Abstract
Non‐union defects of bone are a major problem in orthopedics, especially for patients with a low healing capacity. Fixation devices and osteoconductive materials are used to provide a stable environment for osteogenesis and an osteogenic component such as autologous human bone marrow (hBM) is then used, but robust bone formation is contingent on the healing capacity of the patients. A safe and rapid procedure for improvement of the osteoanabolic properties of hBM is, therefore, sought after in the field of orthopedics, especially if it can be performed within the temporal limitations of the surgical procedure, with minimal manipulation, and at point‐of‐care. One way to achieve this goal is to stimulate canonical Wingless (cWnt) signaling in bone marrow‐resident human mesenchymal stem cells (hMSCs), the presumptive precursors of osteoblasts in bone marrow. Herein, we report that the effects of cWnt stimulation can be achieved by transient (1–2 hours) exposure of osteoprogenitors to the GSK3β‐inhibitor (2′Z,3′E)‐6‐bromoindirubin‐3′‐oxime (BIO) at a concentration of 800 nM. Very‐rapid‐exposure‐to‐BIO (VRE‐BIO) on either hMSCs or whole hBM resulted in the long‐term establishment of an osteogenic phenotype associated with accelerated alkaline phosphatase activity and enhanced transcription of the master regulator of osteogenesis, Runx2. When VRE‐BIO treated hBM was tested in a rat spinal fusion model, VRE‐BIO caused the formation of a denser, stiffer, fusion mass as compared with vehicle treated hBM. Collectively, these data indicate that the VRE‐BIO procedure may represent a rapid, safe, and point‐of‐care strategy for the osteogenic enhancement of autologous hBM for use in clinical orthopedic procedures. stem cells translational medicine 2018;7:342–353
Keywords: Bone marrow, Spine fusion, Bromo‐indirubin‐monooxime, Osteogenesis, Stem cells
Significance Statement.
Non‐union defects of bone are a major problem in orthopedics, especially for patients with a low healing capacity. Autologous human bone marrow (hBM) is often used as a source for osteogenic stem cells, but efficacy is contingent on the healing capacity of the patients. Herein, this study shows that exposure of hBM to a glycogen‐synthease‐kinase inhibitor for a very short time, initiates an irreversible cascade that increases the osteogenic capacity of resident stem cells. Importantly, the BIO‐treated hBM has the capacity to generate stronger and more compact fusion masses when tested in a rodent spine fusion model. This approach could represent a feasible and cost‐effective strategy for functional enhancement of autologous hBM for orthopedic applications.
Introduction
Non‐union defects of bone are a major problem in orthopedics, especially for those with a low capacity for healing. Of the 13 million yearly fractures that occur in the U.S., about 10% fail to repair and up to 25% of spinal fusion procedures fail 1, 2, 3. Fixation devices are frequently used to restore the proper alignment and load bearing capacity of bone immediately, and osteoconductive materials such as synthetic or processed bone fillers are used in an attempt to bridge large bone deficits and provide a solid substrate for osteoprogenitor cell migration, angiogenesis, and callus formation 4. This is usually accompanied by an osteoinductive component such as recombinant bone morphogenic proteins (BMPs) and/or autologous cell preparations 5. Live, autologous bone grafts are frequently used for this purpose, and they are highly effective, but the approach is associated with donor site morbidity and the volume of available graft material is limited 6. Autologous human bone marrow (hBM) concentrate administered with an appropriate scaffold represents a promising alternative to bone graft and can be prepared in relatively large quantities 7. However, when compared with bone graft, hBM contains a minute proportion of osteogenic and angiogenic progenitors and osteoanabolic potential can be limited 8. Success is also contingent on the healing capacity of the patients’ donated tissue which can be significantly affected by age, disease, and the use of tobacco or alcohol 9, 10, 11. A safe and rapid procedure for improvement of the osteoanabolic properties of hBM is, therefore, highly sought after in the field of orthopedics, especially if it can be performed within the temporal limitations of the surgical procedure, with minimal manipulation of the specimen, and at point‐of‐care. One way to achieve this goal is to stimulate canonical Wingless (cWnt) signaling in bone marrow‐resident human mesenchymal stem cells (hMSCs), the presumptive precursors of osteoblasts in bone marrow 12, 13, 14, 15, 16. In this pathway, extracellular cWnt ligands bind to the transmembrane receptor frizzled (Frz) and the co‐receptor lipoprotein‐related protein 5 and 6 (LRP‐5/6) on the surface of the target cell. Activation of Frz recruits the cytoplasmic bridging molecule, disheveled (Dsh), so as to inhibit the action of glycogen‐synthease‐kinase‐3‐beta (GSK3β). Inhibition of GSK3β decreases phosphorylation of β‐catenin, preventing its degradation by the ubiquitin‐mediated pathway. The stabilized β‐catenin acts on the nucleus by activating T‐cell factor (TCF) mediated transcription of target genes 17, 18, 19 that elicit a variety of effects including induction of the early stages of osteogenic differentiation 15, 20, 21, 22, 23. In vitro studies have demonstrated that cWnt signal transduction from the membrane to the nucleus occurs very rapidly in the order of minutes and hours 24, 25, 26, 27 suggesting that the initial stages of cWnt signaling could be achieved over a duration compatible with standard orthopedic procedures, thus raising the attractive notion of a point‐of‐care procedure for osteogenic enhancement of autologous bone marrow. Janeczek et al. took the first steps developing this approach by demonstrating that a rapid (24 hours) and transient exposure of human bone marrow to Wnt3A resulted in the generation of an osteogenically enhanced sub‐population of STRO‐1high/GlycophorinAneg skeletal stem cells 28. Herein, we report that the effects of cWnt stimulation can be achieved by transient exposure of subcultured hMSCs to the GSK3β‐inhibitor (2′Z,3′E)‐6‐bromoindirubin‐3′‐oxime (BIO) 29, 30 for 1–2 hours followed by withdrawal of BIO and washing. In addition, very‐rapid‐exposure‐to‐BIO (VRE‐BIO) on either hMSCs or whole unfractionated hBM resulted in the long‐term establishment of a persistent osteogenic phenotype associated with accelerated alkaline phosphatase (ALP) activity and enhanced transcription of the master regulator of osteogenesis, Runx2. When VRE‐BIO treated hBM was tested for efficacy in a rat posterolateral spinal fusion model, VRE‐BIO treatment caused the formation of a denser, more interconnected bone matrix that was more resistant to deformation as compared with vehicle‐treated hBM. Collectively, these data indicate that the VRE‐BIO procedure may represent a rapid, safe, and point‐of‐care strategy for the osteogenic enhancement of autologous hBM for use in clinical orthopedic procedures.
Materials and Methods
Detailed methods are provided in Supporting Information.
Human MSCs
Bone marrow (2–5 ml) was collected with informed consent from donors undergoing spine fusion procedures at Baylor Scott and White Hospital (Temple, TX) in accordance with an institutionally approved protocol. Human MSCs were cultured by plastic adherence from the nucleated fraction of bone marrow as described 31.
Human BM
The mononuclear fraction of hBM was prepared by Ficoll density gradient centrifugation and frozen at −180°C in 5 × 107—cell aliquots containing α‐MEM, 50% (vol/vol) FCS and 5% dimethyl‐sulfoxide, and 20 units per ml DNAse I (Sigma).
VRE‐BIO Procedure
Plastic adherent hMSCs were cultured to 50%–60% confluence (5,000–10,000 cells per cm2) in complete culture media (CCM) and hBM (1 × 107 cells) was suspended in CCM at 1 × 106 cells ml−1. One thousand‐fold stocks of BIO (Sigma, St. Louis, MO) were prepared in dimethyl‐sulfoxide (DMSO) and added to media at the desired working concentration.
Immunocytochemistry for β‐Catenin
Human MSCs were seeded at 1,000 cells per cm2 in 4 cm2 chamber slides (Nunc Lab‐Tek II, Thermo Scientific, Waltham, MA). When the hMSCs established a monolayer of about 60% confluence, the cultures were subjected to VRE‐BIO procedures. Slides were then processed and visualized as previously described 14.
