Abstract
Stem cell therapy is a promising strategy for tissue regeneration. The therapeutic benefits of cell therapy are mediated by both direct and indirect mechanisms. However, the application of stem cell therapy in the clinic is hampered by several limitations. This concise review provides a brief introduction into stem cell therapies for ischemic heart disease. It summarizes cell‐based and cell‐free paradigms, their limitations, and the benefits of using them to target disease. stem cells translational medicine 2018;7:354–359
Keywords: Myocardial injury, Stem cell therapy, Synthetic stem cells, Biomaterials
Significance Statement.
The review provides an introduction to current barriers and limitations in stem cell therapies for treating ischemic heart diseases. These limitations include low retention rate, tumor growth risks, off‐target migration, short‐life in storage and so on. This review then provide potential solutions by summarizing the latest technological developments used to improve cell retention, reduce transplantation risk, and target cells to the injury.
Introduction
Cardiovascular disease is a major cause of morbidity and mortality in the world, as well as its major health care burden 1. In the U.S., cardiovascular disease has a mortality rate of nearly 801,000 people per year, and is listed as the country's leading cause of death. Ischemic heart disease (IHD), including myocardial infarction (MI), is an especially devastating type of cardiovascular disease. Insufficient blood supply to the heart muscle can lead to permanent and progressive damage to the myocardium, which can further develop into heart failure.
Pharmacological treatments, such as angiotensin receptor blockers, aldosterone antagonists, and β‐blockers have improved clinical outcomes for patients with heart failure, but they are not able to reduce the size of established scar tissue on the heart 2, 3, 4. Heart transplantation is usually the last option, but is limited by the availability of donor organs. Regenerative medicine strategies, including stem cell therapies, have gained attention as promising treatment options for IHD.
Stem Cell Therapies in Ischemic Heart Disease
Decades ago, the heart was considered a terminally differentiated organ with limited intrinsic regenerative capacity 5. A paradigm shift emerged when intrinsic cardiac stem cells and cardiomyocyte turnover were reported by various groups worldwide 6. Cardiomyocyte renewal accelerates when injury occurs. Nonetheless, the spontaneous regenerative capacity of mature heart alone is insufficient to compensate for the pathological loss of cardiac myocytes during a big injury such as a MI 5. Multiple types of stem/progenitor‐like cells have been reported to contribute to cardiac repair in IHD. These include noncardiac resident cells such as bone marrow‐derived cells 7, mesenchymal stem cells (MSCs) 8 and cardiac resident cells, which includes c‐Kit+ cardiac progenitor cells (CPCs) 9, 10, Sca‐1+ CPCs 11, 12, side population cells 13, and cardiosphere‐derived cells (CDCs) 14, 15, 16. However, the differentiation of stem cells after transplantation and the paracrine strategies are unlikely to be effective or just show modest efficacy in long‐term, randomized clinical trials, which are in stark contrast to the exciting scientific progress in preclinical models 4, 17, 18, 19. In 2017, Nature Biotechnology published an editorial “A futile cycle in cell therapy” 20. In that paper, the editors expressed a severe concern on the none‐to‐marginal benefits of cardiac cell therapy trials and argued that cardiac cell therapy is “far from getting approval” and “much more preclinical data needs to be performed before any new clinical trials.” With such embarrassing outcomes from clinical trials and concerns from both regulatory and funding agencies, one may wonder: is cardiac cell therapy dead? Or to be more positive, we should ask: what can we do next?
In this review, we will limit our discussion to adult (multipotent) stem cells only as these cells are the majority in current clinical trials 21. We agree that pluripotent stem cell therapy including embryonic stem cells (ES) and induced pluripotent stem cells (iPS) 22, 23, 24 represent the future of regenerative medicine. Nonetheless, the regulatory hurdles for such riskier candidates will likely to be high and the use of such cells in the clinic is still limited.
