Abstract
Rule-based category learning was examined in youths with Down syndrome (DS), youths with intellectual disability (ID), and typically developing (TD) youths. Two tasks measured category learning: the Modified Card Sort task (MCST) and the Concept Formation test of the Woodcock–Johnson-III (Woodock, McGrew, & Mather, 2001). In regression-based analyses, DS and ID groups performed below the level expected for their nonverbal ability. In cross-sectional developmental trajectory analyses, results depended on the task. On the MCST, the DS and ID groups were similar to the TD group. On the Concept Formation test, the DS group had slower cross-sectional change than the other 2 groups. Category learning may be an area of difficulty for those with ID, but task-related factors may affect trajectories for youths with DS.
Keywords: Down syndrome, category learning, developmental trajectory
Understanding of categories is crucial to negotiating the world in everyday life. For example, it is important to know whether an object is edible or not, if a particular color means stop or go, whether a person is a friend or potentially harmful. Understanding of categories dictates one’s response to them, which in turn determines one’s well-being. This is true for people with intellectual disability as well as those without. Category learning begins early in life and continues to develop through the lifespan. According to the COVIS model (Ashby, Alfonso-Reese, Turken, & Waldron, 1998), at least two categorization systems exist. One is a rule-based system that involves relatively discrete or simple categories whose rules are easily verbalized (e.g., color, shape, number, size). Ashby and colleagues cite the Wisconsin Card Sort Task (Heaton, Chelune, Talley, Kay, & Curtis, 1993) as a good example of a task that reflects rule-based category learning (Ashby & Ell, 2001; Ashby & O’Brien, 2005). The other is an information integration system that involves complex categories that combine stimulus dimensions and whose rules cannot be readily stated. Within the rule-based system, category learning is explicit, highly conscious, and accomplished by way of hypothesis testing. Within the information integration system, category learning is unintentional, implicit, and accomplished by integration of covariances that are experienced over many exemplars. The present study focuses only on the rule-based system by examining two rule-based category learning tasks that have different cognitive demands. These tasks were completed by young people with Down syndrome (DS) in comparison to those with typical development (TD) and mixed-etiology intellectual disability (ID).
DS is the most common genetic disorder that results in ID and is caused by an extra copy of chromosome 21 (i.e., trisomy 21). DS affects approximately 13.65 per 10,000 live births, or 1 in 733 (Canfield et al., 2006). It drastically affects cognitive, emotional, and physical development. Intellectually, people with DS are usually moderately to severely delayed, with an IQ range of 25 to 55, and they also experience an increased risk for congenital heart disease, respiratory infections, loss of vision and hearing, and early-onset Alzheimer’s disease (Van Allen, Fung, & Jurenka, 1999). As a result of such health risks, people with DS typically experience a decreased life expectancy compared with TD people, though life expectancy is increasing for the DS population (Bittles & Glasson, 2004). In addition, DS has a distinct cognitive–linguistic profile. Those with DS struggle with verbal memory relative to spatial memory, expressive language relative to receptive language, and syntax relative to vocabulary (for reviews, see Chapman & Hesketh, 2000; Davis, 2008; Moldavsky, Lev, & Lerman-Sagie, 2001; Silverman, 2007). The present study investigated whether rule-based category learning might be an area of special challenge in DS.
Developmentally, the information integration system of category learning is believed to be dependent on early developing subcortical frontal–striatal structures, and so it should be in place within the first year of life. Although the rule-based system involves the basal ganglia, it is heavily dependent on later developing cortical structures—especially the prefrontal cortex and anterior cingulate cortex (Ashby et al., 1998; Ashby & Ell, 2001; Schnyer et al., 2009). Thus, there should be a clear developmental trajectory in rule-based category learning, though perhaps not in information integration category learning.
Indeed, several studies have shown increases in rule-based category learning from childhood through young adulthood (e.g., Huang-Pollock, Maddox, & Karalunas, 2011; Minda, Desroches, & Church, 2008; Schmittman, Visser, & Raijmakers, 2006). Some researchers have examined learning processes to pinpoint reasons for the developmental increase in rule-based category learning. Huang-Pollock et al. (2011) found that children age 9–13 were more likely to use the dimensions that were irrelevant to the category than were college-age adults. They asked participants to categorize 400 Gabor patches that differed in line orientation and spatial frequency into one of two groups. In the rule-based condition, the categorization was based on spatial frequency alone; that is, line orientation was irrelevant to the correct categorization. They found that children were more likely to respond on the basis of line orientation, which was irrelevant to the category, rather than on spatial frequency, which defined the category. Further, children’s use of the relevant dimension (e.g., spatial frequency) was less consistent over trial blocks. Huang-Pollock et al. suggested that increases in rule-based category learning from childhood to young adulthood are due to increased ability to inhibit irrelevant categories.
Minda et al. (2008) found that rule-based category learning improves with age, and this improvement likely depends on the fact that verbal working memory improves with development, allowing persons to handle increasingly complex rule learning tasks. Children age 3–8 years and college-age adults completed three rule-based category learning tasks that increased in complexity. Based on the COVIS model, all three tasks were believed to rely on the rule-based category system, yet they varied in the demand they placed on working memory. In the single-dimension task, categories were defined by a single dimension such as color (e.g., if Black then Category = 1). In the disjunctive task, categories were complex but clearly could be defined by a verbalizable rule (e.g., if Black triangle OR White square then Category = 1). In the “nonlinearly separable task,” categories were defined by a rule plus at least one exception (e.g., if Black OR the small white triangle, then Category = 1). Minda et al. found a developmental increase on the single-dimension task from age 3 to 5. From age 5 on, participants quickly learned the categories and performed at ceiling. Also, there was a developmental increase on both the disjunctive and the nonlinearly separable tasks from age 8 to adulthood. Only adults could clearly learn the categories in these more demanding tasks. Minda et al. suggested that children’s more limited verbal working memory is responsible for age-related differences in rule-based category learning.
Thus, both Huang-Pollack et al. (2011) and Minda et al. (2008) suggested that developmental increases in rule-based category learning correspond to developmental increases in executive functioning associated with the prefrontal cortex, either through inhibition of the irrelevant dimension (Huang-Pollack et al., 2011) or increased verbal working memory (Minda et al., 2008). There also may be other aspects of executive functioning that underlie developmental improvement on rule-based category learning tasks (e.g., set-shifting), though we are aware of no studies that have demonstrated this specifically. Several studies have shown that children and adults with DS perform below their developmental level on a variety of tasks measuring inhibition (e.g., Atkinson & Braddock, 2012; Borella, Carretta, & Lanfranchi, 2013; Kogan et al., 2002; Lanfranchi, Jerman, Dal Pont, Alberti, & Vianello, 2010; Rowe, Lavender, & Turk, 2006; Wilding, Cornish, & Munir, 2002; but see Carney, Brown, & Henry, 2013; Lee et al., 2011; Pennington, Moon, Edgin, Stedron, & Nadel, 2003; Randolf & Burack, 2000) and working memory (e.g., Carney et al., 2013; Lanfranchi, Cornoldi, & Vianello, 2004; Lanfranchi, Jerman, & Vianello, 2009; Lanfranchi et al., 2010; Munir, Cornish, & Wilding, 2000; Vicari, Carlesimo, & Caltagirone, 1995; but see Edgin, Pennington, & Mervis, 2010). Although few studies have examined set-shifting directly in DS, some evidence points to special difficulty in this skill (Carney et al., 2013; Lanfranchi et al., 2010; Rowe et al., 2006). Because DS affects development of the prefrontal cortex (e.g., Jernigan, Bellugi, Sowell, Doherty, & Hesselink, 1993; Raz, et al., 1995), these findings are not surprising.