Immunoblotting
Triton‐insoluble/soluble subcellular fractions were prepared using a previously described protocol 32 with modifications 14. Samples were immunoblotted as previously described 33. Chemiluminescence was detected using a Versadoc Imaging System and analyzed using QuantityOne software (BioRad, Hercules, CA).
β‐Catenin Enzyme Linked Immunosorbent Assay
One million hMSCs at a density of approximately 8,000 cells per cm2 were incubated in CCM containing BIO or vehicle for 1 hour. Cultures were washed in phosphate buffered saline (PBS) and recovered by trypsinization. After centrifugation and further washing in PBS, pellets were lysed in 200 μl PBS containing 1% (vol/vol) Nonidet P‐40 (Sigma), 5 mM EDTA, 150 mM NaCl, and protease inhibitor cocktail (Roche, Basel, Switzerland) for 30 minutes at 4°C. Lysates were subjected to centrifugation at 2,000g followed by protein quantification using a standard Bradford assay (BioRad). Concentrations were between 0.8 and 1.6 mg/ml, 100 μl of the sample was used per well of an enzyme linked immunosorbent assay (ELISA) plate (soluble beta catenin assay, R&D Systems, Minneapolis, MN).
Quantitative RT‐PCR
Total RNA was extracted from 1 × 106 hMSCs or 1 × 107 bone marrow cells using a total RNA isolation kit (High Pure, Roche). Yields were in the range of 500–800 μg per sample for hMSCs and 100–500 μg per sample for bone marrow. The 260/280 nm ratios ranged between 1.7 and 1.9. For quantitative RT‐PCR, 1 μg of total RNA was used to synthesize cDNA (Superscript III cDNA kit, Invitrogen, Carlsbad, CA). One half‐μg of cDNA was amplified in a 25 μl reaction containing SYBR‐green PCR master mix (Fast SYBR Green, Applied Biosystems Invitrogen) on a C1000 thermocycler fitted with a real‐time module (CFX96, Biorad). Expression data were calculated using the 2‐delta,deltaCT method using human GAPDH as a reference [ 34, 35]. Experimental variation was quantified by comparing the mean fold change between controls with each separate control sample, thereby defining the range of variation for a fold‐change measurement of 1. Amplimers were as follows; GAPDH for: ctctctgctcctcctgttcgac, GAPDH rev: tgagcgatgtggctcggct 36. Runx2 for: gcaaggttcaacgatctgaga, Runx2 rev: ttcccgaggtccatctactg 37. Osx for: gtgggcagctagaagggagt, Osx rev: aattagggcagtcgcagga 37. All annealing temperatures were set to 60°C.
ALP Colorimetric Assay
Human MSCs were plated in 12‐well plates at 100 cells per cm2 and cultured with CCM until reaching about 8,000 cells per cm2. VRE‐BIO was performed on the monolayers followed by washing in PBS and replacement with osteogenic base media (OBM) consisting of CCM containing 50 μg/ml ascorbic acid and 5 mM β‐glycerol phosphate. For assay of adherent cells from hBM, 1 × 107 cells were subjected to VRE‐BIO followed by washing in PBS and plating in 4 cm2 tissue culture wells in the presence of CCM. After 24 hours, the nonadherent cells were washed away and media was replaced with OBM. Media was changed every 2 days for 8 days following measurement of ALP activity as previously described 38. The rates were normalized against cell number and statistically analyzed using one‐way analysis of variance (ANOVA) and Dunnett's post‐test for multiple comparisons with control.
Osteoprotegerin ELISA
Osteoprotegerin ELISA was performed according to the manufacturer's instructions (R&D Systems) on 1‐day (hMSC monolayers) or 2‐day (hBM‐derived monolayers) conditioned media diluted at 1 in 10 with phosphate buffered saline containing 0.1% (vol/vol) Tween 20.
Quantification of Cells
Cells in monolayers were enumerated using the CyQuantGR fluorescent nucleic acid labeling system (Invitrogen) using a previously described extended processing protocol to counteract the effects of high extracellular matrix concentrations 39.
Posterolateral Lumbar Fusion Model in Rats
All procedures were performed in accordance with an approved animal use protocol from the Baylor Scott & White Animal Care and Use Committee. Ten 6‐week‐old (approximately 135 g) female athymic nude rats (Hsd:RH‐Foxnrnu) (n = 5 per group) were acquired from Harlan Laboratories (Indianapolis, IN). A bilateral intertransverse process fusion at L5‐L6 was performed on them as previously described 40, but with some modifications 41.
Manual Palpation
After 8 weeks, rats were humanely euthanized under deep anesthesia. The spines (L3 to sacral vertebrae, with ilium) were carefully dissected with associated muscle tissue and placed in cold PBS. The explanted spines were manually tested for intersegmental motion by the three blinded observers in accordance with a standardized experimental protocol 42. Due to the potential subjectivity of the manual fusion assessments, inter‐observer variation was evaluated statistically by Kappa test 43, 44, 45, 46, 47. In all cases, Kappa criteria (>0.9) were satisfied and fusion and palpation data were analyzed for significance using a Fisher exact test (summing small p‐values method). Fusion was further confirmed for each specimen. Palpation results were then compared with visual interpretation of micro‐computed tomographic scans to further confirm fusion. Complete correlation between scans and palpation results were observed.
Micro‐Computed Tomography
Spines were subjected to tomographic scanning using a Skyscan 1174 high resolution specimen imager (Bruker, Kontich, Belgium). Images were scanned at 26 W, 661 μA, 39 kV with 33 μm/pixel resolution. Axial images were generated using NRecon software (Bruker, Billerica, MA), with thresholding set to 500–10,000 Hounsfield Units. In most cases, it was impossible to distinguish the new bone from the mature transverse processes because outgrowths were completely contiguous with original bone. We therefore used previously described methods that compared total volumes of fused vertebrae 41. For histomorphometric analysis of fusion beds, specimens were scanned at 18 μm/pixel resolution with image smoothing and ring artifact compensation set to maximum. To maximize resolution, 10 images were averaged per degree for 360°. Axial images were generated with thresholding set to 300–3,200 Hounsfield Units. A region of interest consisting of one hundred axial images taken from the left fusion bed, in line with the central 1.8 mm of the L5 vertebra, was selected and subjected to a comprehensive 3D histomorphometric analysis. Values from the BIO‐treated and control groups were analyzed statistically by Student's t test and p‐values <.05 were considered statistically significant. Three‐dimensional images were rendered by CTvol software (Bruker).
Uniaxial Compression Testing
Excised spines were cleared of connective tissue by proteinase K digestion and fine dissection and allowed to dry in a desiccated environment for 15 hours at 21°C–25°C. Using a 22 mm diameter, 0.12 mm thick diamond coated rotary blade (Strauss Diamond, Palm Coast FL) fitted to a dental drill, a 4 mm‐thick axial slice was prepared that included the fusion mass at the intervertebral space between the L5 and L6 vertebrae. Slices were immobilized with the cut surface of the fusion beds exposed. Following a previous experimental procedure 48, an ADMET eXpert 7600 single column testing system equipped with a 10 lb transducer and 1 mm indentation probe was used to apply a preload of 0.1N to immobilize and maintain indenter contact with the specimen. A constant indentation rate was then applied (1 mm minute−1) and mean force/displacement measured so as to calculate the modulus (kPa) from the linear elastic region (0.05–0.2 strain).
Statistics
Parametric data were analyzed by one‐way ANOVA with Tukey's or Dunnett's post‐test where indicated. Binary (fusion) data were tested by 2‐tailed Fisher Exact test. For ANOVA and Fisher tests, a p‐value of <.05 was considered statistically significant. Testing of interobserver agreement was performed with the Cohen's Kappa test.
