Abstract
Background
Calprotectin is a marker of inflammation, but its clinical utility in dogs with chronic inflammatory enteropathies (CIE) is unknown.
Objective
Evaluation of fecal calprotectin in dogs with biopsy‐confirmed CIE.
Animals
127 dogs.
Methods
Prospective case‐control study. Dogs were assigned a canine chronic enteropathy clinical activity index (CCECAI) score, and histologic lesions severity was assessed. Fecal calprotectin, fecal S100A12, and serum C‐reactive protein (CRP) were measured. Food‐ or antibiotic‐responsive cases (FRE/ARE, n = 13) were distinguished from steroid‐/immunosuppressant‐responsive or ‐refractory cases (SRE/IRE, n = 20). Clinical response to treatment in SRE/IRE dogs was classified as complete remission (CR), partial response (PR), or no response (NR).
Results
Fecal calprotectin correlated with CCECAI (ρ = 0.27, P = .0065) and fecal S100A12 (ρ = 0.90, P < .0001), some inflammatory criteria, and cumulative inflammation scores, but not serum CRP (ρ = 0.16, P = .12). Dogs with SRE/IRE had higher fecal calprotectin concentrations (median: 2.0 μg/g) than FRE/ARE dogs (median: 1.4 μg/g), and within the SRE/IRE group, dogs with PR/NR had higher fecal calprotectin (median: 37.0 μg/g) than dogs with CR (median: 1.6 μg/g). However, both differences did not reach statistical significance (both P = .10). A fecal calprotectin ≥15.2 μg/g separated both groups with 80% sensitivity (95% confidence interval [95%CI]: 28%‐100%) and 75% specificity (95%CI: 43%‐95%).
Conclusions and Clinical Importance
Fecal calprotectin could be a useful surrogate marker of disease severity in dogs with CIE, but larger longitudinal studies are needed to evaluate its utility in predicting the response to treatment.
Keywords: antibiotic‐responsive enteropathy, biomarker, calgranulin, canine, food‐responsive enteropathy, inflammatory bowel disease
Abbreviations
- ARE
antibiotic‐responsive enteropathy
- AUROC
area under the ROC curve
- CCECAI
canine chronic enteropathy clinical activity index
- ΔCCECAI
change in CCECAI
- CD
Crohn's disease
- CI
confidence interval
- CIBDAI
canine IBD activity index
- CIE
chronic inflammatory enteropathy
- CR
complete remission
- CRP
C‐reactive protein
- FRE
food‐responsive enteropathy
- GI
gastrointestinal
- IBD
inflammatory bowel disease
- IQR
interquartile range
- IRE
immunosuppressant‐responsive/‐refractory enteropathy
- ΜΦ
macrophage(s)
- NF‐κB
nuclear factor‐kappa B
- NR
no response
- OR
odds ratio
- PR
partial response
- ROC
receiver operating characteristic
- SRE
steroid‐responsive/‐refractory enteropathy
- TAMU
Texas A&M University
- TLR
Toll‐like receptor
- UC
ulcerative colitis
1. INTRODUCTION
Chronic inflammatory enteropathies (CIE) comprise an important group of disorders in dogs1, 2 that are characterized by chronic persistent or recurrent gastrointestinal (GI) signs and histopathologic evidence of primary intestinal mucosal inflammation.1, 3, 4 Based on the response to treatment, canine non‐infectious CIE are classified as food‐responsive enteropathy (FRE), antibiotic‐responsive enteropathy (ARE), or steroid‐/immunosuppressant‐responsive (or ‐refractory) enteropathy (SRE/IRE).1, 2
A diagnosis of canine SRE/IRE can be challenging as it involves a comprehensive diagnostic investigation to exclude other causes of chronic GI signs (eg, hypoadrenocorticism, exocrine pancreatic insufficiency, endoparasites), to document mucosal inflammation based on histopathology, to demonstrate an inadequate clinical response to appropriately designed dietary and antibiotic treatment trials, and to show clinical improvement with anti‐inflammatory/immunosuppressive therapy (or lack thereof in refractory cases).1, 2, 3, 4 The pathogenesis of canine SRE/IRE is poorly understood, and an impaired immunoregulation and overt inflammatory response against dietary and bacterial antigens appears to play a central role, together with the presence of a genetic predisposition.3, 5
Recent advances in the diagnosis of CIE include the development of a 6‐parameter6 (considering the dog's attitude/activity, appetite, vomiting, feces consistency, feces frequency, and weight loss; the cumulative score can range from 0 to 18) and a 9‐parameter clinical scoring system7 (which considers the dog's attitude/activity, appetite, vomiting, feces consistency, feces frequency, weight loss, serum albumin concentration, ascites/peripheral edema, and pruritus; the cumulative score can range from 0 to 27), an endoscopic lesion score,8 and a consensus for classifying CIE based on histopathologic criteria.3, 9 However, only a few minimally or noninvasive biomarkers of inflammation have been evaluated in dogs with CIE,10, 11, 12, 13, 14, 15, 16, 17, 18 none of which are currently routinely used in clinical practice.
Canine calprotectin, the S100A8/A9 protein complex, and also S100A12 are Ca2+‐binding proteins of the S100/calgranulin family that have been shown to be associated with acute and chronic inflammation and with malignant transformation.5 These proteins are involved in the regulation of cell proliferation and metastasis, and after their extracellular release function as endogenous danger‐signaling molecules (alarmins).19 Calprotectin and S100A12 have potential as markers of inflammation in dogs.11, 15, 16, 19, 20, 21 In canine CIE, the expression of mucosal S100‐mRNA was shown to be increased 11‐fold.22 Fecal calprotectin and S100A12 have been shown to be correlated with the clinical disease activity,15, 20, 21 and the latter also correlated with the severity of endoscopic lesions15 and the response to treatment in dogs with CIE.16
C‐reactive protein (CRP) is a positive acute‐phase protein of the pentraxin family that is produced by the liver in response to IL‐6 and IL‐1β during states of infection, inflammation, or cancer.23 Serum CRP concentration has been suggested to serve as a marker of disease progression and response to treatment in dogs with SRE/IRE.6, 10, 11
We hypothesized that fecal calprotectin concentrations (1) are associated with the severity of clinical signs, histologic lesions, or both (2) predict the response to different forms of treatment, and (3) correlate with the concentration of other biomarkers of intestinal inflammation. To prove or disprove these hypotheses, the objectives of our study were to evaluate fecal calprotectin concentrations in dogs with CIE in relation to (1) the severity of clinical signs and histologic lesions, (2) disease classification (SRE/IRE versus FRE/ARE) and response to treatment (complete remission [CR] versus partial or no response [NR]), and (3) the concentration of serum CRP and fecal S100A12. In addition, the possibility of an association between fecal calprotectin and the concentration of calprotectin and S100A12 in serum was tested.
