Abstract
Borrelia burgdorferi infection is common in horses living in Lyme endemic areas and the geographic range for exposure is increasing. Morbidity after B. burgdorferi infection in horses is unknown. Documented, naturally occurring syndromes attributed to B. burgdorferi infection in horses include neuroborreliosis, uveitis, and cutaneous pseudolymphoma. Although other clinical signs such as lameness and stiffness are reported in horses, these are often not well documented. Diagnosis of Lyme disease is based on exposure to B. burgdorferi, cytology or histopathology of infected fluid or tissue and antigen detection. Treatment of Lyme disease in horses is similar to treatment of humans or small animals but treatment success might not be the same because of species differences in antimicrobial bioavailability and duration of infection before initiation of treatment. There are no approved equine label Lyme vaccines but there is strong evidence that proper vaccination could prevent infection in horses.
Keywords: Disease, Equine, Lyme, Seroprevalence, Treatment, Vaccination
Abbreviations
- FISH
fluorescent in situ hybridization
- Osp
outer surface protein
- VLsE
variable major protein‐like sequence expressed
The motile spirochete Borrelia burgdorferi sensu lato complex is believed to be the predominant cause of Lyme disease.1, 2, 3 The primary North American genospecies is B. burgdorferi sensu stricto, with Ixodid ticks being the vector for transmission.4, 5, 6 The disease is common in humans and occurs sporadically in dogs but in both species some aspects of the disease remain controversial.7, 8 Clinical signs associated with the infection in humans include arthritis, carditis, erythema migrans, cutaneous pseudolymphoma, neurological involvement, and perhaps, chronic infection leading to malaise and fatigue.9 In dogs, glomerulonephritis is an additional syndrome of Lyme disease.10
The exact pathogenesis of disease after B. burgdorferi infection in the horse is not known. After exposing ponies to B. burgdorferi‐infected ticks and necropsy 9 months later, the organism was cultured most commonly from skin near the tick bite as well as from connective tissue and muscle and around nerves and blood vessels near synovial membranes.11, 12, 13 There were no obvious clinical signs associated with infection in any of the ponies. Microscopic lesions were restricted to the skin and peripheral lymph nodes near the tick attachment sites in most ponies, but some ponies had mild, nonsuppurative synovitis, perineuritis, or meningitis. A lymphocytic plasmacytic reaction within infected tissues is common after B. burdorferi infection in humans14 and in experimentally infected ponies this reaction was consistently observed and associated with the highest concentration of the Borrelia organism.11, 12, 13
The immunopathology of Lyme disease in people is still being elucidated, but many human patients have increased markers of inflammation and there is a role for Th1, Th2, Th9, Th17, and T‐reg in the immunopathology of the disease.14, 15
Experimental equine infection studies11, 12, 13, 16 and case reports17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 are sparse, limiting our understanding of both B. burgdorferi infection and Lyme disease in horses. The high seroprevalence for B. burgdorferi in adult horses in some areas of North America28, 29, 30, 31 and the paucity of documented cases of Lyme disease have made B. burgdorferi infection and Lyme disease an extremely controversial topic in equine practice. The purpose of this consensus statement is to examine the entire body of literature regarding B. burgdorferi infection and Lyme disease in horses and when evidence is sufficient, to make recommendations regarding diagnosis, treatments and prevention. Grade of recommendation or level of evidence criteria is listed in Table 1.
Table 1.
Grade of recommendation or level of evidence criteria.
| Strong/Level 1—Evidence from a large retrospective study or experimental study combined with comparative controlled trials or multiple high‐quality studies—further research unlikely to change results. |
| Moderate/Level 2—Case reports supported by high quality comparative studies—further research may change level. |
| Modest/Level 3—Clinical experiences by respected veterinarians and authors supported by comparative studies—further research could completely disapprove the interpretation. |
Biology of Infection
Borrelia burgdorferi bacteria are not free‐living organisms and they quickly die outside of a host. They are maintained in a 2‐year enzootic life cycle that involves mammals and Ixodid ticks: Ixodes scapularis in eastern North America and Ixodes pacificus on the North American west coast.32 The white‐footed mouse in the east and gray squirrels in the west serve as common reservoir hosts for the spirochete and provide a source of spirochetes for Ixodes larvae and nymph infection with a continuation of the infection in adult ticks, whereas deer or other large wild mammals help maintain the adult Ixodes tick.33, 34, 35, 36 Borrelia burgdorferi is transferred from the tick gut to animals during blood meals. After tick attachment, several hours are believed to be required to successfully transfer the organism to a mammalian host.37 This time is needed for the organism to down‐regulate outer membrane lipoprotein OspA, which is important for survival in the tick gut but its down regulation is also important in transmission of infection to a mammalian host.38 Conversely, B. burgdorferi outer surface lipoproteins OspC and variable major protein‐like sequence expressed (VLsE) are up‐regulated and are important in the establishment of acute and chronic infections, respectively.39, 40 OspC expression is not necessary to maintain infection and, after early infection, OspC is down‐regulated whereas VLsE and other outer surface proteins (Osp) such as OspF expression increases.14, 41, 42 The VLsE protein has both invariant (eg, C6 peptide) and genetically variable regions, with the variable regions providing an important mechanism for immune evasion and persistence of infection.41, 42 After infection the organism spreads locally through connective tissue and in blood, allowing both local and systemic dissemination to preferred tissues where it colonizes and replicates.43
Seroprevalence
The seroprevalence of B. burgdorferi in dogs, horses and humans is increasing nationwide, as is the range of the Ixodid tick.30, 44, 45, 46, 47, 48, 49 Data strongly support the regional spread of Ixodes ticks, and animal and human exposure to B. burgdorferi in the Midwest, Pacific, and East Coasts of the United States. A recent review of 6 different literature searches, documented an increased incidence and prevalence of Ixodes scapularis and I. pacificus in the United States within the last 20 years (Fig 1).50 Spread of Ixodes has predominantly occurred in the Northeast from increased numbers in New York and northward into Vermont, New Hampshire, Maine, and southern Ontario, Canada. Ixodes distribution has also expanded into Western Pennsylvania and Ohio. In the South, Ixodes has increased in West Virginia, Virginia, and North Carolina. In the Midwest, spread is predominantly thought to have begun in Wisconsin, and then spread to Illinois and Minnesota and from Indiana to both Illinois and Michigan. The greatest factors thought to affect spread were suitable climate and habitats that support white tailed deer and mice, and included waterways, river valleys and forests.50 In addition to studies performed that collected ticks nationwide, regional and/or statewide data are available in some cases.51, 52, 53 Part of the challenge in reviewing the literature is that there has been no standardized method or organization responsible for studying some of the important changes in Ixodes populations and B. burgdorferi presence or infection rates in dogs and horses and, as a result, data sets are more limited than is ideal. There are data on the temporal changes in seroprevalence of B. burgdorferi in dogs.54 Based on 66,582 samples submitted to the Vector Borne Disease Lab at North Carolina State University, data supports a temporal increase in B. burgdorferi infection in dogs in the southern United States between 2008 and 2010.54 A baseline B. burgdorferi antibody prevalence map (Fig 2) for dogs has been developed by a Bayesian spatio‐temporal model.45 It might be expected that horses would have a similar or even higher seroprevalence than dogs in the same geographic areas55 because horses often have equal or greater exposure to tick habitat and are less likely to have tick preventative control treatments.
Figure 1.
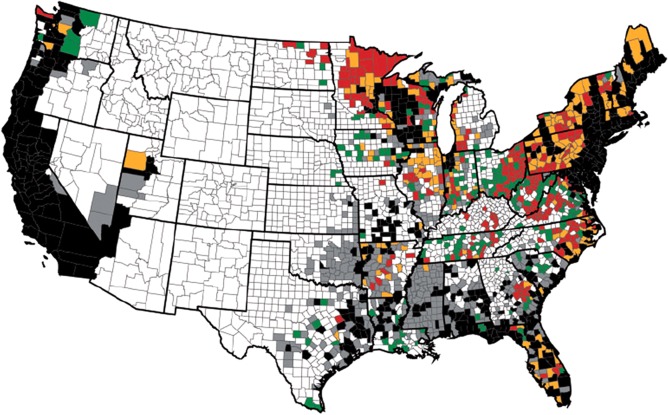
From Eisen et al J Med Entomol 201650 with permission. Changes in county status for Ixodes scapularis and Ixodes pacificus from December 1996 to August 2015. Black color indicates that county status already was established (black) or reported (gray) for I. scapularis or I. pacificus and considered to be the same in this study. Red or orange color indicates that the status of a county changed from no records to established (red) or from reported to established (orange). Green color indicates that the status of a county changed from no records to reported.
Figure 2.
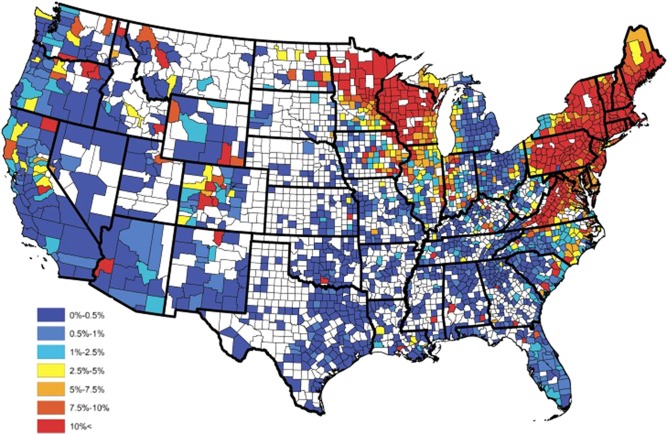
Observed Borrelia burgdorferi antibody prevalence in domestic dogs for 2015.45 From Stella C. Watson et al PLoS One 2017 with permission.
