Abstract
Severe acute graft‐versus‐host disease (GVHD) is a life‐threatening complication after allogeneic hematopoietic stem cell transplantation (HSCT). The placenta protects the fetus from the mother's immune system. We evaluated placenta‐derived decidua stromal cells (DSCs), which differ from bone marrow mesenchymal stromal cells (BM‐MSCs), as a treatment for severe acute GVHD. DSCs were obtained from term placentas. The DSCs were given to 38 patients with severe acute GVHD; 25 were steroid refractory (SR). DSCs were thawed and infused in buffer supplemented with either 10% AB plasma (group 1, n = 17), or 5% albumin (group 2, n = 21). The viability of cells was higher when thawed in albumin rather than AB plasma (p < .001). Group 1 received a higher cell dose (p < .001), cells of lower passage number (p < .001), and fewer infusions (p = .002) than group 2. The GVHD response (no/partial/complete) was 7/5/5 in group 1 and 0/10/11 in group 2 (p = .01). One‐year survival in the two groups was 47% (95% confidence interval [CI] 23–68) and 76% (95% CI 51–89), respectively (p = .016). For the SR patients, 1‐year survival was 73% (95% CI 37–90) in SR group 2 (n = 11), which was better than 31% (95% CI 11–54) in SR group 1 (n = 13; p = .02), 20% (95% CI 5–42) in BM‐MSC treated (n = 15; p = .0015), and 3% (95% CI 0–14) in historic controls (n = 32; p < .001). DSCs are a promising new treatment for severe acute GVHD. Prospective randomized trials are needed for evaluation of efficacy. (Clinical trial NCT‐02172937.) stem cells translational medicine 2018;7:325–332
Keywords: Decidua stromal cells, Acute graft‐versus‐host disease, Allogeneic hematopoietic stem cell transplantation, Mesenchymal stromal cells
Significance Statement.
There has been no effective therapy for severe acute graft‐versus‐host disease (GVHD), a life‐threatening complication after allogeneic hematopoietic stem cell transplantation. Bone marrow‐derived mesenchymal stromal cells were introduced as a novel therapy for acute GVHD, which cured some, but not all, patients with severe acute GVHD. The placenta plays an important role in fetomaternal tolerance and has been used in Africa for 100 years to successfully treat burn injuries. It was found that placenta‐derived decidua stromal cells (DSCs) are immunosuppressive in vitro and in vivo and may cure severe acute GVHD. In this pilot study, an optimal protocol was found using DSCs at 1 × 106 cells/kg dissolved in saline with 5% human albumin instead of 10% AB‐plasma, given at least one dose a week. All patients receiving this treatment showed partial or complete responses and the best one‐year survival. This was a small pilot study, but all patients with severe acute GVHD were cured using the new protocol. There were no major side effects. In conclusion, DSCs are a novel, promising therapy for acute GVHD and other inflammatory immunological disorders.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is a well‐established treatment for advanced leukemias and severe hematological and metabolic diseases 1, 2. Graft‐versus‐host disease (GVHD) is a major cause of morbidity and mortality after HSCT 3, 4, 5. There is no effective treatment for severe acute GVHD, and the outcome has been poor for patients with acute GVHD that are refractory to steroids 4, 6, 7, 8. The use of mesenchymal stromal cells (MSCs) to treat GVHD was introduced by us more than a decade ago 9, 10, 11. Despite promising results initially, long‐term overall survival was not any better than in the controls, which was not so encouraging 12, 13. A meta‐analysis found a survival rate of 63% at 6 months in patients with severe acute GVHD that responded completely to MSC therapy 14. However, the outcome is poor in partial responders and nonresponders 10.
The placenta protects the fetus from the mother's immune system during pregnancy and provides a readily available source of stromal cells 15, 16. We have isolated decidua stromal cells (DSCs), which are of maternal origin and inhibit alloreactive T cells in vitro better than other sources of stromal cells 17, 18. DSCs induce FOXP3‐positive regulatory T cells and inhibit alloreactivity in vitro in a contact‐dependent manner and not by soluble factors like MSCs 19. DSCs are half the size of MSCs and do not differentiate well to chondrocytes and osteocytes 20, 21. In the allogeneic setting, DSCs promote an anti‐inflammatory cytokine profile 5, 19. DSCs also have stronger hemostatic properties than MSCs. DSCs have typical MSCs surface markers, but a stronger expression of programmed death‐ligand 1 (PD‐L1), PD‐L2, and CD49d (a marker for homing to inflamed tissue) than MSCs from bone marrow 18. Taken together, these differences may explain why DSCs have a stronger immunomodulatory effect as opposed to other sources of MSCs. Here we report our experience using DSCs for treatment of severe acute GVHD. This is a pilot study using DSCs for severe acute GvHD, in which two specified protocols have been explored.
Materials and Methods
Patients
This is a retrospective analysis of the safety and efficacy of DSC treatment for acute GVHD. Between 2011 and 2015, 38 patients were treated with DSCs for acute GVHD after HSCT (Table 1), in keeping with the Declaration of Helsinki. The patients and donors of DSCs gave their written, informed consent. Patients with severe GVHD of grade 2–4 were included, based on clinical evaluation by the treating physician and with inclusion criteria stated below. They were all consecutive patients, and no patient declined to be enrolled in the DSC study. The patients were treated with prednisolone and calcineurin inhibitors but no other immunosuppressive therapy. The last follow‐up was on November 12, 2015. Eight patients have been reported previously 18. The cells were previously named fetal membrane cells.
