Abstract
Colorectal cancer (CRC) is one of the leading causes of cancer-related deaths worldwide. As society ages, the number of elderly patients with CRC will increase. The percentage of patients with right-sided colon cancer and the incidence of microsatellite instability are higher in elderly than in younger patients with CRC. Moreover, the higher incidence of comorbid diseases in elderly patients indicates the need for less invasive treatment strategies. For example, care should be taken in performing additional surgery after endoscopic submucosal dissection for elderly patients with high-risk T1 CRC. Minimally invasive surgery, such as laparoscopic colectomy, would be preferable for elderly patients with CRC. Chemotherapy for elderly patients requires careful monitoring for adverse events. The aim of this review is to summarize the clinicopathological features of CRC in elderly patients, optical surgical strategies, including endoscopic and laparoscopic resection, and chemotherapeutic strategies, including postoperative adjuvant chemotherapy and systemic chemotherapy for unresectable CRC.
1. Introduction
Expansion of the worldwide population and elevation of life expectancy have increased the number of elderly individuals, resulting in aging of the population. According to the United Nations Population Fund (UNFPA), life expectancy around the world elevated from 64.8 years to 70 years over the past 20 years. Moreover, by 2050, people aged ≥60 years will account for almost 22% of the world's population, reaching over 2 billion people [1]. Because the incidence of many cancers is higher in patients aged ≥65 years, the number of elderly patients with cancers is expected to increase markedly.
Colorectal cancer (CRC) is one of the most common causes of cancer deaths worldwide [2–4], and the global incidence of CRC continues to increase [5]. In clinical practice, increased numbers of elderly patients with CRC undergo surgery and/or receive chemotherapy. These individuals are more likely than young patients to have comorbidities, such as cardiovascular disease, respiratory disease, renal dysfunction, and/or liver dysfunction, making treatment riskier. Physical activity is usually evaluated by measuring the performance status (PS) scoring of Eastern Cooperative Oncology Group (ECOG), but it is sometimes difficult to determine [6]. Furthermore, aging itself can reduce physiological recuperative power. In fact, aging is an independent risk factor for both in-hospital morbidity and mortality after colorectal surgery [7–9]. Therefore, designing appropriate treatment strategies for elderly patients with CRC requires comprehensive understanding of the characteristics of CRC in these patients. Here, we would like to review clinicopathological features and molecular alterations of CRC in elderly patients, as well as the optimal surgical approaches (i.e., endoscopic resection and laparoscopic surgery), and chemotherapy.
2. Clinicopathological Features and Genetic Background of CRC in Elderly Patients
2.1. Clinical Characteristics
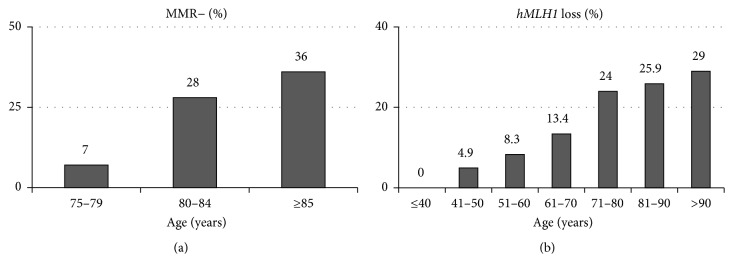
One of the most prominent clinical characteristics of elderly, compared to younger, patients with CRC is their higher frequency of right-sided colon cancer. This incidence increases with patient age, reaching about 50% in patients with CRC aged ≥80 years (Figure 1(a)) [10, 11]. Moreover, the incidence of right-sided colon cancer is about 10% higher in women than in men aged ≥80 years [11]. Although elderly patients tend to have large and locally invasive CRC, the frequency of lymph nodes metastasis is lower compared to that in younger ones (Figures 1(b) and 1(c)) [10]. Mismatch repair- (MMR-) deficient cancer with microsatellite instability (MSI) is more frequent in elderly patients with CRC, being present in 36% of patients aged ≥85 years, with an especially high frequency in women (Figure 2(a)) [12, 13]. In these right-sided and MSI-high CRC developed in the elderly women, hMLH1 gene promoter is frequently methylated and its protein expression is silenced (Figure 2(b)) [13–15].
Figure 1.
(a) Proportion of tumor location at indicated ages [10]. (b) Proportion of pathological T factor at indicated ages [10]. (c) Proportion of lymph node metastases at indicated ages [10].
Figure 2.
(a) Proportion of MMR-deficient CRC at indicated ages [12]. (b) Proportion of hMLH1 loss in CRC at indicated ages [13].
In a mouse model of MSI colonic adenoma that carries loxP sites flanking exon 14 of Adenomatous polyposis coli (Apc) gene regulated by CDX2 promoter and a long mononucleotide tract (CDX2P9.5-G22Cre;Apcflox/flox), most of the adenomatous lesions can be developed in the proximal colon [16]. These data are consistent with the fact that the incidence of MSI-high CRC is higher in right-sided colon than in left-sided colon or rectum in elderly patients [17, 18].