Horses—Seroprevalence
In comparison with regional or national studies on B. burgdorferi infections in dogs and people, meta‐analysis comprehensive nationwide studies have not been performed in horses. Additionally, biases were present in many of the published equine investigations, with most having analyzed samples from animals with clinical signs thought to be consistent with Lyme. In one report, suspect Borrelia or Anaplasma phagocytophilum infected horses from Connecticut and New York during 1985–1999 were tested by whole cell enzyme‐linked immunosorbent assay (ELISA) and 59% were seropositive for Borrelia.47 During a 1 year period (2011–2012), 8% of equine samples from Lyme suspect horses in New York were positive for OspC antibody suggesting recent infection in those horses.48 An increased seroprevalence by indirect fluorescent antibody (IFA) testing of suspected disease horses in Minnesota over a 10‐year period (2001–2010) was reported, with a mean of 58.7% of the tested horses being positive during this time period.30 By Luciferase immunoprecipitation systems for antibody detection, a 94% seropositive rate was reported from suspect clinical cases in Maryland horses.55 In contrast, other studies assessing seroprevalence on nonclinically biased samples ranged from 0.2% seroprevalence in Texas in 1991,56 14.8% of horses from the Pacific Northwest,31 45% of normal horses from the Northeast28 and more recently, 33% of presumed healthy horses in southwest Virginia tested positive.29 While nationwide B. burgdorferi studies assessing both nonclinically affected and suspected clinically affected horses do not exist, the above seroprevalence data support both a high and in some reports an increasing seroprevalence in horses in several areas of the United States.
There is strong evidence that equine B. burgdorferi exposure is common in several geographic areas of the United States. There is also strong evidence that the geographic range for exposure is increasing in horses. Serologic surveys have demonstrated that clinically normal horses living in endemic areas often have detectable antibody levels against B. burgdorferi (Level 1).
Diagnosis of Infection
Diagnosis of current or previous infection with B. burgdorferi is most commonly achieved via serologic testing. Positive test results indicate presence of antibodies against B. burgdorferi at the sampling time point and might represent different infection stages depending on the test utilized. Commercially available laboratory tests currently include indirect fluorescent antibody test (IFAT),19, 57 ELISA whole cell,11, 58, 59, 60 Western blot (WB),11, 13, 58, 61, 62 and a bead‐based multiple antigen ELISA assay (Multiplex).63, 64 A point of care ELISA kit (C6 SNAP) marketed for dogs has also been utilized for testing horses.65 None of the tests consistently detect antibody until 3 or more weeks after infection. There was no consensus to recommend a specific test for diagnosis of B. burgdorferi infection or as a measure of response to treatment. Specific test information is summarized in Table 2.
Table 2.
Serologic tests for Borrelia burgdorferi exposure in horses.
| Test | Laboratory | Antibody Targets | Interpretation | Pros | Cons |
|---|---|---|---|---|---|
|
ELISA, IFAT (Serum, CSF, joint fluid) |
U. Conn. Vet. Diag. Lab. Also available at other labsa |
|
|
|
|
|
WB (Serum, CSF, joint fluid) |
U. Conn. Vet. Diag. Lab. Also available at other labsa |
|
|
|
|
|
Equine Multiplex Assay (Serum and CSF)—not synovial fluid |
AHDC, Cornell University | Three recombinant antigens:
|
|
|
|
|
SNAP4Dx (Serum, plasma, or anti‐coagulated whole blood) |
IDEXX |
|
|
|
|
AHDC, Cornell, Animal Health Diagnostic Center, Cornell University College of Veterinary Medicine; Bb, Borrelia burgdorferi; U. Conn. Vet. Diag. Lab., Connecticut Veterinary Medical Diagnostic Laboratory, University of Connecticut; ELISA, enzyme‐linked immunosorbent assay; IFAT, indirect fluorescent antibody test; IR, immunodominant region; Osp, outer surface protein; VlsE, Vmp‐like sequence, expressed; WB, Western blot.
Quality control might vary between different laboratories.
A positive test result in the absence of previous vaccination indicates exposure from either a current or previous infection. Regardless of test methodology, a positive result does not prove causation of current clinical signs (clinical infection) nor does a positive result predict whether infection is likely to cause clinical signs in the future. There is no known correlation between magnitude of titer and likelihood of disease. The WB and Multiplex assays may assist in determining stage of infection (acute versus chronic) and vaccination status.11, 63 Antibody against the OspC protein is believed to develop within 3–5 weeks after infection and generally disappears by 4–5 months as OspC expression is down‐regulated after infection is established. Possibly because of antigenic variation in OspC, some dogs did not have detectable OspC antibody after infection when tested with the Multiplex assay66 and the same could be true for horses. OspF antibody is first detected 5–8 weeks post infection and may persist for many months or years, with or without treatments.29, 63 Whole cell lysate assays such as IFAT and ELISA do not distinguish between infection stages or between natural infection and vaccination. Additionally, evidence from dogs and horses suggests that whole cell lysate assays can give false positive results attributable to cross‐reactivity with antibodies against common bacterial antigens such as flagellar proteins,67, 68, 69 and positive IFAT or ELISA results therefore require confirmation of infection by WB. Serologic surveys have demonstrated that clinically normal horses living in endemic areas often have detectable antibody levels against B. burgdorferi.28, 29, 70 Therefore, a positive serologic test generally indicates current or past infection but has low positive predictive value of disease.28, 29, 70
Many horses, including a high percentage of those that receive antimicrobial treatments for B. burgdorferi, continue to have positive serologic tests for several months or even years.29, 70 It has yet to be determined if the prolonged positive serology is caused by persistence of infection with B. burgdorferi (either as motile spirochete or non‐motile persister forms), reinfection in some cases, or continued IgG production against Borrelia antigens after elimination of the organism. All ponies that were experimentally infected with B. burgdorferi and untreated had persistent whole cell ELISA and WB antibody and remained infected with live culturable organisms 9 months later, confirming that chronic infection is possible in horses. Horses can become whole cell ELISA and C6 negative after antimicrobial treatment.12, 29, 65, 70 In the pony experimental infection, whole cell ELISA and C6 titers went from positive to negative by 4 months after antibiotic treatments and corresponded to elimination of the organism as documented by postmortem culture and polymerase chain reaction (PCR) testing.12, 65 Therefore, based upon the experimental pony studies, it seems possible that horses with persistently increased antibody levels are chronically infected. However, naturally infected horses might also maintain increased antibody levels caused by reinfection/exposure or continued immune response in the absence of infection. Although OspA is generally considered to be a vaccine‐induced antibody, early, transient and small increases may occur after natural infection and in a small number of non‐vaccinated horses, high and persistent OspA antibody levels may be found.29 The clinical importance of chronically high OspA antibody in non‐vaccinated horses is undetermined but in humans it been associated with chronic infection and disease development.71
Recommendation for serologic testing of horses at select examinations (eg, purchase exams or wellness exams) in the absence of compelling clinical signs compatible with Lyme disease is not supported by current literature or research data. This might be explained to the owner as being similar to equine protozoal myeloencephalitis (Sarcocystis neurona) testing in healthy horses. High seroprevalence after natural infection in healthy horses in many regions, absence of knowledge on vaccination status in some horses and persistence of antibody levels posttreatment makes such testing of low positive predictive value for current or future disease.11, 28, 29, 70, 72, 73, 74
Negative test results are believed to have a high negative predictive value unless the horse is acutely infected (less than 1 month), immune‐compromised or if infection is localized to an immune privileged site such as the eye and central nervous systems.21, 23, 26
Strain variation in North American B. burgdorferi exists but its importance in diagnostic testing and treatment success is unknown.5 Whole cell ELISA, WB, and C6 assays used to detect B. burgdorferi antibodies might also detect antibodies to B. mayonii, a recently reported Lyme borreliosis organism found, so far, only in the mid‐western United States.75 Antibodies to B. miyamotoi, a non‐Lyme disease spirochete found in Ixodes ticks in the Northeastern United States, will not be detected by current serologic tests used for B. burgdorferi antibody detection.76 Although B. miyamotoi, B. bissettii, and B. mayonii have been found in Ixodes ticks in parts of the United States, there are currently no reports of equine infection with any of the 3 organisms. Testing for B. miyamotoi (WB antibody and PCR) can be performed at some Northeastern veterinary diagnostic laboratories.
Antigen detection tests can be used when potentially infected tissues or samples such as; skin biopsy, cerebrospinal fluid, ocular fluid, or synovial fluid are available. Multiple techniques have been utilized, including PCR, immunohistochemistry, fluorescent in situ hybridization (FISH), and silver staining. Culture can also be attempted but has proven to be difficult. The sensitivity and specificity of these techniques in clinical equine cases have not been evaluated.
Regardless of serologic test methodology, a positive result does not prove causation of clinical signs nor does it predict whether infection is likely to cause clinical signs in the future. Therefore, a positive serologic test can be interpreted as evidence of current or past infection but has low positive predictive value of clinical disease (Level 1).
The authors believe that serologic testing should in general not be recommended in healthy horses in high seroprevalence areas (Level 2).
Evidence is not available to absolutely determine if the prolonged positive serology observed in many treated and untreated horses is due to persistence of infection with the Borrelia organism.
Although OspA antibody is commonly associated with prior vaccination, a small number of unvaccinated horses have persistently high OspA antibody levels.
Clinical Disease
The best‐documented naturally occurring syndromes attributed to B. burgdorferi infection in horses include neuroborreliosis, uveitis, and cutaneous pseudolymphoma. Both sporadic case reports and a more recent retrospective study with postmortem examination and ancillary testing document clinical findings indicative of neuroborreliosis.18, 20, 21, 22, 26, 27 Clinical findings and disease course in these studies were variable but clinical signs included; atrophy of spinous muscles, dysphagia, laryngeal dysfunction resulting in respiratory distress, facial paresis, spinal cord ataxia and paresis, behavioral changes, hyperesthesia, fasciculations, neck and back stiffness with pain. Fever was inconsistent and most often absent in the reported cases of neuroborreliosis.26 Duration of neurologic dysfunction before death ranged from 2 to 730 days, with a median of 120 days.26 Additional systemic signs in horses with neuroborreliosis might also be present, including uveitis, joint effusion, and cardiac arrhythmias. Although signs of cranial nerve dysfunction, radiculoneuritis, and meningitis are the most common clinical presentations, these findings can mimic several other equine neurologic disorders.
Uveitis occurs when spirochetes infect the eye.17, 20, 23, 26 Clinical findings usually include severe and most often bilateral ocular disease consistent with chronic uveitis, including a yellow‐green fibroid aqueous humor, aqueous flare, synechiae, miosis, preiridal fibrovascular membrane formation, and other iris changes such as rubeosis iridis and loss of corpora nigra. Horses with Borrelia‐associated uveitis may simultaneously show signs of neuroborreliosis or subsequently develop neurologic disease; 8/9 horses with uveitis in published reports had neuro‐ocular borreliosis.17, 20, 23, 26
Cutaneous pseudolymphoma associated with B. burgdorferi infection has been reported in 1 horse and was characterized by dermal, papular to nodular lesions that occurred at the site of the tick bite.24 Histologic evaluation was suggestive of lymphoma but immunohistochemistry revealed mixed lymphoid hyperplasia and Borrelia PCR performed on the tissue was positive. This lesion was also found in experimentally infected ponies.11 Borrelia‐associated cutaneous pseudolymphoma should be considered for horses in endemic regions with focal infiltrative skin lesions, particularly if the site corresponds to that of a known tick bite.