Results
Rapid and Transient Exposure of hMSCs to BIO Stimulates Endogenous Markers of cWnt Signaling
We aimed to develop a clinical point‐of‐care protocol for the stimulation of bone marrow osteoprogenitor activity based on rapid acceleration of cWnt signaling. During the assays, exposure conditions were limited to a maximum of 4 hours, a duration compatible with the viability of hBM aspirates 49 and also orthopedic surgical procedures. To avoid the need for fresh human BM preparations, we performed initial experiments with monolayers of hMSCs. To determine whether BIO had the capacity to stimulate cWnt signaling after short exposure times, we screened BIO stimulation conditions with concentrations ranging between 100 and 800 nM BIO and durations between 30 minutes and 4 hours. We selected this range of concentrations based on previous studies on the effects of BIO on hMSCs 16, 38, 50. After withdrawal of BIO and replacement of standard media, we subjected the hMSCs to various recovery times (1–4 hours) and measured β‐catenin and GSK3β levels in detergent‐soluble (cytosolic) and insoluble subcellular fractions. The detergent‐insoluble fraction contained nuclei, cytoskeleton, and various other membranous structures 14. We reasoned that upregulated cWnt signaling would result in nuclear accumulation of β‐catenin and depletion of cytosolic GSK3β over a relatively short time course 24, 26, 27. From these assays, we ascertained that 1 hour of BIO exposure at a concentration between 200 and 800 nM consistently upregulated levels of β‐catenin in soluble fractions while downregulating the presence of soluble GSK3β (Fig. 1A, 1B). This effect was concentration dependent and sustained for the duration of the assay (4 hours). Levels of β‐catenin in detergent insoluble fractions followed a similar pattern, but tended to diminish at higher BIO concentrations after 4 hours (Fig. 1B); the reason for these reduced levels are unclear, but potent feedback mechanisms are a likely explanation for this observation. Upregulated β‐catenin levels in hMSCs after VRE‐BIO treatment could be confirmed by immuno‐cytochemistry (Fig. 1C top left and right) in the majority of cells treated. High power imaging also demonstrated a nuclear/perinuclear distribution of β‐catenin in those cells that responded to the BIO but cells with very low β‐catenin immunoreactivity were evident in BIO treated cultures, indicating that VRE‐BIO treatment affects a subpopulation of cells within cultures of hMSCs. Upregulation of β‐catenin levels in VRE‐BIO treated hMSC cultures could also be demonstrated when cell lysates were subjected to ELISA assays but the relative increases were surprisingly modest when compared with the immunofluorescence data (Fig. 1D). This discrepancy is probably attributed to the tendency of immunocytochemistry to detect insoluble targets whereas ELISA is better suited to measuring soluble targets. Collectively, these data indicate that 1 hour of transient BIO exposure modulates the subcellular distribution and steady‐state levels of GSK3β and β‐catenin in a manner indicative of accelerated cWnt signaling.
Figure 1.
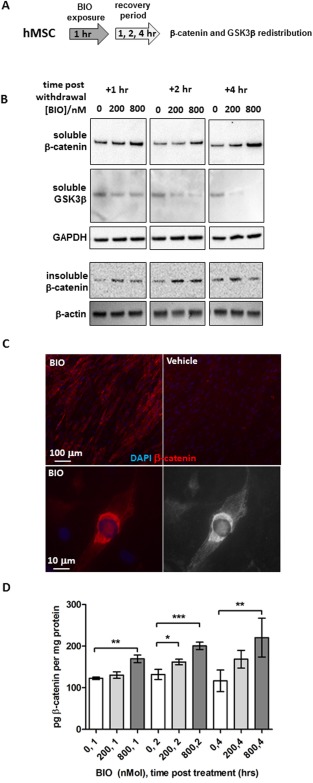
VRE‐BIO exposure on hMSCs causes downregulation of cytosolic GSK3β and upregulation of β‐catenin. (A): Experimental scheme. (B): Immunoblot assays for β‐catenin and GSK3β on soluble and insoluble fractions of BIO‐treated hMSCs at various time points during the recovery period. (C): Immunocytochemistry for β‐catenin on monolayers of hMSCs treated for 2 hours with 800 nM BIO followed a 2‐hour recovery period (top left) as compared with a vehicle treated control (top right). High power image of BIO‐treated hMSC with peri‐nuclear accumulation of β‐catenin (bottom left) with traces of immunoreactive material in the nucleus (bottom right). Red color indicates β‐catenin immunoreactivity and blue indicated nuclear staining with DAPI. (D): Enzyme linked immunosorbent assay measurement of β‐catenin levels in hMSC extracts treated with BIO. Statistics are ANOVA with Dunnett's post‐test (*, p < .05; **, p < .01; ***, p < .005). Abbreviations: BIO, GSK3β‐inhibitor (2′Z,3′E)‐6‐bromoindirubin‐3′‐oxime; DAPI, 4′,6‐diamidino‐2‐phenylindole; GSK3β, glycogen‐synthease‐kinase‐3‐beta; hMSCs, human mesenchymal stem cells.
Rapid and Transient Exposure of hMSCs to BIO Stimulates Early Markers of Osteogenic Differentiation
We next questioned whether VRE‐BIO exposure could stimulate osteogenic differentiation in hMSCs. For this purpose, hMSCs were transiently exposed to BIO for 1 hour and exposed to osteogenic differentiation media for up to 8 days (Fig. 2A). Following treatment, transcription of the master regulator of osteogenic differentiation, runt‐related transcription factor 2 (Runx2) was significantly upregulated after 800 nM BIO exposure followed by 24 hours of recovery (Fig. 2B). In contrast, transcription of osterix, another key regulator of osteogenic differentiation was not significantly affected by VRE‐BIO. After a 4‐day recovery period in osteogenic conditions, secretion of the osteogenic protein ligand osteoprotegerin (OPG) was measured by ELISA, and we observed that pre‐exposure to 200–800 nM BIO for 1 hour resulted in enhanced OPG output (Fig. 2D). A similar observation was made for the osteogenic marker ALP after 8 days when enzyme activity was measured on intact monolayers by colorimetric assay (Fig. 2E).
Figure 2.
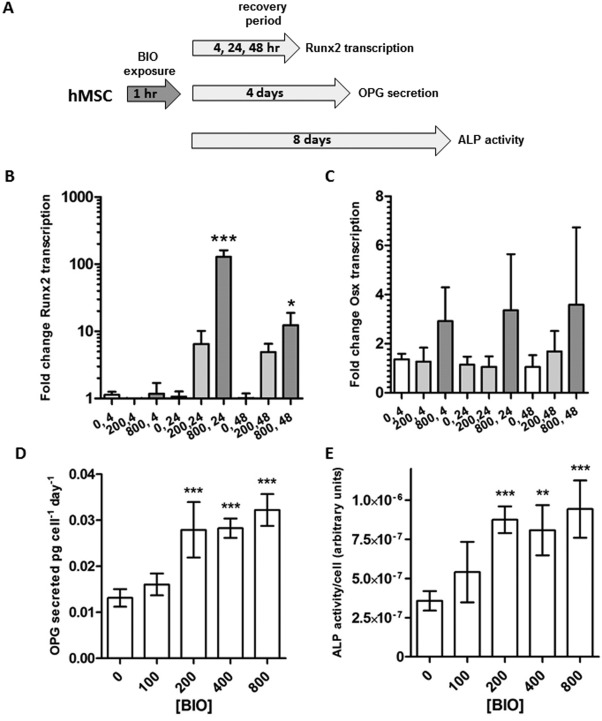
VRE‐BIO on hMSCs upregulates ALP activity, OPG secretion, and Runx2 transcription. (A): Experimental scheme. (B): Runx2 transcription in monolayers of hMSCs subjected to VRE‐BIO treatment (0, 200, or 800 nM BIO) followed by 4, 24, and 48 hours in the presence of osteogenic stimulus. Fold‐change transcription is normalized to vehicle only treatment after a 4‐hour recovery period. (C): Osx transcription in monolayers of hMSCs subjected to VRE‐BIO treatment (conditions as B). (D): OPG secretion by monolayers of hMSCs subjected to VRE‐BIO treatment followed by 4 days in the presence of osteogenic stimulus. Activity is normalized to cell number. (E): ALP activity on monolayers of hMSCs subjected to VRE‐BIO treatment followed by 8 days in the presence of osteogenic stimulus. Activity is normalized to cell number and media conditioning time. For all Panels, statistics are ANOVA with Dunnett's post‐test (*, p < .05; **, p < .01; ***, p < .005) with comparison against 0 nM BIO. Abbreviations: ALP, alkaline phosphatase; BIO, GSK3β‐inhibitor (2′Z,3′E)‐6‐bromoindirubin‐3′‐oxime; hMSCs, human mesenchymal stem cells; OPG, osteoprotegerin.
