THINKING IN REVERSE
In the simplest terms, conventional benchtop‐to‐bedside therapeutics research starts in the laboratory and ends in a clinical trial. The process is linear, with a distinct beginning and end, fraught with many pitfalls, and, as we have learned from experience, has a high probability of failure.
Benchtop‐to‐Bedside Research Paradigm laboratory discovery → clinical trial.
In contrast, reverse translation, also called bedside‐to‐benchtop research, begins with actual, real‐life patient experiences in the clinic, or during a clinical trial, and works backward to uncover the mechanistic basis for these experiences and clinical observations. In the reverse translation paradigm, research becomes a seamless, continuous, cyclical process, in which each new patient observation stimulates new testable hypotheses that help refine and direct the next iteration of benchtop therapeutics research, which, in turn, leads to the next clinical trial and the next human experience (Figure 1).1
Figure 1.
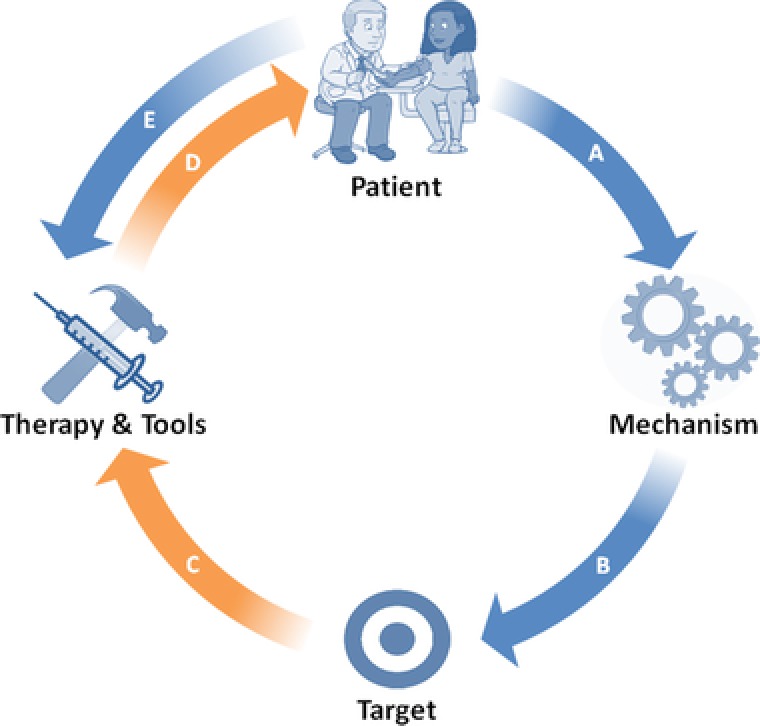
Reverse translation research paradigm. Republished with permission from Wagner, J.A. Patient‐centered reverse translation. Clin. Pharmacol. Ther. (2017). https://doi.org/10.1002/cpt.902.
The beauty of reverse translation, unlike benchtop‐to‐bedside research, is that there is no such thing as a failed clinical trial; only expected and unexpected therapeutic outcomes, and the inevitable variability in the observed human therapeutic response that needs further explanation and exploration. The reverse translation issue of Clinical and Translational Science embraces this broad view of reverse translation, which encompasses a wide range of topics highly relevant to clinical pharmacology and patient‐centered translational therapeutics research.
DRUG REPURPOSING
A classic example of reverse translation is drug repurposing, an opportunity that arises from clinical observations of a drug exerting a therapeutic effect unrelated to its primary therapeutic indication. This phenomenon can result from a drug acting off‐target, or as an unforeseen downstream consequence of a drug acting on‐target, as proposed by McWilliam et al.,2 for the cholesterol lowering, 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase inhibitors, rosuvastatin, and its newly discovered renal‐protective potential for children with cystic fibrosis. The ongoing odyssey of repurposing statins for kidney protection illustrates the pivotal role of reverse translation in uncovering mechanisms of drug action and highlights how real‐life clinical observations direct benchtop research and the next iteration of therapeutic experiments.