2.2. Pathological Characteristics and Genetic Background
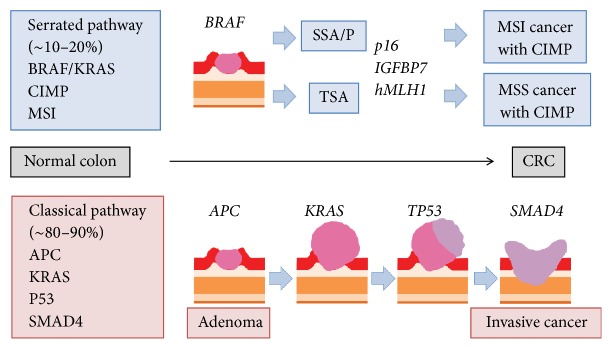
Mucinous carcinoma and serrated adenocarcinoma are often found in elderly patients [19, 20]. The serrated pathway is one of the evolutionary steps of CRC carcinogenesis (Figure 3) [21]. The serrated pathway starts from the progression of serrated polyps, including traditional serrated adenomas (TSAs) and sessile serrated adenomas/polyp (SSA/P) [22]. TSA tends to develop in the left-sided colon and rectum, whereas SSA/P tends to develop in the right-sided colon. TSAs can have two types of molecular characteristics: one having KRAS mutations and the other having BRAF mutations [22]. CRCs that develop from TSAs seldom exhibit MSI, but develop into microsatellite stable (MSS) tumors. In contrast, SSA/Ps can give rise to CRCs with high MSI [23]. Most SSA/Ps are primed by BRAF mutations, followed by bearing CpG island methylator phenotype (CIMP), and it finally becomes MSI-high CRC [22–24]. Although CIMP is a genome-wide phenotype, methylation of the p16, insulin growth factor binding protein 7 (IGFBP7), and hMLH1 is important for the development of MSI-high CRC [24]. Acquiring the MSI phenotype is the key step of malignant progression from SSA/P, as this phenotype increases the likelihood of mutations in the microsatellite genomic region, resulting in an invasive phenotype.
Figure 3.
Schematic representation of the hypothesis of CRC carcinogenesis. Upper section shows serrated pathway, and lower one shows classical pathway.
2.3. Medullary Adenocarcinoma
Medullary adenocarcinoma is a rare pathological type of CRC. This type of poorly differentiated adenocarcinoma has a phenotype indicative of minimal or no glandular differentiation [25]. The clinicopathological characteristics of medullary adenocarcinoma include its predominant location in the right-sided colon, its higher incidence in elderly women, and its relatively better prognosis despite its poorly differentiated phenotype. MSI is high in these tumors, along with hMLH1 promoter methylation [25]. Histologically, medullary carcinomas consist of a small uniform population of tumor cells with prominent nucleoli and eosinophilic cytoplasm. These cells grow in a solid-sheet structure, often containing Crohn's-like lymphoid reaction (CLR) and intratumoral lymphocytic infiltration [26]. CLR represents peritumoral lymphoid aggregates located couple of millimeters beyond the advancing tumor fronts [27]. Tumor-infiltrating lymphocytes (TILs) consist of T-cell population, and are frequently found in CRCs with high MSI [28]. The presence of CLR and TILs reflects strong antitumor immunity, and it is a good prognostic indicator for CRC after adjustment of traditional staging [29]. These data are consistent with the recent finding that immune checkpoint inhibitors are effective in the patients with MSI-high CRC [30].
3. Surgical Approaches for Elderly Patients with CRC
3.1. Endoscopic Resection
Endoscopic resection is a minimally invasive approach for adenomas and early cancers. Endoscopic submucosal dissection (ESD) is ideal because of its en bloc resection. Although less invasive than surgery, ESD still carries risks of perforation (6%) and bleeding (1%) [43]. Therefore, caution should be exercised in performing ESD for elderly patients, as they are more likely to have comorbidities that can exacerbate post-ESD complications. However, aging itself is not a contraindication for ESD, as it has been shown to be effective and safe for elderly patients with CRC, with en block resection rates of 81.2–96.3%, perforation rates of 1.8–6.1%, and bleeding rates of 3.0–3.7% [44–46]. Moreover, the 5-year disease specific survival (DFS) rates in the elderly populations have been reported to be almost 100% when appropriately managed [45].
Some patients with early CRC who undergo endoscopic resection require additional colectomy with lymph node dissection, because about 10% of patients with T1 CRC have lymph node metastases [47]. Indications for additional surgery in patients with T1 tumors include (1) depth of submucosal invasion ≥ 1000 μm, (2) vascular invasion (i.e., lymphatic or venous invasion) positive, (3) poorly differentiated adenocarcinoma, signet-ring cell carcinoma, or mucinous carcinoma, or (4) grade 2/3 budding at the site of deepest invasion [47, 48]. To date, there is no consensus regarding whether additional surgery is really effective and reasonable for elderly patients who have T1 CRC with such signs as described above.
