Abstract
Background
Sucralfate is a gastroprotectant with no known systemic effects. The efficacy of sucralfate for prevention and treatment of stress‐related mucosal diseases (SRMD) in dogs is unknown.
Hypothesis/Objectives
To develop a canine ex vivo model of SRMD and to determine the effect of sucralfate on mucosal barrier function in this model.
Animals
Gastric antral mucosa was collected immediately postmortem from 29 random‐source apparently healthy dogs euthanized at a local animal control facility.
Methods
Randomized experimental trial. Sucralfate (100 mg/mL) was applied to ex vivo canine gastric mucosa concurrent with and after acid injury. Barrier function was assessed by measurement of transepithelial electrical resistance (TER) and radiolabeled mannitol flux.
Results
Application of acidified Ringers solution to the mucosal side of gastric antrum caused a reduction in gastric barrier function, and washout of acidified Ringers solution allowed recovery of barrier function (TER: 34.0 ± 2.8% of control at maximum injury, 71.3 ± 5.5% at recovery, P < .001). Sucralfate application at the time of injury or after injury significantly hastened recovery of barrier function (TER: 118.0 ± 15.2% of control at maximum injury, P < .001 and 111.0 ± 15.5% at recovery, P = .35).
Conclusions and Clinical Importance
Sucralfate appeared effective at restoring defects in gastric barrier function induced by acid and accelerating repair of tissues subjected to acid in this model, suggesting that sucralfate could have utility for the treatment and prevention of SRMD in dogs.
Keywords: barrier function, stress ulcer, transepithelial electrical resistance, Ussing chamber
Abbreviations
- CDAD
clostridium difficile‐associated diarrhea
- H2RA
histamine‐2 receptor antagonist
- ICU
intensive care unit
- Isc
short circuit current
- NSAID
non‐steroidal anti‐inflammatory drug
- PD
potential difference
- PPI
proton pump inhibitor
- H&E
hematoxylin and eosin
- SRMD
stress‐related mucosal disease
- TER
transepithelial electrical resistance
- SUP
stress ulcer prophylaxis
1. INTRODUCTION
During critical illness, several factors contribute to the loss of gastric mucosal barrier defenses. Splanchnic hypoperfusion occurs,1, 2, 3 reducing gastric motility,4 and prolonging exposure of gastric mucosa to acid and other irritants. Decreased gastric microcirculation diminishes acid‐buffering capacity and mucus and bicarbonate secretion.1, 2, 3 Nonsteroidal anti‐inflammatory drugs (NSAIDs) and corticosteroids inhibit prostaglandin release, impeding mucosal healing and recovery.5 These combined factors lead to development of gastric erosions, ulcers, and gastrointestinal hemorrhage, which are collectively termed stress‐related mucosal disease (SRMD).
Before the widespread use of SRMD prophylaxis in ICUs, SRMD occurred in up to 100% of human ICU patients, 8% of which had clinically relevant hemorrhage, and this hemorrhage was associated with a 5‐times increased risk of death.6, 7, 8 ICU‐hospitalized dogs had a similar rate (7%) of clinically relevant hemorrhage associated with 5‐fold increased risk of death; this included both dogs with melena (consistent with SRMD) and hematochezia, so the prevalence of SRMD cannot be extrapolated from this study.9 Forty‐nine percent of Alaskan sled dogs develop SRMD.10 There are no models of the condition relevant to dogs.
In people, stress ulcer prophylaxis (SUP) has become the standard of care for prevention of SRMD in high‐risk patient populations. Human ICUs largely prescribe either proton pump inhibitors (PPIs, 66% of SUP protocols) or histamine2 receptor antagonists (H2RAs, 30% of SUP protocols).11 Acid suppression with omeprazole leads to proliferation of bacteria in the gastric lumen in both species.12, 13 PPI administration in people carries a 3.5‐fold risk for the development of Clostridium difficile‐associated diarrhea (CDAD), which is independently associated with longer ICU stays and increased mortality.11, 14 The prevalence of infection by pathogenic Clostridia species in dogs receiving PPI treatment is unknown. Overall, a meta‐analysis concluded that the evidence for SUP in human ICU patients is limited and further work is needed to determine the role of various SUP protocols on outcome.15
A recent review of SRMD in dogs recommended standard use of SUP, particularly PPIs, in critically ill dogs.16 Just as the efficacy of SUP for critically ill dogs has not been established, the adverse effects of acid suppression in critically ill dogs is unknown. There might be as yet unidentified risk factors for SRMD in dogs, as in people, that would be an indication for SUP.