To the extent that rule-based category learning heavily involves inhibitory processes, verbal working memory, and possibly set-shifting and because these are associated with the prefrontal cortex, we expect individuals with DS to have difficulty in rule-based category learning beyond that predicted by their general developmental level. To test this hypothesis in the present study, we compared performance of young people with DS on two different rule-based category learning tasks with that of TD children who were in the same range of nonverbal ability. To determine whether any pattern of performance in DS is etiology specific, we also compared their performance with that of young people with non-DS ID.
Few studies have examined rule-based category learning in individuals with DS. Using novel animal categories, Klinger and Dawson (2001) found that participants with DS (ages 7–19, mental ages [MAs] 6–7) did not differ on simple feature-based category learning from participants with autism spectrum disorder (ages 5–21) and TD (ages 4–11) who were matched on receptive vocabulary. In contrast, using a variant of the Wisconsin Card Sorting task, Lanfranchi et al. (2010) found that participants with DS (ages 11–18, MAs 4–6) performed more poorly than TD participants (ages 4–6) who were matched on nonverbal reasoning. Although tasks in both studies reflected rule-based category learning, they differed in two potentially important ways. First, the Klinger and Dawson task was a two-choice task, whereas the Lanfranchi task was a four-choice task. The four-choice task might have been more demanding on working memory because there was more information to be evaluated and held in mind while making decisions. Second, the Klinger and Dawson task used very different stimuli from one category learning problem to the next (i.e., different animals), whereas the Lanfranchi task used the same stimuli from one category learning problem to the next (cards with colored shapes on them). Using the same stimuli may have placed more demand on inhibitory processes and/or set-shifting because participants had to stop responding to the previous category-defining dimension (e.g., color) in order to respond to the new category-defining dimension (e.g., shape). To the extent that participants with DS have working memory and inhibitory processing difficulties, and possibly set-shifting difficulties, these task variations could create the difference in results across the two studies.
In the present study, we used two measures that reflect rule-based category learning to compare adolescents with DS to children with TD and adolescents with ID in the same range of nonverbal ability. One was the Modified Card Sort Task (MCST; Nelson, 1976), which was used by Lanfranchi et al. (2010), and the other was the Concept Formation subtest of the Woodcock Johnson Test of Cognitive Abilities–III (Woodcock, McGrew, & Mather, 2001). In the MCST, participants use cards, each containing a number of colored shapes (e.g., two green stars, four blue circles, etc.). They try to discover the examiner’s sorting rule (i.e., number, color, or shape) by sorting each card into one of four piles and receiving feedback. After six correct card sorts in a row, the participant is told that there is now a new rule to discover, different from the last. In the Woodcock Johnson-III Concept Formation subtest, participants look at colored shapes that vary in color, shape, size, and/or number. Some of the shapes are shown in a box, whereas others are shown outside the box. Participants try to discover the rule that defines the shapes in the box, and then verbally state the rule. In early trials, the rule is simple (e.g., big), but in later trials, the rule is compound (e.g., big and red, big or red).
Both tests use colored shapes as stimuli and require attention to various dimensions of the stimuli (shape, color, number, size) and comparison across the values of these dimensions. Both tasks also require participants to be flexible enough to change the basis on which they compare stimuli (e.g., by color vs. by shape). To the extent that both tasks reflect rule-based category learning, we would expect to find results similar to those of Lanfranchi et al. (2010) on both tasks. The present study was also able to address etiology specificity because it included a mixed-etiology ID group, which was not part of the previous studies.
However, the two tasks have some key differences. The Concept Formation test uses all four dimensions listed above, whereas the MCST uses only shape, color, and number. Also, the Concept Formation test asks participants to identify the different stimulus, whereas the MCST asks participants to find the same (matching) stimulus. The Concept Formation test graduates to more difficult comparisons that require use of compound rules, whereas the MCST has a consistent structure throughout. The more consistent structure of the MCST, though, might be more likely to encourage adoption of a set, and thus may be more demanding in terms of inhibition and/or set shifting. Finally, at one point in the Concept Formation test, verbal responses are required, whereas in the MCST verbal responses are never required. We were interested in whether these task differences would be important enough to produce different results across DS, ID, and TD groups. On balance, the Concept Formation test seems to present more challenges that are specific to DS than the MCST. Thus, we expected that, if anything, group contrasts would be greater on the Concept Formation test than the MCST.
In addition to examining group differences in rule-based category learning, in the present study we examined cross-sectional developmental trajectories. It is fairly well documented that in DS, growth in overall cognitive ability, or MA, does not keep pace with increases in chronological age (e.g., see Vicari, 2006). Also, for a variety of cognitive abilities, growth relative to MA appears even slower for participants with DS than for those with TD, including verbal memory span (Frenkel & Bourdin, 2009; Hulme & Mackenzie, 1992; Mackenzie & Hulme,1987), visuo-spatial memory span (Frenkel & Bourdin, 2009), holistic face recognition (Annaz, Karmiloff-Smith, Johnson, & Thomas, 2009), and some aspects of attention (Cornish, Scerif, & Karmiloff-Smith, 2007). However, growth in visual pattern span over MA seems similar in DS and TD groups (Frenkel & Bourdin, 2009). If growth in rule-based concept learning is also slow relative to MA in DS, in the age and ability range of our participants, this should be apparent in the cross-sectional developmental trajectory analyses.
We expected that participants with DS would perform below the level expected for their nonverbal ability and that they would show a flatter slope in their cross-sectional developmental trajectory. This hypothesis was based on the finding that the development of rule-based category learning depends on the development of inhibitory processes and verbal working memory, and on the involvement of the prefrontal cortex. To the extent that this difficulty is specific to DS, we expected the group with DS to perform below the group with ID, and to have a flatter slope in their cross-sectional developmental trajectory. Because the Concept Formation test seemed to present more challenges that may be specific to DS, compared with the MCST, we expected that the pattern of results would be more pronounced on this measure of rule-based category learning.
Methods
Participants
Participants were 41 individuals with DS, 25 individuals with ID, and 28 TD individuals in the same range of nonverbal ability as determined by the Leiter International Performance Test-Revised brief form (Leiter-R; Roid & Miller, 1997) growth score value. The current study was a part of a larger study examining the cognitive predictors of language impairment in DS. Participants were recruited for the larger study in Alabama and Wisconsin through multiple avenues, including local schools and agencies as well as research participant registries at both sites. To be eligible for the larger study, all participants had to be native English speakers, use speech as their primary means of communication, and have use of their hands to manipulate cards. To be included in the present analysis, participants had to be able to complete both rule-based category learning measures that were administered in the study and score between 4 and 9 years in nonverbal MA on the Leiter-R brief form. See Table 1 for participant characteristics.
