tree and Iterative Dichotomiser (ID3) methods to analyze data collected from HD symptoms such as trembling in the legs, arms, hands, impaired speech articulation, and production difficulties [11]. Hybrid models combine different AI and ML approaches for reproducing intelligent human reasoning process [12]. By using information fusion, hybrid models combine heterogeneous ML approaches and improve quality of reasoning for complex regression and classification problems [13]. Neurofuzzy systems combine neural network and fuzzy logic paradigms to avoid the limitations of neural network explanations to reach decision and limitations of fuzzy logic to automatically acquire the rules used for making those decisions [14]. Fuzzy expert systems such as neurofuzzy system (ANFIS) can be applied in assessment of Parkinson's disease with a noninvasive screening system for quantitative evaluation and analysis by using amplitude, frequency, spectral characteristics, and trembling localization parameters of input data [15]. Hybrid model is adapted in designing a decision support system (DSS) for the intelligent identification of Alzheimer where neurofuzzy system explores approximation techniques from neural networks to find the parameter of a fuzzy system [16]. Hybrid systems are also used as a classifier fusion strategy (Bayesian, SVM, k-nearest neighbours) in the prevalence of age-related diseases like Alzheimer's and dementia [17], diagnostics and measurement [18] with wavelet transform (WT) and norm entropy feature extraction methods. The DSS that uses MLP and RBFNN is applied for monitoring patients with neurological disorders [19]. The data is collected using noninvasive smart devices (modified mouse and 3-axis accelerometer sensor). Integration of neurofuzzy networks and information fusion for multimodal human cognitive state recognition is described in [20]. Projection-based learning for metacognitive radial basis function network (PBL-McRBFN) is applied to predict Parkinson's disease [21]. Other hybrid systems and applications include nonlinear adaptive system, which fuses brain and gait information algorithmically using multistate Markov model [22]. Accurate Parkinson disease diagnosis model based on cluster analysis uses random tree, classification and regression tree (C-RT), ID3, binary logistic regression, k-NN, partial least square regression (PLS), support vector machines (SVM) [23], and fuzzy c-means clustering (FCM) [24]. Table 1 provides a summary of methods used by other authors.
Table 1.
Comparison of various ML methods adapted for neurodegenerative disorders such as Huntington or Parkinson disease to solve prediction and classification problems.
| Work ref. | ML method | Learning approach | ML problem | Size of data set | Number of test subjects | Target group | Involve HD patients |
|---|---|---|---|---|---|---|---|
| [8] | ANN, MLP | Supervised | Classification | — | 21 | PD, healthy | No |
| [9] | RBFNN | Supervised | Regression | — | — | PD | No |
| [10] | DNN | Supervised | Classification | — | 12 | PD (8), healthy (4) | No |
| [11] | Decision tree, ID3 | Supervised | Classification | 195 | 31 | PD (23), healthy (8) | No |
| [15] | Adaptive neurofuzzy | Hybrid | 100 | — | PD | No | |
| [16] | Neurofuzzy system | Hybrid | — | — | ALS | No | |
| [17] | Fusion of classifiers (Bayesian, SVM, k-nearest neighbor) | Hybrid | 640 | ALS (13), PD (15), HD (16), healthy (16) | ALS, PD, HD | Yes | |
| [18] | Neurofuzzy system | Hybrid | — | — | Only survey was done | No | |
| [19] | ANN + MLP, RBFNN | Hybrid | — | — | PD | No | |
| [20] | Neurofuzzy system | Hybrid | — | — | — | No | |
| [21] | PBL-McRBFN | Supervised | Classification | 22,283 | 72 (50 PD, 22 healthy) | PD, healthy | No |
| [22] | Multistate Markov model | Hybrid | 2500 | 72 (82 PD, 62 healthy) | PD, healthy | No | |
| [23] | Random tree, (C-RT), ID3, binary logistic regression, k-NN, (PLS), (SVM) | Supervised | Classification | 195 | 31 (23 PD, 8 healthy) | PD, healthy | No |
| [24] | FCM | Unsupervised | Clustering | 195 | — | PD | No |
Our previous work included the development of text input-based system for evaluating the condition of Huntington's patients [25]. The use of ANN for predicting the functional capacity of a Huntington's patient was proposed in [26].
The aim of this paper is to create a computerized behavioural model, which predicts an impaired reaction condition for HD patients. We develop a mobile application to collect a dataset using finger touch coordinates and reaction time features extracted from test subjects (healthy and HD patients); create an ANN to predict the functional capacity level and fuzzy logic system (FLS) to determine the reaction condition (stage) for individual person; combine ANN with FLS into a hybrid model to predict the impaired reaction condition for HD patients; and simulate an experimental setup for test subjects to perform a provided exercise (test) at the different moments in time in order to predict a possibly impairing reaction condition with the help of the proposed hybrid model.
