Abstract
Background
The Zika virus was first discovered in 1947. It was neglected until a major outbreak occurred on Yap Island, Micronesia, in 2007. Teratogenic effects resulting in microcephaly in newborn infants is the greatest public health threat. In 2016, the Zika virus epidemic was declared as a Public Health Emergency of International Concern (PHEIC). Consequently, mathematical models were constructed to explicitly elucidate related transmission dynamics.
Survey Methodology
In this review article, two steps of journal article searching were performed. First, we attempted to identify mathematical models previously applied to the study of vector-borne diseases using the search terms “dynamics,” “mathematical model,” “modeling,” and “vector-borne” together with the names of vector-borne diseases including chikungunya, dengue, malaria, West Nile, and Zika. Then the identified types of model were further investigated. Second, we narrowed down our survey to focus on only Zika virus research. The terms we searched for were “compartmental,” “spatial,” “metapopulation,” “network,” “individual-based,” “agent-based” AND “Zika.” All relevant studies were included regardless of the year of publication. We have collected research articles that were published before August 2017 based on our search criteria. In this publication survey, we explored the Google Scholar and PubMed databases.
Results
We found five basic model architectures previously applied to vector-borne virus studies, particularly in Zika virus simulations. These include compartmental, spatial, metapopulation, network, and individual-based models. We found that Zika models carried out for early epidemics were mostly fit into compartmental structures and were less complicated compared to the more recent ones. Simple models are still commonly used for the timely assessment of epidemics. Nevertheless, due to the availability of large-scale real-world data and computational power, recently there has been growing interest in more complex modeling frameworks.
Discussion
Mathematical models are employed to explore and predict how an infectious disease spreads in the real world, evaluate the disease importation risk, and assess the effectiveness of intervention strategies. As the trends in modeling of infectious diseases have been shifting towards data-driven approaches, simple and complex models should be exploited differently. Simple models can be produced in a timely fashion to provide an estimation of the possible impacts. In contrast, complex models integrating real-world data require more time to develop but are far more realistic. The preparation of complicated modeling frameworks prior to the outbreaks is recommended, including the case of future Zika epidemic preparation.
Keywords: Deterministic, Intervention, Import risk, Stochastic, Epidemic model, Zika
Introduction
Zika is a single-stranded RNA flavivirus, a member of the Flaviviridae family (Lopes, Miyaji & Infante, 2016). The virus is genetically related to some others responsible for encephalitis in humans, including chikungunya, dengue, Japanese encephalitis, West Nile, and the yellow fever virus (Lucey & Gostin, 2016; Goeijenbier et al., 2016; Vest, 2016). Zika is one of the arboviruses transmitted by Aedes mosquitoes. The main vectors are Aedes aegypti and Aedes albopictus (Al-Qahtani et al., 2016). These mosquitoes are mostly found in tropical and subtropical regions (Petersen et al., 2016).
The Zika virus was first discovered in rhesus monkeys in 1947 while researchers were studying yellow fever in Zika Forest, Uganda, and it was isolated from Aedes africanus mosquitoes the subsequent year (Dick, Kitchen & Haddow, 1952). The first human isolation was recorded in Nigeria six years later (MacNamara, 1954; Petersen et al., 2016). For decades, the viral infection was sporadically reported in Africa and Southeast Asia (Hayes, 2009; Goeijenbier et al., 2016). The first large outbreaks occurred on Yap Island, Federated States of Micronesia, in 2007 (Duffy et al., 2009). In this epidemic, 49 confirmed cases were found together with another 59 probable cases. It was estimated that up to 73% of the Yap Island residents were asymptomatically infected (Duffy et al., 2009; Kindhauser et al., 2016). The episodes of large-scale Zika virus outbreaks happened in 2013, when the virus migrated to French Polynesia, a French territory located in the South Pacific. This outbreak was the largest recorded at the time (Cao-Lormeau et al., 2014; Cao-Lormeau et al., 2016; Musso, 2015). Overall, 19,000 suspected cases were estimated throughout the epidemic’s course (Cao-Lormeau et al., 2014). The first evidence of Guillain-Barré syndrome related to the Zika virus was also seen in this historic outbreak (Cao-Lormeau et al., 2016). Subsequently, the virus from French Polynesia dispersed to many countries in the Pacific Ocean, finally reaching Easter Island, Chile, in 2014 (Tognarelli et al., 2015). The virus seems to have established well on the continent, especially in Latin American countries (Shi et al., 2016). For example, the autochthonous transmission was first confirmed in Brazil in 2015 (Zanluca et al., 2015) and the Brazilian Ministry of Health estimated the number of suspected cases at 440,000 to 1,300,000 that year. The Zika infection was also linked to the unusual rising incidence of microcephaly in newborn infants (Mlakar et al., 2016; De Oliveira & Da Costa Vasconcelos, 2016; Heymann et al., 2016) together with some other neurological disorders including Guillain-Barré syndrome (De Oliveira et al., 2017). On February 1, 2016, the World Health Organization (WHO) Director-General declared Zika virus outbreaks in Latin American countries as a Public Health Emergency of International Concern (PHEIC) (Heymann et al., 2016). As of March 9, 2017, vector-borne Zika virus transmission was found in 84 countries, territories, or subnational areas (WHO, 2017).
In addition to Zika, in the twenty-first century many emerging and reemerging infectious diseases threaten the human race. With rapid globalization, these diseases are often disseminated at unprecedented speed. The epidemics of severe acute respiratory syndrome (SARS) in 2003 and the H1N1 influenza pandemic of 2009 are excellent evidence in the first decade (Mackey & Liang, 2012). More recently, we face new threats almost every year, for example, the Middle East respiratory syndrome coronavirus (MERS-CoV) in Saudi Arabia in 2012 (De Groot et al., 2013), the Ebola virus in the West African region in 2014 (WHO Ebola Response Team, 2014). In such epidemics, the real-time evaluation of the ongoing situation is vitally important. To serve this purpose, mathematical modeling has been exploited to monitor the outbreak progression, predict the trend of disease transmission, and tailor related control strategies (McVernon, McCaw & Mathews, 2007; De Jong & Hagenaars, 2009).
Infectious disease modeling is an interdisciplinary approach. Modelers are obligated to comprehend not only the mathematical frameworks but also the biological knowledge behind the epidemics (Rock et al., 2014). Recently, mathematical modeling has been well established as an epidemiological tool. It has been used to combat many infectious diseases. The very first mathematical model was traced back to the work of Daniel Bernoulli in the eighteenth century. Bernoulli employed a simple model to estimate life expectancy due to variolation practices in smallpox epidemics (Bernoulli, 1766). However, the modern era of infectious disease modeling was actually initiated a century ago with a mosquito-borne model proposed by Sir Ronald Ross. Ross developed a set of mathematical equations to illustrate how malaria parasites were transmitted between mosquitoes and humans (Ross, 1911). The model was later complemented by the work of Macdonald (MacDonald, 1952), and finally became the well-known Ross-Macdonald models. This modeling framework still plays an important role in research on malaria and other mosquito-borne diseases (Smith et al., 2012). Nevertheless, there were also many other scientists working on malaria transmission dynamics. For instance, Kermack and McKendrick incorporated the law of mass action into the Ross model and proposed new and modern compartmental models (Kermack & McKendrick, 1927; Kermack & McKendrick, 1932; Kermack & McKendrick, 1933) that later became the most widely used basic structures in infectious disease modeling.
The present review aims to provide an overview of mathematical modeling methods, particularly those developed for Zika virus transmission. However, it is not possible to cover, in a review, all kinds of mathematical models applied to infectious disease studies. In this review, we describe some common models developed thus far. We explain different approaches ranging from simple compartments to sophisticated models integrating real-word data. The idea is to provide some basic knowledge of mathematical projections before further exploring the models, particularly those developed for Zika virus transmission. We also discuss recent advances and trends of research in the infectious disease modeling.
Survey Methodology
We attempt to cover different types of mathematical models applied to the study of vector-borne disease, particularly the Zika virus. First, we provide basic knowledge on methodological approaches in order to facilitate non-mathematical background readers. We therefore initiated our survey to investigate previously published modeling frameworks. Subsequently, we further explore specifically the use of models in the study of the Zika virus. In our publication survey, we used the Google Scholar (https://scholar.google.com/) and PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) databases to search for the relevant peer-reviewed journal articles. In the first step, we used the search terms “dynamics,” “mathematical model,” “modeling,” and “vector-borne” together with the names of vector-borne diseases including chikungunya, dengue, malaria, West Nile, and Zika. Then, we expanded our search to include the related models identified by the prior screening. The secondary search terminologies included “compartmental,” “spatial,” “metapopulation,” “network,” “individual-based,” and “agent-based.” In the second step, we examined only the models applied to Zika virus simulations. We strictly searched for publications focusing on the applications of mathematical modeling in Zika virus research. The search terms were then designated as the names of the modeling techniques described earlier AND “Zika.” We consistently excluded unrelated studies throughout the review process. For the publications that met our criteria, we intensively reviewed their modeling methods, categorized into the modeling types and compared to other related studies we found. The papers with irrelevant methodology were then removed.
As we tried to capture all available studies, the publication year was unrestricted. However, the mathematical modeling approach in the Zika virus study has recently emerged. Hence, most of the research was recently published. We have collected research articles that were published before August 2017 based on our search criteria.
Results
We found five basic model architectures previously applied to vector-borne research. These include compartmental, spatial, metapopulation, network, and individual-based models. We reviewed these accordingly.
Basic compartmental model
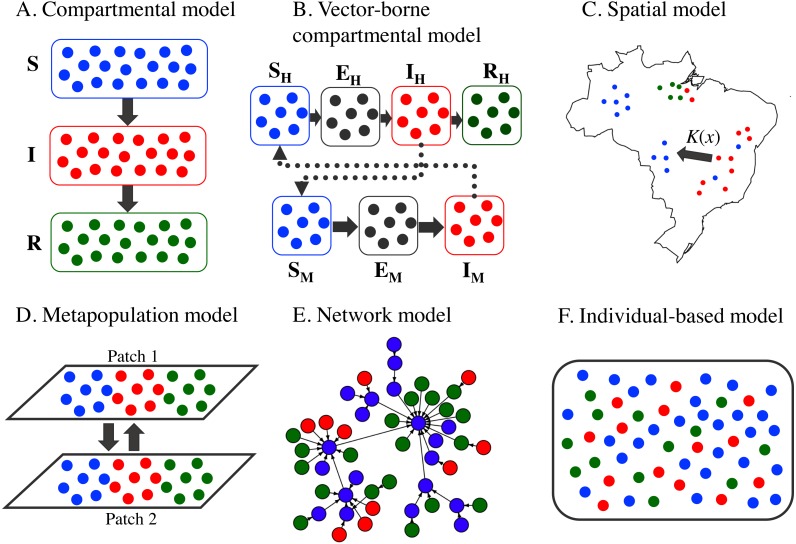
In the classical compartmental model, the whole population is divided into groups according to individual health status (Hethcote, 2000). For example, in the SIR model, the population is split into the compartments of susceptible (S, healthy individuals), infectious (I, diseased and contagious individuals), and recovered (R, immune individuals). During the course of disease transmission, each individual may progress across the compartments, with the rate illustrated by these ordinary differential equations (Fig. 1A):
| (1) |
where β denotes the transmission rate, dictating the speed at which susceptible individuals become infectious, and γ represents the recovery rate, which defines how fast the infectious individuals recovered from the disease. The force of infection in this case is defined as βI∕N, where N is the total population. In this simplest case, it is assumed that the dynamics of disease transmission are much faster than the dynamics of demographic processes, for example, births, deaths, and migration; hence, these demographic dynamics can be ignored. In addition to SIR, other forms of compartmental models exist, for instance, SI, SIS, SIRS, SEIR, SEIRS, MSIR, MSEIR, and MSEIRS, among others. E and M are acronyms for exposed (individuals already exposed to the disease but not yet infectious) and maternal (those with maternal immunity), respectively. The inclusion of different compartments is based on the nature of the diseases (Hethcote, 2000). The models have been applied to many emerging infectious diseases, for example, avian influenza (De Jong & Hagenaars, 2009), Ebola (Browne, Gulbudak & Webb, 2015; Khan et al., 2015; Santermans et al., 2016; Asher, 2017), HIV/AIDS (Akpa & Oyejola, 2010; Luo et al., 2015), and many others.
