Abstract
Sensory processing deficits are core features of schizophrenia, reflected in impaired generation of event-related potential (ERP) measures such as auditory mismatch negativity (MMN) and visual P1. To understand the potential time course of development of deficits in schizophrenia, we obtained MMN to unattended duration, intensity and frequency deviants, and visual P1 to attended LSF stimuli, in 43 healthy individuals ages 6 to 25 years (mean 17), and compared results to data from 30 adult schizophrenia patients (mean age 38). We analyzed “time-domain” measures of amplitude and latency, and event-related spectral perturbation (ERSP, “time-frequency”) to evaluate underlying neurophysiological mechanisms.
Duration and intensity MMN amplitudes increased from childhood to late adolescence, while frequency MMN reached maximum amplitude during early development. As reported previously, in ERSP analyses, MMN activity corresponded primarily to theta-band (4-7 Hz) activity, while responses to standards occurred primarily in alpha (8-12 Hz) across age groups. Both deviant-induced theta and standard-induced alpha activity declined significantly with age for all deviant types. Likewise, visual P1 also showed an amplitude decline over development, reflecting a reduction in both evoked power and ITC.
While MMN “difference” waveform ERP data suggest failure of maturation in schizophrenia, MMN ERSP analyses instead support a neurodegenerative process, as these isolate responses to deviants and standards, showing large low-frequency evoked power for both in children. Neurodegenerative processes are also supported by large visual P1 amplitudes and large low-frequency evoked power in children, in contrast with adult schizophrenia. Sensory processing deficits in schizophrenia may be related to accelerated synaptic pruning.
Keywords: ERP, ERSP, development, mismatch negativity, adolescence, visual-evoked potentials
1. Introduction
Deficits in sensory processing are core characteristics of schizophrenia (rev. in Javitt, 2015). These deficits may be assessed objectively using event-related potentials (ERP) such as auditory mismatch negativity (MMN) and visual P1, which index neurophysiological processing within auditory and visual sensory cortices, respectively. Deficits in both MMN and visual P1 have been extensively replicated in schizophrenia (e.g. Friedman et al., 2012). The present study evaluates MMN and visual P1 in a cohort of normally developing children and adolescents in order to permit interpretation of ERP abnormalities in schizophrenia within a neurodevelopmental framework.
We have previously shown that higher cognitive abilities as reflected in attention/vigilance or composite scores on the MATRICS consensus cognitive battery (MCCB) increase dramatically from late childhood to adulthood, whereas sensory measures such as face (FER) or auditory (AER) emotion recognition tend to develop earlier and then plateau (Corcoran et al., 2015). Theories of schizophrenia pathogenesis based upon higher order cognitive assessment might suggest a “developmental arrest” during late adolescence, with schizophrenia patients retaining the cognitive abilities, in general, of 12-16 year olds (Fuller et al., 2002). However, studies of sensory systems might provide complementary information and assist in identifying both early neurodevelopmental and late neurodegenerative processes.
MMN is typically assessed within the context of an auditory “oddball” paradigm, in which less frequent deviant stimuli interrupt a series of repetitive standard stimuli (Friedman et al., 2012). Deviants typically differ from standards in duration, intensity or frequency, though can vary in any domain, such as spatial location or phonological features (Kantrowitz et al., 2015). The most common analysis of auditory oddball data is in the time-domain, in which the mismatch negativity (MMN) event-related potential (ERP) is calculated as the difference waveform in frontocentral and other regions between averaged responses to deviants (larger in amplitude) and standards (smaller in amplitude).
Reduction in auditory MMN ERP amplitude is among the most replicated biomarkers of schizophrenia(Javitt, 2015), and it is an early core characteristic of schizophrenia, evident even in prodromal stages of illness (Bodatsch et al., 2011; Higuchi et al., 2013; Kayser et al., 2014; Perez et al., 2014; Shaikh et al., 2012; van Tricht et al., 2015). Auditory MMN may further deteriorate after illness onset in initially high functioning subjects (Devrim-Ucok et al., 2008; Kaur et al., 2013; Salisbury et al., 2007), although others show stable deficits following onset (Koshiyama et al., 2017; Light and Braff, 2005).
It has been reported that over normal development, auditory MMN tends to increase gradually from childhood through adolescence, specifically for deviants of higher frequency (Bishop et al., 2011; Cooray et al., 2016; Mahajan and McArthur, 2015), suggesting its reduction in schizophrenia may represent a failure of maturation in line with that observed for higher cognitive functions (Corcoran et al., 2015; Fuller et al., 2002; Hood et al., 1989). Nevertheless, literature on normal MMN development during the at-risk period for development of schizophrenia (early teens-late twenties) is sparse -especially for non-tonal deviants such as duration or intensity - limiting the degree to which the MMN literature in schizophrenia can be assessed within a developmental framework.