2. Materials and Methods
2.1. Subjects
The study included ten (10) Huntington disease (HD) patients living in Lithuania. Each HD patient agreed to participate and allowed the data collected during the test to be used for scientific purposes. Every HD patient fall in the early clinical descriptor category of Huntington disease, that is, I and II stages according to Shoulson–Fahn evaluation system [27]. Such HD patients have hand tremors, body movement distractions, but are capable to perform the test on a mobile application without extra help, for example, from medical personnel, nurses, or family members. Other ten (10) participants were healthy people with no signs of any neurological or neurodegenerative disorder.
2.2. Procedure
The test can be performed using various mobile devices that support Android OS. The mobile application randomly generates circular shape objects (2, 3, and 5 circles at time) of particular color that are generated on the mobile device's screen. Each circle is located in different positions of the screen, thus no possible collisions (overlapping) between two particular circles are possible. An active circle that needs to be touched is marked by a black contour so as to differ from other objects.
The subjects are instructed to touch every object, starting from first in sequence, by finger as close to center and as quickly as possible. When subject finishes the test, collected data is stored in external mobile device storage and sent to the database using the internet connection.
2.3. Dataset
The collected dataset consists of 3032 data examples collected from 20 test subjects (10—healthy and 10—HD patients). The dataset (see a sample in Table 2) contains the ground truth coordinates of the generated object, the coordinates of subject's touch, subject's reaction time, subject's label, and the marker of Huntington's disease.
Table 2.
Collected data from mobile application (random sample data of 5 records).
| x | y | xt | yt | nC | rt | delta | User | IsSick |
|---|---|---|---|---|---|---|---|---|
| 126 | 871 | 125 | 872 | 5 | 1.665 | 1.414 | 1 | 0 |
| 411 | 403 | 390 | 408 | 3 | 3.886 | 21.587 | 1 | 0 |
| 243 | 609 | 299 | 592 | 3 | 0.573 | 58.523 | 2 | 1 |
| 580 | 377 | 618 | 449 | 5 | 0.545 | 81.413 | 2 | 1 |
| 501 | 634 | 437 | 585 | 2 | 0.741 | 56.436 | 3 | 1 |
x,y: screen coordinates of the center of circular object to touch; xt, yt: screen coordinates of user touch; nC: number of circular objects rendered on the device screen; rt: user's reaction time in seconds; delta: the Euclidean distance between object's center and touch position. User: user ID; IsSick: indicates if test subject has Huntington disease (1 yes, 0 otherwise).
2.4. Feature Extraction and Class Labelling
The subject's reaction time (rt) and the Euclidian distance between the two points of true and touched positions (delta) serve as features which are incorporated as input variables to ANN. We assume that smaller rt and delta values indicate better functional capacity level. The bigger delta value can show stronger hand tremoring, whereas higher rt value is an indicator of body stagnancy.
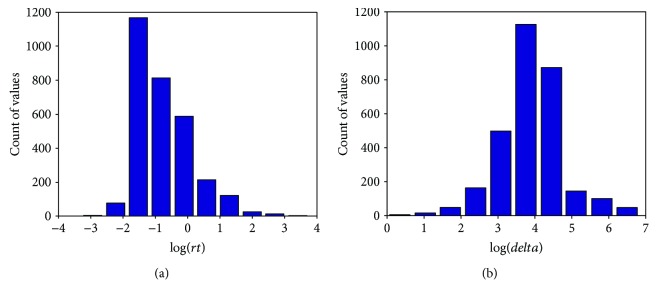
The statistical analysis of the rt and delta values has revealed that the values are not normally distributed, but after the applying the log transformation, which is commonly used in regression analysis of biological data with highly skewed distribution [28], the values become normal as confirmed by visual inspection in Figure 1 and skewness γ and kurtosis κ tests (γrt = 1.046, κrt = 4.239 and γdelta = 0.028, κdelta = 4.779). For data samples greater than 300, values |γ| < 2 and |κ| < 7 are considered as acceptable for normality [29].
Figure 1.
Statistical distribution of log-transformed rt (a) and delta (b) values.
To analyze the power of rt and delta values to correctly predict the healthy or sick state of the subject, we have performed feature evaluation using the relative entropy (also known as the Kullback–Leibler distance or divergence) criterion, considering different number of objects presented at the screen. The results are presented in Figure 2. In all cases, delta feature has larger discriminative power than rt, and the features from 3 and 5 objects test are more statistically discriminative.
Figure 2.
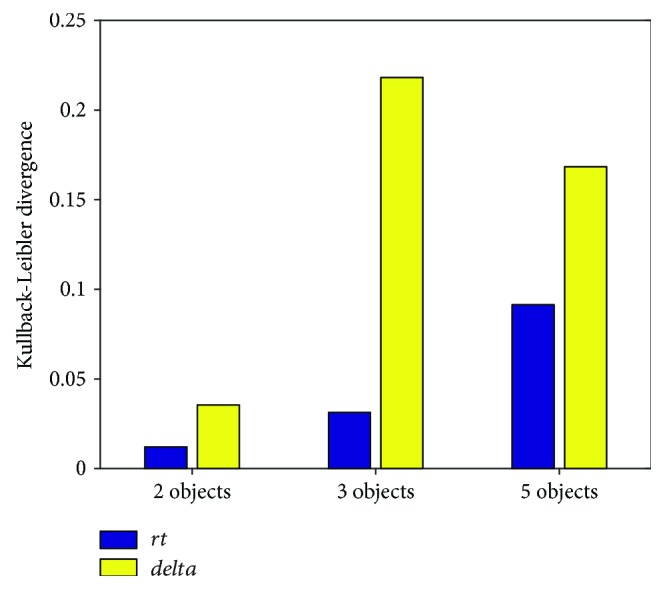
Feature ranking according to the Kullback–Leibler distance.