Figure 1. Conceptual frameworks of different epidemic models.
The colors represent epidemiological status: susceptible (S, blue), exposed (E, gray), infectious (I, red), and recovered (R, green). (A) Basic SIR compartmental model. Individuals are assumed to be well-mixed and are classified only according to their epidemiological status. (B) Vector-borne compartmental model. The subscripts H and M denote human and mosquito, respectively. Both host and vector individuals are assumed to be well-mixed and are classified only according to their epidemiological status. (C) Spatial model. Individuals are located at different locations. The transmission of infection between an infectious individual and a susceptible individual at distance x may occur with probability K(x). (D) Metapopulation model. The entire population is divided into two distinct subpopulations, each with independent disease transmission dynamics, together with interactions between subpopulations. The subpopulation in each patch is mixed homogeneously. (E) Network model. The model is formed by at least two basic components: vertex and edge. Vertices are connected by edges defined by the relationship of interest such as trade or travel. Infectious diseases are modeled to spread via the edges in this model. (F) Individual-based model. In this most complicated model, the stochastic epidemiological dynamics for each individual can be explicitly simulated with a set of characteristics including epidemiological status, spatial location, interaction preference, behavior traits, etc.
One of the most important parameters that is always measured in the compartmental model is the basic reproduction number R0. The R0 is defined as “the average number of secondary cases produced by a single infectious individual in a totally susceptible population in the initial stage of the outbreak” (Hethcote, 2000; Rock et al., 2014). The R0 is regarded as a threshold at which the epidemic is still progressing. The infection may persist and the transmission continues if the R0 is greater than 1, whereas the epidemic is going to cease in the long term when the R0 is otherwise (Hethcote, 2000; Rock et al., 2014; Sidiki & Tchuente, 2014). This parameter is estimated by β∕γ in the SIR framework (Rock et al., 2014). Nonetheless, the R0 varies considerably from disease to disease. For example, the approximate R0 for measles, mumps, and polio is 16, 12, and 5, respectively (Glomski & Ohanian, 2012). Furthermore, the R0 values are also different in the same disease but at a different place and time. For example, during the 2014–2015 Ebola virus outbreaks, the R0 values were 1.71 for Guinea, 1.83 for Liberia, and 2.02 for Sierra Leone (WHO Ebola Response Team, 2014). Therefore, the R0 is not likely referable across spatiotemporal entities.
Vector-borne compartmental model
The models applied for vector-borne diseases are still globally based on the standard compartmental model. Nonetheless, the compartments designed to visualize the dynamics of vector populations are always incorporated. Indeed, the vector-borne model accounts for a multi-species approach involving interspecies disease transmission. Hosts and vectors must be present; otherwise, the pathogen cannot spread. The most notable model may refer to the Ross-Macdonald models (Ross, 1911; MacDonald, 1952) for the transmission of malaria. However, the vector-borne models are often represented in the SEIR and SEI frameworks for human and vector compartments, respectively (Fig. 1B). Here, we demonstrate a model developed to illustrate Zika virus transmission (Funk et al., 2016):
Hosts
| (2) |
Mosquitoes
where the subscripts H and M stand for the host and mosquito, respectively. The parameters λ, δ, ϑ, and μ represent the force of infection, incubation rate, birth rate, and death rate, respectively. The forces of infection for humans and mosquitoes were calculated as:
| (3) |
where a is the mosquito biting rate, pH is the probability that a bite from an infectious mosquito will lead to infection in human, pM is the probability of a mosquito being infected from biting an infectious human, NH is the number of human individuals, and m represents the number of mosquitoes contacting one human.
To get a better understanding of the range of dynamics in these vector-borne diseases, we calculate the number of secondary human cases generated from an average human case, incorporating the cycle of transmission through the vector. To do so, we start with one freshly infected human. From this primary human case, the expected number of infected mosquitoes is the product of the infectious duration in humans, the rate of disease transmission, and the probability that a newly infected mosquito will progress to the infectious stage . Similarly, the expected number of human individuals infected by an infectious mosquito is calculated as . Thus, the R0 is given by the product of these two terms (Rock et al., 2014)
| (4) |
It is noteworthy that this value of R0 that includes a complete cycle of transmission is the square of the value calculated using the next-generation matrix approach (Diekmann, Heesterbeek & Roberts, 2010); however, they agree on the invasion threshold.
Like direct-contact diseases, the R0 for vector-borne epidemics varies across space–time settings. For example, the R0 for the dengue virus in Brazil ranged from 2–103 in different epidemics in the country from 1996–2003 (Tabachnick, 2016). For Zika, the R0 in the outbreaks on Yap Island was estimated between 4.3 and 5.8 in 2007, whereas the value was found at 1.8–2.0 in the French Polynesian epidemics in 2013–2014 (Nishiura et al., 2016a). In the recent Zika virus epidemic in Columbia in 2015–2016, the R0 was approximately 2.2–14.8 (Nishiura et al., 2016b).
Spatial epidemic model
According to the first law of geography proposed by Waldo Tobler, “everything is related to everything else, but near things are more related than distant things” (Tobler, 1970). This idea has become a fundamental concept of spatial studies. Spatial epidemiology is a field concerning the geographical distributions of disease incidences (Lawson, 2013). The most primitive tool is disease mapping. However, spatial modeling is much more advanced. This method incorporates the spatial features of disease occurrences and disease transmission behaviors. In many cases, diseases were observed to spread around the index case. One of the best examples is the airborne virus foot-and-mouth disease (FMD). The FMD virus is capable of transmission by air up to 60 km on land and up to 250 km above water bodies (Lee et al., 2013). In addition, spatial cluster causes closer places to become more vulnerable (Lessler et al., 2016a; Lessler, 2016b). To calculate the spatial probability, the transmission kernel is calculated. The transmission kernel is defined as the probability distribution of distances between the infectious premise and other related places (Lessler et al., 2016a; Lessler, 2016b). The estimation of this parameter can be performed using various forms, for example, exponential (E), Gaussian (G), and fat-tailed (F) methods, which are demonstrated as
| (5) |
where α denotes the kernel parameter (Szmaragd et al., 2009).
The spatial epidemic models have also been applied to vector-borne diseases. For instance, in the studies of dengue (Delmelle et al., 2016; Sardar & Saha, 2017; Vincenti-Gonzalez et al., 2017), West Nile (Crowder et al., 2013; Harrigan et al., 2014; Lin & Zhu, 2017), and Zika (Fitzgibbon, Morgan & Webb, 2017), different modeling approaches were used. In the study of the dengue virus, a power-law form time-dependent transmission kernel (Sardar & Saha, 2017), hot-spot detection and risk factor analysis (Vincenti-Gonzalez et al., 2017), and geographically weighted regression model (Delmelle et al., 2016) were used to illustrate how the virus spreads. In the West Nile virus study, a weighted ensemble model (Harrigan et al., 2014) and a spatially explicit model incorporating land-use and climate variables (Crowder et al., 2013) as well as a reaction–diffusion model using a spatial–temporal risk index (Lin & Zhu, 2017) were constructed to explain the spatial diffusion of the virus under different circumstances. For Zika, spatially dependent differential equations were employed to describe the 2015–2016 Zika outbreak in Rio de Janeiro Municipality in Brazil (Fitzgibbon, Morgan & Webb, 2017). A location-specific projection was also performed to estimate the magnitude of Zika virus infections in childbearing women on the American continent (Perkins et al., 2016).
Metapopulation model
The term “metapopulation” was coined by Richard Levins in 1969 (Levins, 1969) to systematically delineate the dynamics of insect pest population in farms. However, the term and its concepts have been widely expanded to different scientific communities including epidemiology. Metapopulation assumes that the whole population is divided into different discrete spatial subgroups called “patches.” The subpopulation mixes homogeneously whereas the contact between the patches only occurs at some rates (Rock et al., 2014). Consequently, we can fit compartmental models such as SIR and SEIR into each patch to better project how the disease of interest spreads within the subgroups (Rock et al., 2014; Wang & Li, 2014). At this point, the metapopulation becomes the combination of compartmental and spatial epidemic models. This approach allows us to simulate a very large population with a well-defined spatial distribution (Banos et al., 2015).
For a SIR-based metapopulation model, a suitable modified version of the classical SIR approach, Eq. (1), would be
| (6) |
where the subscript i indicates the parameters and variables that are particular to patch i. The force of infection, λi, incorporates transmission from both the infectious individuals within patch i and the infectious individuals from patch j. The exact formula of λi depends on the assumed mechanism of transmission and the strength of the interaction between the patches. In general, the force of infection is expressed as (Rock et al., 2014; Sornbundit, Triampo & Modchang, 2017)
| (7) |
where βij is the transmission rate from the infectious individuals in patch j to the susceptible individuals in patch i, Nj is the total number of individuals in patch j, and n is the number of patches.
In vector-borne disease modeling, the ideas of metapopulation have already been deeply imbedded. The models always involve different subgroups, that is, the hosts and vectors. In many cases, spatial distribution patterns were concurrently considered. As demonstrated in a previous dengue study (Lee & Castillo-Chavez, 2015), a two-patch model was constructed to explore the influence of between-patch human movements on viral transmission dynamics. In the patches, the SEIR and SEI models were architected for human and mosquito populations, respectively. This was done to imitate how diseases spread within subpopulations. Another example is a study on the impact of human movement on the dynamics and persistence of vector-borne diseases at the city scale (Adams & Kapan, 2009). The authors constructed metapopulation models which assume that human population lives in a home patch free of mosquitoes but moves to and fro patches with immobile mosquito subpopulations. Different human movement patterns were represented by different connection patterns between human and mosquito subpopulations. It was found that more variable human movement pattern increases the influence of the large vector population patches in establishing new foci of transmission and enhances pathogen persistence (Adams & Kapan, 2009). In Zika virus research, a metapopulation-typed model was constructed to investigate the effects of sexual transmission and human migration in the spread of the virus (Baca-Carrasco & Velasco-Hernández, 2016). Recently, Zhang et al. expanded the Global Epidemic and Mobility Model (GLEAM) (Balcan et al., 2010), a metapopulation model integrating real-world demographic data and human mobility patterns, to incorporate data on mosquito density and entomological-related parameters. The expanded GLEAM model was employed to analyze the spread of the Zika virus in the Americas. It was estimated that the first introduction of the virus to Brazil may have occurred between August 2013 and April 2014 (Zhang et al., 2017).
