Abstract
Background
Paroxysmal gluten‐sensitive dyskinesia (PGSD) in border terriers (BTs) results from an immunologic response directed against transglutaminase (TG)2 and gliadin. Recent evidence suggests that PGSD is only one aspect of a range of possible manifestations of gluten sensitivity in the breed.
Hypothesis/Objectives
Gluten sensitivity in BTs is a heterogeneous disease process with a diverse clinical spectrum; to characterize the phenotype of PGSD using TG2 and gliadin autoantibodies as diagnostic markers.
Animals
One hundred twenty‐eight client‐owned BTs with various disorders.
Methods
Prospective study. BTs with paroxysmal episodes and a normal interictal examination were phenotyped using footage of a representative episode and assigned to 3 groups: idiopathic epilepsy (IE), paroxysmal dyskinesia (PD), or other. Owners of each dog completed a questionnaire to obtain information regarding clinical signs. Healthy BTs formed a control group. Serum antibodies against TG2 and AGA were measured in all dogs.
Results
One hundred twenty‐eight BTs were enrolled; 45 with PD, 28 with IE, 35 with other conditions, and 20 controls. Three overlapping phenotypes were identified; PD, signs suggestive of gastrointestinal disease, and dermatopathy. AGA‐IgG concentrations were increased in PD, compared with IE (P = 0.012), controls (P < 0.0001) and other (P = 0.018) conditions. Anti‐canine TG2‐IgA concentrations were increased in PD, compared with IE (P < 0.0001), controls (P < 0.0001) and other (P = 0.012) conditions. Serological markers are highly specific for PGSD but lack sensitivity.
Conclusions
PGSD appears part of a syndrome of gluten intolerance consisting of episodes of transient dyskinesia, signs suggestive of gastrointestinal disease, and dermatological hypersensitivity.
Keywords: Canine epileptoid cramping syndrome, Gluten‐free diet, Movement disorder, Neurology
Abbreviations
- AGA IgG
anti‐canine gliadin IgG
- BT
border terrier
- CD
coeliac disease
- GTCS
generalized tonic‐clonic seizure
- IE
idiopathic epilepsy
- NCGS
non‐coeliac gluten sensitivity
- PD
paroxysmal dyskinesia
- PGSD
paroxysmal gluten‐sensitive dyskinesia
- TG
transglutaminase
- TG2 IgA
anti‐canine transglutaminase‐2‐IgA
Canine epileptoid cramping syndrome is a paroxysmal gluten‐sensitive dyskinesia (PGSD) of border terriers (BTs) that is analogous to paroxysmal non‐kinesigenic dyskinesias in people.1, 2, 3 PGSD is characterized by episodic combinations of dystonia, chorea, ballism, tremor, and athetosis occurring at rest with no loss of consciousness.2 Episodes begin between 6 weeks and 7 years of age. The episodes might last a few minutes up to a few hours, with some owners reporting an association with stress or excitement and others reporting signs during waking from sleep. One interesting feature is the association in around 50% of dogs with signs suggestive of mild gastrointestinal involvement, such as vomiting, diarrhea or borborygmi,2 and a recent report suggests that PGSD is only one aspect of a range of possible manifestations of gluten sensitivity in the breed.4 Measurement of serum AGA IgG and anti‐canine transglutaminase‐2‐IgA (TG2 IgA) was increased in 5 affected dogs, and a clinical and serological improvement was identified on institution of a gluten‐free diet.3 A clinical diagnosis previously relied on exclusion of other causes for the paroxysmal episodes by recognition of the typical features of PGSD by observing a video recording of a representative episode2 and performing diagnostic testing to rule‐out extracranial and intracranial structural disease. Stratification of individuals meant that diagnosis was limited to those with the typical signs of the disease. We speculated that there is more phenotypic heterogeneity within this condition than has been reported. Diagnostic biomarkers for PGSD have now been discovered, opening the way for more detailed studies of phenotype and underlying pathobiology.3
The discovery of this canine gluten sensitivity has implications for people. Sensitivity to gluten results in a systemic immune‐mediated disease that can present with diverse manifestations. Coeliac disease (CD) is triggered in genetically susceptible people by the ingestion of gluten.5 However, this term is reserved for those patients with gluten‐sensitive enteropathy. The more recently coined term, non‐coeliac gluten sensitivity (NCGS), comprises a gamut of conditions in people, all of which are characterized by an immune response to the ingestion of gluten, but with diverse multi‐organ manifestations6 including the gut, skin (dermatitis herpetiformis),7 and brain (gluten ataxia; GA).8
Our aim was to confirm the use of serum AGA IgG and TG2 IgA as a diagnostic test for PGSD. In this verification process, we aimed to identify and describe the broad spectrum of clinical signs resulting in PGSD in the BT and to determine if these antibodies could delineate epileptic seizures from PGSD.