Table 1.
Patient characteristics for all the patients treated with DSCs
| Characteristics | Group 1, n = 17 | Group 2, n = 21 | p value |
|---|---|---|---|
| Sex (M/F) | 9/8 | 16/5 | .18 |
| Age at GVHD, years, median (range) | 54.5 (0.9–65.6) | 48.9 (1.6–72.4) | .33 |
| Children (<19 years of age) | 2 | 3 | .33 |
| Diagnosis (malignant/nonmalignant) | 14/3 | 17/4 | 1.00 |
| Disease status (high risk/low risk) | 9/8 | 14/7 | .51 |
| Conditioning (MAC/RIC) | 8/9 | 4/17 | .09 |
| ATG (yes/no) | 10/7 | 14/7 | .74 |
| GVHD prophylaxis | .63 | ||
| CsA/MTX | 13 | 13 | |
| TAC/SIR | 3 | 6 | |
| CsA/MTX/Cy | 1 | 2 | |
| Donor SIB/MUD/CB/haplo | 6/10/1/0 | 6/14/0/1 | .52 |
| Graft source (PBSCs/BM/CB) | 14/2/1 | 16/5/0 | .36 |
| GVHD grade at time of intervention (2/3) | 2/15 | 6/15 | .26 |
| GVHD localization (gut and other/only liver) | 17/0 | 21/0 | 1.00 |
| Fungal prophylaxis (yes/no) | 17/0 | 21/0 | 1.00 |
| CMV (double‐neg./any pos.) | 3/14 | 7/14 | .46 |
| GVHD after DLI (yes/no) | 0/17 | 2/19 | .49 |
| HSCT/DLI steroids, days (range) | 59 (10–375) | 64 (5–265) | .97 |
| Days with steroids median (range) | 13 (1–37) | 7 (0–35) | .09 |
| Number of infusions (range) | 1 (1–5) | 2 (1–6) | .002 |
| Cell dose (range) | 2.0 (0.9–2.8) | 1.2 (0.9–2.9) | <.001 |
| Cell passage (range) | 2 (2–4) | 4 (2–4) | <.001 |
| Viability, % (range) | 90 (70–97) | 95 (69–100) | <.001 |
Abbreviations: ATG, antithymocyte globulin; BM, bone marrow; CB, cord blood; CMV, cytomegalovirus; CsA, cyclosporine A; Cy, cyclophosphamide; DLI, donor lymphocyte infusion; DSCs, decidua stromal cells; F, female; GVHD, graft‐versus‐host disease; HSCT, allogeneic hematopoietic stem cell transplantation; M, male; MAC, myeloablative conditioning; MTX, methotrexate; MUD, matched unrelated donor; neg., negative; PBSCs, peripheral blood stem cells; pos., positive; RIC, reduced‐intensity conditioning; SIB, sibling donor; SIR, sirolimus; TAC, tacrolimus.
The regional ethical committee of Stockholm approved the donation and isolation of DSCs (entry nos. 2009/418‐31/4 and 2010/2061‐32) and the use of DSCs for GVHD (entry nos. 2010/452‐31/4 and 2014/2132‐32).
Procedures and Definitions
Before HSCT, the patients received either myeloablative or reduced‐intensity conditioning. The conditioning was myeloablative in 12 patients who were given cyclophosphamide (120 mg/kg) combined with busulfan (16 mg/kg), or fractionated whole‐body irradiation (12 Gy). Twenty‐six patients had reduced‐intensity conditioning regimens with fludarabine phosphate combined with various cytotoxic drugs such as busulfan or treosulfan, or 2 Gy whole‐body irradiation.
As GVHD prophylaxis, most patients received cyclosporine combined with four doses of intravenous methotrexate. In addition, three patients were treated with two doses of post‐transplantation cyclophosphamide (100 mg/kg). Nine patients were treated with tacrolimus and sirolimus as part of a randomized trial 22. Twenty‐four recipients of hematopoietic stem cells from unrelated donors were treated with antithymocyte globulin. Patients were treated in reversed isolation, or at home during the pancytopenic phase if they lived close to the hospital 23. Patient care procedures and the transplantation procedures have been published previously in detail 23.
Acute GVHD was graded according to the Seattle criteria 3. All patients had gastrointestinal GVHD, and the diagnosis was confirmed by histological analysis of biopsies taken during colonoscopy or gastroscopy prior to therapy. No post‐DSC biopsies were performed. Six patients developed acute GVHD after donor lymphocyte infusion. Steroid‐refractory acute GVHD was defined as disease progressive after 3 days despite prednisolone 1 or 2 mg/kg/day or lack of response after 7 days. Some patients were included with lack of response of steroids after 3 days due to high age and/or comorbidities. They were treated with DSCs because they were not considered able to tolerate long‐term immunosuppressive therapy with high‐dose steroids. The infusion schedule was as follows. Group 1 received one dose. If complete response (CR) was seen, no additional DSC doses were given. Patients in group 2 were scheduled to receive a second dose after 1 week even if complete response was seen. Patients with active acute GVHD got additional weekly DSC doses until complete response or acceptable, stable, or partial response (PR) was achieved and the patient could be sent home. Fungal prophylaxis with posaconazole was given to all patients.