Together, these data demonstrate that VRE‐BIO treatment has the potential to enhance osteogenic responses by hMSCs when also subjected to standard osteogenic culture conditions.
Rapid and Transient Exposure of Whole BM to BIO Stimulates Early Markers of Osteogenic Differentiation
We then tested the effects of VRE‐BIO treatment on whole BM in vitro. Aliquots of 1 × 107 hBM cells were incubated for 1 hour in the presence of 200 nM or 800 nM BIO and subjected to a recovery period of up to 8 days in osteogenic media (Fig. 3A). After 24 and 48 hours of recovery under suspension conditions, qRT‐PCR demonstrated that Runx2 transcription was upregulated as compared with untreated controls, but to a lesser degree than observed with monolayer hMSCs (Fig. 3B). In some experiments, 1 × 107 cells were plated in a 4 cm2 tissue culture well after VRE‐BIO treatment, and adherent cells were allowed to attach under standard culture conditions for 24 hours. Cultures were then washed to remove suspension cells, and allowed to expand and differentiate in the presence of osteogenic media for 8 days (Fig. 3A). Compared with untreated controls, VRE‐BIO at a concentration of 800 nM resulted in enhanced OPG secretion and ALP activity by the plastic adherent, hMSC‐containing, component of the bone marrow (Fig. 3C, 3D). VRE‐BIO did not appear to affect cell yields, with monolayers reaching about 60,000 cells per well in each case. Assuming a fibroblastic colony forming unit frequency of about 3 × 10−5 51, this suggests an average doubling time of approximately 25 hours. Trials (n = 30) on hBM confirmed that 1 hour of exposure to BIO had no detrimental effect on the viability of hMSC‐containing colony forming units (CFU).
Figure 3.
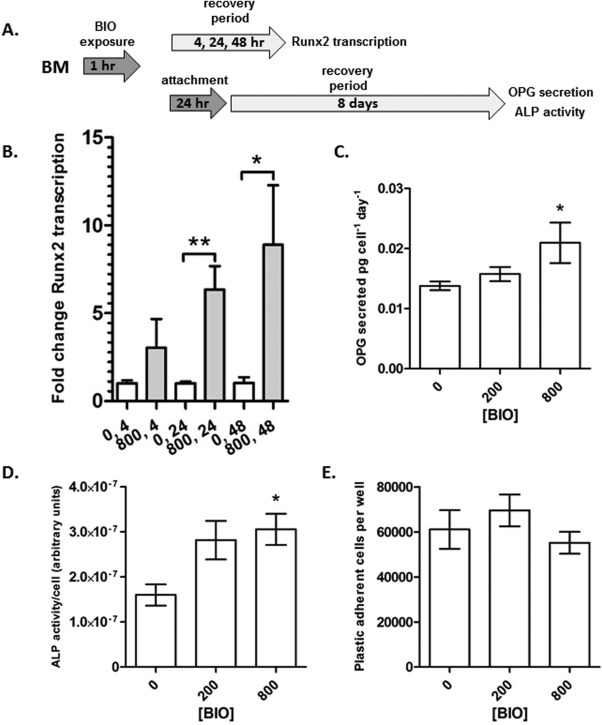
VRE‐BIO exposure on human BM upregulates overall Runx2 transcription, ALP activity and OPG secretion in resident plastic adherent human mesenchymal stem cells (hMSCs). (A): Experimental scheme. (B): Runx2 transcription in suspended BM subjected to VRE‐BIO treatment followed by 4, 24, and 48 hours in the presence of osteogenic stimulus. Fold‐change transcription is normalized to vehicle only treatment after a 4‐hour recovery period. (C): OPG secretion by monolayers of hMSCs subjected to VRE‐BIO treatment followed by a 24‐hours attachment period, then 4 days in the presence of osteogenic stimulus. Activity is normalized to cell number and media conditioning time. (D): ALP activity on monolayers of hMSCs subjected to VRE‐BIO treatment followed by a 24‐hours attachment period, then 8 days in the presence of osteogenic stimulus. Activity is normalized to cell number. (E): The number of plastic adherent cells present after 24‐hours attachment period and 8 days of osteogenic stimulus. For (B), statistics are Student's t test on arcsine transformed data (*, p < .05; **, p < .01). For (C, D, E), statistics are ANOVA with Dunnett's post‐test (*, p < .05) with comparison against 0 nM BIO. Abbreviations: ALP, alkaline phosphatase; BIO, GSK3β‐inhibitor (2′Z,3′E)‐6‐bromoindirubin‐3′‐oxime; BM, bone marrow; OPG, osteoprotegerin.
These results demonstrate that 800 nM VRE‐BIO treatment for 1 hour on unfractionated hBM results in upregulated transcription of the osteogenic master‐regulator Runx2, and sustained osteogenic enhancement of the plastic adherent, hMSC‐containing subpopulation.
VRE‐BIO‐Treated hBM Generates More Compact De Novo Bone with Greater Trabecular Connectivity and Increased Stiffness When Tested in an Experimental Rodent Posterolateral Lumbar Fusion Model
To examine whether VRE‐BIO treatment improved the osteogenic capacity of hBM in vivo, we used a spinal fusion model in immune compromised rats. For this purpose, hBM was subjected to VRE‐BIO for 1 hour with 800 nM BIO because these conditions resulted in the most robust upregulation of osteogenic parameters tested. The hBM was then combined with a radiolucent scaffold we developed consisting of porcine gelatin foam with hMSC‐derived extracellular matrix and positioned over decorticated the posterolateral processes of the L4‐L6 vertebrae followed by an 8 week (period of healing 41, 52) (Fig. 4A). In a previous study, we demonstrated that the ECM scaffold exhibits no inherent healing capacity without supplementation with an appropriate cell source 41. When compared with untreated hBM, VRE‐BIO treated hBM did not affect fusion frequency (5/5 fused in each case) or the volume of the fusion masses (Fig. 4B). When fusion masses were subjected to high resolution scanning, classical histomorphometric parameters such as trabecular spacing, thickness, and number were also unaffected (data not shown). However, we did observe that de novo bone generated by VRE‐BIO treated hBM had a significantly lower surface: volume ratio (Fig. 4C), fractal dimension (Fig. 4D), and trabecular pattern factor (Fig. 4E). Reduced values for these parameters tend to describe a denser, less diffuse bone structure with greater interconnectivity 53, 54, 55, 56. Qualitatively, this appeared to be the case upon inspection of 3D‐reconstructed high‐resolution scans of fusion masses (Fig. 4G). Histological examination of the fusion masses confirmed the μCT data, demonstrating a greater level of de novo bone with greater connectivity in BIO‐treated fusion masses as compared with controls (Fig. 4H). The histological staining also indicated that the de novo bone in BIO‐treated masses consisted of a greater degree of blue‐stained mature bone (Fig. 4H, below) rather than the predominance of immature osteoid present in control specimens (Fig. 4H, above). BIO‐treated fusion masses also exhibited a greater degree of bone marrow cellularity as compared with controls. When the fusion masses were subjected to biomechanical indentation testing, the BIO‐treated fusion masses withstood significantly higher compressive force than the controls, further supporting the observation that the BIO‐treated fusion masses were mechanically stronger and probably denser than control samples (Fig. 4F).
Figure 4.