Materials and Methods
Animals
Cases were solicited via the veterinary media (The Veterinary Times and Veterinary Record), internet (solicitation on BT forums and Facebook), international press (radio and newspaper driven), and also by personal contacts within the BT breed societies, asking primary veterinarians and owners to contact us regarding BTs presenting with abnormal paroxysmal episodes, including epileptic seizures and paroxysmal dyskinesia (PD). However, other disorders associated with BTs were included. Clinical assessment and serum antibody measurements were performed independently and before data analysis. The target population consisted of 4 groups: BTs with PD, BTs with idiopathic epilepsy (IE), BTs with other conditions, and healthy control BTs. Dogs fed a gluten‐free diet prior to accession were excluded from the study.
Inclusion Criteria
Video footage of a representative episode was submitted by all owners (groups 1–3) and was reviewed to determine the likely origin of the episode based on the observed phenomenology and accompanying clinical history, ie, IE (group 1) versus PD (group 2). If the diagnosis was uncertain, or if the episode related to another problem, then the dog was assigned to a third group (“Other”; group 3); hence, this group included dogs with less stereotypical signs of PD, behavioral disorders, ambiguous paroxysmal episodes, and other conditions. A 4th group comprised healthy BTs with no known concurrent illness reported in the last 6 months; the control group. Dogs on immunosuppressive therapy and those already receiving a gluten‐free or restricted diet were excluded from the study.
Inclusion criteria for assignment as PD or IE were that BTs be in good general health with an interictal neurological examination that detected no abnormalities and a history of abnormal episodes (≥3) for at least 1 year. If this was not fulfilled and transient episodes were observed and confirmed by video, BTs were grouped into the “Other” category.
Pedigrees, medical history, and litter information were collected. In the cases recruited via primary veterinarians, a full history was also requested and reviewed. If the owner had contacted us directly and was included in the study, consent was obtained to request and review the history from their primary veterinarian. This history supplemented the information gained from the questionnaire. Data were acquired from January 2014 to July 2017.
Presumptive Diagnosis
A diagnosis of PD was determined using clinical phenomenology of an episode from video footage (assessed by ML), an owner's account of the episode, and historical information using guidelines described in a review on the subject.9 Specifically, BTs were diagnosed based on a combination of standard criteria that included no loss of consciousness despite motor manifestations in more than 1 limb, the absence of autonomic signs (specifically urination, defecation, and hypersalivation), and no postictal period. Involuntary movements matching the above description that were induced by sudden movements (such as rising from a period of lying down) increased the clinical suspicion of PD. Video 1 demonstrates the typical signs expected with PD. The diagnosis of IE was achieved based on video evidence demonstrating a typical generalized tonic‐clonic seizure (GTCS) and including the presence of autonomic signs (specifically urination, defecation, and hypersalivation), a loss of awareness, and the observation of a postictal period (assessed by ML). GTCS in BTs with interictal abnormalities were excluded from the IE group and placed in the “Other” group. Episodes occurring from rest or sleep were considered more likely to reflect an epileptic seizure if they had the defining semiology described. Video 2 demonstrates the typical features of a GTCS. A review by 2 of the authors describes this decision process in more detail.9
Questionnaire
Before completion of the questionnaire, an interview via phone or email was performed in which an open description of the paroxysmal episodes was given by the owner. Having received the video, performed the interview, and undergone group assignation, owners were invited to complete a questionnaire. The questionnaire was presented online utilizing the online survey software tool SurveyMonkey (http://www.surveymonkey.com). It consisted of open‐ended questions, to which the answer could be recorded verbatim, followed by specific leading questions. The majority of questions were closed questions with multiple choice answers. Questions directed toward the types and characteristics of the episodes also included an opportunity to provide a description verbatim of the episodes. Where more than 1 type of episode was observed, participants were asked to characterize each individually. The questions were designed to provide detailed phenotypic information in terms of the signalment of BTs, possible precipitating factors, current and previous diets, general health (including but not limited to signs of gastrointestinal, dermatological, or neurological disease), history of medication, and the characteristics of the episodes. Its purpose was to gather further information on the diagnosis achieved by video footage and initial interview, rather than to ensure correct group designation had been performed. Questions were randomized to prevent owners from drawing conclusions about the expected answer.
