Abstract
We assessed the current status of Asian global clinical trials (GCTs) and factors, such as therapeutic areas, main metabolic enzymes targeted, approval status in the United States or the European Union, development strategies, influencing drug‐development strategies in Asian GCTs and in general GCTs for drug approval in Japan. The findings suggested that region‐specific diseases in Asia, intrinsic and extrinsic ethnic differences between Japanese/Asian and Caucasian populations, and the status of the drugs’ development in the United States or the European Union all affected Asian GCTs. These factors may influence the drug development process in Asian GCTs. Furthermore, we found that pharmacokinetics data in Asian GCTs comparing similarities between Japanese and other Asian populations were mainly obtained from late‐phase trials, which may delay the identification of potential differences in ethnic factors.
Study Highlights
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
✓ The numbers of global clinical trials (GCTs) in Asia have increased in recent years. However, the specific characteristics of Asian GCTs have not been determined to date.
WHAT QUESTION DID THIS STUDY ADDRESS?
✓ We identified factors that influence drug development strategies in Asian GCTs by comparing Asian GCT‐based and general GCT‐based drug approvals in Japan.
WHAT THIS STUDY ADDS TO OUR KNOWLEDGE
✓ We found that the conduct of Asian GCTs may correlate with characteristics of the disease treated, including regional prevalence, ethnic differences between Japanese/Asian and Caucasian populations, and the status of the drug's development in the United States/European Union. Pharmacokinetics data from Japanese subjects and other Asian ethnicities were obtained from late‐phase trials.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE
✓ Our findings may identify factors to consider in conducting Asian GCTs.
Japanese participation in global clinical trials (GCTs) increased gradually following the 2007 publication of a Japanese guidance document entitled “Basic principles on Global Clinical Trials.”1, 2 The percentage of GCTs conducted only in Asian regions (Asian GCTs) has also increased after FY2009, and the pivotal clinical trial data of ∼2–4% of new drugs approved in Japan.3 Several reasons have been suggested for this increase: The similarities in type and frequency of metabolic enzyme polymorphisms and gene profiles among Asian ethnic groups, emerging drug markets, the improvement of clinical trial environments, and the high prevalence of certain diseases in Asia. Thus, Asia now plays an important role in general drug development.
The current status and characteristics of GCTs in Japan has been discussed in many reports. For example, Ichimaru et al. reported that the major target diseases of the GCTs were cancer and cardiovascular disease, and the trials were conducted mainly as confirmatory studies of phase III trials.2 Furthermore, Ando and Uyama indicated that the proportion of the Japanese sample size relative to the total sample size in GCTs was less than 0.2 in many cases.4 However, very little has been published about Asian GCTs. Although the characteristics of Asian GCTs in terms of sample size have been reported, comprehensive evaluations of the characteristics of Asian GCTs, such as therapeutic areas, main metabolic enzymes targeted, and development strategies, have not yet been performed. 5
We therefore attempted to elucidate the current status of Asian GCTs, and to identify factors that influence development strategies in Asian GCTs, by comparing the latter with general GCTs. We also discuss the most appropriate drug‐development strategies in Asian GCTs with respect to the factors identified in this study.
METHODS
Data sources and information collected
We collected data from a list of approved products on the website of the Pharmaceuticals & Medical Devices Agency (PMDA, Japanese regulatory agency)6 and identified drugs that were approved between FY2007 and FY2015 (April 2007–March 2016) on the basis of a GCT as a pivotal clinical trial in Japan. “Pivotal clinical trial” was defined as an important factor in evaluating efficacy and safety for making an approval decision, and was marked “evaluation data” in the PMDA review reports. If a clinical trial was marked “reference data” in a review report, it was not classified as a pivotal clinical trial. We collected other information (geographical location, sample size, target therapeutic area, metabolic enzymes targeted, development strategy, and approval status in the United States and/or the European Union (EU)) from the following online sources: The PMDA website,7 Clinicaltrials.gov,8 and the National Institutes of Health Clinical (NIHC) Trials Search platform.9 Data on sample size were collected from phase II and phase III studies. One case lacking a description of geographical location and Japanese sample size was excluded. The target therapeutic area was categorized based on ICD‐10 codes (v. 2015).