Anecdotal reports and a 2009 survey of equine practitioners in the Northeastern United States suggest a plethora of not—well—documented clinical signs associated with Lyme disease in horses, most commonly stiffness and shifting leg or intermittent lameness.28, 47, 77 There is little research or overt clinical data to document generalized lameness and stiffness as an equine Lyme disease syndrome. The frequent clinical association of these signs with Lyme disease might be plausible though, based on our knowledge that B. burgdorferi is commonly found in synovial membranes after equine experimental infection and abnormal lympho‐plasmacytic synovitis was found in 1 experimentally infected pony.11, 13 There are 4 clinical case reports documenting B. burgdorferi infection with lympho‐plasmacytic synovitis and lameness in horses.18, 19, 20, 25 All of the horses in these clinical reports had marked joint or tendon sheath fluid distention, which is not what is commonly reported in the majority of presumed but unconfirmed equine Lyme lameness cases.28, 77 In a survey of equine practitioners regarding Lyme disease in horses, behavioral changes, hyperesthesia, and muscle wasting were also reported and could be attributed to neuroborreliosis or to lymphocytic‐histiocytic and plasmacytic inflammation of the deep dermis, muscle, and the panniculus.13, 22, 26 Although anecdotal, web‐based, reports of equine Lyme disease causing laminitis, headshaking, hepatitis, nephritis, or fistulous withers can be found, there is little research and no overt clinical data to support the claims. The range of specific clinical signs associated with Lyme disease is certainly in need of further experimental and epidemiological evaluation, but future progress might well be on a case by case report basis.
The best—documented, naturally occurring syndromes attributed to B. burgdorferi infection include neuroborreliosis, uveitis, and cutaneous pseudolymphoma.
The association of B. burgdorferi infection with stiffness and lameness in horses is not well documented and there is no evidence of the infection causing laminitis.
The actual range and specific clinical signs associated with Lyme disease needs further experimental and epidemiological evaluation.
Diagnosis of Disease
Definitive diagnosis of clinical Lyme disease in horses is challenging. Positive serology merely confirms past exposure or present infection but does not confirm clinical disease. Many other equine diseases share similar clinical signs to Lyme disease, making the diagnosis difficult and raising the strong possibility that Lyme disease is over‐diagnosed in areas with a high seroprevalence.78
Several criteria for making a diagnosis of Lyme disease in humans have been suggested and are listed in the following sentences.79 Possibility of exposure to Borrelia‐infected ticks, based on geographical location or travel history, should be a prerequisite for consideration of clinical Lyme disease. Clinical signs caused by Lyme disease are variable based on the clinical syndrome present. Ruling out other diseases that might cause similar clinical signs is likely the most important aspect (Level 1) of working toward a diagnosis of Lyme disease (Fig 3). When considering equine neuroborreliosis, diseases with similar neurologic signs such as equine protozoal myeloencephalitis, viral encephalitis, and other causes of radiculoneuritis must be ruled out. When considering ocular or dermatologic manifestations of Lyme disease in horses, diseases such as Leptospira spp. associated uveitis and cutaneous lymphoma, respectively, must be ruled out. Unlike with human Lyme disease, the classic cutaneous erythema migrans has not been noted in the equine after experimental or natural infection. Other causes of subtle gait abnormalities, stiffness, or lameness, should also be ruled thoroughly out before even a tentative diagnosis of Lyme disease is made in horses with those signs. This is especially important in sport horses that may have one or more of these signs caused by a variety of infectious or noninfectious causes. Borrelia burgdorferi co‐infection with A. phagocytophilum is reported in horses and signs characteristic of A. phagocytophilum infection such as fever, partial anorexia, and sometimes ataxia followed by leg edema, and icterus should not be mistaken as signs of Lyme disease.28 When these clinical signs are reported, the diagnostic priority would be to perform PCR testing of whole blood for A. phagocytophilum rather than Lyme testing.
Figure 3.
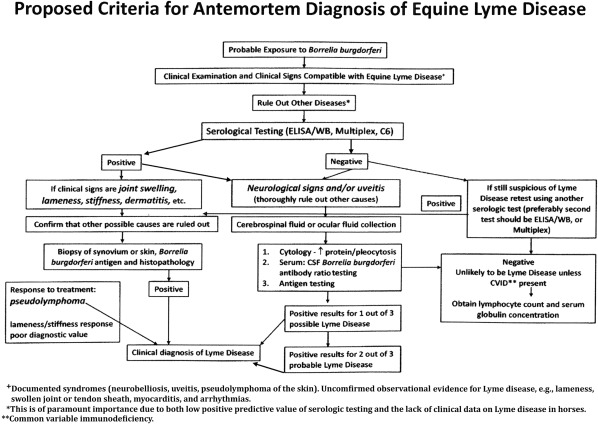
Criteria for diagnosing equine Lyme disease.
Evidence for prior or current infection with B. burgdorferi, as demonstrated by positive serology, is present in most Lyme cases but the positive predictive value is very low because of the high incidence of exposure in many geographic locations. Some horses with clinical disease, especially neuroborreliosis, are seronegative.22, 23, 26 Causes for absence of seroconversion are frequently unknown, but include location of B. burgdorferi in an immune privileged site within the host such as the eye or central nervous system, recent infection (<1 month), and abnormal host response as documented in cases with common variable immunodeficiency syndrome.21, 26
Demonstration of intrathecal antibody production may be supportive in cases of neuroborreliosis but can be misleading in many cases.26 Laboratory dilution of serum and cerebrospinal fluid (CSF) must be considered in the calculation, depending upon the serologic methods used. For example, when testing with the Multiplex assay, the serum is tested at a dilution of 1:400, whereas CSF is tested undiluted. Therefore, to estimate the serum to CSF ratio after Multiplex testing, 1 group proposed accounting for this dilution by multiplying the serum results by 400 and then dividing by the CSF result. If the ratio is less than 130:1, which is the approximate normal serum to CSF IgG ratio,80 then intrathecal antibody production against B. burgdorferi is a consideration.26 However, there are assumptions made both on the linearity of the antibody ratio testing and permeability of the blood‐brain barrier, which is likely abnormal in most neuroborreliosis cases, in making this calculation. Additionally, ratio cutoffs have not been validated. Therefore, using serum to CSF B. burgdorferi antibody ratio in the diagnosis of neuroborreliosis can be confusing and should not be considered a gold standard test (Level 1).
Cytology of specific fluid samples can be supportive of Lyme disease. Cerebrospinal fluid analysis is often abnormal, and either a neutrophilic or lymphocytic pleocytosis, increased total protein, and/or xanthochromia are found in most neuroborreliosis cases (Level 2).26, 27 In humans with neuroborreliosis, lymphocytic pleocytosis is the predominant cytologic finding; this might not be the case in horses with the disease.26, 81
Spirochetes might be identified on cytology of the vitreous humor from Lyme uveitis cases but have not been observed in the CSF of horses with Lyme neuroborreliosis.23 Antigen testing should be performed when suspect infected tissue or fluid samples are available. Positive PCR testing of affected fluids such as CSF, synovial fluid, or ocular fluids and skin biopsy confirms the presence of the organism or its DNA. Polymerase chain reaction testing of CSF in suspected cases of Lyme neuroborreliosis is recommended although the sensitivity is low.21, 26, 27 A possible explanation for this is that the organism resides mostly in the meninges or parenchyma of the central nervous system and not in the CSF. Synovial fluid PCR testing before antibiotic treatment is typically positive in human patients with Lyme arthropathy82 but there are no similar studies on antigen testing or even cytologic examination of synovial fluid in lame horses suspected to have Lyme disease.
Histologic lesions observed in infected and diseased tissues are predominantly lymphohistiocytic and plasmacytic infiltrates,1, 11, 18, 20, 22, 24 although necrosuppurative lesions have also been described.22 These histopathologic changes along with immunohistochemical, PCR or FISH detection of Borrelia ssp. spirochetes or DNA have been found in synovium, skin, meninges, and less commonly in brain on postmortem examination of horses with signs consistent with Lyme disease.18, 20, 22, 24, 26 These histopathologic and sometimes grossly visualized meningeal lesions found in horses with neuroborreliosis are generally quite different from those found in other North American equine infectious neurologic disorders and when these are observed, B. burgdorferi antigen testing is strongly recommended. Histopathology with antigen testing of synovial membrane biopsies obtained antemortem in suspected clinical cases of Lyme borreliosis has not been described but is a possible avenue for diagnostic testing. Histopathologic features of synovium in infected dogs have been described and shown to be reliable indicators of Borrelia infection.66 If synovial biopsies are performed in horses suspected to have Lyme disease, both histopathology and antigen testing should be performed, as presence of the organism alone in the synovium would not be sufficient to prove causation of disease.10, 11
A positive response to treatment with tetracyclines of suspected cases of Lyme disease has been reported in clinical practice.83, 84 Tetracycline drugs have, however, been demonstrated to have anti‐inflammatory properties due in part to a reduction of synovial matrix metalloproteinase‐13.85, 86 In horses with stiffness or lameness suspected to be caused by Lyme disease, the anti‐inflammatory effects of these drugs might result in improvement regardless of Lyme status. Based on this information, response to treatment with tetracyclines is rarely recommended as a diagnostic modality for confirmation of most Lyme disease syndromes in horses (Level 2). Horses with neuroborreliosis and ocular manifestations of Lyme disease typically have a poor response to antibiotic therapy, therefore using response to treatment as a diagnostic test in these cases is also likely to be misleading.26
Many equine diseases share similar clinical signs to Lyme disease making the diagnosis difficult and raising the possibility that Lyme disease is over‐diagnosed in areas with a high seroprevalence in the horse population.
Ruling out other diseases that might cause the clinical signs in the horse being examined should be a high priority before making a diagnosis of Lyme disease (Level 1).
In addition, antigen detection when possible should be used to help confirm a diagnosis. Response to treatment can rarely be used as a diagnostic modality.