Serum Antibodies
Serum samples were collected from all BTs and samples were stored at −70°C until assayed. All serum samples from the control group were derived from routine clinical investigation. Consent was granted by clients on the clinical consent form for admission to the centers enrolled. Serum AGA IgG and anti‐canine TG2 IgA concentrations were determined by ELISA,1 according to the manufacturer's instructions. Briefly, for detection of antibodies against canine tissue TG2, the antigen was coated onto a microtiter plate. The plate surface was blocked with bovine serum albumin prior to use with canine sera and control samples. Bound antibodies against canine TG2 were detected by incubation with peroxidase‐conjugated secondary antibody against canine IgA. In the last step, the peroxidase converts a substrate (tetramethylbenzidine) into a blue product, which that upon addition of the stop solution (0.5 M H2SO4) turns yellow. Negative control values were recorded using a buffer and conjugate.
Regarding IgG anti‐gliadin antibodies, the gliadin‐coated microtiter plate from the ELISA for the determination of antibodies (IgG) against gliadin from Steffens Biotechnische Analysen was used.2 Detection of bound antibodies against gliadin was obtained by incubation with peroxidase conjugated secondary antibody against canine IgG1. Negative control values were recorded using a buffer and conjugate.
Statistical Analysis
For comparison of continuous variables, the independent t‐test or Mann‐Whitney U‐test was performed. For comparison of categorical variables, the χ2 test or Fisher exact test was employed. The association of AGA IgG and TG2 IgA with disease characteristics was assessed by Spearman's rank of order. Sensitivity, specificity, and likelihood ratios were determined. Sensitivity was defined as the probability of a positive test result for PD in a dog with the disease. Specificity was defined as the probability of having a negative result in a dog without PD. The likelihood ratio is a measure of the validity of a test, given its sensitivity and specificity. It is calculated as the ratio of the probability of getting a result in dogs with PD to the probability of getting that same result in dogs without the condition. For all statistical analyses, P values of less than 0.05 were considered statistically significant. All statistical tests were performed using a commercial software package.3
Results
Clinical Characteristics
Controls
Twenty dogs were included as control subjects, with 9 female dogs (4 neutered) and 11 male dogs (5 neutered). The median age of control dogs was 29.5 months (range 12–85 months).
BTs with presumed PD
This group comprised 45 dogs, with 19 female (7 spayed) and 26 male (9 neutered) dogs. Median age at first onset was 36 months (range 9–96 months). Episodes typically lasted from 1 minute up to 1 hour; their frequency ranged from once every 2–3 months up to once per year. General clinical and neurological examinations were normal between attacks in all affected dogs.
Clinical features of the episodes included sustained muscular hypertonicity throughout (45/45), difficulty or an inability to stand and walk (40/45) and dystonia of 1 or more limbs (31/45). Other features reported included signs consistent with atopy (ie, dry skin, erythema, pruritus, chewing the pads, and reverse sneezing) (27/45) and signs suggestive of mild gastrointestinal disease (ie, intermittent vomiting, diarrhea, and borborygmi) (31/45). Therefore 20/45 (44%) dogs had both signs suggestive of gastrointestinal disease and a dermatopathy, 11/45 (24%) had just signs suggestive of gastrointestinal disease, and 7/27 (26%) had only signs suggestive of dermatological disease. The signs suggestive of gastrointestinal disease all predated the first episodes of dyskinesia. The signs suggestive of dermatological disease all occurred after the signs suggestive of gastrointestinal diseases, but might have preceded (8/27; 30%) or followed (19/27; 70%) the neurological signs.