Table 1.
Descriptive Statistics
| M | SD | Range | |
|---|---|---|---|
| Down syndrome | |||
| Chronological age | 15.29 | 3.21 | 10.25–21.92 |
| Nonverbal IQ | 44.66 | 8.83 | 36–71 |
| Nonverbal ability (GSV) | 467.46 | 9.94 | 453–492 |
| MCST (learning efficiency score) | 12.00 | 7.10 | 0–30 |
| Concept Formation test (raw score) | 5.12 | 4.15 | 0–16 |
| Intellectual disability | |||
| Chronological age | 15.85 | 2.54 | 10.25–20.67 |
| Nonverbal IQ | 54.20 | 10.18 | 36–77 |
| Nonverbal ability (GSV) | 476.92 | 8.93 | 458–487 |
| MCST (learning efficiency score) | 18.56 | 14.61 | 0–44 |
| Concept Formation test (raw score) | 11.16 | 6.74 | 2–24 |
| Typically developing | |||
| Chronological age | 7.41 | 2.67 | 4.25–13.50 |
| Nonverbal IQ | 97.39 | 13.30 | 76–129 |
| Nonverbal ability (GSV) | 477.18 | 13.18 | 459–498 |
| MCST (learning efficiency score) | 26.11 | 12.25 | 12–45 |
| Concept Formation test (raw score) | 13.64 | 9.37 | 1–34 |
Note. GSV = growth score value; MCST = Modified Card Sort Task.
Participants with DS
In addition to the general eligibility criteria, participants with DS had to be between 10 and 21 years old and pass an autism screener (i.e., the Social Communication Questionnaire). Of 44 participants with DS in the larger study who met criteria for the present study, 41 were included in the final data analysis (18 males; 34 White, 4 White Hispanic, 1 Native American Indian and White, 1 Asian/Pacific Islander and White, 1 other race). Of those who were excluded from the present analyses, one demonstrated signs of fatigue and frustration and did not appear to be engaged during the MCST and the Concept Formation test, and two were extreme outliers on the MCST. All but four participants with DS were diagnosed by obtaining a copy of the chromosomal analysis from the parents or, if this was unavailable, by having a confirmation of diagnosis from the individual’s doctor. Such confirmation could not be obtained for the remaining four participants, and they were diagnosed on the basis of parent report.
Participants with ID
In addition to the general eligibility criteria, participants in the ID group had to be between 10 and 21 years old, have a school classification or clinical diagnosis of ID, and score below 75 on the nonverbal IQ test given in the study. Also, they had to pass an autism screener. Of 27 participants with DS in the larger study who met criteria for the present study, 25 were included in the final data analysis (11 males; 21 White, 3 African American, 1 White Hispanic). Of the participants who were excluded from analyses, one had physical limitations that compromised performance on the tests, and one’s true birth date was unknown, making it impossible to compute standard scores for the Leiter-R.
TD participants
In addition to the general eligibility criteria, to be included in the TD group, participants had to be at least 4 years old; ineligible for special services in school including those for learning disability, speech and language services, and giftedness; and not have a diagnosis of attention-deficit/hyperactivity disorder or an autism spectrum disorder. Of 28 participants with TD in the larger study who met criteria for the present study, all were included in the final data analysis (17 males; 14 White, 8 African American, 3 White Hispanic, 1 Black Hispanic, 1 White and African American, 1 other race).
Measures
Nonverbal ability
The Leiter-R brief form (Roid & Miller, 1997) was used to provide estimates of nonverbal ability. Nonverbal intelligence, as opposed to verbal intelligence, was used in the current study due to the known verbal deficits in DS. The Leiter-R is a published standardized norm-referenced test designed for ages 2 years through 21 years. We administered the four subtests that make up the Brief IQ battery: Figure Ground, Form Completion, Sequential Order, and Repeated Patterns. These subtests measure visual spatial and inductive reasoning skills typically classified as fluid intelligence. We used the MA (or age equivalence) score to determine eligibility for the present analysis. However, we used the growth score value (GSV) in the data analyses. The GSV is the conversion of the raw score in which scale corrections are made for variability in item difficulty, and it ranges from approximately 380 to 560. The Leiter-R brief form correlates .85 with both the full version of the Leiter-R and the Wechsler Intelligence Scale for Children–Third Edition IQ test, and reliability ranges from .75 to .88 (Roid & Miller, 1997).
MCST (Nelson, 1976)
The MCST was one of two tasks used to measure rule-based category learning. It is an adaptation of the Wisconsin Card Sorting Test (Berg, 1948; see also Heaton et al., 1993), introduced by Nelson (1976) and developed by Cianchetti, Corona, Foscoliano, Contu, and Siannio-Fancello (2007). The adaptation makes the task more appropriate for children as young as 4 years, and also emphasizes category learning more so than set-shifting by explicitly telling participants when the category has changed. The standard Wisconsin Card Sorting Test does not alert examinees to the category changes, but instead requires examinees to discover these shifts on their own. The MCST uses stimulus cards containing triangles, stars, crosses, or circles. Each card has from one to four shapes on it (all the same shape on a card) and the shapes are in one of four colors (all the same color on a card). After showing the participant the different colors, shapes, and number of shapes on the cards, the examiner lays out four standard cards—(1) one red triangle, (2) two green stars, (3) three yellow crosses, and (4) four blue circles—and hands the participant one card at a time from a stack of 48 cards, which contain different combinations of shape, color, and number. The examiner asks the participant to match the card to one of four standard cards to try to figure out the examiner’s “rule.” The participant’s first choice is always correct, which means that it determines what the rule will be (color, shape, or number). The examiner gives feedback after each trial, telling the participant if the match was correct or incorrect. The task continues until the participant correctly sorts six cards in a row. The cards are then picked up and the examiner says that the participant must try to figure out the examiner’s new rule.
The game is played six times, with each of the three categories (color, shape, and number) being a matching rule two times. Each category is used once as the rule in Games 1–3, and again in Games 4–6 in the same order. The participant chooses the rule in Game 1 on the basis of what he or she matched on first. The rule in Game 2 can be either of the two unused categories, and the rule in Game 3 is the final unused category. The task ends when the participant has run out of cards or completes all six games, whichever is first. We used the learning efficiency score (Cianchetti et al., 2007) as the main measure from this task. This is the total number of categories completed (i.e., six cards in a row correct) multiplied by 6 plus the number of unplayed cards (if any). Spearman–Brown split half reliability was .96 for the entire sample, and above .90 for each of the three participant groups.