Deficits in visual processing are also evident in schizophrenia, specifically impairment in magnocellular-based processing of low spatial frequency (LSF) stimuli, indexed by the visual P1 ERP, a bilateral positivity at 100-120 ms over dorsal occipital scalp (Javitt, 2015). Visual P1 amplitude reduction is a replicated finding in schizophrenia (Javitt, 2015) that appears stable after illness onset (Oribe et al., 2015). Cross-sectional behavioral studies show abnormal visual processing in clinical high-risk samples (Kimhy et al., 2007; Mittal et al., 2015), that may significantly predict conversion to schizophrenia (Corcoran et al., 2015). As visual P1 amplitudes significantly decrease over normal development (Batty and Taylor, 2006; Hileman et al., 2011; MacNamara et al., 2016; Meaux et al., 2014), the small visual P1 amplitude observed in schizophrenia may be due to either early maturational or late neurodegenerative processes. (Friedman et al., 2012)
In addition to conventional time-domain analysis of the ERP data, we also conducted time-frequency analyses (event-related spectral perturbation, ERSP), which provide additional data regarding underlying neurophysiological mechanisms (Javitt, 2015; Lee et al., 2017). Auditory stimuli normally evoke power in the low-frequency range, with deviants evoking theta (4-7 Hz) and standards primarily evoking alpha (7- 12 Hz) power; individuals with schizophrenia have reduction in low-frequency evoked power (and intertrial coherence) to both standards and deviants (Lee et al., 2017). As opposed to MMN amplitude measured in the time-domain, evoked theta to MMN deviants is high in children and decreases over development (Bishop et al., 2011; Ehlers et al., 2014), potentially reflecting normal developmental pruning and refinement of sensory maps. To the extent that this is true, reductions in MMN theta amplitude may serve as an index of the accelerated pruning that may contribute to development of neurocognitive impairments in schizophrenia (Feinberg, 1990). We have also recently observed that deficits in visual P1 are associated with theta-band dysfunction (Martinez et al., 2015). The developmental trajectory of visual-evoked theta responses, however, remains to be determined.
To gain insight into the potential time course over which auditory and visual processing deficits develop in schizophrenia, we administered a passive auditory oddball paradigm, with attended visual stimuli (Friedman et al., 2012) to young people ages 6 to 25 in the NKI-Rockland community-ascertained cohort (Nooner et al., 2012). This paradigm was previously administered to schizophrenia patients, finding reductions in auditory MMN and visual P1 (Friedman et al., 2012), and significant reduction in low-frequency evoked power for both auditory MMN deviants and standards(Lee et al., 2017). Therefore, we can contextualize these findings of abnormal auditory and visual processing in schizophrenia in respect to their normal developmental trajectory, using the same paradigm. For auditory processing, we expected to replicate increases in auditory MMN ERP in the context of decreases in low-frequency evoked power over development (Bishop et al., 2011; Ehlers et al., 2014; MacNamara et al., 2016). Likewise, we expected a decrease in visual P1 over development (MacNamara et al., 2016), and hypothesized large low-frequency evoked power in children, similar to that previously seen for auditory stimuli (Bishop, Hardiman et al. 2011). Together, these findings would evaluate the utility of MMN and other sensory ERP for investigation of neurodevelopmental vs. neurodegenerative processes in schizophrenia.
2. Methods
2.1 Participants
Subjects were 43 young people ascertained from the NKI-Rockland community-ascertained cohort (Nooner et al., 2012), ranging in age from 6 to 25, mean (±SD) age = 17.4±5.5 years, 58% male. Also, EEG data for the same auditory oddball paradigm were available from thirty adults with schizophrenia (mean(SD) = 38.0 (10.7)), previously studied within the Schizophrenia Research Division at the Nathan Kline Institute(Friedman et al., 2012; Lee et al., 2017). Written informed consent was obtained from adults, and for parents of minors, who themselves provided written assent. Exclusion criteria for all participants included organic brain disorders, mental retardation, past drug or alcohol dependence, current drug or alcohol abuse, and hearing/vision impairments. The study was approved by the Institutional Review Boards of Nathan Kline Institute and Columbia University.
2.2 Procedure
The paradigm is as described previously (Friedman et al., 2012): a series of tones were presented at random with stimulus onset asynchrony (SOA) of 500-505 ms. Standards occurred with a sequential probability of 70%, and were harmonic tones of three superimposed sinusoids of 500, 1000 and 15000 Hz, that were 100 ms in duration, 85dB and with a 5 ms rise and fall. Each of the three deviants had a sequential probability of 10%. The frequency deviant was 10% lower in pitch; the duration deviant was 50 ms longer in duration; and the intensity deviant was 10 dB lower in loudness.
During auditory stimulus presentation, participants attended to a sequence of visual stimuli, which consisted of 39% HSF (5 Hz) and 39% LSF (1 Hz) horizontal gratings, viewed at 114 cm, with a stimulus field subtended 6.1 – 4.6 degrees of visual angle. Stimuli were presented centrally against a 50% gray background isoluminant with mean luminance of the sinewave gratings, with SOA 875 ±25 ms.