2.5. ANN for Functional Capacity Level Prediction
We have analyzed the following neural network models: (1) feed-forward backpropagation (FFBP); (2) feed-forward time delay neural network (FFTD); (3) cascade-forward backpropagation (CFBP); (4) nonlinear autoregressive exogenous model (NARX); (5) Elman neural network; (6) layer recurrent neural network (RNN); and (7) generalized regression neural network (GRNN).
FFBP is a simple neural network without any cycle connections between neurons [30]. FFTD has no internal state and adds delayed copies as other inputs as an input signal to obtain time-shift invariance [31]. In CFBP, the input values calculated after every hidden layer are backpropagated and the weights adjusted [32]. NARX have a limited feedback, which comes only from the output neuron rather than from hidden layer [33]. Elman network additionally has context units, which are connected to the hidden units, thus providing the network with memory [34]. RNN represent an architecture where connections between units form a directed cycle [35]. GRNN has only one (smoothness) parameter, and its convergence is guaranteed; fast and stable [36].
Each neural network has 2 inputs (rt, delta) and 1 output (Y). Neural network is composed of single neurons that are treated as a simple unit carrying signals (data) to each other or different layers via transfer functions, which correspond to sum of input signal. Training function is the optimization algorithm used for finding global minimum of a function. The outputs of ANN are class labels for determining the functional capacity of a person (the larger value indicates that a person is more capable to do motoric activities). Such scenario imitates the TFC scale measurement system for Huntington disease patients presented in Table 3 [27].
Table 3.
Total functional capacity score (TFC) and its relationship to Shoulson–Fahn stages and clinical descriptors [27].
| Descriptor | TFC | Stage |
|---|---|---|
| Early | 11–13 | I |
| 7–10 | II | |
| Moderate or mid | 4–6 | III |
| Advanced or late | 1–3 | IV |
| 0 | V |
Table 4 illustrates the setup for analyzed ANN models with their parameters.
Table 4.
Summary of different neural network models and their configuration parameters.
| Network | Hidden layer (neurons) | Transfer function | Training function | Number of weight elements | Time delay |
|---|---|---|---|---|---|
| FFBP | 1 (10) | Log-sigmoid, linear | Gradient descent with adaptive learning rate backpropagation | 41 | − |
| FFTD | 1 (10) | Tan-sigmoid | Levenberg–Marquardt | 101 | + |
| CFBP | 1 (10) | Tan-sigmoid | Levenberg–Marquardt | 43 | − |
| NARX | 1 (10) | Tan-sigmoid | Levenberg–Marquardt | 81 | + |
| Elman, RNN | 1 (10) | Tan-sigmoid | Levenberg–Marquardt | 141 | + |
| GRNN | 1 (size of dataset) | Radial basis, linear | Levenberg–Marquardt | 800 | − |
2.6. Training and Testing
The dataset was randomly divided into 3 sets: training, validation, and testing. Training set uses all samples from 70% of users. Validation set (15%) is used to measure network generalization and to stop training when necessary. Testing set (15%) provides independent performance of the network afterwards. We also analyzed a different partition of the dataset (40% for training, 30% for validation, and 30% for testing); however, there were no significant differences in the performance of ANN.
Overfitting was prevented by using the early stopping technique, which controls error on the validation set which is monitored during training process: when error increases for a specified number of iterations then the training is stopped and the weights and biases at the minimum of the validation error are returned.
For each neural network model, we have repeated the training and testing process for 20 times in order to allow calculation of statistical characteristics (mean, standard deviation) of ANN performance measures and to perform statistical comparison.
2.7. Reaction Stage Determination Using Fuzzy Logic System (FLS)
The aim of the FLS system is to determine the reaction stage of a patient (test subject) according to some predefined parameters. The FLS consists of three main parts: fuzzification block, inference mechanism, and defuzzification block. Membership functions, linguistic variables are created in fuzzification module. Inference engine is responsible for applying logical rules (fuzzy rule base) to the knowledge base and deduce new knowledge. Defuzzification module converts all the fuzzy terms created by the rule base of the controller to crisp terms (numerical values). The FLS uses triangular membership Mamdani-type functions with fuzzy set inference mechanism (minimum implication, maximum aggregation, minimum AND operator, maximum OR operator) and centroid defuzzification method.