The most common histopathologic lesion of Lyme disease in horses is a lymphohistiocytic and plasmacytic infiltrate. Gross thickening of the meninges may be observed with equine neuroborreliosis.
Treatment
The ideal treatment regimen for equine Lyme disease is unknown. Investigation is hampered by lack of a disease model as well as the difficulty in establishing a definitive antemortem diagnosis. Therefore, treatment recommendations have been based on in vitro B. burgdorferi antibiotic susceptibility, extrapolation from human treatment guidelines, available antibiotic pharmacokinetic data in horses, and a single treatment trial in experimentally infected ponies.12, 87, 88, 89, 90, 91, 92, 93
Recommended treatments for Lyme disease in people vary depending on stage of infection and whether neurologic or cardiac involvement is present.94 For early human Lyme disease manifesting as erythema migrans and associated clinical signs, PO administered doxycycline, amoxicillin, or cefuroxime are most commonly used with high success. Practice guidelines for early stage human Lyme disease suggest a 2‐week treatment period, with longer periods generally having no additional benefit.94, 95, 96 Macrolides such as azithromycin, clarithromycin, or erythromycin are sometimes used for patients intolerant of the first‐line antimicrobials. If meningitis and other manifestations of early neurologic Lyme disease are observed, parenteral ceftriaxone, cefotaxime, or penicillin G are frequently administered for 2 weeks or more.97 Similar drug recommendations apply for late Lyme disease manifesting with arthritis or other signs but treatment duration is often extended to 4 weeks. Oral doxycycline is reported to be as effective as parenteral administered beta lactam drugs in many studies on human neuroborreliosis or chronic stages of Lyme disease.96, 98 This might not be true in horses, though, because of the low bioavailability of PO administered tetracyclines in the horse in comparison to the very high bioavailability in humans.88, 91, 99
Consistent with human guidelines, tetracyclines and β‐lactam drugs are most commonly used to treat equine Lyme disease. Available evidence does not clearly support use of one drug over the others. Although one of the experimental pony trials investigated antibiotic treatment,12 it is difficult to draw firm conclusions from that study because there were small numbers in each treatment group, none of the ponies displayed clinical signs, and the drug dosages utilized were somewhat different than what are currently used in clinical practice. In that study, ponies were experimentally infected and then treated with antibiotics for 28 days, starting approximately 3 months after tick exposure. Four ponies were administered tetracycline (5 mg/kg/day IV), 4 ponies were administered doxycycline hyclate (10 mg/kg once daily PO), 4 ponies were administered ceftiofur sodium (2.2 mg/kg/day IM), and 4 ponies were untreated.12 Based on serology returning to baseline and postmortem tissue sample culture and PCR results, infection was eliminated in 4/4 tetracycline‐treated ponies, 2/4 ceftiofur‐treated ponies, 1/4 doxycycline‐treated, and none of the untreated ponies.12 These results led some practitioners to recommend a month‐long course of IV tetracycline or oxytetracycline for suspect cases of equine Lyme disease. Anecdotally, however, veterinarians and owners perceive clinical treatment success in suspected cases of Lyme disease with similar durations of treatment with either doxycycline (10 mg/kg PO q 12 h) or minocycline (4 mg/kg PO q 12 h) with less risk of an adverse event. Likewise, β‐lactam drugs such as penicillin and cephalosporins are effective against Borrelia and theoretically appropriate for equine Lyme disease, although these drugs require parenteral administration, may be cost‐prohibitive and also have some risk of toxicity.16 In the absence of neurologic or ocular disease, antibiotic choice for equine Lyme disease can be based upon the recommended antibiotics for human Lyme disease, information from the experimental pony studies, availability, ease of administration, and pharmacokinetics of each drug in horses in addition to cost and likelihood of adverse effects (Table 3).
Table 3.
Minimum inhibitory concentrations of antimicrobials for Borrelia burgdorferi and comments regarding use in horses with Lyme disease.
| Drug | Dosage (reference) | MICa (µg/mL) for B. burgdorferi (reference) | Comments |
|---|---|---|---|
| Amikacin | 32–>128 (Hunfeld 2006) | NOT recommended (not effective against Borrelia) | |
| Amoxicillin |
0.05–0.39 (Kim 2006) 0.03–2 (Hunfeld 2006) |
NOT recommended for adult horses (low oral bioavailability Ensink 1992) | |
| Azithromycin | 0.003–0.03 (Hunfeld 2006) | NOT recommended for adult horses (risk of colitis) | |
| Cefotaxime | 25 mg/kg IV q6h (Orsini 2004) |
≤ 0.125 (Ates 2010) 0.01–1 (Hunfeld 2006) |
Expensive drug; higher dosages (eg, 50 mg/kg IV q 6 h) might be more effective for neuroborreliosis |
| Ceftiofurb |
Ceftiofur sodium 2.2 mg/kg IV q12h Ceftiofur crystalline free acid (CFA) 6.6 mg/kg IM Day 1, 4, then q7d |
< 0.04–0.08 (Caol 2017) | Serum ceftiofur and desfuroylceftiofur (DCA) combined concentrations remain >0.22 μg/mL throughout CFA administration at 1, 4, 7 days and weekly. Tissue concentrations of DCA in the uterus were maintained between 0.1 and 0.2 μg/g. (Scofield 2014) Concentrations in other tissues not reported. |
| Ceftriaxone | 25–50 mg/kg IV q12h (Ringger 1996) |
0.03 (Ates 2010) <0.01–0.125 (Hunfeld 2006) |
CAUTION: reported to cause life‐threatening gastrointestinal disease and anaphylaxis in some adult horses |
| Chloramphenicol | 50 mg/kg PO q6h | 1.25–2 (Hunfeld 2006) | No data on clinical use for borreliosis |
| Doxycyclineb | 10 mg/kg PO q12h (Bryant 2000) |
0.125–0.25 (Ates 2010) 0.06–2 (Hunfeld 2006) |
Commonly used for Lyme borreliosis in humans and horses. Peak synovial fluid concentrations can be similar or greater than serum concentrations. (Maher 2014, Schnabel 2010) Results in low or undetectable levels in the CSF or ocular fluids of healthy adult horses. (Bryant 2000) (Gilmour 2005) |
| Enrofloxacin | 12.5–50.0 (Kim 2006) | NOT recommended (not effective against Borrelia) | |
| Erythromycin | <0.007–1 (Hunfeld 2006) | NOT recommended for adult horses (risk of colitis) | |
| Metronidazole | 15–25 mg/kg PO q6–8h |
0.06–32 (Sapi 2011) 0.25–0.50 (Cao1 2017) |
No data on clinical use for equine borreliosis; theoretically more effective than other drugs at reducing round body (cystic) forms but clinical relevance uncertain. Poor in vitro efficacy against motile B. burgdorferi. |
| Minocyclineb | 4 mg/kg PO q12h (Schnabel 2012) |
0.03–1 (Hunfeld 2006) 0.4–0.8 (Caol 2017) |
Superior aqueous humor and CSF penetration to doxycycline. Mean CSF concentration is 69% of corresponding peak plasma concentration. Aqueous concentration was 0.9 μg/mL in healthy horse eyes. (Schnabel 2012) Reported trough synovial fluid concentrations of minocycline in horses were 0.33 μg/mL or less. |
| Penicillin Gb | 22,000–44,000 IU/kg IV q6h | 0.03–8 (Hunfeld 2006) | Highest dosage recommended for neuroborreliosis |
| Tetracyclineb | Oxytetracycline 5.0–6.6 mg/kg IV q12 or 24h (Brown 1981, Dowling 2000) |
0.25 (Ates 2010) 0.01–20 (Hunfeld 2006) |
IV oxytetracycline often used for Borrelia infection in horses. Oral tetracycline/oxytetracycline NOT recommended. |
| Tilmicosin | ≤0.01 (Kim 2006) | NOT recommended in horses—fatalities reported after injection; no data on clinical use for equine borreliosis | |
| Trimethoprim/sulfa‐methoxazole | 25 mg/kg PO q12h | >256 (Baradaran‐Dilmaghani, 1996) | NOT recommended for treatment of motile B. burgdorferi. Sulfonamides may have some activity against B. burgdorferi persisters (Feng 2014) |
Measurements of MIC can serve as a treatment guide but in vitro results cannot be directly applied to in vivo situations because of differences in pharmacokinetics and dynamics of each drug must be considered along with the immune responses of the patient.
Antibiotics with MIC values <1 μg/mL against B. burgdorferi and having good safety data for use in adult horses.
In the authors' experience and based on reports in the literature, successful treatment of equine neuroborreliosis is difficult.26 This assertion is certainly biased, as definitive diagnosis is often based on postmortem findings. However, horses that have succumbed to neuroborreliosis have often been treated for long periods of time (months) before death with drugs that in theory should be effective (doxycycline, minocycline). An explanation for this lack of treatment response in horses may be the poor bioavailability of oral doxycycline and minocycline in horses compared to humans or that duration of infection and advancement of disease before treatment is likely greater in horses than in humans. There is evidence that chronic B. burgdorferi infections are more difficult to treat than more recent infections.100, 101 Although speculative, it seems reasonable to recommend similar treatment approaches for confirmed equine neuroborreliosis as those used in people. If financially feasible, using parenteral high‐dose penicillin (44,000 U/kg IV q 4–6h) or cefotaxime (25–50 mg/kg IV q 6–8h) might be most effective. Ceftriaxone is commonly used in people with chronic Lyme disease but this drug has been reported to have a high incidence of adverse effects in adult horses and therefore should be used cautiously, if at all (Level 2).16, 90 If these parenteral antibiotic administration options are either cost‐prohibitive or impractical, minocycline (4 mg/kg or greater PO q 12h) is likely to be more effective than doxycycline in treating neuroborreliosis because of better blood‐brain barrier penetration and evidence that minocycline may help protect neuronal cells from inflammation.102, 103 Likewise, horses with ocular involvement should be treated with drugs that obtain levels above B. burgdorferi MIC in ocular fluids. Limited information regarding ocular penetration of systemic antibiotics in horses is available; at currently recommended dosages, minocycline reaches higher levels than doxycycline but its efficacy for ocular borreliosis is unknown.91 Also, horses might have end‐stage ocular disease at the time of diagnosis, with no chance of salvaging vision.
