Abstract
Background
Tetrastarch can cause acute kidney injury (AKI) in humans with sepsis, but less likely to result in tissue edema than lactated Ringer's solution (LRS).
Objectives
Compare effects of volume replacement (VR) with LRS and 6% tetrastarch solution (TS) on extravascular lung water (EVLW) and markers of AKI in hemorrhaged dogs.
Animals
Six healthy English Pointer dogs (19.7–35.3 kg).
Methods
Prospective crossover study. Animals underwent anesthesia without hemorrhage (Control). Two weeks later, dogs hemorrhaged under anesthesia on 2 occasions (8‐week washout intervals) and randomly received VR with LRS or TS at 3 : 1 or 1 : 1 of shed blood, respectively. Anesthesia was maintained until 4 hour after VR for EVLW measurements derived from transpulmonary thermodilution cardiac output. Neutrophil gelatinase‐associated lipocalin (NGAL) and creatinine concentrations in plasma and urine were measured until 72 hour after VR.
Results
The EVLW index (mL/kg) was lower at 1 hour after TS (10.0 ± 1.9) in comparison with controls (11.9 ± 3.4, P = 0.04), and at 4 hour after TS (9.7 ± 1.9) in comparison with LRS (11.8 ± 2.7, P = 0.03). Arterial oxygen partial pressure‐to‐inspired oxygen fraction ratio did not differ among treatments from 0.5 to 4 hour after VR. Urine NGAL/creatinine ratio did not differ among treatments and remained below threshold for AKI (120,000 pg/mg).
Conclusions and Clinical Importance
Although TS causes less EVLW accumulation than LRS, neither fluid produced evidence of lung edema (impaired oxygenation). Both fluids appear not to cause AKI when used for VR after hemorrhage in healthy nonseptic dogs.
Keywords: Colloids, Crystalloids, Hydroxyethyl starch, Neutrophil gelatinase‐associated lipocalin
Abbreviations
- AKI
acute kidney injury
- ANH
acute normovolemic hemodilution
- BW
blood withdrawal
- CI
cardiac index
- COP
colloid oncotic pressure
- CVP
central venous pressure
- ETISO
end‐tidal isoflurane concentration
- EVLWI
extravascular lung water index
- GEDVI
global end‐diastolic volume index
- Hct
hematocrit
- HR
heart rate
- LRS
lactated Ringer's solution
- MAP
mean arterial pressure
- NGAL
neutrophil gelatinase‐associated lipocalin
- PaO2/FiO2
arterial oxygen partial pressure‐to‐inspired oxygen fraction ratio
- SVRI
systemic vascular resistance index
- TS
6% tetrastarch solution
- VR
volume replacement
The use of hydroxyethyl starch (HES) solutions for volume replacement (VR) is controversial. Third generation, iso‐oncotic HES solution (6% tetrastarch) was developed because earlier HES generations were associated impaired coagulation and acute kidney injury (AKI).1 Although inhibition of coagulation is minimized when 6% tetrastarch solution (TS) is used for VR, this artificial colloid has also been linked to an increased risk of death and AKI due to renal tubular damage in septic and nonseptic critically ill human patients2, 3, 4 However, when used early for volume replacement in human patients with penetrating trauma, TS could result in favorable outcomes (better lactate clearance and lower incidence of renal injury than physiological saline).5 Recently published meta‐analysis has concluded that the generalized restrictions to the use of TS in humans (except for patients with sepsis) are not supported by evidence.6
In veterinary medicine, there is insufficient data to establish the safety of TS in dogs.7, 8 A hyper‐oncotic (10%) HES solution, with larger molecular weight and larger molar substitution than TS, increased the risk of an adverse outcome, including AKI and death in critically ill dogs.9 However, because of differences in elimination kinetics between HES generations, these results cannot be extrapolated to TS.7, 8 Another recent study reported that TS administration to critically ill dogs [median dose 25 mL/kg/d (range, 12–62 mL/kg/d) with a median of 3 days of administration (range, 1–9 days)] did not have an effect on serum creatinine levels in comparison with isotonic crystalloids.10 However, conclusions regarding the renal safety of TS in this canine population were limited because creatinine has a poor sensitivity to detect early stages of AKI, when compared to other biomarkers of renal damage, such as neutrophil gelatinase‐associated lipocalin (NGAL).11 The urinary NGAL/creatinine ratio has been shown to be a highly sensitive and specific test to detect early stage (mild) AKI in dogs, before increases in serum creatinine concentration and changes in urinalysis parameters take place.11
In spite of its potential adverse effects, volume replacement (VR) with TS could be more advantageous than LRS because it can decrease the risk of tissue/lung edema by supporting colloid oncotic pressure (COP).12 For similar reasons, this fluid can result in a more prolonged increases in cardiac output than LRS when used as a blood substitute during acute normovolemic hemodilution13 or as a VR fluid after hypotensive hemorrhagic shock.14
Extravascular lung water index (EVLWI) is the amount of fluid in the interstitial space and alveolar lumen.15 The EVLWI, estimated from analysis of transpulmonary thermodilution curves, accurately reflects the amount of extravascular lung water in several species, including dogs.15, 16, 17, 18 In lung edema associated with intravenous volume loading, increased EVLWI is associated with oxygenation impairment [decreased arterial partial pressure of oxygen‐to‐inspired oxygen fraction ratio (PaO2/FiO2 ratio)].19 This study aimed to compare the effects of VR with LRS and TS on EVLWI and on PaO2/FiO2 ratio in dogs with acute hemorrhage. The second objective was to compare the effects of these fluids on markers of AKI (NGAL and creatinine measurements in plasma and urine).
Material and Methods
Animals, Study Design, and Hemorrhage Model
This study was approved by the Institutional Animal Care Committee under the protocol number 43/2015. Six healthy purpose‐bred English Pointer dogs (weight range 19.4–35.8 kg), 4 males and 2 females, 47 and 48 months old, were used in this study. Based on EVLWI values recorded from 8 healthy anesthetized dogs (11 ± 2 mL/kg), a sample size of 6 animals was estimated to be necessary to show a 30% increase in EVLWI with a statistical power of 80% and an alpha level of 5%. All animals were healthy based on physical and laboratory investigations (CBC, serum biochemistry, urinalysis, and venous blood gases analysis/electrolytes) within reference ranges. After the end of the study, all animals were adopted by private owners.
This prospective, nonblinded, partially randomized, crossover study was divided into two phases. During phase‐1 animals were anaesthetized for 7 hour without hemorrhage and VR (Control treatment). After a 2‐week washout period, phase‐2 was initiated, and animals were anaesthetized with the same technique for the same time‐period on two occasions, allowing 8‐week washout intervals. During each anesthetic episode of phase‐2, animals were hemorrhaged and randomly assigned1 to receive VR with LRS or TS (LRS and TS treatments, respectively).