PATIENT‐DRIVEN RAPID TRANSLATION
Another contemporary example of reverse translation is the recent quest for expedited Zika virus diagnostics during the 2015–2016 viral outbreak, a state of public health emergency declared by the World Health Organization and the United States Department of Health and Human Services, as outlined by Kurani et al.3 In their commentary, the authors describe how public need pushed forward the development of diagnostics and therapeutics necessary to combat the Zika virus, whereas acknowledging the systems in place that helped facilitate the rapid translation of in vitro diagnostics (e.g., Emergency Use Authorization from the US Food and Drug Administration (FDA)) and the lessons learned from this translational experience during a public health crisis.
TURNING THE TABLES ON THERAPEUTIC FAILURE
Therapeutic failure is one of the most feared outcomes in research and clinical practice. However, in the context of reverse translation, therapeutic failure represents an opportunity to identify new therapeutic targets and novel biomarkers of drug response. This change in attitude toward therapeutic failure is embraced by Becker and Funk4 in their commentary on the active role of reverse translation in the advancement of therapeutics for the treatment of rare rheumatologic disorders.
Shifting the focus from therapeutic failures to recent anticancer successes at the regulatory level, Faucette et al.5 distills the comprehensive biopharmacologic evidence submitted to the FDA for approval of oncology new molecular entities to a practical framework intended to expedite the clinical development and regulatory submission of future oncology drugs. The framework advocates for careful consideration of the benefit‐to‐risk profile and dose justification of new molecular entities for the general population, as well as for select patient subpopulations based on age, race, organ function, genotype and concomitant medication use, recognizing the importance of real‐world interindividual variability to drug response.
In addition to successfully integrating regulatory science, reverse translation also encompasses quantitative systems biology, as explained by Edginton6 in her commentary on the successful application of physiologically based pharmacokinetic models to inform variability in drug response, including adverse events and potentially fatal toxicities.
EMBRACING VARIABILITY IN DRUG RESPONSE
Dissecting the origins of the inherent variability in the human drug response is the cornerstone of reverse translation. Thus, it comes as no surprise that genome‐wide and phenome‐wide association studies fall under the umbrella term “reverse translation.” In their timely review of the application of genome‐wide and phenome‐wide association studies, methodologies to real‐world data, Robinson et al.7 eloquently explain how the electronic health record, the bioinformatics engine of today's busy medical practice, can be leveraged as a readily accessible, powerful, convenient tool for the collection of real‐world longitudinal pharmacokinetic and pharmacodynamic data to inform drug development, personalization, and repurposing. Expect more to come from this emerging reverse translation discipline of electronic health record/real‐world data‐informed therapeutics research in the near future.
In summary, Nobel Prize laureate, Sydney Brenner, provides the best contextualization for reverse translation: “we don't have to look for model organisms anymore, because we are the model organism.”1 The reverse translation issue of Clinical and Translational Science honors this ode to Homo sapiens.
Conflict of Interest
The author declared no conflicts of interest.
References
- 1. Ledford, H. Translational research: the full cycle. Nature 453, 843–845 (2008). [DOI] [PubMed] [Google Scholar]
- 2. McWilliam, S.J. , Antoine, D.J. & Pirmohamed, M. Repurposing statins for renal protection: is it a class effect? Clin. Transl. Sci. 11, 100–102 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kurani, S. , Theel, E. , Greenberg‐Worisek, A . Diagnostic testing for Zika: observing rapid translation during a public health emergency. Clin. Transl. Sci. 11, 103–105 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Becker, M.L. & Funk, R.S. Reverse translation in advancing pharmacotherapy in pediatric rheumatology: a logical approach in rare diseases with limited resources. Clin. Transl. Sci. 11, 106–108 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Faucette, S. , Wagh, S. , Trivedi, A. , Venkatakrishnan, K. & Gupta, N. Reverse translation of US Food and Drug Administration reviews of oncology new molecular entities approved in 2011‐2017: lessons learned for anticancer drug development. Clin. Transl. Sci. 11, 123–146 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Edginton, A.N. Using physiologically based pharmacokinetic modeling for mechanistic insight: cases of reverse translation. Clin. Transl. Sci. 11, 109–111 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Robinson, J.R. , Denny, J.C. , Roden, D.M. & Van Driest, S.L. Genome‐wide and phenome‐wide approaches to understand variable drug actions in electronic health records. Clin. Transl. Sci. 11, 112–122 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]