There was no stimulus overlap, and auditory and visual stimuli were presented in 250 s blocks, with eight blocks per participant.
2.3 Data acquisition
Continuous EEG data, with digital timing tags, were acquired using a 64-channel ANT with standard reference and ground procedures. Sample rate was 512 Hz. Eyeblinks were removed using independent component analysis (ICA) in EEGLAB (< 10%). Using BESA, data were epoched (-500 to 1000 msec) with baseline (-500 to 0) correction, and then high-pass filtered (0.30 Hz, 6 db/oct, forward). Epochs with activity exceeding ±120 microvolts were rejected, resulting in 68% of 250 trials retained. Interpolation of channels occurred at <10%. Epochs were averaged offline for each stimulus type for each participant.
2.4 ERP analyses
For ERP analyses, waveforms were averaged by stimulus type and baseline-corrected relative to pre-stimulus baseline. MMN waveforms were calculated as the difference between deviant and standard waveforms. Latency ranges for duration, intensity and frequency deviants were respectively 130-230 ms, 130-200 ms and 90-190 ms post-stimulus onset. Amplitudes were measured at frontocentral electrodes relative to average mastoids. Visual P1 ERP to LSF stimuli were obtained in the range of 90 – 125 ms. Electrodes for each component were chosen based on visual inspection of data and published distributions of auditory and visual ERP's.
2.5 ERSP analyses
ERSP analyses are as previously described (Lee et al., 2017). For evoked analyses, ERP waves were transformed by multitaper methods with Hanning window implemented with Fieldtrip Open Toolbox with 50 ms time resolution and 1 Hz of frequency resolution. For single-trial analyses, intertrial coherence and baseline-corrected evoked power was obtained from BESA 5.1 using a complex demodulation procedure with 2 Hz frequency resolution and 25 ms time resolution.
2.6 Statistical Analyses
Following Bishop and colleagues (Bishop et al., 2011), we stratified the developmental data by four age range bins: 1) children or “kids”: ages 6-12 (N=9); 2) “teens”: ages 13-16 (N=11); 3) “young adults”: ages 17-21 (N=11); 4) “older adults”: ages 22- 26 (N=12). We calculated grand average ERP waveforms (auditory MMN and visual P1) for each age group in the normal developmental cohort, and for schizophrenia patients. An arc-tangent transform was applied to ERP data to improve normality. The relationship between variables in the developmental cohort was assessed by linear regression or analysis of variance (ANOVA) as appropriate. All statistics were two-tailed with alpha set at p < .05. Data for schizophrenia patients were not included in statistical analyses with the developmental cohort, but are provided for comparison.
3. Results
3.1 Auditory processing
3.1.1. ERP
Duration (r=-.41, p=.006) and intensity (r=-.42, p=.005) both increased significantly with age (i.e. more negative potential), whereas the relationship between age and frequency MMN was non-significant (r=.2, p=.2). Healthy controls showed larger frequency MMN amplitudes than schizophrenia patients at all age ranges (Figure 1).
Figure 1. Auditory mismatch negativity ERP's in schizophrenia and across development.
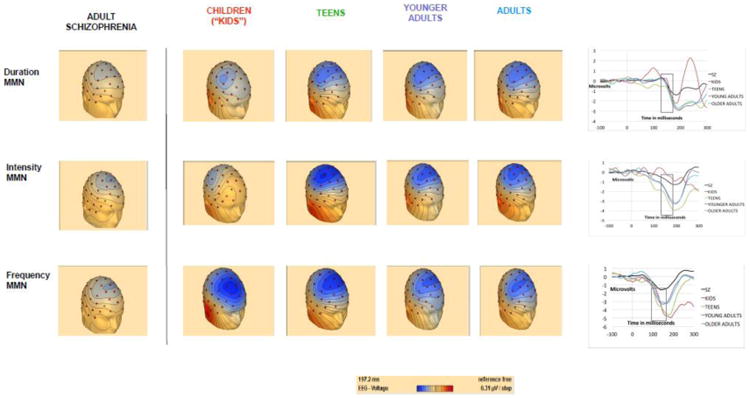
These head maps and plotting of MMN amplitudes at Fz show a normal age-related increase in MMN amplitude from childhood through adolescence for duration and intensity, but not frequency, MMN. Schizophrenia headmaps are included for comparison.
3.1.2. ERSP
Evoked power in theta in the time window of 50-250 msec showed a significant decrease over normal development for each of the MMN deviants: duration (r = -0.37, p = .015), intensity (r = -0.43, p = .004) and frequency (r = -0.46, p = .002) (Figure 2). Similarly, evoked power in alpha showed a significant decrease over normal development for standards (r = -0.46, p = .002). There was also an increase in intertrial coherence for deviants (p > .05) but a decrease for standards (p < .05) over normal development (Supplementary Figure). Of note, there was no association with age of the ERP/ERSP measures in the schizophrenia cohort (data not shown).
Figure 2. Evoked power for MMN deviants and standards across development.