3.2. Laparoscopic Surgery for Elderly Patients with CRC
Aging is an independent risk factor in major digestive surgery [49]. Laparoscopic surgery for CRC was widely adopted in the late 1990s to 2000s because it was regarded as minimally invasive. However, at first, application for laparoscopic surgery was limited and sometimes elderly and/or high-risk patients were excluded just because this surgery required techniques different from those of open surgery and standardized procedure had not been established. Recent randomized controlled trials have reported that laparoscopic surgery for CRC has an equivalent oncological result and better short-term outcomes compared with open surgery [50–56]. Moreover, analyses of large databases have found that laparoscopic surgery is an independent predictor of reduced mortality after CRC surgery [57–61]. Taken together, these studies emphasize the benefits of laparoscopic colectomy over open surgery, including reduced invasiveness, lower mortality rates, shorter hospital stay, and lower costs, with comparable oncological outcomes.
Minimally invasive laparoscopic colectomy is therefore indicated for elderly patients with CRC. Some observational studies have shown that laparoscopic surgery has better short-term outcomes than open surgery for elderly patients with CRC (Table 1) [31–35, 62, 63]. Most of these studies have reported that postoperative hospital stay is shorter after laparoscopic than after open surgery, suggesting that laparoscopic surgery may reduce surgical complications. Similar to nonelderly patients, elderly patients should undergo curative laparoscopic colectomy with D3 lymph node dissection, when an operation under the general anesthesia is possible due to a lack of severe comorbidities. Moreover, care should be taken, especially in elderly patients, to maintain postoperative activities of daily living (ADL) and quality of life (QOL). In particular, the anus-sparing surgery for low rectal cancer (i.e., low anterior resection or intersphincteric resection) can cause the postoperative defecation dysfunction, and so we need to determine the operative method considering the preoperative anal function.
Table 1.
Representative studies comparing laparoscopic colectomy and open surgery for elderly CRC patients.
| Author (year) | Age | Hospital stay (days)∗ | P | OS∗∗ | P |
|---|---|---|---|---|---|
| Cummings (2012) [31] | ≥65 | 8.3 ± 6.2 versus 10 ± 8.9 | <0.001 | 55.8% versus 50.05% (5 y) | 0.095 |
| Mukai (2014) [32] | ≥85 | 14.7 versus 21.7 | <0.0001 | – | – |
| Vallribera Valls (2014) [33] | 75–84 | 10 versus 14.3 | 0.001 | – | – |
| ≥85 | 11.4 versus 15.4 | 0.077 | – | – | |
| Nakamura (2014) [34] | ≥85 | 10 versus 19 | <0.0001 | – | – |
| Hinoi (2015) [35] | ≥80 | 12 versus 13.0 (colon) | <0.001 | 85.5% versus 81.2% (colon, 3 y) | 0.916 |
| 19 versus 18 (rectum) | 0.990 | 78.6% versus 70.2% (rectum, 3 y) | 0.765 |
∗Laparoscopic surgery versus open surgery; ∗∗percentage of survival at indicated years in parentheses. y, years; –, not mentioned in the article.
4. Chemotherapy for Elderly Patients with CRC
4.1. General Management of Chemotherapy
Particular attention is required when planning chemotherapy for elderly cancer patients, because of reductions in organ function and preexisting comorbidities. The kidneys and livers are the most important organs involved in the pharmacokinetics and pharmacodynamics of chemotherapy agents. For example, doses of capecitabine and TS-1, both of which are frequently used to treat CRC, should be reduced or omitted in patients with renal dysfunction [64, 65], and doses of irinotecan should be reduced in patients with hepatic dysfunction [66]. Bevacizumab, an antivascular endothelial growth factor (VEGF) neutralizing antibody, sometimes causes proteinuria in a dose-dependent manner, requiring its reduction or discontinuation [67].
4.2. Adjuvant Chemotherapy (Table 2)
Table 2.
Representative studies of adjuvant chemotherapy for stage II and/or stage III CRC.
| Author (year) | Regimen | DFS∗∗ | P | OS∗∗ | P |
|---|---|---|---|---|---|
| Moertel (1995) [36] | 5-FU/LEV versus none | 63% versus 47% (3.5 y) | <0.0001 | 71% versus 55% (3.5 y) | 0.0064 |
| Francini (1994) [37] | 5-FU/LV versus none | 74% versus 59% (5 y) | 0.005 | 79% versus 65% (5 y) | 0.0044 |
| IMPACT (1995) [38] | 5-FU/LV versus none | 71% versus 62% (3 y) | <0.0001 | 83% versus 78% (3 y) | 0.018 |
| O'Connell (1997) [39] | 5-FU/LV versus none | 74% versus 58% (5 y) | 0.001 | 74% versus 63% (5 y) | 0.01 |
| André (2004) [40] | FL + Oxali versus FL | 78% versus 73% (3 y) | 0.002 | – | – |
| Kuebler (2007) [41] | FLOX versus FULV | 73% versus 67% (4 y) | 0.0034 | – | – |
| André (2009) [42] | FOLFOX4 versus LV5FU2 | 66% versus 59% (5 y) | 0.005 | 73% versus 69% (6 y) | 0.023 |
∗∗Percentage of survival at indicated years in parentheses. y, years; –, not mentioned in the article.