Sucralfate, an alternative prophylactic treatment for SRMD, is a complex of sucrose and aluminum salts. Sucralfate binds to negatively charged subepithelial proteins exposed during mucosal injury, forming a viscous layer that protects the vascular bed and proliferative zone, allowing for epithelial restitution.17 Sucralfate absorbs and reduces the activity of pepsin, and is cytoprotective as well as antiapoptotic.18, 19 Sucralfate stimulates mucus synthesis and secretion and bicarbonate secretion.20, 21 Compared with H2RAs and PPIs, this drug does not increase bacterial colonization and therefore has a lower likelihood of CDAD and is protective against the development of nosocomial pneumonia in people.14 It was as effective as H2RAs for prevention of overt gastric bleeding events in critically ill people with lower rates of ventilator‐associated pneumonia and gastric colonization.22 Sucralfate does not affect CYP450 enzymes, so there is no effect on the therapeutic effect of concurrently administered medications. No SUP protocol has been examined for treatment or prevention of SRMD in dogs.
The objectives of this study were to develop an ex vivo SRMD model of canine gastric mucosa and to examine the effect of sucralfate on mucosal barrier function. Sucralfate was administered concurrently with acid injury and immediately after the injury with the aim of determining its efficacy both as a protective and reparative drug. The antral and pyloric regions of gastric mucosa were used as they are the most frequent sites for gastric ulceration in dogs.13, 23
2. MATERIALS AND METHODS
2.1. Gastric tissue acquisition
Tissue samples were obtained from dogs that were euthanized at a local animal shelter for the purpose of local population control. The investigators had no influence on the selection of dogs for euthanasia or the timing of euthanasia. The NC State University Institutional Animal Care and Use Committee reviewed the study, and waived approval of the study because investigators solely collected tissues from euthanized dogs, and did not take part in the euthanasia of dogs. Shelter staff used an overdose of pentobarbital to euthanize dogs. Dogs that were surrendered for illness or had obvious signs of systemic disease were excluded.
The precise age of the dogs was unknown in most cases, but ranged from ∼8 months to 10 years‐of‐age. The dogs were typically mixed breed, ranging in size from ∼10 to 30 kg. All dogs were euthanized with an overdose of sodium pentobarbital. Immediately after euthanasia, the entire antral‐pyloric section of the stomach was excised, then incised along the greater curvature and placed mucosa side down in oxygenated (95% O2, 5% CO2) Ringer's solution (Ringer's solution additives, in mM: 114.0 NaCl, 5.0 KCl, 1.25 CaCl2, 1.10 MgCl2, 25.0 NaHCO3, 0.3 NaH2PO4, and 1.65 Na2HPO4) at room temperature. After a 20‐ to 25‐minute transport time to the laboratory, the tissue was transferred to oxygenated Ringer's solution at room temperature and the seromuscular layer was removed via blunt dissection. The remaining antral mucosa tissue was mounted on Ussing chambers (1.1 cm2 diameter). Tissues were mounted on Ussing chambers from each animal for all control and treatment groups. Tissue samples from various areas of the antral mucosa were randomly assigned as either control or treatment groups.
2.2. Ussing chambers
Mucosa was bathed on both the mucosal and serosal sides of the tissue mounted on the chambers with 10 mL of oxygenated Ringer's solution maintained at 37°C by water‐jacketed reservoirs. As previously described,24 10 mmol/L glucose was added to the serosal bathing solution, which was balanced with the addition of 10 mmol/L mannitol in the mucosal bathing solution. Treatments were applied to individual tissues after a 30‐minute incubation period.
2.3. Objective 1: SRMD model development
2.3.1. Stage 1: Optimization of injury model
Ringer's solution, titrated to one of three pH concentrations: 1.1, 1.2, 1.3, was applied to the mucosal side of the gastric mucosa for either 30 or 45 minutes. Each of these treatments was applied to the gastric mucosa from 4 dogs. Tissue from each dog was used in all three treatment groups and the control.
2.3.2. Stage 2: Hydrochloric acid injury model validation
After selection of the optimal injury model (pH 1.2 for 45 minutes) based on transepithelial electrical resistance (TER) levels, tissue from an additional 25 dogs was used in studies to further evaluate this model and as has been previously described.25 Tissue from each dog was treated on Ussing chambers with the acid injury model with additional tissue from each dog mounted without injury as control (neutral pH Ringer's solution only). Outcome measures were: TER, 3H‐mannitol flux (see below for description), and histological evaluation.
2.4. Objective 2: Model responsiveness: Effect of sucralfate
2.4.1. Stage 1: Sucralfate + acid injury concurrently
Ringer's solution was titrated to pH 1.2 with hydrochloric acid. Sucralfate was dissolved in dimethyl sulfoxide (DMSO) at 1 g/mL as it was the maximum dose that was soluble; one milliliter was added to the 10‐mL chamber to give a final dose of 100 mg/mL, or a total of 1 g. DMSO was used as the recommended vehicle by the manufacturer. One gram was selected as it is the recommended dose in vivo.26 Sucralfate + acidified Ringer's solution was applied to the mucosal aspect of the tissue for 45 minutes. After this injury period, acidified Ringer's solution was removed and replaced with neutral Ringer's solution. When acidified Ringer's was removed, sucralfate was added with the neutral Ringer's solution to maintain a 100 mg/mL concentration throughout this experiment. One Ussing chamber was maintained with neutral Ringer's solution and another treated with acidified Ringer's solution without sucralfate as controls. Each of these treatments was applied to the gastric mucosa of 9 dogs. Tissue from each dog was used in both treatment and control groups. Outcome measures were TER, 3H‐mannitol flux, and histological evaluation.