Mechanisms of Stem Cell‐Mediated Heart Repair
Before we admit the failures and propose a new direction, we should first be looking for the modes of actions (MOAs) that elucidate the mechanisms behind cardiac cell therapy. FDA requires clear MOAs for approving new chemical and small molecule drugs 25. Even for the recently developed biologic drugs such as antibody drugs and CAR‐T therapies, the MOAs are well defined 26. However, this is not the case for cardiac cell therapy or stem cell therapies in general. The mechanisms for stem cell‐mediated heart repair are complicated. The initial thoughts are injected stem cells repair the host tissue by direct tissue replacement (i.e., cardiac stem cell differentiation) 27. However, the limited stem cell engraftment and direct differentiation of transplanted cells into newly born cardiomyocytes and vascular cells, either by transdifferentiation or cell fusion, could not explain the obvious cardiac benefits comprehensively 27, 28, 29. Later on, secretion of soluble factors, exosomes, and non‐coding RNAs, were viewed as the major contributors to the functional benefits of stem cell based therapies 30, 31. These paracrine substances promote cardiac repair by activating endogenous precursors, promoting neovascularization, modulating extracellular matrix, cytoprotection, and inhibiting apoptosis/fibrosis/inflammation 32, 33. Growth factors secreted by adult stem cells (such as CDCs and MSCs) include vascular endothelial growth factor (VEGF), hepatocyte growth factor, stromal‐derived factor‐1 (SDF‐1), and insulin‐like growth factor‐1 (IGF‐1), among many others 34, 35, 36. In particular, IGF‐1 could inhibit apoptosis of cardiomyocytes in addition to recruiting endogenous stem cells and promoting angiogenesis 37. SDF‐1, VEGF, basic fibroblast growth factor, connective tissue growth factory‐β, and angiogenin‐1 can also be secreted by stem cells, which exhibit enhancement to angiogenesis 38, 39, 40, 41. In addition, Xie et al. reported that cell–cell contact was pivotal to the functional benefits of cell therapies 42. These results indicate that on top of soluble factors, cell membranes play an important role in stem cell‐mediated regeneration.
To date, advances in cardiovascular therapies have focused on the heart immediately after injury, while the most urgent target for therapies is advanced cardiomyopathy, as those patients don't have any other options besides heart transplant. Many in the field believe that paracrine effects are not able to treat advanced heart failure. However, given the regulatory hurdles for pluripotent stem cells, adult stem cells still remain the most viable cell therapy products.
Barriers in Stem Cell Therapies for Heart Repair
We name a few concerns that one should be taken into consideration: (a) tumorigenicity; (b) immunogenicity; (c) retention/engraftment; (d) tissue targeting; (e) storage/shipping stability; (f) appropriate (large) animal models.
Tumorigenicity
The risk of tumorigenicity is a salient concern for both pluripotent stem cells (such as ES cells and iPS cells) 43, 44. This is less of a concern when using adult stem cells. There are only a few reports of tumor formation from adult stem cells 43. Nonetheless, as living agents, the risk of tumor formation in injected stem cells should never be neglected.
Immunogenicity
Immunologic intolerance of host is another major point to be considered as this would affect the function of stem cells 43. Autologous products can obviate rejection, but the process to generate autologous cells is expensive and time consuming. In addition, the manufacturing process of stem cells can cause immunological issues, such as fetal bovine serum and sialic acid derivative Neu5G from mouse feeder layers have both been shown to alter the immunogenicity of stem cells 43.
Retention/Engraftment
Stem cell transplantations into the heart are hampered by poor survival and engraftment rate 45, 46, limiting the long‐term efficacy of stem cells in the injured heart. The harsh microenvironment after the ischemia/reperfusion injury is the major barrier for cell survival and engraftment after delivery. What's worse, reperfusion causes secondary injury due to reactive oxygen species and inflammatory cells 47.
Tissue Targeting
One way to delivery cells is to directly inject into the faulty tissue (e.g., intramyocardial injection of stem cells into the infarct border zone of the heart). However, this usually requires open‐chest surgery which is less ideal for patients with mild‐to‐moderate heart diseases. Intravascular routes (such as intravenous or intracoronary injections by catheter) are safer but the challenge then becomes systematically targeting the delivery of cells to the injured heart.
Storage/Shipping Stability
Obviously, there is also the issue of cell liability affected by the freezing/thawing process. As a “living” drug, cells need to be carefully preserved and processed before clinical applications. Off‐the‐shelf availability isn't normally the case.