The parameters of the FLS are derived from the ANN output corresponding to the functional capacity level, so in the FLS design process, the model input and output values need to be considered accordingly. There are three input and one output variable in the FLS. The input parameters are AVG1, AVG2, and AVG3, which correspond to the average of ANN output values when test subject is working with two, three, and five objects, respectively. All three inputs can have values in range [0; 10].
The linguistic variables (terms) for AVG1, AVG2, and AVG3 are
LOW [0 2 4];
AVERAGE [3.6 5.5 7];
HIGH [6.6; 8.5 10].
The model has one output parameter ReactionStage can have one of five values: close to peaks 1, 3, 5, 7, or 9, that is, each peak corresponds to particular linguistic variable of ReactionStage. The terms for output parameter ReactionStage are
ADVANCED [0 1 2];
LATE [1.5 3 4];
AVERAGE [3.5 5 6];
EARLY [5.5 7 8];
HEALTHY/PRECLINICAL [7.5 9 10].
The FLS rule base is formed from 27 fuzzy rules. Table 5 illustrates the principles of constructing fuzzy rule base. These can be interpreted as general fuzzy IF-THEN rules containing only fuzzy logical AND operators, for example,
Table 5.
FLS rule base (5 random examples chosen for each reaction stage).
| AVG1 | AVG2 | AVG3 | Reaction stage |
|---|---|---|---|
| HIGH | HIGH | HIGH | HEALTHY/PRECLINICAL |
| HIGH | LOW | HIGH | EARLY |
| AVERAGE | AVERAGE | AVERAGE | AVERAGE |
| LOW | LOW | HIGH | LATE |
| LOW | LOW | LOW | ADVANCED |
IF AVG1 is LOW AND AVG2 is LOW AND AVG3 is LOW
THEN ReactionStage is ADVANCED.
3. Proposed Hybrid Model
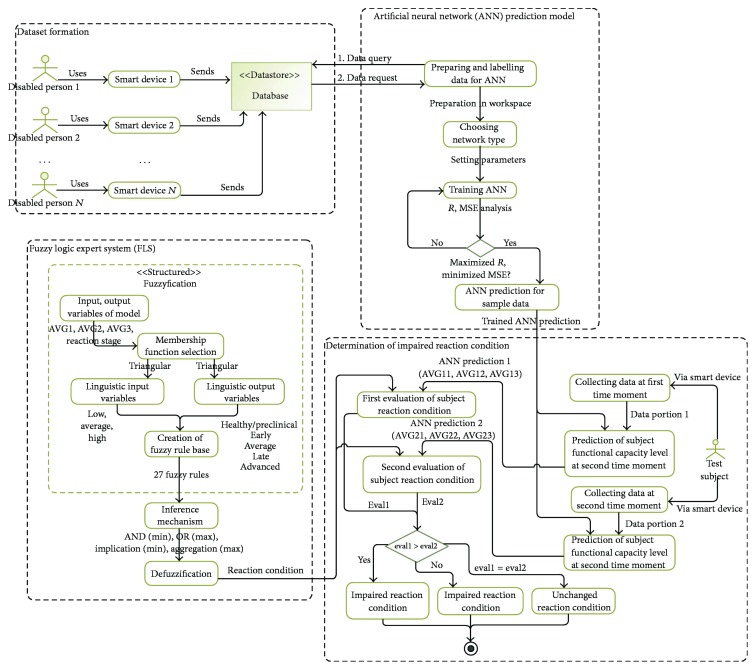
The hybrid model (see Figure 3) is composed of four sub models: (1) dataset formation; (2) ANN prediction model; (3) fuzzy logic expert system (FLS); and (4) decision module for determination of person's condition.
Figure 3.
Schema of prototype hybrid model to forecast impaired reaction condition.
During dataset formation, test subjects (under the supervision of a healthy person—a medical doctor or a nurse) use smart devices to perform reaction and accuracy test experiments with their fingers. The collected data is stored in the database. The ANN submodel predicts the functional capacity level of a person using the data from the database. The network is trained by observing regression (R), that is, correlation measurement between outputs and targets and mean squared error (MSE) values. Once the network is trained, it can make predictions on new sample data. Finally, to evaluate the reaction condition of a test subject, the test session is repeated at a different time and the ANN predictions are aggregated, and the reaction stage of a person is evaluated using a fuzzy rules system.
4. Experimental Results
The hybrid model was implemented with MATLAB Neural Network and Fuzzy Logic Toolbox software (MathWorks Inc.). The results of regression and comparison of the prediction results of the analyzed ANN models is presented in Table 6, whereas the performance of neural networks in terms of means and 95% confidence intervals of R and MSE is given in Table 7. The “TFC” field indicates the ground truth evaluation of the patient state provided by a medical neurologist expert according to the TFC scale. The R metric measures the correlation between output and targets, whereas the MSE metric is the average squared difference between outputs and targets.
Table 6.