Concept Formation test (Woodcock et al., 2001)
The Concept Formation subtest of the Woodcock Johnson–III Test of Cognitive Abilities was used as a second measure of rule-based category learning. This published standardized test is normed for individuals between 2 and 90 years of age. It requires participants to look at colored shapes that vary on up to four dimensions (color, shape, size, and number). On the first five items, participants are shown a series of shapes on the left and an empty box on the right. They are asked to point to the shape that is different and are told that the different shape goes in the box. On the remaining items, there is a series of shapes on the left and a box (or boxes) on the right that contains a particular shape or shapes. Participants decide how the shapes in the box (or boxes) are similar to one another and different from those on the left; in other words, they try to discover the rule that defines the shapes in the box. On the first set of items, this rule is just a single dimension (e.g., red, big, two), and participants must verbally state the rule. Then participants complete a set of items where the rule is compounded by the word and (e.g., big and red, two and small). In these items, the shapes in the box are defined by both dimensions, and participants must verbally state both dimensions and include the word and in their response. Next participants complete a set of items where the rule is compounded by the word or (e.g., big or red, two or small). In these items, the shapes in the box are defined by either one dimension or the other, and participants must verbally state both dimensions and include the word or in their response. Finally, participants complete a set of items that combines all of the different types of items they have completed (i.e., single dimension or compound dimension). They are not told which type of item they are completing but must determine on their own if the item’s rule is a single dimension or a compound dimension using and or or. The examiner steps the participant through the early trials of each type of item and provides feedback so that the participant learns how to discover the rules. Throughout the task, there are cut-off points that the participant must pass in order to move on to the next set of items; if the participant did not correctly answer enough items, then the task is stopped at that cut-off point. The Concept Formation subtest of the Woodcock–Johnson-III has a reliability coefficient of .94. Participants get a point for each item they answer correctly. Total points awarded (raw scores) were used in data analyses.
Procedure
For the larger study, participants completed a battery of learning, memory, and language tasks, divided into two to four testing sessions. Total testing time was 3–7 hr, depending on the individual characteristics of the participant and the participant’s ability to remain attentive and engaged. The Leiter-R and MCST were always administered during the first session, and the Concept Formation test was always administered at the end of the final testing session. The Leiter-R took approximately 30 min. The MCST took approximately 10 min, and the Concept Formation test took approximately 15 min.
Results
Analytic Approach
For each of the two rule-based category learning measures, we examined performance relative to nonverbal ability, and we performed a developmental trajectory analysis. To examine performance of participants with DS and with ID relative to that expected on the basis of their nonverbal ability, we first computed the regression equation of nonverbal ability predicting each of the category learning measures within the TD group. Then we computed residual scores for each participant. To determine whether performance of the DS and ID groups was below that predicted for nonverbal ability, we computed one-sample t tests comparing group mean residual scores to zero. Then we used one-way analysis of variance and post hoc tests to compare residual scores across groups. Compared with a matched groups approach, the regression-based approach has the advantage that it does not require groups to be matched precisely on, in this case, nonverbal ability (Jarrold & Brock, 2004).
To compare linear regressions (i.e., trajectories) across groups, we used the method described by Thomas et al. (2009), which adapts the analysis of covariance (ANCOVA) function within SPSS’s General Linear Model. An important assumption in the traditional use of the ANCOVA is that the covariate has the same relation to the dependent variable in each group. Traditionally, ANCOVA tests this assumption by examining whether there is a significant Group × Covariate interaction (there should be none to meet the assumption). For the present trajectory analysis, we were specifically interested in whether nonverbal ability has a different relation to the performance variable (e.g., category learning) across groups. Thus, the ANCOVA was used to test group differences in slope. Group differences in intercept were indicated by the effect of group, and group differences in slope were indicated by the Group × Nonverbal Ability interaction.
Preliminary Analyses
Means, standard deviations, and ranges for all variables are listed in Table 1, and the correlations among all variables, using Pearson product-moment correlation coefficients, are listed in Table 2. There were no serious violations of normality; however, two participants from the DS group scored outside ± 3 SD on the MCST. As mentioned in the Participants section, these two participants were removed from further analyses.
Table 2.
Correlations
| Group and variable | CA | Nonverbal ability (GSV) | MCST (learning efficiency score) |
|---|---|---|---|
| Down syndrome | |||
| CA | — | — | — |
| Nonverbal ability (GSV) | .16 | — | — |
| MCST (learning efficiency score) | .26 | .67** | — |
| Concept Formation test (raw score) | −.17 | .49** | .48** |
| Intellectual disability | |||
| CA | — | — | — |
| Nonverbal ability (GSV) | .26 | — | — |
| MCST (learning efficiency score) | .23 | .28 | — |
| Concept Formation test (raw score) | .29 | .58** | .78** |
| Typically developing | |||
| CA | — | — | — |
| Nonverbal ability (GSV) | .84** | — | — |
| MCST (learning efficiency score) | .78** | .77** | — |
| Concept Formation test (raw score) | .66** | .73** | .61** |
Note. CA = chronological age; GSV = growth score value; MCST = Modified Card Sort Task.
p < .05, two-tailed;
p < .01, two-tailed.
To prepare for the regression-based analyses, we generated scatterplots within each group showing the function of category learning over nonverbal ability. Each scatterplot was analyzed by visual inspection for linearity, and there were no clear nonlinear trends. Also, the extra sum-of-squares test was used to confirm linearity of the data. For the current data, the linear function was found to be the best model for both measures of category learning over nonverbal ability for all groups (DS, ID, and TD). To determine whether any points were exerting undue influence on the regressions, Cook’s D values were generated separately within each group for each of the category learning measures. In no case was Cook’s D above 1.00, so no points were removed from the analyses. For interpretability of the intercept in the trajectory analyses, the Leiter GSV (nonverbal ability) variable was transformed so that lowest score in the analysis was set equal to zero.
MCST
Group performance relative to nonverbal ability
For the MCST, nonverbal ability accounted for 58.9% of the variance in the TD group, F(1, 26) = 37.27, p < .001. Residual scores of both groups with DS and ID were significantly below zero, t(40) = 7.97, p < .001, d = 1.24, and t(24) = 2.59, p = .016, d = 0.52, respectively, indicating performance below that expected for nonverbal ability (DS M = −7.17, SD = 5.77; ID M = −7.36, SD = 14.20; TD M = 0; SD = 7.85). Also, there was a significant effect of group, F(2, 91) = 6.00, p = .004, η2 = 0.12. As a result of unequal variances across groups, Games–Howell post hoc analysis was used for pairwise comparisons. As expected, the DS group’s residual scores were significantly lower than those of the TD group (p < .001). However, they were equivalent to those of the ID group (p = .998). The ID group’s residual scores were lower than those of the TD group, but the difference fell short of statistical significance (p = .069).
Developmental trajectory analysis
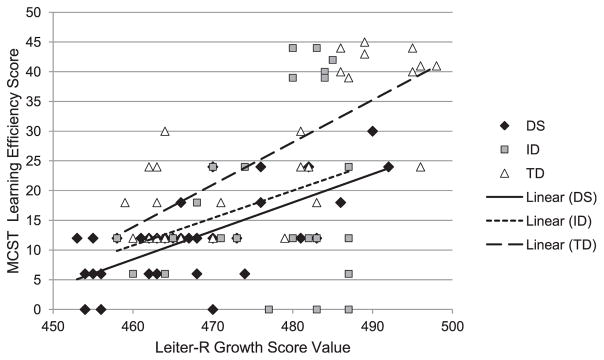
The developmental trajectories for the MCST over nonverbal ability are shown in Figure 1 (and see Table 3 for regression equations). There was no overall effect of group, F(2, 88) = 0.37, p = .689, ηp2 = .008, and no significant Group × Nonverbal Ability interaction, F(2, 88) = 0.89, p = .414, ηp2 = .020. Thus, at the lowest nonverbal ability score in the data set (equivalent to an approximate MA of 4 years), the regression lines for each group were not significantly different. Also, contrary to expectations, growth in MCST as a function of nonverbal ability was similar across the three groups. All groups showed similar growth, though there was marked variability, especially in the ID group. As a result of the high variability of the ID group, this analysis was conducted with just the DS and TD groups to see if the variability of the ID group was masking the difference between these groups. However, there was still no overall effect of group or significant Group × Nonverbal Ability interaction.