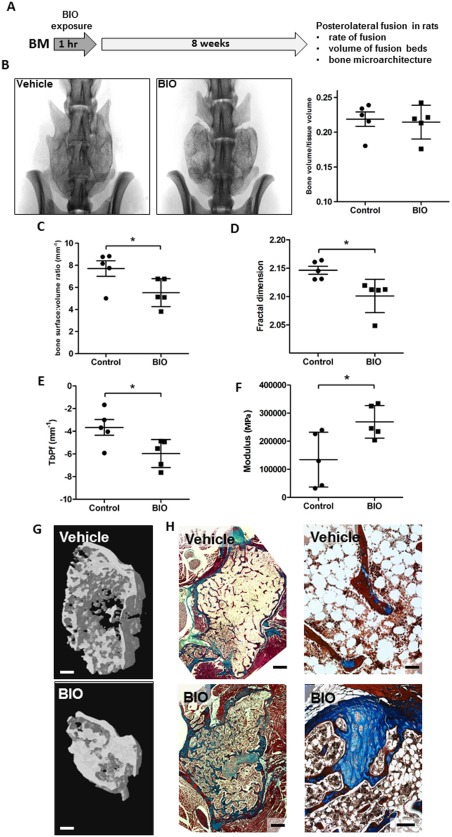
VRE‐BIO exposure on hBM improves histomorphometric parameters of de novo bone when cells are administered in a preclinical model of posterolateral lumbar spine fusion. (A): Experimental scheme. (B): Scans of representative specimens demonstrating the presence of fusion masses between L4, 5 and 6 in both control and BIO‐treated groups (left) volumetric measurements of fusion beds demonstrate that bone formed in each case is equivalent between control and BIO‐treated groups (right). (C): Bone surface to volume ratio in fusion masses is lower in BIO‐treated group suggesting a more compact conformation. (D): Fractal dimension calculations on fusion masses demonstrate a denser conformation in BIO‐treated specimens. (E): Trabecular pattern factor measurements demonstrate bone in BIO‐treated fusion masses exhibit greater connectivity than controls. (F): Compression testing demonstrates that BIO‐treated fusion masses are stiffer than control masses. (G): Three dimensional renderings of representative regions of interest indicate a denser, more interconnected bone conformation in BIO‐treated fusion masses as compared with controls, but equivalent overall bone volume. Bar = 1 mm. (H): Micrographs of Masson's trichrome stained axial sections of fusion masses at low (left) and high (right) power. BIO‐treated fusion masses appear to harbor a greater level of mature bone and increased cellularity. For low power, bar = 0.75 mm, for high power, bar = 100 μm and 125 μm for vehicle and BIO, respectively. Statistics for (B–F) calculated with Student's t test (*, p < .05). Abbreviations: BIO, GSK3β‐inhibitor (2′Z,3′E)‐6‐bromoindirubin‐3′‐oxime; BM, bone marrow; Tbpf, trabecular bone pattern factor.
Discussion
GSK3β antagonists are thought to modulate cWnt signaling by preventing the formation of a functional β‐catenin destruction complex. The increased level of free intracellular β‐catenin activates the osteogenic differentiation of some types of adult stem/progenitor cells 23. In a previous study, we observed that long‐term incubation of confluent hMSCs for up to 8 days in the presence of BIO resulted in upregulation endogenous markers of cWnt signaling, thereby enhancing markers of osteogenic activity in vitro in a manner very similar to the results reported herein 38. Long‐term BIO‐treated hMSCs were viable after 8 days, at the time of harvest, but when implanted into calvarial lesions generated in nude mice, the cells did not exhibit osteogenic efficacy, nor did they engraft. These observations were deemed BIO‐dependent because hMSC‐treatment conditions that did not involve BIO exhibited osteogenic efficacy and long‐term engraftment. It is likely that a combination of sustained cWnt signaling and the potential cross‐reactivity of BIO with several cyclin dependent kinases 30 could have contributed to the failure of the hMSCs. Because the transduction of cWnt signals from the receptor to the nucleus is reported to be in the order of minutes 24, we postulated that a cellular response could be detected after exposure to a cWnt agonist for hours rather than days. This is attractive for cell‐based therapies because newly recovered autologous cell preparations could be osteogenically enhanced at the point of care for durations compatible with surgical procedures. This dismisses the need for a local clinical good manufacturing practice (cGMP) culture facility and the need for a dedicated visit by the patient to provide hBM. If the activating agent is washed from the cell preparations prior to administration, it also minimizes exposure to potent bioactive agents. Initial studies performed by Janeczek et al. 28 demonstrated the feasibility of this approach by exposing hBM to Wnt3a ligand for 24 hours prior to assays of clonogenicity and osteogenesis. Wnt stimulation for 24 hours resulted in elevated presence of cWnt signaling markers in the STRO‐1 positive component of hBM that harbors the hMSCs thought to contribute to osteogenesis. Wnt3a exposure also upregulated the frequency of STRO‐1 cells, increased the formation of osteogenic single cell derived colonies, and enhanced in vitro osteogenic potential. The authors attributed their results to the Wnt‐mediated expansion of a STRO‐1 positive/glycophorin A negative osteogenic progenitor sub‐population in hBM. Interestingly, and in agreement with our previous observations with BIO and hMSCs, long‐term (14 days) exposure of hBM cultures to Wnt3a inhibited osteogenesis.
Herein, we used BIO to enhance cWnt signaling through inhibition of GSK3β rather than by directly agonizing the Frz and LRP receptors with Wnt ligands. The rationale for this approach was based on the superior availability of small molecule GSK3β inhibitors and the relative ease of clinical translation as compared with recombinant Wnt ligands. In initial experiments, we exposed semiconfluent, culture‐expanded, plastic adherent hMSCs to various concentrations of BIO for short durations ranging from 1 to 4 hours. It is generally accepted that plastic adherent hMSCs contain STRO‐1 positive osteogenic progenitors, and the use of hMSCs minimized the need for extensive volumes of donated hBM. Under these conditions, it was found that a single hour of exposure to 200–800 nM BIO caused a dose‐dependent upregulation of cytosolic and insoluble β‐catenin and reduced the detection of cytosolic GSK3β that was detectable 4 hours after withdrawal of BIO. The observed accumulation and distribution of β‐catenin and GSK3β is typical of conditions where cWnt signaling is amplified in hMSCs 38 and in agreement with the rapid kinetics predicted by simulations 25 and experimental studies on cultured cells 24, 26, 27. As predicted by previously published observations 57, the rapid cytosolic and nuclear accumulation of β‐catenin was sufficient to upregulate transcription of the master osteogenic regulator Runx2 58 that was maximally detected 24 hours after transient incubation with 800 nM BIO with a slight decline after 48 hours. Runx2 upregulation was also observed at the lower dose of 200 nM BIO, but was not deemed statistically significant based on the criteria set for the study. Likewise, the osteogenic transcription factor Osterix 59 appeared to be transcriptionally upregulated with 800 nM BIO, but inter‐assay variation was high. We have previously reported that osteogenic hMSCs upregulate secretion of the osteogenic ligand OPG after about 4 days of osteogenic stimulus followed my maximal activity of ALP 2–6 days thereafter 39. When pre‐established cultures of hMSCs were transiently treated with 200–800 nM BIO for 1 hour, we observed that OPG and ALP was upregulated at day 4 and 8 respectively when compared with untreated controls. We also observed that hMSCs recovered from hBM by plastic adherence exhibited increased OPG secretion and ALP activity if the hBM was exposed to 800 nM BIO for 8 hours. Collectively, these data indicate that transient BIO exposure activates elements of the cWnt pathway that initiates the early stages of osteogenic differentiation. The induced osteogenic phenotype persists for days after cessation of the original BIO stimulus, a phenomenon that could be explained by several previously reported mechanisms. One possible cause for the longevity of transient BIO activation could arise from reported cooperativity between the BMP/SMAD and cWnt axes and the establishment of feed‐forward signaling loop between the two pathways resulting in Runx2 synthesis 12, 60. Once initiated, Runx2 signaling can persist for long periods or even become irreversible, causing accumulation of epigenetic modifications that are retained post‐mitotically [47–49]. This signaling is further strengthened through the action of BIO on GSK3β, which has the capacity to attenuate Runx2 by direct phosphorylation 46. The current literature and the work herein therefore support a model where transient cWnt activation “locks in” the osteogenic phenotype of hMSCs and their daughter cells through a mechanism involving a BMP/SMAD/cWnt feed‐forward loop, increased stability of existing Runx2, and epigenetic stimulation of Runx2 expression. Of interest was the observation that while cWnt upregulation could be observed in the presence of standard complete media, upregulation of later factors such as OPG and ALP, required the presence of OBM. The specific reason for this requirement is unclear, but it appears that the persistence of the sustained osteogenic phenotype is dependent the presence of basal osteogenic factors such as a source of ascorbate and phosphate commonly found in healing bone tissue.