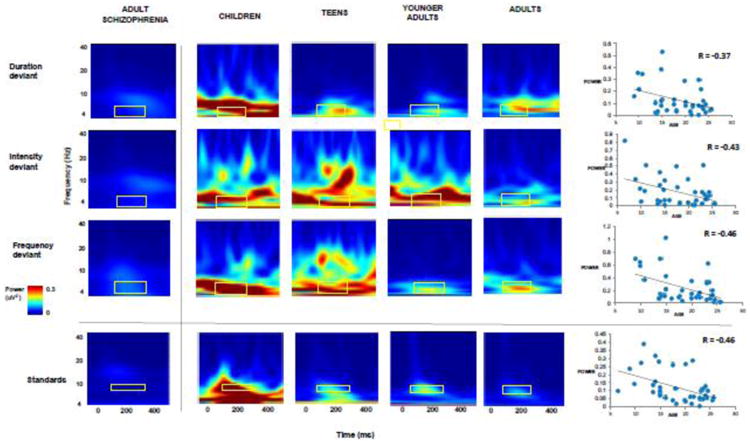
These time frequency plots across age groups for auditory deviant stimuli (duration, intensity and frequency) illustrate a progressive reduction in theta evoked power (4-8 Hz) at Fz across development, in the time window of 50-250 msec, confirmed by scatterplots that show significant age-related decreases. Time frequency plots for evoked power in schizophrenia are included for comparison.
3.2 Visual processing
3.2.1 ERP
Visual P1 amplitudes declined significantly over normal development (r = -0.67, p < .001) from childhood to adulthood (Figure 3), with adults showing significantly smaller amplitudes than children (p < .05). Visual P1 amplitudes in schizophrenia patients were markedly smaller than that seen in children (p < .05) (Figure 3).
Figure 3. Visual P1 across development: ERP, evoked power, and intertrial coherence.
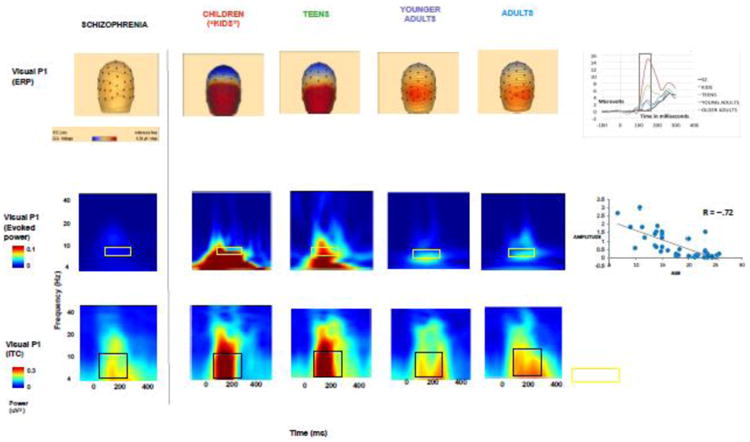
These head maps and time-frequency plots show a decrease over normal development in visual P1 amplitudes (90-125 msec), and associated evoked power of alpha, in the time window of 50-250 msec, and intertrial coherence. Visual P1 ERP's and time frequency plots for schizophrenia are included for comparison.
3.2.2 ERSP
Evoked power in alpha range significantly decreased over development (r = -0.72, p < .0001) (Figure 3). Similarly, intertrial coherence in both theta and alpha in 50-250 ms declined over development (p < .05) (Figure 3). Of note, there was no association with age of the ERP/ERSP measures in the schizophrenia cohort (data not shown).
4. Discussion
Deficits in sensory processing are well-established core characteristics of schizophrenia related to its functional morbidity, evident even during early prodromal stages (rev. in Javitt, 2015). To map the sensory processing deficits of schizophrenia along a normal developmental trajectory, we administered a passive auditory oddball paradigm, with attended visual stimuli (Friedman et al., 2012), to a community-ascertained normal developmental cohort with an age range of 6 to 25 (Nooner et al., 2012).
This paradigm has previously been used by our group to show that schizophrenia patients have substantial reductions in ERP amplitudes across sensory domains, including auditory MMN to all deviants, and visual P1 to low-spatial frequency (Friedman et al., 2012). Further, in ERSP analyses, it has been used by our group to demonstrate that decreased auditory MMN ERP in schizophrenia is accounted for by a smaller difference between reduced low-frequency power for both deviants and standards (Lee et al., 2017). If these deficits in schizophrenia were consequent to failure of maturation, we would expect similar patterns of relative reductions in both ERP amplitudes and evoked power in normal children and in schizophrenia.
4.1 Auditory mismatch
An analysis of auditory MMN ERP's alone in our developmental cohort would support a failure of maturation, as amplitudes increase gradually over normal development for both duration and intensity MMN, and normal individuals in all age groups have greater MMN amplitudes than do patients with schizophrenia (Figure 1). Whereas we did not find an association of age with frequency MMN ERP amplitudes, using deviants of lower frequency, prior studies that used deviants of higher frequency found an increase in frequency MMN ERP amplitudes from childhood through late adolescence (Bishop et al., 2011; Cooray et al., 2016; Mahajan and McArthur, 2015).