2.4.2. Stage 2: Sucralfate after acid injury
Acid injury was induced as previously described. Immediately after acid injury (75 minutes), 1 mL of 1 g/mL of sucralfate (sucrose octasulfate) in DMSO was added to neutral Ringer's solution on the mucosal aspect of the tissue to give a final dose of 100 mg/mL. Controls included uninjured control, acid injury control, uninjured control + sucralfate, and injured control + vehicle (DMSO). Outcome measures were TER, 3H‐mannitol flux, and histological evaluation. These treatments were applied to mucosa from 8 dogs. Tissue from each dog was used in both treatment and control groups.
2.5. Transepithelial resistance
The spontaneous potential difference (PD) was measured with Ringer‐agar bridges connected to calomel electrodes, and the PD was short‐circuited through silver‐silver chloride electrodes with a voltage clamp that corrected for fluid resistance. TER (Ω·cm2) was calculated from the spontaneous PD and short circuit current (Isc). If the spontaneous PD was between −1 and 1 mV, tissues were current clamped at ± 100 μA for 5‐seconds and the PD was recorded. The Isc and PD were recorded every 15 minutes for 210 minutes. Data were entered into spreadsheets that calculated TER from Isc and PD using Ohm's law.
2.6. 3H‐mannitol flux
As a second indicator of gastric permeability, flux of 3H‐labeled mannitol across the mucosa was measured. Two‐hundred molar of 3H‐radiolabeled mannitol was added to the mucosal reservoir. Samples were taken from both the serosal and mucosal reservoirs after 3 minutes to establish baseline radioactivity. As is standardly performed,24 two 1‐hour mucosal‐to‐serosal fluxes were performed by sampling serosal bathing solutions at 1 and 2 hours after addition of radiolabeled mannitol.
2.7. Histological examination
Gastric mucosal samples were taken for each dog before mounting on Ussing chambers. After 210 minutes, the tissues were collected from each treatment group in Carnoy's fixative for 24 hours and transferred to 70% ethanol. Samples were sectioned at 5 µm, stained with hematoxylin and eosin (H&E), and viewed with a light microscope. Additional sections from each treatment was stained with Alcian blue and periodic acid‐Schiff (PAS) stain to stain the gastric mucus layer and viewed with a light microscope.
2.8. Statistical analysis
A 2‐way repeated measures ANOVA was used to compare TER data. If two groups were compared, a t‐test (parametric data) or rank sum test (nonparametric data) was used. If there were more than two groups, a one‐way ANOVA was used to analyze prostanoid and flux data; a Kruskal‐Wallis ANOVA on ranks was used to analyze nonparametric prostanoid and flux data. The Tukey's post hoc test was used to detect differences among treatments and time when significance was detected during the initial ANOVA. Histologic data was descriptively reported. Significance was set at P < .05. Data are represented at means ± SE.
3. RESULTS
3.1. Objective 1: SRMD model development
3.1.1. Stage 1: Optimization of injury model
Gastric mucosa from 4 dogs was subjected to HCl on its mucosal surface within Ussing chambers at one of three pH concentrations: 1.1, 1.2, or 1.3 for either 30‐ (Figure 1A) or 45‐ (Figure 1B) minutes. All treatments induced a significant decrease in TER during the period of injury, and dose‐dependent reductions in gastric barrier function were noted. Once acidified Ringer's was replaced with neutral Ringer's solution, TER partially recovered in all treatment groups. Based on these experimental results, an acid exposure time of 45 minutes was selected for further study because of the more gradual recovery of TER (Figure 1B). In addition, an acid concentration of pH 1.2 was selected because it induced reliable reductions in barrier function in all dogs without irreversible injury that resulted in an inability to gain an electrical reading. The latter occurred in 1 dog with pH 1.1 for 45 minutes.
Figure 1.