To test the potential efficacy of the VRE‐BIO approach in vivo, hBM from a single human donor was incubated in media containing 800 nM BIO for 1 hour during preparation of a posterolateral fusion bed in nude rats. The 800‐nM concentration was chosen because it was well tolerated by the cells and resulted in robust effects on the osteogenic parameters tested. The hBM was washed in PBS followed by mixing with a previously described scaffold generated from the gelatin foam coated with extracellular matrix derived from cultured hMSCs 41. Controls received no BIO but were treated in exactly the same way. After 8 weeks of healing, we observed that fusion frequency was equally high in both groups when assayed by manual palpation, the standard approach of subjectively moving the vertebral joint to assess motion in all directions. When carefully performed by trained, blinded observers, and statistically analyzed for interobserver variation, this method is currently regarded as the most reliable method for assessment of fusion when combined with an appropriate radiographic technique such as computational tomography 42, 61. Computational and biomechanical 62 approaches have been proposed to assess fusion 63, but while these protocols promise to reduce subjectivity in the evaluation of fusion, they require specialized equipment and expertise and their advantages over manual palpation have not been well‐characterized to date. While the rate of fusion and also the volume of bone generated was comparable, the fusion beds in the VRE‐BIO subjects exhibited key differences when compared with untreated controls. We found that while the control fusion beds exhibited a more hollow, trabecular conformation bounded by a thin outer layer of bone, the VRE‐BIO bone was more compact. These differences could be detected qualitatively by 3D renderings of μCT scans and histology, but also by measurable parameters of compactness, trabecularity, and interconnectivity such as surface:volume ratio, fractal dimension, and trabecular pattern factor. When the fusion beds were excised and subjected to indentation testing, the VRE‐BIO specimens were harder to deform, exhibiting increased stiffness. According to the indentation analysis, the stiffness of bone in the fusion mass was increased by approximately twofold, from 134.5 MPa to 270 MPa. These values are relatively low as compared with studies on intact vertebrae from healthy and osteoporotic rats (200–900 MPa) 64, but the de novo bone in the fusion beds had not undergone the successive rounds of remodeling that strengthens newly formed bone. Nevertheless, a twofold increase in biomechanical stiffness is likely to contribute to the durability of the fusion 65 and reduce the probability of hardware loosening. This would be especially advantageous in cases where bone healing could be compromised by age, disease and/or tobacco use 66, 67.
The enhanced in vitro osteogenic activity of hMSCs grown out of BIO‐treated hBM samples suggests that BIO improves osteogenic capabilities of hBM by acting in part on hMSCs, but this does not necessarily exclude the possibility that BIO acts on other cell types within hBM. For example, while the frequency of osteoclasts was not measured in this study, the predicted increase of BMP, cWnt ligand, and OPG caused by BIO could theoretically result in their reduced numbers and an overall reduction in bone resorption 68. Closely related to osteoclasts, macrophages can constitute between 2.5% and 25% of the nucleated component of hBM depending on donor, physiological status and macrophage phenotype 69, 70, 71. Macrophages have the capacity to polarize into the inflammatory M1 subclass 71 or a more immunomodulatory M2 70 subclass with regenerative and remodeling capacity 72. Interestingly, cWnt signaling has been recently shown to drive macrophages to the M2 subtype 73, and bioactive factors secreted by M2 macrophages have osteogenic effects on osteoprogenitors 74, 75. In a recent study, BIO has also been shown to stimulate the proliferation of mandibular chondrocytes in vivo 76.
While hMSCs do indeed represent a minute subpopulation of the total cellular component of hBM (< 0.1%), the resident hMSCs in the administered hBM could proliferate to physiologically significant numbers during the experiment, and extremely rapid proliferation of mesenchymal cells have been observed in the early stages of bone healing 77. In this study, we observed extremely rapid outgrowth of hMSCs from BIO‐treated hBM with an average doubling time of about 25 hours. With an average colony forming unit frequency of 1 per 3 × 105 bone marrow cells, and assuming 50% viability, this could result in 7 × 108 cells per fusion mass over 4 weeks based on the dose of hBM cells administered. Given the average volume of a fibroblast (2,000 μm3), this could generate a condensed cell mass of 1,000 mm3 which could account for the fusion masses observed.
The data presented herein suggest that VRE‐BIO methodology is likely to be useful for the improvement of recovered hBM used at point of care for orthopedic procedures, but it may also be of use for stimulation of osteogenic potential in expanded hMSCs too. In vivo experiments are certainly warranted to test this approach, but strategies that use cultured hMSCs are subject to several future challenges before implementation in the clinic. Significant milestones include optimization of protocols for careful characterization, addressing FDA concerns related to manipulated cell sources as well as the costs and logistical challenges associated with hMSC expansion in the cGMP setting. The direct treatment of whole, uncultured hBM, therefore, represents a more rapid path to clinical translation.
Conclusion
The data presented here suggest that transient exposure of hBM to 800 nM GSK3β inhibitor, BIO for 1 hour, has a stimulatory effect on the osteoprogenitor cells therein. When BIO‐treated hBM is used in a rodent model of spine fusion, the resultant fusion mass is more compact and stiffer than controls that received untreated hBM. BIO is a member of the tyrian purple indirubin family of molecules which have a long record of safety in humans 78, 79, 80 and have a high oral LD50 in rats, canines and mice 50, 81. The exposure conditions for the VRE‐BIO method are temporally compatible with the majority of surgical procedures and could be performed readily at the point of care with minimal additional training. We therefore propose that the VRE‐BIO method could represent a feasible and cost‐effective strategy for functional enhancement of autologous hBM for orthopedic applications.
Author Contributions
B.C.: Collection and/or assembly of data, data analysis and interpretation, final approval of manuscript. S.Z.: Collection and/or assembly of data, data analysis and interpretation, administrative support, final approval of manuscript. U.K.: Conception and design, collection and/or assembly of data, data analysis and interpretation, final approval of manuscript. C.C.: Conception and design, provision of study material or patients, financial support final approval of manuscript. L.C.: Collection and/or assembly of data, administrative support, final approval of manuscript. A.G.: Conception and design, collection and/or assembly of data, data analysis and interpretation, financial support, final approval of manuscript. C.G.: Conception and design, collection and/or assembly of data, data analysis and interpretation, financial support, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
C.G. declared research funding with NIH, CPRIT, NSF, TAMU, and ownership interest in Theodent holdings. The other authors indicated no potential conflicts of interest.
Supporting information
Supporting Information
Acknowledgments
We acknowledge Kunal Shah for his assistance in designing the indentation testing probe. This work was supported by a research grant from the North American Spine Society (C.A.G.), The Scott & White Research Grants Program (C.A.G., C.D.C.), The Texas Engineering Experiment Station Strategic Initiative (C.A.G., A.K.G.), an R01 AR066033 from NIAMS (C.A.G.) and an R03 EB023454 from NIBIB (A.K.G.).
References
- 1. Rosemont IL. United States Bone and Joint Decade: The Burden of Musculoskeletal Diseases and Musculoskeletal Injuries: American Academy of Orthopedic Surgeons. Rosemont, IL: American Academy of Orthopaedic Surgeons, 2008.