Some Lyme researchers blame treatment failures on development of antibiotic resistant non‐motile forms of B. burgdorferi known as spheroplasts, round bodies, L‐forms, or cysts. These forms are thought to be less susceptible to certain antibiotics such as tetracycline and β‐lactam drugs but might be susceptible to metronidazole.104, 105 However, current evidence does not support specific treatment of these morphologic variants106, 107 and metronidazole has poor in vitro activity against motile B. burgdorferi.93
Because of the myriad and often obscure clinical signs attributed to Lyme disease in horses and the lack of clinical trials evaluating and defining “treatment strategies,” the recommendations for treatment duration are not well defined. Current laboratory and human patient research supports a lack of resistance of motile Borrelia to antibiotic therapy, that serological testing sometimes remain positive after treatment, and that reinfection posttreatment might occur and will affect serological results.108 Therefore, use of serological markers such as a multiplex assay, western blot, or C6 antibody in the determination of treatment duration might not be that beneficial. However, negative serological tests (eg, C6, ELISA) in ponies have been noted after treatment with tetracycline, doxycycline, and ceftiofur12 when treatment was begun 3 months after experimental infection. A continual decline to negative range in antibody beginning 2–3 months after starting antibiotic treatment would suggest successful elimination of the organism but treatment decisions, either initial or prolonged, should not be based solely on a quantitative antibody test (Level 2). It is apparent that additional clinical studies need to be completed to determine guidelines for the duration of treatment of equine Lyme disease and if there is any clinical significance of post treatment serologic results.
In comparison to treatment of Lyme disease in humans, treatment of the disease in horses is complicated by the difficulty in confirming the diagnosis, poor bioavailability of oral antibiotics commonly used for treating Lyme disease in horses and the longer duration of infection in horses prior to beginning antimicrobial treatment.
Oral and parenterally administered tetracycline antibiotics and a select number of parenterally administered β‐lactam antibiotics are the consensus recommendation for treatment of horses with confirmed Lyme disease (Level 2).
Duration of treatment is not well defined in the horse but should be based upon clinical response and to a lesser degree decline in serum antibody level. Treatment should not be based solely on positive serology (Level 1).
Lyme Consensus—Ancillary Treatments
While antibiotic therapy remains the primary recommended treatment for horses and other species afflicted with Lyme disease, ancillary treatments have been suggested. The use of NSAIDs in horses with pain or neurologic signs suspected to be because of Lyme disease remains common practice but evidence of efficacy is lacking. In vivo experimental studies in rhesus monkeys have shown that meloxicam does not decrease levels of inflammatory mediators, dorsal root ganglia‐apoptosis, and inflammatory neurodegenerative lesions in the nerve roots and dorsal root ganglia of B. burgdorferi‐infected cells.109, 110 Dexamethasone treatment in humans with B. burdorferi infection has likely been associated with both beneficial and harmful outcomes,110 with worse long‐term outcomes reported in one study.111 Although clinical signs could improve transiently, the committee does not recommend corticosteroids for equine Lyme disease except in some cases of uveitis or neuroborreliosis that are both acute and severe (Level 2). Acupuncture and herbal treatments have been used as adjunctive treatments in horses with Lyme disease. In an uncontrolled study of suspect equine Lyme cases there was observational evidence that these treatments subjectively decreased cutaneous hyperesthesia believed to have occurred secondary to B. burgdorferi‐induced myofascial syndrome and neuritis.84 In searching both veterinary and human peer reviewed articles, we could not find data to confirm efficacy of these treatments.
Treatment of Nonclinical Horses
It is assumed that horses in high‐risk environments are exposed to B. burgdorferi on a regular basis. Studies have indicated an approximate exposure rate of 33% in presumed normal horses in southwest Virginia.29 After titers in these horses did not change nor did clinical signs develop after 5 months, indicating the low positive predictive value of serologic testing for clinical signs of Lyme disease. Treatment of nonclinical horses will result in treatment of many horses when there is no indication for such, unnecessary expense, increased risk of adverse events and inappropriate use of antimicrobials. Therefore, it is in general recommended that horses with clinical signs consistent with Lyme disease for which other potential causes have been excluded be the only seropositive animals selected for antimicrobial treatment.
Treatment of nonclinical, seropositive horses will result in the unnecessary treatment of many horses resulting in unnecessary expense, increased risk of adverse events and inappropriate use of antimicrobials (Level 1).
Prognosis
Making even modest recommendations regarding the prognosis for horses with Lyme disease is complicated by the difficulty of an antemortem definitive diagnosis and the difficulty in experimentally reproducing clinical disease. The prognosis for horses with Lyme disease appears variable depending upon localization of infection and possible chronicity of infection.
The prognosis for human cases of Lyme disease is generally good with early antibiotic treatment, with human patients treated with antibiotics for Lyme disease having cure rates around 90%.112 Approximately 10% of cases continue to show nonspecific signs such as fatigue and joint pain. A subset of human patients with persistent clinical signs leads to a diagnosis of chronic Lyme disease, more commonly called posttreatment Lyme disease syndrome. Most studies conclude that long‐term antibiotic treatment of patients with what is presumed to be chronic Lyme disease does not affect outcome, although this is an issue of frequent debate.113
The prognosis for seropositive horses that are treated for vague or undocumented clinical signs believed to be Lyme disease is unclear and in most cases Lyme disease is not confirmed. It is also unclear whether antibiotic treatment reliably eliminates the infection because many horses remain seropositive for many months or even years after antibiotic treatments.70 Clinical improvement reportedly occurs in many adult horses suspected to have Lyme disease despite persistent seropositivity; this also occurs in people who can remain seropositive for 10 years after clinical recovery.114 Therefore, persistence of positive serologic results alone should not be linked to poor prognosis and may be explained by persistence of antigen without disease, reinfection, or long‐term serologic memory.
The prognosis for horses treated for neuroborreliosis is generally poor, with only a single case report of successful treatment in the literature.27 The prognosis for horses with Lyme‐induced uveitis is poor for restoration of vision.23 Antibiotic treatment provided an excellent outcome in a horse with Lyme pseudolymphoma.24 Differences in prognosis, poorer in horses versus humans with confirmed Lyme syndromes, are likely related to duration of infection before treatment and species differences in bioavailability of the administered antibiotics.
Tick Control, Tick Protection, and Vaccination
Borrelia burgdorferi might be transmitted to humans and small animals by adult, nymph, or larval stages of the infected Ixodid ticks but in large mammals, adult female, and nymphal Ixodes ticks are thought to be responsible for a higher percentage of the B. burgdorferi transmission.115 The adult females predominantly quest and feed in early spring or late fall, times that horses would be at greatest risk for infections. This is dependent upon environmental temperatures in a region and in some colder parts of the United States, adult female Ixodes sp. ticks may be found on horses in the winter after a thaw. Adult males are not thought to take a blood meal or transmit the infection. Horse owners should be encouraged to meticulously check their horses for attached ticks and shown how to properly remove attached ticks. Feeding Ixodes sp. ticks, once removed, can be tested by PCR for B. burgdorferi but this is rarely recommended. Studies evaluating the effectiveness of environmental management for tick control in horses have been relatively sparse. A great deal of tick control measures is not equine‐specific, nor are they specific to the Borrelia vectored species of tick, but are based on information from other species in regards to tick control. “Tickscaping” practices should be a part of the environmental control. Well maintained, dry, sunlit, regularly disturbed, and clean areas tend to have fewer ticks. Reduced exposure to woodland and woodland edges is recommended.116 Pasture mowing, leaf and debris clearing from pastures and if possible, exclusion of deer from the surrounding area may be helpful.117 However, a major reduction in risk of exposure is not expected from environmental control.116 Ticks can survive in stalls and in pastures regardless of winter conditions. Freezing and thawing have a more detrimental effect on ticks than do consistently cold temperatures.118 It is reasonable to assume that stalls can be tick preserves, even in the harshest winter conditions. The use of environmental acaricides controlling ticks in the equine environment would be expensive, potentially toxic and unlikely to be highly effective.
Chemical protection of horses against tick attachment includes wipe‐on, pour‐on and spray‐on products containing cypermethrin, permethrin, pyrethrins, or piperonyl butoxide, which can provide at least several hours of protection. Dust, dirt, perspiration, and water shorten protection time, making reapplication a necessity. Permethrin, and other spot‐on products have been subjectively successful in repelling ticks.116 There are commercially available equine‐specific “spot on” products that contain the above chemicals. Better prophylactic treatments for tick control in horses are needed.
The conclusion drawn from the results of one vaccination study, using a challenge model, was that ponies could be protected from B. burgdorferi infection when using an aluminum adjuvanted recombinant outer‐surface protein A (rOspA) vaccine.13 A recent publication identified that antibody concentration for OspA was markedly increased in horses after administration of a nonadjuvanted “off label” canine approved rOspA vaccine but there was a marked decrease in antibody concentration by 4 months post vaccination.119 Another study evaluating horse response to 3 different canine Lyme vaccines (rOspA antigen and 2 whole‐cell bacterins) also showed that increased antibodies persisted for less than 16 weeks after the initial vaccination series.120 Antibodies to the OspA vaccine were prolonged to 20 weeks when a booster vaccine was given after the initial series.120 Antibody responses were not affected by route of vaccination (subcutaneous versus intramuscular), but were increased with 2 mL versus 1 mL dose.120 In both studies, serum antibody concentrations dropped significantly within 4 months after the last vaccination suggesting that if vaccination is used in horses the timing of the vaccine should coincide with time of peak adult female feeding which is typically fall or early spring. Currently, information on a protective OspA antibody titer are lacking, although in vitro inhibition of B. burgdorferi was shown to diminish with waning serum OspA antibody level.13 Lyme vaccines containing immunogenic OspA antigen have a unique feature of inhibiting the Borrelia organism in the tick, preventing natural exposure. The recommendation for use of a safe vaccine that protects against B. burgdorferi infection is somewhat dependent upon morbidity in infected horses, data that is currently not available.