Figure 1.
Growth rate of category task over Leiter-R GSV.
Table 3.
Linear Trajectory Statistics for Each Category Learning Measure Over Nonverbal Ability in Each Group
| Group | Regression results | Regression equation |
|---|---|---|
| MCST | ||
| Down syndrome | R2 = .449, F(1, 39) = 31.72, p < .001 | y = 0.48x + 5.08 |
| Intellectual disability | R2 = .079, F(1, 23) = 1.98, p = .173 | y = 0.46x + 7.54 |
| Typically developing | R2 = .589, F(1, 26) = 37.27, p < .001 | y = 0.71x + 8.85 |
| Concept Formation test | ||
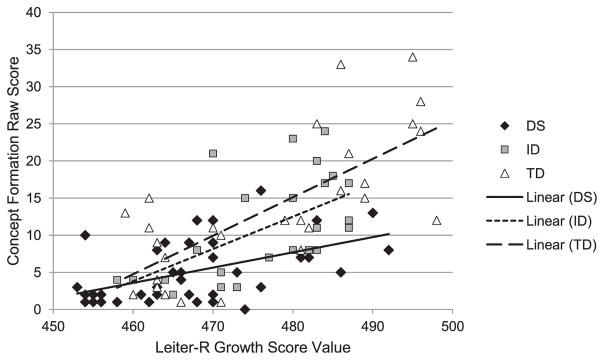
| Down syndrome | R2 = .242, F(1, 39) = 12.47, p = .001 | y = 0.21x + 2.15 |
| Intellectual disability | R2 = .331, F(1, 23) = 11.39, p = .003 | y = 0.44x + 0.76 |
| Typically developing | R2 = .529, F(1, 26) = 29.16, p < .001 | y = 0.52x + 1.14 |
Note. MCST = Modified Card Sort Task.
Concept Formation Test
Performance relative to nonverbal ability
For the Concept Formation test, nonverbal ability accounted for 52.9% of the variance in the TD group, F(1, 26) = 29.16, p < .001. Similar to the MCST, residual scores of both groups with DS and ID were significantly below zero, t(40), = 4.71, p < .001, d = 0.73, and t(24) = 2.11, p = .045, d = 0.42, respectively (DS M = −3.50, SD = 4.76; ID M = −2.35, SD = 5.56; TD M = 0; SD = 6.44). Also, there was a significant effect of group on residual scores, F(2, 91) = 3.37, p = .039, η2 = 0.07. Tukey’s honestly significant difference test indicated that, similar to the MCST, the DS group’s residual scores were significantly lower than those of the TD group (p = .030) but equivalent to those of the ID group (p = .692). The ID group’s residual scores were lower but not significantly different from those of the TD group (p = .273).
Cross-sectional developmental trajectory analysis
The cross-sectional developmental trajectories for the Concept Formation test over nonverbal ability are shown in Figure 2 (and see Table 3 for regression equations). Similar to MCST, there was no overall effect of group, F(2, 88) = 0.13, p = .875, ηp2 = .003; however, there was a significant Group × Nonverbal Ability interaction, F(2, 88) = 3.97, p = .022, ηp2 = .083. Follow-up developmental trajectories were compared across two groups to further analyze the significant interaction. The slope for the DS group was flatter than that of both the TD group, F(1, 65) = 8.26, p = .005, ηp2 = .113, and the ID group, F(1, 62) = 3.36, p = .072, ηp2 = .051, though the latter comparison did not quite reach statistical significance. The TD and ID groups had similar slopes, F(1, 49) = 0.24, p = .623, ηp2 = .005. Thus, at the lowest nonverbal ability measured (equivalent to an approximate MA of 4 years), the regression line for the DS group was not lower than that for the other groups; however, the DS group showed less growth with increasing nonverbal ability than the other two groups.
Figure 2.
Growth rate of WJ-III Concept Formation over Leiter-R GSV.
Within-Group Trajectory Analysis
Next, we compared slopes of the two category learning tasks within each group. To put the scores from the two tasks on the same scale, we computed z-scores based on within-group means and standard deviations. Then, for each group, we conducted an ANCOVA comparing nonverbal ability-based task trajectories, again following the method described by Thomas et al. (2009). Task was the within-subjects factor, nonverbal ability was the covariate, and Task × Nonverbal Ability was the interaction term. Across six analyses, there was only one significant effect: in the DS group, the Concept Formation slope was flatter than the MCST slope, F(1, 39) = 9.50, p = .004, ηp2 = .196. For the TD and ID groups, growth was similar in the two category learning tasks.
Discussion
The purpose of the current study was to examine performance in rule-based learning by young people with DS in comparison with developmental level expectations. On the basis of previous studies that suggested involvement of inhibitory processes, working memory, and prefrontal cortex, we expected participants with DS to perform below the level expected for their nonverbal ability. Also, we expected that, if the special challenges of this type of learning are associated with the etiology of DS, above and beyond intellectual disability, the group with DS would perform lower than the group with mixed-etiology ID.
Results of the present study showed that, indeed, participants with DS performed below the level expected on the basis of their nonverbal ability on both the MCST and the Concept Formation task. This finding replicates Lanfranchi et al. (2010), using a regression-based approach and a larger sample size. However, in the present study, the ID group also performed below the level expected on the basis of their nonverbal ability, and the DS and ID groups were comparable. Thus, in terms of groupwise performance in rule-based category learning, the difficulty seems more related to intellectual disability than to the etiology of DS. Although the present study was not able to do so, future studies should examine underlying reasons for poor category learning (e.g., inhibitory processes, verbal working memory, and set-shifting) in persons with DS and ID.
When making the comparison between the DS and ID groups, our results showed similar performance. There are several reasons such a similarity could result. Possibly, the groups showed similar difficulty because rule-based category learning requires an array of basic cognitive abilities and brain structures, not all of which are uniquely affected by DS. Some of these abilities and structures are affected by DS as well as by other etiologies of ID. For example, inhibitory processes, verbal working memory, and prefrontal cortex are compromised in some other ID syndromes besides DS, such as Fragile X syndrome (Conners, Moore, Loveall, & Merrill, 2011; Cornish et al., 2004). Also, some of the abilities and structures involved in category learning are not clearly affected by DS, but may be affected by other etiologies of ID. For example, the basal ganglia play a role in rule-based category learning (e.g., Ashby & Ell, 2001; Ell, Marchant, & Ivry, 2006) and are affected by fetal alcohol syndrome more so than DS (Mattson et al., 1996; Roussotte et al., 2012). Unfortunately, the size of our ID sample precludes analysis by etiology, but this would be an important avenue for future research. These results point out the need for contrasting mixed- or idiopathic-etiology ID groups in DS research, particularly if the goal is to help define the etiology-specific phenotype.