Definitions
In this study, a GCT was defined as a single clinical trial conducted in multiple countries (including Japan) and according to a common clinical trial protocol. “General GCTs” were defined as clinical trials conducted in multiple countries and regions (Asia, America, Europe, Oceania, and Africa). “Asian GCTs” were defined as clinical trials conducted in Asian countries and regions (East Asia, Southeast Asia, and South Asia). Clinical trials for drug development conducted in both Asian and general settings were classified as Asian GCTs. A “bridging study” was defined based on the ICH E5 guideline10 as a supplemental study performed in a new region to provide pharmacodynamics data that describe the relation of the effect to dose or drug concentration, or clinical data on efficacy, safety, dosage, and dose regimen that would allow extrapolation of the original clinical data to the new region. A “simultaneous‐submission drug” was defined as a drug approved in Japan and in the United States or European Union within a 2‐year period and included under review in the United States or European Union. An “approved drug” was defined as a drug already approved in the United States or European Union for more than 2 years.
RESULTS
Characteristics of drugs approved based on GCTs in Japan
We identified drugs newly approved based on data from GCTs according to the criteria shown in Figure 1, which excluded bridging studies and Japanese‐only studies. Between FY2007 and FY2015, 1,017 new drugs were approved in Japan, and 121 drugs (11.9%) were approved based on data from GCTs (Supplementary Table 1). Of the 121 GCT‐based approvals, 31 approvals were based on Asian GCTs (Japan‐based companies: eight drugs; US/EU‐based companies: 23 drugs), and the other 90 approvals were based on general GCTs (Japan‐based companies: 12 drugs; US/EU‐based companies: 78 drugs). There were 175 GCTs for the 121 drugs (Asian GCTs: 36 studies; general GCTs: 139 studies), as some drugs were tested in two or more GCTs. The 121 drugs that received approvals based on data from GCTs were mainly categorized as drugs with new active ingredients (49.6%, 60/121 drugs; 16 of 60 drugs in Asian GCTs, and 44 of 60 drugs in general GCTs), established drugs with a new dosage and indication (19.0%, 23/121 drugs; 5 of 23 drugs in Asian GCTs, and 18 of 23 drugs in general GCTs), and drugs with a new indication (12.4%, 15/121 drugs; 5 of 15 drugs in Asian GCTs, and 10 of 15 drugs in general GCTs). The percentage of these three categories were similar between Asian GCTs (83.9%, 26/31 drugs) and general GCTs (80.0%, 72/90 drugs).
Figure 1.
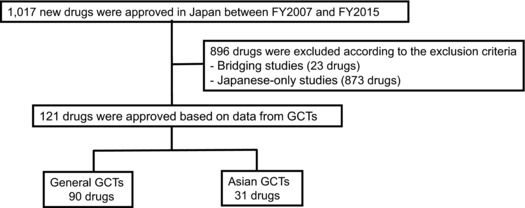
Identification of drugs newly approved in Japan based on data from general or Asian global clinical trials (GCTs). We collected data of newly approved drugs from the website of the Pharmaceuticals & Medical Devices Agency and identified drugs which were approved between FY2007 and FY2015 on the basis of a GCT as a pivotal clinical trial in Japan.
The aim of the 2007 Japanese guideline was to offer points to consider in planning and conducting GCTs, and to encourage Japanese participation in such trials. To examine the effect of the guideline, we assessed the yearly trends of new drug applications (NDAs) that received GCT‐based approvals and bridging studies that included Japanese subjects between FY2007, when the guideline was issued, and FY2015 (Figure 2). We found that the absolute numbers and percentage of GCTs increased greatly after FY2009 (i.e., 2 years after the publication of the guideline), although the absolute numbers and percentage of drugs approved based on bridging studies were greater than those of drugs approved based on GCTs in FY2007 and FY2008. Since FY2009, the percentage of GCT‐based approvals has been larger than that of drug approvals based on bridging studies, probably as a result of the transition of the drug development strategy from sequential bridging studies to GCTs. After 2014, 20% or more of new drugs approved in Japan were GCT‐based approvals (FY2014: 27.7% (33/119 drugs); FY2015: 20.0% (23/115 drugs)). The percentage of GCTs conducted only in Asian regions has also increased over the past few years, and the pivotal clinical trial data of ∼5% of new drugs approved in Japan were collected from Asian GCTs (Figure 2).