Unfortunately, some patients experience recurrence/metastasis even after complete resection of the primary CRC. The likelihood of recurrence after curative resection may be reduced by administration of adjuvant chemotherapy. A randomized trial sponsored by the National Cancer Institute reported, in 1990, that adjuvant therapy with fluorouracil plus levamisole (5-FU/LEV) reduced recurrence risk by 41% over surgery alone for patients with stage III (metastatic lymph node-positive) CRC [68]. Based on the results of this trial and other following trials, a regimen of 5-FU plus folic acid (leucovorin) (5-FU/LV) became a standard treatment for stage III CRC [37–39]. Many randomized controlled trials have tested other adjuvant chemotherapy regimens. For example, the addition of oxaliplatin to 5-FU/LV resulted in acceptable tolerance and a better DFS and/or overall survival (OS) rate in patients with stage III CRC patients (MOSAIC trial [40, 42] and NSABP C-07 [41]). Results of the MOSAIC trial, however, found that elderly patients aged ≥65 years did not benefit from adding oxaliplatin to 5-FU/LV, suggesting the need for care in applying the results of these randomized trials to adjuvant chemotherapy for elderly patients with CRC.
Most of these randomized trials did not include many elderly patients. For example, the two major trials described above included only 25 (1%) and 131 (5%) patients aged ≥75 years, respectively [69]. A large cohort study, published in 2002, of elderly patients aged ≥67 years with stage III CRC reported a survival benefit of 5-FU-based adjuvant chemotherapy over surgery alone [70]. Another cohort study published in 2006 also reported its benefit in patients aged ≥65 years [71]. However, the age predilection of CRC suggests that patients in their late 60s are not “elderly.” In 2012, Sanoff et al. reported a cohort study combining four large databases of patients diagnosed as stage III CRC between 2004 and 2007. A total of 5,489 patients with stage III CRC aged ≥75 years were analyzed using covariate adjusted and propensity score-matched proportional hazards models. Compared with surgery alone, 5-FU-based adjuvant chemotherapy had significant survival benefit, whereas the addition of oxaliplatin to 5-FU-based chemotherapy provided no significant benefit over 5-FU alone, although it tended to improve prognosis [69].
4.3. Chemotherapy for Unresectable CRC
Because of the discovery of novel drugs, including molecular targeting reagents, systemic chemotherapy for advanced/metastatic CRC dramatically has increased median overall survival by 2-3 years these days. However, most of these clinical trials did not include patients with CRC aged ≥75 years, because these trials were usually designed for the patients without any comorbidities. It is uncertain, therefore, whether the results of these clinical trials can be applicable to elderly patients with CRC.
A pooled analysis of the safety and efficacy of oxaliplatin in elderly patients with CRC was reported in 2006 [72]. Although this analysis mixed trials of the FOLFOX4 regimen as adjuvant, first-line, and second-line settings, it included 614 patients with CRC aged ≥70 years. That analysis found that the incidence of grade ≥3 hematologic toxicities (neutropenia and thrombocytopenia) was significantly higher in elderly than in other patients. In contrast, the incidence of other adverse events, such as neurotoxicity, infection, diarrhea, nausea/vomiting, and fatigue, and the overall incidence of grade ≥3 toxicity were not associated with older age. Moreover, the benefits of FOLFOX over control treatment, as determined by response rate, progression-free survival (PFS), DFS, and OS, were not associated with age, suggesting that oxaliplatin-containing chemotherapy is efficient and safe for the elderly patients with CRC.
The randomized trial (MRC FOCUS2) was designed for the elderly and frail CRC patients who needed the reduced dosage of chemotherapy regimen [73]. In this trial, 42% (191/459) were aged ≥76 years, and the starting doses were 80% of the standard doses, with discretionary escalation to full dose after 6 weeks. They identified that adding oxaliplatin onto a 5-FU-based regimen exhibited some improvement of PFS, although not statistically significant.
5. Conclusion
Aging is one of the factors we need to take into account in determining a comprehensive strategy of CRC treatment. Several studies reported that aging itself was an independent prognostic factor in these patients. To date, there is not enough evidence to develop a standardized treatment of elderly patients with CRC. A personalized strategy is required, considering each patient's comorbidities, performance status, and life styles.