We were also interested in whether participants with DS would show a flatter cross-sectional developmental trajectory in rule-based category learning over nonverbal ability, as some previous studies have shown for other cognitive skills. Cross-sectional developmental trajectories provide an approximation of longitudinal trajectories based on a sample that varies widely in developmental age. As in similar investigations focusing on groups with ID, the cross-sectional developmental trajectories presented here provide information on change not over chronological age, but over an index of developmental age (in our case, nonverbal ability). In our analyses, the DS group showed similar cross-sectional change over nonverbal ability on the MCST compared with both the TD and ID groups, but slower change on the Concept Formation test. Further, for TD and ID groups, cross-sectional change was similar for the two tasks, but for the DS group, change was slower in the Concept Formation test than the MCST.
Thus, the comparison of the group with DS to groups with TD or ID produced mixed results. On the MCST, the group with DS showed a similar rate of change to the other two groups. This result is consistent with that for visual pattern span (Frenkel & Bourdin, 2009). Though their performance (and that of the ID group) was low for their nonverbal ability, their improvement on the MCST with increasing nonverbal ability was very similar to that of the TD group. This suggests that when the learning task has a consistent format throughout and requires only manual responses, young people with DS and ID can keep the same pace of improvement as those with TD. However, change on the Concept Formation test with increasing nonverbal ability was not as great for participants with DS as for participants with TD or ID. This result is consistent with those of several studies examining other cognitive measures (Annaz et al., 2009; Cornish et al., 2007; Frenkel & Bourdin, 2009; Hulme & Mackenzie, 1992; Mackenzie & Hulme, 1987). In combination with the results from the MCST, this suggests that task structure and/or task demands can slow learning selectively for those with DS.
The pattern of similar cross-sectional change on the MCST but slower change on the Concept Formation test suggests that the two tests differ in a way that is relevant to DS above and beyond intellectual disability. Specifically, some aspect of the Concept Formation test limits growth for young people with DS more so than for young people with mixed-etiology ID. For example, the Concept Formation test requires participants to find the category that is different, whereas the MCST requires participants to match on a category that is the same. Although the concept different is more advanced than same, we have no compelling reason to think that this would be more relevant to DS than to other ID. Also, the Concept Formation test includes compound categories, which are harder than simple categories. Again, we have no compelling reason to believe that compound categories would be relatively more difficult than simple categories for participants with DS versus participants with ID. These could be investigated in future research.
Another difference between the two tasks is that the Concept Formation test changes format, whereas the MCST has a consistent format across the entire task. The more consistent format of the MCST might be more likely to encourage adoption of a set, and therefore place more demand on inhibition or set shifting. However, because there are special difficulties in inhibition and possibly set-shifting associated with DS, this would result in findings opposite to what actually occurred. That is, the DS group would have shown slower cross-sectional change on the MCST relative to the ID group, and perhaps a similar rate of change on the Concept Formation test.
Also, the number of stimulus dimensions involved in the task differs across the two tasks. The Concept Formation test has four dimensions (color, shape, number, and size), whereas the MCST has only three dimensions (color, shape and number). An additional dimension could mean more demand on working memory in the Concept Formation test. Because working memory—in both verbal and visuospatial domains—is an etiology-specific characteristic of DS, the task demands on working memory could be a reason for slower cross-sectional change over nonverbal ability in the DS group compared with the ID and TD groups. Future research could examine the role of working memory in the different cross-sectional developmental trajectories for the two tasks.
A further possible limiting aspect of the Concept Formation test is that it requires verbally expressed responses, whereas the MCST does not. This may be an aspect of the test that added difficulty for participants with DS more so than for participants with ID in general. The first five trials of the Concept Formation test require participants to point to the shape that is different, but after these five trials, participants are required to verbally state what is different about the shape(s) in the box versus the shapes outside of the box (e.g., small, big, red, yellow, two, one). In contrast, the MCST does not require any type of verbal response; rather, participants must simply match the card to one of the four standard cards. Due to the known etiology-specific expressive language impairments in DS (Boudreau & Chapman, 2000; Chapman, Seung, Schwartz, & Bird, 1998; Dykens, Hodapp, & Evans, 1994; Finestack & Abbeduto, 2010; Price et al., 2008; Rosin, Swift, Bless, & Kluppel Vetter, 1988), the verbal component of the Concept Formation test may be limiting the DS group’s performance on this task, resulting in a slower rate of development and a lack of variability in this group’s scores compared with the scores on the MCST, and compared with the TD and ID groups. Although we did not include a measure of expressive language in the present analysis to confirm this explanation, it should be investigated in the future.
The present study has certain limitations that warrant mention. Although the DS sample was a good size, the TD and ID samples were somewhat smaller. In addition, it should be pointed out that there was quite a bit of variability, particularly in the ID group in the MCST developmental trajectory. This was the only regression that was not statistically significant, with only 8% of the variance in MCST accounted for by nonverbal ability. Participants in this group scored from floor to ceiling, and this was not explained by etiology, chronological age, or any other covariate we examined. Further, this pattern of variance occurred only on the MCST and not nearly as much on the Concept Formation test; therefore, we attribute it to error variance rather than systematic variance. For these reasons, the results of the present study need to be replicated using a separate sample. The use of cross-sectional data to examine growth in rule-based category learning is a good first step, but ultimately, longitudinal data will be needed to confirm that the observed trends represent actual change over time. The present study focused on category learning based on familiar stimulus dimensions (color, shape, number, and size), but not on learning of novel categories. Although the present study demonstrated that growth patterns might depend on the exact task being used, it was not able to fully explain the task-related differences. Further research will be needed to do so.
On the basis of the current analyses and discussion, we conclude that rule-based category learning poses a special challenge for young people with ID, beyond what would be expected for their level of nonverbal ability. This is true for those with DS as well as those with non-DS ID. These young people need extra supports to learn the many categories essential to increased independence. For example, in school, children learn to categorize line drawings as letters versus numbers versus pictures; they learn to categorize those drawings that are letters into specific letter categories. Learning processes like these may be more difficult than we would expect for children with ID. They may need more explicit instruction in hypothesis testing—how to guess a rule, try it, and decide if it is correct. Further, for those with DS, growth in the ability to learn categories may be dampened if factors such as expressive language response requirements are imposed. For these individuals, learning may improve when this type of barrier is minimized. Research using education-relevant materials in classroom settings will be important for making this translation.
Acknowledgments
The authors would like to thank the participants and their families; collaborators Len Abbeduto, Jamie DeCoster, and Laura Klinger; and the testers at the University of Alabama, the University of California Davis MIND Institute, and the University of Wisconsin-Madison Waisman Center. This research was supported by E. K. Shriver National Institute of Child Health and Human Development Grant HD055345.
Contributor Information
B. Allyson Phillips, University of Alabama, Psychology.
Frances A. Conners, University of Alabama, Psychology
Edward Merrill, University of Alabama, Psychology.