Network model
In fact, the interactions between actors in mathematical models are governed by the concept of the contact network. It is assumed in the homogeneous compartmental model that all individuals are linked by a regular random pattern (Bansal, Grenfell & Meyers, 2007). On the other hand, the heterogeneous models, namely spatial and metapopulation, possess different assumptions that take into account the higher realistic contact structures. The idea of a contact network emerged from the mathematical graph theory and was first used in social sciences. Two fundamental components that form a network are called “vertex” and “edge” (Lanzas & Chen, 2015). A vertex is a unit of interest for an individual, a group of people, a village, a city, or even an entire country. An edge is the link between a pair of vertices. The edge represents the bond between vertices, which is important in disease transmission, such as animal movement or human transportation. The interaction is further divided into directed and undirected (Martínez-López, Perez & Sánchez-Vizcaíno, 2009), of which the directed links dictate the incoming and outgoing edges; for example, flight itineraries, whereas the undirected approach does not consider directions, such as co-author networks. In epidemiology, contact network modeling has often been used to investigate disease transmission in both humans (Vazquez-Prokopec et al., 2013; Machens et al., 2013) and animals (Craft, 2015; Rossi et al., 2017). The network structure exploration is helpful for targeting risk actors and tailoring prevention and control strategies.
Determining a “real” network structure requires knowledge of all individuals in a population and all possible relationships among them. In large networks, this is an impractical and time-consuming task. However, several techniques have been exploited to approximate the structure of the network, for example, a radio-based wearable device was used to identify high-resolution close proximity interactions (less than 1.5 m) among 75 individuals dwelling in 5 different households in rural Kenya (Kiti et al., 2016). The study makes it possible to collect a high-resolution human contact data without any direct observations. Similar wireless sensor was also used to explore social contact interactions among students, teachers and staff in an American high school (Salathé et al., 2010). The network structure can also be approximated using movement data, for example, airline route maps (Hufnagel, Brockmann & Geisel, 2004) or livestock movement patterns (Wiratsudakul et al., 2014; Chintrakulchai, Vuttichai & Wiratsudakul, 2017; Khengwa et al., 2017). However, these data sources have the disadvantage that the network generally links sub-populations or groups of hosts rather than being a network between individuals. Alternatively, the spatial contact proximity can be detected from mobile phones (Eagle, Pentland & Lazer, 2009) or other Global Positioning System (GPS) data-loggers (Vazquez-Prokopec et al., 2013). Data retrieved from these devices make the contact network to be more realistic which further improve the accuracy of related epidemic models.
Besides using approximated “real” networks, several forms of computer-generated networks have also been employed in previous studies. Examples of these “idealized” networks include a random network (Erdös & Rényi, 1959; Gilbert, 1959), in which each pair of nodes is connected randomly, and a scale-free network (Barabási & Albert, 1999), where the probability that a node is connected is proportional to its degree. These computer-generated networks are proven to be useful in some aspects of infectious disease transmission (Keeling & Eames, 2005; Pastor-Satorras et al., 2015). A bipartite network, a network whose nodes are divided into two separate groups with a scale-free degree distribution, was also used to simulate vector-borne disease transmission (Bisanzio et al., 2010). The authors found that the spread of disease strongly depends on the degree distribution of the two classes of nodes.
The contact network has also been used to describe disease transmission patterns in mosquito-borne diseases. For instance, a previous study employed a contact-tracing investigation to identify possible contact-site clusters. The authors suggested that house-to-house human movement was likely to indicate how the dengue virus spread spatially (Stoddard et al., 2013). This contact-identification technique is applicable to other mosquito-borne diseases, including Zika (Scatà et al., 2016; Saad-Roy, Van den Driessche & Ma, 2016).
Individual-based model
The individual-based approach, also known as the agent-based model, allows us to mimic the complexity of individual interactions. Each individual can be explicitly simulated with a set of characteristics including spatial location, interaction preference, behavior traits, etc. Moreover, these state variables dictate how individuals interact with each other. However, they can change over time (DeAngelis & Grimm, 2014). Exploitation of the micro-level pattern (the bottom-up method) can prevent the rough estimation that inevitably occurs from the top-down approaches, for example, the compartmental model. The individual-based model is powerful for the integration of different scales and datasets. Therefore, it has been applied to various fields of scientific studies (El-Sayed et al., 2012; Merler et al., 2015; Matheson, Satterthwaite & Highlander, 2017). However, the trade-off between the model complexity and technological requirements must be considered. The realistic models integrating large-scale real-word data apparently demand more sophisticated machines (Lanzas & Chen, 2015). Individual-based models have been extensively applied to diseases that require highly unique individual features such as HIV/AIDS (White et al., 2014), influenza (Eichner et al., 2014), tuberculosis (Graciani Rodrigues, Espíndola & Penna, 2015), and Ebola (Merler et al., 2015). In mosquito-borne diseases, individual-based models were previously used to describe the transmission dynamics of the chikungunya virus (Dommar et al., 2014), the dengue virus (Chao, Longini & Halloran, 2013), malaria (Pizzitutti et al., 2013), and the Zika virus (Matheson, Satterthwaite & Highlander, 2017). It is noteworthy that state-of-the-art structures, including the individual-based, metapopulation, and network models, are not necessarily more realistic than compartmental models. Indeed, the advantage of these modeling structures is that modelers are allowed to fully integrate the models with large-scale real-world data. Consequently, such models are believed to be highly realistic (Lessler et al., 2016a; Lessler, 2016b). A graphical presentation of the basic models described in this review is illustrated in Fig. 1.
Mathematical modeling for Zika virus epidemics
The Zika virus has been circulating among human beings for more than 70 years. However, it has been in the sights of modelers for just a decade following a series of outbreaks on Yap Island. Since then, a number of models have been proposed. This study compared some examples based on model structures and discussed the uses of mathematical models in Zika import risk estimation and intervention planning.
Model architectures
As shown in Table 1, it is noticeable that Zika models carried out for early epidemics were less complicated compared to the more recent ones. To our knowledge, the compartmental approach was a fundamental framework for other sophisticated models that were recently developed. In Zika modeling, all early works were fit into compartmental structures. It was relatively fast and convenient to start with existing knowledge from other related diseases and change the relevant parameters for the Zika virus. However, the compartmental model was still regularly used as a backbone for later models.
Table 1. Examples of mathematical models used in Zika virus studies, 2007–2017.
Note that a model is marked as “compartmental” only when the population is divided into groups according to only their health status.
| Period | Location (Country/ Region/Continent) | Population (Compartments) | Model architecture | References | ||||
|---|---|---|---|---|---|---|---|---|
| Compartmental | Spatial | Metapopulation | Network | Indv.—based | ||||
| 2007–2012 | Micronesia | Human (SEIR) Mosquito (SEI) |
X | Funk et al. (2016) | ||||
| 2007, 2013–2014, 2014 | Micronesia, French Polynesia, New Caledonia | Human (SEIR) Mosquito (SEI) |
X | Champagne et al. (2016) | ||||
| 2013–2014 | French Polynesia | Human (SEIR) Mosquito (SEI) |
X | Kucharski et al. (2016) | ||||
| 2013–2016 | French Polynesia, French West Indies | Human (SIR) | X | Riou, Poletto & Boëlle (2017) | ||||
| 2014–2017 | American continent | Human (SEIR) Mosquito (SEI) |
X | Zhang et al. (2017) | ||||
| 2015 | American continent | Human (SIR) | X | Perkins et al. (2016) | ||||
| 2015–2016 | Brazil | Human (SI) Mosquito (SI) |
X | Fitzgibbon, Morgan & Webb (2017) | ||||
| 2015–2016 | Brazil | Human (ND) | X | Zinszer et al. (2017) | ||||
| 2015–2016 | Brazil, Colombia, El Salvador | Human (SEAIR) Mosquito (SEI) |
X | Gao et al. (2016) | ||||
| 2016 | United States | Human (SEIR) | X | Castro et al. (2017) | ||||
| 2016 | Brazil | Human (SEIR) Mosquito (SEIR) |
X | Matheson, Satterthwaite & Highlander (2017) | ||||
| ND | Brazil | Non-human primates (SIR) Mosquito (SI) |
X | Althouse et al. (2015) | ||||
| ND | Worldwide | Human (ND) Mosquito (ND) |
X | Alaniz, Bacigalupo & Cattan (2017) | ||||
| ND | ND | Human (SIR/SEIR) Mosquito (SI) |
X | X | Baca-Carrasco & Velasco-Hernández (2016) | |||
| ND | ND | Human (SIR) Mosquito (SI) |
X | X | Saad-Roy, Van den Driessche & Ma (2016) | |||
| ND | ND | Human (SIR, SipIR, UAF) | X | Scatà et al. (2016) | ||||
Notes.
- ND
- Not designated
Compartmentally, the crisscross transmission between humans and mosquitoes has been popularly simulated. However, the models specially designed for only one (Monaghan et al., 2016; Riou, Poletto & Boëlle, 2017; Scatà et al., 2016) or even another species (Althouse et al., 2015) were also observed. Focusing on the model architecture, SEIR was usually used for humans whereas SEI was commonly used for mosquitos. In addition, a model focusing only on human compartments was recently proposed (Castro et al., 2017). Nonetheless, other compartmental orientations were occasionally proposed, for example, the susceptible-infectious-recovered (SIR) (Perkins et al., 2016), the susceptible-exposed-asymptomatic-infectious-recovered (SEAIR) (Gao et al., 2016), the susceptible-preventive isolated-infectious-recovered (SipIR), and the unaware-aware-faded (UAF) models (Scatà et al., 2016).
Spatial models were developed to demonstrate how the Zika virus moves across geographical spaces. Frequently, the spatial framework was complementarily driven by other types of models (Zinszer et al., 2017; Fitzgibbon, Morgan & Webb, 2017). The most prominent advantage of the spatial models is their visualizing power. Apparently, the maps generated from spatial modeling were the most comprehensible tools for the general public compared to other model outputs. Hence, their final products, viral distribution maps, were frequently exploited in public communication through various channels such as governmental authorities, mainstream media, and even informal online platforms. The disease maps were widely used to increase public awareness and to design specific prevention and control strategies for Zika (Rodriguez-Morales et al., 2016) and other emerging diseases (Coburn & Blower, 2013; Emmanuel, Isac & Blanchard, 2013; Koch, 2015).