However, resolution of the MMN difference waveform through separate ERSP analyses of standards and deviants in our developmental cohort tell a different story, as children have significantly larger evoked power for both standards and deviants, as compared with normal adults (Figure 2), and by extension, adult schizophrenia. Thus, the deficits in evoked power for auditory MMN in schizophrenia identified using this same paradigm (Lee et al., 2017) may be neurodegenerative or consequent to accelerated aging, as suggested by neurodegenerative hypotheses (Feinberg, 1982, 1990). Such theories would be particularly consistent with studies that show reductions in auditory MMN over time in schizophrenia, even following illness onset (Devrim-Ucok et al., 2008; Kaur et al., 2013; Salisbury et al., 2007).
Our finding of a trajectory of increasing (i.e. more negative) MMN ERP in the context of decreasing evoked theta power replicates prior studies (Bishop et al., 2011), and provides the first data for duration and intensity deviants. This apparent paradox is resolved by the finding that increases in phase-synchronization of theta over normal development (Bishop et al., 2011; Muller et al., 2009; Shahin et al., 2010; Yordanova and Kolev, 2008) accounts for age-related increase in MMN amplitude, as phase-locking in theta is larger for deviant than for standard stimuli (Fuentemilla et al., 2008; Muller et al., 2009). Correspondingly, we found that over development, intertrial coherence within theta increases for deviants but decreases for standards (Supplementary Figure), which contributes to the developmental increase in the MMN difference waveform. This normal maturational decrease in evoked theta power shows the same curvilinear decline in gray matter in frontal and parietal cortices observed within a separate cohort, which has been posited to be due to normal synaptic pruning (Whitford et al., 2007). Thus, “accelerated aging” of evoked theta power in schizophrenia may be an objective index of the excessive synaptic pruning argued to be inherent to schizophrenia pathophysiology (Cannon, 2015; Feinberg, 1982), leading to disturbances in glutamatergic neurotransmission that may be central to the pathophysiology of schizophrenia.
4.2 Early visual processing
In respect to visual processing, we found a significant normal maturational decrease in visual P1 amplitude from childhood through adulthood, replicating prior studies (Batty and Taylor, 2006; Hileman et al., 2011; MacNamara et al., 2016; Meaux et al., 2014), a trajectory considered to reflect greater automaticity of visual processing with maturation. We also provided the first demonstration of reduction in visual-induced theta over time and demonstrated significant impairments in both P1-related theta power and ITC. (Friedman et al., 2012; Javitt, 2015). Taken together, these findings also suggest accelerated aging or neurodegenerative effects on visual processing in schizophrenia, which may occur particularly early, given deficits in visual processing in clinical high-risk cohorts (Kimhy et al., 2007; Mittal et al., 2015) and the stability of V1 amplitude reduction in schizophrenia (Oribe et al., 2015).
Visual P1 reduction in schizophrenia is considered to be a trait vulnerability marker, as it correlates poorly with functional measures and illness duration (Friedman et al., 2012). In addition to serving as a biomarker of visual sensory dysfunction, it may also contribute significantly to poor outcome. For example, the visual P1 is tied to face emotion recognition. Deficits in face emotion recognition in turn are observed in schizophrenia across illness stages, with accuracy lower than that achieved by a normal 10 year old (Corcoran et al., 2015). These disturbances therefore may both contribute to prodromal symptoms in at-risk individuals and predict progression to schizophrenia. (Martinez et al., 2015).
4.3 Overall
The main limitations in this study include its small sample size, increasing risk for both Type 1 and Type 2 error, and the older age of the schizophrenia patients, as compared with the developmental cohort. Another potential limitation in the analysis of the developmental trajectory of auditory MMN ERP is that children's waveforms tend to have longer latency, subtle differences in topography, and a more pronounced late discriminative negativity (LDN) emerging around 300 msec, as is evident in the children's topographies and waveforms in Figure 1, and as described in prior studies (Bishop et al., 2011; Mahajan and McArthur 2015; Cooray et al., 2016). These differences may account in part for the small amplitude with large variance in auditory MMN event-related potentials in children.
Nonetheless, the present study provides a critical developmental framework to aid in the interpretation of neurocognitive findings in schizophrenia. As opposed to higher order neurocognitive processes that mature late during development, both MMN-and visual P1-related neurophysiological activity declines over development and may therefore index normal synaptic elimination processes. Neurophysiological deficits seen in schizophrenia may therefore reflect either early insult, relating to failures in initial synapse development, or “overpruning” leading to reduced synaptic integrity. By applying the same auditory oddball paradigm with attended visual stimuli to both a normal developmental cohort and to a schizophrenia sample, we were able to provide ERP and ERSP data that together suggest neurodegenerative or “accelerated aging” effects on sensory processing in schizophrenia. These findings were most clear when neuro-oscillatory “time-frequency” measures were used to assess sensory-related activity, and suggest increased utilization of ERSP, along with ERP, approaches in neurophysiological data analysis.