The volume of shed blood was calculated on the basis of a formula used for acute normovolemic hemodilution,20 with the aim of decreasing the hematocrit to 33% after VR with LRS or TS, as follows: volume of shed blood (mL) = 80 × body weight (kg) × [(Hcttarget − Hctbaseline)/(Hctaverage)]; where 80 represents the estimated total blood volume (mL/kg); Hcttarget, Hctbaseline, and Hctaverage, the target Hct (33%), the Hct obtained before hemorrhage, and the arithmetic average of the Hcttarget and Hctbaseline, respectively.
The calculated amount of shed blood was withdrawn over a 30‐minute period from a 20‐gauge catheter2 placed in the dorsal pedal artery. The blood was immediately transferred into collection bags containing sodium citrate, phosphate, dextrose, and adenine.3 The bags with the proper amount of anticoagulant were weighed throughout the hemorrhage procedure to control the exact volume of shed blood. Immediately after the calculated amount of blood was withdrawn, VR with 3 mL LRS and 1 mL of TS for each mL of shed blood was performed over a 30‐minute period with 60 mL syringes. The 3 : 1 and 1 : 1 ratios of VR with LRS and tetrastrach, respectively, were chosen based on the concept that the volume expansion efficiency (defined as the ratio of expanded plasma volume/amount of infused fluid) of LRS and TS would approach 33% and 100%, respectively.14
Instrumentation and Cardiopulmonary Variables Recorded
After food was withheld for 12 hour, animals were premedicated with morphine4 (0.5 mg/kg, IM) before insertion of a 20‐gauge catheter2 into a cephalic vein. After induction of anesthesia with isoflurane5 by face mask, an orotracheal tube was inserted and the animals were positioned on the surgical table in dorsal recumbency. Anesthesia was maintained with isoflurane and IV remifentanil hydrochloride6 (0.15 μg/kg/min), administered by means of a circle breathing circuit of the anesthesia apparatus7 and by a syringe pump,8 respectively. Synchronous intermittent mandatory ventilation7 was initiated with an inspired oxygen fraction (FiO2) of 0.40. The expired tidal volume and the inspiration‐to‐expiration ratio were maintained constant (12 mL/kg and 1:1.5, respectively). The respiratory rate was adjusted to maintain the arterial partial pressure of carbon dioxide (PaCO2) close to 40 mmHg throughout the study.
An 18‐gauge, 20‐cm‐long, single lumen central venous catheter9 was aseptically inserted into the jugular vein until its tip was positioned at the level of the second thoracic rib for monitoring central venous pressure (CVP). A second 3‐French, 7‐cm‐long, thermodilution catheter10 was aseptically inserted into the femoral artery, 2.5 cm away from the inguinal fold to monitor cardiac output as previously described.21 Both catheters were connected to fluid‐filled pressure transducers11 zeroed and leveled at the base of the heart for monitoring central venous pressure (CVP) and mean arterial pressure (MAP), respectively.
End‐tidal isoflurane concentrations (ETISO), recorded by an infrared gas analyzer incorporated into the anesthesia apparatus7, were adjusted to provide immobility and to maintain MAP between 60 and 70 mmHg throughout anesthesia. Esophageal temperature was maintained between 37.5 and 38.5°C by means of a forced warm air device.12 A constant rate infusion of LRS (2 mL/kg/h) was administered during anesthesia by means of a peristaltic pump.13
Heart rate (HR) was recorded according to a lead II ECG with a multiparameter monitor.14 Cardiac output was measured by the transpulmonary thermodilution technique15 with 5‐mL boluses of ice‐cold physiological saline (≤5°C) injected over 2–3 seconds into the central venous catheter.21 The temperature of the injectate was monitored by an inline thermistor placed between the injection port and the central venous catheter. A thermistor located at the tip of the femoral artery catheter detected the change in blood temperature over time. Based on each thermodilution curve generated by ice‐cold thermal indicator, the monitor calculated cardiac output, global end‐diastolic volume, and extravascular lung water.22 For each data sampling time, these variables water were averaged from 3 serial measurements. Hemodynamic variables were indexed to body surface area [BSA (m2) = weight (grams)2/3 • 10.1 • 10−4] as follows: CI = cardiac output/BSA; stroke index (SI) = CI/heart rate; systemic vascular resistance index (SVRI) = (MAP‐CVP)/CI × 79.9, global end‐diastolic volume index (GEDVI) = global end‐diastolic volume/BSA. Extravascular lung water index (EVLWI) was indexed to body weight (extravascular lung water/body weight) based on the literature.15, 16, 17, 18
Arterial blood samples (1.0 mL) were drawn from the femoral catheter into heparinized syringes and immediately analyzed16 for temperature‐corrected pH, PaCO2, and the PaO2/FiO2. The same samples were placed in microhematocrit tubes and subsequently centrifuged17 at 14,500 g for 5 minutes for measuring the Hct, and for measuring total plasma protein (TPP) using a refractometer.18
Biomarkers of Renal Injury
Concentrations of NGAL and creatinine were determined in plasma and urine. Urine samples (20 mL) were collected from 8‐ or 10‐French polyethylene urinary catheters aseptically placed during anesthesia. These catheters were removed upon recovery from anesthesia and, if spontaneous micturition failed to yield samples in awake animals, another urinary catheter was temporarily placed and immediately removed after urine sampling. Blood samples (20 mL) were collected in tubes containing EDTA from the central venous catheter in anesthetized and conscious animals. Plasma and urine supernatant samples, obtained by centrifugation19 (1,000 g) at 2–8°C for 20 minutes, were stored at −70°C until analyzed.
Plasma and urine creatinine concentrations were measured by colorimetry.20 , 21 Plasma and urine NGAL concentrations were measured by a species‐specific sandwich ELISA tests.22 Plasma and urine samples were thawed, and NGAL was measured in duplicate in 96‐well microplates after the manufacturer's recommendations. After the reaction was stopped by adding diluted sulfuric acid, color intensity was determined by a microplate reader23 at 450 nm wavelength. The NGAL concentrations were determined by standard curves generated by 8 predefined concentrations of the peptide. If the coefficient of variation of duplicate samples was >20%, a second duplicate analysis was performed and averaged.
Experimental Protocol (Fig 1)
Figure 1.
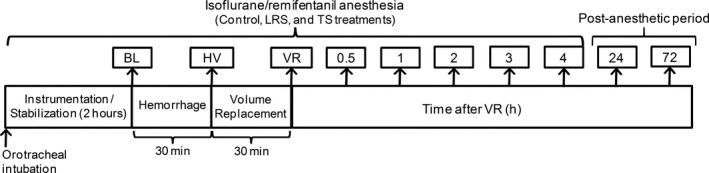
Experimental protocol. Animals underwent anesthesia without hemorrhage (Control treatment). After a 2‐week washout period, dogs were hemorrhaged under anesthesia on two occasions (8‐week washout intervals) and randomly received volume replacement (VR) with lactated Ringer's solution (LRS treatment) or 6% tetrastarch solution (TS treatment) at 3 : 1 or 1 : 1 of shed blood, respectively. BL, baseline; BW, blood withdrawal.