2. MATERIALS AND METHODS
2.1. Ethics approval
The study design was reviewed and approved by the Clinical Research Review Committee (CRRC# 2009‐06 and 2010‐05) and the Institutional Animal Care and Use Committee (IACUC, AUP# 2012‐083) at Texas A&M University (TAMU). The owner of each enrolled dog had to give written consent to the study before the inclusion of the dog. Based on our preliminary data,24 a sample size of at least 20 dogs per group was calculated to be required to reveal a difference in fecal canine calprotectin concentrations of at least 20 μg/g with a statistical power of >80% (α = 0.05, β = 0.2; enrollment ratio = 1:1).25
2.2. Sampling population
Dogs (n = 225) >6 months of age presenting for routine diagnostic evaluation of chronic (≥2–3 weeks duration) signs of GI disease (ie, vomiting, diarrhea, hypo‐ or anorexia, abdominal pain, weight loss), including a planned endoscopic or surgical collection of GI tissue biopsies, were prospectively enrolled in the study between August 2009 and July 2015 (Figure 1) at the Veterinary Teaching Hospitals at TAMU or Purdue University, or at 1 of several other referral hospitals across the United States. All dogs underwent a routine diagnostic evaluation according to current guidelines,3 which included a CBC, serum chemistry profile, urinalysis, and additional diagnostics to rule out other causes of chronic signs of GI disease (eg, endoparasites based on a fecal examination, hypoadrenocorticism based on a serum baseline cortisol measurement, and an adrenocorticotropic hormone stimulation test if indicated). The severity of the dog's clinical signs at presentation was assessed by the attending clinician using the 9‐variable canine chronic enteropathy clinical activity index (CCECAI) scoring system, and a CCECAI of ≥1 (ie, minimal to mild clinical disease severity) was required for inclusion in the study.7 A study questionnaire evaluating the dog's overall health, diet, and medication history also had to be completed by the owner. Treatment of each individual dog, including dietary management, antibiotic treatment, and supplements (including prebiotics and probiotics), anti‐inflammatory or immunosuppressant therapy, or all was at the discretion of the attending clinician. Sequential treatment trials (elimination diet for a minimum of 2–3 weeks each, antibiotic trial for at least 10–14 days) were required to be performed to further characterize the disease process,1, 3 with a deviation from this scheme being allowed for dogs with very severe clinical signs or marked abnormal clinicopathologic findings (ie, hypoalbuminemia or panhypoproteinemia).3, 7 The time point of GI tissue specimen collection was also at the clinician's discretion. Dogs were only included in the study if they had not received any anti‐inflammatory/immunosuppressive treatment during the 2 weeks before enrollment and initial sample collection.
Figure 1.
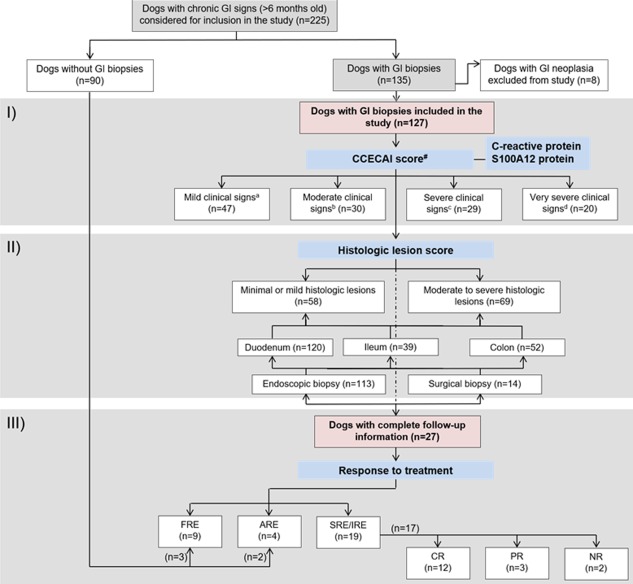
Study flow chart. Flow diagram summarizing the group distribution of the 225 dogs considered for inclusion in the study. The 3 different parts of the study (gray shaded areas) were: evaluation of (I) fecal calprotectin in relation to clinical disease severity (CCECAI score); (II) fecal calprotectin and microscopic lesion severity (histologic lesion score); and (III) fecal calprotectin in relation to disease classification and response to treatment. GI, gastrointestinal; #not recorded from 1 dog; aCCECAI score ≤5; bCCECAI score 6–8; cCCECAI score 9–11; dCCECAI score ≥12
Exclusion criteria for the study that were applied retrospectively, were the lack of intestinal biopsies submitted from a dog or if intestinal biopsies submitted were of inadequate quality (n = 90). Further, dogs were excluded if neoplasia was identified on GI histopathology (n = 8).
Follow‐up information was available from some dogs (n = 32) using the same study questionnaire including a CCECAI scoring sheet, and was used to characterize the disease (ARE: clinical response to tylosin or metronidazole defined as a CCECAI = 0 or a percentage of change in the CCECAI score [ΔCCECAI] >75% at the time of recheck; FRE: clinical response to an easily digestible diet, a novel protein diet, or a diet containing hydrolyzed protein with CCECAI = 0 or ΔCCECAI >75% at the time of recheck; SRE/IRE: requiring treatment with anti‐inflammatory drug, immunosuppressive drugs, or both).1, 2 Five dogs that did not have biopsies collected and were categorized as either FRE or ARE based on complete follow‐up information (follow‐up time 6–169 weeks) were included in the group comparison (Figure 1). Further, the response to treatment in 17 dogs with SRE/IRE was determined based on the ΔCCECAI as either CR (ΔCCECAI >75% or clinical signs resolved [ie, CCECAI = 0]), partial response (PR; ΔCCECAI 25%‐75% or clinical signs improved), or NR (ΔCCECAI <25% or clinical signs same or worse).10, 16
2.3. Sample collection
Single whole blood (ethylenediaminetetraacetic acid was used as anticoagulant), serum, and urine specimens were collected from each dog at the first presentation for diagnostic work‐up and, if a reevaluation was performed according to the study protocol, at the respective recheck(s). Whole blood and serum were collected after food was withheld for at least 12 hours. Fecal samples (an aliquot of approximately 1 g each) were collected from 3 consecutive days.26 Endoscopic (n = 113) or surgical (n = 14) GI tissue biopsies were obtained from each dog (Figure 1) with the method of tissue collection being left at the discretion of the attending clinician. Within 1 day of collection, all specimens, including GI tissues (if obtained as part of the diagnostic work‐up), collected outside of TAMU were shipped to the Gastrointestinal Laboratory at TAMU overnight on ice packs.
Follow‐up data were included from dogs that were clinically reevaluated as described and sample sets were collected at the time of recheck evaluation.
2.4. Sample analyses
Whole blood was used for routine hematology (performed at the institution recruiting the case for the study or the Texas Veterinary Medical Diagnostic Laboratory). Serum was used for a blood chemistry profile (LiquiColor, Sirrus clinical chemistry analyzer, Stanbio Laboratory, Boerne, Texas), and for measurement of serum cobalamin (Immulite 2000, Vitamin B12, Siemens Healthcare Diagnostics Inc., Deerfield, Illinois), folate (Immulite 2000, Folic Acid, Siemens Healthcare Diagnostics Inc.), and CRP (Tri‐Delta Phase CRP, Tri‐Delta Diagnostic, Boonton Township, New Jersey)27 concentrations. Urine samples were used for routine urinalysis (performed at the institution recruiting the respective case for the study or the TAMU Clinical Pathology service) and, if indicated, further diagnostic testing (ie, urine culture, urine protein/creatinine ratio).
Fecal and serum calprotectin concentrations were measured using a species‐specific sandwich ELISA.26 The working range of the assay for serum is 0.3–26.8 mg/L and is 3.2–267.6 μg/g for fecal extracts. The assay was demonstrated to not cross‐react with the canine S100A12 protein.26 Intra‐ and inter‐assay coefficients of variation (%CV) of the assay are ≤12.7% and ≤17.2% for serum samples and ≤10.0% and ≤12.3% for fecal extracts.26 The 3‐day mean fecal calprotectin concentration was calculated26 and used for statistical analyses. Fecal and serum S100A12 concentrations were measured by a species‐specific in‐house sandwich ELISA,28 and the 3‐day mean fecal S100A12 concentration was also calculated and used for analyses.
2.5. Histopathologic evaluation of gastrointestinal tissue biopsies
Histopathologic evaluation of GI tissue biopsies was performed by 1 of 7 board‐certified pathologists (Gastrointestinal Histopathology service, Gastrointestinal Laboratory at Texas A&M University, College Station, Texas; median of 14 dogs evaluated by each pathologist, range: 1–34) using the World Small Animal Veterinary Association Gastrointestinal Standardization grading system.3 The severity of morphologic lesions and inflammatory changes in the duodenum, ileum, and colon were recorded using a 4‐point grading system (0 = normal, 1 = mild lesions, 2 = moderate lesions, and 3 = severe lesions). Individual and cumulative lesion scores (calculated as the sum of individual lesion scores) were considered for statistical analyses. Gastric biopsies were evaluated to rule out evidence of neoplastic disease, but inflammatory changes were not graded.