Supplementary Material
These time-frequency plots illustrate an increase in intertrial coherence in theta for deviants, but a decrease in intertrial coherence for standards in alpha across development, in the time window of 50-250 msec.
Table 1. Auditory and visual ERP's and ERSP (evoked power) across normal age groups.
| Children (N = 9) | Teens (N = 11) | Young Adults (N = 11) | Adults (N = 12) | Schizophrenia (N=68) | |
|---|---|---|---|---|---|
| Auditory MMN ERP (Amplitude) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Duration (130-230 msec) | -0.77 (3.48) | -1.59 (1.33) | -1.53 (0.74) | -1.42 (0.59) | -1.10 (0.80) |
| Intensity (130-200 msec) | -1.24 (2.86) | -2.91 (1.29) | -2.08 (0.95) | -2.03 (0.99) | -1.20 (0.96) |
| Frequency (90-190 msec) | -4.03 (2.65) | -3.14 (1.86) | -1.95 (1.13) | -1.98 (1.00) | -1.22 (0.87) |
| Auditory MMN Evoked Power (50-250 msec; theta) | |||||
| Duration | 0.27 (0.10) | 0.15 (0.17) | 0.09 (0.08) | 0.10 (0.09) | 0.03(0.04) |
| Intensity | 0.35 (0.28) | 0.15 (0.14) | 0.14 (0.16) | 0.13 (0.13) | 0.04(0.06) |
| Frequency | 0.57 (0.14) | 0.29 (0.31) | 0.14 (0.08) | 0.18 (0.19) | 0.05(0.09) |
| Auditory standard (50-250 msec; alpha) | 0.23 (0.12) | 0.14 (0.09) | 0.11 (0.11) | 0.07 (0.03) | 0.02(0.02) |
| Visual P1 | |||||
| ERP (90- 125 msec; amplitude) | 7.99 (4.24) | 4.73 (2.14) | 2.11 (2.43) | 2.20 (1.94) | 0.8(0.62) |
| Evoked power (50-250 msec; alpha) | 9 .78 (0.89) | 14.5 (0.53) | 0.26 (0.32) | 0.33 (0.45) | 0.04(0.07) |
Acknowledgments
Neurophysiological and demographic data were ascertained from the Nathan Kline Institute-Rockland Sample (NKI-RS), a lifespan sample dataset funded by the NIMH.
Funding. This work was supported by NIMH R01 MH107558 (Corcoran, PI).
Footnotes
Contributors. Dr. Javitt designed the study. Drs. Corcoran and Stoops completed data analyses with assistance from Drs. Lee, Dias, Sehatpour and Martinez. Dr. Corcoran conducted the literature searches and wrote the manuscript, with editing by Dr. Javitt. All authors contributed to and have approved the final manuscript.
Conflict of interest. All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Batty M, Taylor MJ. The development of emotional face processing during childhood. Dev Sci. 2006;9(2):207–220. doi: 10.1111/j.1467-7687.2006.00480.x. [DOI] [PubMed] [Google Scholar]
- Bishop DV, Hardiman MJ, Barry JG. Is auditory discrimination mature by middle childhood? A study using time-frequency analysis of mismatch responses from 7 years to adulthood. Dev Sci. 2011;14(2):402–416. doi: 10.1111/j.1467-7687.2010.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodatsch M, Ruhrmann S, Wagner M, Muller R, Schultze-Lutter F, Frommann I, Brinkmeyer J, Gaebel W, Maier W, Klosterkotter J, Brockhaus-Dumke A. Prediction of psychosis by mismatch negativity. Biological psychiatry. 2011;69(10):959–966. doi: 10.1016/j.biopsych.2010.09.057. [DOI] [PubMed] [Google Scholar]
- Cannon TD. How Schizophrenia Develops: Cognitive and Brain Mechanisms Underlying Onset of Psychosis. Trends Cogn Sci. 2015;19(12):744–756. doi: 10.1016/j.tics.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooray GK, Garrido MI, Brismar T, Hyllienmark L. The maturation of mismatch negativity networks in normal adolescence. Clin Neurophysiol. 2016;127(1):520–529. doi: 10.1016/j.clinph.2015.06.026. [DOI] [PubMed] [Google Scholar]
- Corcoran CM, Keilp JG, Kayser J, Klim C, Butler PD, Bruder GE, Gur RC, Javitt DC. Emotion recognition deficits as predictors of transition in individuals at clinical high risk for schizophrenia: a neurodevelopmental perspective. Psychol Med. 2015;45(14):2959–2973. doi: 10.1017/S0033291715000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devrim-Ucok M, Keskin-Ergen HY, Ucok A. Mismatch negativity at acute and post-acute phases of first-episode schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2008;258(3):179–185. doi: 10.1007/s00406-007-0772-9. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wills DN, Desikan A, Phillips E, Havstad J. Decreases in energy and increases in phase locking of event-related oscillations to auditory stimuli occur during adolescence in human and rodent brain. Dev Neurosci. 2014;36(3-4):175–195. doi: 10.1159/000358484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17(4):319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Feinberg I. Cortical pruning and the development of schizophrenia. Schizophr Bull. 1990;16(4):567–570. doi: 10.1093/schbul/16.4.567. [DOI] [PubMed] [Google Scholar]