After a 2‐hour equilibration period, where ETISO concentrations were adjusted to mimic typical pressures of anesthetized patients (MAP between 60 and 70 mmHg) and provide immobility, baseline (BL) data were recorded and the hemorrhage/VR procedure was initiated. Through the urethral catheter, the bladder was fully expressed after the first urine sample collection at BL. Cardiopulmonary data, Hct, and TPP were recorded at BL, immediately after blood withdrawal (BW), immediately after VR, and at 0.5, 1, 2, 3, and 4 hour after VR in the LRS and TS treatments. Data were collected at the same time points in the Control treatment. Animals were recovered from anesthesia after the 4‐hour time point. Plasma and urine concentrations of creatinine, NGAL, and the urine NGAL/creatinine ratio were measured during anesthesia at BL and 4 hour after VR, and in conscious animals at 24 and 72 hour after VR.
Recovery from Anesthesia
After the last data collection, the femoral artery, the dorsal pedal, and the cephalic vein catheters were removed and the isoflurane/remifentanil administration was interrupted. The central venous catheter was maintained until 24 hour after anesthesia. The times elapsed from the interruption of isoflurane administration until the animals were able to remain in standing position were recorded.
Data Analysis
Data were analyzed by commercial statistical software.24 Shapiro‐Wilk and Kolmogorov‐Smirnov tests were used to verify the symmetry of data distribution. For Hct, PPT, cardiopulmonary variables, and plasma creatinine comparisons among treatments were made by a two‐way ANOVA for repeated measures by both factors (time and treatment), followed by a Tukey's test adjusted for the repeated measure design. A paired t test was used to compare the total amount of blood withdrawn in the LRS and TS treatments.
Visual inspection of the histogram of urine creatinine, plasma and urine NGAL, and urine NGAL/creatinine ratio) revealed a substantial pattern of asymmetry, which resulted in large coefficient of variation values (>50%). In this case, a nonparametric test was applied for comparisons among treatment groups (Friedman followed by a Dunn's multiple comparison test). For all variables, the significance level was set at P ≤ 0.05.
Results
The total volume of shed blood did not differ between the LRS and TS treatments (25 ± 7 mL/kg and 23 ± 4 mL/kg, respectively). The total volumes of LRS and TS infused during the 30‐minutes posthemorrhage period were 75 ± 14 mL/kg and 23 ± 4 mL/kg (P < 0.001).
Effects of LRS and TS on EVLWI, PaO2/FiO2 ratio, Hct, and TPP (Fig 2)
Figure 2.
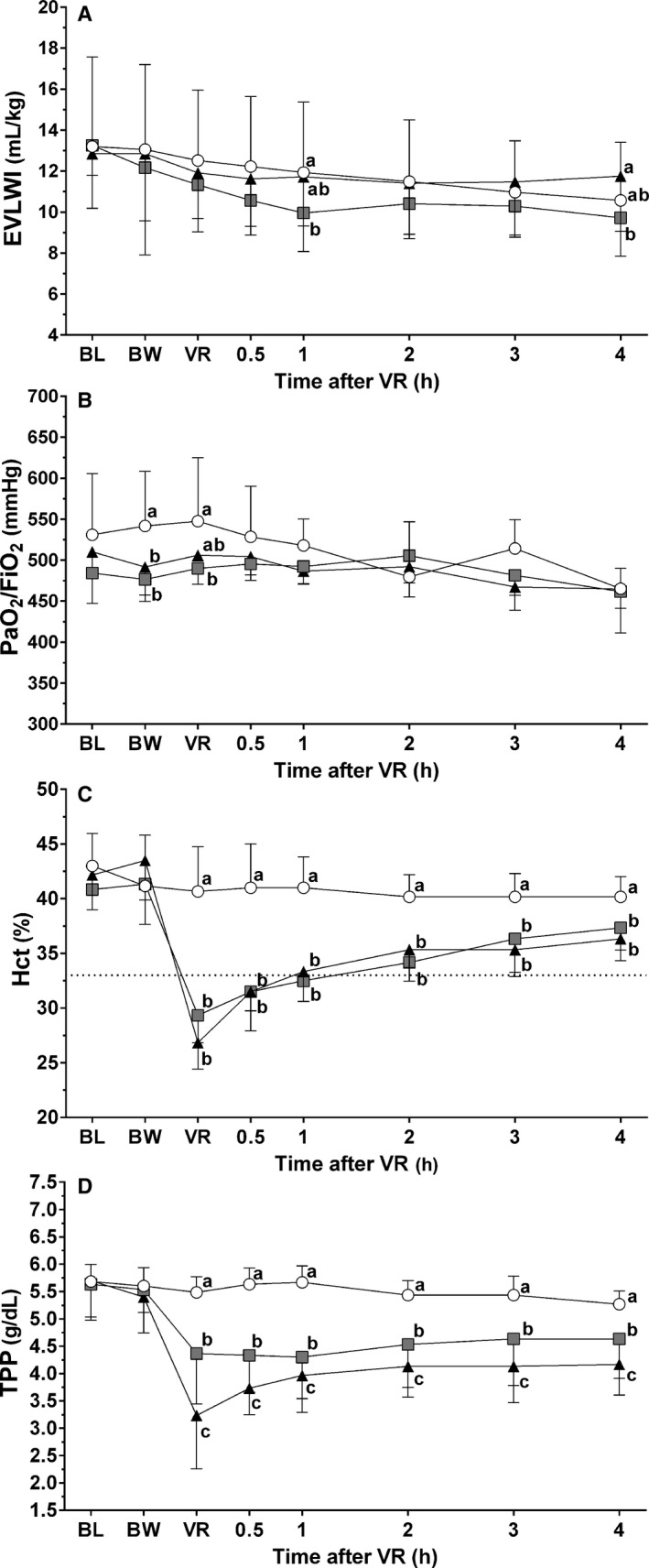
(A) Extravascular lung water index (EVLWI), (B) arterial oxygen partial pressure/inspired oxygen fraction ratio (PaO2/FiO2), (C) Hematocrit (Hct), and (D) total plasma protein (TPP) of six anesthetized dogs (mean ± SD) that did not undergo any intervention (Control treatment, open circles) or that underwent hemorrhage and randomly received volume replacement with lactated Ringer's solution (LRS treatment, triangles) or with 6% tetrastarch (TS treatment, squares) at a ratio of 3 : 1 or 1 : 1 the volume of shed blood, respectively. Variables recorded before hemorrhage (BL), immediately after blood withdrawal over 30 minutes (BW), immediately after volume replacement over 30 minutes (VR), and during 4 hour after VR. a, b, c Significant difference between treatment groups are shown by different letters (P ≤ 0.05). Dashed line in (A) represents the target Hct (33%).