2.6. Data analyses
Statistical software packages were used for all statistical analyses (JMP v13.0, SAS Institute, Cary, North Carolina, GraphPad Prism v7.0, GraphPad Software, San Diego, California). Continuous variable data were first assessed for normality of their distribution and equality of the variances using a Shapiro‐Wilk W test and a Brown‐Forsythe test, respectively. Summary statistics for continuous variables are reported as medians and interquartile ranges (IQR). Categorical data are presented as counts (n) and percentages.
A non‐parametric Wilcoxon rank‐sum test was utilized to compare continuous variables between dogs with SRE/IRE and the group of dogs with FRE/ARE, and also in SRE/IRE dogs with CR versus PR/NR. A likelihood ratio test or a Fisher's exact test (as appropriate) was used to test the association between categorical variables and disease group or response to treatment. A non‐parametric Spearman rank correlation coefficient ρ was calculated to test for any possible correlation between fecal calprotectin concentrations and the CCECAI scores, individual and cumulative histologic lesion scores, and the concentrations of serum CRP, fecal and serum S100A12, and serum calprotectin. The Spearman ρ was interpreted as indicating a very strong (0.8–1.0), strong (0.6–0.8), moderate (0.4–0.6), weak (0.2–0.4), or very weak (0–0.2) correlation. A Wilcoxon signed rank test served to compare fecal calprotectin concentrations before and after treatment. Statistical significance was set at P < .05, and a Bonferroni correction for multiple statistical comparisons (P corr = P ÷ n) at the same level was applied, if indicated.
A receiver operating characteristic (ROC) curve analysis served to calculate the sensitivity and specificity at optimum cut‐off concentrations (determined by the Youden index) to differentiate (1) dogs with SRE/IRE from those dogs with FRE/ARE and (2) SRE/IRE dogs with PR/NR from those dogs with CR. A Fisher's exact test, with calculation of the odds ratio (OR) and 95% confidence interval (95%CI), served to test the possibility and the odds of an association between a fecal calprotectin concentration of ≥15.2 μg/g, a serum CRP concentration of ≥9.1 mg/L (or ≥12.9 mg/L), a CCECAI score ≥8 (or ≥12), or different combinations of these and (1) a diagnosis of SRE/IRE versus FRE/ARE and (2) a PR/NR versus CR in dogs with SRE/IRE. The probabilistic solution was used to estimate the OR with zero values.29
3. RESULTS
3.1. Study population
A total of 127 dogs diagnosed with CIE (median age: 7 years, IQR: 4–9 years; 64 males/63 females) were included in the study (Figure 1, Table 1). Median CCECAI score was 7 (moderate disease; IQR: 5–10) and median histologic lesion score was 2 (moderate lesions; IQR: 1–3) (Figure 1, Table 1). Median serum cobalamin and folate concentrations were 326 ng/L and 12.9 μg/L, respectively. Hypocobalaminemia (serum cobalamin concentration ≤250 ng/L30) was detected in 38 dogs (30%), of which 16 dogs (13%) had an undetectable serum cobalamin concentration (≤149 ng/L). Hypofolatemia (serum folate concentration ≤7.7 μg/L31) was seen in 17 dogs (14%), and hyperfolatemia (serum folate concentration ≥24.4 μg/L31) in 6 dogs (5%). Breeds most commonly represented in our study included German Shepherd dogs (n = 10), Yorkshire terriers (n = 9), and mixed breed dogs (n = 22).
Table 1.
Dog characteristics, clinical findings, and clinicopathologic parameters in dogs with chronic inflammatory enteropathies (CIE) included in the study
| Group characteristic | CIE (all) | FRE/ARE a | SRE/IRE | P value b | P corr c |
|---|---|---|---|---|---|
| Total number, n | 127 | 13 | 19 | – | – |
| Dog characteristics | |||||
| Age in years, median (IQR) | 7 (4–9) | 6 (3–9) | 8 (7–10) | .13 | 1.00 |
| Sex, male/female | 64/63 | 9/4 | 10/9 | .34 | 1.00 |
| Body weight in kg, median (IQR) | 15.1 (6.6–26.2) | 13.2 (5.3–26.7) | 16.4 (4.7–25.0) | .80 | 1.00 |
| Breed, n (%) | .79 | 1.00 | |||
| ‐ Pure‐bred | 105 (83%) | 12 (92%) | 17 (89%) | ||
| ‐ Mixed breed | 22 (17%) | 1 (8%) | 2 (11%) | ||
| Follow‐up duration in weeks, median (IQR) | – | 13 (5–21) | 8 (4–11) | .08 | .96 |
| Response to treatment, d n (%) | – | – | |||
| ‐ Complete remission (CR) | – | – | 12 (70%) | ||
| ‐ Partial response (PR) | 3 (18%) | ||||
| ‐ No response (NR) | 2 (12%) | ||||
| Biopsy type, n (%) | |||||
| ‐ Endoscopic | 113 (89%) | 7 (54%) | 18 (95%) | ||
| ‐ Surgical | 14 (11%) | 1 (8%) | 1 (5%) | .53 | 1.00 |
| Number of sites biopsied, median (IQR) | 2 (2–3) | 3 (2–4) | 2 (2–3) | .49 | 1.00 |
| Number of biopsies per site, median (IQR) | |||||
| ‐ Stomach (not analyzed) | 12 (8–15) | 10 (7–15) | 10 (9–19) | .65 | 1.00 |
| ‐ Duodenum | 12 (6–17) | 9 (6–12) | 12 (11–14) | .11 | 1.00 |
| ‐ Ileum | 4 (1–11) | 1 (1–4) | 6 (1–11) | .35 | 1.00 |
| ‐ Colon | 12 (8–17) | 12 (10–17) | 11 (6–17) | .76 | 1.00 |
| Histologic lesion score, median (IQR) | 2 (1–3) | 2 (1–2) | 2 (1–2) | .84 | 1.00 |
| Clinical parameters | |||||
| Disease duration in months, median (IQR) | 6 (3–17) | 4 (2–11) | 3 (1–9) | .52 | 1.00 |
| Clinical signs, n (%) | |||||
| ‐ Vomiting | 78 (61%) | 8 (62%) | 9 (47%) | .43 | 1.00 |
| ‐ Diarrhea | 96 (76%) | 10 (77%) | 15 (79%) | .89 | 1.00 |
| ‐ Weight loss | 78 (61%) | 7 (54%) | 9 (47%) | .72 | 1.00 |
| ‐ Hypo‐ or anorexia | 65 (51%) | 2 (15%) | 11 (58%) | .013 | .090 |
| CCECAI score, e median (IQR) | 7 (5–10) | 5 (3–7) | 9 (5–11) | .0025 | .018 |
| Clinical disease severity, e n (%) | |||||
| ‐ Mild (CCECAI score ≤ 5) | 47 (37%) | 7 (58%) | 6 (31%) | .026 | .18 |
| ‐ Moderate (CCECAI score 6–8) | 30 (24%) | 4 (33%) | 2 (11%) | ||
| ‐ Severe (CCECAI score 9–11) | 29 (23%) | 1 (9%) | 9 (47%) | ||
| ‐ Very severe (CCECAI score ≥ 12) | 20 (16%) | – | 2 (11%) | ||
| Clinicopathologic parameters | |||||
| Serum cobalamin in ng/L, f median (IQR) | 326 (217–653) | 439 (248–877) | 301 (194–345) | .14 | 1.00 |
| Hypocobalaminemia, f , g n (%) | 38 (30%) | 3 (23%) | 8 (42%) | .33 | 1.00 |
| Serum folate in μg/L, f median (IQR) | 12.9 (9.5–16.6) | 14.2 (9.7–21.4) | 15.8 (11.3–19.5) | 1.00 | 1.00 |
| Hypofolatemia, f , h n (%) | 17 (14%) | 0 (0%) | 3 (16%) | .077 | .54 |
| Serum albumin in g/dL, i median (IQR) | 3.0 (2.5–3.3) | 3.2 (2.9–3.5) | 2.3 (1.7–3.5) | .0012 | .0084 |
| Hypoalbuminemia, i , j n (%) | 50 (40%) | 1 (8%) | 9 (47%) | .0082 | .057 |
| Hypoalbuminemia severity, i n (%) | |||||
| ‐ Minimal (albumin 2.0–2.4 g/dL) | 15 (12%) | 0 (0%) | 2 (10.5%) | .056 | .39 |
| ‐ Mild (albumin 1.5–1.99 g/dL) | 17 (14%) | 0 (0%) | 4 (21%) | ||
| ‐ Moderate (albumin 1.2‐1.49 g/dL) | 10 (8%) | 1 (8%) | 1 (5%) | ||
| ‐ Severe (albumin <1.2 g/dL) | 8 (6%) | 0 (0%) | 2 (10.5%) | ||
| Biomarkers of inflammation | |||||
| Fecal calprotectin in μg/g, median (IQR) | 1.6 (0.04–15.3) | 1.4 (0.5–2.4) | 2.0 (1.1–37.0) | .10 | .52 |
| Fecal S100A12 in ng/g, median (IQR) | 148 (22–899) | 138 (35–248) | 242 (71–1,709) | .12 | .58 |
| Serum CRP in mg/L, median (IQR) | 8.3 (1.2–25.8) | 1.8 (0.2–4.5) | 14.3 (5.4–37.8) | .0028 | .014 |
| Serum calprotectin in μg/L, median (IQR) | 6,389 (4,114–8,903) | 5,083 (2,982–13,172) | 9,320 (6,169–11,907) | .45 | 1.00 |
| Serum S100A12 in μg/L, median (IQR) | 206 (144–328) | 137 (77–252) | 239 (155–412) | .14 | .71 |
Abbreviations: CCECAI, canine chronic enteropathy clinical activity index; FRE/ARE, food‐ or antibiotic‐responsive enteropathy; SRE/IRE, steroid‐ or immunosuppressant‐responsive (or ‐refractory) enteropathy; IQR, interquartile range.