We found that the early metapopulation, network, and individual-based models were mostly structured without geographical or timeframe references (Table 1) (Scatà et al., 2016; Baca-Carrasco & Velasco-Hernández, 2016; Saad-Roy, Van den Driessche & Ma, 2016). It seemed difficult to immediately fit the real-world data into these sophisticated frameworks. However, not long after the Zika epidemic started in Brazil, a data-driven metapopulation model incorporating large-scale real-world data was presented (Zhang et al., 2017). The GLEAM model (Balcan et al., 2010) was expanded to incorporate data on mosquito density and other entomological-related parameters (Zhang et al., 2017). Inclusion of these real-world data into the model is believed to improve the ability of the model to reproduce the observed data and reliably predict future epidemic dynamics.
Import risk model
Disease transmission models are developed to explore how a pathogen spreads in an epidemic zone. However, the disease, especially a virus, may spread across the globe overnight. Therefore, an import risk model is used in this assessment. A particular framework is designed to quantitatively assess the likelihood of viral importation into a certain territory. Such a model was previously built to evaluate the importation risk of different emerging diseases, for instance, the Ebola virus (Chen et al., 2014; Wiratsudakul et al., 2016), MERS-CoV (Nishiura et al., 2015; Nah et al., 2016), and severe acute respiratory syndrome (SARS) (Goubar et al., 2009). For the Zika virus, the imported cases were well documented in many countries on different continents (Pyke et al., 2014; Bachiller-Luque, 2016; Jang et al., 2016; Sokal et al., 2016; Zhong et al., 2016; Hashimoto et al., 2017; Xiang et al., 2017). The import risk models are necessary to foresee the probability of Zika importation into other unaffected countries. As Brazil was recently in the spotlight for Zika epidemics, models focused on the Zika virus escaping the country were increasingly produced. In particular, models considering the risk of mass gatherings for international events such as the Olympic games were recently proposed (Grills et al., 2016; Massad, Coutinho & Wilder-Smith, 2016; Burattini et al., 2016). Herein, we described three basic methods used in import risk estimation, deterministic and stochastic risk estimation and risk estimation by force of infection.
Deterministic risk estimation
This method roughly calculated the probability of Zika virus importation into different countries around the world via commercial air travel. In a previous model, the virus was designated to spread from Brazil (Quam & Wilder-Smith, 2016). The risk was formulated as RI = T × I × P, where the risk (RI) is the product of the number of air passengers (T) who traveled from the Zika epidemic areas, the estimated infectious incidence per individual (I), and the probability of infection in the travel period (P). The results suggested that 584–1,786 Zika cases may have been exported from Brazil during the 2014–2015 epidemics.
Stochastic risk estimation
This process takes into account the stochasticity of travel volumes. The model was previously employed to estimate the risk of Ebola virus importation into the top 20 destination countries of travelers departing from the three Ebola epidemic countries in West Africa (Wiratsudakul et al., 2016). The risk was estimated using the binomial distribution Rn,e,t = Binom(Tn,e,t × In,e,t), where Rn,e,t represents the risk of viral importation into country n from affected country e at time t whereas Tn,e,t and In,e,t denote the corresponding number of flight travelers and outbreak country incidence, respectively. This simulation indicated that the risk of importing the Ebola virus during the peak of the epidemics could have reached 0.73 in Ghana, where the highest number of air passengers were observed.
Risk estimation by force of infection
This method was previously used in the import risk assessment for dengue virus diffusion from Brazil to other countries during the 2016 summer Olympic games (Ximenes et al., 2016). First, the force of infection λ was estimated from a Gaussian function , where C1 determines the highest incidence, C2 is the peak incidence time, and C3 is the period of incidence function. F(t) represents the ad hoc function, which is written in a logistic form as , where C4 and C5 are the rate of incidence acceleration and the initial infection time, respectively. Subsequently, λ was used to calculate the risk of dengue infection during times t1 and t2 as . This model scenario indicated that the number of asymptomatic dengue cases among tourists may have reached 206 during the study period.
Intervention model
Apart from disease dynamic illustration, mathematical models also functioned as a basic framework to assess the effectiveness of different interventional strategies. For example, a simulated outbreak scenario was examined for the performance of control measures against highly pathogenic avian influenza in Ontario, Canada (Lewis et al., 2015). An import risk model was tested for the mitigation capability of pandemic Ebola outbreaks through commercial air travel restrictions (Wiratsudakul et al., 2016). The intervention models were also constructed for Zika. A previous study exploited the prior knowledge of rubella control to construct a Zika virus simulation. Rubella is a classic example of teratogenic agents causing viruses in humans. In addition, the body of knowledge on rubella in terms of virology, epidemiology, and mathematical modeling has been well documented (Metcalf & Barrett, 2016). This is an excellent example of a modeling framework derived from other well-known diseases. An intervention model for a related emerging disease could be well supported in a timely fashion using such solid mathematical environments.
Theoretical network modeling was also used in the strategic planning for Zika virus outbreak alleviation (Scatà et al., 2016). The model selectively removed some specific vertices in the network based on the eigenvector-like centrality and awareness values. Their findings highlighted the importance of heterogeneity and public awareness in the control of infectious diseases under different socioeconomic conditions. Prospectively, the authors planned to include an analogy of HIV epidemics into the sexual transmission of Zika as well as an economic impact evaluation of the disease (Scatà et al., 2016). For the economic aspects, a model addressing the cost-effectiveness of Zika control interventions was proposed (Alfaro-Murillo et al., 2016). The research team created a user-friendly online tool that was flexible enough to include new parameters and provided a real-time analysis. The program facilitated the financial allocation and assessed its effectiveness. Another example is the economic appraisement of a newly established policy. In the United States, blood centers were ordered to test for the Zika virus to prevent transfusion-transmitted infection. An economic model was built to assess the implementation costs and to suggest alternatives to reduce them (Ellingson et al., 2017). The model was essential for predicting the overall investments and selecting the most cost-effective one. In addition to financial management, other supporting facilities should also be considered. A previous study used a modeling approach to assess the requirement of healthcare resources in real-time (Andronico et al., 2017). The authors claimed that their model could provide an accurate prediction.
According to our examples, there are several ways to simulate strategic manipulation using mathematical models. Most modelers designed their models based on existing or newly established policies in order to guide policy makers and precisely meet the needs of societies. However, the field data accuracy and baseline simulations directly affect the prediction power of strategic models. One must seriously consider these factors before translating models into practices.
Perspectives on Zika virus epidemic models
We noticed that the compartmental model is still commonly used for the timely assessment of epidemics. However, much more complex modeling frameworks (metapopulation, network, and individual-based models) have been of increasing interest due to the recent availability of large-scale real-world data and computational power. As we know, the computational capacity of modern computers is presently very high. This allows us to deploy a sophisticated model to observe the changes and predict the trends of disease dynamics in real-time. Moreover, the model will help policy makers choose the most appropriate intervention to fight outbreaks and further assess the corresponding results in a timely manner. In Zika virus modeling, state-of-the-art structures (the metapopulation, network, and individual-based models) have been increasingly developed using advanced computational capacity. In other diseases such as Ebola, the real-word data was placed into a complicated individual-based modeling framework. The study made it possible to produce a more realistic output reflecting the actual outbreak situations (Merler et al., 2015).
“Big data” is an emerging field arising from the extremely large amount of data available together with the advancement in computer infrastructures. In biomedical informatics, large-scale health-related data shared among health professionals are undoubtedly beneficial for the unprecedented development of healthcare services (Bellazzi, 2014). Automated modeling could be enabled with the integration of big data and machine learning (Furqan et al., 2017). The future of infectious disease modeling including vector-borne diseases may alter the classical methods. Multiple modeling outputs may be generated automatically right after raw data are entered into computers. However, there are some challenges, for example, the reproducibility of the results as well as privacy and data reuse issues (Bellazzi, 2014).
In epidemiology, big data are increasingly being used to estimate disease spread and investigate effectiveness of interventions. Recent works in infectious disease dynamics have been characterized by an increasing focus on data-driven approaches. For example, the mobile call data records (CDRs) have been used to explain the dynamics of large-scale Ebola outbreaks in West Africa (Wesolowski et al., 2014). The CDR-based transmission models have also been employed to analyze the spread of rubella disease in Kenya (Wesolowski et al., 2015). The individual-based model that integrates detailed geographical and demographic data, and movements of individuals was used to estimate the transmission of Ebola virus and investigate the effectiveness of interventions in Liberia (Merler et al., 2015). For Zika, the data-driven metapopulation model integrating real-world demographic, human mobility, socioeconomic, temperature, and vector density data has also been used to analyze the spread of the Zika virus in the Americas (Zhang et al., 2017). These emerging data-driven approaches further allow the metapopulation, network, and individual-based models better simulating the real epidemics.
In general, epidemic models can be used either as predictive tools or as a means of understanding fundamental epidemiological processes. However, prediction is perhaps the most obvious use of epidemic models. These allow us to predict the population-level epidemic dynamics from an individual-level knowledge of epidemiological factors, and assess the effectiveness of intervention strategies. As the trends in modeling of infectious diseases have been shifting towards data-driven approaches (Lessler et al., 2016a; Lessler, 2016b), the model complexity itself may hamper the use of models by nonspecialists and public health practitioners. These complex modeling architectures should be translated into a comprehensible environment. The modelers may adopt some strategies taught in classes on translational medicine to evaluate how to turn epidemic models into practices. Alternatively, user-friendly interfaces are helpful for health professionals to include mathematical models in their strategic plans. For example, the GLEAM framework provides a user-friendly and easy-to-use graphical tool for general modelers and public health agencies (http://www.gleamviz.org). This is an excellent initiation of the translation of complex mathematical models into a touchable framework. The results presented by Zhang et al. (2017) were also delivered via user-friendly and easy-to-read graphics on a web application (http://www.zika-model.org/). Therefore, an alliance with computer and graphical scientists is encouraged. Moreover, some educational mobile applications are suggested to acquaint the general public and especially younger generations with epidemic simulations. An excellent example is a simulation game available at the App Store and Google Play called Plague Inc. (Ndemic Creations, Bristol, UK). The game makes mathematical models feel touchable and not too difficult, leading to more familiarity and acceptance.
To fully implement mathematical modeling, one must persuade policy makers to include the methods and try to prove that they are necessary. In this case, the translation of mathematical language into political contexts is crucial. Moreover, simple and complex models should be exploited differently. Simple models can be produced in a timely fashion to provide an estimation of the possible impacts. In contrast, complex models require more time to develop but are far more realistic. The models are much more powerful in terms of predictive capability. The preparation of complicated models before outbreaks is recommended.
Conclusions
Mathematical models can be used either as predictive tools or as a means of understanding fundamental epidemiological processes. This review provides basic knowledge of different mathematical models used in studies of disease dynamics. We demonstrated how the models were applied during the course of Zika virus outbreaks and discussed the uses of mathematical models in Zika import risk estimation and intervention planning. We found that Zika models carried out for early epidemics were less complicated compared to the more recent ones. The compartmental model is still commonly used for the timely assessment of epidemics. However, more complex modeling frameworks including metapopulation, network, and individual-based models have been of increasing interest due to the recent availability of large-scale real-world data and computational power. Inclusion of these real-world data into the model is believed to improve the ability of the model to reproduce the observed data and reliably predict future epidemic dynamics.