Abbreviations
- Apc:
Adenomatous polyposis coli
- ADL:
Activity of daily living
- CIMP:
CpG island methylator phenotype
- CLR:
Crohn's-like lymphoid reaction
- CRC:
Colorectal cancer
- DFS:
Disease-free survival
- ECOG:
Eastern Cooperative Oncology Group
- ESD:
Endoscopic submucosal dissection
- IGFBP:
Insulin growth factor binding protein
- MMR:
Mismatch repair
- MSI:
Microsatellite instability/instable
- MSS:
Microsatellite stable
- OS:
Overall survival
- PFS:
Progression-free survival
- PS:
Performance status
- QOL:
Quality of life
- SSA/P:
Sessile serrated adenoma/polyp
- TIL:
Tumor-infiltrating lymphocyte
- TSA:
Traditional serrated adenomas
- VEGF:
Vascular endothelial growth factor.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding this paper.
References
- 1.(UNFPA) U.N.P.F., http://www.unfpa.org/ageing.
- 2.Malvezzi M., Bertuccio P., Levi F., La Vecchia C., Negri E. European cancer mortality predictions for the year 2014. Annals of Oncology. 2014;25(8):1650–1656. doi: 10.1093/annonc/mdu138. [DOI] [PubMed] [Google Scholar]
- 3.Hori M., Matsuda T., Shibata A., Katanoda K., Sobue T., Nishimoto H. Cancer incidence and incidence rates in Japan in 2009: a study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Japanese Journal of Clinical Oncology. 2015;45(9):884–891. doi: 10.1093/jjco/hyv088. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A., Ward E. M., Johnson C. J., et al. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival. Journal of the National Cancer Institute. 2017;109(9) doi: 10.1093/jnci/djx030.djx030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold M., Sierra M. S., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):p. 683. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 6.Common Toxicity Criteria (CTC) http://ecog-acrin.org/resources/ecog-performance-status.
- 7.Alley P. Surgery for colorectal cancer in elderly patients: a systematic review. The Lancet. 2000;356(9234):956–974. doi: 10.1016/S0140-6736(00)02707-0. [DOI] [PubMed] [Google Scholar]
- 8.Turrentine F. E., Wang H., Simpson V. B., Jones R. S. Surgical Risk Factors, Morbidity, and Mortality in Elderly Patients. Journal of the American College of Surgeons. 2006;203(6):865–877. doi: 10.1016/j.jamcollsurg.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 9.Jafari M. D., Jafari F., Halabi W. J., et al. Colorectal cancer resections in the aging US population: A trend toward decreasing rates and improved outcomes. JAMA Surgery. 2014;149(6):557–564. doi: 10.1001/jamasurg.2013.4930. [DOI] [PubMed] [Google Scholar]
- 10.Kotake K., Asano M., Ozawa H., Kobayashi H., Sugihara K. Tumour characteristics, treatment patterns and survival of patients aged 80 years or older with colorectal cancer. Colorectal Disease. 2015;17(3):205–215. doi: 10.1111/codi.12826. [DOI] [PubMed] [Google Scholar]
- 11.Siegel R. L., Miller K. D., Fedewa S. A., et al. Colorectal cancer statistics. CA: A Cancer Journal for Clinicians. 2017;67(3):177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 12.Aparicio T., Schischmanoff O., Poupardin C., et al. Deficient mismatch repair phenotype is a prognostic factor for colorectal cancer in elderly patients. Digestive and Liver Disease. 2013;45(3):245–250. doi: 10.1016/j.dld.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Kakar S., Burgart L. J., Thibodeau S. N., et al. Frequency of loss of hMLH1 expression in colorectal carcinoma increases with advancing age. Cancer. 2003;97(6):1421–1427. doi: 10.1002/cncr.11206. [DOI] [PubMed] [Google Scholar]
- 14.Kane M. F., Loda M., Gaida G. M., et al. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Research. 1997;57(5):808–811. [PubMed] [Google Scholar]
- 15.Malkhosyan S. R., Yamamoto H., Piao Z., Perucho M. Late onset and high incidence of colon cancer of the mutator phenotype with hypermethylated hMLH1 gene in women [3] Gastroenterology. 2000;119(2):p. 598. doi: 10.1053/gast.2000.16154. [DOI] [PubMed] [Google Scholar]
- 16.Akyol A., Hinoi T., Feng Y., Bommer G. T., Glaser T. M., Fearon E. R. Generating somatic mosaicism with a Cre recombinase-microsatellite sequence transgene. Nature Methods. 2008;5(3):231–233. doi: 10.1038/nmeth.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thibodeau S. N., Bren G., Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260(5109):816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- 18.Jernvall P., Mäkinen M. J., Karttunen T. J., Mäkelä J., Vihko P. Microsatellite instability: Impact on cancer progression in proximal and distal colorectal cancers. European Journal of Cancer. 1999;35(2):197–201. doi: 10.1016/S0959-8049(98)00306-2. [DOI] [PubMed] [Google Scholar]