The EVLWI was decreased from controls in the TS treatment at 1 hour after VR (P = 0.04) (Fig 2A). The EVLWI in TS treatment was lower in comparison with the LRS treatment at 4 hour after VR (P = 0.03).
The PaO2/FiO2 ratio was lower immediately after BW in the LRS (P = 0.04) and TS treatments (P = 0.005) in comparison with controls (Fig 2B). Immediately after VR, the PaO2/FiO2 ratio was lower in the TS treatment (P = 0.02) than in controls. There was no difference in PaO2/FiO2 ratio among treatment groups from 0.5 to 4 hour after VR and PaO2/FiO2 ratio remained above 400 mmHg in all animals throughout the study in all treatments.
The Hct decreased in comparison with controls immediately after VR and from 0.5 to 4 hour after VR in the LRS (P = <0.001–0.004) and TS treatments (P = <0.001–0.04) (Fig 2C). The Hct reached the target value (33%) 1 hour after VR in the LRS and TS treatments.
Immediately after VR and from 0.5 to 4 hour after VR, TPP was decreased from controls in the LRS and TS treatments (P = <0.001) (Fig 2D). Immediately after VR, and from 0.5 to 4 hour after VR, TPP was higher in the TS treatment in comparison with the LRS treatment (P = <0.001–0.01).
Effects of LRS and TS on Markers of AKI (Table 1)
Table 1.
Plasma creatinine (mean ± SD), urine creatinine, plasma and urine neutrophil gelatinase‐associated lipocalin (NGAL), and urine NGAL/creatinine of six dogs (median and upper–lower range) that did not undergo any intervention (Control treatment) or that underwent hemorrhage and randomly received volume replacement (VR) with lactated Ringer's solution (LRS treatment) or with 6% tetrastarch (TS treatment) at a ratio of 3 : 1 or 1 : 1 the volume of shed blood, respectively. Variables recorded during anesthesia, before hemorrhage (BL), and 4 hour after VR. Anesthesia was interrupted 4 hour after VR, and data were collected 24 and 72 hour after VR
| Variable | Treatment | BL | Time after VR (hour) | ||
|---|---|---|---|---|---|
| 4 | 24 | 72 | |||
| Plasma Creatinine (mg/dL) | Control | 0.77 ± 0.12 | 0.77 ± 0.12a | 0.70 ± 0.04 | 0.76 ± 0.15 |
| LRS | 0.72 ± 0.11 | 0.70 ± 0.08b | 0.65 ± 0.07 | 0.77 ± 0.08 | |
| TS | 0.70 ± 0.10 | 0.71 ± 0.11ab | 0.67 ± 0.07 | 0.74 ± 0.11 | |
| Urine Creatinine (mg/dL) | Control | 226 (162–353) | 272 (182–370)a | 99 (75–139) | 184 (75–259) |
| LRS | 217 (61–365) | 180 (119–318)b | 53 (38–79) | 243 (105–465) | |
| TS | 134 (82–299) | 218 (129–233)ab | 116 (43–189) | 153 (42–371) | |
| Plasma NGAL (pg/mL) | Control | 6,396 (3,562–14,618) | 5,871 (3,671–12,979) | 8,084 (4,134–15,298) | 7422 (5,085–23,968) |
| LRS | 9,318 (5,653–17,963) | 7,740 (4,179–17,321) | 11,737 (6,095–19,581) | 12,214 (5,677–25,550) | |
| TS | 9,169 (3,751–28,978) | 7,576 (3,445–31,850) | 11,294 (4,417–36,926) | 13,118 (4,529–31,187) | |
| Urine NGAL (pg/mL) | Control | 919 (320–5,084) | 1,684 (921–3,524) | 867 (580–5,978) | 1,062 (398–11,096) |
| LRS | 1,626 (249–4,586) | 606 (73–4,545) | 431 (206–3332) | 1,074 (138–9,133) | |
| TS | 286 (261–17,093) | 948 (260–20,827) | 1,140 (238–4,738) | 887 (262–8,527) | |
| Urine NGAL/Creatinine (pg/mg) | Control | 538 (122–1,442) | 621 (249–1,470) | 822 (632–6,069) | 671 (267–4,483) |
| LRS | 721 (143–3,264) | 366 (23–2,631) | 863 (260–5,976) | 381 (132–2,720) | |
| TS | 279 (87–9,128) | 514 (115–16,114) | 870 (418–6,494) | 373 (246–4,418) | |
a,b,cWithin each column. Treatment group means followed by different letters are significantly different from each other (Tukey's test, P ≤ 0.05).
Plasma and urine creatinine values were significantly lower in the LRS treatment (P = 0.04 and P = 0.01, respectively) than in controls at 4 hour after VR. There was no difference among treatments for plasma NGAL, urine NGAL, and urine NGAL/creatinine ratio (P = 0.25–0.99).
Effects of LRS and TS on ETISO and hemodynamics (Table 2)
Table 2.
End‐tidal isoflurane (ETISO) and hemodynamic parameters of six anesthetized dogs (mean ± SD) that did not undergo intervention (Control treatment) or that underwent hemorrhage and randomly received volume replacement with lactated Ringer's solution (LRS treatment) or with 6% tetrastarch (TS treatment) at a ratio of 3 : 1 or 1 : 1 the volume of shed blood, respectively. Variables recorded before hemorrhage (BL), immediately after blood withdrawal over 30 minutes (BW), immediately after volume replacement over 30 minutes (VR), and during 4 hour after VR
| Variable | Treatment | BL | BW | VR | Time after VR (hour) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 0.5 | 1 | 2 | 3 | 4 | |||||
| ETISO (%) | Control | 1.35 ± 0.51 | 1.37 ± 0.53 | 1.32 ± 0.48ab | 1.33 ± 0.49 | 1.32 ± 0.45 | 1.43 ± 0.34 | 1.48 ± 0.34 | 1.37 ± 0.43 |
| LRS | 1.37 ± 0.33 | 1.27 ± 0.21 | 1.12 ± 0.29a | 1.20 ± 0.06 | 1.33 ± 0.23 | 1.38 ± 0.31 | 1.52 ± 0.34 | 1.42 ± 0.29 | |
| TS | 1.53 ± 0.36 | 1.42 ± 0.37 | 1.35 ± 0.33b | 1.40 ± 0.32 | 1.42 ± 0.25 | 1.48 ± 0.31 | 1.48 ± 0.35 | 1.48 ± 0.33 | |
| HR (beats/min) | Control | 96 ± 27 | 99 ± 29a | 97 ± 28 | 97 ± 28a | 93 ± 24a | 96 ± 25a | 95 ± 25a | 99 ± 22a |