Regression and prediction result comparison of different ANN models.
| Functional capacity level predictions (data sample of 10 records from 1 test subject) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| rt | 2.65 | 0.25 | 0.67 | 0.26 | 0.24 | 0.78 | 0.4 | 0.29 | 0.34 | 0.25 |
| delta | 22.36 | 108.3 | 87.8 | 267 | 60.1 | 20.8 | 37 | 113 | 41.4 | 68.8 |
| TFC | 8 | 3 | 4 | 1 | 5 | 8 | 7 | 3 | 7 | 5 |
| FFBP | 8.02 | 3.31 | 3.90 | 1.32 | 5.39 | 8.55 | 7.25 | 3.19 | 6.85 | 4.83 |
| FFTD | 7.99 | 3.26 | 3.94 | 0.97 | 5.41 | 8.51 | 7.24 | 3.15 | 6.83 | 4.87 |
| CFBP | 7.94 | 3.32 | 3.59 | 1.28 | 5.45 | 8.84 | 7.15 | 3.16 | 6.83 | 4.87 |
| NARX | 8.06 | 3.28 | 3.83 | 1.20 | 5.43 | 8.81 | 7.24 | 3.16 | 6.85 | 4.87 |
| Elman | 8.01 | 3.27 | 3.87 | 1.19 | 5.40 | 8.79 | 7.30 | 3.16 | 6.88 | 4.84 |
| RNN | 7.97 | 3.28 | 3.86 | 1.15 | 5.42 | 8.54 | 7.27 | 3.15 | 6.87 | 4.87 |
| GRNN | 8.20 | 2.92 | 3.86 | 0.99 | 5.18 | 8.93 | 6.83 | 2.91 | 6.88 | 4.87 |
Table 7.
Performance comparison of analyzed ANN models.
| Neural network model | Mean R | 95% confidence intervals of R | Mean MSE | 95% confidence intervals of MSE |
|---|---|---|---|---|
| FFBP | 0.9876 | [0.9871, 0.9880] | 0.0809 | [0.0782, 0.0835] |
| FFTD | 0.9861 | [0.9855, 0.9867] | 0.0906 | [0.0865, 0.0948] |
| CFBP | 0.9827 | [0.9787, 0.9868] | 0.1125 | [0.0860, 0.1389] |
| NARX | 0.9868 | [0.9868, 0.9869] | 0.0858 | [0.0855, 0.0860] |
| Elman | 0.9868 | [0.9868, 0.9868] | 0.0857 | [0.0856, 0.0857] |
| RNN | 0.9870 | [0.9870, 0.9870] | 0.0845 | [0.0845, 0.0845] |
| GRNN | 0.9849 | [0.9841, 0.9858] | 0.0977 | [0.0926, 0.1029] |
Nonparametric Friedman test was conducted to compare the performance results (MSE) among ANN models. Results show that there is a significant difference in performance among all ANN models (chi-square = 133.15; p = 2 · 10−26). Posthoc Nemenyi tests further reveal that the performance of FFBP is the best among all ANN models (Figure 4).
Figure 4.
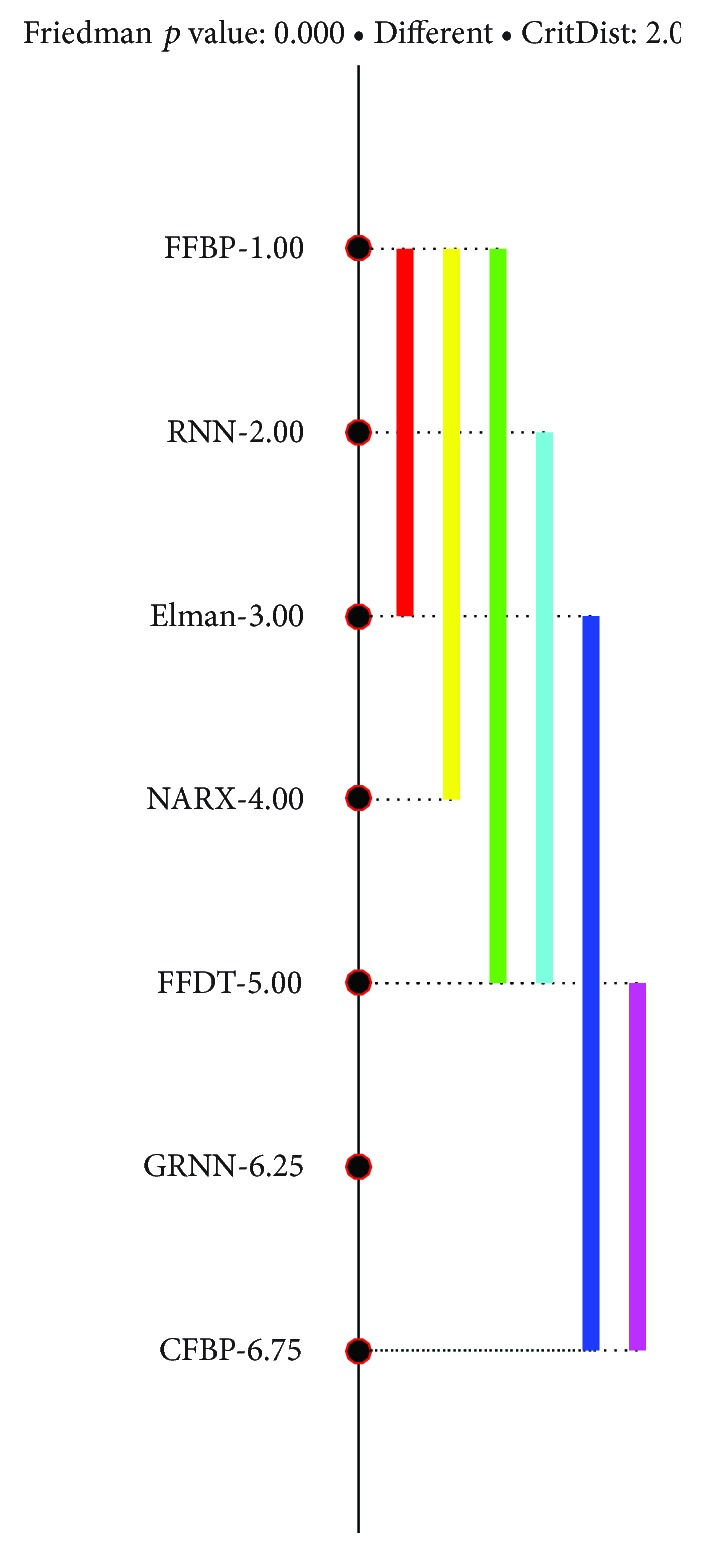
Results of Nemenyi test on performance (MSE) of ANN models.
Figure 5 shows an example of FFBP best performance equal to R = 0.993 and MSE = 0.094 on the validation set.
Figure 5.
FFBP performance evaluation using R (a) and MSE (b) metrics.
Table 8 illustrates impaired reaction condition simulation example on a single test subject using the FLS system. In order to make comparison, data samples were collected at different time moments. Feature (rt1, delta1, rt2, and delta2) values are presented in all three modes (10 attempts), thus giving two separate ANN (in the example provided, FFBP model was used) prediction outputs, which are used to calculate average values and evaluate the reaction condition in the FLS.