- 19.Arai T., Takubo K. Clinicopathological and molecular characteristics of gastric and colorectal carcinomas in the elderly. Pathology International. 2007;57(6):303–314. doi: 10.1111/j.1440-1827.2007.02101.x. [DOI] [PubMed] [Google Scholar]
- 20.Whitehall V. L. J., Leggett B. A. The serrated pathway of colorectal carcinogenesis. Current Colorectal Cancer Reports. 2009;5(2):75–83. doi: 10.1007/s11888-009-0012-y. [DOI] [Google Scholar]
- 21.Jass J. R., Whitehall V. L. J., Young J., Leggett B. A. Emerging concepts in colorectal neoplasia. Gastroenterology. 2002;123(3):862–876. doi: 10.1053/gast.2002.35392. [DOI] [PubMed] [Google Scholar]
- 22.Leggett B., Whitehall V. Role of the Serrated Pathway in Colorectal Cancer Pathogenesis. Gastroenterology. 2010;138(6):2088–2100. doi: 10.1053/j.gastro.2009.12.066. [DOI] [PubMed] [Google Scholar]
- 23.Jass J. R. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50(1):113–130. doi: 10.1111/j.1365-2559.2006.02549.x. [DOI] [PubMed] [Google Scholar]
- 24.Toyota M., Ahuja N., Ohe-Toyota M., Herman J. G., Baylin S. B., Issa J. J. CpG island methylator phenotype in colorectal cancer. Proceedings of the National Acadamy of Sciences of the United States of America. 1999;96(15):8681–8686. doi: 10.1073/pnas.96.15.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arai T., Esaki Y., Sawabe M., Honma N., Nakamura K.-I., Takubo K. Hypermethylation of the hMLH1 promoter with absent hMLH1 expression in medullary-type poorly differentiated colorectal adenocarcinoma in the elderly. Modern Pathology. 2004;17(2):172–179. doi: 10.1038/modpathol.3800018. [DOI] [PubMed] [Google Scholar]
- 26.Lanza G., Gafà R., Matteuzzi M., Santini A. Medullary-type poorly differentiated adenocarcinoma of the large bowel: a distinct clinicopathologic entity characterized by microsatellite instability and improved survival. Journal of Clinical Oncology. 1999;17(8):2429–2438. doi: 10.1200/JCO.1999.17.8.2429. [DOI] [PubMed] [Google Scholar]
- 27.Graham D. M., Appelman H. D. Crohn's-like lymphoid reaction and colorectal carcinoma: a potential histologic prognosticator. Modern Pathology. 1990;3(3):332–335. [PubMed] [Google Scholar]
- 28.Takemoto N., Konishi F., Yamashita K., et al. The correlation of microsatellite instability and tumor-infiltrating lymphocytes in hereditary non-polyposis colorectal cancer (HNPCC) and sporadic colorectal cancers: The significance of different types of lymphocyte infiltration. Japanese Journal of Clinical Oncology. 2004;34(2):90–98. doi: 10.1093/jjco/hyh018. [DOI] [PubMed] [Google Scholar]
- 29.Rozek L. S., Schmit S. L., Greenson J. K., et al. Tumor-Infiltrating lymphocytes, Crohn's-like lymphoid reaction, and survival from colorectal cancer. Journal of the National Cancer Institute. 2016;108(8) doi: 10.1093/jnci/djw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le D. T., Uram, Wang H., et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. The New England Journal of Medicine. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cummings L. C., Delaney C. P., Cooper G. S. Laparoscopic versus open colectomy for colon cancer in an older population: A cohort study. World Journal of Surgical Oncology. 2012;10, article no. 31 doi: 10.1186/1477-7819-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukai T., Akiyoshi T., Ueno M., et al. Outcomes of laparoscopic surgery for colorectal cancer in oldest-old patients. Surgical Laparoscopy Endoscopy & Percutaneous Techniques. 2014;24:366–369. doi: 10.1097/SLE.0b013e31829012ca. [DOI] [PubMed] [Google Scholar]
- 33.Vallribera Valls F., Landi F., Espín Basany E., et al. Laparoscopy-assisted versus open colectomy for treatment of colon cancer in the elderly: morbidity and mortality outcomes in 545 patients. Surgical Endoscopy. 2014;28(12):3373–3378. doi: 10.1007/s00464-014-3597-4. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura T., Sato T., Miura H., et al. Feasibility and outcomes of surgical therapy in very elderly patients with colorectal cancer. Surgical Laparoscopy Endoscopy & Percutaneous Techniques. 2014;24(1):85–88. doi: 10.1097/SLE.0b013e3182a83477. [DOI] [PubMed] [Google Scholar]
- 35.Hinoi T., Kawaguchi Y., Hattori M., et al. Laparoscopic Versus Open Surgery for Colorectal Cancer in Elderly Patients: A Multicenter Matched Case–Control Study. Annals of Surgical Oncology. 2015;22(6):2040–2050. doi: 10.1245/s10434-014-4172-x. [DOI] [PubMed] [Google Scholar]
- 36.Moertel C. G., Fleming T. R., Macdonald J. S., et al. Intergroup study of fluorouracil plus levamisole as adjuvant therapy for stage II/Dukes' B2 colon cancer. Journal of Clinical Oncology. 1995;13(12):2936–2943. doi: 10.1200/JCO.1995.13.12.2936. [DOI] [PubMed] [Google Scholar]