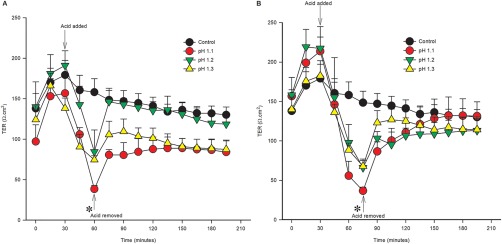
Acid injury titration‐time experiment. TER was measured as an index of gastric barrier function. A, pH 1.1, 1.2, and 1.3 were applied to the mucosal side of the tissue for 30 minutes. Acid injury caused a significant decrease in TER at 60 minutes (*, P < .05). B, pH 1.1, 1.2, and 1.3 were applied to the mucosal side of the tissue for 45 minutes. Acid injury caused a significant decrease in TER at 75 minutes (1B) (*, P < .05). After replacement with neutral Ringer's solution, TER returned to control values within 15–30 minutes. Acid injury of pH 1.2 for 45 minutes was selected for further investigation. Values represent means ± SE. n = 4
3.1.2. Stage 2: Hydrochloric acid injury model validation
The acid injury model of pH 1.2 for 45 minutes was applied to an additional 25 dogs' gastric mucosa to assess its consistency (Figure 2). This model produced a reliable decrease in barrier function, measured both by TER and by mucosal‐to‐serosal flux of 3H‐mannitol. After 45 minutes of acid injury, TER in acid‐injured tissue was 34.0 ± 2.8% of control TER. After the 45‐minute acid injury period, barrier function partially recovered but remained significantly decreased compared with control; TER of injured tissue at 210 minutes was 71.3 ± 5.5% of control tissue at 210 minutes (Figure 2A, P < .001). Flux of radiolabeled mannitol in injured tissue was significantly increased to 183.4 ± 38.4% that of control (Figure 2B, P = .006).
Figure 2.
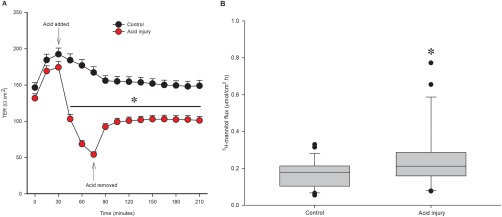
Acid injury model. For selected injury, acid Ringer's solution pH 1.2 was applied to mucosal side for 45 minutes then replaced with neutral Ringer's solution. A, TER is significantly lower in acid‐injured tissue during injury and recovery periods (*, P < .001, n = 29). B, Flux of 3H‐mannitol is increased with acid injury, indicating a change in paracellular permeability (µmol of mannitol flux per cm2 per hour, *, P = .006, n = 29)
Hematoxylin and eosin staining of tissues showed little effect of Ussing chamber incubation for the duration of the experiment on tissue morphology (Figure 3A,B). Alternatively, acid‐injured tissue consistently had moderate to marked sloughing of gastric epithelial cells in all samples (Figure 3C). By the end of the experiment, gastric mucosa appeared grossly to have a thickened mucus layer in acid injured tissue as compared with control tissue (Figure 3D). Because of this observation, PAS/Alcian blue stains were performed to evaluate the gastric mucus layer (Figure 3). Uninjured control tissue demonstrated a thin layer of gastric mucus (Figure 3E), whereas acid‐injured tissue uniformly had a thicker mucus layer, with sloughed gastric epithelial cells trapped within the mucus in injured tissue (Figure 3F).
Figure 3.
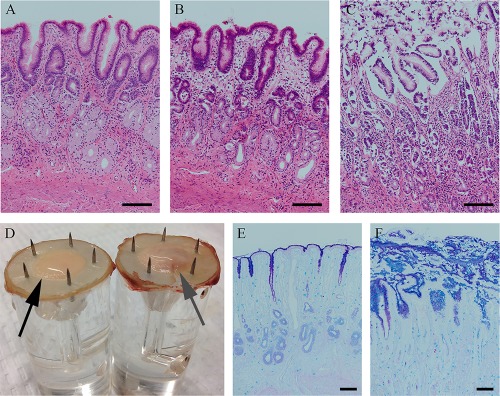
Appearance of tissue subjected to acid in Ussing chambers. A‐C, Hematoxylin and eosin stain. Tissue processed at the time of euthanasia (A) is similar to control tissue (B) processed at the end of the experiment, showing no alteration by placement on Ussing chambers. Acid‐injured tissue showed moderate to marked sloughing of gastric epithelial cells superficially (C). D, Grossly, acid‐injured tissue (right, gray arrow) had a subjectively thicker mucus layer than control tissue (left, black arrow). E,F, Periodic acid‐Schiff/Alcian blue stain. Uninjured control tissue (E) had a thin layer of mucus. Acid‐injured tissue (F) had a thick layer of mucus, which included sloughed epithelial cells within the mucus layer. These selections demonstrate characteristic changes seen consistently in all samples. Bar = 100 µm
3.2. Objective 2: Model responsiveness: Effect of sucralfate
3.2.1. Stage 1: Sucralfate + acid injury concurrently: Administration of sucralfate at the time of acid application ameliorates injury
Initially, sucralfate and acid were applied concurrently to the mucosal side of the Ussing chamber using the same acid injury model (pH 1.2 for 45 minutes) from 9 dogs. Sucralfate (100 mg/mL) was administered in acidified Ringer's solution, at a dose previously established during in vitro studies.27 When acidified Ringer's was removed, sucralfate was added with the neutral Ringer's solution to maintain 100 mg/mL throughout the experiment. When sucralfate treatment was added at the same time as the commencement of acid injury, sucralfate attenuated the decrease in TER induced by acid (Figure 4A, P < .001). At 75 minutes, TER of this subset of acid‐injured tissues was 42.5 ± 11.1% of control; TER of sucralfate treated, acid injured tissue at 75 minutes was 118.0 ± 15.2% of control. By the end of the recovery period at 210 minutes, acid‐injured tissue was 71.4 ± 11.6% of control; sucralfate treatment significantly increased this recovery to 119.1 ± 12.0% of control.