Figure 2.
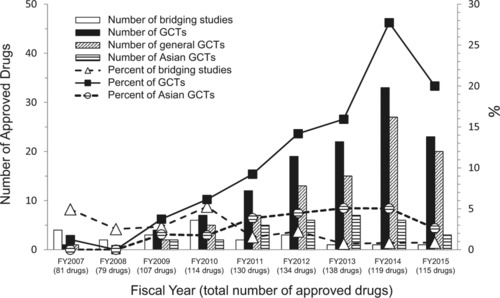
Trends in new drug application approvals and related pivotal clinical trial data. The numbers of newly approved drugs whose pivotal clinical trial data were collected from global clinical trials between FY2007 and FY2015 are shown. The bars denote the number of drugs approved based on specific types of clinical trials. Squares, triangles, and circles denote the percentages of drugs approved based on specific types of clinical trials.
Figure 3 shows the number of Asian regions participating in GCTs by study initiation time. Both general GCTs and Asian GCTs were conducted mainly in East Asia. However, the number of trials in Southeast and South Asia countries was relatively low in Asian GCTs compared with general GCTs, except in trials initiated in 2006 and 2008. In East Asia, the number of trials in China was relatively low for Asian GCTs compared with general GCTs. This finding indicates variability in Asian countries and regions’ participation in general GCTs and Asian GCTs.
Figure 3.
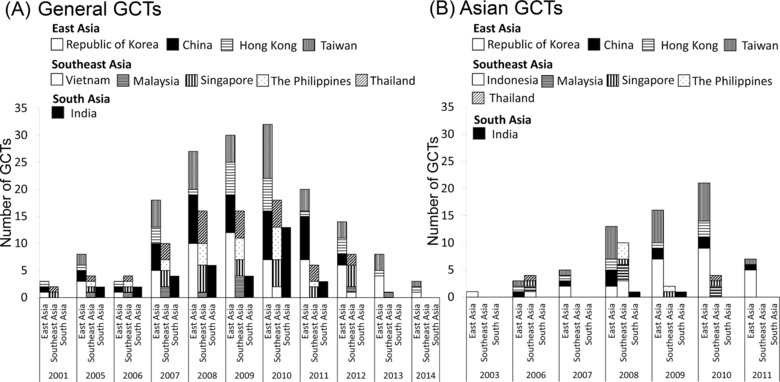
Global clinical trials (GCTs) by location and year initiated. The numbers of GCTs in each Asian region at the study initiation time are shown. Asian regions were classified as East Asia (Republic of Korea, China, Hong Kong, Taiwan), Southeast Asia (Vietnam, Indonesia, Malaysia, Singapore, The Philippines, Thailand), and South Asia (India). (A) General GCTs and (B) Asian GCTs are shown.
In general, the numbers of Japanese subjects in GCTs are assumed to be relatively high in the exploratory trials and relatively low in large‐scale confirmatory trials. We therefore analyzed the proportion of the Japanese sample size relative to the total sample size (Figure 4). Japanese subjects enrolled in general GCTs comprised 2–50% of the total sample. In contrast, Japanese subjects enrolled in Asian GCTs comprised 20–90% of the total sample. The average percentage of Japanese subjects was 12.4% in general GCTs and 51.0% in Asian GCTs. Higher proportions of Japanese subjects (>50%) in Asian GCTs were seen in trials for infectious and parasitic diseases and for mental and behavioral disorders. The large‐scale trials (>1,000 subjects) were more prevalent in general GCTs (Asian GCTs: 8.3%, 3/36 studies; general GCTs: 22.3%, 31/139 studies).
Figure 4.