Funding Statement
This review study was financially supported by the National Science and Technology Development Agency, Thailand (Project ID P-16-50695) and the Centre of Excellence in Mathematics, the Commission on Higher Education, Thailand. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Anuwat Wiratsudakul and Charin Modchang conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Parinya Suparit analyzed the data, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The research in this article did not generate any data or code (literature review).
References
- Adams & Kapan (2009).Adams B, Kapan DD. Man bites mosquito: understanding the contribution of human movement to vector-borne disease dynamics. PLOS ONE. 2009;4:e6763. doi: 10.1371/journal.pone.0006763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akpa & Oyejola (2010).Akpa OM, Oyejola BA. Modeling the transmission dynamics of HIV/AIDS epidemics: an introduction and a review. The Journal of Infection in Developing Countries. 2010;4:597–608. doi: 10.3855/jidc.542. [DOI] [PubMed] [Google Scholar]
- Al-Qahtani et al. (2016).Al-Qahtani AA, Nazir N, Al-Anazi MR, Rubino S, Al-Ahdal MN. Zika virus: a new pandemic threat. Journal of Infection in Developing Countries. 2016;10:201–207. doi: 10.3855/jidc.8350. [DOI] [PubMed] [Google Scholar]
- Alaniz, Bacigalupo & Cattan (2017).Alaniz AJ, Bacigalupo A, Cattan PE. Spatial quantification of the world population potentially exposed to Zika virus. International Journal of Epidemiology. 2017;46:966–975. doi: 10.1093/ije/dyw366. [DOI] [PubMed] [Google Scholar]
- Alfaro-Murillo et al. (2016).Alfaro-Murillo JA, Parpia AS, Fitzpatrick MC, Tamagnan JA, Medlock J, Ndeffo-Mbah ML, Fish D, Avila-Aguero ML, Marin R, Ko AI, Galvani AP. A cost-effectiveness tool for informing policies on Zika virus control. PLOS Neglected Tropical Diseases. 2016;10:e0004743. doi: 10.1371/journal.pntd.0004743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althouse et al. (2015).Althouse BM, Hanley KA, Diallo M, Sall AA, Ba Y, Faye O, Diallo D, Watts DM, Weaver SC, Cummings DAT. Impact of climate and mosquito vector abundance on sylvatic arbovirus circulation dynamics in Senegal. The American Journal of Tropical Medicine and Hygiene. 2015;92:88–97. doi: 10.4269/ajtmh.13-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andronico et al. (2017).Andronico A, Dorléans F, Fergé J-L, Salje H, Ghawché F, Signate A, Daudens-Vaysse E, Baudouin L, Dub T, Aubry M, Cao-Lormeau V-M, Ledrans M, Noel H, Mallet H-P, Fontanet A, Cabié A, Cauchemez S. Real-time assessment of health-care requirements during the Zika virus epidemic in martinique. American Journal of Epidemiology. 2017;10:1–10. doi: 10.1093/aje/kwx008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher (2017).Asher J. Forecasting Ebola with a regression transmission model. Epidemics. 2017 doi: 10.1016/j.epidem.2017.02.009. Epub ahead of print Feb 27 2017. [DOI] [PubMed] [Google Scholar]
- Baca-Carrasco & Velasco-Hernández (2016).Baca-Carrasco D, Velasco-Hernández JX. Sex, mosquitoes and epidemics: an evaluation of Zika disease dynamics. Bulletin of Mathematical Biology. 2016;78:2228–2242. doi: 10.1007/s11538-016-0219-4. [DOI] [PubMed] [Google Scholar]
- Bachiller-Luque (2016).Bachiller-Luque P, Domínguez-Gil González M, Álvarez-Manzanares J, Vázquez A, De Ory F, Sánchez-Seco Farin¯as MP. First case of imported Zika virus infection in Spain. Enfermedades Infecciosas Y Microbiologia Clinica. 2016;34:243–246. doi: 10.1016/j.eimc.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Balcan et al. (2010).Balcan D, Gonçalves B, Hu H, Ramasco JJ, Colizza V, Vespignani A. Modeling the spatial spread of infectious diseases: the global epidemic and mobility computational model. Journal of Computational Science. 2010;1:132–145. doi: 10.1016/j.jocs.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banos et al. (2015).Banos A, Corson N, Gaudou B, Laperrière V, Coyrehourcq S. The importance of being hybrid for spatial epidemic models:a multi-scale approach. Systems. 2015;3:309–329. doi: 10.3390/systems3040309. [DOI] [Google Scholar]
- Bansal, Grenfell & Meyers (2007).Bansal S, Grenfell BT, Meyers LA. When individual behaviour matters: homogeneous and network models in epidemiology. Journal of the Royal Society Interface. 2007;4:879–891. doi: 10.1098/rsif.2007.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabási & Albert (1999).Barabási A-L, Albert R. Emergence of scaling in random networks. Science. 1999;286:509–512. doi: 10.1126/science.286.5439.509. [DOI] [PubMed] [Google Scholar]
- Bellazzi (2014).Bellazzi R. Big data and biomedical informatics: a challenging opportunity. IMIA Yearbook. 2014;9:8–13. doi: 10.15265/IY-2014-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernoulli (1766).Bernoulli D. Essai d’une nouvelle analyse de la mortalité causée par la petite vérole. Mémoires de Mathématiques et de Physique, Académie Royale des Sciences. 1766;1:1–45. [Google Scholar]
- Bisanzio et al. (2010).Bisanzio D, Bertolotti L, Tomassone L, Amore G, Ragagli C, Mannelli A, Giacobini M, Provero P. Modeling the spread of vector-borne diseases on bipartite networks. PLOS ONE. 2010;5:e13796. doi: 10.1371/journal.pone.0013796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne, Gulbudak & Webb (2015).Browne C, Gulbudak H, Webb G. Modeling contact tracing in outbreaks with application to Ebola. Journal of Theoretical Biology. 2015;384:33–49. doi: 10.1016/j.jtbi.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Burattini et al. (2016).Burattini MN, Coutinho FAB, Lopez LF, Ximenes R, Quam M, Wilder-Smith A, Massad E. Potential exposure to Zika virus for foreign tourists during the 2016 Carnival and Olympic Games in Rio de Janeiro, Brazil. Epidemiology and Infection. 2016;144:1904–1906. doi: 10.1017/S0950268816000649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao-Lormeau et al. (2016).Cao-Lormeau V-M, Blake A, Mons S, Lastère S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P, Vial A-L, Decam C, Choumet V, Halstead SK, Willison HJ, Musset L, Manuguerra J-C, Despres P, Fournier E, Mallet H-P, Musso D, Fontanet A, Neil J, Ghawché F. Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. The Lancet. 2016;387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao-Lormeau et al. (2014).Cao-Lormeau V-M, Roche C, Teissier A, Robin E, Berry A-L, Mallet H-P, Sall AA, Musso D. Zika virus, French polynesia, South pacific, 2013. Emerging Infectious Diseases. 2014;20:1085–1086. doi: 10.3201/eid2006.140138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro et al. (2017).Castro LA, Fox SJ, Chen X, Liu K, Bellan SE, Dimitrov NB, Galvani AP, Meyers LA. Assessing real-time Zika risk in the United States. BMC Infectious Diseases. 2017;17:284. doi: 10.1186/s12879-017-2394-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne et al. (2016).Champagne C, Salthouse DG, Paul R, Cao-Lormeau VM, Roche B, Cazelles B. Structure in the variability of the basic reproductive number (R0) for Zika epidemics in the Pacific islands. eLife. 2016;5:e19874. doi: 10.7554/eLife.19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao, Longini & Halloran (2013).Chao DL, Longini IM, Halloran ME. The effects of vector movement and distribution in a mathematical model of dengue transmission. PLOS ONE. 2013;8:e76044. doi: 10.1371/journal.pone.0076044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2014).Chen T, Ka-Kit Leung R, Liu R, Chen F, Zhang X, Zhao J, Chen S. Risk of imported Ebola virus disease in China. Travel Medicine and Infectious Disease. 2014;12:650–658. doi: 10.1016/j.tmaid.2014.10.015. [DOI] [PubMed] [Google Scholar]
- Chintrakulchai, Vuttichai & Wiratsudakul (2017).Chintrakulchai P, Vuttichai S, Wiratsudakul A. Goat movement network analysis and its implications for caprine brucellosis propagation in Nonthaburi Province, Thailand. Asian Pacific Journal of Tropical Disease. 2017;7:477–481. doi: 10.12980/apjtd.7.2017D7-85. [DOI] [Google Scholar]
- Coburn & Blower (2013).Coburn BJ, Blower S. Mapping HIV epidemics in sub-Saharan Africa with use of GPS data. The Lancet Global Health. 2013;1:e251–e253. doi: 10.1016/S2214-109X(13)70084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft (2015).Craft ME. Infectious disease transmission and contact networks in wildlife and livestock. Philosophical Transactions of the Royal Society B: Biological Sciences. 2015;370 doi: 10.1098/rstb.2014.0107. Article 20140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder et al. (2013).Crowder DW, Dykstra EA, Brauner JM, Duffy A, Reed C, Martin E, Peterson W, Carrière Y, Dutilleul P, Owen JP. West nile virus prevalence across landscapes is mediated by local effects of agriculture on vector and host communities. PLOS ONE. 2013;8:e55006. doi: 10.1371/journal.pone.0055006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeAngelis & Grimm (2014).DeAngelis DL, Grimm V. Individual-based models in ecology after four decades. F1000Prime Report. 2014;6 doi: 10.12703/P6-39. Article 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot et al. (2013).De Groot RJ, Baker SC, Baric RS, Brown CS, Drosten C, Enjuanes L, Fouchier RAM, Galiano M, Gorbalenya AE, Memish ZA, Perlman S, Poon LLM, Snijder EJ, Stephens GM, Woo PCY, Zaki AM, Zambon M, Ziebuhr J. Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. Journal of Virology. 2013;87:7790–7792. doi: 10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong & Hagenaars (2009).De Jong MCM, Hagenaars TJ. Modelling control of avian influenza in poultry: the link with data. Revue scientifique et technique (International Office of Epizootics) 2009;28:371–377. doi: 10.20506/rst.28.1.1858. [DOI] [PubMed] [Google Scholar]
- Delmelle et al. (2016).Delmelle E, Hagenlocher M, Kienberger S, Casas I. A spatial model of socioeconomic and environmental determinants of dengue fever in Cali, Colombia. Acta Tropica. 2016;164:169–176. doi: 10.1016/j.actatropica.2016.08.028. [DOI] [PubMed] [Google Scholar]