Figure 4.
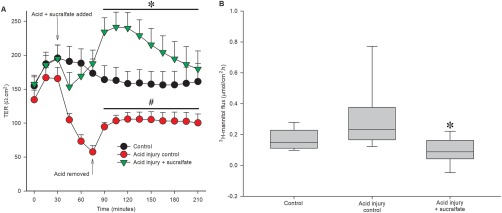
Administration of sucralfate ameliorates acid injury. A, Acid Ringer's solution induced a significant decrease in TER as compared with uninjured control (*P = .003). Sucralfate treatment concurrent with acid injury significantly attenuated the decrease in barrier function induced by acid Ringer's solution (#, P < .001, n = 9 for treatment and control). B, There was a significant effect of treatment on flux of radiolabeled mannitol across canine gastric mucosa (P = .008). Sucralfate administered concurrently with acid injury significantly decreased 3H‐mannitol flux compared with acid injury control (n = 9 for treatment and control)
Sucralfate also attenuated the decrease in gastric permeability, as determined by mucosal‐to‐serosal flux of 3H‐mannitol (Figure 4B, P = .008). Permeability to this molecule was increased in acid‐injured tissue (control flux: 0.17 ± 0.02 µmol/cm2·h, acid injury flux: 0.29 ± 0.07 µmol/cm2·h). When sucralfate was applied with acid Ringer's solution, flux of 3H‐mannitol was significantly decreased (sucralfate + acid injury flux: 0.09 ± 0.03 µmol/cm2·h, P = .008).
The effect of sucralfate on uninjured tissue was also examined. Transepithelial resistance and mannitol flux were not different between control tissue and uninjured control with sucralfate added at 75 minutes (data not shown). In addition, DMSO, the vehicle for sucralfate delivery, was administered after acid injury; acid injury with the addition of DMSO showed similar TER recovery to acid‐injured tissues in the absence of DMSO (data not shown).
Histologically, control tissues maintained largely unchanged morphology compared with baseline tissues taken at the time of tissue collection with only occasional apoptotic cells (Figure 5A,B). However, exposure to HCl induced moderate to marked sloughing of the superficial gastric epithelium with associated thickening of the mucus layer (Figure 5C). When sucralfate was administered concurrently with acid, little to no tissue sloughing or apoptosis was noted in any samples although the mucus/sucralfate layer was of a similar thickness to the acid‐injured control tissue (Figure 5D).
Figure 5.
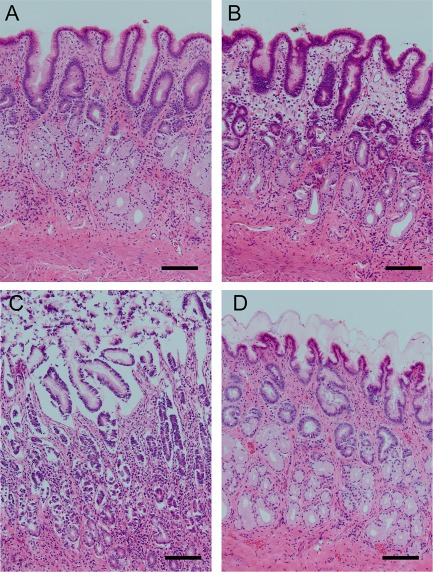
Histological appearance of acid‐injured tissues pretreated with or without sucralfate. Tissues processed at the time of euthanasia (baseline, A) are similar to control tissues (B) mounted on Ussing chambers with occasional apoptotic epithelial cell noted. Acid injury (C) induced moderate to marked epithelial cell sloughing. Treatment with sucralfate concurrent with injury (D) protected against morphologic change induced by acid. These selections demonstrate characteristic changes seen consistently in all samples. Bar = 100 µm
3.2.2. Stage 2: Sucralfate after acid injury: Sucralfate enhances recovery when administered after acid injury
To evaluate sucralfate as a treatment for pre‐existing alterations in barrier function, sucralfate was applied after the initial acid injury in gastric tissues from n = 8 dogs. The acid injury was applied as described previously. Immediately after acid injury (at the completion of the 45‐minute injury period), sucralfate was applied to the mucosal bathing reservoir to give a concentration of 100 mg/mL in neutral Ringer's solution. In this subset of dogs, sucralfate increased TER during recovery (Figure 6A; acid injury: 75.1 ± 4.8% of control at 210 minutes, sucralfate + acid injury: 111.0 ± 15.5% of control at 210 minutes, P < .035). Transepithelial resistance of sucralfate‐treated, acid‐injured tissue was higher than acid‐injured control tissue from 150 minutes until the end of the experiment. In this group of 8 canine tissues, there was not an overall significant effect of treatment on mucosal permeability to radiolabeled mannitol (Figure 6B; control flux: 0.13 ± 0.01 µmol/cm2 h, acid injury flux: 0.15 ± 0.02 µmol/cm2 h, sucralfate + acid injury flux: 0.12 ± 0.01 µmol/cm2 h). Similar to sucralfate administered at the time of injury, sucralfate administered at the beginning of the recovery period had a continuous layer of epithelium, whereas in the absence of sucralfate treatment, there was notable evidence of epithelial sloughing (Figure 7).