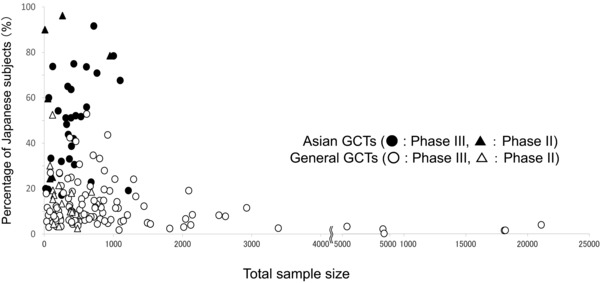
Proportion of Japanese subjects to total subjects in global clinical trials (GCTs). Relationship between total sample size and the proportion of Japanese subjects in the sample is shown. Closed circles and triangles represent the pivotal clinical trial data from Asian GCTs, and open circles and triangles represent the pivotal clinical trial data from general GCTs.
Evaluation of factors that influence drug development strategies in Asian GCTs
Among the 121 drugs with GCT‐based approvals, the most common target therapeutic area was neoplasms (32.2%, 39/121 drugs; 6 of 39 drugs in Asian GCTs and 33 of 39 drugs in general GCTs) (Table 1). Development of orphan drugs was a target of 35.9% of GCTs in the neoplasms category (three of six drugs in Asian GCTs and 11 of 33 drugs in general GCTs). The second most common target therapeutic areas were endocrine, nutritional, and metabolic diseases (16.5%, 20/121 drugs; 6 of 20 drugs in Asian GCTs and 14 of 20 drugs in general GCTs). Major products of GCTs focusing on the endocrine, nutritional, and metabolic diseases areas comprised insulin preparations and treatments for type 2 diabetes. The focus on antidiabetic drugs was more evident in Asian GCTs than in general GCTs (Asian GCTs: 83.3%, 5/6 drugs; general GCTs: 57.1%, 8/14 drugs). Most (four out of five) antidiabetic drugs in Asian GCTs had been approved almost simultaneously in Japan and in the United States or European Union (exenatide, insulin‐degludec, insulin‐degludec+aspart, and lixisenatide). Drug development for rare diseases in the endocrine, nutritional, and metabolic diseases areas was conducted only in general GCTs (four drugs: elosulfase alfa, eliglustat, asfotase alfa, and sebelipase alfa).
Table 1.
Target therapeutic areas and main metabolic enzymes targeted in general global clinical trials (GCTs) and Asian GCTs
| General GCTs (90 drugs) | Asian GCTs (31 drugs) | Total (121 drugs) | ||
|---|---|---|---|---|
| Target therapeutic area | Neoplasms | 33 (36.7) | 6 (19.4) | 39 (32.2) |
| Endocrine, nutritional, and metabolic diseases | 14 (15.6) | 6 (19.4) | 20 (16.5) | |
| Diseases of the respiratory system | 12 (13.3) | 1 (3.2) | 13 (10.7) | |
| Diseases of the blood and blood‐forming organs, and certain disorders involving immune mechanisms | 8 (8.9) | 0 (0.0) | 8 (6.6) | |
| Diseases of the circulatory system | 7 (7.8) | 1 (3.2) | 8 (6.6) | |
| Diseases of the eye and adnexa | 5 (5.6) | 1 (3.2) | 6 (5.0) | |
| Diseases of the nervous system | 4 (4.4) | 3 (9.7) | 7 (5.8) | |
| Certain infectious and parasitic diseases | 2 (2.2) | 3 (9.7) | 5 (4.1) | |
| Mental and behavioral disorders | 1 (1.1) | 5 (16.1) | 6 (5.0) | |
| Diseases of the genitourinary system | 0 (0.0) | 2 (6.5) | 2 (1.7) | |
| Other diseases | 4 (4.4) | 3 (9.7) | 7 (5.8) | |
| Metabolic enzyme | None | 38 (42.2) | 12 (38.7) | 50 (41.3) |
| CYP3A | 29 (32.2) | 4 (12.9) | 33 (27.3) | |
| UDP‐glucuronosyltransferases (UGTs) | 10 (11.1) | 5 (16.1) | 15 (12.4) | |
| CYP2D6 | 4 (4.4) | 4 (12.9) | 8 (6.6) | |
| Multiple CYP isoenzymes | 4 (4.4) | 0 (0.0) | 4 (3.3) | |
| CYP2C8 | 2 (2.2) | 0 (0.0) | 2 (1.7) | |
| CYP2C19 | 1 (1.1) | 1 (3.2) | 2 (1.7) | |
| CYP1A2 | 1 (1.1) | 1 (3.2) | 2 (1.7) | |
| Other enzymes | 1 (1.1) | 4 (12.9) | 5 (4.1) |
Numbers in parentheses denote percentages.