Mark R. Klinger, University of North Carolina
References
- Annaz D, Karmiloff-Smith A, Johnson MH, Thomas MSC. A cross-syndrome study of the development of holistic face recognition in children with autism, Down syndrome, and Williams syndrome. Journal of Experimental Child Psychology. 2009;102:456–486. doi: 10.1016/j.jecp.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Alfonso-Reese LA, Turken AU, Waldron EM. A neuropsychological theory of multiple systems in category learning. Psychological Review. 1998;105:442–481. doi: 10.1037/0033-295x.105.3.442. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Ell SW. The neurobiology of human category learning. Trends in Cognitive Sciences. 2001;5:204–210. doi: 10.1016/s1364-6613(00)01624-7. [DOI] [PubMed] [Google Scholar]
- Ashby FG, O’Brien JB. Category learning and multiple memory systems. Trends in Cognitive Sciences. 2005;9:83–89. doi: 10.1016/j.tics.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Atkinson J, Braddock O. Visual attention in the first years: Typical development and developmental disorders. Developmental Medicine and Child Neurology. 2012;54:589–595. doi: 10.1111/j.1469-8749.2012.04294.x. [DOI] [PubMed] [Google Scholar]
- Berg EA. A simple objective technique for measuring flexibility in thinking. Journal of General Psychology. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- Bittles AH, Glasson EJ. Clinical, social, and ethical implications of changing life expectancy in Down syndrome. Developmental Medicine and Child Neurology. 2004;46:282–286. doi: 10.1017/s0012162204000441. [DOI] [PubMed] [Google Scholar]
- Borella E, Carretta B, Lanfranchi S. Inhibitory mechanisms in Down syndrome: Is there a specific or general deficit? Research in Developmental Disabilities. 2013;34:65–71. doi: 10.1016/j.ridd.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Boudreau DM, Chapman RS. The relationship between event representation and linguistic skill in narratives of children and adolescents with Down syndrome. Journal of Speech, Language, and Hearing Research. 2000;43:1146–1159. doi: 10.1044/jslhr.4305.1146. [DOI] [PubMed] [Google Scholar]
- Canfield MA, Honein MA, Yuskiv N, Xing J, Mai CT, Collins JS, … Kirby RS. National estimates and race/ethnic-specific variation of selected birth defects in the United States, 1999–2001. Birth Defects Research (Part A) 2006;76:747–756. doi: 10.1002/bdra.20294. [DOI] [PubMed] [Google Scholar]
- Carney PJ, Brown JH, Henry LA. Executive function in Williams and Down syndromes. Research in Developmental Disabilities. 2013;34:46–55. doi: 10.1016/j.ridd.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Chapman RS, Hesketh LJ. Behavioral phenotype of individuals with Down syndrome. Mental Retardation and Developmental Disabilities Research Reviews. 2000;6:84–95. doi: 10.1002/1098-2779(2000)6:2<84::AID-MRDD2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Chapman RS, Seung H, Schwartz SE, Bird E. Language skills of children and adolescents with Down syndrome II: Production deficits. Journal of Speech, Language, and Hearing Research. 1998;41:861–873. doi: 10.1044/jslhr.4104.861. [DOI] [PubMed] [Google Scholar]
- Cianchetti C, Corona S, Foscoliano M, Contu D, Siannio-Fancello G. Modified Wisconsin Card Sorting Test (MCST, MWCST): Normative data in children 4–13 years old, according to classical and new types of scoring. Clinical Neuropsychologist. 2007;21:456–478. doi: 10.1080/13854040600629766. [DOI] [PubMed] [Google Scholar]
- Conners FA, Moore MS, Loveall SJ, Merrill EJ. Memory profiles of Down, Williams, and fragile X syndromes: Implications for reading development. Journal of Developmental and Behavioral Pediatrics. 2011;32:405–417. doi: 10.1097/DBP.0b013e3182168f95. [DOI] [PubMed] [Google Scholar]
- Cornish K, Scerif G, Karmiloff-Smith A. Tracing syndrome-specific trajectories of attention across the lifespan. Cortex. 2007;43:672–685. doi: 10.1016/s0010-9452(08)70497-0. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Turk J, Wilding J, Sudhalter V, Munir F, Kooy F, Hagerman R. Deconstructing the attention deficit in fragile X syndrome: A developmental neuropsychological approach. Journal of Child Psychology and Psychiatry. 2004;45:1042–1053. doi: 10.1111/j.1469-7610.2004.t01-1-00297.x. [DOI] [PubMed] [Google Scholar]
- Davis AS. Children with Down syndrome: Implications for assessment and intervention in the school. School Psychology Quarterly. 2008;23:271–281. [Google Scholar]
- Dykens EM, Hodapp RM, Evans EW. Profiles and development of adaptive behavior in children with Down syndrome. American Journal of Mental Retardation. 1994;98:580–587. [PubMed] [Google Scholar]
- Edgin JO, Pennington BF, Mervis CB. Neuropsychological components of intellectual disability: The contributions of immediate, working, and associative memory. Journal of Intellectual Disability Research. 2010;54:406–417. doi: 10.1111/j.1365-2788.2010.01278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ell SW, Marchant NL, Ivry RB. Focal putamen lesions impair learning in rule-based, but not information-integration categorization tasks. Neuropsychologia. 2006;44(10):1737–1751. doi: 10.1016/j.neuropsychologia.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Finestack LH, Abbeduto L. Expressive language profiles of verbally expressive adolescents and young adults with Down syndrome or fragile X syndrome. Journal of Speech, Language, and Hearing Research. 2010;53:1334–1348. doi: 10.1044/1092-4388(2010/09-0125). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel S, Bourdin B. Verbal, visual, and spatio-sequential short-term memory: Assessment of the storage capacities of children and teenagers with Down’s syndrome. Journal of Intellectual Disability Research. 2009;53:152–160. doi: 10.1111/j.1365-2788.2008.01139.x. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtis G. Wisconsin Card Sorting Test manual: Revised and expanded. Odessa, FL: Psychological Assessment Resources; 1993. [Google Scholar]
- Huang-Pollock CL, Maddox WT, Karalunas SL. Development of implicit and explicit category learning. Journal of Experimental Child Psychology. 2011;109:321–335. doi: 10.1016/j.jecp.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme C, Mackenzie S. Working memory and severe learning difficulties. Hove, England: Erlbaum; 1992. [Google Scholar]
- Jarrold C, Brock J. To match or not to match? Methodological issues in autism-related research. Journal of Autism and Developmental Disorders. 2004;34:81–86. doi: 10.1023/b:jadd.0000018078.82542.ab. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Bellugi U, Sowell E, Doherty S, Hesselink JR. Cerebral morphologic distinctions between Williams and Down syndromes. Archives of Neurology. 1993;50:186–191. doi: 10.1001/archneur.1993.00540020062019. [DOI] [PubMed] [Google Scholar]