All affected BTs had PD when awake. Attacks were usually evoked by sudden movements: eg, standing up suddenly after a long period of sitting or running suddenly. Some dogs experienced attacks when their owner perceived they had encountered stress or anxiety, such as if another dog came into the home or just before the administration of deworming tablets. Although reported to arise after sudden movements, all episodes occurred exclusively at or after rest, and never ensued after prolonged exercise or during sleep. Furthermore, only 1 owner reported their dog having an episode away from the home.
Involuntary intermittent movements, which could be unilateral, alternating, or bilateral, involved the head, neck, extremities, and trunk. No involuntary movements or twitching of the face were reported in any BT with PD. The single most common phenotypic presentation in our cases was that of dystonia, mirroring previous data,2 followed by tremors (fine oscillation of the muscle fibers) and whole body or head jerks (myoclonus). However, most cases presented with a combination of at least 2 movement disorders (eg, dyskinesia, myoclonus, and tremors) and 11 dogs presented with additional more complex movement disorders that were difficult to classify, involving manifestations such as head tremors10 and a trance‐like syndrome,11 as defined in a previous study.
Twenty‐one dogs exhibited postural dystonia, in that episodes were relieved on sitting or lying down. Sixteen dogs had stiffness of all 4 limbs and could not move during an episode. Unilateral muscle tightness was often the first observed sign in the majority of dogs, followed by ipsilateral choreoathetotic movements of 1 limb, subsequently spreading to hemidystonia/choreoathetosis and frequently progressing to generalized dystonic spasms and choreoathetotic movements in all 4 limbs. In dogs where limb involvement was not the first sign, head tremors or “bobbing” were frequently the preceding sign that would progress to result in dystonic and choreoathetotic movements of 1 or more limbs. Tremor, when present, would resolve on movement of the affected body part and was never observed as an isolated movement disturbance. Ballism was also a clinical sign occasionally observed in some of the affected dogs, although this was rare. A trance‐like syndrome was observed in 2 dogs, but this was never associated with the episodes of dyskinesia.
BTs with presumed IE
This group comprised 28 dogs with 12 female (5 spayed) and 16 male (5 neutered) dogs. Seizure onset was between 12 and 85 months with a median of 48 months. All 28 dogs had GTCS confirmed by video footage. Twelve dogs were reported by the owners to also have partial seizures, although none had been captured on video. Of these 12 dogs with partial seizures, all exhibited secondary generalization. Therefore, no dog was included in the study with isolated partial seizures.
Approximately half the dogs had seizures more than once a month; the frequency of seizures in the remaining dogs ranged from 1 every 2 months to 1 every 12 months. The median frequency was 1 every 81 days. Clinical features observed during seizures, corroborated by video footage, included loss of consciousness (28/28; 100%), recumbency (28/28; 100%), salivation (26/28; 93%), urination (6/28; 21%), and defecation (4/28; 14%). Regarding the focal seizures (12/28), all owners reported episodes consisting of facial twitching that might or might not spread to involve 1 or other forelimb, although these reports could not be confirmed by video. Salivation was reported by the owners in 8/12 dogs (66%) with suspected partial seizures. Nineteen of the 28 dogs were receiving antiepileptic medication at the time of analysis. No dogs were found to suffer from epileptic seizures and PD.
BTs with other conditions**
Thirty‐five BTs were diagnosed with other conditions. Eighteen dogs were female (7 neutered) and 17 were male (7 neutered) dogs. Median age at first onset was 24 months (range 4–152 months). Other conditions included dogs with gastrointestinal complaints (18/35), skin disease (12/35), and episodes that were considered neurological but did not fulfill the clinical criteria to be dyskinesia or IE (12/35). Such episodes included myoclonus (3/35), intermittent tremors (3/35), GTCS associated with a brain tumor (2/35), head bobbing (2/35), trance‐like syndrome (2/35), suspected sleep disorder (1/35), and myokymia (1/35).
Epidemiological analyses
Male and female dogs were equally distributed between groups. There was a significant difference in age onset between dogs with PGSD and IE (P < 0.013), with cases of PGSD presenting younger. There was also a significant difference in mean episode frequency and duration between IE and PGSD (P < 0.041 and P < 0.002, respectively) with epileptic seizures being more frequent but shorter in duration.