The main differences between Asian and general GCTs were observed in five therapeutic areas. Specifically, neoplasms, diseases of the respiratory system, and diseases of the blood and blood‐forming organs, and certain disorders involving the immune mechanism were more frequent target areas in general GCTs. Certain infectious and parasitic diseases and mental and behavioral disorders were more frequent target areas in Asian GCTs. Drug development for infectious diseases in Asian GCTs focused on antiviral agents against influenza (peramivir, laninamivir, and favipiravir), and these drugs had not been approved at the time in other global regions. Development of orphan drugs was more prevalent in general GCTs (Asian GCTs: 9.7%, 3/31 drugs; general GCTs: 22.2%, 20/90 drugs).
Because many articles have reported that the ethnic differences in the variant frequencies of enzymes between East Asian and Caucasian populations affect efficacy and safety, although the types and frequency of metabolic enzyme polymorphisms are thought to be similar among Asian ethnicities,11 we identified the main enzymes involved in metabolizing the 121 drugs that received GCT‐based approvals. Most GCTs did not test drugs with a specific enzyme target, or tested drugs that did not undergo extensive biotransformation (41.3%, 50/121 drugs; 12 of 50 drugs in Asian GCTs, and 38 of 50 drugs in general GCTs); these drugs are considered insensitive to ethnicity, and mostly are antibody products or protein products. CYP3A, which does not exhibit ethnic differences, was tested more in general GCTs (27.3%, 33/121 drugs; 4 of 33 drugs in Asian GCTs, and 29 of 33 drugs in general GCTs) (Table 1). The percentage of drugs mainly metabolized by CYP2D6 was higher in Asian GCTs than in general GCTs. Furthermore, drugs that were substrates of CYP2D6 in Asian GCTs had already been approved for use in the United States or European Union; this suggests that the ethnic difference in CYP2D6 variant frequencies between East Asian and Caucasian populations may affect drug development in GCTs.11
We also assessed drug approval status in the United States or European Union in relation to drug application in Japan. As shown in Figure 5, the percentage of simultaneous‐submission drug applications in Japan and the United States or European Union was higher in general GCTs than in Asian GCTs (Asian GCTs: 16.1%, 5/31 drugs; general GCTs: 82.2%, 74/90 drugs). In contrast, the percentage of drugs already approved in the United States or European Union was higher in Asian GCTs than in general GCTs (Asian GCTs: 58.1%, 18/31 drugs; general GCTs: 11.1%, 10/90 drugs). The 8 of 31 drugs approved based on Asian GCTs that had not been approved for use in the United States or European Union (peramivir, laninamivir, edoxaban (approved in April 2011 in Japan), esomeprazole, goserelin, favipiravir, aflibercept (approved in September 2014 in Japan), and darbepoetin alfa) are mostly related to Asia‐specific diseases or diseases with high morbidity in Asia, such as influenza or pathologic myopia. In addition, five simultaneous‐submission of the 31 drugs approved based on Asian GCTs (exenatide, indacaterol, insulin‐degludec, insulin‐degludec+aspart, and lixisenatide) are associated with ethnic differences between Japanese and Caucasian populations, such as susceptibility to type 2 diabetes mellitus. More than half of drugs approved in Japan based on data from Asian GCTs had already been approved in the United States or European Union prior to Japanese approval. Simultaneous‐submission drugs and approvals for drugs not yet approved in the United States or European Union were mostly for drugs to treat type 2 diabetes mellitus and antiviral agents against influenza.
Figure 5.
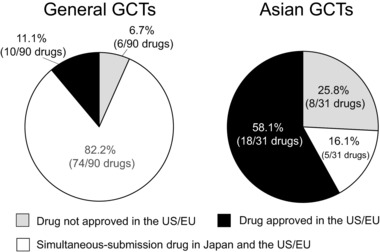
Approval status in the United states or European Union for drugs tested in general global clinical trials (GCTs) and Asian GCTs. A “simultaneous‐submission drug” was defined as a drug approved in Japan and in the United States/European Union within a 2‐year period. An “approved drug” was defined as a drug already approved in the United States or European Union in advance to more than 2 years.