Figure 6.
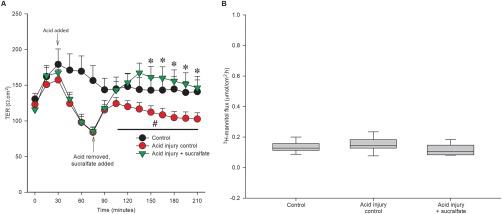
Treatment of acid‐injured tissues with sucralfate enhances recovery. A, Acid injury induced a significant decrease in TER compared with uninjured control (+, P = .002). When sucralfate was administered after acid injury, TER recovered to a higher level than untreated, injured tissue. Acid‐injured tissue treated with sucralfate had a significantly higher TER from 150 minutes until the end of the experiment (*P < .035, n = 8). B, There is not a significant effect of injury or sucralfate treatment on 3H‐mannitol flux (P = 0.214, n = 8)
Figure 7.
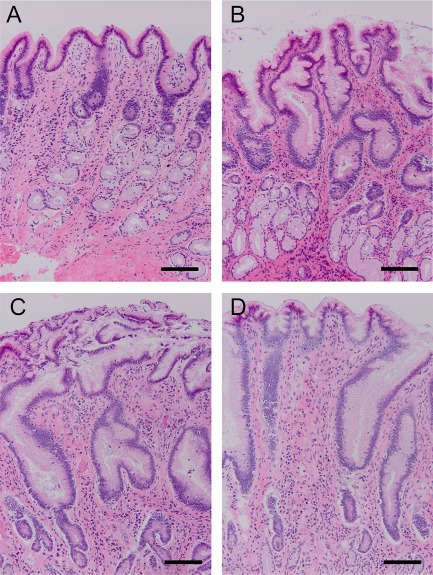
Histological appearance of acid‐injured tissues treated with or without sucralfate. Tissues processed at the time of euthanasia (baseline, A) are similar to control tissues (B) mounted on Ussing chambers with occasional apoptotic epithelial cell noted. Acid injury (C) induced moderate to marked epithelial cell sloughing. Treatment with sucralfate after injury (D) protected against morphologic change induced by acid. These selected tissues demonstrate characteristic changes seen consistently in all samples. Bar = 100 µm
4. DISCUSSION
Using a novel gastric canine ex vivo model of SRMD, a single dose of sucralfate administered at the time of injury attenuated changes in mucosal barrier function and tissue morphology. When administered immediately after acid injury, sucralfate accelerated recovery from acid injury. These findings suggest potential utility for SUP using sucralfate in dogs.
The Ussing chamber mimics the alteration in submucosal blood flow that precipitates SRMD, resulting in mucosal injury at a physiologically relevant pH.28 Because pH 1.2 is in the range of daily pH variation, gastric ulceration would not necessarily be expected in vivo. However, in the present experiments, tissues in the Ussing chamber model are isolated from a blood supply, removing an important gastroprotective mechanism, likely allowing for tissue to be more easily injured than in vivo, similar to injury that occurs during splanchnic hypoperfusion. The model of pH 1.2 for 45 minutes was selected from a number of time and acid injury combinations because of its reliability of producing marked but recoverable acid injury.
When sucralfate was applied to the gastric mucosa at the completion of acid injury, treatment accelerated recovery of barrier function and normalized tissue morphology. Flux of 3H‐mannitol as a measurement of gastric permeability was not altered by sucralfate treatment in this stage of the investigation, which is not surprising given that mannitol fluxes detect changes in barrier function over larger blocks of time (1 hour versus 15 minutes) and as such, the remaining time post‐injury might not have been sufficient to detect a difference in barrier function. This model of injury more closely mimics treatment with sucralfate in patients with pre‐existing damage to the gastric mucosa, implying that sucralfate could be used to treat pre‐existing SRMD.