- Friedman T, Sehatpour P, Dias E, Perrin M, Javitt DC. Differential relationships of mismatch negativity and visual p1 deficits to premorbid characteristics and functional outcome in schizophrenia. Biological psychiatry. 2012;71(6):521–529. doi: 10.1016/j.biopsych.2011.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentemilla L, Marco-Pallares J, Munte TF, Grau C. Theta EEG oscillatory activity and auditory change detection. Brain Res. 2008;1220:93–101. doi: 10.1016/j.brainres.2007.07.079. [DOI] [PubMed] [Google Scholar]
- Fuller R, Nopoulos P, Arndt S, O'Leary D, Ho BC, Andreasen NC. Longitudinal assessment of premorbid cognitive functioning in patients with schizophrenia through examination of standardized scholastic test performance. Am J Psychiatry. 2002;159(7):1183–1189. doi: 10.1176/appi.ajp.159.7.1183. [DOI] [PubMed] [Google Scholar]
- Higuchi Y, Sumiyoshi T, Seo T, Miyanishi T, Kawasaki Y, Suzuki M. Mismatch negativity and cognitive performance for the prediction of psychosis in subjects with at-risk mental state. PLoS One. 2013;8(1):e54080. doi: 10.1371/journal.pone.0054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hileman CM, Henderson H, Mundy P, Newell L, Jaime M. Developmental and individual differences on the P1 and N170 ERP components in children with and without autism. Dev Neuropsychol. 2011;36(2):214–236. doi: 10.1080/87565641.2010.549870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood WF, Compton RP, Monahan JB. D-cycloserine: a ligand for the N-methyl-D-aspartate coupled glycine receptor has partial agonist characteristics. Neurosci Lett. 1989;98(1):91–95. doi: 10.1016/0304-3940(89)90379-0. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Neurophysiological models for new treatment development in schizophrenia: early sensory approaches. Ann N Y Acad Sci. 2015;1344:92–104. doi: 10.1111/nyas.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz JT, Hoptman MJ, Leitman DI, Moreno-Ortega M, Lehrfeld JM, Dias E, Sehatpour P, Laukka P, Silipo G, Javitt DC. Neural Substrates of Auditory Emotion Recognition Deficits in Schizophrenia. J Neurosci. 2015;35(44):14909–14921. doi: 10.1523/JNEUROSCI.4603-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur M, Lagopoulos J, Lee RS, Ward PB, Naismith SL, Hickie IB, Hermens DF. Longitudinal associations between mismatch negativity and disability in early schizophrenia- and affective-spectrum disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:161–169. doi: 10.1016/j.pnpbp.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, Kroppmann CJ, Alschuler DM, Fekri S, Ben-David S, Corcoran CM, Bruder GE. Auditory event-related potentials and alpha oscillations in the psychosis prodrome: Neuronal generator patterns during a novelty oddball task. Int J Psychophysiol. 2014;91(2):104–120. doi: 10.1016/j.ijpsycho.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimhy D, Corcoran C, Harkavy-Friedman JM, Ritzler B, Javitt DC, Malaspina D. Visual form perception: a comparison of individuals at high risk for psychosis, recent onset schizophrenia and chronic schizophrenia. Schizophr Res. 2007;97(1-3):25–34. doi: 10.1016/j.schres.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiyama D, Kirihara K, Tada M, Nagai T, Koike S, Suga M, Araki T, Kasai K. Duration and frequency mismatch negativity shows no progressive reduction in early stages of psychosis. Schizophr Res. 2017 doi: 10.1016/j.schres.2017.03.015. [DOI] [PubMed] [Google Scholar]
- Lee M, Sehatpour P, Hoptman MJ, Lakatos P, Dias EC, Kantrowitz JT, Martinez AM, Javitt DC. Neural mechanisms of mismatch negativity dysfunction in schizophrenia. Mol Psychiatry. 2017 doi: 10.1038/mp.2017.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light GA, Braff DL. Stability of mismatch negativity deficits and their relationship to functional impairments in chronic schizophrenia. Am J Psychiatry. 2005;162(9):1741–1743. doi: 10.1176/appi.ajp.162.9.1741. [DOI] [PubMed] [Google Scholar]
- MacNamara A, Verges A, Kujawa A, Fitzgerald KD, Monk CS, Phan KL. Age-related changes in emotional face processing across childhood and into young adulthood: Evidence from event-related potentials. Dev Psychobiol. 2016;58(1):27–38. doi: 10.1002/dev.21341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan Y, McArthur G. Maturation of mismatch negativity and P3a response across adolescence. Neurosci Lett. 2015;587:102–106. doi: 10.1016/j.neulet.2014.12.041. [DOI] [PubMed] [Google Scholar]