We investigated the clinical development strategies of GCTs for drugs with new active ingredients. The data for interethnic pharmacokinetics (PK) comparisons between Japanese and non‐Japanese populations had been collected from two sources: Standard PK early‐phase studies (e.g., Cmax and area under the curve (AUC) values), or late‐phase studies such as GCTs (e.g., trough level). Figure 6A shows the sources of data for PK comparisons between Japanese and non‐Japanese populations. The percentage of standard PK studies conducted to assess similarities between Japanese and non‐Japanese subjects was higher in general GCTs than in Asian GCTs (Asian GCTs: 25.0%, 4/16 drugs; general GCTs: 75.0%, 33/44 drugs). In contrast, the percentage of drugs for which PK data had been obtained in the late phase was higher in Asian GCTs than in general GCTs (Asian GCTs: 56.3%, 9/16 drugs; general GCTs: 25.0%, 11/44 drugs). In some Asian GCTs, PK data on non‐Japanese subjects (other Asian subjects) were not obtained (18.7%, 3/16 drugs).
Figure 6.
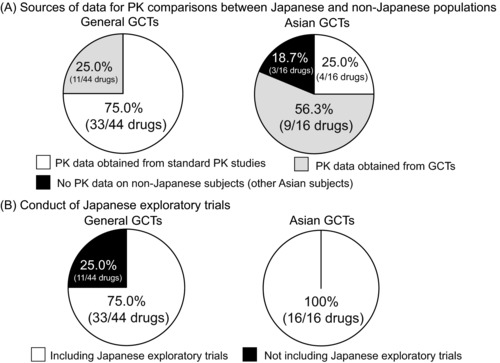
Clinical development strategies for drugs with new active ingredients in general global clinical trials (GCTs) and Asian GCTs. (A) The ratios of source data for the ethnic comparisons of the pharmacokinetics (PK) in the general GCTs and Asian GCTs are shown. The PK data had been collected from two sources, standard PK early‐phase studies or the late‐phase studies such as GCTs. (B) The performance ratios of exploratory trials for the Japanese population including clinical pharmacology studies in the general GCTs and Asian GCTs are shown.
We looked at whether Japanese exploratory trials (phase I and II studies) were included in the clinical development strategies of GCTs (Figure 6B). Most drug trials, both Asian and general GCTs, included Japanese exploratory trials (Asian GCTs: 100%, 16/16 drugs; general GCTs: 75.0%, 33/44 drugs). In some general GCTs, Japanese exploratory trials had not been conducted (25.0%, 11/44 drugs). These 11 drugs were intended to treat rare diseases (10 of 11 drugs) or were topically active products (one drug).
DISCUSSION
In this study we investigated the current state of Asian and general GCTs, and identified factors influencing development strategies in Asian GCTs. The number of trials in Asian GCTs has steadily increased. To address this increase, the supplement document “Basic principles on Global Clinical Trials (Reference Cases)” was published in 2012.12 The document was designed to further promote an understanding of the 2007 guideline, and to ensure Japan's successful participation in global drug‐development activities from an early stage and an effective and appropriate conduct of GCTs in Asia, where an increase in such trials is expected. Thus, Asian GCTs play an important role in drug approval in Japan.