The mechanism by which sucralfate exerted is protective and reparative effects to injured mucosa was not specifically examined in the current study. Histologically, PAS/Alcian blue demonstrated a thick proteoglycan layer in sucralfate treated tissues (data not shown), implying a potential role of sucralfate in increasing mucus production. However, we could not conceive of an accurate method to measure significant differences in the mucus layer. For example, in Figure 3, injury causes an apparent increased thickness of the mucus layer, but the irregular nature of this thickening precluded measuring and statistically analyzing these changes. Instead, we relied on our primary goal of measuring gastric barrier function in treated tissues.
There are several limitations to the use of the Ussing chamber model to study gastric injury and specifically SRMD. It allows only for the study of peracute injury (induced within an hour) because of the viability of canine gastric mucosa ex vivo, so the duration of efficacy of sucralfate could not be examined. Drugs that have a delayed effect on tissues, such as a PPI, would be difficult to investigate without pretreatment of dogs before euthanasia. The Ussing chamber model is not an ideal replication of splanchnic hypoperfusion and does not allow for the interaction of other host factors, such as gastric motility or concurrent medications. However, it has the advantage of allowing for the examination of barrier function in a more comprehensive manner than would be possible with an in vitro or in vivo model.
This investigation was completed using tissue from dogs that were previously scheduled for euthanasia and included a variety of breeds and ages. The effect of age, sex, and neuter status did not affect baseline barrier function (data not shown). However, there was a large degree of inter‐dog variability that might be in part explained by the variation in ages and breeds. Nonetheless, no research animals were used and in spite of the individual variation among dogs, a significant protective and reparative effect of sucralfate was demonstrated.
This ex vivo model of SRMD provided an avenue to investigate the pathophysiology and potential treatment of this disease syndrome in dogs, and demonstrated that sucralfate effectively prevented and accelerated healing of acid‐induced mucosa. We conclude that sucralfate could be an effective alternative SUP in dogs that can tolerate oral medications. Further work is needed to examine the efficacy of sucralfate as a prophylactic and treatment for SRMD through in vivo studies and with critically ill dogs.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
Hill TL, Lascelles BDX, Blikslager AT. Effect of sucralfate on gastric permeability in an ex vivo model of stress‐related mucosal disease in dogs. J Vet Intern Med. 2018;32:670–678. https://doi.org/10.1111/jvim.15076
Funding information Novartis Animal Health; National Institutes of Health, Grant/Award Number: T32 OD011130; National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Number: P30 DK034987
REFERENCES
- 1. Fennerty MB. Pathophysiology of the upper gastrointestinal tract in the critically ill patient: rationale for the therapeutic benefits of acid suppression. Crit Care Med. 2002;30:S351–S355. [DOI] [PubMed] [Google Scholar]
- 2. Takeda S, Sato N, Tomaru T. Haemodynamic and splanchnic organ blood flow responses during sevoflurane‐induced hypotension in dogs. Eur J Anaesthesiol. 2002;19:442–446. [DOI] [PubMed] [Google Scholar]
- 3. Lagoa CE, de Figueiredo L, Cruz RJ Jr, Silva E, Rocha e Silva M. Effects of volume resuscitation on splanchnic perfusion in canine model of severe sepsis induced by live Escherichia coli infusion. Crit Care. 2004;8:R221–R228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stupak DP, Abdelsayed GG, Soloway GN. Motility disorders of the upper gastrointestinal tract in the intensive care unit: pathophysiology and contemporary management. J Clin Gastroenterol. 2012;46:449–456. [DOI] [PubMed] [Google Scholar]
- 5. Wooten JG, Blikslager AT, Marks SL, et al. Effect of nonsteroidal anti‐inflammatory drugs with varied cyclooxygenase‐2 selectivity on cyclooxygenase protein and prostanoid concentrations in pyloric and duodenal mucosa of dogs. Am J Vet Res. 2009;70:1243–1249. [DOI] [PubMed] [Google Scholar]
- 6. Laine L, Takeuchi K, Tarnawski A. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology. 2008;135:41–60. [DOI] [PubMed] [Google Scholar]
- 7. DA Peura, Johnson LF. Cimetidine for prevention and treatment of gastroduodenal mucosal lesions in patients in an intensive care unit. Ann Intern Med. 1985;103:173–177. [DOI] [PubMed] [Google Scholar]