- 37.Francini G., Petrioli R., Lorenzini L., et al. Folinic acid and 5-fluorouracil as adjuvant chemotherapy in colon cancer. Gastroenterology. 1994;106(4):899–906. doi: 10.1016/0016-5085(94)90748-X. [DOI] [PubMed] [Google Scholar]
- 38.International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. The Lancet. 1995;345(8955):939–944. doi: 10.1016/s0140-6736(95)90696-7. [DOI] [PubMed] [Google Scholar]
- 39.O'Connell M. J., Mailliard J. A., Kahn M. J., et al. Controlled trial of fluorouracil and low-dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. Journal of Clinical Oncology. 1997;15(1):246–250. doi: 10.1200/JCO.1997.15.1.246. [DOI] [PubMed] [Google Scholar]
- 40.André T., Boni C., Mounedji-Boudiaf L., et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. The New England Journal of Medicine. 2004;350(23):2343–2351. doi: 10.1056/nejmoa032709. [DOI] [PubMed] [Google Scholar]
- 41.Kuebler J. P., et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. Journal of Clinical Oncology. 2007;25(16):2198–2204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 42.André T., Boni C., Navarro M., et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. Journal of Clinical Oncology. 2009;27(19):3109–3116. doi: 10.1200/jco.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 43.Fujishiro M., Yahagi N., Kakushima N., et al. Outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms in 200 consecutive cases. Clinical Gastroenterology and Hepatology. 2007;5(6):678–683. doi: 10.1016/j.cgh.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida N., Naito Y., Sakai K., et al. Outcome of endoscopic submucosal dissection for colorectal tumors in elderly people. International Journal of Colorectal Disease. 2010;25(4):455–461. doi: 10.1007/s00384-009-0841-9. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi Y., Mizuno K.-I., Takahashi K., et al. Long-term outcomes of colorectal endoscopic submucosal dissection in elderly patients. International Journal of Colorectal Disease. 2017;32(4):567–573. doi: 10.1007/s00384-016-2719-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu L., Shen X., Zhu J. Efficacy, Safety, and Overall Quality of Life of Endoscopic Submucosal Dissection for Early Colorectal Cancer in Elderly Patients. Gastroenterology Research and Practice. 2017;2017 doi: 10.1155/2017/2386291.2386291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watanabe T., Muro K., Ajioka Y., et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. International Journal of Clinical Oncology. 2017 doi: 10.1007/s10147-017-1101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ueno H., Mochizuki H., Hashiguchi Y., et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology. 2004;127(2):385–394. doi: 10.1053/j.gastro.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 49.Duron J.-J., Duron E., Dugue T., et al. Risk factors for mortality in major digestive surgery in the elderly: A multicenter prospective study. Annals of Surgery. 2011;254(2):375–382. doi: 10.1097/SLA.0b013e318226a959. [DOI] [PubMed] [Google Scholar]
- 50.Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. The New England Journal of Medicine. 2004;350(20):2050–2059. doi: 10.1056/nejmoa032651. [DOI] [PubMed] [Google Scholar]
- 51.Guillou P. J., Quirke P., Thorpe H., et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. The Lancet. 2005;365(9472):1718–1726. doi: 10.1016/s0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 52.Veldkamp R., Kuhry E., Hop W. C., et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. The Lancet Oncology. 2005;6(7):477–484. doi: 10.1016/S1470-2045(05)70221-7. [DOI] [PubMed] [Google Scholar]
- 53.Jayne D. G., Guillou P. J., Thorpe H., et al. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-Year results of the UK MRC CLASICC trial group. Journal of Clinical Oncology. 2007;25(21):3061–3068. doi: 10.1200/JCO.2006.09.7758. [DOI] [PubMed] [Google Scholar]
- 54.Buunen M., Veldkamp R., Hop W. C. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. The Lancet Oncology. 2009;10(1):44–52. doi: 10.1016/s1470-2045(08)70310-3. [DOI] [PubMed] [Google Scholar]
- 55.Hemandas A. K., Abdelrahman T., Flashman K. G., et al. Laparoscopic colorectal surgery produces better outcomes for high risk cancer patients compared to open surgery. Annals of Surgery. 2010;252(1):84–89. doi: 10.1097/SLA.0b013e3181e45b66. [DOI] [PubMed] [Google Scholar]
- 56.Bagshaw P. F., Allardyce R. A., Frampton C. M., et al. Long-term outcomes of the australasian randomized clinical trial comparing laparoscopic and conventional open surgical treatments for colon cancer: The australasian laparoscopic colon cancer study trial. Annals of Surgery. 2012;256(6):915–919. doi: 10.1097/SLA.0b013e3182765ff8. [DOI] [PubMed] [Google Scholar]