aIncluding 5 dogs with FRE or ARE that did not have gastrointestinal biopsies taken.
bSignificant difference between (or association with) ARE/FRE and SRE/IRE group (bold face values: P < .05).
cBonferroni corrected P value (n = 12, 7, 7, and 5).
dDocumented in n = 20 dogs.
eDocumented in n = 126 dogs.
fDocumented in n = 125 dogs.
gDefined as ≤250 ng/L.
hDefined as ≤7.7 μg/L.
iDocumented in n = 124 dogs
jDefined as ≤2.4 g/dL.
Of the 32 dogs with CIE from which follow‐up information was available, 19 dogs were diagnosed with SRE/IRE and 13 dogs with FRE or ARE (Figure 1, Table 1). In the FRE group, an easily digestible diet was given to 2 dogs, a novel protein diet to 2 dogs, and a diet containing hydrolyzed protein to 4 dogs; 1 FRE dogs was given a home‐prepared limited antigen diet. In the SRE/IRE group, dogs received prednisone/prednisolone (n = 15) or budesonide (n = 4), azathioprine (n = 4), or cyclosporine (n = 1); 5 of the dogs received a combination of 2 medications. Dietary choices in SRE/IRE dogs were an easily digestible or low‐fat diet (n = 3), a novel protein diet (n = 4), and a diet containing hydrolyzed protein (n = 9); 3 SRE/IRE dogs were given a home‐prepared limited antigen diet.
Total (ie, maximum) follow‐up time ranged from 2 to 169 weeks (median: 8 weeks, IQR: 4–13 weeks), with a first follow‐up at a median of 5 weeks (IQR: 3–9 weeks). A first follow‐up with recollection of samples (serum and feces) and data exactly 3–4 weeks after the initial work‐up (ie, collection of biopsies) was only accomplished in 14 of the 32 dogs (44%). About a third of the dogs (11/32) with a first set of follow‐up samples and data had at least 1 additional follow‐up recorded at a later time. The response to treatment was assessed retrospectively based on the longest available follow‐up time.
Sex and age did not differ between dogs diagnosed with SRE/IRE and dogs with FRE/ARE (Table 1). However, dogs with SRE/IRE had significantly higher CCECAI scores (median: 9, IQR: 5–11) than dogs with FRE/ARE (median: 5, IQR: 3–7; P corr = 0.018, Table 1). Sex and CCECAI scores did not differ between SRE/IRE dogs with CR and those dogs with PR/NR (P = .59 and P = .49, respectively), but dogs with PR/NR were significantly older (median = 11 years, IQR = 7–12 years) than those dogs with CR (median = 8 years, IQR: 5–8 years; P = .039).
3.2. Fecal calprotectin concentrations and other biomarkers
Calprotectin concentrations ranged from 0.04 to 1,971.0 μg/g in individual fecal samples from all 127 dogs, with the 3‐day mean fecal calprotectin concentrations ranging from 0.04 to 992.5 μg/g (median: 1.6 μg/g, IQR: 0.5–15.3 μg/g). Serum CRP concentrations ranged from 0.1 to 81.0 mg/L (median: 1.6 mg/L, IQR: 0.1–11.4 mg/L) in all dogs with CIE. Fecal S100A12 concentrations ranged from 1 to 101,980 ng/g in individual fecal samples from all CIE dogs, with the 3‐day mean fecal S100A12 concentrations ranging from 1 to 41,660 ng/g (median: 65 ng/g, IQR: 14–425 ng/g).
Fecal calprotectin concentrations were correlated with fecal S100A12 concentrations (ρ = 0.90, P corr < .0006), but showed no association with serum concentrations of CRP (ρ = 0.16, P corr = .71), calprotectin (ρ = 0.14, P corr = 1.00), or S100A12 (ρ = 0.09, P corr = 1.00) (Table 2).
Table 2.
Correlation among clinical, laboratory, and histologic findings in dogs with CIE
| Spearman ρ correlation coefficient (P corr) a | ||||
|---|---|---|---|---|
| Parameter Correlated with | Fecal canine calprotectin | Fecal canine S100A12 | Serum CRP | CCECAI score |
| CCECAI score | 0.27 (.039) | 0.24 (.097) | 0.42 (<.0006) | – |
| Serum CRP concentration | 0.16 (.71) | 0.15 (.87) | – | 0.42 (<.0006) |
| Fecal canine S100A12 concentration | 0.90 (<.0006) | – | 0.15 (.87) | 0.24 (.097) |
| Serum calprotectin concentration | 0.14 (1.00) | 0.13 (1.00) | 0.19 (.21) | 0.20 (.71) |
| Serum S100A12 concentration | 0.09 (1.00) | 0.14 (1.00) | 0.26 (.0018) | 0.09 (1.00) |
| Histologic lesions (composite score) b | 0.32 (1.00) | 0.21 (1.00) | 0.27 (1.00) | 0.06 (1.00) |
| Morphologic criteria b | 0.07 (1.00) | 0.05 (1.00) | 0.35 (.18) | 0.16 (.85) |
| Inflammatory criteria b | 0.52 (.036) | 0.34 (.30) | 0.10 (1.00) | −0.03 (1.00) |
| Duodenum (composite score) | −0.04 (1.00) | 0.10 (1.00) | 0.26 (.018) | 0.07 (1.00) |
| Morphologic criteria (sum) | −0.07 (1.00) | 0.06 (1.00) | 0.29 (.0048) | 0.11 (.52) |
| • Villus stunting | −0.08 (1.00) | −0.03 (1.00) | 0.23 (.033) | 0.19 (.23) |
| • Epithelial injury | 0.01 (1.00) | 0.14 (.88) | 0.16 (.48) | 0.12 (1.00) |
| • Crypt distension | −0.08 (1.00) | 0.02 (1.00) | 0.27 (.024) | 0.11 (1.00) |
| • Lacteal dilatation | 0.01 (1.00) | 0.17 (.57) | 0.18 (.32) | 0.05 (1.00) |
| • Mucosal fibrosis | −0.04 (1.00) | −0.03 (1.00) | 0.02 (1.00) | 0.05 (1.00) |
| Inflammatory criteria (sum) | 0.08 (.91) | 0.10 (.67) | 0.14 (.31) | 0.02 (1.00) |
| • Intraepithelial lymphocytes | 0.30 (.027) | 0.30 (.017) | 0.17 (.39) | 0.05 (1.00) |
| • Lamina propria LPC | −0.03 (1.00) | 0.06 (1.00) | 0.13 (.83) | 0.03 (1.00) |
| • Lamina propria eosinophils | −0.08 (1.00) | −0.16 (.61) | −0.12 (1.00) | −0.18 (.30) |
| • Lamina propria neutrophils | 0.17 (.57) | 0.17 (.53) | 0.24 (.061) | 0.11 (1.00) |
| • Lamina propria ΜΦ | 0.16 (.66) | 0.15 (.82) | 0.18 (.30) | 0.24 (.0495) |
| Ileum (composite score) | 0.25 (.59) | 0.28 (.44) | 0.13 (1.00) | 0.04 (1.00) |
| Morphologic criteria (sum) | 0.25 (.40) | 0.17 (.77) | 0.25 (.32) | 0.07 (1.00) |
| • Villus stunting | 0.07 (1.00) | −0.05 (1.00) | 0.32 (.32) | 0.14 (1.00) |
| • Epithelial injury | 0.21 (1.00) | 0.19 (1.00) | 0.24 (.89) | −0.11 (1.00) |