| LRS | 99 ± 24 | 133 ± 33b | 106 ± 26 | 113 ± 35b | 113 ± 33b | 111 ± 31b | 118 ± 30b | 122 ± 29b | |
| TS | 102 ± 26 | 146 ± 33b | 109 ± 30 | 112 ± 27b | 112 ± 29b | 114 ± 33b | 110 ± 33b | 123 ± 38b | |
| CI (mL/m2) | Control | 2.80 ± 0.45 | 2.97 ± 0.54 | 2.89 ± 0.38a | 3.23 ± 0.48a | 3.31 ± 0.45a | 3.48 ± 0.33 | 3.39 ± 0.50a | 3.68 ± 0.42a |
| LRS | 2.97 ± 0.52 | 2.74 ± 0.17 | 5.38 ± 1.09b | 4.92 ± 1.84b | 4.39 ± 1.17b | 4.10 ± 0.92 | 4.73 ± 0.86b | 4.77 ± 0.92b | |
| TS | 3.20 ± 0.40 | 2.74 ± 0.29 | 4.57 ± 0.61c | 4.10 ± 0.67c | 3.98 ± 0.63ab | 3.69 ± 0.68 | 3.78 ± 0.64a | 3.72 ± 0.49a | |
| SVRI (dynes/sec/cm−5/m2) | Control | 1861 ± 324a | 1789 ± 285a | 1759 ± 228a | 1606 ± 206a | 1556 ± 161a | 1461 ± 130 | 1479 ± 200a | 1372 ± 176a |
| LRS | 1750 ± 271ab | 1990 ± 119b | 891 ± 218b | 1100 ± 318b | 1223 ± 259b | 1294 ± 271 | 1085 ± 168b | 1113 ± 177b | |
| TS | 1644 ± 204b | 1996 ± 176b | 1133 ± 149c | 1252 ± 187b | 1314 ± 242b | 1426 ± 178 | 1351 ± 170a | 1344 ± 158a | |
| MAP (mmHg) | Control | 65 ± 3 | 66 ± 3 | 64 ± 4ab | 65 ± 1 | 65 ± 3 | 65 ± 3 | 63 ± 2 | 63 ± 3 |
| LRS | 65 ± 2 | 67 ± 1 | 61 ± 5a | 63 ± 5 | 65 ± 4 | 65 ± 2 | 64 ± 3 | 65 ± 2 | |
| TS | 65 ± 2 | 67 ± 3 | 65 ± 4b | 64 ± 3 | 64 ± 2 | 64 ± 4 | 62 ± 3 | 61 ± 5 | |
| CVP (mmHg) | Control | 0.3 ± 0.8 | 0.8 ± 1.5a | 1.0 ± 1.3a | 1.3 ± 1.2 | 0.7 ± 1.5 | 1.3 ± 1a | 1.5 ± 1.5a | 0.5 ± 0.8 |
| LRS | 1.2 ± 1.2 | −1.2 ± 2.6b | 2.8 ± 2.0b | 0.8 ± 1.3 | 0.8 ± 1.5 | 0.8 ± 1.2a | 0.7 ± 2.3ab | −0.2 ± 1.5 | |
| TS | 0.3 ± 0.5 | −1.2 ± 1.2b | 1.3 ± 1.2a | 0.8 ± 0.8 | −0.3 ± 1.2 | −0.8 ± 0.8b | −0.5 ± 1.2b | −0.7 ± 1.0 | |
| GEDVI (mL/m2) | Control | 637 ± 145a | 609 ± 125a | 567 ± 91a | 602 ± 108a | 587 ± 100a | 563 ± 75a | 545 ± 77a | 551 ± 96 |
| LRS | 668 ± 91a | 546 ± 92b | 704 ± 55b | 647 ± 82ab | 617 ± 64ab | 603 ± 52ab | 610 ± 44b | 576 ± 34 | |
| TS | 732 ± 164b | 551 ± 121b | 740 ± 187b | 668 ± 128b | 665 ± 177b | 627 ± 126b | 610 ± 139b | 590 ± 128 | |
a, b, cWithin each column. For a given time point, treatment group means followed by different superscript letters are significantly different from each other (Tukey′s test, P ≤ 0.05).
There was no significant difference between variables at BL, except for SVRI, which was lower in the TS treatment in comparison with controls (P = 0.01), and GEDVI, which was higher in the TS treatment in comparison with the Control (P < 0.001) and LRS (P = 0.02) treatments.
Immediately after VR, the ETISO adjusted to maintain MAP between 60 and 70 mmHg was lower in the LRS treatment in comparison with the TS treatment (P = 0.04).
Heart rate was higher in the LRS and TS treatments in comparison with controls immediately after BW (P < 0.001), and from 0.5 to 4 hour after VR (P = <0.001–0.03).
Cardiac index was higher in the LRS treatment in comparison with controls immediately after VR, and from 0.5 to 4 hour after VR (P < 0.001) (except for the 2‐hour time point). The TS treatment presented higher CI values in comparison with controls immediately after VR (P < 0.001) and 0.5 hour after VR (P = 0.01). Cardiac index was higher in the LRS than in the TS treatment immediately after VR, and from 0.5 to 4 hour after VR (P < 0.001–0.01), except for the 1‐ and 2‐hour time points.
In the LRS and TS treatments, SVRI was increased from controls immediately after BW (P = 0.01). In the LRS treatment, SVRI was decreased from controls immediately after VR and from 0.5 to 4 hour after VR (P < 0.001), except for the 2‐hour time point. In the TS treatment, SVRI was decreased from controls immediately after VR, 0.5, and 1 hour after VR (P = <0.001–0.003). The SVRI was decreased in the LRS treatment in comparison with the TS treatment immediately after VR, 3, and 4 hour after VR (P = <0.001–0.004).
Mean arterial pressure was lower immediately after VR in the LRS treatment (P = 0.02) in comparison with the TS treatment. Mean arterial pressure was maintained within the target range (60–70 mmHg) at most data sampling times throughout the study, except for 3 animals that showed MAP <60 mmHg (56–59 mmHg and 54–58 mmHg in the LRS and TS treatments, respectively) at some time points after administration of LRS and TS (same individuals) because ETISO could not be further decreased due to the presence of movement.
Immediately after BW, CVP was lower in the LRS and TS treatments in comparison with controls (P < 0.001). In the LRS treatment, CVP was higher than controls immediately after VR (P = 0.002). In the TS treatment, CVP was lower than in controls at 2 and 3 hour after VR (P < 0.001). Central venous pressure was lower in the TS treatment than in the LRS treatment immediately after VR and 2 hour after VR (P = 0.01).
Compared to controls, GEDVI was lower immediately after BW in the LRS (P = 0.03) and TS treatments (P = 0.05). In the LRS treatment, GEDVI was increased from controls immediately after VR (P < 0.001) and 3 hour after VR (P = 0.02). In the TS treatment, GEDVI was higher than controls immediately after VR (P < 0.001), and from 0.5 to 3 hour after VR (P = >0.004–0.03).