Equine and Human Lyme Disease—Controversies and One Health
Controversies surrounding B. burgdorferi infection and Lyme disease exist in both horses and humans, and this is especially true for those patients with chronic or relapsing nervous system signs, joint or muscle pain and lethargy that remain seropositive for B. burgdorferi after antibiotic therapy. In both species, recurrent or persistent clinical signs and continued positive serology are generally considered inadequate to confirm a diagnosis of chronic Lyme disease.29, 70, 121 Unfortunately, there are no diagnostic tests readily available to the practitioner to confirm the infection status or pathology typical of Lyme disease in those patients. Symptomatic (clinical) improvement after administration of tetracycline antibiotics does not confirm a Lyme diagnosis. The duration of treatment for horses and humans with serologic evidence of chronic infection and with a clinical diagnosis of Lyme disease remains controversial.100 In both humans and horses, prolonged antibiotic therapy often occurs with no clear indication for such in most cases.70, 122 There are clear guidelines for antibiotic treatments in humans with early phase Lyme disease, but antibiotic treatment recommendations made in humans cannot be directly applied to horses because of antibiotic pharmacokinetic differences between the species and that an early disease syndrome is rarely recognized in horses. The high number of confirmed cases of Lyme disease in humans and the strong evidence for geographic expansion of B. burgdorferi infection in both humans and horses, in addition to the low success of tick control measures, have led to the question: is it time for an approved Lyme vaccine?123 An OspA vaccine was previously shown to be effective in experimental ponies and a similar vaccine was approved in the United States for use in humans between 1998 and 2002; canine approved vaccines are currently used off‐label in some horse populations. There will undoubtedly be many questions, some controversial, that arise regarding the need, efficacy, safety, antigen preference, duration of immunity, and cost of an approved human or equine Lyme vaccine.124
In order to have a minimal understanding of Lyme disease in horses, equine veterinarians have relied heavily on the vast amount of published information on human Lyme disease. Knowledge gained from Lyme disease treatments, longitudinal serology studies in both treated and untreated horses, and equine vaccine studies, may be of comparative value toward improved diagnostics and management in human and canine Lyme disease. As horses, dogs and humans are exposed to the same tick species and Borrelia spp. pathogens, continued seroprevalence studies in horses and dogs will likely be a good predictor of future infection surveillance maps of Lyme disease in humans.
Future Directions
Additional experimental and epidemiological studies are needed to determine the morbidity in B. burgdorferi‐infected horses and to identify the range of clinical signs specifically associated with Lyme disease. It will be especially important to determine if stiffness, hyperesthesia, and lameness are common signs of equine Lyme disease. The development of an equine experimental infection model that results in clinical disease would be helpful in determining the range of clinical signs in equine Lyme disease, provide an opportunity for controlled treatment trials, and allow additional evaluations of currently available serologic tests. Highly sensitive and specific antigen detection tests that demonstrate B. burgdorferi within diseased tissue are needed to permit better documentation of Lyme disease. The determination of a vaccine protective antibody level would be of benefit in evaluating vaccination protocols. Finally, infection prevalence and disease investigations of other Lyme or Borrelia spp. organisms that are known to be present in North American Ixodes ticks should be performed.
Acknowledgments
Conflict of Interest Declaration
In the past 5 years Dr Divers has received funds related to Lyme research from Merial and Zoetis. Dr Divers is employed at Cornell University which offers for diagnostic purposes a bead‐based multiple antigen ELISA assay (Multiplex) for detecting B. burgdorferi antibodies. Dr Bertone received financial support from Merial for the purpose of conducting a Lyme vaccine serologic response study.
Off‐Label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
Institutional Animal Care and Use Committee (IACUC) or Other Approval Declaration
Authors declare no IACUC or other approval was needed.
Consensus Statements of the American College of Veterinary Internal Medicine (ACVIM) provide the veterinary community with up‐to‐date information on the pathophysiology, diagnosis, and treatment of clinically important animal diseases. The ACVIM Board of Regents oversees selection of relevant topics, identification of panel members with the expertise to draft the statements, and other aspects of assuring the integrity of the process. The statements are derived from evidence‐based medicine whenever possible and the panel offers interpretive comments when such evidence is inadequate or contradictory. A draft is prepared by the panel, followed by solicitation of input by the ACVIM membership which may be incorporated into the statement. It is then submitted to the Journal of Veterinary Internal Medicine, where it is edited prior to publication. The authors are solely responsible for the content of the statements.
Presented at the 2017 American College of Veterinary Internal Medicine Forum, National Harbor, MD.
References
- 1. Burgdorfer W. Discovery of the Lyme disease spirochete and its relation to tick vectors. Yale J Biol Med 1984;57:515–520. [PMC free article] [PubMed] [Google Scholar]
- 2. Parola P, Raoult D. Tick‐borne bacterial diseases emerging in Europe. Clin Microbiol Infect 2001;7:80–83. [DOI] [PubMed] [Google Scholar]
- 3. Appel MJ, Allan S, Jacobson RH, et al. Experimental Lyme disease in dogs produces arthritis and persistent infection. J Infect Dis 1993;167:651–664. [DOI] [PubMed] [Google Scholar]
- 4. Normal GL, Antig JM, Bigaignon G, et al. Serodiagnosis of Lyme borreliosis by Borrelia burgdorferi sensu stricto, B. garinii, and B. afzelii western blots (immunoblots). J Clin Microbiol 1996;34:1732–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schutzer SE, Fraser‐Liggett CM, Casjens SR, et al. Whole‐genome sequences of thirteen isolates of Borrelia burgdorferi . J Bacteriol 2011;193:1018–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cerar T, Strle F, Stupica D, et al. Differences in genotype, clinical features, and inflammatory potential of Borrelia burgdorferi sensu stricto strains from Europe and the United States. Emerg Infect Dis 2016;22:818–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aguero‐Rosenfeld ME, Wormser GP. Lyme disease: Diagnostic issues and controversies. Expert Rev Mol Diagn 2015;15:1–4. [DOI] [PubMed] [Google Scholar]
- 8. Littman MP, Goldstein RE, Labato MA, et al. ACVIM small animal consensus statement on Lyme disease in dogs: Diagnosis, treatment, and prevention. J Vet Intern Med 2006;20:422–434. [DOI] [PubMed] [Google Scholar]
- 9. Sanchez JL. Clinical manifestations and treatment of Lyme disease. Clin Lab Med 2015;35:765–778. [DOI] [PubMed] [Google Scholar]
- 10. Dambach DM, Smith CA, Lewis RM, et al. Morphologic, immunohistochemical, and ultrastructural characterization of a distinctive renal lesion in dogs putatively associated with Borrelia burgdorferi infection: 49 cases (1987–1992). Vet Pathol 1997;34:85–96. [DOI] [PubMed] [Google Scholar]
- 11. Chang YF, Novosol V, McDonough SP, et al. Experimental infection of ponies with Borrelia burgdorferi by exposure to Ixodid ticks. Vet Pathol 2000;37:68–76. [DOI] [PubMed] [Google Scholar]
- 12. Chang YF, Ku YW, Chang CF, et al. Antibiotic treatment of experimentally Borrelia burgdorferi‐infected ponies. Vet Microbiol 2005;107:285–294. [DOI] [PubMed] [Google Scholar]
- 13. Chang Y, Novosol V, McDonough SP, et al. Vaccination against lyme disease with recombinant Borrelia burgdorferi outer‐surface protein A (rOspA) in horses. Vaccine 1999;18:540–548. [DOI] [PubMed] [Google Scholar]
- 14. Steere AC, Strle F, Wormser GP, et al. Lyme borreliosis. Nat Rev Dis Primers 2016;2:16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pietikäinen A, Maksimow M, Kauko T, et al. Cerebrospinal fluid cytokines in Lyme neuroborreliosis. J Neuroinflammation 2016;13:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Basile RC, Rivera GG, Del Rio LA, et al. Anaphylactoid reaction caused by sodium ceftriaxone in two horses experimentally infected by Borrelia burgdorferi . BMC Vet Res 2015;11:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burgess EC, Gillette D, Pickett JP. Arthritis and panuveitis as manifestations of Borrelia burgdorferi infection in a Wisconsin pony. J Am Vet Med Assoc 1986;189:1340–1342. [PubMed] [Google Scholar]
- 18. Burgess EC, Mattison M. Encephalitis associated with Borrelia burgdorferi infection in a horse. J Am Vet Med Assoc 1987;191:1457–1458. [PubMed] [Google Scholar]
- 19. Magnarelli LA, Anderson JF, Shaw E, et al. Borreliosis in equids in northeastern United States. Am J Vet Res 1988;49:359–362. [PubMed] [Google Scholar]
- 20. Hahn CN, Mayhew IG, Whitwell KE, et al. A possible case of Lyme borreliosis in a horse in the UK. Equine Vet J 1996;28:84–88. [DOI] [PubMed] [Google Scholar]
- 21. James FM, Engiles JB, Beech J. Meningitis, cranial neuritis, and radiculoneuritis associated with Borrelia burgdorferi infection in a horse. J Am Vet Med Assoc 2010;237:1180–1185. [DOI] [PubMed] [Google Scholar]
- 22. Imai DM, Barr BC, Daft B, et al. Lyme neuroborreliosis in 2 horses. Vet Pathol 2011;48:1151–1157. [DOI] [PubMed] [Google Scholar]
- 23. Priest HL, Irby NL, Schlafer DH, et al. Diagnosis of Borrelia‐associated uveitis in two horses. Vet Ophthalmol 2012;15:398–405. [DOI] [PubMed] [Google Scholar]
- 24. Sears KP, Divers TJ, Neff RT, et al. A case of Borrelia‐associated cutaneous pseudolymphoma in a horse. Vet Dermatol 2012;23:153–156. [DOI] [PubMed] [Google Scholar]
- 25. Passamonti F, Veronesi F, Cappelli K, et al. Polysynovitis in a horse due to Borrelia burgdorferi sensu lato infection–Case study. Ann Agric Environ Med 2015;22:247–250. [DOI] [PubMed] [Google Scholar]