Serological Characteristics
Controls
Values for AGA IgG ranged from 0.03 to 0.27 (median 0.11). Values for TG2 IgA ranged from 0.16 to 0.53 (median 0.22). The cut‐off value for negativity was set to 0.27 for AGA IgG and 0.53 for TG2 IgA based on the results of the control dogs.
BTs with presumed PD
Serum concentrations of AGA IgG were increased above the control values in 38/45 BTs (median 0.35; range 0.11–1.68) and of TG2 IgA were increased in 41/45 BTs (median 1.31; range 0.19–2.68) in the PD group. Four BTs had normal values for both AGA IgG and TG2 IgA, but none had a history of signs suggestive of gastrointestinal disease, 3 had a mild dermatopathy.
BTs with presumed IE
AGA IgG and TG2 IgA concentrations were within the control range in 26/28 dogs. Two BTs in the epileptic seizure group had positive concentrations of both AGA IgG and TG2 IgA. One of these dogs was reported to have a persistent skin problem diagnosed at the referring vet as atopy. The other dog had occasional vomiting after eating, but this had never been investigated. Neither of these dogs was reported to have suffered partial seizures. The median concentration for AGA IgA was 0.20 (range 0.06–0.51) and was 0.40 for TG2 IgA (range 0.02–1.07).
BTs with other conditions
AGA IgG concentrations were increased in 16/35 dogs and TG2 IgA concentrations were increased in 19/35 dogs in the “Other” group. All 16 dogs with normal AGA IgG concentrations also had normal TG2 IgA concentrations. All dogs with increased concentrations had signs suggestive of either a dermatopathy, gastrointestinal condition, or both. The remaining 16 dogs with antibody concentrations within the control range exhibited atopy (4/16), intermittent tremors (2/16), myoclonus (2/16), head bobbing (2/16), GTCS associated with a brain tumor (2/16), trance‐like syndrome (2/16), suspected sleep disorder (1/16), and myokymia (1/16). The median concentration for AGA IgA was 0.26 (range 0.04–0.27) and was 0.22 for TG2 IgA (range 0.16–0.53).
Serum antibodies
As shown in Figure 1, AGA IgG were significantly increased in BTs with PD, compared with IE (P = 0.012), controls (P < 0.0001) and other (P = 0.018) conditions. There was no significant difference in AGA IgG concentrations in BT controls compared with IE (P = 1.00) and other (P = 1.00) conditions. A significant difference was also observed in TG2 IgA concentrations in BTs with PD, compared with IE (P < 0.0001), controls (P < 0.0001) and other (P = 0.012) conditions (Fig 2). There was no significant difference in TG2 IgA concentrations in BT controls compared with IE (P = 1.00) and other (P = 1.00) conditions.
Figure 1.
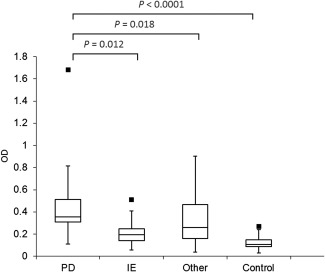
Box plots demonstrating the serum optical density of anti‐gliadin IgG (AGA IgG) in each group of dogs. The box represents the 25–75th percentile range, the line through represents the median, the range lines correspond to the highest and lowest values and outliers are represented by ▪. controls (n = 20); idiopathic epilepsy (IE; n = 28); other (n = 35); paroxysmal dyskinesia (PD; n = 45). optical density (OD).
Figure 2.
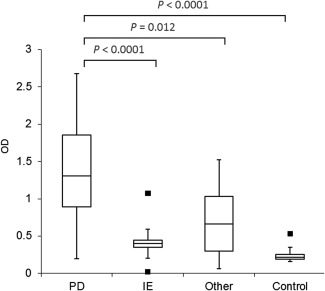
Box plots demonstrating the serum optical density of anti‐transglutaminase‐2 IgA (TG2 IgA) in each group of dogs. The box represents the 25–75th percentile range, the line through represents the median, the range lines correspond to the highest and lowest values and outliers are represented by ▪. controls (n = 20); idiopathic epilepsy (IE; n = 28); other (n = 35); paroxysmal dyskinesia (PD; n = 45). optical density (OD).