Recovery from Anesthesia
Times to recover from anesthesia did not differ among treatment groups. Animals were extubated after 14 ± 8 minutes (mean ± SD) and were standing in 30 (15–136 minutes) [median (lower‐upper range)]. Recovery from anesthesia was uneventful in the Control and TS treatments. From 2 to 4 hour after VR, edema of the eyelids and lips was evident in 3/6 animals of the LRS treatment. In one animal treated with LRS, a transient inspiratory stridor was observed immediately after the animal was standing. These signs were no longer evident from 2 to 4 hour after animals were standing.
Discussion
Although VR with TS resulted in lower EVLWI than LRS at 4 hour after VR, this difference might not be clinically relevant because, at this time point, neither LRS nor TS differed significantly from controls and arterial oxygenation was not impaired (PaO2/FiO2 ratio >400 mmHg). Based on urine NGAL/Creatinine concentrations, LRS and TS did not appear to cause renal tubule damage in healthy dogs, corroborating with a recent report showing that TS did not alter serum creatinine levels in this species.10 Therefore, the general restrictions for the use of TS in humans3, 4 might not be extrapolated to healthy nonseptic dogs. However, because of substantial variation in NGAL concentrations and of the small number of dogs evaluated, further investigations on the renal effects of TS are warranted.
The EVLWI measured by transpulmonary thermodilution is a useful tool to identify lung edema.15, 16, 17, 18, 19 The mean (±SD) EVLWI values recorded in the Control treatment ranged from 11 ± 2 to 13 ± 4 mL/kg. These values were substantially higher than EVLWI values using the same technique reported in mongrel dogs with healthy lungs (9.4 ± 3.5 mL/kg)16 and in humans without diffuse alveolar damage (≤10 mL/kg).15, 18 However, it is unlikely that these values represented an abnormal accumulation of extravascular lung water because controls received a relatively small infusion rate of fluids (2 mL/kg/h of LRS) and the PaO2/FiO2 ratio suggested a near optimal oxygen transfer across the alveolar‐capillary barrier (PaO2/FiO2 ratio >400 mmHg). Volume replacement with TS significantly decreased EVLWI in comparison with controls at 1 hour after VR, and in comparison with the LRS treatment at 4 hour after VR. Because EVLWI values in these treatment groups were not significantly increased from controls and resulted in PaO2/FiO2 ratio values >400 mmHg, neither LRS nor TS produced evidence of lung edema. Further studies are recommended to establish normal EVLWI values in dogs and the cut‐off point of EVLWI to recognize lung edema in canine species.
In the present study, LRS administration caused signs of peripheral edema in 3/6 dogs, and resulted in an increase in EVLWI when compared to TS at 4 hour after VR. Although COP was not measured in the present study, a decrease in COP could have caused the peripheral edema secondary to isotonic crystalloid administration.23, 24 Because TPP was significantly lower after VR in the LRS treatment in comparison with the Control and TS treatments, it is likely that COP was decreased by LRS administration, as nearly 80% of total COP is determined by plasma albumin. Contrastingly, the significant decrease in TPP observed after TS administration in comparison with controls probably does not correspond to lower COP values. In fact, COP is maintained by 6% HES solutions in spite of decreased TPP induced by a dilutional effect.23 Although TPP assessed by refractometry might suggest COP changes under certain circumstances, in critically ill dogs this parameter shows of a large proportion of false negatives (poor sensitivity) to detect hypoalbuminemia/low COP because of an altered albumin/globulin ratio.25
Another factor that could have been determinant to the observation of peripheral edema after LRS administration was the speed of fluid administration. In the present report, lactated Ringer's solution was administered at a faster rate (150 ± 28 mL/kg/h) than the maximum infusion rate of isotonic crystalloids that has been historically recommended for volume replacement in dogs hypovolemic shock (90 mL/kg/h).26 Chemosis and lip edema have been reported in anesthetized normovolemic dogs that received 60 mL/kg/h of isotonic crystalloids (total of 60 mL/kg).24 Immediately after VR with LRS, CVP was significantly increased in comparison with the other treatment groups. However, CVP values recorded at this time point (2.8 ± 2 mmHg) in the LRS treatment were within reference ranges (0–6 mmHg) and cannot be interpreted as a sign of circulating volume overload. In splenectomized dogs with hypotensive hemorrhagic shock, 38% of the infused volume of LRS, administered at a dose of 3 mL for each mL of shed blood, remained within intravascular space at 5 minutes after fluid administration.14 Although these findings are in line with the concept that LRS should be administered at a 3 : 1 ratio to maintain the circulating volume after hemorrhage, the volume expansion efficacy of LRS was short‐lived, as 11% of the infused volume remained within the intravascular space at 90 minutes after VR.14 Therefore, it is evident that a large percentage of LRS can leave the intravascular space and cause peripheral edema, but lung edema is less likely to occur in healthy dogs.
The formula used to determine the amount of shed blood in the present study was developed for estimating the allowable blood loss, based on the expected degree of hemodilution (Hcttarget) when the shed blood is replaced by crystalloids, colloids, or both fluids.20 The degree of hemodilution was similar between LRS and TS, as demonstrated by Hct changes recorded after VR. Regardless of the VR fluid, a greater degree of hemodilution than expected (33%) was observed immediately after VR. This effect was followed by progressive increases in Hct over time, which could have been caused by redistribution of VR fluids from the intravascular space to other compartments.
In the present report, VR with LRS and tetrastarch induced a hyperdynamic state characterized by increases in CI and decreases in SVRI.14, 23 Decreases in afterload, associated with the vasodilatory state and with an improvement in rheological properties of blood (lower Hct), and increases in preload/myocardial contractility are thought to directly contribute to increases in CI observed after fluid administration patients that undergo acute normovolemic hemodilution with colloids and/or crystalloids.13, 27, 28
The hyperdynamic state (increased CI) observed after LRS and TS administration can be explained by the significant increases in HR and preload (GEDVI), and by the significant decrease in afterload (SVRI) observed after VR with both fluids. Changes in preload and afterload could have ben caused by an improvement in rheological properties of blood due to the hemodilution (lower viscosity) induced by fluid administration.28 When compared to controls, VR with TS appeared to induce a more consistent increase in GEDVI (from immediately after VR until 3 hour after VR) than VR with LRS (GEDVI increased immediately after VR and at 3 hour after VR). However, VR with LRS resulted in greater and more prolonged increases in CI than VR with TS. The longer lasting hyperdynamic state induced by LRS cannot be attributed to differences in preload because GEDVI values did not differ between LRS and TS treatments after VR. These results are in contrast with those reported in splenectomized dogs with hypotensive hemorrhagic shock, where TS resulted in longer lasting plasma volume expansion and more sustained increases in CI than a volume 3 times higher of LRS.14 In the present study, the more prolonged decreases in SVRI (reflecting longer lasting decreases in afterload) observed after VR with LRS in comparison with VR with TS might have contributed to the sustained hyperdynamic state induced by the crystalloid. However, a definite explanation for the greater and longer lasting hyperdynamic state induced by LRS in comparison with TS could not be found under the circumstances of the present study.