- 26. Johnstone LK, Engiles JB, Aceto H, et al. Retrospective evaluation of horses diagnosed with neuroborreliosis on postmortem examination: 16 cases (2004–2015). J Vet Intern Med 2016;30:1305–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wagner B, Glaser A, Bartol J, et al. A new sensitive Lyme multiplex assay to confirm neuroborreliosis in horses: A case report. Proc Am Assoc Equine Pract 2011;57:70–75. [Google Scholar]
- 28. Magnarelli LA, Ijdo JW, Van Andel AE, et al. Serologic confirmation of Ehrlichia equi and Borrelia burgdorferi infections in horses from the northeastern United States. J Am Vet Med Assoc 2000;217:1045–1050. [DOI] [PubMed] [Google Scholar]
- 29. Funk RA, Pleasant RS, Witonsky SG, et al. Seroprevalence of Borrelia burgdorferi in horses presented for coggins testing in southwest Virginia and change in positive test results approximately 1 year later. J Vet Intern Med 2016;30:1300–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Durrani AZ, Goyal SM, Kamal N. Retrospective study on seroprevalence of Borrelia burgdorferi antibodies in horses in Minnesota. J Equine Vet Sci 2011;31:427–429. [Google Scholar]
- 31. Metcalf KB, Lilley CS, Revenaugh MS, et al. The prevalence of antibodies against Borrelia burgdorferi found in horses residing in the northwestern United States. J Equine Vet Sci 2008;28:587–589. [Google Scholar]
- 32. Rosa PA. Microbiology of Borrelia burgdorferi . Semin Neurol 1997;17:5–10. [DOI] [PubMed] [Google Scholar]
- 33. Eisen L, Eisen RJ, Mun J, et al. Transmission cycles of Borrelia burgdorferi and B. bissettii in relation to habitat type in northwestern California. J Vector Ecol 2009;34:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barbour AG, Bunikis J, Fish D, et al. Association between body size and reservoir competence of mammals bearing Borrelia burgdorferi at an endemic site in the northeastern United States. Parasit Vectors 2015;30:299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mather TN, Wilson ML, Moore SI, et al. Comparing the relative potential of rodents as reservoirs of the Lyme disease spirochete (Borrelia burgdorferi). Am J Epidemiol 1989;130:143–150. [DOI] [PubMed] [Google Scholar]
- 36. Radolf JD, Caimano MJ, Stevenson B, et al. Of ticks, mice and men: Understanding the dual‐host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol 2012;10:87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cook MJ. Lyme borreliosis: A review of data on transmission time after tick attachment. Int J Gen Med 2015;8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pal U, Montgomery RR, Lusitani D, et al. Inhibition of Borrelia burgdorferi‐tick interactions in vivo by outer surface protein A antibody. J Immunol 2001;166:7398–7403. [DOI] [PubMed] [Google Scholar]
- 39. Tilly K, Rosa PA, Stewart PE. Biology of infection with Borrelia burgdorferi . Infect Dis Clin North Am 2008;22:217–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Caine JA, Coburn J. Multifunctional and redundant roles of Borrelia burgdorferi outer surface proteins in tissue adhesion, colonization, and complement evasion. Front Immunol 2016;7:442–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liang FT, Yan J, Mbow ML, et al. Borrelia burgdorferi changes its surface antigenic expression in response to host immune responses. Infect Immun 2004;72:5759–5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tilly K, Bestor A, Rosa PA. Lipoprotein succession in Borrelia burgdorferi: Similar but distinct roles for OspC and VIsE at different stages of mammalian infection. Mol Microbiol 2013;89:216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Caine JA, Coburn J, Morrison RP. A short‐term Borrelia burgdorferi infection model identifies tissue tropisms and bloodstream survival conferred by adhesion proteins. Infect Immun 2015;83:3184–3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Applegren NC, Kraus CK. Lyme disease: Emergency department considerations. J Emerg Med 2017;52:815–824. [DOI] [PubMed] [Google Scholar]
- 45. Watson SC, Liu Y, Lund RB. A Bayesian spatio‐temporal model for forecasting the prevalence of antibodies to Borrelia burgdorferi, causative agent of Lyme disease, in domestic dogs within the contiguous United States. PLoS One 2017;12:e0174428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kugeler KJ, Farley GM, Forrester JD, et al. Geographic distribution and expansion of human Lyme disease, United States. Emerg Infect Dis 2015;21:1455–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Magnarelli E, Fikrig E. Detection of antibodies to B. burgdorferi in naturally infected horses in the USA by enzyme linked immunosorbent assay using whole cell recombinant antigens. Res Vet Sci 2005;79:99–103. [DOI] [PubMed] [Google Scholar]
- 48. Wagner B, Erb H. Dogs and horses with antibodies to outer‐surface protein C as on‐time sentinels for ticks infected with Borrelia burgdorferi in New York State in 2011. Prev Vet Med 2012;107:275–279. [DOI] [PubMed] [Google Scholar]
- 49. Burtis JC, Sullivan P, Levi T, et al. The impact of temperature and precipitation on blacklegged tick activity and Lyme disease incidence in endemic and emerging regions. Parasit Vectors 2016;9:606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Eisen RJ, Eisen L, Beard CB. County‐scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. J Med Entomol 2016;53:349–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Herrin B, Zajac AM, Little SE. Confirmation of Borrelia burgdorferi sensu stricto and Anaplasma phagocytophilum in Ixodes scapularis, Southwestern Virginia. Vector Borne Zoonotic Dis 2014;14:821–823. [DOI] [PubMed] [Google Scholar]
- 52. Nelder MP, Russell CB, Sheehan NJ, et al. Human pathogens associated with blacklegged tick Ixodes scapularis: A systematic review. Parasit Vectors 2016;9:265–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xu G, Mather TN, Hollingsworth CS, et al. Passive surveillance of Ixodes scapularis, their biting activity, and associated pathogens in Massachusetts. Vector Borne Zoonotic Dis 2016;16:520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Qurollo BA, Chandrashekar R, Hegarty BC, et al. A serological survey of tick‐borne pathogens in dogs in North America and the Caribbean as assessed by Anaplasma phagocytophilum, A. platys, Ehrlichia canis, E. chaffeensis, E. ewingii, and Borrelia burgdorferi species‐specific peptides. Infect Ecol Epidemiol 2014;4:24699–24712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Burbelo P, Bren KE, Ching KH, et al. Antibody profiling of Borrelia burgdorferi infection in horses. Clin Vaccine Immunol 2011;18:1562–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cohen ND, Heck FC, Heim B, et al. Seroprevalence of antibodies to Borrelia burgdorferi in a population of horses in central Texas. J Am Vet Med Assoc 1992;201:1030–1034. [PubMed] [Google Scholar]
- 57. Marcus LC, Patterson MM, Gilfillan RE, et al. Antibodies to Borrelia burgdorferi in New England horses: Serologic survey. Am J Vet Res 1985;46:2570–2571. [PubMed] [Google Scholar]
- 58. Bosler EM, Cohen DP, Schulze TL, et al. Host responses to Borrelia burgdorferi in dogs and horses. Ann N Y Acad Sci 1988;539:221–234. [DOI] [PubMed] [Google Scholar]
- 59. Magnarelli LA, Anderson JF. Class‐specific and polyvalent enzyme‐linked immunosorbent assays for detection of antibodies to Borrelia burgdorferi in equids. J Am Vet Med Assoc 1989;195:1365–1368. [PubMed] [Google Scholar]
- 60. Magnarelli LA, Flavell RA, Padula SJ, et al. Serologic diagnosis of canine and equine borreliosis: Use of recombinant antigens in enzyme‐linked immunosorbent assays. J Clin Microbiol 1997;35:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Carter SD, May C, Barnes A, et al. Borrelia burgdorferi infection in UK horses. Equine Vet J 1994;26:187–190. [DOI] [PubMed] [Google Scholar]
- 62. Dzierzecka M, Kita J. The use of chosen serological diagnostic methods in Lyme disease in horses. Part II. Western blot. Pol J Vet Sci 2002;5:79–84. [PubMed] [Google Scholar]
- 63. Wagner B, Freer H, Rollins A, et al. Development of a multiplex assay for the detection of antibodies to Borrelia burgdorferi in horses and its validation using Bayesian and conventional statistical methods. Vet Immunol Immunopathol 2011;144:374–381. [DOI] [PubMed] [Google Scholar]
- 64. Wagner B, Goodman LB, Rollins A, et al. Antibodies to OspC, OspF and C6 antigens as indicators for infection with Borrelia burgdorferi in horses. Equine Vet J 2013;45:533–537. [DOI] [PubMed] [Google Scholar]
- 65. Johnson AL, Divers TJ, Chang YF. Validation of an in‐clinic enzyme‐linked immunosorbent assay kit for diagnosis of Borrelia burgdorferi infection in horses. J Vet Diagn Invest 2008;20:321–324. [DOI] [PubMed] [Google Scholar]
- 66. Grosenbaugh DA, Rissi DR, Krimer PM. Demonstration of the ability of a canine Lyme vaccine to reduce the incidence of histological synovial lesions following experimentally‐induced canine Lyme borreliosis. Vet Immunol Immunopathol 2016;180:29–33. [DOI] [PubMed] [Google Scholar]
- 67. Lindenmayer J, Weber M, Bryant J, et al. Comparison of indirect immunofluorescent‐antibody assay, enzyme‐linked immunosorbent assay, and Western immunoblot for the diagnosis of Lyme disease in dogs. J Clin Microbiol 1990;28:92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shin SJ, Chang YF, Jacobson RH, et al. Cross‐reactivity between B. burgdorferi and other spirochetes affects specificity of serotests for detection of antibodies to the Lyme disease agent in dogs. Vet Microbiol 1993;36:161–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schvartz G, Epp T, Burgess HJ, et al. Comparison between available serologic tests for detecting antibodies against Anaplasma phagocytophilum and Borrelia burgdorferi in horses in Canada. J Vet Diagn Invest 2015;27:540–546. [DOI] [PubMed] [Google Scholar]
- 70. Divers TJ, Grice AL, Mohammed HO, et al. Changes in Borrelia burgdorferi ELISA antibody over time in both antibiotic treated and untreated horses. Acta Vet Hung 2012;60:421–429. [DOI] [PubMed] [Google Scholar]