The prevalence of increased AGA IgG and TG2 IgA concentrations was evaluated among all dogs. Overall, the prevalence of increased AGA IgG and TG2 IgA concentrations in PD was 84% (38/45) and 91% (41/45), respectively, which was higher than values in IE (AGA IgG, 7%, 2/28; TG2 IgA, 7%, 2/28), other (AGA IgG, 46%, 16/35; TG2 IgA, 54%, 19/35), and control BTs (AGA IgG, 0%, 0/20; TG2 IgA, 0%, 0/20). To further assess the potential role of these autoantibodies in distinguishing BTs with PD from those with IE, P values were calculated between PD BTs and IE BTs. A significantly higher prevalence of increased AGA IgG and TG2 IgA concentrations was detected in BTs with PD (41/45, 91%), compared with BTs with IE (2/28, 7%) (P = 0.0001).
Given that AGA IgG and TG2 IgA demonstrated a good performance in distinguishing PD from IE, we evaluated the predictive power of these 2 antibodies in distinguishing PD from IE once dogs with signs suggestive of gastrointestinal and dermatological manifestations were removed from analysis. This raised the specificity and positive predictive value to 100%, but decreased the sensitivity to 62.5–75% (Table 1). The cut‐off value for negativity was set to 0.27 for AGA IgG and 0.53 for TG2 IgA, based on the results of the control dogs.
Table 1.
Predictive power of serological markers for differentiation among border terriers with paroxysmal gluten‐sensitive dyskinesia and idiopathic epilepsy.
| Sensitivity | Specificity | LR+ | LR− | |
|---|---|---|---|---|
| All dogs | ||||
| AGA IgG | 82.61% (0.70–0.93) | 93.10% (0.75–0.99) | 11.98 (3.09–45.23) | 0.19 (0.08–0.33) |
| TG2 IgA | 89.13% (0.79–0.96) | 93.10% (0.77–0.98) | 12.92 (3.35–48.65) | 0.12 (0.04–0.25) |
| Excluding dogs with signs suggestive of gastrointestinal or dermatological disease | ||||
| AGA IgG | 62.5% (0.51–0.74) | 100% (0.84–1) | – | 0.38 (0.25–0.51) |
| TG2 IgA | 75% (0.69–0.82) | 100% (0.84–1) | – | 0.25 (0.14–0.53) |
Values are given with 95% confidence intervals (in brackets). AGA IgG, anti‐gliadin antibody immunoglobulin G; TG2 IgA, anti‐transglutaminase‐2 immunoglobulin a; LR−, negative likelihood ratio; LR+, positive likelihood ratio.
Discussion
Our study confirmed a multisystem manifestation of gluten sensitivity in BTs. Our results suggest that serological assays are able to reliably identify PGSD in the BT, but that other gluten‐sensitive conditions can give a false‐positive result.
Gluten sensitivity is defined as a state of heightened immunological responsiveness to ingested gluten in genetically susceptible individuals.12 Such responsiveness might find expression in organs other than the gut. The typical presentation of NCGS in people is a combination of signs suggestive of gastrointestinal disease such as abdominal pain, nausea, bloating, flatulence, diarrhea, or constipation followed by manifestations of extraintestinal disease, such as dermatological or neurological complaints. The focus of this study was on neurological manifestations of gluten sensitivity in BTs, so‐called PGSD. It is striking that a proportion of dogs presented with gluten sensitivity manifesting as enteropathy or dermatopathy. Future studies should examine the role of various subtypes of TG in BTs to see if specific subtypes are increased with variable system expression. In the absence of canine specific TG antibodies, it is not currently possible to measure subtypes of TG.