Table 8.
Example of hybrid model results for evaluation of a single test subject.
(a).
| Feature | Mode 1 2 objects |
Mode 2 3 objects |
Mode 3 5 objects |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dataset formation: data portion 1 | ||||||||||
| rt1 | 4.75 | 8.05 | 5.30 | 2.27 | 7.09 | 1.48 | 6.58 | 6.33 | 2.29 | 1.82 |
| delta1 | 3.32 | 2.99 | 4.05 | 19.09 | 0.31 | 19.15 | 0.51 | 19.42 | 5.95 | 10.50 |
| Artificial neural network (ANN) prediction model: ANN prediction 1 | ||||||||||
| 8.94 | 8.00 | 8.43 | 6.68 | 8.99 | 6.79 | 9.00 | 5.46 | 9.03 | 8.10 | |
| Dataset formation: data portion 2 | ||||||||||
| rt2 | 8.62 | 8.96 | 1.89 | 6.60 | 9.41 | 1.48 | 6.58 | 6.33 | 2.29 | 1.82 |
| delta2 | 1.42 | 9.78 | 16.99 | 19.94 | 0.89 | 10.85 | 17.27 | 18.18 | 16.90 | 17.57 |
| Artificial neural network (ANN) prediction model: ANN prediction 2 | ||||||||||
| 8.01 | 7.94 | 7.00 | 5.10 | 8.01 | 7.26 | 7.01 | 6.93 | 5.99 | 7.04 | |
(b).
| Fuzzy logic expert system (FLS) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| AVG11 | AVG12 | AVG13 | AVG21 | AVG22 | AVG23 | eval1 | eval2 | Condition 1 | Condition 2 |
| 8.47 | 8.03 | 7.67 | 7.98 | 6.70 | 6.84 | 9.00 | 7.00 | Healthy/Preclinical | Early |
| Conclusion: Impaired reaction condition. | |||||||||
5. Conclusions
We have presented an actual experimental framework to assess finger-tapping tests performed by patients suffering from the Huntington's disease (HD). The proposed model was validated using a dataset of 3032 data records collected from 20 test subjects (both healthy and HD patients). The reaction condition was determined using the developed Mamdani Type-1 fuzzy logic expert system (FLS) with 3 input (3 linguistic variables), 1 output (5 linguistic variables), triangular membership functions, and 27 fuzzy rules base.
We describe an architecture that combines several artificial neural networks (ANN) of different type (FFBP, FFDT, CFBP, NARX, Elman, RNN, and GRNN) to create a hybrid (neurofuzzy) model, which integrates feature extraction, prediction, and classification routines to forecast the impaired reaction condition for HD patients. The best results were achieved using the feed-forward backpropagation (FFBP) neural network model, which predicts the total functionality capability (TFC score) with high performance results, that is, it has obtained regression R value not less than 0.98 and mean squared error (MSE) values of 0.08, while FLS evaluates several measurements taken time apart to provide a final evaluation of the subject's reaction condition.