- De Oliveira et al. (2017).De Oliveira WK, Carmo EH, Henriques CM, Coelho G, Vazquez E, Cortez-Escalante J, Molina J, Aldighieri S, Espinal MA, Dye C. Zika virus infection and associated neurologic disorders in Brazil. New England Journal of Medicine. 2017;376:1591–1593. doi: 10.1056/NEJMc1608612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Oliveira & Da Costa Vasconcelos (2016).De Oliveira CS, Da Costa Vasconcelos PF. Microcephaly and Zika virus. Jornal de Pediatria. 2016;92:103–105. doi: 10.1016/j.jped.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Dick, Kitchen & Haddow (1952).Dick GW, Kitchen S, Haddow A. Zika virus (I). Isolations and serological specificity. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1952;46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- Diekmann, Heesterbeek & Roberts (2010).Diekmann O, Heesterbeek J, Roberts M. The construction of next-generation matrices for compartmental epidemic models. Journal of the Royal Society Interface. 2010;7:873–885. doi: 10.1098/rsif.2009.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dommar et al. (2014).Dommar CJ, Lowe R, Robinson M, Rodó X. An agent-based model driven by tropical rainfall to understand the spatio-temporal heterogeneity of a chikungunya outbreak. Acta Tropica. 2014;129:61–73. doi: 10.1016/j.actatropica.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy et al. (2009).Duffy MR, Chen T-H, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C, Guillaumot L, Griggs A, Bel M, Lambert AJ, Laven J, Kosoy O, Panella A, Biggerstaff BJ, Fischer M, Hayes EB. Zika virus outbreak on Yap Island, Federated States of Micronesia. The New England Journal of Medicine. 2009;360:2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- Eagle, Pentland & Lazer (2009).Eagle N, Pentland AS, Lazer D. Inferring friendship network structure by using mobile phone data. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15274–15278. doi: 10.1073/pnas.0900282106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner et al. (2014).Eichner M, Schwehm M, Hain J, Uphoff H, Salzberger B, Knuf M, Schmidt-Ott R. 4Flu—an individual based simulation tool to study the effects of quadrivalent vaccination on seasonal influenza in Germany. BMC Infectious Diseases. 2014;14:365. doi: 10.1186/1471-2334-14-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed et al. (2012).El-Sayed AM, Scarborough P, Seemann L, Galea S. Social network analysis and agent-based modeling in social epidemiology. Epidemiologic Perspectives & Innovations. 2012;9 doi: 10.1186/1742-5573-9-1. Article 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingson et al. (2017).Ellingson KD, Sapiano MRP, Haass KA, Savinkina AA, Baker ML, Henry RA, Berger JJ, Kuehnert MJ, Basavaraju SV. Cost projections for implementation of safety interventions to prevent transfusion-transmitted Zika virus infection in the United States. Transfusion. 2017;57:1625–1633. doi: 10.1111/trf.14164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanuel, Isac & Blanchard (2013).Emmanuel F, Isac S, Blanchard JF. Using geographical mapping of key vulnerable populations to control the spread of HIV epidemics. Expert Review of Anti-Infective Therapy. 2013;11:451–453. doi: 10.1586/eri.13.33. [DOI] [PubMed] [Google Scholar]
- Erdös & Rényi (1959).Erdös P, Rényi A. On random graphs, I. Publicationes Mathematicae (Debrecen) 1959;6:290–297. [Google Scholar]
- Fitzgibbon, Morgan & Webb (2017).Fitzgibbon WE, Morgan JJ, Webb GF. An outbreak vector-host epidemic model with spatial structure: the 2015–2016 Zika outbreak in Rio De Janeiro. Theoretical Biology and Medical Modelling. 2017;14 doi: 10.1186/s12976-017-0051-z. Article 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk et al. (2016).Funk S, Kucharski AJ, Camacho A, Eggo RM, Yakob L, Murray LM, Edmunds WJ. Comparative analysis of dengue and Zika outbreaks reveals differences by setting and virus. PLOS Neglected Tropical Diseases. 2016;10:e0005173. doi: 10.1371/journal.pntd.0005173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furqan et al. (2017).Furqan HT, Wahab AA, Sadiq S, Ali Shah PA. Ready mealy, moore & markov mathematical modeling machines for big data. In: Ferreira J, Alam M, editors. Future intelligent vehicular technologies. Future 5V 2016. vol. 185. Springer; Cham: 2017. (Lecture notes of the Institute for Computer Sciences, Social Informatics and Telecommunications Engineering). [DOI] [Google Scholar]
- Gao et al. (2016).Gao D, Lou Y, He D, Porco TC, Kuang Y, Chowell G, Ruan S. Prevention and control of Zika fever as a mosquito-borne and sexually transmitted disease. Nature Publishing Group. 2016;6:1–31. doi: 10.1038/srep28070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert (1959).Gilbert EN. Random graphs. The Annals of Mathematical Statistics. 1959;30:1141–1144. doi: 10.1214/aoms/1177706098. [DOI] [Google Scholar]
- Glomski & Ohanian (2012).Glomski M, Ohanian E. Eradicating a disease: lessons from mathematical epidemiology. The College Mathematics Journal. 2012;43:123–132. doi: 10.4169/college.math.j.43.2.123. [DOI] [Google Scholar]
- Goeijenbier et al. (2016).Goeijenbier M, Slobbe L, Van der Eijk A, De Mendonça Melo M, Koopmans MPG, Reusken CBEM. Zika virus and the current outbreak: an overview. The Netherlands Journal of Medicine. 2016;74:104–109. [PubMed] [Google Scholar]
- Goubar et al. (2009).Goubar A, Bitar D, Cao WC, Feng D, Fang LQ, Desenclos JC. An approach to estimate the number of SARS cases imported by international air travel. Epidemiology and Infection. 2009;137:1019–1031. doi: 10.1017/S0950268808001635. [DOI] [PubMed] [Google Scholar]
- Graciani Rodrigues, Espíndola & Penna (2015).Graciani Rodrigues CC, Espíndola AL, Penna TJP. An agent-based computational model for tuberculosis spreading on age-structured populations. Physica A: Statistical Mechanics and its Applications. 2015;428:52–59. doi: 10.1016/j.physa.2015.02.027. [DOI] [Google Scholar]
- Grills et al. (2016).Grills A, Morrison S, Nelson B, Miniota J, Watts A, Cetron MS. Projected Zika virus importation and subsequent ongoing transmission after travel to the 2016 olympic and paralympic games–country-specific assessment, July 2016. Morbidity and Mortality Weekly Report. 2016;65:711–715. doi: 10.15585/mmwr.mm6528e1. [DOI] [PubMed] [Google Scholar]
- Harrigan et al. (2014).Harrigan RJ, Thomassen HA, Buermann W, Smith TB. A continental risk assessment of West Nile virus under climate change. Global Change Biology. 2014;20:2417–2425. doi: 10.1111/gcb.12534. [DOI] [PubMed] [Google Scholar]
- Hashimoto et al. (2017).Hashimoto T, Kutsuna S, Tajima S, Nakayama E, Maeki T, Taniguchi S, Lim CK, Katanami Y, Takeshita N, Hayakawa K, Kato Y, Ohmagari N. Importation of Zika virus from Vietnam to Japan, November 2016. Emerging Infectious Diseases. 2017;23:1222–1225. doi: 10.3201/eid2307.170519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes (2009).Hayes EB. Zika virus outside Africa. Emerging Infectious Diseases. 2009;15:1347–1350. doi: 10.3201/eid1509.090442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hethcote (2000).Hethcote HW. The mathematics of infectious diseases. SIAM Review. 2000;42:599–653. doi: 10.1137/S0036144500371907. [DOI] [Google Scholar]
- Heymann et al. (2016).Heymann DL, Hodgson A, Sall AA, Freedman DO, Staples JE, Althabe F, Baruah K, Mahmud G, Kandun N, Vasconcelos PFC, Bino S, Menon KU. Zika virus and microcephaly: why is this situation a PHEIC? The Lancet. 2016;387:719–721. doi: 10.1016/S0140-6736(16)00320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufnagel, Brockmann & Geisel (2004).Hufnagel L, Brockmann D, Geisel T. Forecast and control of epidemics in a globalized world. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:15124–15129. doi: 10.1073/pnas.0308344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang et al. (2016).Jang HC, Park WB, Kim UJ, Chun JY, Choi SJ, Choe PG, Jung SI, Jee Y, Kim NJ, Choi EH, Oh MD. First imported case of Zika virus infection into Korea. Journal of Korean Medical Science. 2016;31:1173–1177. doi: 10.3346/jkms.2016.31.7.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling & Eames (2005).Keeling MJ, Eames KTD. Networks and epidemic models. Journal of the Royal Society, Interface/the Royal Society. 2005;2:295–307. doi: 10.1098/rsif.2005.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermack & McKendrick (1927).Kermack WO, McKendrick AG. A contribution to the mathematical theory of epidemics. Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences. 1927;115:700–721. doi: 10.1098/rspa.1927.0118. [DOI] [Google Scholar]
- Kermack & McKendrick (1932).Kermack WO, McKendrick AG. Contributions to the mathematical theory of epidemics. II. The problem of endemicity. Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences. 1932;138:55–83. doi: 10.1098/rspa.1932.0171. [DOI] [Google Scholar]
- Kermack & McKendrick (1933).Kermack WO, McKendrick AG. Contributions to the mathematical theory of epidemics. III. Further studies of the problem of endemicity. Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences. 1933;141:94–122. doi: 10.1098/rspa.1933.0106. [DOI] [Google Scholar]
- Khan et al. (2015).Khan A, Naveed M, Dur-e-Ahmad M, Imran M. Estimating the basic reproductive ratio for the Ebola outbreak in Liberia and Sierra Leone. Infectious Diseases of Poverty. 2015;4 doi: 10.1186/s40249-015-0043-3. Article 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khengwa et al. (2017).Khengwa C, Jongchansittoe P, Sedwisai P, Wiratsudakul A. A traditional cattle trade network in Tak province, Thailand and its potential in the spread of infectious diseases. Animal Production Science. 2017;57:152–160. doi: 10.1071/AN15043. [DOI] [Google Scholar]
- Kindhauser et al. (2016).Kindhauser MK, Allen T, Frank V, Santhana RS, Dye C. Zika: the origin and spread of a mosquito-borne virus. Bulletin of the World Health Organization. 2016;94:675–686C. doi: 10.2471/BLT.16.171082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiti et al. (2016).Kiti MC, Tizzoni M, Kinyanjui TM, Koech DC, Munywoki PK, Meriac M, Cappa L, Panisson A, Barrat A, Cattuto C, Nokes DJ. Quantifying social contacts in a household setting of rural Kenya using wearable proximity sensors. EPJ Data Science. 2016;5 doi: 10.1140/epjds/s13688-016-0084-2. Article 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch (2015).Koch T. Mapping medical disasters: ebola makes old lessons, new. Disaster Medicine and Public Health Preparedness. 2015;9:66–73. doi: 10.1017/dmp.2015.14. [DOI] [PubMed] [Google Scholar]