All patients received first‐line treatment of acute GVHD consisting of oral or intravenous corticosteroids in prednisolone doses of 2 mg/kg/day, which was later changed to 1 mg/kg/day 24. The controls received median 2 mg/kg/day prednisolone at the beginning of this treatment, as opposed to 1.85 mg/kg/day in group 1 and 1.60 mg/kg/day (p < .001 vs. controls) in group 2. In the latter two groups, some patients got 2 mg/kg/day and others got 1 mg/kg/day. In addition, oral budesonide and a calcineurin inhibitor were given to all patients. No other immunosuppressive therapy was given.
Response to the treatment was evaluated 4 weeks after intervention. CR was defined as disappearance of all symptoms of acute GVHD; PR was defined as improvement by at least one organ‐specific grade; and no response was defined as no improvement of GVHD symptoms. Transplantation‐related mortality (TRM) included all deaths associated with transplantation of hematopoietic cells, except for those related to recurrence of underlying disease. Data on corticosteroid treatment were obtained from the patients’ charts. GVHD‐related mortality was defined as the presence of GVHD symptoms at the time of death.
Laboratory Methods
Human term placentas (n = 9) were obtained from healthy mothers during elective Caesarean section births. All donors were seronegative for HIV, hepatitis B and C, and syphilis. The fetal membranes were carefully dissected from the placenta and subsequently cultured as previously described in detail 17, 18. The DSCs were of maternal origin and had limited differentiation capacity 18, 19. They expressed CD29, CD73, CD90, CD105, CD49d, CD44, CD54, human leukocyte antigen (HLA) class I, PD‐L1, and PD‐L2. They were negative for the hematopoietic markers CD45 and CD34, the endothelial marker CD31, and HLA class II. DSCs were stored frozen in liquid nitrogen until use. The cells were thawed rapidly and resuspended in CliniMACS PBS/EDTA buffer (Miltenyi Biotech, Bergisch Gladbach, Germany) supplemented with either 5% human AB plasma (in‐house) or 5% human serum albumin (CSL Behring, King of Prussia, PA). The cells were washed three times and then resuspended in an infusion solution containing NaCl (B. Braun Melsungen AG, Melsungen, Germany) supplemented with either 10% AB plasma or 5% albumin. The infusion solution was then filtered using a 70‐μM cell strainer (BD Bioscience, Franklin Lakes, NJ) before being transferred to a heparinized (Leo Pharma, Ballerup, Denmark) syringe at 2 × 106 cells/mL. The cell suspension was infused intravenously using a central venous line. Before and after infusion with DSCs, the central venous line was flushed with 2–5 mL of NaCl with 50 IE heparin/mL for adults. For children over 15 kg in weight, 25 IE heparin/mL was infused, and for children below 15 kg in weight, 12.5 IE heparin/mL was given. There were three children (<19 years of age) in group 1, two in group 2, none in the MSC group, and six in the historic controls (Table 1).
After the first 17 patients (group 1), three protocol changes were made. The thawing procedure was changed from use of AB plasma to use of albumin as supplement in the thawing and washing solution. DSC clotted during washing when prepared for patient 18. New DSCs were thawed, which were resuspended and washed in PBS and 5% albumin. There was no clotting, and cell viability was improved. Subsequently all DSCs were washed in PBS and 5% albumin. At the same time, we decided to change the protocol, and instead of giving 2 × 106 DSC/kg in one dose, this was divided to two doses of 1 × 106 DSC/kg given 1 week apart. Group 1 received 2 × 106 cells/kg (median) at inclusion. The next 21 patients (group 2) were given a lower dose of cells, aiming at 1 × 106 DSCs/kg. The patients in group 1 were given one infusion of DSCs, whereas all the patients in group 2 were given at least two weekly infusions of DSCs. Thereafter, all patients received additional doses based on the GVHD response. There were no significant differences in patient characteristics between the two groups (Table 1). Acute steroid‐refractory (SR) GVHD was defined as progressive disease after 3 days of steroid therapy, or 7 days with steroids without any improvement.
Severe adverse events (SAEs) occurring after infusion with DSCs during the observation period were analyzed in all patients. For the controls, SAEs were listed 13 days after onset of acute GVHD, which was the median day for DSC treatment after appearance of acute GVHD. Graft failure was defined as absolute neutrophil counts of less than 0.5 × 109 per liter, necessitating boosting of hematopoietic stem cells.
Statistical Analysis
Time to survival and relapse‐free survival were determined with the Lifetable method using the log‐rank (Mantel‐Haenzel) test, taking censored data into account. The incidence of chronic GVHD, GVHD‐related mortality, TRM, and hematological relapse were estimated using a nonparametric estimator of cumulative incidence curves taking competing events into consideration. Competing events were death without GVHD (for GVHD), death from other causes (for GVHD‐related mortality), relapse (for TRM), and TRM (for relapse). Patients were censored at the time of death, relapse, or at last follow‐up. Analyses were performed using the CMPRSK software package (developed by Gray, June 2001), S‐PLUS 6.2 software, and Statistica (StatSoft, Tulsa, OK). The Mann‐Whitney U test was used for unrelated continuous variables comparing two groups, and the Kruskal‐Wallis test was used for continuous variables comparing three groups, followed by Dunn's multiple comparisons test. Fisher's exact test or the chi‐square test was used to compare the distribution of categorical variables.