Available Solutions to Barriers
Solutions to Low Retention/Engraftment
To overcome the low retention and engraftment issue, one straightforward strategy is to apply repeated dosing 48, 49, 50. A single large dose presents a lot of cells at the beginning but soon gets “washed out” with a quick decay. Multiple dosing can create a durable cell persistence and paracrine signal for tissue repair. However, it is noteworthy that repeated dosing is risky for invasive delivery routes such as intramyocardial and intracoronary injections. Systemic delivery such as intravenous injection needs to be proposed. Another strategy to counter rapid washout of injected cells is to encapsulate stem cells in biomaterials. Injectable hydrogels have been used as cell carriers to boost cell retention and attenuate immune reactions 51, 52. Another method to increase cell retention is to deliver therapeutic cells in a cardiac patch sutured or sprayed onto the heart surface 53, 54, 55. The hydrogel and cardiac patch strategies normally require open‐chest surgery, hampering the use of them for mild‐to‐moderate patients with heart failure after MI. Nonetheless, the benefit/risk ratio could be high for patients with advanced heart failure and/or patients who need open‐chest surgery regardless.
Solutions to Injury Targeting
Previously, we reported the application of magnetic targeting to augment cell retention in the heart 56, 57, 58. Stem cells were pre‐labeled with iron particles. During injection, an external magnetic field was placed above the heart to keep the cells in the injected area. One caveat of this strategy is that the fast decay of magnetic field limits the effective distance of this targeting strategy. Also, the placement of a strong magnetic field may represent a threat to the sensitive equipment in the operating room.
As another strategy, studies from our lab and others have also demonstrated that antibodies against cardiac injury biomarkers such as myosin light chain can be used to target stem cells to the injured heart 59, 60. However, a major disadvantage is that such targeting fully relies on that particular biomarker, which is only expressed acutely after the injury. In addition, this strategy requires expensive antibody processing technologies.
In addition, platelet binding molecules or whole platelet membranes can be used to adhere injected stem cells to the injured endothelium 61. Recently, our group has developed a method to employ platelet membranes to guide intravascularly delivered cardiac stem cells to the injured heart 61. These studies shed the light on the development of targeting strategies to direct systemically delivered stem cells to the injured heart.
Solutions to Tumorigenicity/Immunogenicity
As long as live cells are used, the risk of tumorigenicity cannot be completely ignored. Also, immunogenicity is another issue when non‐autologous cells are used. Cell‐free agents have been proposed to replace stem cell therapies for heart repair. Recently, extracellular vesicles, including microvesicles and exosomes, represent the bioactive components (mRNA, miRNAs, proteins) of stem cells, and have been shown to recapitulate the salutary effects of cell therapy on myocardial repair after injury 62. Exosomes are 30–100 nm tiny vesicles secreted by a variety of cell types including adult stem cells 62. The regenerative potential of exosomes treating in heart diseases has been demonstrated by many groups 63, 64, 65, 66, 67. Nevertheless, the extraction of exosomes is still lack standard methods, and only a small number of exosomes can be produced from stem cell‐conditioned media.
New Solutions: Cell Mimicking Microparticles
We recently developed stem cell biomimetic microparticles, namely cell mimicking microparticles (CMMPs), for heart repair 68, 69. It starts with biodegradable and biocompatible polymers such as poly (lactic‐co‐glycolic acid), which has provided a safe and non‐toxic building block for various control‐release systems as a biocompatible and biodegradable polymer 70. Using a double emulsion method 71, the stem cell secretome can be incorporated into the biodegradable polymer to from a drug‐releasing microparticle.