| • Crypt distension | 0.35 (.30) | 0.32 (.48) | 0.27 (.61) | −0.24 (.87) |
| • Lacteal dilatation | −0.07 (1.00) | −0.05 (1.00) | 0.01 (1.00) | 0.34 (.22) |
| • Mucosal fibrosis | N/A | N/A | N/A | N/A |
| Inflammatory criteria (sum) | 0.38 (.082) | 0.33 (.16) | 0.01 (1.00) | 0.01 (1.00) |
| • Intraepithelial lymphocytes | 0.29 (.64) | 0.22 (1.00) | 0.04 (1.00) | 0.18 (1.00) |
| • Lamina propria LPC | 0.41 (.14) | 0.29 (.64) | 0.12 (1.00) | 0.02 (1.00) |
| • Lamina propria eosinophils | 0.13 (1.00) | 0.08 (1.00) | −0.29 (.50) | −0.26 (.68) |
| • Lamina propria neutrophils | 0.12 (1.00) | 0.23 (1.00) | 0.01 (1.00) | −0.07 (1.00) |
| • Lamina propria ΜΦ | 0.24 (1.00) | 0.25 (.95) | 0.24 (.89) | 0.05 (1.00) |
| Colon (composite score) | 0.13 (1.00) | 0.13 (1.00) | 0.12 (1.00) | −0.10 (1.00) |
| Morphologic criteria (sum) | 0.03 (1.00) | 0.05 (1.00) | 0.18 (.48) | −0.07 (1.00) |
| • Epithelial injury | 0.05 (1.00) | 0.11 (1.00) | 0.09 (1.00) | 0.06 (1.00) |
| • Goblet cell loss or hyperplasia | 0.08 (1.00) | 0.15 (1.00) | 0.21 (.66) | −0.11 (1.00) |
| • Crypt dilatation and distortion | 0.04 (1.00) | 0.05 (1.00) | 0.20 (.73) | −0.03 (1.00) |
| • Mucosal fibrosis and atrophy | −0.05 (1.00) | −0.19 (.96) | 0.02 (1.00) | 0.10 (1.00) |
| Inflammatory criteria (sum) | 0.21 (.41) | 0.21 (.38) | 0.08 (1.00) | −0.07 (1.00) |
| • Intraepithelial lymphocytes | 0.19 (1.00) | 0.16 (1.00) | 0.17 (1.00) | −0.13 (1.00) |
| • Lamina propria LPC | 0.14 (1.00) | 0.10 (1.00) | 0.10 (1.00) | 0.01 (1.00) |
| • Lamina propria eosinophils | 0.06 (1.00) | 0.02 (1.00) | −0.31 (.18) | −0.16 (1.00) |
| • Lamina propria neutrophils | −0.03 (1.00) | 0.01 (1.00) | 0.17 (1.00) | 0.05 (1.00) |
| • Lamina propria ΜΦ | 0.06 (1.00) | 0.13 (1.00) | 0.11 (1.00) | 0.10 (1.00) |
Relationship between the 3‐day mean fecal calprotectin concentrations and the clinical disease activity (CCECAI) score, concentrations of other inflammatory markers (serum CRP, serum and 3‐day mean fecal S100A12, and serum calprotectin), and the severity of morphologic and inflammatory histologic lesions in the duodenum, ileum, and colon in dogs with CIE (n = 127).
Abbreviations: LPC, lymphocytes/plasma cells; ΜΦ, macrophages; N/A, not applicable; P corr, Bonferroni corrected P value (P corr = P × n).
aOrange shaded cells: significant (bold face values indicate P < .05) only without Bonferroni correction; blue shaded cells: significance (bold face values indicate P < .05) remaining after Bonferroni correction (n = 2, 3, 4, or 5).
bCalculated only when duodenum, ileum, and colon were sampled and evaluated.
3.3. Fecal calprotectin concentrations and clinical disease severity
Three‐day mean fecal calprotectin concentrations were weakly correlated with the CCECAI score at the time of enrollment (ρ = 0.27, P corr = .039).
3.4. Other biomarkers and clinical disease severity
Correlation with the CCECAI score was also seen for serum CRP concentrations (ρ = 0.42, P corr < .0006), but not for fecal S100A12 concentrations (ρ = 0.24, P corr = .097).
3.5. Fecal calprotectin concentrations and microscopic lesion severity
Fecal calprotectin concentrations were correlated with the number of intraepithelial lymphocytes in the duodenum (ρ = 0.30, P corr = .027) and with the overall cumulative inflammatory lesion score (ρ = 0.52, P = .018; Table 2), whereas the correlation with the lamina propria lymphoplasmacytic infiltration (ρ = 0.41, P corr = .14) and the overall inflammatory lesion score in the ileum (ρ = 0.38, P corr = .08) did not remain significant after correction for multiple comparisons.
3.6. Other biomarkers and microscopic lesion severity
Serum CRP concentrations were associated with the severity of morphologic lesions in the duodenum (Table 2). However, there was no significant correlation between serum CRP concentrations and the severity of microscopic lesions in the ileum or colon; neither did serum CRP concentrations correlate with the cumulative histologic lesion scores (Table 2). Fecal S100A12 concentrations were significantly correlated with the number of duodenal lamina propria intraepithelial lymphocytes (ρ = 0.30, P corr = .017), whereas the relationship with other inflammatory criteria did not reach significance (Table 2).
3.7. Fecal calprotectin concentrations and disease classification
Fecal calprotectin concentrations were numerically higher in dogs diagnosed with SRE/IRE (median: 2.0 μg/g, IQR: 1.1–37.0 μg/g) compared to dogs with FRE or ARE (median: 1.4 μg/g, IQR: 0.5–2.4 μg/g), but the difference did not reach significance (P = .10) (Figure 2). Using a cut‐off fecal calprotectin concentration of ≥15.2 μg/g yielded a sensitivity of 37% (95%CI: 18%–57%) and a specificity of 100% (95%CI: 70%–100%; area under the ROC curve [AUROC]: 67%, 95%CI: 49%–86%) for the diagnosis of SRE/IRE (Table 3, Figure 2).
Figure 2.

Fecal calprotectin concentrations, serum CRP concentrations, and CCECAI scores in relation to disease classification in dogs with CIE. (A) Fecal calprotectin concentrations were numerically higher in dogs with SRE/IRE (n = 19) compared to dogs with FRE/ARE (n = 13), but the difference was not significant (P = .10). (B) Serum CRP concentrations and (C) CCECAI scores were significantly higher in dogs with SRE/IRE than in dogs with FRE/ARE (P = .0028 and .0025, respectively). the optimum cut‐off values for sensitivity and specificity calculation determined by ROC curve analyses were (D) a fecal calprotectin concentration ≥15.2 μg/g, (E) a serum CRP concentration ≥9.1 mg/L, and (F) a CCECAI score ≥8
Table 3.