There continues to be controversy about using phenomenological characteristics to distinguish an epileptic seizure from a PD in dogs and people. This study has shown that PGSD and epileptic seizures can be clearly separated from video footage and historical information gleaned from the owner, with this division being confirmed with serological testing. Epilepsy remains a clinical diagnosis—the history provides critical diagnostic information in most patients but it is the recognition of the epileptic event by the attending clinician that guides diagnosis. In dogs, some consider PGSD to be one of the differential diagnoses for partial‐onset motor seizures, although they are distinguishable by their reproducible triggers (eg, stress, startle), lack of facial involvement, and absence of secondary generalization. Attack semiology is also different with dystonia, chorea, ballism, or a mixture, and frequent bilateral involvement, as opposed to the unilateral focal clonus of a single limb and the involuntary facial contractions of partial‐onset motor seizures. Unfortunately, we did not identify dogs suffering only partial‐onset motor seizures in this study and instead had dogs with relatively characteristic signs of GTCS. The devil's advocate would state that we did indeed include partial‐onset motor seizures but incorrectly included them as PGSD and these dogs were represented in the results by the 4 BTs with normal serological values for TG2 and gliadin antibodies. However, partial‐onset motor seizures involve only 1 body part, as opposed to the 4 seronegative BTs in the PD group that exhibited involvement of all 4 limbs during an episode, excluding them from the definition of partial‐onset motor seizures. Electroencephalography, despite its limitations, might help to confirm these findings but our study shows the robust nature of using semiological characteristics alone to differentiate GTCS and PDs.
Paroxysmal movement disorders are being increasingly recognized in dogs. Despite their prevalence, there are a number of knowledge gaps with regards to their recognition, pathophysiology, management, and long‐term outcome. One of the key advancements made in this study is the refinement of the diagnostic work‐up to focus more on positive criteria (rather than by exclusion of organic causes), with serological markers being diagnostic for PGSD in BTs. However, biomarkers can change in response to a particular therapeutic intervention and in this case the institution of a gluten‐free diet can normalize, or at least reduce, concentrations of gluten‐specific antibodies.3 This means the utility of antibody markers to gluten might become diminished in dogs already receiving a gluten‐free diet. This part of the history taking is therefore integral to obtaining a representative result when performing gluten serology. The advantage of such specificity is in monitoring the compliance of dietary therapy and so repeated serum testing is indicated once the correct diet is implemented. Furthermore, sensitivity of these markers is just below 90% due to the fact that these markers are not exclusive to neurological disease. The degree by which a gluten‐free diet might affect the serological data was not investigated here due to the exclusion of dogs already receiving the aforementioned diet.
It seems appropriate to label our observations as gluten sensitivity in the BT. The BTs described herein demonstrate that the phenotype of gluten sensitivity might range from an enteropathy to extraintestinal disease. It remains obscure why some BTs experience PD, mild signs suggestive of gastrointestinal disease, and no skin involvement, whereas some experience mild atopy with often only mild or perhaps subclinical enteropathy with or without neurological signs. In people, CD and dermatitis herpetiformis share the same genetic involvement, further confirming that these entities are not separate disorders but belong to the same spectrum of genetic gluten intolerance.13 It seems likely a similar genetic phenomenon underlies our observations given the apparent restriction of this condition to the BT. The identification of 4 BTs with the PGSD phenotype that were serologically negative for gliadin and TG2 antibodies suggests that the presence of PD in this breed should not automatically infer a gluten‐related sensitivity. Instead, a separate pathogenesis should be considered to explain this phenomenon. We currently have no data on the clinical response of these dogs to a gluten‐free diet.
Several limitations in this study need to be highlighted. Age‐matched controls were not possible given the apparent prevalence of signs potentially relating to cutaneous, neurological, and gastrointestinal conditions in the BTs included. Dogs were not given a gluten‐free diet to monitor the serological and clinical response of the clinical signs observed. Therefore, many of these clinical signs could be gluten‐independent and any potential correlation of antibody titer to PD might only have a spurious relationship. However, the association of such clinical signs with increased antibody concentrations against gliadin and TG‐2 is supportive of such an association. It is also uncertain where the line between neurological signs and signs suggestive of gastrointestinal disease should be drawn. Dyskinesia is relatively easy to identify where involuntary movements of 1 or more limb are observed. However, it is our belief that esophageal reflux can result in pain and hence make a dog tremor. Tremors are frequently considered to be neurological but in this circumstance and in the dogs seen here it is more likely they were secondary to the gastrointestinal condition.