Main drug development trends in Asian GCTs focused on region‐specific diseases, including influenza. Because infection with novel influenza strains, such as highly pathogenic avian influenza viruses, is usually identified first in Southeast Asia, seasonal and region‐specific diseases such as influenza may accelerate new drug development for these diseases in Asia. These drugs received approval in Asia in advance of approval in the United states or European Union. Another example is the topically active drug aflibercept (approved in September 2014 in Japan) to treat pathologic myopia, which is more prevalent in Asia than in the United States and European Union.13, 14 Region‐specific diseases and diseases with high morbidity in Asia were rational choices for drug development in Asian GCTs. Another notable target therapeutic area in Asian GCTs was mental and behavioral disorders. Psychiatric disorders may involve extrinsic ethnic factors such as culture and lifestyle that may affect the efficacy and safety of drugs. Asian GCTs would therefore offer an opportunity to test these extrinsic ethnic factors in a controlled manner. Asian GCTs also focused on the treatment of diabetes mellitus, which has been linked to intrinsic ethnic factors of insulin secretion and insulin resistance among Japanese and Caucasian populations.15
In addition, we categorized the main enzymatic targets of the 121 drugs. The percentage of drugs that were substrates for CYP2D6 in Asian GCTs was higher than in general GCTs. The types and frequency of metabolic enzyme polymorphisms are thought to be similar among Asian ethnicities.16, 17 Therefore, ethnic differences in the variant frequencies of enzymes such as CYP2D6 between East Asian and Caucasian populations may affect the targets selected in GCTs; diseases associated with ethnic factors would be rational targets for drug development in Asian GCTs. Since intrinsic ethnic factors are thought to be similar among Asian populations,11 if extrinsic factors such as diagnostic methods and concomitant drug use are controlled, GCTs conducted in Asian countries may have a higher probability of achieving consistent results than general GCTs. More than half of drugs approved in Japan based on Asian GCTs had already been approved for use in the United States or European Union. These results suggested that drug development in Asia may be behind the United States or European Union. Therefore, the conduct of Asian GCTs may correlate with status of development in the United States or European Union.
The proportion of the Japanese sample size to the total sample size in Asian GCTs tended to be higher than in general GCTs. These findings could reflect the number of participating countries/regions, the conduct of large‐scale trials, and the development phase of the drugs. Furthermore, this trend suggested that Japan actively contributes to drug development in Asia.
We investigated the clinical development strategy in GCTs for drugs with new active ingredients. PK data in Asian GCTs assessing PK similarities between Japanese and non‐Japanese subjects were usually obtained from late‐phase trials such as GCTs, while PK data in general GCTs were mainly obtained from early‐phase research, such as standard PK studies. This trend may be attributable to the similarity of ethnic factors in drug response among Asian populations, and it is difficult to compare PK parameters from all Asian subjects. Furthermore, if there are no marked PK differences between Japanese and Caucasian populations, it may be supposed that there are no major differences between Japanese and other Asian populations either. However, differences in ethnic factors may affect the efficacy and safety of drugs even within Asia; carbamazepine‐induced Stevens–Johnson syndrome/toxic epidermal necrolysis is one such example.18–21 Therefore, to ensure appropriate planning in the drug‐development process, PK comparisons should be performed during the early stages, to identify potential differences in ethnic factors that may affect the drug's efficacy and safety. GCTs in Asia should be designed and conducted based on prior sufficient evaluation of the effect of ethnic differences on the efficacy and safety of drugs.
In conclusion, we determined that the conduct of Asian GCTs may correlate with characteristics of the diseases targeted, ethnic differences between Japanese/Asian and Caucasian populations, and the drug's development status in the United States or European Union. Drug‐development strategies in Asian and general GCTs should be determined in consideration of these influencing factors. We believe that, in the future, the lag in drug development between Japan and the United States/European Union will be smaller, and Asian GCTs may shift from follow‐up to simultaneous development with the United States/European Union. Furthermore, although the Asian GCTs we analyzed had been conducted mainly in East Asia, more Asian countries will be involved in future Asian GCTs.
Conflict of Interest
The authors declared no conflicts of interest.
Supporting information
Supporting Information
Author Contributions
K.A. and M.T. wrote the article; K.A., Y.U., and M.T. designed the research; K.A. and Y.U. analyzed the data.
Disclosure
The views expressed herein are the result of independent work and do not necessarily represent the views and findings of the Pharmaceuticals & Medical Devices Agency.