- Kucharski et al. (2016).Kucharski AJ, Funk S, Eggo RM, Mallet HP, Edmunds WJ, Nilles EJ. Transmission dynamics of Zika virus in island populations: a modelling analysis of the 2013–2014 French polynesia outbreak. PLOS Neglected Tropical Diseases. 2016;10:e0004726. doi: 10.1371/journal.pntd.0004726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzas & Chen (2015).Lanzas C, Chen S. Complex system modelling for veterinary epidemiology. Preventive Veterinary Medicine. 2015;118:207–214. doi: 10.1016/j.prevetmed.2014.09.012. [DOI] [PubMed] [Google Scholar]
- Lawson (2013).Lawson AB. Statistical methods in spatial epidemiology. Wiley; Hoboken: 2013. [Google Scholar]
- Lee et al. (2013).Lee SY, Baek GY, Choi EG, Kim CH, Kim TH, Son WG, Kim KY, Kim HT. Analysis of FMD(Foot and Mouth Disease) diffusion route by using GIS in gyeongbuk area. IFAC Proceedings Volumes. 2013;46:176–180. doi: 10.3182/20130327-3-JP-3017.00040. [DOI] [Google Scholar]
- Lee & Castillo-Chavez (2015).Lee S, Castillo-Chavez C. The role of residence times in two-patch dengue transmission dynamics and optimal strategies. Journal of Theoretical Biology. 2015;374:152–164. doi: 10.1016/j.jtbi.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Lessler et al. (2016a).Lessler J, Azman AS, Grabowski MK, Salje H, Rodriguez-Barraquer I. Trends in the mechanistic and dynamic modeling of infectious diseases. Current Epidemiology Reports. 2016a;3:212–222. doi: 10.1007/s40471-016-0078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessler (2016b).Lessler J, Salje H, Grabowski MK, Cummings DAT, Buddhari D, Nisalak A, Silva A de. Measuring spatial dependence for infectious disease epidemiology. PLOS ONE. 2016b;11:e0155249. doi: 10.1371/journal.pone.0155249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levins (1969).Levins R. Some demographic and genetic consequences of environmental heterogeneity for biological control. Bulletin of the Entomological Society of America. 1969;15:237–240. doi: 10.1093/besa/15.3.237. [DOI] [Google Scholar]
- Lewis et al. (2015).Lewis N, Dorjee S, Dubé C, Vanleeuwen J, Sanchez J. Assessment of effectiveness of control strategies against simulated outbreaks of highly pathogenic avian influenza in Ontario, Canada. Transboundary and Emerging Diseases. 2015;64:938–950. doi: 10.1111/tbed.12461. [DOI] [PubMed] [Google Scholar]
- Lin & Zhu (2017).Lin Z, Zhu H. Spatial spreading model and dynamics of West Nile virus in birds and mosquitoes with free boundary. Journal of Mathematical Biology. 2017;75:1381–1409. doi: 10.1007/s00285-017-1124-7. [DOI] [PubMed] [Google Scholar]
- Lopes, Miyaji & Infante (2016).Lopes MH, Miyaji KT, Infante V. Zika virus. Revista da Associação Médica Brasileira. 2016;62:4–9. doi: 10.1590/1806-9282.62.01.4. [DOI] [PubMed] [Google Scholar]
- Lucey & Gostin (2016).Lucey DR, Gostin LO. The emerging Zika pandemic: enhancing preparedness. Journal of the American Medical Association. 2016;315:865–866. doi: 10.1001/jama.2016.0904. [DOI] [PubMed] [Google Scholar]
- Luo et al. (2015).Luo S, Han L, Lu H, Dou Z, Tao Q, Khoshnood K, Wu Z, Xu J. Evaluating the impact of test-and-treat on the HIV epidemic among MSM in China using a mathematical moodel. PLOS ONE. 2015;10:e0126893. doi: 10.1371/journal.pone.0126893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald (1952).MacDonald G. The analysis of equilibrium in malaria. Tropical Diseases Bulletin. 1952;49:813–829. [PubMed] [Google Scholar]
- Machens et al. (2013).Machens A, Gesualdo F, Rizzo C, Tozzi AE, Barrat A, Cattuto C. An infectious disease model on empirical networks of human contact: bridging the gap between dynamic network data and contact matrices. BMC Infectious Diseases. 2013;13:185. doi: 10.1186/1471-2334-13-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey & Liang (2012).Mackey TK, Liang BA. Lessons from SARS and H1N1/A: employing a WHO–WTO forum to promote optimal economic-public health pandemic response. Journal of Public Health Policy. 2012;33:119–130. doi: 10.1057/jphp.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara (1954).MacNamara FN. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1954;48:139–145. doi: 10.1016/0035-9203(54)90006-1. [DOI] [PubMed] [Google Scholar]
- Martínez-López, Perez & Sánchez-Vizcaíno (2009).Martínez-López B, Perez AM, Sánchez-Vizcaíno JM. Social network analysis. Review of general concepts and use in preventive veterinary medicine. Transboundary and Emerging Diseases. 2009;56:109–120. doi: 10.1111/j.1865-1682.2009.01073.x. [DOI] [PubMed] [Google Scholar]
- Massad, Coutinho & Wilder-Smith (2016).Massad E, Coutinho FAB, Wilder-Smith A. Is Zika a substantial risk for visitors to the Rio de Janeiro Olympic Games? Lancet. 2016;388:25. doi: 10.1016/S0140-6736(16)30842-X. [DOI] [PubMed] [Google Scholar]
- Matheson, Satterthwaite & Highlander (2017).Matheson T, Satterthwaite B, Highlander HC. Modeling the spread of the Zika virus at the 2016 olympics. Spora: A Journal of Biomathematics. 2017;3:29–44. [Google Scholar]
- McVernon, McCaw & Mathews (2007).McVernon J, McCaw C, Mathews J. Model answers or trivial pursuits? The role of mathematical models in influenza pandemic preparedness planning. Influenza and Other Respiratory Viruses. 2007;1:43–54. doi: 10.1111/j.1750-2659.2007.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merler et al. (2015).Merler S, Ajelli M, Fumanelli L, Gomes MFC, Piontti APY, Rossi L, Chao DL, Longini IM, Halloran ME, Vespignani A. Spatiotemporal spread of the 2014 outbreak of Ebola virus disease in Liberia and the effectiveness of non-pharmaceutical interventions: a computational modelling analysis. The Lancet Infectious Diseases. 2015;15:204–211. doi: 10.1016/S1473-3099(14)71074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf & Barrett (2016).Metcalf CJE, Barrett A. Invasion dynamics of teratogenic infections in light of rubella control: implications for Zika virus. PLOS Currents Outbreaks. 2016 doi: 10.1371/currents.outbreaks.873427b89ab9c75eb90c8ddb8d8c7c90. 2016 Aug 16. Edition 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlakar et al. (2016).Mlakar J, Korva M, Tul N, Popović M, Poljšak-Prijatelj M, Mraz J, Kolenc M, Resman Rus K, Vesnaver Vipotnik T, Fabjan Vodušek V, Vizjak A, Pižem J, Petrovec M, Avšič Županc T. Zika virus associated with microcephaly. New England Journal of Medicine. 2016;374:951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- Monaghan et al. (2016).Monaghan AJ, Morin CW, Steinhoff DF, Wilhelmi O, Hayden M, Quattrochi DA, Reiskind M, Lloyd AL, Smith K, Schmidt CA, Scalf PE, Ernst K. On the seasonal occurrence and abundance of the Zika virus vector mosquito aedes aegypti in the contiguous United States. PLOS Currents. 2016 doi: 10.1371/currents.outbreaks.50dfc7f46798675fc63e7d7da563da76. 2016 Mar 16. Edition 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso (2015).Musso D. Zika virus transmission from french polynesia to Brazil. Emerging Infectious Diseases. 2015;21:1887. doi: 10.3201/eid2110.151125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nah et al. (2016).Nah K, Otsuki S, Chowell G, Nishiura H. Predicting the international spread of Middle East respiratory syndrome (MERS) BMC Infectious Diseases. 2016;16:356. doi: 10.1186/s12879-016-1675-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura et al. (2016a).Nishiura H, Kinoshita R, Mizumoto K, Yasuda Y, Nah K. Transmission potential of Zika virus infection in the South Pacific. International Journal of Infectious Diseases. 2016a;45:95–97. doi: 10.1016/j.ijid.2016.02.017. [DOI] [PubMed] [Google Scholar]
- Nishiura et al. (2015).Nishiura H, Miyamatsu Y, Chowell G, Saitoh M. Assessing the risk of observing multiple generations of middle east respiratory syndrome (MERS) cases given an imported case. Eurosurveillance. 2015;20:6–11. doi: 10.2807/1560-7917.ES2015.20.27.21181. [DOI] [PubMed] [Google Scholar]
- Nishiura et al. (2016b).Nishiura H, Mizumoto K, Villamil-Gómez WE, Rodríguez-Morales AJ. Preliminary estimation of the basic reproduction number of Zika virus infection during Colombia epidemic, 2015–2016. Travel Medicine and Infectious Disease. 2016b;14:274–276. doi: 10.1016/j.tmaid.2016.03.016. [DOI] [PubMed] [Google Scholar]
- Pastor-Satorras et al. (2015).Pastor-Satorras R, Castellano C, Van Mieghem P, Vespignani A. Epidemic processes in complex networks. Reviews of Modern Physics. 2015;87:925–979. doi: 10.1103/RevModPhys.87.925. [DOI] [Google Scholar]
- Perkins et al. (2016).Perkins TA, Siraj AS, Ruktanonchai CW, Kraemer MUG, Tatem AJ. Model-based projections of Zika virus infections in childbearing women in the Americas. Nature Microbiology. 2016;1 doi: 10.1038/nmicrobiol.2016.126. Article 16126. [DOI] [PubMed] [Google Scholar]
- Petersen et al. (2016).Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika Virus. The New England Journal of Medicine. 2016;374:1552–1563. doi: 10.1056/NEJMra1602113. [DOI] [PubMed] [Google Scholar]
- Pizzitutti et al. (2013).Pizzitutti F, Pan W, Barbieri A, Miranda JJ, Feingold B, Guedes GR, Alarcon-Valenzuela J, Mena CF. Malaria Policy Advisory Committee to the WHO: conclusions and recommendations of March 2013 meeting. Malaria Journal. 2013;12 doi: 10.1186/1475-2875-12-213. Article 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke et al. (2014).Pyke AT, Daly MT, Cameron JN, Moore PR, Taylor CT, Hewitson GR, Humphreys JL, Gair R. Imported Zika virus infection from the cook islands into australia, 2014. PLOS Currents. 2014 doi: 10.1371/currents.outbreaks.4635a54dbffba2156fb2fd76dc49f65e. 2014 Jun 2. Edition 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quam & Wilder-Smith (2016).Quam MB, Wilder-Smith A. Estimated global exportations of Zika virus infections via travellers from Brazil from 2014 to 2015. Journal of Travel Medicine. 2016;23 doi: 10.1093/jtm/taw059. Article taw059. [DOI] [PubMed] [Google Scholar]
- Riou, Poletto & Boëlle (2017).Riou J, Poletto C, Boëlle PY. A comparative analysis of Chikungunya and Zika transmission. Epidemics. 2017;19:43–52. doi: 10.1016/j.epidem.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Rock et al. (2014).Rock K, Brand S, Moir J, Keeling MJ. Dynamics of infectious diseases. Reports on Progress in Physics. 2014;77 doi: 10.1088/0034-4885/77/2/026602. Article 26602. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Morales et al. (2016).Rodriguez-Morales AJ, Patiño-Cadavid LJ, Lozada-Riascos CO, Villamil-Gómez WE. Mapping Zika in municipalities of one coastal department of Colombia (Sucre) using geographic information systems during the 2015–2016 outbreak: implications for public health and travel advice. International Journal of Infectious Diseases. 2016;48:70–72. doi: 10.1016/j.ijid.2016.05.012. [DOI] [PubMed] [Google Scholar]