To make the microparticle more biomimetic, we sought to coat the particle with stem cell membranes. It has been well established for the methods of coating polymer nanoparticles with cell membranes derived from red blood cells 72, platelets 73, and cancer cells 74. We coated the microparticles with cardiac stem cell membranes (Fig. 1) to make the final product CMMPs 68. Inheriting the major functional components of stem cells, these CMMPs act as synthetic cardiac stem cells, displaying therapeutic benefits similar to real cardiac stem cells in rodent models of MI. CMMPs overcome several major limitations of live stem cells (i.e., difficulty of cryopreservation, tumorigenicity). This strategy can be applied to other cell types such as MSCs 69. We fabricated synthetic MSC particles (Fig. 2). Similarly, these agents could undergo freezing/thawing process without changes in their properties. In addition, synthetic MSCs could endure lyophilization processes without changing their properties or causing inflammation in the heart. As summarized in Table 1, CMMPs differ from exosomes in several ways.
Figure 1.
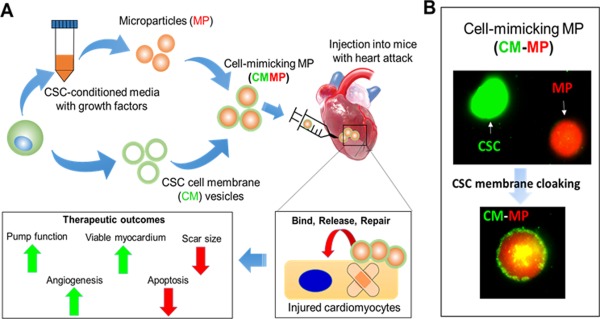
Fabrication of CMMPs. (A): Illustration showing the fabrication of CMMPs and the application of CMMPs for therapeutic heart regeneration. (B): CMMPs are formed with a core polymer particle containing stem cell‐secreted factors and a coat from stem cell membranes (modified from Ref. [68]). Abbreviation: CSC, cardiac stem cell.
Figure 2.
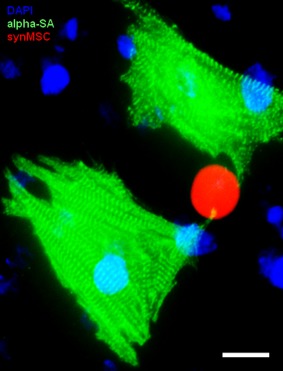
Interaction between synthetic MSCs and cardiomyocytes (modified from Ref. [69]). Scale bar, 20 um. Abbreviation: MSCs, mesenchymal stem cells.
Table 1.
Summary of the difference between CMMP and exosome
| Size | Coat | Cargo | Backbone | Stability in the body | |
|---|---|---|---|---|---|
| Exosome | 30–100 nm | CD9, CD63, CD81, Alix, Flotillin‐1, Tsg101 | microRNAs mRNAs proteins | None | Minutes of blood half‐life; untaken by cells |
| CMMP | >10 μm | Whatever on the cell membrane | Exosomes and other proteins | Biodegradable polymers | Days to weeks in the heart |
Abbreviation: CMMP, cell mimicking microparticles.
The future development of CMMPs for clinical application still faces several challenges. First, the manufacturing of synthetic stem cells still requires cell processing. Nonetheless, since cells are only used as “production lines” rather than the “final products,” steps for cell harvest and cell packaging are eliminated. In addition, final formulation for cell‐free products is far less challenging than that for cellular products. Also, more compact systems such as bioreactors and fibercells can be used to produce conditioned media to make synthetic stem cells. Second, the current sizes for synthetic stem cells are at the micron level. Systemic delivery is an issue. In the studies of Tang et al. and Luo et al. 68, 69, the microparticles were delivered by direct intramyocardial injection. Despite the fact that mechanisms for extravasation of micro‐sized particles do exist (i.e., angiopellosis 75), embolization risks remain for vascular delivery. Future efforts should focus on developing nano‐sized and targeted synthetic stem cells for systemic delivery. Even though targeted infusion using coronary catheters usually results in better engraftment, systemic delivery such as intravenous injection is more convenient and has already been established as a possible conduit for therapy 76.
Conclusion
In summary, after 17 years of testing, cardiac cell therapy is not dying and should not die. Despite the challenges, new solutions are emerging to move the field forward (Fig. 3). Millions of patients all over the world are looking for new alternatives to improve their quality of life and extend their life expectancy. The development of new technologies, such as bioengineering/biomaterials tools, exosome therapies, synthetic stem cells, hold the potential to revitalize this field.
Figure 3.