- 8. Cook D, Heyland D, Griffith L, Cook R, Marshall J, Pagliarello J. Risk factors for clinically important upper gastrointestinal bleeding in patients requiring mechanical ventilation. Crit Care Med. 1999;27:2812–2817. [DOI] [PubMed] [Google Scholar]
- 9. Swann JW, Maunder CL, Roberts E, McLauchlan G, Adamantos S. Prevalence and risk factors for development of hemorrhagic gastro‐intestinal disease in veterinary intensive care units in the United Kingdom. J Vet Emerg Crit Care (San Antonio). 2016;26:419–427. [DOI] [PubMed] [Google Scholar]
- 10. Davis MS, Willard MD, Nelson SL, et al. Prevalence of gastric lesions in racing Alaskan sled dogs. J Vet Intern Med. 2003;17:311–314. [DOI] [PubMed] [Google Scholar]
- 11. Krag M, Perner A, Wetterslev J, et al. Stress ulcer prophylaxis in the intensive care unit: an international survey of 97 units in 11 countries. Acta Anaesthesiol Scand. 2015;59:576–585. [DOI] [PubMed] [Google Scholar]
- 12. Garcia‐Mazcorro JF, Suchodolski JS, Jones KR, et al. Effect of the proton pump inhibitor omeprazole on the gastrointestinal bacterial microbiota of healthy dogs. FEMS Microbiol Ecol. 2012;80:624–636. [DOI] [PubMed] [Google Scholar]
- 13. Mowat C, Williams C, Gillen D, et al. Omeprazole, Helicobacter pylori status, and alterations in the intragastric milieu facilitating bacterial N‐nitrosation. Gastroenterology. 2000;119:339–347. [DOI] [PubMed] [Google Scholar]
- 14. Buendgens L, Bruensing J, Matthes M, et al. Administration of proton pump inhibitors in critically ill medical patients is associated with increased risk of developing Clostridium difficile‐associated diarrhea. J Crit Care. 2014;29:696.e11–695. [DOI] [PubMed] [Google Scholar]
- 15. Krag M, Perner A, Wetterslev J, et al. Stress ulcer prophylaxis in the intensive care unit: is it indicated? A topical systematic review. Acta Anaesthesiol Scand. 2013;57:835–847. [DOI] [PubMed] [Google Scholar]
- 16. Monnig AA, Prittie JE. A review of stress‐related mucosal disease. J Vet Emerg Crit Care (San Antonio). 2011;21:484–495. [DOI] [PubMed] [Google Scholar]
- 17. Szabo S, Hollander D. Pathways of gastrointestinal protection and repair: mechanisms of action of sucralfate. Am J Med. 1989;86:23–31. [DOI] [PubMed] [Google Scholar]
- 18. Matsuu‐Matsuyama M, Shichijo K, Okaichi K, et al. Protection by polaprezinc against radiation‐induced apoptosis in rat jejunal crypt cells. J Radiat Res. 2008;49:341–347. [DOI] [PubMed] [Google Scholar]
- 19. Kaya N, Boyunapa H, Baris S, et al. Protective effects of sucralfate and omeprazole on gastric mucosal damage induced by ethanol in rats. Wien Klin Wochenschr. 1998;110:96–100. [PubMed] [Google Scholar]
- 20. Shorrock CJ, Rees WD. Effect of sucralfate on human gastric bicarbonate secretion and local prostaglandin E2 metabolism. Am J Med. 1989;86:2–4. [DOI] [PubMed] [Google Scholar]
- 21. Takahashi S, Okabe S. Effects of sucralfate and its components on indomethacin‐induced damage to cultured rabbit gastric mucosal cells. J Physiol Pharmacol. 1996;47:611–619. [PubMed] [Google Scholar]
- 22. Huang J, Cao Y, Liao C, et al. Effect of histamine‐2‐receptor antagonists versus sucralfate on stress ulcer prophylaxis in mechanically ventilated patients: a meta‐analysis of 10 randomized controlled trials. Crit Care. 2010;14:R194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stanton ME, Bright RM. Gastroduodenal ulceration in dogs retrospective study of 43 cases and literature‐review. J Vet Intern Med. 1989;3:238–244. [DOI] [PubMed] [Google Scholar]
- 24. Blikslager AT, Zimmel DN, Young KM, et al. Recovery of ischaemic injured porcine ileum: evidence for a contributory role of COX‐1 and COX‐2. Gut. 2002;50:615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hill TL, Lascelles BD, Law JM, et al. The effect of tramadol and indomethacin coadministration on gastric barrier function in dogs. J Vet Intern Med. 2014;28:793–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. KuKanich K, KuKanich B, Harris A, et al. Effect of sucralfate on oral minocycline absorption in healthy dogs. J Vet Pharmacol Ther. 2014;37:451–456. [DOI] [PubMed] [Google Scholar]
- 27. Kato S, Nishiwaki H, Konaka A, et al. Mucosal ulcerogenic action of monochloramine in rat stomachs: effects of polaprezinc and sucralfate. Dig Dis Sci. 1997;42:2156–2163. [DOI] [PubMed] [Google Scholar]
- 28. Tolbert K, Bissett S, King A, et al. Efficacy of oral famotidine and two omeprazole formulations for the control of intragastric pH in dogs. J Vet Int Med. 2010;24:722. [DOI] [PubMed] [Google Scholar]