Diagnostic accuracy of the biomarkers in dogs with CIE
| Parameter | Sensitivity (%) | Specificity (%) | OR (95%CI) | P value a | P corr b |
|---|---|---|---|---|---|
| Classification of CIE—diagnosis of SRE/IRE (n = 19) versus FRE/ARE (n = 13) | |||||
| Fecal calprotectin ≥15.2 μg/g | 37 | 100 | 7.5 (1.0–90.0) c | .059 | .18 |
| Serum CRP ≥9.1 mg/L | 72 | 100 | 22.3 (2.9–253.4) c | .0006 | .0018 |
| CCECAI score ≥8 | 68 | 92 | 26 (3.3–295.2) | .0009 | .0027 |
| Fecal calprotectin ≥15.2 μg/g or serum CRP ≥9.1 mg/L | 78 | 100 | 48.8 (5.5–542.9) c | <.0001 | <.0003 |
| Fecal calprotectin ≥15.2 μg/g or CCECAI score ≥8 | 79 | 92 | 45.0 (5.0–503.9) | <.0001 | <.0003 |
| Serum CRP ≥9.1 mg/L or CCECAI score ≥8 | 79 | 92 | 58.7 (5.7–656.0) | <.0001 | <.0003 |
| Fecal calprotectin ≥15.2 μg/g or CCECAI score ≥8 or serum CRP ≥9.1 mg/L | 88 | 92 | 93.5 (7.4–1,035.0) | <.0001 | <.0001 |
| Response to treatment—PR/NR (n = 5) versus CR (n = 12) (dogs with SRE/IRE only) | |||||
| Fecal calprotectin ≥15.2 μg/g | 80 | 75 | 20.0 (1.3–261.0) | .028 | .08 |
| Serum CRP ≥12.9 mg/L | 60 | 36 | 0.9 (0.1–6.5) | 1.00 | ns |
| CCECAI score ≥12 | 40 | 100 | 13 (1.3–177.8) c | .074 | .22 |
Performance and cut–off levels for fecal calprotectin, serum CRP, and the clinical activity index (CCECAI) to predict SRE/IRE versus FRE/ARE in dogs with CIE (n = 32) and to predict PR/NR versus CR in dogs with SRE/IRE (n = 19).
aFisher's exact test.
bBonferroni corrected P value; blue shaded cells: best performing biomarker (or combination of biomarkers) to distinguish the different groups of dogs; values in bold face indicate significance at P < .05; ns: not significant (P > 1.0000).
cEstimated using the probabilistic solution to dealing with zero counts.29
3.8. Other biomarkers and disease classification
A serum CRP concentration of ≥9.1 mg/L had a sensitivity of 72% (95%CI: 47%–90%) and a specificity of 100% (95%CI: 74%–100%; AUROC: 83%, 95%CI: 68%–98%) for the diagnosis of SRE/IRE versus FRE/ARE, whereas a CCECAI score of ≥8 had a sensitivity of 68% (95%CI: 44%–87%) and a specificity of 92% (95%CI: 64%–100%; AUROC: 82%, 95%CI: 68%–96%) to distinguish these 2 groups of dogs.
A combination of at least 2 of these markers yielded an increased sensitivity (about 80%), the highest sensitivity, and specificity being reached for the combination of fecal calprotectin and serum CRP concentration (78% and 100%, respectively; Table 3).
3.9. Fecal calprotectin concentrations and response to treatment
Fecal calprotectin concentrations were also numerically higher in dogs diagnosed with SRE/IRE that showed only PR or NR (median: 37.0 μg/g, IQR: 8.3–124.4 μg/g) compared to SRE/IRE dogs that reached CR (median: 1.6 μg/g, IQR: 0.9–15.6 μg/g), but the difference did not reach statistical significance (P = .10) (Figure 3). A fecal calprotectin concentration ≥15.2 μg/g was the only disease marker that, in dogs with SRE/IRE could discriminate between dogs with PR/NR and those dogs with CR, with a sensitivity of 80% (95%CI: 28%–100%) and a specificity of 75% (95%CI: 43%–95%; AUROC: 77%, 95%CI: 53%–100%) (Table 3). Whether fecal calprotectin can also distinguish SRE/IRE dogs with PR from those with NR could not be tested because of the small number of individuals in these groups of dogs.
Figure 3.
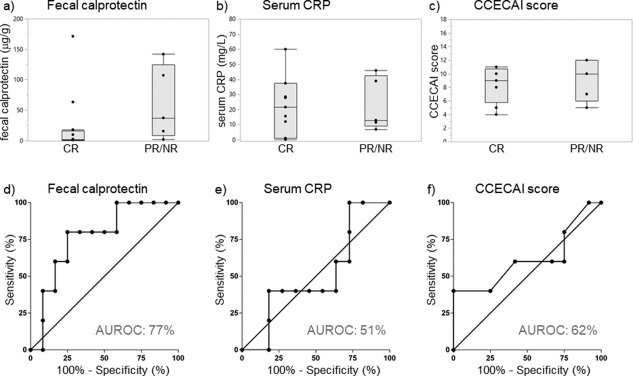
Fecal calprotectin concentrations, serum CRP concentrations, and CCECAI scores in relation to the response to treatment in SRE/IRE dogs. (A) Fecal calprotectin concentrations were numerically higher in SRE/IRE dogs with PR/NR (n = 9) compared to those dogs with CR (n = 11), but the difference was not significant (P = .10). Between SRE/IRE dogs with PR/NR and dogs with CR there was no significant difference in (B) serum CRP concentrations (P = 1.00) and (C) CCECAI scores (P = .49). The optimum cut‐off values for sensitivity and specificity calculation determined by ROC curve analyses were (D) a fecal calprotectin concentration ≥15.2 μg/g, (E) a serum CRP concentration ≥12.9 mg/L, and (F) a CCECAI score ≥12
Fecal calprotectin concentrations decreased significantly from before treatment initiation (median: 1.5 μg/g, IQR: 0.7–29.0 μg/g) to after treatment (median: 0.04 μg/g, IQR: 0.04‐0.2 μg/g; P = .0039) in SRE/IRE dogs with CR, but the change in fecal calprotectin concentrations could not be evaluated in those SRE/IRE dogs with PR/NR because of the small sample size of these subgroups.
4. DISCUSSION
The aim of our study was to evaluate the potential clinical utility of measuring fecal canine calprotectin concentration as a noninvasive marker for the diagnosis and monitoring of dogs with CIE. Fecal calprotectin concentrations correlated with the severity of clinical signs and with fecal S100A12 but not with serum CRP concentrations. Fecal calprotectin was also moderately correlated with the severity of intestinal (duodenal) inflammation. Dogs with SRE/IRE, and particularly dogs with PR/NR, had higher fecal calprotectin concentrations than dogs diagnosed with FRE or ARE. Age, sex, and breed distribution in our study were found to be similar to those reported by others.7, 10, 21, 32
Serum CRP concentration, together with fecal calprotectin concentration, belongs to the most widely used markers in human inflammatory bowel disease (IBD).33, 34, 35 Serum CRP concentrations were also evaluated in our study and were found to not correlate with fecal calprotectin concentrations. This is consistent with some studies in human IBD patients showing no correlation between both biomarkers in patients with ulcerative colitis (UC)36 but contrasts the findings of others where CRP was moderately correlated with fecal calprotectin in patients with Crohn's disease (CD)33, 35, 36, 37 or UC,35, 37 and it also agrees with the lack of a correlation between serum calprotectin and CRP in dogs with idiopathic IBD.11 These findings suggest that the intestinal inflammation in dogs with CIE is not related to the systemic inflammatory response. However, a high‐sensitivity CRP test, which was shown to be superior to standard CRP assays for the detection and differentiation of low serum concentrations of CRP (<5 mg/L) in humans,38 is currently not available for use in dogs. Also, a high biological variability of serum CRP concentrations within individual dogs39 and nonspecific increases in serum CRP concentration in response to other inflammatory conditions (eg, pancreatitis)40 can limit the clinical usefulness of this biomarker for diagnosing or monitoring CIE in dogs. Being a biomarker that is measured in fecal samples, it is reasonable to assume that fecal calprotectin is specific for the GI tract.19 Calprotectin has also been shown to be stable in naturally passed fecal samples for at least 7 days,41 and thus can easily be measured in samples collected in the dog's home environment by the owner.