- Klinger LG, Dawson G. Prototype formation in autism. Development and Psychopathology. 2001;13:111, 124. doi: 10.1017/s0954579401001080. [DOI] [PubMed] [Google Scholar]
- Kogan CS, Boutet I, Cornish K, Graham GE, Berry-Kravis E, Drouin A, Milgram NW. A comparative neuropsychological test battery differentiates cognitive signatures of fragile X and Down syndromes. Journal of Intellectual Disability Research. 2002;53:125–142. doi: 10.1111/j.1365-2788.2008.01135.x. [DOI] [PubMed] [Google Scholar]
- Lanfranchi S, Cornoldi C, Vianello R. Verbal and visuospatial working memory deficits in children with Down syndrome. American Journal on Mental Retardation. 2004;109:456–466. doi: 10.1352/0895-8017(2004)109<456:VAVWMD>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Lanfranchi S, Jerman O, Dal Pont E, Alberti A, Vianello R. Executive function in adolescents with Down syndrome. Journal of Intellectual Disability Research. 2010;54:308–319. doi: 10.1111/j.1365-2788.2010.01262.x. [DOI] [PubMed] [Google Scholar]
- Lanfranchi S, Jerman O, Vianello R. Working memory and cognitive skills in individuals with Down syndrome. Child Neuropsychology. 2009;15:397–416. doi: 10.1080/09297040902740652. [DOI] [PubMed] [Google Scholar]
- Lee NR, Fidler DJ, Blakeley-Smith A, Daunhauer L, Robinson C, Hepburn SL. Caregiver report of executive functioning in a population-based sample of young children with Down syndrome. American Journal on Intellectual and Developmental Disabilities. 2011;116:290–304. doi: 10.1352/1944-7558-116.4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie S, Hulme C. Memory span development in Down’s syndrome, severely subnormal and normal subjects. Cognitive Neuropsychology. 1987;4:303–319. [Google Scholar]
- Mattson SN, Riley EP, Sowell ER, Jernigan TL, Sobel DF, Jones KL. A decrease in the size of the basal ganglia in children with fetal alcohol syndrome. Alcoholism: Clinical and Experimental Research. 1996;20:1088–1093. doi: 10.1111/j.1530-0277.1996.tb01951.x. [DOI] [PubMed] [Google Scholar]
- Minda JP, Desroches AS, Church BA. Learning rule-described and non -rule-described categories: A comparison of children and adults. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34:1518–1533. doi: 10.1037/a0013355. [DOI] [PubMed] [Google Scholar]
- Moldavsky M, Lev D, Lerman-Sagie T. Behavioral phenotypes of genetic syndromes: A reference guide for psychiatrists. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:749–761. doi: 10.1097/00004583-200107000-00009. [DOI] [PubMed] [Google Scholar]
- Munir F, Cornish KM, Wilding J. A neuropsychological profile of attention deficits in young males with fragile X syndrome. Neuropsychologia. 2000;38:1261–1270. doi: 10.1016/S0028-3932(00)00036-1. [DOI] [PubMed] [Google Scholar]
- Nelson HE. A modified card sorting test sensitive to frontal lobe defects. Cortex. 1976;12:313–324. doi: 10.1016/s0010-9452(76)80035-4. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Moon J, Edgin J, Stedron J, Nadel L. The neuropsychology of Down syndrome: Evidence for hippocampal dysfunction. Child Development. 2003;74:75–93. doi: 10.1111/1467-8624.00522. [DOI] [PubMed] [Google Scholar]
- Price J, Roberts J, Hennon EA, Berni MC, Anderston KL, Sideris J. Syntactic complexity during conversation of boys with fragile X syndrome and Down syndrome. Journal of Speech, Language, and Hearing Research. 2008;51:3–15. doi: 10.1044/1092-4388(2008/001). [DOI] [PubMed] [Google Scholar]
- Randolf B, Burack JA. Visual filtering and covert orienting in persons with Down syndrome. International Journal of Behavioral Development. 2000;24:167–172. [Google Scholar]
- Raz N, Torres IJ, Briggs SD, Spencer WD, Thornton AE, Loken WJ, … Acker JD. Selective neuroanatomic abnormalities in Down’s syndrome and their cognitive correlates: Evidence from MRI morphometry. Neurology. 1995;45:356–366. doi: 10.1212/wnl.45.2.356. [DOI] [PubMed] [Google Scholar]
- Roid G, Miller L. Leiter International Performance Scale–Revised. Wood Dale, IL: Stoelting; 1997. [Google Scholar]
- Rosin MM, Swift E, Bless D, Kluppel Vetter D. Communication profiles of adolescents with Down syndrome. Journal of Childhood Communication Disorders. 1988;12:49–64. [Google Scholar]
- Roussotte FF, Sulik KK, Mattson SN, Riley EP, Jones KL, Adams CM, … Sowell ER. Regional brain volume reductions relate to facial dysmorphology and neurocognitive function in fetal alcohol spectrum disorders. Human Brain Mapping. 2012;33:920–937. doi: 10.1002/hbm.21260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe J, Lavender A, Turk V. Cognitive executive function in Down’s syndrome. British Journal of Clinical Psychology. 2006;45:5–17. doi: 10.1348/014466505X29594. [DOI] [PubMed] [Google Scholar]
- Schmittman VD, Visser I, Raijmakers MEJ. Neuropsychologia. 2006;44:2079–2091. doi: 10.1016/j.neuropsychologia.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Schnyer DM, Maddox WT, Ell S, Davis S, Pacheco J, Verfaellie M. Prefrontal contributions to rule-based and information-integration category learning. Neuropsychologia. 2009;47:2995–3006. doi: 10.1016/j.neuropsychologia.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman W. Down syndrome: Cognitive phenotype. Mental Retardation and Developmental Disabilities Research Reviews. 2007;13:228–236. doi: 10.1002/mrdd.20156. [DOI] [PubMed] [Google Scholar]
- Thomas MS, Annaz D, Ansari D, Scerif G, Jarrold C, Karmiloff-Smith A. Using developmental trajectories to understand developmental disorders. Journal of Speech, Language, and Hearing Research. 2009;52:336–358. doi: 10.1044/1092-4388(2009/07-0144). [DOI] [PubMed] [Google Scholar]
- Van Allen MI, Fung J, Jurenka SB. Health care concerns and guidelines for adults with Down syndrome. American Journal of Medical Genetics. 1999;89:100–110. [PubMed] [Google Scholar]
- Vicari S. Motor development and neuropsychological patterns in persons with Down syndrome. Behavior Genetics. 2006;36:355–364. doi: 10.1007/s10519-006-9057-8. [DOI] [PubMed] [Google Scholar]
- Vicari S, Carlesimo A, Caltagirone C. Short-term memory in persons with intellectual disabilities and Down’s syndrome. Journal of Intellectual Disability Research. 1995;39:532–537. doi: 10.1111/j.1365-2788.1995.tb00574.x. [DOI] [PubMed] [Google Scholar]
- Wilding J, Cornish K, Munir F. Further delineation of the executive deficit in males with fragile-x syndrome. Neuropsychologia. 2002;40:1343–1349. doi: 10.1016/s0028-3932(01)00212-3. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock Johnson III Tests of Cognitive Ability. Rolling Meadows, IL: Riverside Publishing Company; 2001. [Google Scholar]