- 57.Cone M. M., Herzig D. O., Diggs B. S., et al. Dramatic decreases in mortality from laparoscopic colon resections based on data from the nationwide inpatient sample. JAMA Surgery. 2011;146(5):594–599. doi: 10.1001/archsurg.2011.79. [DOI] [PubMed] [Google Scholar]
- 58.Webb S., Rubinfeld I., Velanovich V., Horst H. M., Reickert C. Using national surgical quality improvement program (NSQIP) data for risk adjustment to compare Clavien 4 and 5 complications in open and laparoscopic colectomy. Surgical Endoscopy. 2012;26(3):732–737. doi: 10.1007/s00464-011-1944-2. [DOI] [PubMed] [Google Scholar]
- 59.Stefanou A. J., Reickert C. A., Velanovich V., Falvo A., Rubinfeld I. Laparoscopic colectomy significantly decreases length of stay compared with open operation. Surgical Endoscopy. 2012;26(1):144–148. doi: 10.1007/s00464-011-1840-9. [DOI] [PubMed] [Google Scholar]
- 60.Vaid S., Tucker J., Bell T., Grim R., Ahuja V. Cost analysis of laparoscopic versus open colectomy in patients with colon cancer: Results from a large nationwide population database. The American Surgeon. 2012;78(6):635–641. [PubMed] [Google Scholar]
- 61.Kuroyanagi H., Inomata M., Saida Y., et al. Gastroenterological Surgery: Large intestine. Asian Journal of Endoscopic Surgery. 2015;8(3):246–262. doi: 10.1111/ases.12222. [DOI] [PubMed] [Google Scholar]
- 62.Okamura R., Hida K., Hasegawa S., et al. Impact of intraoperative blood loss on morbidity and survival after radical surgery for colorectal cancer patients aged 80 years or older. International Journal of Colorectal Disease. 2016;31(2):327–334. doi: 10.1007/s00384-015-2405-5. [DOI] [PubMed] [Google Scholar]
- 63.Devoto L., Celentano V., Cohen R., Khan J., Chand M. Colorectal cancer surgery in the very elderly patient: a systematic review of laparoscopic versus open colorectal resection. International Journal of Colorectal Disease. 2017;32(9):1237–1242. doi: 10.1007/s00384-017-2848-y. [DOI] [PubMed] [Google Scholar]
- 64.Superfin D., Iannucci A. A., Davies A. M. Commentary: Oncologic drugs in patients with organ dysfunction: A summary. The Oncologist. 2007;12(9):1070–1083. doi: 10.1634/theoncologist.12-9-1070. [DOI] [PubMed] [Google Scholar]
- 65.Shiroyama T., Kijima T., Komuta K., et al. Phase II tailored S-1 regimen study of first-line chemotherapy in elderly patients with advanced and recurrent non-small cell lung cancer. Cancer Chemotherapy and Pharmacology. 2012;70(6):783–789. doi: 10.1007/s00280-012-1958-z. [DOI] [PubMed] [Google Scholar]
- 66.Raymond E., Boige V., Faivre S., et al. Dosage adjustment and pharmacokinetic profile of irinotecan in cancer patients with hepatic dysfunction. Journal of Clinical Oncology. 2002;20(21):4303–4312. doi: 10.1200/jco.2002.03.123. [DOI] [PubMed] [Google Scholar]
- 67.Wu S., Kim C., Baer L., Zhu X. Bevacizumab increases risk for severe proteinuria in cancer patients. Journal of the American Society of Nephrology. 2010;21(8):1381–1389. doi: 10.1681/ASN.2010020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moertel C. G., Fleming T. R., Macdonald J. S., et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. The New England Journal of Medicine. 1990;322(6):352–358. doi: 10.1056/NEJM199002083220602. [DOI] [PubMed] [Google Scholar]
- 69.Sanoff H. K., Carpenter W. R., Sturmer T., et al. Effect of adjuvant chemotherapy on survival of patients with stage III colon cancer diagnosed after age 75 years. Journal of Clinical Oncology. 2012;30(21):2624–2634. doi: 10.1200/jco.2011.41.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Iwashyna T. J., Lamont E. B. Effectiveness of adjuvant fluorouracil in clinical practice: A population-based cohort study of elderly patients with stage III colon cancer. Journal of Clinical Oncology. 2002;20(19):3992–3998. doi: 10.1200/JCO.2002.03.083. [DOI] [PubMed] [Google Scholar]
- 71.Neugut A. I., Matasar M., Wang X., et al. Duration of adjuvant chemotherapy for colon cancer and survival among the elderly. Journal of Clinical Oncology. 2006;24(15):2368–2375. doi: 10.1200/JCO.2005.04.5005. [DOI] [PubMed] [Google Scholar]
- 72.Goldberg R. M., Tabah-Fisch I., Bleiberg H., et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. Journal of Clinical Oncology. 2006;24(25):4085–4091. doi: 10.1200/JCO.2006.06.9039. [DOI] [PubMed] [Google Scholar]
- 73.Seymour M. T., Thompson L. C., Wasan H. S., et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): An open-label, randomised factorial trial. The Lancet. 2011;377(9779):1749–1759. doi: 10.1016/S0140-6736(11)60399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]