Future work will focus on the validation of the proposed system using a larger dataset, which includes the data collected from the Parkinson's and Alzheimer's patients as well, the analysis and use of more sophisticated finger-tapping features, and the comparison of the ANN results with those of SVM regression.
Acknowledgments
Authors would like to thank the president of Lithuania Huntington disease association, Dr. Zivile Navikiene, for contacting family members of HD patients to help carry out experiments for the investigation described in this paper, as well as for practical support and advice.
Additional Points
Human Studies. Research on human subjects was approved by the Institutional Review Board of the Faculty of Informatics of Kaunas University of Technology.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
References
- 1.Roos R. A. C. Huntington’s disease: a clinical review. Orphanet Journal of Rare Diseases. 2010;5(1):p. 40. doi: 10.1186/1750-1172-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haddad M. S., Cummings J. L. Huntington’s disease. The Psychiatric Clinics of North America. 1997;20(4):791–807. doi: 10.1016/S0193-953X(05)70345-2. [DOI] [PubMed] [Google Scholar]
- 3.Jones C., Busse M., Quinn L., et al. The societal cost of Huntington’s disease: are we underestimating the burden? European Journal of Neurology. 2016;23(10):1588–1590. doi: 10.1111/ene.13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennasar M., Hicks Y., Clinch S., et al. Huntington’s disease assessment using tri axis accelerometers. Procedia Computer Science. 2016;96:1193–1201. doi: 10.1016/j.procs.2016.08.163. [DOI] [Google Scholar]
- 5.Wiecki T. V., Antoniades C. A., Stevenson A., et al. A computational cognitive biomarker for early-stage Huntington’s disease. PLoS One. 2016;11(2, article e0148409) doi: 10.1371/journal.pone.0148409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulsen J. S., Langbehn D. R., Stout J. C., et al. Detection of Huntington’s disease decades before diagnosis: the Predict-HD study. Journal of Neurology, Neurosurgery & Psychiatry. 2008;79(8):874–880. doi: 10.1136/jnnp.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aylward E. H., Sparks B. F., Field K. M., et al. Onset and rate of striatal atrophy in preclinical Huntington disease. Neurology. 2004;63(1):66–72. doi: 10.1212/01.wnl.0000132965.14653.d1. [DOI] [PubMed] [Google Scholar]
- 8.Engin M., Demirag S., Engin E., et al. The classification of human tremor signals using artificial neural network. Expert Systems with Applications. 2007;33(3):754–761. doi: 10.1016/j.eswa.2006.06.014. [DOI] [Google Scholar]
- 9.Wu D., Warwick K., Ma Z., et al. Prediction of Parkinson’s disease tremor onset using a radial basis function neural network based on particle swarm optimization. International Journal of Neural Systems. 2010;20(02):109–116. doi: 10.1142/S0129065710002292. [DOI] [PubMed] [Google Scholar]
- 10.Cole B. T., Roy S. H., De Luca C. J., Nawab S. H. Dynamic neural network detection of tremor and dyskinesia from wearable sensor data. 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology; 2010; Buenos Aires, Argentina. pp. 6062–6065. [DOI] [PubMed] [Google Scholar]
- 11.Chandrashekhar A., Jain S., Kumar Jha V. Design and analysis of data mining based prediction model for Parkinson’s disease. International Journal of Computer Science Engineering (IJCSE) 2014;3(03) [Google Scholar]
- 12.Furuhashi T., Tano S., Jacobsen H.-A. Deep Fusion of Computational and Symbolic Processing. Vol. 59. New York, NY, USA: Publish in Physica, Fuzziness and Soft Computing; 2012. [DOI] [Google Scholar]
- 13.Kazienko P., Lughofer E., Trawinski B. Hybrid and ensemble methods in machine learning. Published in Journal of Universal Computer Science. 2013;19(4):457–461. [Google Scholar]
- 14.Fuller R. Advances in Intelligent and Soft Computing. Vol. 2. Springer Science & Business Media (2013); 2013. Introduction to neuro-fuzzy systems. [Google Scholar]
- 15.Geman O. Parkinson’s disease assessment using fuzzy expert system and nonlinear dynamics. Advances in Electrical and Computer Engineering. 2013;13(1):41–46. doi: 10.4316/aece.2013.01007. [DOI] [Google Scholar]
- 16.Obi J. C., Imainvan A. A. Decision support system for the intelligent identification of Alzheimer using neuro fuzzy logic. International Journal on Soft Computing (IJSC) 2011;2(2):25–38. doi: 10.5121/ijsc.2011.2203. [DOI] [Google Scholar]
- 17.Iram S., Fergus P., Al–Jumeily D., Hussain A., Randles M. A classifier fusion strategy to improve the early detection of neurodegenerative diseases. International Journal of Artificial Intelligence and Soft Computing (IJAISC) 2015;5(1):23–44. doi: 10.1504/IJAISC.2015.067525. [DOI] [Google Scholar]