- 2. Marsh D. Concepts of fracture union, delayed union, and nonunion. Clin Orthop Relat Res 1998;355S:S22–S30. [DOI] [PubMed] [Google Scholar]
- 3. Boden SD. Biology of lumbar spine fusion and use of bone graft substitutes: Present, future, and next generation. Tissue Eng 2000;6:383–399. [DOI] [PubMed] [Google Scholar]
- 4. Kao ST, Scott DD. A review of bone substitutes. Oral Maxillofac Surg Clin North Am 2007;19:513–521. [DOI] [PubMed] [Google Scholar]
- 5. Burkus JK, Heim SE, Gornet MF. Is INFUSE bone graft superior to autograft bone? An integrated analysis of clinical trials using the LT‐CAGE lumbar tapered fusion device. J Spinal Disord Tech 2003;16:113–122. [DOI] [PubMed] [Google Scholar]
- 6. de Boer HH. The history of bone grafts. Clin Orthop Relat Res 1988;226:292–298. [PubMed] [Google Scholar]
- 7. Hernigou P, Poignard A, Beaujean F et al. Percutaneous autologous bone‐marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am 2005;87:1430–1437. [DOI] [PubMed] [Google Scholar]
- 8. Cuomo AV, Virk M, Petrigliano F et al. Mesenchymal stem cell concentration and bone repair: Potential pitfalls from bench to bedside. J Bone Joint Surg Am 2009;91:1073–1083. [DOI] [PubMed] [Google Scholar]
- 9. Chakkalakal DA. Alcohol‐induced bone loss and deficient bone repair. Alcohol Clin Exp Res 2005;29:2077–2090. [DOI] [PubMed] [Google Scholar]
- 10. Scolaro JA, Schenker ML, Yannascoli S et al. Cigarette smoking increases complications following fracture: A systematic review. J Bone Joint Surg Am 2014;96:674–681. [DOI] [PubMed] [Google Scholar]
- 11. Oei L, Rivadeneira F, Zillikens MC et al. Diabetes, diabetic complications, and fracture risk. Curr Osteoporos Rep 2015;13:106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rawadi G, Vayssiere B, Dunn F et al. BMP‐2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J Bone Miner Res 2003;18:1842–1853. [DOI] [PubMed] [Google Scholar]
- 13. Bain G, Muller T, Wang X et al. Activated beta‐catenin induces osteoblast differentiation of C3H10T1/2 cells and participates in BMP2 mediated signal transduction. Biochem Biophys Res Commun 2003;301:84–91. [DOI] [PubMed] [Google Scholar]
- 14. Gregory CA, Perry AS, Reyes E et al. Dkk‐1‐derived synthetic peptides and lithium chloride for the control and recovery of adult stem cells from bone marrow. J Biol Chem 2005;280:2309–2323. [DOI] [PubMed] [Google Scholar]
- 15. Gregory CA, Gunn WG, Reyes E et al. How Wnt signaling affects bone repair by mesenchymal stem cells from the bone marrow. Ann N Y Acad Sci 2005;1049:97–106. [DOI] [PubMed] [Google Scholar]
- 16. Gunn WG, Conley A, Deininger L et al. A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and interleukin‐6: A potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells 2006;24:986–991. [DOI] [PubMed] [Google Scholar]
- 17. Kikuchi A, Yamamoto H, Sato A. Selective activation mechanisms of Wnt signaling pathways. Trends Cell Biol 2009;19:119–129. [DOI] [PubMed] [Google Scholar]
- 18. Rao TP, Kühl M. An updated overview on Wnt signaling pathways: A prelude for more. Circ Res 2010;106:1798–1806. [DOI] [PubMed] [Google Scholar]
- 19. Katoh M. Network of WNT and other regulatory signaling cascades in pluripotent stem cells and cancer stem cells. Curr Pharm Biotechnol 2011;12:160–170. [DOI] [PubMed] [Google Scholar]
- 20. Agholme F, Aspenberg P. Wnt signaling and orthopedics, an overview. Acta Orthop 2011;82:125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rudnicki MA, Williams BO. Wnt signaling in bone and muscle. Bone 2015;80:60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gregory CA, Green A, Lee N et al. The promise of canonical Wnt signaling modulators in enhancing bone repair. Drug News Perspect 2006;19:445–452. [DOI] [PubMed] [Google Scholar]
- 23. Krause U, Gregory CA. Potential of modulating Wnt signaling pathway toward the development of bone anabolic agent. Curr Mol Pharmacol 2011;5:164–173. [DOI] [PubMed] [Google Scholar]
- 24. Hannoush RN, Astier Y. Kinetics of Wnt‐driven beta‐catenin stabilization revealed by quantitative and temporal imaging. PLoS One 2008;3:e3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee E, Salic A, Krüger R. The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol 2003;1:E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hernández AR, Klein AM, Kirschner MW. Kinetic responses of β‐catenin specify the sites of Wnt control. Science 2012;338:1337–1340. [DOI] [PubMed] [Google Scholar]
- 27. Kafri P, Hasenson SE, Kanter I et al. Quantifying β‐catenin subcellular dynamics and cyclin D1 mRNA transcription during Wnt signaling in single living cells. Elife 2016;5:pii:e16748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Janeczek AA, Tare RS, Scarpa E et al. Transient canonical Wnt stimulation enriches human bone marrow mononuclear cell isolates for osteoprogenitors. Stem Cells 2016;34:418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Damiens E, Baratte B, Marie D et al. Anti‐mitotic properties of indirubin‐3'‐monoxime, a CDK/GSK‐3 inhibitor: Induction of endoreplication following prophase arrest. Oncogene 2001;20:3786–3797. [DOI] [PubMed] [Google Scholar]
- 30. Meijer L, Skaltsounis AL, Magiatis P et al. GSK‐3‐selective inhibitors derived from Tyrian purple indirubins. Chem Biol 2003;10:1255–1266. [DOI] [PubMed] [Google Scholar]
- 31. Gregory CA, Prockop DJ. Fundamentals of culture and characterization of mesenchymal stem/progenitor cells from bone marrow stroma In: Freshney RIS, Auerbach GN, JM., eds. Culture of Human Stem Cells. Hoboken, NJ: Wiley‐Liss, 2007:208. [Google Scholar]
- 32. Ko K, Arora P, Lee W et al. Biochemical and functional characterization of intercellular adhesion and gap junctions in fibroblasts. Am J Physiol Cell Physiol 2000;279:C147–C157. [DOI] [PubMed] [Google Scholar]
- 33. Gregory CA, Singh H, Perry AS et al. The Wnt signaling inhibitor dickkopf‐1 is required for reentry into the cell cycle of human adult stem cells from bone marrow. J Biol Chem 2003;278:28067–28078. [DOI] [PubMed] [Google Scholar]
- 34. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) Method. Methods 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 35. Pfaffl MW. A new mathematical model for relative quantification in real‐time RT‐PCR. Nucleic Acids Res 2001;29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carraro G, Albertin G, Forneris M et al. Similar sequence‐free amplification of human glyceraldehyde‐3‐phosphate dehydrogenase for real time RT‐PCR applications. Mol Cell Probes 2005;19:181–186. [DOI] [PubMed] [Google Scholar]
- 37. Schaap‐Oziemlak AM, Raymakers RA, Bergevoet SM et al. MicroRNA hsa‐miR‐135b regulates mineralization in osteogenic differentiation of human unrestricted somatic stem cells. Stem Cells Dev 2010;19:877–885. [DOI] [PubMed] [Google Scholar]
- 38. Krause U, Harris S, Green A et al. Pharmaceutical modulation of canonical Wnt signaling in multipotent stromal cells for improved osteoinductive therapy. Proc Natl Acad Sci USA 2010;107:4147–4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Krause U, Seckinger A, Gregory CA. Assays of osteogenic differentiation by cultured human mesenchymal stem cells. Methods Mol Biol 2011;698:215–230. [DOI] [PubMed] [Google Scholar]
- 40. Grauer JN, Bomback DA, Lugo R et al. Posterolateral lumbar fusions in athymic rats: Characterization of a model. Spine J 2004;4:281–286. [DOI] [PubMed] [Google Scholar]
- 41. Clough BH, McNeill EP, Palmer D et al. An allograft generated from adult stem cells and their secreted products efficiently fuses vertebrae in immunocompromised athymic rats and inhibits local immune responses. Spine J 2017;17:418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Boden SD, Schimandle JH, Hutton WC. An experimental lumbar intertransverse process spinal fusion model. Radiographic, histologic, and biomechanical healing characteristics. Spine (Phila Pa 1976) 1995;20:412–420. [DOI] [PubMed] [Google Scholar]