The canine IBD activity index (CIBDAI)6 or CCECAI7 scoring systems are often used for semi‐objective assessment of clinical disease activity and response to treatment. Similar to previous studies finding lower clinical disease scores in dogs with FRE compared to dogs with SRE/IRE,2, 32 CCECAI scores in dogs with FRE/ARE were lower compared to dogs diagnosed with SRE/IRE in our study. The relationship between fecal calprotectin concentrations and clinical disease activity (CCECAI) scores is also consistent with our previous results in dogs with chronic diarrhea where higher CCECAI scores were associated with higher fecal calprotectin concentrations and a fecal calprotectin concentration ≥48.9 μg/g predicted severe clinical signs (CCECAI scores ≥12) with moderate sensitivity and high specificity.20 Our results also agree with studies in children and human adults with IBD,33, 35, 42, 43, 44, 45, 46 where the correlation between fecal calprotectin and clinical disease activity indices was shown to be generally weaker in dogs with CD33, 35, 45 than in those with UC.42, 43, 44, 45
The minimum amount of time that clinical signs had to be present was 2–3 weeks, which is slightly shorter than the time reported in the 2010 consensus statement.3 The inclusion of dogs in the study when GI signs were noticeable to the owner for at least 2 weeks (n = 4 dogs, 3%) was considered an appropriate criterion for dogs with severe clinical signs or marked clinicopathologic findings (ie, hypoalbuminemia/panhypoproteinemia and hypocobalaminemia, both of which were shown to be negative prognostic factors7) indicating intestinal protein loss. All 4 dogs had moderate to marked lesions on histopathologic examination of intestinal (duodenal) biopsies in addition to hypoalbuminemia and hypofolatemia, hypocobalaminemia, or both.
Histopathologic evaluation of GI tissue biopsies, in combination with clinical signs and systematic therapeutic trials, is currently the gold standard for the diagnosis of CIE. The severity of clinical signs (based on CIBDAI6 or CCECAI scores7) has been shown to not reflect the severity of histologic lesions,7, 15 which is also supported by the findings of the present study. Correlation of fecal calprotectin concentrations with histologic inflammatory lesions in the small intestine and the extent of intestinal inflammation (ie, cumulative lesion score) also agrees with the results of studies in children and adult human patients with IBD, where fecal calprotectin concentrations correlated with the severity of histologic inflammation and cut‐off concentrations between 100 and 170 μg/g had strong predictive value for histologic remission.44, 45, 47, 48 These findings suggest that fecal calprotectin testing could be a good surrogate marker to evaluate disease severity.
Fecal calprotectin concentrations were correlated with histologic inflammatory lesions, particularly lamina propria lymphocytes in the ileum, but not with the number of intestinal lamina propria neutrophils and macrophage(s) (ΜΦ) (especially in the colon). The latter was an unexpected finding as calprotectin is predominantly expressed by activated neutrophils and ΜΦ19, 49 and because calprotectin correlates with the number of infiltrating neutrophils in human IBD.50 A possible explanation could be that, while the inflammatory infiltrate in canine CIE is primarily lymphoplasmacytic with an eosinophilic component in some cases, neutrophils and eosinophils can be very difficult to identify depending on tissue processing and staining.51, 52 In line with this, histopathologic changes (primarily the number of T lymphocytes in the lamina propria) poorly correlate with clinical disease or response to treatment.53, 54, 55 An alternative explanation for this finding could be that calprotectin expression does not merely reflect the number of intestinal lamina propria neutrophils and ΜΦ, but rather reflects the activity of these cells. In human IBD, it is believed that activated ΜΦ contribute to the production of inflammatory cytokines that lead to altered chemotactic signals and an increased recruitment of lymphocytes.56 In dogs with CIE, only few cytokines have been evaluated based on mRNA analysis and the results have been inconsistent.57, 58 However, numbers of cells staining positive for leukocyte protein‐1 (ie, ΜΦ and neutrophils)59 and activated ΜΦ (determined by staining for activated nuclear factor‐kappa B, NF‐κB60) in the lamina propria were shown to be increased in canine IBD. Also, mucosal Toll‐like receptor (TLR)2‐ and TLR4‐mRNA expression was upregulated in canine IBD.5, 61, 62 Interestingly, calprotectin is an endogenous ligand for TLR4 and NF‐κB is involved in the expression of proinflammatory mediators downstream of TLR4.49 Calprotectin has also been shown to be induced in epithelial cells in humans49 and whether this is also true in dogs warrants further study.
The heterogenous response to treatment in dogs with CIE is the basis for classification of the disease into FRE, ARE, and SRE/IRE, whose features are often indistinguishable on endoscopy or histopathology.1, 2, 32 A noninvasive test that could identify dogs likely to fail treatment trials with antibiotics, elimination diets, or both before a treatment decision is made, would be a useful tool in clinical practice. Fecal calprotectin concentrations were numerically higher in dogs with SRE/IRE compared to dogs with FRE or ARE in our study, but significance was not reached likely because the comparison was underpowered. A cut‐off fecal calprotectin concentration of ≥15.2 μg/g was able to discriminate between both groups with moderate sensitivity (37%) and high specificity (100%). Diagnostic accuracy to detect dogs with SRE/IRE was improved when fecal calprotectin was used in combination with serum CRP (cut‐off concentration: ≥9.1 mg/L), the CCECAI score (cut‐off score: ≥8), or both. These findings are in line with our previous investigations showing calprotectin concentrations to be increased in serum from dogs with SRE/IRE compared to healthy dogs11 and also in pretreatment fecal samples from dogs with SRE/IRE compared to healthy controls.21 Measuring higher fecal calprotectin concentrations in dogs with SRE/IRE is also consistent with studies in human medicine showing that increased fecal calprotectin concentrations can identify dogs with active IBD from either non‐IBD controls or IBD dogs in remission,35, 44, 45, 63 and is also similar to the results reported for fecal S100A12 concentrations in dogs.16 The current study suggests that a pretreatment fecal calprotectin concentration of ≥15.2 μg/g might signal the need for more aggressive (ie, immunosuppressive, anti‐inflammatory, or both) treatment in CIE dogs, but a combination with serum CRP, CCECAI score, or both yielded a higher diagnostic accuracy.
Disease classification in dogs with CIE is based on the response to treatment,1, 2, 7, 32 and the best time to perform more invasive diagnostics (ie, endoscopy with collection of GI tissue biopsies) is often debated in clinical practice.64 This is also reflected in the dog population of our study, where 8 dogs with FRE/ARE (62%) had GI tissue biopsies taken and 5 dogs included in the FRE/ARE group (38%) did not have biopsies taken. While intestinal inflammation was not documented in these 5 dogs, the mild clinical signs (CCECAI scores of 2, 3, 3, 5, and 6) and relatively long follow‐up time (6, 13, 24, 52, and 169 weeks) render FRE/ARE the most likely diagnosis. However, another intestinal disease process cannot be entirely excluded in these dogs.
Fecal calprotectin concentrations were numerically higher in SRE/IRE dogs with PR/NR compared to those dogs with CR, but significance was not reached likely because of the low statistical power. Higher pretreatment fecal calprotectin concentrations in SRE/IRE dogs with only partial or no response to treatment (PR/NR) is consistent with the results of studies in children and human adults with IBD showing lower fecal calprotectin concentrations to predict clinical remission44 or to predict sustained clinical remission over 1 year.65, 66 This finding also agrees with the results for fecal S100A12 in dogs with CIE where fecal S100A12 concentrations ≥2,700 ng/g identified dogs with IBD that were refractory to anti‐inflammatory/immunosuppressive treatment.16 In the present study, the diagnostic accuracy of fecal calprotectin to predict PR/NR was also superior to that of serum CRP and the CCECAI score, and the lack of a relationship between clinical disease severity and outcome contrasts the findings of other studies that showed the individual outcome to be significantly associated with higher clinical disease activity scores.7, 32 Thus, fecal calprotectin could be a clinically useful surrogate marker for the diagnosis and management of canine CIE. However, further research is needed to evaluate the time course of changes in fecal calprotectin concentrations in dogs with CIE, particularly in dogs with SRE/IRE before, during, and after clinical flares. Further studies are also needed to assess the utility of fecal calprotectin as a surrogate marker to guide diagnostic and clinical decision‐making in dogs with CIE.