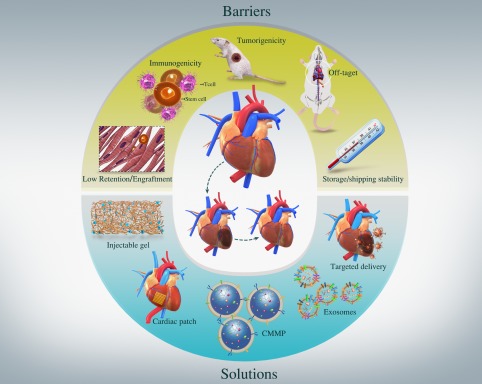
Challenges to the field of cardiac cell therapy and emerging new solutions.
Author Contributions
J.T., J.C., K.H., X.C., L.L., J.Z., T.L., and L.Q.: conception and design, manuscript writing; K.C.: manuscript writing, financial support, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Acknowledgments
This work was supported by funding from National Institute of Health (HL123920, HL137093), NC State University Chancellor's Faculty Excellence Program, NC State Chancellor's Innovation Fund, University of North Carolina General Assembly Research Opportunities Initiative grant, a Grant‐in‐Aid from the Ministry of Education, Science, Sports, Culture and Technology of Japan, a Program of the network‐type joint Usage/Research Center for Radiation Disaster Medical Science of Hiroshima University, Nagasaki University and Fukushima Medical University, University‐College Joint Cultivation Fund of Zhengzhou University (2016‐BSTDJJ‐19), and National Natural Science Foundation of China 81370216.
References
- 1. Benjamin EJ, Blaha MJ, Chiuve SE et al. Heart disease and stroke statistics‐2017 update: A report from the American Heart Association. Circulation 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wong M, Staszewsky L, Latini R et al. Severity of left ventricular remodeling defines outcomes and response to therapy in heart failure: Valsartan heart failure trial (Val‐HeFT) echocardiographic data. J Am Coll Cardiol 2004;43:2022–2027. [DOI] [PubMed] [Google Scholar]
- 3. Kramer DG, Trikalinos TA, Kent DM et al. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: A meta‐analytic approach. J Am Coll Cardiol 2010;56:392–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Madonna R, Van Laake LW, Davidson SM et al. Position Paper of the European Society of Cardiology Working Group Cellular Biology of the Heart: Cell‐based therapies for myocardial repair and regeneration in ischemic heart disease and heart failure. Eur Heart J 2016;37:1789–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Madonna R, Ferdinandy P, De Caterina R et al. Recent developments in cardiovascular stem cells. Circ Res 2014;115:e71–e78. [DOI] [PubMed] [Google Scholar]
- 6. Kajstura J, Gurusamy N, Ogorek B et al. Myocyte turnover in the aging human heart. Circ Res 2010;107:1374–1386. [DOI] [PubMed] [Google Scholar]
- 7. Orlic D, Kajstura J, Chimenti S et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001;410:701–705. [DOI] [PubMed] [Google Scholar]
- 8. Gnecchi M, He H, Noiseux N et al. Evidence supporting paracrine hypothesis for akt‐modified mesenchymal stem cell‐mediated cardiac protection and functional improvement. FASEB J 2006;20:661–669. [DOI] [PubMed] [Google Scholar]
- 9. Bolli R, Tang XL, Sanganalmath SK et al. Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation 2013;128:122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bolli R, Chugh AR, D'Amario D et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): Initial results of a randomised phase 1 trial. Lancet 2011;378:1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11. Matsuura K, Honda A, Nagai T et al. Transplantation of cardiac progenitor cells ameliorates cardiac dysfunction after myocardial infarction in mice. J Clin Invest 2009;119:2204–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takamiya M, Haider KH, Ashraf M et al. Identification and characterization of a novel multipotent sub‐population of Sca‐1+ cardiac progenitor cells for myocardial regeneration. PLoS One 2011;6:e25265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pfister O, Mouquet F, Jain M et al. CD31‐ but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res 2005;97:52–61. [DOI] [PubMed] [Google Scholar]
- 14. Makkar RR, Smith RR, Cheng K et al. Intracoronary cardiosphere‐derived cells for heart regeneration after myocardial infarction (CADUCEUS): A prospective, randomised phase 1 trial. Lancet 2012;379:895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Malliaras K, Makkar RR, Smith RR et al. Intracoronary cardiosphere‐derived cells after myocardial infarction: Evidence of therapeutic regeneration in the final 1‐year results of the CADUCEUS trial (CArdiosphere‐Derived aUtologous stem CElls to reverse ventricUlar dySfunction). J Am Coll Cardiol 2014;63:110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheng K, Ibrahim A, Hensley MT et al. Relative roles of CD90 and c‐kit to the regenerative efficacy of cardiosphere‐derived cells in humans and in a mouse model of myocardial infarction. J Am Heart Assoc 2014;3:e001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martin‐Rendon E, Brunskill SJ, Hyde CJ et al. Autologous bone marrow stem cells to treat acute myocardial infarction: A systematic review. Eur Heart J 2008;29:1807–1818. [DOI] [PubMed] [Google Scholar]