- 43. Miyazaki M, Sugiyama O, Tow B et al. The effects of lentiviral gene therapy with bone morphogenetic protein‐2‐producing bone marrow cells on spinal fusion in rats. J Spinal Disord Tech 2008;21:372–379. [DOI] [PubMed] [Google Scholar]
- 44. Huang RC, Khan SN, Sandhu HS et al. Alendronate inhibits spine fusion in a rat model. Spine (Phila Pa 1976) 2005;30:2516–2522. [DOI] [PubMed] [Google Scholar]
- 45. Qvistgaard E, Rasmussen J, Lætgaard J et al. Intra‐observer and inter‐observer agreement of the manual examination of the lumbar spine in chronic low‐back pain. Eur Spine J 2007;16:277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–174. [PubMed] [Google Scholar]
- 47. Landis JR, Koch GG. An application of hierarchical kappa‐type statistics in the assessment of majority agreement among multiple observers. Biometrics 1977;33:363–374. [PubMed] [Google Scholar]
- 48. Cross LM, Shah K, Palani S et al. Gradient Nanocomposite Hydrogels for Interface Tissue Engineering. Nanomedicine: Nanotechnology, Biology and Medicine. 2017. doi:10.1016/j.nano.2017.02.022. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Badrinath R, Bohl DD, Hustedt JW et al. Only prolonged time from abstraction found to affect viable nucleated cell concentrations in vertebral body bone marrow aspirate. Spine J 2014;14:990–995. [DOI] [PubMed] [Google Scholar]
- 50. Gunn WG, Krause U, Lee N et al. Pharmaceutical inhibition of glycogen synthetase kinase‐3beta reduces multiple myeloma‐induced bone disease in a novel murine plasmacytoma xenograft model. Blood 2011;117:1641–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Le Nail LR, Stanovici J, Fournier J et al. Percutaneous grafting with bone marrow autologous concentrate for open tibia fractures: Analysis of forty three cases and literature review. Int Orthop 2014;38:1845–1853. [DOI] [PubMed] [Google Scholar]
- 52. Clough BH, McCarley MR, Krause U et al. Bone regeneration with osteogenically enhanced mesenchymal stem cells and their extracellular matrix proteins. J Bone Miner Res 2015;30:83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dalle Carbonare L, Valenti MT, Bertoldo F et al. Bone microarchitecture evaluated by histomorphometry. Micron 2005;36:609–616. [DOI] [PubMed] [Google Scholar]
- 54. Hahn M, Vogel M, Pompesius‐Kempa M et al. Trabecular bone pattern factor–a new parameter for simple quantification of bone microarchitecture. Bone 1992;13:327–330. [DOI] [PubMed] [Google Scholar]
- 55. Chappard D, Stancu IC. Porosity imaged by a vector projection algorithm correlates with fractal dimension measured on 3D models obtained by microCT. J Microsc 2015;258:24–30. [DOI] [PubMed] [Google Scholar]
- 56. Pothuaud L, Benhamou CL, Porion P et al. Fractal dimension of trabecular bone projection texture is related to three‐dimensional microarchitecture. J Bone Miner Res 2010;15:691–699. [DOI] [PubMed] [Google Scholar]
- 57. Gaur T, Lengner CJ, Hovhannisyan H et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem 2005;280:33132–33140. [DOI] [PubMed] [Google Scholar]
- 58. Komori T. Regulation of osteoblast differentiation by Runx2. Adv Exp Med Biol 2010;658:43–49. [DOI] [PubMed] [Google Scholar]
- 59. Nakashima K, Zhou X, Kunkel G et al. The novel zinc finger‐containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002;108:17–29. [DOI] [PubMed] [Google Scholar]
- 60. Zhang R, Oyajobi BO, Harris SE et al. Wnt/β‐catenin signaling activates bone morphogenetic protein 2 expression in osteoblasts. Bone 2013;52:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yee AJ, Bae HW, Friess D et al. Accuracy and interobserver agreement for determinations of rabbit posterolateral spinal fusion. Spine (Phila Pa 1976) 2004;29:1308–1313. [DOI] [PubMed] [Google Scholar]
- 62. Robinson ST, Svet MT, Kanim LA et al. Four‐point bending as a method for quantitatively evaluating spinal arthrodesis in a rat model. Comp Med 2015;65:46–50. [PMC free article] [PubMed] [Google Scholar]
- 63. Chung CG, James AW, Asatrian G et al. Human perivascular stem cell‐based bone graft substitute induces rat spinal fusion. Stem Cells Translational Medicine 2014;3:1231–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Müller R, Kampschulte M, Khassawna TE et al. Change of mechanical vertebrae properties due to progressive osteoporosis: Combined biomechanical and finite‐element analysis within a rat model. Med Biol Eng Comput 2014;52:405–414. [DOI] [PubMed] [Google Scholar]
- 65. Kaufman HH, Jones E. The principles of bony spinal fusion. Neurosurgery 1989;24:264–270. [DOI] [PubMed] [Google Scholar]
- 66. Gruskay JA, Fu M, Basques BA et al. Factors affecting length of stay and complications after elective anterior cervical discectomy and fusion: A study of 2164 patients from The American College of Surgeons National Surgical Quality Improvement Project Database (ACS NSQIP). Clin Spine Surg 2016;29:E34–E42. [DOI] [PubMed] [Google Scholar]
- 67. Hadley MN, Reddy SV. Smoking and the human vertebral column: A review of the impact of cigarette use on vertebral bone metabolism and spinal fusion. Neurosurgery 1997;41:116–124. [DOI] [PubMed] [Google Scholar]
- 68. Glass DA 2nd, Bialek P, Ahn JD et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 2005;8:751–764. [DOI] [PubMed] [Google Scholar]
- 69. Song JX, Dian ZJ, Wen Y et al. Assessment of the number and phenotype of macrophages in the human BMB samples of CML. Biomed Res Int 2016;2016:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rőszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm 2015;2015:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep 2014;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Brown BN, Sicari BM, Badylak SF. Rethinking regenerative medicine: A macrophage‐centered approach. Front Immunol 2014;5:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sha H, Zhang D, Zhang Y et al. ATF3 promotes migration and M1/M2 polarization of macrophages by activating tenascinC via Wnt/betacatenin pathway. Mol Med Rep 2017;16:3641–3647. [DOI] [PubMed] [Google Scholar]
- 74. He XT, Li X, Yin Y et al. The effects of conditioned media generated by polarized macrophages on the cellular behaviours of bone marrow mesenchymal stem cells. J Cell Mol Med 2017. doi:10.1111/jcmm.13431. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cordova LA, Loi F, Lin TH et al. CCL2, CCL5, and IGF‐1 participate in the immunomodulation of osteogenesis during M1/M2 transition in vitro. J Biomed Mater Res A 2017;105:3069–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jiang YY, Wen J, Gong C et al. BIO allieviated compressive mechanical force‐mediated mandibular cartilage pathological changes through Wnt/beta‐catenin signaling activation. J Orthop Res 2017. doi:10.1002/jor.23748. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 77. Einhorn TA. The cell and molecular biology of fracture healing. Clin Orthop Relat Res 1998;355S:S7–21. [DOI] [PubMed] [Google Scholar]
- 78. Han R. Highlight on the studies of anticancer drugs derived from plants in China. Stem Cells 1994;12:53–63. [DOI] [PubMed] [Google Scholar]
- 79. Hoessel R, Leclerc S, Endicott JA et al. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin‐dependent kinases. Nat Cell Biol 1999;1:60–67. [DOI] [PubMed] [Google Scholar]
- 80. Ma MZ, Yao BY. Progress in indirubin treatment of chronic myelocytic leukemia. J Tradit Chin Med 1983;3:245–248. [PubMed] [Google Scholar]
- 81. Ji XJ, Zhang FR, Lei JL et al. [Studies on the antineoplastic action and toxicity of synthetic indirubin (author's transl)]. Yao Xue Xue Bao 1981;16:146–148. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