- 71. Akin E, McHugh GL, Flavell FA, et al. The immunoglobulin (IgG) antibody response to OspA and OspB correlates with severe and prolonged Lyme arthritis and the IgG response to P35 correlates with mild and brief arthritis. Infect Immun 1999;67:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Leeflang MM, Ang CW, Berkhout J, et al. The diagnostic accuracy of serological tests for Lyme borreliosis in Europe: A systematic review and meta‐analysis. BMC Infect Dis 2016;16:140–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ebani VV, Bertelloni F, Pinzauti P, et al. Seroprevalence of Leptospira spp. and Borrelia burgdorferi sensu lato in Italian horses. Ann Agric Environ Med 2012;19:237–240. [PubMed] [Google Scholar]
- 74. Wieneke C, Lovrich SD, Callister SM, et al. Evaluation of whole‐cell and OspC enzyme‐linked immunosorbent assays for discrimination of early lyme borreliosis from OspA vaccination. J Clin Microbiol 2000;38:313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pritt BS, Mead PS, Johnson DKH, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: A descriptive study. Lancet Infect Dis 2016;16:556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Krause PJ, Narasimhan S, Wormser GP, et al. Borrelia miyamotoi sensu lato seroreactivity and seroprevalence in the northeastern United States. Emerg Infect Dis 2014;20:1183–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. DeVilbiss BA, Mohammed HO, Divers TJ. Perception of equine practitioners regarding the occurrence of selected equine neurologic diseases in the northeast over a 10‐year period. J Equine Vet Sci 2009;29:237–246. [Google Scholar]
- 78. Bartol J. Is Lyme disease overdiagnosed in horses?. Equine Vet J 2013;45:529–530. [DOI] [PubMed] [Google Scholar]
- 79. Cutler SJ, Rudenko N, Golovchenko M, et al. Diagnosing borreliosis. Vector Borne Zoonotic Dis 2017;17:2–11. [DOI] [PubMed] [Google Scholar]
- 80. Furr M, Howe D, Reed S, et al. Antibody coefficients for the diagnosis of equine protozoal myeloencephalitis. J Vet Intern Med 2011;25:138–142. [DOI] [PubMed] [Google Scholar]
- 81. Djukic M, Schmidt‐Samoa C, Lange P, et al. Cerebrospinal fluid findings in adults with acute Lyme neuroborreliosis. J Neurol 2012;259:630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am 2015;29:269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Browning A, Carter SD, Barnes A, et al. Lameness associated with Borrelia burgdorferi infection in the horse. Vet Rec 1993;132:610–611. [DOI] [PubMed] [Google Scholar]
- 84. Schoen A. Equine immune‐mediated myofascial syndrome and its relation to Wei Qi syndromes. Am J Trad Chinese Vet Med 2007;2:75–78. [Google Scholar]
- 85. Maher MC, Schnabel LV, Cross JA, et al. Plasma and synovial fluid concentration of doxycycline following low‐dose, low‐frequency administration, and resultant inhibition of matrix metalloproteinase‐13 from interleukin‐stimulated equine synoviocytes. Equine Vet J 2014;46:198–202. [DOI] [PubMed] [Google Scholar]
- 86. Bernardino AL, Kaushal D, Philipp MT. The antibiotics doxycycline and minocycline inhibit inflammatory responses to the Lyme disease spirochete Borrelia burgdorferi . J Infect Dis 2009;199:1379–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ates L, Hanssen‐Hubner C, Norris DE, et al. Comparison of in vitro activities of tigecycline, doxycycline, and tetracycline against the spirochete Borrelia burgdorferi . Ticks Tick Borne Dis 2010;1:30–34. [DOI] [PubMed] [Google Scholar]
- 88. Bryant JE, Brown MP, Gronwall RR, et al. Study of intragastric administration of doxycycline: Pharmacokinetics including body fluid, endometrial and minimum inhibitory concentrations. Equine Vet J 2000;32:233–238. [DOI] [PubMed] [Google Scholar]
- 89. Dowling PM, Russell AM. Pharmacokinetics of a long‐acting oxytetracycline‐polyethylene glycol formulation in horses. J Vet Pharmacol Ther 2000;23:107–110. [DOI] [PubMed] [Google Scholar]
- 90. Gardner SY, Aucoin DP. Pharmacokinetics of ceftriaxone in mares. J Vet Pharmacol Ther 1994;17:155–156. [DOI] [PubMed] [Google Scholar]
- 91. Schnabel LV, Papich MG, Divers TJ, et al. Pharmacokinetics and distribution of minocycline in mature horses after oral administration of multiple doses and comparison with minimum inhibitory concentrations. Equine Vet J 2012;44:453–458. [DOI] [PubMed] [Google Scholar]
- 92. Kim D, Kordick D, Divers T, et al. In vitro susceptibilities of Leptospira spp. and Borrelia burgdorferi isolates to amoxicillin, tilmicosin, and enrofloxacin. J Vet Sci 2006;7:355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Caol S, Divers T, Crisman M, et al. In vitro susceptibility of Borrelia burgdorferi isolates to three antibiotics commonly used for treating equine Lyme disease. BMC Vet Res 2017;13:293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006;43:1089–1134. [DOI] [PubMed] [Google Scholar]
- 95. Berende A, ter Hofstede HJ, Vos FJ, et al. Randomized trial of longer‐term therapy for symptoms attributed to Lyme disease. New Engl J Med 2016;374:1209–1220. [DOI] [PubMed] [Google Scholar]
- 96. Halperin JJ. Chronic Lyme disease: Misconceptions and challenges for patient management. Infect Drug Resist 2015;8:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cadavid D, Auwaerter PG, Rumbaugh J, et al. Antibiotics for the neurological complications of Lyme disease. Cochrane Database Syst Rev 2016;12:CD006978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ogrinc K, Logar M, Lotric‐Furlan S, et al. Doxycycline versus ceftriaxone for the treatment of patients with chronic Lyme borreliosis. Wien Klin Wochenschr 2006;118:696–701. [DOI] [PubMed] [Google Scholar]
- 99. Agwuh KN, MacGowan A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J Antimicrob Chemother 2006;58:256–265. [DOI] [PubMed] [Google Scholar]
- 100. Stricker RB. Counterpoint: Long‐term antibiotic therapy improves persistent symptoms associated with lyme disease. Clin Infect Dis 2007;45:149–157. [DOI] [PubMed] [Google Scholar]
- 101. Girschick HJ, Morbach H, Tappe D. Treatment of Lyme borreliosis. Arthritis Res Ther 2009;11:258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Grace PM, Hutchinson MR, Maier SF, et al. Pathological pain and the neuroimmune interface. Nat Rev Immunol 2014;14:217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gegelashvili G, Bjerrum OJ. High‐affinity glutamate transporters in chronic pain: An emerging therapeutic target. J Neurochem 2014;131:712–730. [DOI] [PubMed] [Google Scholar]
- 104. Brorson O, Brorson SH. An in vitro study of the susceptibility of mobile and cystic forms of Borrelia burgdorferi to metronidazole. Acta Path Micro Im Scand 1999;107:566–576. [DOI] [PubMed] [Google Scholar]
- 105. Sapi E, Kaur N, Anyanwu S, et al. Evaluation of in‐vitro antibiotic susceptibility of different morphological forms of Borrelia burgdoferi . Infect Drug Resist 2011;4:97–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lantos PM, Wormser GP. Chronic coinfections in patients diagnosed with chronic lyme disease: A systematic review. Am J Med 2014;127:1105–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bockenstedt LK, Mao J, Hodzic E, et al. Detection of attenuated, noninfectious spirochetes in Borrelia burgdorferi‐infected mice after antibiotic treatment. J Infect Dis 2002;186:1430–1437. [DOI] [PubMed] [Google Scholar]
- 108. Kowalski TJ, Tata S, Berth W, et al. Antibiotic treatment duration and long‐term outcomes of patients with early lyme disease from a lyme disease‐hyperendemic area. Clin Infect Dis 2010;50:512–520. [DOI] [PubMed] [Google Scholar]
- 109. Ramesh G, Meisner OC, Philipp MT. Anti‐inflammatory effects of dexamethasone and meloxicam on Borrelia burgdorferi‐induced inflammation in neuronal cultures of dorsal root ganglia and myelinating cells of the peripheral nervous system. J Neuroinflammation 2015;12:240–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ramesh G, Martinez AN, Martin DS, et al. Effects of dexamethasone and meloxicam on Borrelia burgdorferi‐induced inflammation in glial and neuronal cells of the central nervous system. J Neuroinflammation 2017;14:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Jowett N, Gaudin RA, Banks CA, et al. Steroid use in Lyme disease‐associated facial palsy is associated with worse long‐term outcomes. Laryngoscope 2017;127:1451–1458. [DOI] [PubMed] [Google Scholar]
- 112. Shapiro ED. Lyme disease. New Engl J Med 2014;371:684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lantos PM. Chronic Lyme disease. Infect Dis Clin North Am 2015;29:325–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hammers‐Berggren S, Lebech AM, Karlsson M, et al. Serological follow‐up after treatment of patients with erythema migrans and neuroborreliosis. J Clin Microbiol 1994;32:1519–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Butler CM, Sloet van Oldruitenborgh‐Oosterbaan MM, Werners AH, et al. Borrelia burgdorferi and Anaplasma phagocytophilum in ticks and their equine hosts: A prospective clinical and diagnostic study of 47 horses following removal of a feeding tick. Pferdeheilkunde 2016;32:335–345. [Google Scholar]
- 116. Stafford KC III. Tick Management Handbook. New Haven, CT: Connecticut Agricultural Experiment Station; 2004. [Google Scholar]
- 117. Deblinger RD, Wilson ML, Rimmer DW, et al. Reduced abundance of immature Ixodes dammini (Acari: Ixodidae) following incremental removal of deer. J Med Entomol 1993;30:144–150. [DOI] [PubMed] [Google Scholar]
- 118. Herrmann C, Gern L. Survival of Ixodes ricinus (Acari:Ixodidae) nymphs under cold conditions is negatively influenced by frequent temperature variations. Ticks Tick Borne Dis 2013;4:445–451. [DOI] [PubMed] [Google Scholar]
- 119. Slaughter KM, Halland SK, Schur LA, et al. Humoral response of Borrelia burgdorferi outer surface protein A (OspA) vaccination in equids. Equine Vet Educ 2017;29:572–576. [Google Scholar]
- 120. Guarino C, Asbie S, Rohde J, et al. Vaccination of horses with Lyme vaccines for dogs induces short‐lasting antibody responses. Vaccine 2017;35:4140–4147. [DOI] [PubMed] [Google Scholar]
- 121. Halperin JJ, Baker P, Wormser GP. Common misconceptions about Lyme disease. Am J Med 2013;126:264.e1–264.e7. [DOI] [PubMed] [Google Scholar]
- 122. Lantos PM. Chronic Lyme disease: The controversies and the science. Expert Rev anti Infect Ther 2011;9:787–797. [DOI] [PubMed] [Google Scholar]
- 123. Plotkin SA. Need for a new Lyme disease vaccine. New. Engl J Med 2016;375:911–913. [DOI] [PubMed] [Google Scholar]
- 124. Šmit R, Postma MJ. Lyme borreliosis: Reviewing potential vaccines, clinical aspects and health economics. Expert Rev Vaccines 2015;14:1549–1561. [DOI] [PubMed] [Google Scholar]