- 18.Viharos Z. J., Kis K. B. Survey on neuro-fuzzy systems and their applications in technical diagnostics and measurement. Measurement. 2015;67:126–136. doi: 10.1016/j.measurement.2015.02.001. [DOI] [Google Scholar]
- 19.Chiuchisan I., Geman O. An approach of a decision support and home monitoring system for patients with neurological disorders using internet of things concepts. WSEAS Transactions on Systems. 2014;13:460–469. [Google Scholar]
- 20.Yang G., Lin Y., Bhattacharya P. Multimodality inferring of human cognitive states based on integration of neuro-fuzzy network and information fusion techniques. EURASIP Journal on Advances in Signal Processing. 2007;2008(1, article 371621) doi: 10.1155/2008/371621. [DOI] [Google Scholar]
- 21.Sateesh Babu G., Suresh S. Parkinson’s disease prediction using gene expression – a projection based learning meta-cognitive neural classifier approach. Expert Systems with Applications. 2013;40(5):1519–1529. doi: 10.1016/j.eswa.2012.08.070. [DOI] [Google Scholar]
- 22.Geman O. Towards an inclusive Parkinson’s screening system. 2014 18th International Conference on System Theory, Control and Computing (ICSTCC); 2014; Sinaia, Romania. pp. 470–475. [DOI] [Google Scholar]
- 23.Ramani G. R., Sivagami G. Parkinson disease classification using data mining algorithms. International Journal of Computer Applications. 2011;32(9):17–22. [Google Scholar]
- 24.Rustempasic I., Can M. Diagnosis of Parkinson’s disease using fuzzy c-means clustering and pattern recognition. Southeast Europe Journal of Soft Computing. 2013;2:42–49. doi: 10.21533/scjournal.v2i1.44. [DOI] [Google Scholar]
- 25.Gelsvartas J., Simutis R., Maskeliunas R. User adaptive text predictor for mentally disabled Huntington’s patients. Computational Intelligence and Neuroscience. 2016;2016:6. doi: 10.1155/2016/3054258.3054258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lauraitis A., Maskeliūnas R. Investigation of predicting functional capacity level for Huntington disease patients. In: Damaševičius R., Mikašytė V., editors. Information and Software Technologies. ICIST 2017. Communications in Computer and Information Science. Vol. 756. Cham: Springer; 2017. pp. 142–149. [DOI] [Google Scholar]
- 27.Shoulson I., Fahn S. Huntington disease: clinical care and evaluation. Neurology. 1979;29(1):1–3. doi: 10.1212/WNL.29.1.1. [DOI] [PubMed] [Google Scholar]
- 28.Gustavsson S., Fagerberg B., Sallsten G., Andersson E. M. Regression models for log-normal data: comparing different methods for quantifying the association between abdominal adiposity and biomarkers of inflammation and insulin resistance. International Journal of Environmental Research and Public Health. 2014;11(12):3521–3539. doi: 10.3390/ijerph110403521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim H.-Y. Statistical notes for clinical researchers: assessing normal distribution (2) using skewness and kurtosis. Restorative Dentistry & Endodontics. 2013;38(1):52–54. doi: 10.5395/rde.2013.38.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rumelhart D. E., Hinton G. E., Williams R. J. Learning representations by back-propagating errors. Nature. 1986;323(6088):533–536. doi: 10.1038/323533a0. [DOI] [Google Scholar]
- 31.Waibel A., Hanazawa T., Hinton G., Shikano K., Lang K. J. Phoneme recognition using time-delay neural networks. IEEE Transactions on Acoustics Speech and Signal Processing. 1989;37(3):328–339. doi: 10.1109/29.21701. [DOI] [Google Scholar]
- 32.Goyal S., Goyal G. K. Cascade and feedforward backpropagation artificial neural network models for prediction of sensory quality of instant coffee flavored sterilized drink. Canadian Journal on Artificial Intelligence, Machine Learning and Pattern Recognition. 2011;2(6):78–82. [Google Scholar]
- 33.Billings S. Nonlinear System Identification: NARMAX Methods in the Time, Frequency, and Spatio-Temporal Domains. Chichester, UK: Wiley; 2013. [DOI] [Google Scholar]
- 34.Elman J. L. Distributed representations, simple recurrent networks, and grammatical structure. Machine Learning. 1991;7(2-3):195–225. doi: 10.1007/BF00114844. [DOI] [Google Scholar]
- 35.Goller C., Küchler A. Learning task-dependent distributed representations by backpropagation through structure. Neural Networks, 1996, IEEE International Conference on; 1996; Washington, DC, USA. pp. 347–352. [DOI] [Google Scholar]
- 36.Specht D. F. A general regression neural network. IEEE Transactions on Neural Networks. 1991;2(6):568–576. doi: 10.1109/72.97934. [DOI] [PubMed] [Google Scholar]