Results
Characteristics of DSC Treatment
The first 17 patients received DSCs that had been thawed and infused in buffer supplemented with AB plasma (group 1), which was the standard protocol that had been used at this center previously 9, 10, 11, 12, 13. The next 21 patients received DSCs that had been thawed and infused in albumin‐supplemented buffer (group 2). The albumin‐thawed cells had significantly higher viability than the plasma‐thawed cells (Table 1). The patients in group 1 received significantly fewer doses, a higher number of cells per dose, and stromal cells from a lower passage number than group 2 (Table 1).
Response and Survival
The GVHD response (no/partial/complete) was 7/5/5 in group 1 and 0/10/11 in group 2 (p = .013). Group 2 had a significantly higher survival (76%; 51–89) at 1 year than group 1 (47%; 23–68; Fig. 1A). The probability of relapse and chronic GVHD was similar in the two groups (Fig. 1B, 1C). The cumulative incidence of chronic GVHD at 1.5 years was 36% (12–61) in group 1 and 31% (12–53) in group 2, respectively (ns). Of 14 patients in group 1 who were alive beyond day 100, 5, 1, and 1 developed mild, moderate, and severe chronic GVHD, respectively. In group 2, of the 21 patients, 6 developed mild chronic GVHD, 2 developed moderate chronic GVHD, and none developed severe chronic GVHD. The death rate from acute GVHD was 41% (95% confidence interval [CI] 18–64) in group 1 and 5% (95% CI 0–20) in group 2 (Fig. 1D; p = .016).
Figure 1.
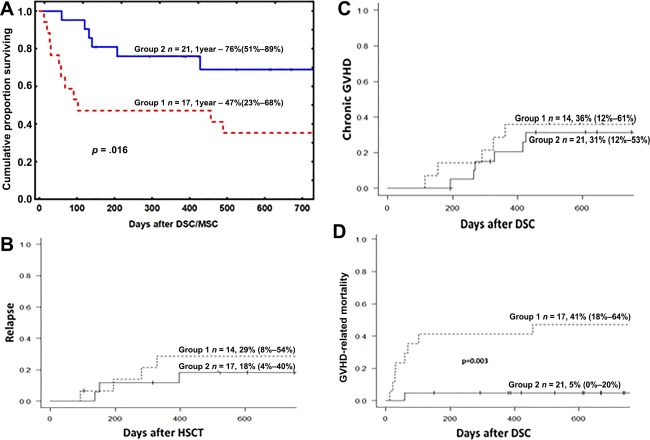
(A): Kaplan‐Meier estimate of the overall survival of patients with severe acute GVHD who were treated with DSCs. The patients were divided into two groups based on differences in the cell handling procedure (Table 1). Group 2 had a significantly higher chance of survival than group 1 (p = .016). There were no significant differences in the relapse incidence (B) or incidence of chronic GVHD (C) between the two groups. (D): The relative risk of having GVHD symptoms at the time of death was significantly higher for the patients in group 1 (p = .016). Abbreviations: DSC, decidua stromal cell; GVHD, graft‐versus‐host disease; HSCT, allogeneic hematopoietic stem cell transplantation; MSC, mesenchymal stromal cell.
Steroid‐Refractory GVHD
The patients with GVHD that was strictly steroid refractory in each group were compared with retrospective controls from our unit, during the period 2000–2010, who had acute steroid‐refractory GVHD (Table 2). Patients treated with mesenchymal stromal cells (MSCs 1 × 106 MSC/kg, n = 15) were also reported. Compared with the DSC patients, the historic controls not given stromal cells were younger (p = .02), all had had malignant disorders (p = .02), and all had received cyclosporine and methotrexate as GVHD prophylaxis (p = .005); in addition, fewer control patients who were cytomegalovirus (CMV) seronegative had had a CMV‐seronegative donor (p = .05). In the MSCs group, 13 of 15 received bone marrow graft, which differed from all other groups (p < .001). The MSCs patients more often had GVHD grade 3 at intervention time, which differed from group 2 and historic control (p < .05). There were no other significant differences between the groups.
Table 2.