The NGAL is a protein expressed in neutrophils, epithelial cells of renal tubules, and other organs, whose concentration in urine increases after ischemic renal damage in dogs.29 Synthetic colloids can cause AKI due to an osmotic swelling/lysis of proximal tubular cells secondary to vacuolization/absorption of colloid molecules.7, 8 Renal damage secondary to hyper‐oncotic colloids (10% HES solutions) can also be caused by hyperviscosity/stasis of tubular flow secondary to an increase in colloid oncotic pressure of glomerular arterioles.7, 8, 30 Stasis of tubular flow might be the mechanism involved in the increased risk of death and AKI associated with the administration of hyper‐oncotic (10%) HES solutions to critically ill dogs.9 Based on creatinine and NGAL measurements, no evidence of renal compromise was found with the use of LRS and TS in the present study.
In spite of the wide variation in plasma and urine NGAL concentrations, values were close to the range usually found in dogs without AKI.11, 29, 31 A wide variation in urine NGAL concentrations has also been reported in people, which has made difficult to stablish a reference interval in humans.32, 33 Therefore, increases in urine NGAL from baseline values could be more useful for detecting early stages of AKI than single measurements. Normalization of urine NGAL based on urine creatinine concentrations (urine NGAL/creatinine ratio) has been used to control for changes in urine flow rate and reduce the variability of urine NGAL.34 Maximum urine NGAL/creatinine concentrations were recorded after VR with TS (16,114 pg/mg). However, urine NGAL/creatinine in that animal was already higher at BL (9,128 pg/mg) and values were found to be substantially lower than the cut‐off point of urine NGAL/creatinine ratio that would predict the development of azotemic forms of AKI in dogs (120,000 pg/mg).11
The main limitation of the present report was the small number of animals. Because of the wide range of NGAL concentrations, the study was underpowered to detect differences in NGAL between treatments, increasing the probability of type II error. However, even if a statistical difference might exist among NGAL/creatinine ratios recorded in the LRS, TS, and Control treatments, it might not be clinically relevant, given the reported threshold of NGAL/creatinine ratio for detecting AKI.11 The present study is also limited because results cannot be extrapolated to critically ill animals, or animals with renal compromise. Finally, it should be mentioned that the circumstances of this study (one time administration of TS) might be not be reflective of ongoing use (CRI infusion, dosing over multiple days) as was the case in the human studies/meta‐analyses that investigated the renal implications of TS administration.2, 3, 4
Conclusion
In healthy dogs with acute hemorrhage, VR with LRS and tetrastarch resulted in a hyperdynamic state with no evidence of lung edema or oxygenation impairment. In this study, the use of TS in healthy dogs with acute hemorrhage did not reveal evidence of AKI for up to 72 after its administration. However, the wide variation in NGAL concentrations suggests that additional studies evaluating the effects of TS on renal function are necessary in canine species.
Acknowledgments
Conflict of Interest Declaration
Authors declare no conflict of interest.
Off‐label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
This work was performed at the “Faculdade de Medicina Veterinária e Zootecnia, Universidade Estadual Paulista, Botucatu, São Paulo, Brazil, CEP 18618‐970.”
Funding was provided by “Fundação de Amparo à Pesquisa de São Paulo” (2014/25115‐8, 2015/04810‐2, and 2017/19711‐5) and by a fellowship from CNPq (306342/2015‐4).
Footnotes
Urbaniak, G C, & Plous, S (2013). Research Randomizer (Version 4.0) [Computer software]. Retrieved on June 22, 2013, from Hcttp://www.randomizer.org/
Insyte, Becton Dickinson, Juiz de Fora, MG, Brazil
CPDA blood collection bag, JP Industria Farmacêutica, Ribeirão Preto, SP, Brazil
Dimorf, Cristália, Itapira, SP, Brazil
Isoforine, Cristália, Itapira, SP, Brazil
Ultiva, GlaxoSmithKline, Rio de Janeiro, RJ, Brazil
Dräger Primus, Drägerwerk AG & Co, Lübeck, Germany
SR 8X, Digicare, Boynton Beach, FL
Venoseld single lumen, Rehlinger‐Siersburg, Germany
PiCCO Catheter PV2013L07N, Pulsion Medical Systems, Munich, Germany
TruWave PX 260, Edwards Lifesciences Irvine, CA
Bair Hugger, Arizant Healthcare, Minneapolis, MN
LP 8X, Digicare, Boynton Beach, FL
DX 2020, Dixtal Biomédica, São Paulo, SP, Brazil
PiCCO module, Pulsion Medical Systems/Dixtal, São Paulo, SP, Brazil
pH/Blood Gas Analyzer Model 348, Siemens, Halstead, UK
Micro Hematócrito MH, Celm, São Caetano do Sul, SP, Brazil
Refratômetro, Megabrix, São Paulo, SP, Brazil
Sigma 2‐16KL, Sigma Laborzentrifugen, Osterod am Harz, Germany
Cobas, Mira Plus, Roche, Indianapolis, IN
SB‐199, Celm, São Caetano do Sul, SP, Brazil
Canine NGAL (Lipocalin‐2) ELISA Kit, MyBioSource, San Diego, CA
Synergy HCTX Multi‐Mode Microplate Reader, Biotek, Winooski, VT
Prism 6.02, GraphPad, San Diego, CA
References
- 1. Westphal M, James MF, Kozek‐Langenecker S, et al. Hydroxyethyl starches: Different products‐different effects. Anesthesiology 2009;111:187–202. [DOI] [PubMed] [Google Scholar]
- 2. Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med 2012;367:124–134. [DOI] [PubMed] [Google Scholar]
- 3. Mutter TC, Ruth CA, Dart AB. Hydroxyethyl starch (HES) versus other fluid therapies: Effects on kidney function. Cochrane Database Syst Rev 2013;23:CD007594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zarychanski R, Abou‐Setta AM, Turgeon AF, et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: A systematic review and meta‐analysis. JAMA 2013;309:678–688. [DOI] [PubMed] [Google Scholar]
- 5. James MF, Michell WL, Joubert IA, et al. Resuscitation with hydroxyethyl starch improves renal function and lactate clearance in penetrating trauma in a randomized controlled study: The FIRST trial (Fluids in Resuscitation of Severe Trauma). Br J Anaesth 2011;107:693–702. [DOI] [PubMed] [Google Scholar]
- 6. Qureshi SH, Rizvi SI, Patel NN, et al. Meta‐analysis of colloids versus crystalloids in critically ill, trauma and surgical patients. Br J Surg 2016;103:14–26. [DOI] [PubMed] [Google Scholar]