References
- 1. Ministry of Health , Labour and Welfare. Basic principles on Global Clinical Trials. (2007). http://www.pmda.go.jp/files/000157900.pdf Accessed 4 August 2017. [Google Scholar]
- 2. Ichimaru, K. , Toyoshima, S. & Uyama, Y. Effective global drug development strategy for obtaining regulatory approval in Japan in the context of ethnicity‐related drug response factors. Clin. Pharmacol. Ther. 87, 362–366 (2010). [DOI] [PubMed] [Google Scholar]
- 3. Asano, K. , Tanaka, A. , Sato, T. & Uyama, Y. Regulatory challenges in the review of data from global clinical trials: The PMDA perspective. Clin. Pharmacol. Ther. 94, 195–198 (2013). [DOI] [PubMed] [Google Scholar]
- 4. Ando, Y. & Uyama, Y. Multiregional clinical trials: Japanese perspective on drug development strategy and sample size for Japanese subjects. J. Biopharm. Stat. 22, 977–987 (2012). [DOI] [PubMed] [Google Scholar]
- 5. Shirotani, M. , Kurokawa, T. & Chiba, K. Comparison of global versus Asian clinical trial strategies supportive of registration of drugs in Japan. J. Clin. Pharmacol. 54, 753–764 (2014). [DOI] [PubMed] [Google Scholar]
- 6. Pharmaceuticals and Medical Devices Agency . List of Approved Products. http://www.pmda.go.jp/english/review-services/reviews/approved-information/drugs/0002.html Accessed 4 August 2017.
- 7. Pharmaceuticals and Medical Devices Agency . Information on approved products (in Japanese). http://www.pmda.go.jp/PmdaSearch/iyakuSearch/ Accessed 4 August 2017.
- 8. Clinical Trials.gov . https://www.clinicaltrials.gov/ Accessed 4 August 2017.
- 9. National Institute of Public Health Clinical (NIPH) Trials Search . http://rctportal.niph.go.jp/en/ Accessed 4 August 2017.
- 10. International Conference on Harmonization . (1998). E5 Guideline: Ethnic Factors in the Acceptability of Foreign Clinical Data (R1). http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E5_R1/Step4/E5_R1__Guideline.pdf Accessed 4 August 2017.
- 11. Kurose, K. , Sugiyama, E. & Saito, Y. Population differences in major functional polymorphisms of pharmacokinetics/pharmacodynamics‐related genes in Eastern Asians and Europeans: Implications in the clinical trials for novel drug development. Drug Metab. Pharmacokinet. 27, 9–54 (2012). [DOI] [PubMed] [Google Scholar]
- 12. Ministry of Health, Labour and Welfare . Basic Principles on Global Clinical Trials (Reference Cases). (2012). http://www.pmda.go.jp/files/000157520.pdf Accessed 4 August 2017.
- 13. Morgan, I.G. , Ohno‐Matsui, K. & Saw, S.M. Myopia. Lancet 379, 1739–1748 (2012). [DOI] [PubMed] [Google Scholar]
- 14. Pan, C.W. , Ramamurthy. D. & Saw, S.M. Worldwide prevalence and risk factors for myopia. Ophthalmic. Physiol. Opt. 32, 3–16 (2012). [DOI] [PubMed] [Google Scholar]
- 15. Ma, R.C. & Chan, J.C . Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann. N. Y. Acad. Sci. 64–91 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. The International Hap Map Consortium . A haplotype map of the human genome. Nature 437, 1299–1320 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Myrand, S.P. et al Pharmacokinetics/genotype associations for major cytochrome P450 enzymes in native and first‐ and third‐generation Japanese populations: comparison with Korean, Chinese, and Caucasian populations. Clin. Pharmacol. Ther. 84, 347–361 (2008). [DOI] [PubMed] [Google Scholar]
- 18. Ozaki, T. et al Genome‐wide association study identifies HLA‐A*3101 allele as a genetic risk factor for carbamazepine‐induced cutaneous adverse drug reactions in Japanese population. Hum. Mol. Genet. 20, 1034–1041 (2011). [DOI] [PubMed] [Google Scholar]
- 19. Chung, W.H. et al Medical genetics: a marker for Stevens‐Johnson syndrome. Nature 428, 486 (2004). [DOI] [PubMed] [Google Scholar]
- 20. Hung, S.I. et al Genetic susceptibility to carbamazepine‐induced cutaneous adverse drug reactions. Pharmacogenet. Genomics 16, 297–306 (2006). [DOI] [PubMed] [Google Scholar]
- 21. Middleton, D. , Menchaca, L. , Rood, H. & Komerofsky, R. New allele frequency database: http://www.allelefrequencies.net. Tissue Antigens 61, 403–407 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