Patient characteristics for all steroid‐refractory DSC‐treated patients and controls
| Characteristics | SR group 1, n = 13 | SR group 2, n = 11 | SR MSC, n = 15 | SR controls, n = 32 |
|---|---|---|---|---|
| Sex (M/F) | 6/7 | 7/4 | 11/4 | 18/14 |
| Age at GVHD, years, median (range) | 54.8 (16.4–64.4) | 42.4 (1.6–53.9) | 57 (34–65) | 40.65 (3.7–67.7) |
| Diagnosis (malignant/nonmalignant) | 11/2 | 8/3 | 15/0 | 32/0 |
| Disease status (high risk/low risk) | 8/5 | 6/5 | 6/7 | 17/12 |
| Conditioning (MAC/RIC) | 7/6 | 3/8 | 8/7 | 20/12 |
| ATG (yes/no) | 6/7 | 7/4 | 9/6 | 20/12 |
| GVHD prophylaxis | ||||
| CsA/MTX | 10 | 6 | 14 | 25 |
| CsA/MMF | 0 | 0 | 1 | 7 |
| TAC/SIR | 2 | 3 | 0 | 0 |
| CsA/MTX/Cy | 1 | 2 | 0 | 0 |
| Donor SIB/MUD/CB/haplo | 6/7/0/0 | 4/6/0/1 | 9/5/1/0 | 11/19/2/0 |
| Graft source (PBSCs/BM/CB) | 11/2/0 | 8/3/0/1 | 1/13/1 | 25/5/2 |
| GVHD grade at time of intervention (2/3) | 2/11 | 4/7 | 0/15 | 9/23 |
| GVHD localization (gut and other/only liver) | 13/0 | 11/0 | 15/0 | 27/5 |
| CMV (double‐neg./any pos.) | 2/11 | 4/7 | 1/14 | 2/30 |
| GVHD after DLI (yes/no) | 0/13 | 1/10 | 5/10 | 5/27 |
| HSCT/DLI steroids, days (range) | 33 (10–375) | 27 (5–200) | 28 (11–94) | 25 (8–171) |
| Steroids DSCs, days (range) | 18 (7–37) | 7 (3–23) | 23 (3–90) | N/A |
| Number of infusions (range) | 1 (1–3) | 3 (2–6) | 1 (1–3) | N/A |
| Cell dose (range) | 2.0 (0.9–2.8) | 1.2 (1.0–2.9) | 1.5(0.7–2.0) | N/A |
| Cell passage (range) | 2 (2–3) | 4 (2–4) | 3 (2–3) | N/A |
| Viability, % (range) | 90 (70–97) | 94 (69–100) | >95 | N/A |
Abbreviations: ATG, antithymocyte globulin; BM, bone marrow; CB, cord blood; CMV, cytomegalovirus; CsA, cyclosporine A; Cy, cyclophosphamide; DLI, donor lymphocyte infusion; DSCs, decidua stromal cells; F, female; GVHD, graft‐versus‐host disease; HSCT, allogeneic hematopoietic stem cell transplantation; M, male; MAC, myeloablative conditioning; MTX, methotrexate; MUD, matched unrelated donor; N/A, not applicable; neg., negative; PBSCs, peripheral blood stem cells; pos., positive; RIC, reduced‐intensity conditioning; SIB, sibling donor; SIR, sirolimus; SR, steroid refractory; TAC, tacrolimus.
Among the steroid‐refractory patients, overall response at 4 weeks after the DSCs intervention was 100% in SR group 2 (n = 11), 46% in SR group 1 (n = 13; p = .013), and 25% in the controls (n = 32; p < .001).
SR group 2 had a significantly higher survival rate than SR group 1 (p = .02), the MSCs group (p = .0015), and the controls (p < .001, Fig. 2A). SR group 1 also had a higher survival rate than the controls (p = .02). None of the steroid‐refractory patients in group 2 died from GVHD, as opposed to 54% (95% CI 23–77) of the patients in SR group 1 (p < .01), 60% (95% CI 30–81) in MSC group (p < .01), and 81% (95% CI 62–92) of the controls (p < .001; Fig. 2B). The survival in all patients in group 2 was comparable to the survival in all the patients who underwent HSCT at our center between 2010 and 2015 and significantly better than historic control treated with conventional immunosuppression between 2000 and 2010 (p < .001; Fig. 3).
Figure 2.
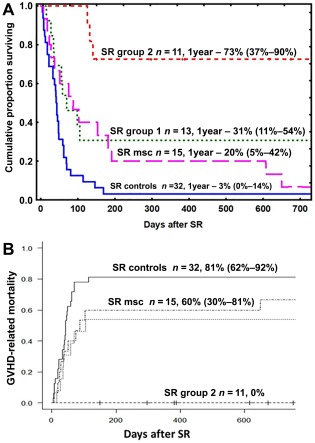
(A): Kaplan‐Meier estimate of the overall survival of patients with acute SR GVHD treated with decidua stromal cells and SR controls. SR group 2 had a significantly higher chance of survival than SR group 1 (p = .02), MSC‐treated patients (p = .0015), and the SR controls (p < .001). (B): The relative risk of having GVHD symptoms at the time of death was significantly higher for SR group 1, MSC group, and the SR controls than for SR group 2, (p < .01; p < .01, and p < .001, respectively). Abbreviations: GVHD, graft‐versus‐host disease; SR, steroid refractory; MSC, mesenchymal stromal cell.
Figure 3.
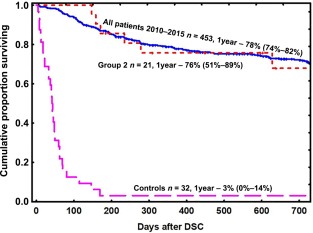
Kaplan‐Meier estimate of the overall survival of patients with severe acute graft‐versus‐host disease treated with DSCs (group 2) and historic controls (2000–2010) compared with that in all patients who were transplanted at our center in the period 2010–2015. The chance of survival for group 2 was similar to that for all the patients treated at our center and was significantly better than historical control group (p < .001). Abbreviations: DSC, decidua stromal cell.
Severe Adverse Events and Causes of Death
Severe adverse events in the DSC patients (n = 38) and in the control group (n = 32) included the following: relapse (8/10), pneumonia (5/9), proven or probable invasive fungal infection (6/5), bacterial infection (2/6), graft failure (3/3), multiple organ failure (1/5), viral infection (2/3), central nervous system complication (2/3), septicemia (2/2), skin squamous cell carcinoma (2/0), interstitial pneumonitis (0/1), acute pancreatitis (1/0), and cardiac failure (0/1). Adverse events in DSC patients and laboratory values are reported in detail in a separate article 25.
Causes of death (in DSC‐treated patients/controls) were acute GVHD (9/18), relapse (2/4), bacterial infection (2/6), multiple organ failure (0/1), viral infection (0/1), invasive fungal infection (1/1), liver failure (1/0), hemorrhaging (1/0), and secondary malignancy (1/0).