- 18. Hirsch A, Nijveldt R, van der Vleuten PA et al. Intracoronary infusion of mononuclear cells from bone marrow or peripheral blood compared with standard therapy in patients after acute myocardial infarction treated by primary percutaneous coronary intervention: Results of the randomized controlled HEBE trial. Eur Heart J 2011;32:1736–1747. [DOI] [PubMed] [Google Scholar]
- 19. Nguyen PK, Neofytou E, Rhee JW et al. Potential strategies to address the major clinical barriers facing stem cell regenerative therapy for cardiovascular disease: A review. JAMA Cardiol 2016;1:953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.A futile cycle in cell therapy. Nat Biotechnol 2017;35:291. [DOI] [PubMed] [Google Scholar]
- 21. Khanabdali R, Rosdah AA, Dusting GJ et al. Harnessing the secretome of cardiac stem cells as therapy for ischemic heart disease. Biochem Pharmacol 2016;113:1–11. [DOI] [PubMed] [Google Scholar]
- 22. Kadota S, Pabon L, Reinecke H et al. In vivo maturation of human induced pluripotent stem cell‐derived cardiomyocytes in neonatal and adult rat hearts. Stem Cell Reports 2017;8:278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee WH, Chen WY, Shao NY et al. Comparison of non‐coding RNAs in exosomes and functional efficacy of human embryonic stem cell‐versus induced pluripotent stem cell‐derived cardiomyocytes. Stem Cells 2017;35:2138–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matsa E, Burridge PW, Yu KH et al. Transcriptome profiling of patient‐specific human iPSC‐cardiomyocytes predicts individual drug safety and efficacy responses in vitro. Cell Stem Cell 2016;19:311–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Overington JP, Al‐Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov 2006;5:993–996. [DOI] [PubMed] [Google Scholar]
- 26. Kimmelman J, Heslop HE, Sugarman J et al. New ISSCR guidelines: Clinical translation of stem cell research. Lancet 2016;387:1979–1981. [DOI] [PubMed] [Google Scholar]
- 27. Sanganalmath SK, Bolli R. Cell therapy for heart failure: A comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res 2013;113:810–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hendrikx M, Hensen K, Clijsters C et al. Recovery of regional but not global contractile function by the direct intramyocardial autologous bone marrow transplantation: Results from a randomized controlled clinical trial. Circulation 2006;114:I‐101–I‐107. [DOI] [PubMed] [Google Scholar]
- 29. Yoon YS, Wecker A, Heyd L et al. Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. J Clin Invest 2005;115:326–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mazhari R, Hare JM. Mechanisms of action of mesenchymal stem cells in cardiac repair: Potential influences on the cardiac stem cell niche. Nat Clin Pract Cardiovasc Med 2007;4:S21–S26. [DOI] [PubMed] [Google Scholar]
- 31. Hatzistergos KE, Quevedo H, Oskouei BN et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res 2010;107:913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gnecchi M, Zhang Z, Ni A et al. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res 2008;103:1204–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chimenti I, Smith RR, Li TS et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere‐derived cells transplanted into infarcted mice. Circ Res 2010;106:971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rota M, Padin‐Iruegas ME, Misao Y et al. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ Res 2008;103:107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lefer DJ, Marbán E. Is Cardioprotection Dead? Circulation 2017;136:98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tang XL, Rokosh G, Sanganalmath SK et al. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30‐day‐old infarction. Circulation 2010;121:293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nagaya N, Kangawa K, Itoh T et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation 2005;112:1128–1135. [DOI] [PubMed] [Google Scholar]