- Martinez A, Gaspar PA, Hillyard SA, Bickel S, Lakatos P, Dias EC, Javitt DC. Neural oscillatory deficits in schizophrenia predict behavioral and neurocognitive impairments. Front Hum Neurosci. 2015;9:371. doi: 10.3389/fnhum.2015.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaux E, Hernandez N, Carteau-Martin I, Martineau J, Barthelemy C, Bonnet-Brilhault F, Batty M. Event-related potential and eye tracking evidence of the developmental dynamics of face processing. Eur J Neurosci. 2014;39(8):1349–1362. doi: 10.1111/ejn.12496. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Gupta T, Keane BP, Silverstein SM. Visual context processing dysfunctions in youth at high risk for psychosis: Resistance to the Ebbinghaus illusion and its symptom and social and role functioning correlates. J Abnorm Psychol. 2015;124(4):953–960. doi: 10.1037/abn0000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller V, Gruber W, Klimesch W, Lindenberger U. Lifespan differences in cortical dynamics of auditory perception. Dev Sci. 2009;12(6):839–853. doi: 10.1111/j.1467-7687.2009.00834.x. [DOI] [PubMed] [Google Scholar]
- Nooner KB, Colcombe SJ, Tobe RH, Mennes M, Benedict MM, Moreno AL, Panek LJ, Brown S, Zavitz ST, Li Q, Sikka S, Gutman D, Bangaru S, Schlachter RT, Kamiel SM, Anwar AR, Hinz CM, Kaplan MS, Rachlin AB, Adelsberg S, Cheung B, Khanuja R, Yan C, Craddock CC, Calhoun V, Courtney W, King M, Wood D, Cox CL, Kelly AM, Di Martino A, Petkova E, Reiss PT, Duan N, Thomsen D, Biswal B, Coffey B, Hoptman MJ, Javitt DC, Pomara N, Sidtis JJ, Koplewicz HS, Castellanos FX, Leventhal BL, Milham MP. The NKI-Rockland Sample: A Model for Accelerating the Pace of Discovery Science in Psychiatry. Front Neurosci. 2012;6:152. doi: 10.3389/fnins.2012.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oribe N, Hirano Y, Kanba S, Del Re E, Seidman L, Mesholam-Gately R, Goldstein JM, Shenton M, Spencer KM, McCarley RW, Niznikiewicz M. Progressive reduction of visual P300 amplitude in patients with first-episode schizophrenia: an ERP study. Schizophr Bull. 2015;41(2):460–470. doi: 10.1093/schbul/sbu083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez VB, Woods SW, Roach BJ, Ford JM, McGlashan TH, Srihari VH, Mathalon DH. Automatic auditory processing deficits in schizophrenia and clinical high-risk patients: forecasting psychosis risk with mismatch negativity. Biological psychiatry. 2014;75(6):459–469. doi: 10.1016/j.biopsych.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64(5):521–529. doi: 10.1001/archpsyc.64.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahin AJ, Trainor LJ, Roberts LE, Backer KC, Miller LM. Development of auditory phase-locked activity for music sounds. J Neurophysiol. 2010;103(1):218–229. doi: 10.1152/jn.00402.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh M, Valmaggia L, Broome MR, Dutt A, Lappin J, Day F, Woolley J, Tabraham P, Walshe M, Johns L, Fusar-Poli P, Howes O, Murray RM, McGuire P, Bramon E. Reduced mismatch negativity predates the onset of psychosis. Schizophr Res. 2012;134(1):42–48. doi: 10.1016/j.schres.2011.09.022. [DOI] [PubMed] [Google Scholar]
- van Tricht MJ, Nieman DH, Koelman JT, Mensink AJ, Bour LJ, van der Meer JN, van Amelsvoort TA, Linszen DH, de Haan L. Sensory gating in subjects at ultra high risk for developing a psychosis before and after a first psychotic episode. World J Biol Psychiatry. 2015;16(1):12–21. doi: 10.3109/15622975.2012.680911. [DOI] [PubMed] [Google Scholar]
- Whitford TJ, Rennie CJ, Grieve SM, Clark CR, Gordon E, Williams LM. Brain maturation in adolescence: concurrent changes in neuroanatomy and neurophysiology. Hum Brain Mapp. 2007;28(3):228–237. doi: 10.1002/hbm.20273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanova J, Kolev V. Event-Related Brain Oscillations in Normal Development. Developmental Psychophysiology: Theory, Systems, and Methods. 2008:15–68. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
These time-frequency plots illustrate an increase in intertrial coherence in theta for deviants, but a decrease in intertrial coherence for standards in alpha across development, in the time window of 50-250 msec.