A 64‐year‐old woman with myelodysplastic syndrome died from squamous cell carcinoma 4 years after HSCT complicated by GVHD, which had been treated with two doses of DSCs 18. She was a heavy smoker and had sun‐tanned extensively.
A 67‐year‐old man with chronic lymphocytic leukemia was treated with four doses of DSCs for acute GVHD. One year after HSCT, he was operated on for squamous cell carcinoma and basalioma at the site of a pretransplant actinic keratosis in the face. Two years after HSCT, a malignant melanoma was removed radically from his back.
Discussion
Here we present data on 38 patients with severe acute gastrointestinal GVHD treated with placenta‐derived DSCs. The treatment protocol was changed after the first 17 patients, because the cell‐handling procedures were optimized during the intervention. With our current protocol (corresponding to group 2), the survival of patients with steroid‐refractory acute GVHD was similar to that in all the patients who underwent HSCT at our center in the last 5 years. It should be noted that patients who survived severe acute GVHD, treated with conventional immunosuppressive therapy, have a significantly worse outcome than all other HSCT patients 26. In contrast, patients who were treated with DSCs for severe acute GVHD had much better survival. These promising results are based only on 11 patients. If confirmed in a larger prospective trial in future, this will be a breakthrough in the treatment of severe acute GVHD.
The use of active human AB plasma as a supplement in the thawing and infusion solution for DSCs resulted in a significantly lower viability of the cells compared with the use of albumin. Group 1 received almost twice as many cells, so the amounts of infused, viable DSCs might be expected to have been comparable in the two groups. Thus, the improved survival for the patients in group 2 was probably not because of a higher number of viable cells. It is tempting to speculate that active human AB plasma contains functional complement factors that might bind to the DSCs during thawing, thus priming them for lysis during intravenous infusion. The role of complement in lysing MSCs was previously investigated by Li et al. 27. The MSCs were washed and dissolved in AB plasma, and it cannot be excluded that MSC therapy may also be improved by being dissolved in albumin.
A previous study from our center showed that patients who received MSCs at a lower passage number had a better survival than those who received MSCs at a higher passage number 13. Despite the higher passage number of DSCs used in group 2, the clinical outcome was better than in group 1. This suggests that passage number may be less important for DSCs than for MSCs regarding treatment of GVHD. All of the steroid‐refractory patients in group 2 responded to DSC treatment, and 7 of 11 patients had a complete response after 4 weeks. In group 1, response rates and survival were comparable to the MSCs group in this study and what has been shown previously using MSCs 10, 11, 12, 13, 14, 28. In the controls, one fourth of the patients with steroid‐refractory acute GVHD showed a response to steroids after 4 weeks (Fig. 2). However, these responses did not result in improved survival (Fig. 3A). In patients with severe acute GVHD who were treated with MSCs, the survival of those with a partial response was not improved 11. This is in contrast to group 2, in which survival was improved in those with a partial response.
Based on the findings in this study and the safety report, it appears that treatment with DSCs is safe 25. The causes of SAEs and deaths were infections, relapse, and other common complications seen among patients undergoing HSCT, especially those with severe acute GVHD. Two patients had squamous cell carcinoma―one of whom died. Squamous cell carcinoma and other skin malignancies are common secondary malignancies in patients who have undergone HSCT or organ transplantation 29. In addition to transplantation, these two patients had risk factors for squamous cell carcinoma. Three patients had graft failure, which is relatively common after HSCT in patients receiving reduced‐intensity conditioning 30. Six patients had invasive fungal infections despite prophylaxis. Given the nature of GVHD and the different immunosuppressive therapies used, patients with acute GVHD can be expected to be heavily immunocompromised. Therapies that have immunomodulatory effects―including DSCs and MSCs―can be expected to give an increased risk of infections. We also saw a high frequency of invasive fungal infection in the controls and in patients treated with MSCs 12. In a safety study, we found that the side effects and causes of death were similar in patients with GVHD and hemorrhagic cystitis who were treated with DSCs and in controls treated with other therapies 25. Because the patients treated with DSCs have survived longer than expected, they have had more time to experience severe adverse events after HSCT complicated by acute GVHD and heavy immunosuppressive treatment. Many more long‐term survivors of acute GVHD will be required to determine whether any particular causes of death and severe adverse events are associated with stromal cell therapy.
The mechanism by which stromal cells overall exert their immunosuppressive effects has not yet been fully investigated. Homing to the spleen and mobilization of macrophages to exert an anti‐inflammatory and immunosuppressive effect is one mechanism for bone marrow‐derived stromal cells 31. Similar effects are possible but not studied using DSCs. DSCs are dependent on cell‐to‐cell contact to perform their immunomodulatory function in vitro 19. In mixed lymphocyte cultures, regulatory T cells were increased in the presence of DSCs, a mechanism that was contact dependent 19. Blocking experiments suggest that interferon‐γ, prostaglandin E2, indoleamine dioxygenase, and PD‐L1 are involved in the immunosuppressive mechanism of DSCs 19. DSCs are more advantageous than many other stromal cells, as the use of term placentas provides an almost unlimited supply of cells, and there is no need for any invasive procedure for isolation.
MSCs also have functions to differentiate along several mesenchymal cell lineages and, in addition, have immunosuppressive properties. The main function for DSCs seems to be to protect the fetus from the mother's HLA‐incompatible T cells and have little, if any, differentiation capacity 21. Stronger expression of PD‐L1, PD‐L2, and CD49d may explain why DSCs are more immunosuppressive than MSCs from bone marrow 18.