- 38. Mathieu M, Bartunek J, El Oumeiri B et al. Cell therapy with autologous bone marrow mononuclear stem cells is associated with superior cardiac recovery compared with use of nonmodified mesenchymal stem cells in a canine model of chronic myocardial infarction. J Thorac Cardiovasc Surg 2009;138:646–653. [DOI] [PubMed] [Google Scholar]
- 39. Askari AT, Unzek S, Popovic ZB et al. Effect of stromal‐cell‐derived factor 1 on stem‐cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet 2003;362:697–703. [DOI] [PubMed] [Google Scholar]
- 40. Urbich C, Aicher A, Heeschen C et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol 2005;39:733–742. [DOI] [PubMed] [Google Scholar]
- 41. Shintani Y, Fukushima S, Varela‐Carver A et al. Donor cell‐type specific paracrine effects of cell transplantation for post‐infarction heart failure. J Mol Cell Cardiol 2009;47:288–295. [DOI] [PubMed] [Google Scholar]
- 42. Xie Y, Ibrahim A, Cheng K et al. Importance of cell‐cell contact in the therapeutic benefits of cardiosphere‐derived cells. Stem Cells 2014;32:2397–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Heslop JA, Hammond TG, Santeramo I et al. Concise review: Workshop review: Understanding and assessing the risks of stem cell‐based therapies. Stem Cells Translational Medicine 2015;4:389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Andrews PW, Matin MM, Bahrami AR et al. Embryonic stem (ES) cells and embryonal carcinoma (EC) cells: Opposite sides of the same coin. Biochem Soc Trans 2005;33:1526–1530. [DOI] [PubMed] [Google Scholar]
- 45. Zeng L, Hu Q, Wang X et al. Bioenergetic and functional consequences of bone marrow‐derived multipotent progenitor cell transplantation in hearts with postinfarction left ventricular remodeling. Circulation 2007;115:1866–1875. [DOI] [PubMed] [Google Scholar]
- 46. Hong KU, Li QH, Guo Y et al. A highly sensitive and accurate method to quantify absolute numbers of c‐kit+ cardiac stem cells following transplantation in mice. Basic Res Cardiol 2013;108:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sanada S, Komuro I, Kitakaze M. Pathophysiology of myocardial reperfusion injury: Preconditioning, postconditioning, and translational aspects of protective measures. Am J Physiol Heart Circ Physiol 2011;301:H1723–H1741. [DOI] [PubMed] [Google Scholar]
- 48. Guo Y, Wysoczynski M, Nong Y et al. Repeated doses of cardiac mesenchymal cells are therapeutically superior to a single dose in mice with old myocardial infarction. Basic Res Cardiol 2017;112:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bolli R. Repeated cell therapy: A paradigm shift whose time has come. Circ Res 2017;120:1072–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tokita Y, Tang XL, Li Q et al. Repeated administrations of cardiac progenitor cells are markedly more effective than a single administration: A new paradigm in cell therapy. Circ Res 2016;119:635–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hernandez MJ, Christman KL. Designing acellular injectable biomaterial therapeutics for treating myocardial infarction and peripheral artery disease. JACC Basic Transl Sci 2017;2:212–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tang J, Cui X, Caranasos TG et al. Heart repair using nanogel‐encapsulated human cardiac stem cells in mice and pigs with myocardial infarction. ACS Nano 2017;11:9738–9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ogle BM, Bursac N, Domian I et al. Distilling complexity to advance cardiac tissue engineering. Sci Transl Med 2016;8:342ps13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tang J, Vandergriff A, Wang Z et al. A regenerative cardiac patch formed by spray painting of biomaterials onto the heart. Tissue Eng Part C Methods 2017;23:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gao L, Kupfer ME, Jung JP et al. Myocardial tissue engineering with cells derived from human‐induced pluripotent stem cells and a native‐like, high‐resolution, 3‐dimensionally printed scaffold. Circ Res 2017;120:1318–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cheng K, Malliaras K, Li TS et al. Magnetic enhancement of cell retention, engraftment, and functional benefit after intracoronary delivery of cardiac‐derived stem cells in a rat model of ischemia/reperfusion. Cell Transplant 2012;21:1121–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vandergriff AC, Hensley TM, Henry ET et al. Magnetic targeting of cardiosphere‐derived stem cells with ferumoxytol nanoparticles for treating rats with myocardial infarction. Biomaterials 2014;35:8528–8539. [DOI] [PubMed] [Google Scholar]