- 7. Glover PA, Rudloff E, Kirby R. Hydroxyethyl starch: A review of pharmacokinetics, pharmacodynamics, current products, and potential clinical risks, benefits, and use. J Vet Emerg Crit Care 2014;24:642–661. [DOI] [PubMed] [Google Scholar]
- 8. Cazzolli D, Prittie J. The crystalloid‐colloid debate: Consequences of resuscitation fluid selection in veterinary critical care. J Vet Emerg Crit Care 2015;25:6–19. [DOI] [PubMed] [Google Scholar]
- 9. Hayes G, Benedicenti L, Mathews K. Retrospective cohort study on the incidence of acute kidney injury and death following hydroxyethyl starch (HES 10% 250/0.5/5:1) administration in dogs (2007–2010). J Vet Emerg Crit Care 2016;26:35–40. [DOI] [PubMed] [Google Scholar]
- 10. Yozova ID, Howard J, Adamik KN. Retrospective evaluation of the effects of administration of tetrastarch (hydroxyethyl starch 130/0.4) on plasma creatinine concentration in dogs (2010–2013): 201 dogs. J Vet Emerg Crit Care 2016;26:568–577. [DOI] [PubMed] [Google Scholar]
- 11. Segev G, Palm C, LeRoy B, et al. Evaluation of neutrophil gelatinase‐associated lipocalin as a marker of kidney injury in dogs. J Vet Intern Med 2013;27:1362–1367. [DOI] [PubMed] [Google Scholar]
- 12. Gauthier V, Holowaychuk MK, Kerr CL, et al. Effect of synthetic colloid administration on hemodynamic and laboratory variables in healthy dogs and dogs with systemic inflammation. J Vet Emerg Crit Care 2014;24:251–258. [DOI] [PubMed] [Google Scholar]
- 13. Otsuki DA, Fantoni DT, Margarido CB, et al. Hydroxyethyl starch is superior to lactated Ringer as a replacement fluid in a pig model of acute normovolaemic haemodilution. Br J Anaesth 2007;98:29–37. [DOI] [PubMed] [Google Scholar]
- 14. Barros JM, do Nascimento P Jr, Marinello JL, et al. The effects of 6% hydroxyethyl starch‐hypertonic saline in resuscitation of dogs with hemorrhagic shock. Anesth Analg 2011;112:395–404. [DOI] [PubMed] [Google Scholar]
- 15. Jozwiak M, Teboul JL, Monnet X. Extravascular lung water in critical care: Recent advances and clinical applications. Ann Intensive Care 2015;5:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Katzenelson R, Perel A, Berkenstadt H, et al. Accuracy of transpulmonary thermodilution versus gravimetric measurement of extravascular lung water. Crit Care Med 2004;32:1550–1554. [DOI] [PubMed] [Google Scholar]
- 17. Fernández‐Mondéjar E, Rivera‐Fernández R, García‐Delgado M, et al. Small increases in extravascular lung water are accurately detected by transpulmonary thermodilution. J Trauma 2005;59:1420–1424. [DOI] [PubMed] [Google Scholar]
- 18. Tagami T, Kushimoto S, Yamamoto Y, et al. Validation of extravascular lung water measurement by single transpulmonary thermodilution: Human autopsy study. Crit Care 2010;14:R162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aman J, Groeneveld AB, van Nieuw Amerongen GP. Predictors of pulmonary edema formation during fluid loading in the critically ill with presumed hypovolemia. Crit Care Med 2012;40:793–799. [DOI] [PubMed] [Google Scholar]
- 20. Gross JB. Estimating allowable blood loss: Corrected for dilution. Anesthesiology 1983;58:277–280. [DOI] [PubMed] [Google Scholar]
- 21. Garofalo NA, Teixeira‐Neto FJ, Rodrigues JC, et al. Comparison of transpulmonary thermodilution and calibrated pulse contour analysis with pulmonary artery thermodilution cardiac output measurements in anesthetized dogs. J Vet Intern Med 2016;30:941–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sakka SG, Reuter DA, Perel A. The transpulmonary thermodilution technique. J Clin Monit Comput 2012;26:347–353. [DOI] [PubMed] [Google Scholar]
- 23. Muir WW, Wiese AJ. Comparison of lactated Ringer's solution and a physiologically balanced 6% hetastarch plasma expander for the treatment of hypotension induced via blood withdrawal in isoflurane‐anesthetized dogs. Am J Vet Res 2004;65:1189–1194. [DOI] [PubMed] [Google Scholar]
- 24. Valverde A, Gianotti G, Rioja‐Garcia E, et al. Effects of high‐volume, rapid‐fluid therapy on cardiovascular function and hematological values during isoflurane‐induced hypotension in healthy dogs. Can J Vet Res 2012;76:99–108. [PMC free article] [PubMed] [Google Scholar]
- 25. Hayes GM, Mathews K, Floras A, et al. Refractometric total plasma protein measurement as a cage‐side indicator of hypoalbuminemia and hypoproteinemia in hospitalized dogs. J Vet Emerg Crit Care 2011;21:356–362. [DOI] [PubMed] [Google Scholar]
- 26. Rozanski EA, Rush JE. Shock In: Rozanski EA, Rush JE, eds. Small Animal Emergency and Critical Care Medicine: A Color Handbook, 1st ed London, UK: Manson Publishing Ltd; 2007:13–20. [Google Scholar]
- 27. Habler OP, Kleen MS, Podtschaske AH, et al. The effect of acute normovolemic hemodilution (ANH) on myocardial contractility in anesthetized dogs. Anesth Analg 1996;83:451–458. [DOI] [PubMed] [Google Scholar]
- 28. Fantoni DT, Otsuki DA, Ambrósio AM, et al. A comparative evaluation of inhaled halothane, isoflurane, and sevoflurane during acute normovolemic hemodilution in dogs. Anesth Analg 2005;100:1014–1019. [DOI] [PubMed] [Google Scholar]
- 29. Davis J, Raisis AL, Cianciolo RE, et al. Urinary neutrophil gelatinase‐associated lipocalin concentration changes after acute haemorrhage and colloid‐mediated reperfusion in anaesthetized dogs. Vet Anaesth Analg 2016;43:262–270. [DOI] [PubMed] [Google Scholar]
- 30. Simon TP, Schuerholz T, Hüter L, et al. Impairment of renal function using hyperoncotic colloids in a two hit model of shock: A prospective randomized study. Crit Care 2012;16:R16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cobrin AR, Blois SL, Abrams‐Ogg AC, et al. Neutrophil gelatinase‐associated lipocalin in dogs with chronic kidney disease, carcinoma, lymphoma and endotoxaemia. J Small Anim Pract 2016;57:291–298. [DOI] [PubMed] [Google Scholar]
- 32. Clerico A, Galli C, Fortunato A, Ronco C. Neutrophil gelatinase‐associated lipocalin (NGAL) as biomarker of acute kidney injury: A review of the laboratory characteristics and clinical evidences. Clin Chem Lab Med 2012;50:1505–1517. [DOI] [PubMed] [Google Scholar]
- 33. Smertka M, Chudek J. Using NGAL as an early diagnostic test of acute kidney injury. Ren Fail 2012;34:130–133. [DOI] [PubMed] [Google Scholar]
- 34. Waikar SS, Sabbisetti VS, Bonventre JV. Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int 2010;78:486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]