When a novel therapy is introduced in the clinic, it is often first tried in end‐stage or terminally ill patients, which was the case when we used MSCs and DSCs 9, 10, 18. According to the Declaration of Helsinki, doctors have the possibility to try a therapy that may help a dying patient even though clinical documentation is missing. When a positive effect is seen with acceptable side effects, the therapy is subsequently given earlier and earlier, with successively improved results. Such an effect is probably also seen using DSCs as well as MSCs. The earlier you treat, even in the case of severe GVHD, the more likely you are to rescue the patient. The two protocols of DSC1 versus 2 and the comparison with MSCs is obscured by the timing of therapy. When prospective trials are planned, timing must be considered in order to save as many patients with severe acute GVHD as possible.
Some limitations of the study were, apart from timing, the retrospective approach with a small, heterogeneous patient population. The controls were historic, and HSCT therapy has improved in more recent years 32. The controls were significantly younger, and young age is important for survival of severe acute GVHD 4. Therefore, the data should be interpreted with caution.
Conclusion
This study has shown promising results in treatment of severe acute GVHD with DSCs. To further assess safety and efficacy, a larger, prospective trial will be necessary. If an effective therapy for severe acute GVHD is indeed found and validated, it will increase the usefulness of HSCT, with a possible broadening of indications.
We also used DSCs successfully to treat acute respiratory distress syndrome and hemorrhagic cystitis 33, 34.
Author Contributions
O.R.: initiation of the studies of MSCs and DSCs, study design, data collection, data analysis, manuscript writing; A.B.: data collection and analysis; M.R.: data collection, statistical analysis; B.G. and J.W.: clinical data; B.K.: study performance, analysis, design; G.M.: data, expertise; L.K.: evaluation of fungal infection; M.W.: organization and support of the logistics for retrieval of the placentas; B.S. study performance, analysis, and design. All the authors contributed to interpretation of the results and to the final preparation of the article.
Disclosure of Potential Conflicts of Interest
L.K. received honoraria from ABBOTT and Gilead for presentations at meetings sponsored by the companies. The other authors indicated no potential conflicts of interest.
Acknowledgments
This study is dedicated to the Seattle team who taught us to perform HSCT. We thank the staff of the participating departments for compassionate and competent care of patients. We want to thank Gunilla Tillinger and Inger Holmström for help in preparing this manuscript. This study was supported by grants to O.R. from the Swedish Cancer Society (CAN2013/671), the Swedish Research Council (K2014‐64X‐05971‐34‐4), the Swedish Childhood Cancer Foundation (PR2013‐0045), the Cancer Society in Stockholm (111293), Stockholm County, and Karolinska Institutet.
The sponsor of this study had no role in the study design, in the collection, analysis, and interpretation of data, or in the writing of the report. The corresponding author and the first author had full access to all the data and had final responsibility for the decision to submit for publication.
References
- 1. Thomas ED, Buckner CD, Banaji M et al. One hundred patients with acute leukemia treated by chemotherapy, total body irradiation, and allogeneic marrow transplantation. Blood 1977;49:511–533. [PubMed] [Google Scholar]
- 2. Hoogerbrugge PM, Brouwer OF, Bordigoni P et al. Allogeneic bone marrow transplantation for lysosomal storage diseases. The European Group for Bone Marrow Transplantation. Lancet 1995;345:1398–1402. [DOI] [PubMed] [Google Scholar]
- 3. Storb R, Thomas ED. Graft‐versus‐host disease in dog and man: The Seattle experience. Immunol Rev 1985;88:215–238. [DOI] [PubMed] [Google Scholar]
- 4. Ringdén O, Nilsson B. Death by graft‐versus‐host disease associated with HLA mismatch, high recipient age, low marrow cell dose, and splenectomy. Transplantation 1985;40:39–44. [DOI] [PubMed] [Google Scholar]
- 5. Ferrara JL, Levine JE, Reddy P et al. Graft‐versus‐host disease. Lancet 2009;373:1550–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dignan FL, Clark A, Amrolia P et al. Diagnosis and management of acute graft‐versus‐host disease. Br J Haematol 2012;158:30–45. [DOI] [PubMed] [Google Scholar]
- 7. Martin PJ, Inamoto Y, Flowers ME et al. Secondary treatment of acute graft‐versus‐host disease: A critical review. Biol Blood Marrow Transplant 2012;18:982–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Westin JR, Saliba RM, De Lima M et al. Steroid‐refractory acute GVHD: Predictors and outcomes. Adv Hematol 2011;2011:601953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Le Blanc K, Rasmusson I, Sundberg B et al. Treatment of severe acute graft‐versus‐host disease with third party haploidentical mesenchymal stem cells. Lancet 2004;363:1439–1441. [DOI] [PubMed] [Google Scholar]
- 10. Ringdén O, Uzunel M, Rasmusson I et al. Mesenchymal stem cells for treatment of therapy‐resistant graft‐versus‐host disease. Transplantation 2006;81:1390–1397. [DOI] [PubMed] [Google Scholar]