- Ross (1911).Ross R. The prevention of malaria. John Murray; London: 1911. pp. 651–686. [Google Scholar]
- Rossi et al. (2017).Rossi G, Smith RL, Pongolini S, Bolzoni L. Modelling farm-to-farm disease transmission through personnel movements: from visits to contacts, and back. Scientific Reports. 2017;7:2375. doi: 10.1038/s41598-017-02567-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad-Roy, Van den Driessche & Ma (2016).Saad-Roy CM, Van den Driessche P, Ma J. Estimation of Zika virus prevalence by appearance of microcephaly. BMC Infectious Diseases. 2016;16:754. doi: 10.1186/s12879-016-2076-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salathé et al. (2010).Salathé M, Kazandjieva M, Lee JW, Levis P, Feldman MW, Jones JH. A high-resolution human contact network for infectious disease transmission. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:22020–22025. doi: 10.1073/pnas.1009094108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santermans et al. (2016).Santermans E, Robesyn E, Ganyani T, Sudre B, Faes C, Quinten C, Van Bortel W, Haber T, Kovac T, Van Reeth F, Testa M, Hens N, Plachouras D. Spatiotemporal evolution of ebola virus disease at sub-national level during the 2014 West Africa Epidemic: model scrutiny and data meagreness. PLOS ONE. 2016;11:e0147172. doi: 10.1371/journal.pone.0147172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardar & Saha (2017).Sardar T, Saha B. Mathematical analysis of a power-law form time dependent vector-borne disease transmission model. Mathematical Biosciences. 2017;288:109–123. doi: 10.1016/j.mbs.2017.03.004. [DOI] [PubMed] [Google Scholar]
- Scatà et al. (2016).Scatà M, Di Stefano A, Liò P, La Corte A. The impact of heterogeneity and awareness in modeling epidemic spreading on multiplex networks. Scientific Reports. 2016;6:37105. doi: 10.1038/srep37105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi et al. (2016).Shi W, Zhang Z, Ling C, Carr MJ, Tong Y, Gao GF. Increasing genetic diversity of Zika virus in the Latin American outbreak. Emerging Microbes & Infections. 2016;5:e68. doi: 10.1038/emi.2016.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidiki & Tchuente (2014).Sidiki A, Tchuente M. The basic reproduction number for cellular SIR networks. Acta Biotheoretica. 2014;62:417–427. doi: 10.1007/s10441-014-9231-y. [DOI] [PubMed] [Google Scholar]
- Smith et al. (2012).Smith DL, Battle KE, Hay SI, Barker CM, Scott TW, McKenzie FE. Ross, macdonald, and a theory for the dynamics and control of mosquito-transmitted pathogens. PLOS Pathogens. 2012;8:e1002588. doi: 10.1371/journal.ppat.1002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal et al. (2016).Sokal A, D’Ortenzio E, Houhou-Fidouh N, Brichler S, Dorchies J, Cabras O, Leparc-Goffart I, Yazdanpanah Y, Matheron S. Zika virus infection: report of the first imported cases in a Paris travel centre. Journal of Travel Medicine. 2016;24 doi: 10.1093/jtm/taw066. Article taw066. [DOI] [PubMed] [Google Scholar]
- Sornbundit, Triampo & Modchang (2017).Sornbundit K, Triampo W, Modchang C. Mathematical modeling of diphtheria transmission in Thailand. Computers in Biology and Medicine. 2017;87:162–168. doi: 10.1016/j.compbiomed.2017.05.031. [DOI] [PubMed] [Google Scholar]
- Stoddard et al. (2013).Stoddard ST, Forshey BM, Morrison AC, Paz-Soldan VA, Vazquez-Prokopec GM, Astete H, Reiner RC, Vilcarromero S, Elder JP, Halsey ES, Kochel TJ, Kitron U, Scott TW. House-to-house human movement drives dengue virus transmission. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:994–999. doi: 10.1073/pnas.1213349110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmaragd et al. (2009).Szmaragd C, Wilson AJ, Carpenter S, Wood JLN, Mellor PS, Gubbins S. A modeling framework to describe the transmission of bluetongue virus within and between farms in Great Britain. PLOS ONE. 2009;4:e7741. doi: 10.1371/journal.pone.0007741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick (2016).Tabachnick WJ. Ecological effects on arbovirus-mosquito cycles of transmission. Current Opinion in Virology. 2016;21:124–131. doi: 10.1016/j.coviro.2016.09.008. [DOI] [PubMed] [Google Scholar]
- Tobler (1970).Tobler WR. A computer movie simulating urban growth in the detroit region. Economic Geography. 1970;46:234–240. doi: 10.2307/143141. [DOI] [Google Scholar]
- Tognarelli et al. (2015).Tognarelli J, Ulloa S, Villagra E, Lagos J, Aguayo C, Fasce R, Parra B, Mora J, Becerra N, Lagos N, Vera L, Olivares B, Vilches M, Fernández J. A report on the outbreak of Zika virus on Easter Island, South Pacific, 2014. Archives of Virology. 2015;161:665–668. doi: 10.1007/s00705-015-2695-5. [DOI] [PubMed] [Google Scholar]
- Vazquez-Prokopec et al. (2013).Vazquez-Prokopec GM, Bisanzio D, Stoddard ST, Paz-Soldan V, Morrison AC, Elder JP, Ramirez-Paredes J, Halsey ES, Kochel TJ, Scott TW, Kitron U. Using GPS technology to quantify human mobility, dynamic contacts and infectious disease dynamics in a resource-poor urban environment. PLOS ONE. 2013;8:e58802. doi: 10.1371/journal.pone.0058802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vest (2016).Vest KG. Zika virus: a basic overview of an emerging arboviral infection in the Western hemisphere. Disaster Medicine and Public Health Preparedness. 2016;10:707–712. doi: 10.1017/dmp.2016.43. [DOI] [PubMed] [Google Scholar]
- Vincenti-Gonzalez et al. (2017).Vincenti-Gonzalez MF, Grillet M-E, Velasco-Salas ZI, Lizarazo EF, Amarista MA, Sierra GM, Comach G, Tami A. Spatial analysis of dengue seroprevalence and modeling of transmission risk factors in a dengue hyperendemic city of venezuela. PLOS Neglected Tropical Diseases. 2017;11:e0005317. doi: 10.1371/journal.pntd.0005317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang & Li (2014).Wang L, Li X. Spatial epidemiology of networked metapopulation: an overview. Chinese Science Bulletin. 2014;59:3511–3522. doi: 10.1007/s11434-014-0499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesolowski et al. (2014).Wesolowski A, Buckee CO, Bengtsson L, Wetter E, Lu X, Tatem AJ. Commentary: containing the ebola outbreak—the potential and challenge of mobile network data. PLOS Currents Outbreaks. 2014 doi: 10.1371/currents.outbreaks.0177e7fcf52217b8b634376e2f3efc5e. 2014 Sep 29. Edition 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesolowski et al. (2015).Wesolowski A, Metcalf CJE, Eagle N, Kombich J, Grenfell BT, Bjørnstad ON, Lessler J, Tatem AJ, Buckee CO. Quantifying seasonal population fluxes driving rubella transmission dynamics using mobile phone data. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:11114–11119. doi: 10.1073/pnas.1423542112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White et al. (2014).White PJ, Fox J, Weber J, Fidler S, Ward H. How Many HIV infections may be averted by targeting primary infection in men who have sex with men? Quantification of changes in transmission-risk behavior, using an individual-based model. The Journal of Infectious Diseases. 2014;210:S594–S599. doi: 10.1093/infdis/jiu470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Ebola Response Team (2014).WHO Ebola Response Team Ebola virus disease in west africa–the first 9 months of the epidemic and forward projections. New England Journal of Medicine. 2014;371:1481–1495. doi: 10.1056/NEJMoa1411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiratsudakul et al. (2014).Wiratsudakul A, Paul MC, Bicout DJ, Tiensin T, Triampo W, Chalvet-Monfray K. Modeling the dynamics of backyard chicken flows in traditional trade networks in Thailand: implications for surveillance and control of avian influenza. Tropical Animal Health and Production. 2014;46:845–853. doi: 10.1007/s11250-014-0575-8. [DOI] [PubMed] [Google Scholar]
- Wiratsudakul et al. (2016).Wiratsudakul A, Triampo W, Laosiritaworn Y, Modchang C. A one-year effective reproduction number of the 2014–2015 Ebola outbreaks in the widespread West African countries and quantitative evaluation of air travel restriction measure. Travel Medicine and Infectious Disease. 2016;14:481–488. doi: 10.1016/j.tmaid.2016.06.011. [DOI] [PubMed] [Google Scholar]
- WHO (2017).World Health Organization (WHO) http://www.who.int/emergencies/zika-virus/situation-report/10-march-2017/en/ [9 May 2017];Zika situation report. 2017
- Xiang et al. (2017).Xiang B, Gao P, Kang Y, Ren T. Importation of Zika virus in China: a significant risk in southern China. Journal of Infection. 2017;74:328–330. doi: 10.1016/j.jinf.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Ximenes et al. (2016).Ximenes R, Amaku M, Lopez LF, Coutinho FAB, Burattini MN, Greenhalgh D, Wilder-Smith A, Struchiner CJ, Massad E. The risk of dengue for non-immune foreign visitors to the 2016 summer olympic games in Rio de Janeiro, Brazil. BMC Infectious Diseases. 2016;16:186. doi: 10.1186/s12879-016-1517-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanluca et al. (2015).Zanluca C, Melo VCA de, Mosimann ALP, Santos GIV dos, Santos CND dos, Luz K, Zanluca C, Melo VCA de, Mosimann ALP, Santos GIV dos, Santos CND dos, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Memórias do Instituto Oswaldo Cruz. 2015;110:569–572. doi: 10.1590/0074-02760150192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2017).Zhang Q, Sun K, Chinazzi M, Pastore y Piontti A, Dean NE, Rojas DP, Merler S, Mistry D, Poletti P, Rossi L, Bray M, Halloran ME, Longini IM, Vespignani A. Spread of Zika virus in the Americas. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:E4334–E4343. doi: 10.1073/pnas.1620161114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong et al. (2016).Zhong BY, Liu XQ, Deng YC, Xu PH, Zhong GR, Zhang W. First case of laboratory-confirmed Zika virus infection imported into China. Chinese Medical Journal. 2016;129:2013–2014. doi: 10.4103/0366-6999.187864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinszer et al. (2017).Zinszer K, Morrison K, Brownstein JS, Marinho F, Santos AF, Nsoesie EO. Reconstruction of Zika virus introduction in Brazil. Emerging Infectious Diseases. 2017;23:92–94. doi: 10.3201/eid2301.161274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The following information was supplied regarding data availability:
The research in this article did not generate any data or code (literature review).