- 58. Cheng K, Li TS, Malliaras K et al. Magnetic targeting enhances engraftment and functional benefit of iron‐labeled cardiosphere‐derived cells in myocardial infarction. Circ Res 2010;106:1570–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cheng K, Shen D, Hensley MT et al. Magnetic antibody‐linked nanomatchmakers for therapeutic cell targeting. Nat Commun 2014;5:4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lee RJ, Fang Q, Davol PA et al. Antibody targeting of stem cells to infarcted myocardium. Stem Cells 2007;25:712–717. [DOI] [PubMed] [Google Scholar]
- 61. Lo CY, Weil BR, Palka BA et al. Cell surface glycoengineering improves selectin‐mediated adhesion of mesenchymal stem cells (MSCs) and cardiosphere‐derived cells (CDCs): Pilot validation in porcine ischemia‐reperfusion model. Biomaterials 2016;74:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kishore R, Khan M. More than tiny sacks: Stem cell exosomes as cell‐free modality for cardiac repair. Circ Res 2016;118:330–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kishore R, Khan M. Cardiac cell‐derived exosomes: Changing face of regenerative biology. Eur Heart J 2017;38:212–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Agarwal U, George A, Bhutani S et al. Experimental, systems, and computational approaches to understanding the microRNA‐mediated reparative potential of cardiac progenitor cell‐derived exosomes from pediatric patients. Circ Res 2017;120:701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liang Y, Sahoo S. Exosomes explosion for cardiac resuscitation. J Am Coll Cardiol 2015;66:612–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ibrahim A, Marbán E. Exosomes: Fundamental biology and roles in cardiovascular physiology. Annu Rev Physiol 2016;78:67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vandergriff AC, de Andrade JB, Tang J et al. Intravenous cardiac stem cell‐derived exosomes ameliorate cardiac dysfunction in doxorubicin induced dilated cardiomyopathy. Stem Cells Int 2015;2015:960926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tang J, Shen D, Caranasos TG et al. Therapeutic microparticles functionalized with biomimetic cardiac stem cell membranes and secretome. Nat Commun 2017;8:13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Luo L, Tang J, Nishi K et al. Fabrication of synthetic mesenchymal stem cells for the treatment of acute myocardial infarction in mice. Circ Res 2017;120:1768–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hu CM, Fang RH, Luk BT et al. Polymeric nanotherapeutics: Clinical development and advances in stealth functionalization strategies. Nanoscale 2014;6:65–75. [DOI] [PubMed] [Google Scholar]
- 71. Iqbal M, Zafar N, Fessi H et al. Double emulsion solvent evaporation techniques used for drug encapsulation. Int J Pharm 2015;496:173–190. [DOI] [PubMed] [Google Scholar]
- 72. Luk BT, Hu CM, Fang RH et al. Interfacial interactions between natural RBC membranes and synthetic polymeric nanoparticles. Nanoscale 2014;6:2730–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hu CM, Fang RH, Wang KC et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 2015;526:118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fang RH, Kroll AV, Zhang L. Nanoparticle‐based manipulation of antigen‐presenting cells for cancer immunotherapy. Small 2015;11:5483–5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Allen TA, Gracieux D, Talib M et al. Angiopellosis as an alternative mechanism of cell extravasation. Stem Cells 2017;35:170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Karantalis V, Hare JM. Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res 2015;116:1413–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]