- 11. Le Blanc K, Frassoni F, Ball L et al. Mesenchymal stem cells for treatment of steroid‐resistant, severe, acute graft‐versus‐host disease: A phase II study. Lancet 2008;371:1579–1586. [DOI] [PubMed] [Google Scholar]
- 12. Remberger M, Ringdén O. Treatment of severe acute graft‐versus‐host disease with mesenchymal stromal cells: A comparison with non‐MSC treated patients. Int J Hematol 2012;96:822–824. [DOI] [PubMed] [Google Scholar]
- 13. von Bahr L, Sundberg B, Lönnies L et al. Long‐term complications, immunologic effects, and role of passage for outcome in mesenchymal stromal cell therapy. Biol Blood Marrow Transplant 2012;18:557–564. [DOI] [PubMed] [Google Scholar]
- 14. Hashmi S, Ahmed M, Murad MH et al. Survival after mesenchymal stromal cell therapy in steroid‐refractory acute graft‐versus‐host disease: Systematic review and meta‐analysis. Lancet Haematol 2016;3:e45–e52. [DOI] [PubMed] [Google Scholar]
- 15. Brooke G, Rossetti T, Pelekanos R et al. Manufacturing of human placenta‐derived mesenchymal stem cells for clinical trials. Br J Haematol 2009;144:571–579. [DOI] [PubMed] [Google Scholar]
- 16. In 't Anker PS, Scherjon SA, Kleijburg‐van der Keur C et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 2004;22:1338–1345. [DOI] [PubMed] [Google Scholar]
- 17. Karlsson H, Erkers T, Nava S et al. Stromal cells from term fetal membrane are highly suppressive in allogeneic settings in vitro. Clin Exp Immunol 2012;167:543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ringdén O, Erkers T, Nava S et al. Fetal membrane cells for treatment of steroid‐refractory acute graft‐versus‐host disease. Stem Cells 2013;31:592–601. [DOI] [PubMed] [Google Scholar]
- 19. Erkers T, Nava S, Yosef J et al. Decidual stromal cells promote regulatory T cells and suppress alloreactivity in a cell contact‐dependent manner. Stem Cells Dev 2013;22:2596–2605. [DOI] [PubMed] [Google Scholar]
- 20. Moll G, Ignatowicz L, Catar R et al. Different procoagulant activity of therapeutic mesenchymal stromal cells derived from bone marrow and placental decidua. Stem Cells Dev 2015;24:2269–2279. [DOI] [PubMed] [Google Scholar]
- 21. Erkers T, Kaipe H, Nava S et al. Treatment of severe chronic graft‐versus‐host disease with decidual stromal cells and tracing with (111)indium radiolabeling. Stem Cells Dev 2015;24:253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Törlén J, Ringdén O, Garming‐Legert K et al. A prospective randomized trial comparing cyclosporine/methotrexate and tacrolimus/sirolimus as graft‐versus‐host disease prophylaxis after allogeneic hematopoietic stem cell transplantation. Haematologica 2016;101:1417–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Svahn BM, Remberger M, Myrbäck KE et al. Home care during the pancytopenic phase after allogeneic hematopoietic stem cell transplantation is advantageous compared with hospital care. Blood 2002;100:4317–4324. [DOI] [PubMed] [Google Scholar]
- 24. Mielcarek M, Storer BE, Boeckh M et al. Initial therapy of acute graft‐versus‐host disease with low‐dose prednisone does not compromise patient outcomes. Blood 2009;113:2888–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baygan A, Aronsson‐Kurttila W, Moretti G et al. Safety and side effects of using placenta‐derived decidual stromal cells for graft‐versus‐host disease and hemorrhagic cystitis. Front Immunol 2017;8:795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ringdén O, Labopin M, Sadeghi B et al. What is the outcome in patients with acute leukaemia who survive severe acute graft‐versus‐host disease? J Intern Med 2018;283:166–177. [DOI] [PubMed] [Google Scholar]
- 27. Li Y, Lin F. Mesenchymal stem cells are injured by complement after their contact with serum. Blood 2012;120:3436–3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao K, Lou R, Huang F et al. Immunomodulation effects of mesenchymal stromal cells on acute graft‐versus‐host disease after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2015;21:97–104. [DOI] [PubMed] [Google Scholar]
- 29. Witherspoon RP, Fisher LD, Schoch G et al. Secondary cancers after bone marrow transplantation for leukemia or aplastic anemia. N Engl J Med 1989;321:784–789. [DOI] [PubMed] [Google Scholar]
- 30. Olsson R, Remberger M, Schaffer M et al. Graft failure in the modern era of allogeneic hematopoietic SCT. Bone Marrow Transplant 2013;48:537–543. [DOI] [PubMed] [Google Scholar]
- 31. Parekkadan B, Upadhyay R, Dunham J et al. Bone marrow stromal cell transplants prevent experimental enterocolitis and require host CD11b+ splenocytes. Gastroenterology 2011;140:966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Remberger M, Ackefors M, Berglund S et al. Improved survival after allogeneic hematopoietic stem cell transplantation in recent years. A single‐center study. Biol Blood Marrow Transplant 2011;17:1688–1697. [DOI] [PubMed] [Google Scholar]
- 33. Ringdén O, Solders M, Erkers T et al. Successful reversal of acute lung injury using placenta‐derived decidual stromal cells. J Stem Cell Res Ther 2014;4:244. [Google Scholar]
- 34. Aronsson‐Kurttila W, Baygan A, Moretti G et al. Placenta‐derived decidua stromal cells for hemorrhagic cystitis after stem cell transplantation. Acta Haematol 2018;139:106–114. [DOI] [PubMed] [Google Scholar]