Conclusions
Gluten sensitivity is a condition of BTs in which the leading manifestation is neurological signs. However, recognition of PGSD has identified a wide spectrum of additional clinical presentations, with signs suggestive of dermatological and gastrointestinal disease also being observed. This might have implications in the diagnosis of PGSD based on serology alone. If a BT is presented with signs suggestive of gastrointestinal and/or dermatological disease, then this might represent a manifestation of NCGS and antibody testing could be positive. Therefore, positive serological assay results for PGSD should only be interpreted in light of the other clinical signs present.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article:
Video 1. A Border terrier with the typical signs expected with paroxysmal dyskinesia (PD). Note the low frequency involuntary muscle contractions in the limbs whilst the dog maintains awareness.
Video 2. Demonstrates the typical clinical signs of a generalized tonic‐clonic seizure (GTCS). Note the high frequency paddling movements in all four legs and loss of consciousness.
Acknowledgments
Grant support
This study was funded by the Kennel Club Charitable Trust, UK.
Conflict of Interest Declaration
Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
Institutional Animal Care and Use Committee (IACUC) or Other Approval Declaration
Authors declare no IACUC or other approval was needed.
The work was done at Dovecote Veterinary Hospital, Derby DE74 2LJ, England.
Footnotes
ZEDIRA GmbH, Darmstadt, Germany
Steffens Biotechnische Analysen GmbH, Ebringen, Germany
XLStats; Excel, Windows
References
- 1. Jankovic J, Demirkiran M. Classification of paroxysmal dyskinesias and ataxias. Adv Neurol 2002;89:387–400. [PubMed] [Google Scholar]
- 2. Black V, Garosi L, Lowrie M, et al. Phenotypic characterisation of canine epileptoid cramping syndrome in the border terrier. J Small Anim Pract 2014;55:102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lowrie M, Garden OA, Hadjivassiliou M, et al. The clinical and serological effect of a gluten‐free diet in border terriers with epileptoid cramping syndrome. J Vet Intern Med 2015;29:1564–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lowrie M, Hadjivassiliou M, Sanders DS, et al. A presumptive case of gluten sensitivity in a border terrier: A multisystem disorder? Vet Rec 2016;179:573 [DOI] [PubMed] [Google Scholar]
- 5. Catassi C, Fasano A. Celiac disease. Curr Opin Gastroenterol 2008;24:687–691. [DOI] [PubMed] [Google Scholar]
- 6. Sapone A, Lammers KM, Mazzarella G, et al. Differential mucosal IL‐17 expression in two gliadin‐induced disorders: Gluten sensitivity and the autoimmune enteropathy celiac disease. Int Arch Allergy Immunol 2002;152:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marks J, Shuster S, Watson AJ. Small‐bowel changes in dermatitis herpetiformis. Lancet 1966;10:120–1282. [DOI] [PubMed] [Google Scholar]
- 8. Hadjivassiliou M, Grünewald R, Sharrack B, et al. Gluten ataxia in perspective: Epidemiology, genetic susceptibility and clinical characteristics. Brain 2003;126:685–691. [DOI] [PubMed] [Google Scholar]
- 9. Lowrie M, Garosi L. Classification of involuntary movements in dogs: Paroxysmal dyskinesias. Vet J 2017;220:65–71. [DOI] [PubMed] [Google Scholar]
- 10. Guevar J, De Decker S, Van Ham LM, et al. Idiopathic head tremor in English bulldogs. Mov Disord 2014;29:191–194. [DOI] [PubMed] [Google Scholar]
- 11. Lowrie M, Smith PM, De Keuster T, et al. Trance‐like syndrome in bull terriers. Vet Rec 2015;177:223 [DOI] [PubMed] [Google Scholar]
- 12. Marsh MN. The natural history of gluten sensitivity: Defining, refining and re‐defining. Q J Med 1995;85:9–13. [PubMed] [Google Scholar]
- 13. Spurkland A, Ingvarsson G, Falk ES, et al. Dermatitis herpetiformis and celiac disease are both primarily associated with the HLA‐DQ (alpha 1*0501, beta 1*02) or the HLA‐DQ (alpha 1*03, beta 1*0302) heterodimers. Tissue Antigens 1997;49:29–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article:
Video 1. A Border terrier with the typical signs expected with paroxysmal dyskinesia (PD). Note the low frequency involuntary muscle contractions in the limbs whilst the dog maintains awareness.
Video 2. Demonstrates the typical clinical signs of a generalized tonic‐clonic seizure (GTCS). Note the high frequency paddling movements in all four legs and loss of consciousness.