The present study aimed to determine the value of measuring fecal calprotectin concentrations in dogs with intestinal inflammatory lesions, and dogs diagnosed with GI cancer were excluded from the study. However, increased fecal calprotectin concentrations have also been detected in people with GI malignancies,67, 68 and the possibility that fecal calprotectin concentrations are also altered in dogs with alimentary neoplasms cannot be excluded. Thus, further evaluation of fecal calprotectin concentrations in canine GI neoplasia is warranted. Until more data is available, fecal calprotectin might be seen as a good surrogate biomarker of GI inflammation but will not be a replacement for biopsy or other clinicopathologic tests.
Our study had some limitations. First, the clinical response to treatment was used as the primary endpoint of the study, and the possibility of a correlation with mucosal healing (deep remission) was not evaluated. Second, single spot fecal samples were used to measure fecal calprotectin concentrations, but the biological variation of fecal calprotectin concentrations has only been estimated in a small number of dogs with CIE.24 Third, long‐term outcome (ie, a follow‐up period of >12 months) was not evaluated in the majority of dogs in our study. Fourth, the small sample size in subgroup analyses (ie, disease classification and response to treatment) has the potential for a type I or type II error. Fifth, several different clinicians were involved in evaluating the CCECAI score, which may add some variability. However, the CCECAI scoring system is 1 of the 2 standardized semi‐objective methods for evaluation of clinical disease severity in dogs with chronic enteropathies, and it has been used in a number of investigations. Further, histopathologic evaluation of GI tissue biopsies was performed by 1 of 7 different pathologists (though with a special expertise in GI pathology) and interobserver variability exists even with the use of standardized criteria.69 Further, given the multicenter nature of our clinical observational study, it was not feasible to standardize treatment and follow‐up for all dogs, and some flexibility in the diagnostic evaluation was needed to accommodate dogs with severe clinical signs, marked clinicopathological abnormalities (eg, severe hypoalbuminemia), or both. Also, choices and doses of immunosuppressives were not standardized in our study. Lastly, the possibility of missing a diffusely infiltrating neoplasm (ie, alimentary lymphoma) in dogs diagnosed with CIE cannot be excluded as biopsies were not available from all section of the GI tract in all dogs and because endoscopy does not allow evaluation of the entire small intestine.
5. CONCLUSIONS
We conclude that fecal calprotectin appears to be a potentially useful variable as a surrogate to assess the severity of GI inflammation in dogs with CIE. Before treatment, fecal calprotectin concentrations had a good ability to distinguish between dogs with SRE/IRE and dogs with FRE or ARE, but CCECAI scores and serum CRP concentrations as a single variable outperformed the fecal calprotectin test. Assessing fecal calprotectin in combination with serum CRP, the CCECAI score, or both increased the ability to differentiate these conditions. Lower pretreatment concentrations of fecal calprotectin in dogs that have failed dietary and antibiotic treatment trials could indicate a higher likelihood of a response to anti‐inflammatory/immunosuppressive treatment. Thus, a prospective evaluation of the clinical utility of fecal calprotectin in determining the disease classification and predicting the response to treatment in CIE dogs undergoing a standardized diagnostic and treatment regimen is warranted.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The study design was reviewed and approved by the Clinical Research Review Committee (CRRC# 2009‐06 and 2010‐05) and the Institutional Animal Care and Use Committee (IACUC, AUP# 2012‐083) at Texas A&M University (TAMU).
ACKNOWLEDGMENTS
Our study was supported by a Research Grant from the Comparative Gastroenterological Society (CGS)/Waltham. We also acknowledge support from the German Research Foundation (DFG) and Leipzig University within the program of Open Access Publishing.
Heilmann RM, Berghoff N, Mansell J, et al. Association of fecal calprotectin concentrations with disease severity, response to treatment, and other biomarkers in dogs with chronic inflammatory enteropathies. J Vet Intern Med. 2018;32:679–692. https://doi.org/10.1111/jvim.15065
Funding information Comparative Gastroenterological Society (CGS)/Waltham, Grant/Award Number: 2011 CGS; German Research Foundation; Leipzig University
Dogs were enrolled in the study and the samples collected at the Gastrointestinal Laboratory at Texas A&M University, the College of Veterinary Medicine at Purdue University, or at one of several other referral hospitals across the United States. Sample analyses were performed at Texas A&M University and Purdue University. Data analysis and manuscript writing were done at the College of Veterinary Medicine at the University of Leipzig.
Part of these data were presented as a research report at the 2017 American College of Veterinary Internal Medicine Forum, National Harbor, MD.
REFERENCES
- 1. Dandrieux JR. Inflammatory bowel disease versus chronic enteropathy in dogs: are they one and the same? J Small Anim Pract. 2016;57:589–599. [DOI] [PubMed] [Google Scholar]
- 2. Allenspach K, Culverwell C, Chan D. Long‐term outcome in dogs with chronic enteropathies: 203 cases. Vet Rec. 2016;178:368. [DOI] [PubMed] [Google Scholar]
- 3. Washabau RJ, Day MJ, Willard MD, et al. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med. 2010;24:10–26.; [DOI] [PubMed] [Google Scholar]
- 4. Jergens AE, Simpson KW. Inflammatory bowel disease in veterinary medicine. Front Biosci. 2012;4:1404–1419. [DOI] [PubMed] [Google Scholar]
- 5. Heilmann RM, Allenspach K. Pattern recognition receptors: signaling pathways and dysregulation in canine chronic enteropathies – brief review. J Vet Diagn Invest. 2017;29:781–787. [DOI] [PubMed] [Google Scholar]
- 6. Jergens AE, Schreiner CA, Frank DE, et al. A scoring index for disease activity in canine inflammatory bowel disease. J Vet Intern Med. 2003;17:291–297. [DOI] [PubMed] [Google Scholar]
- 7. Allenspach K, Wieland B, Gröne A, Gaschen F. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J Vet Intern Med. 2007;21:700–708. [DOI] [PubMed] [Google Scholar]
- 8. Slovak JE, Wang C, Sun Y, et al. Development and validation of an endoscopic activity score for canine inflammatory bowel disease. Vet J. 2015;203:290–295. [DOI] [PubMed] [Google Scholar]
- 9. Day MJ, Bilzer T, Mansell J, et al. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: a report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J Comp Pathol. 2008;138:S1–S43. [DOI] [PubMed] [Google Scholar]
- 10. Jergens AE, Crandell J, Morrison JA, et al. Comparison of oral prednisone and prednisone combined with metronidazole for induction therapy of canine inflammatory bowel disease: a randomized‐controlled trial. J Vet Intern Med. 2010;24:269–277. [DOI] [PubMed] [Google Scholar]
- 11. Heilmann RM, Jergens AE, Ackermann MR, et al. Serum calprotectin concentrations in dogs with idiopathic inflammatory bowel disease. Am J Vet Res. 2012;73:1900–1907. [DOI] [PubMed] [Google Scholar]
- 12. McCann TM, Ridyard AE, Else RW, Simpson JW. Evaluation of disease activity markers in dogs with idiopathic inflammatory bowel disease. J Small Anim Pract. 2007;48:620–625. [DOI] [PubMed] [Google Scholar]
- 13. Berghoff N, Hill S, Parnell NK, et al. Fecal and urinary N‐methylhistamine concentrations in dogs with chronic gastrointestinal disease. Vet J. 2014;201:289–294. [DOI] [PubMed] [Google Scholar]
- 14. Anfinsen KP, Berghoff N, Priestnall SL, et al. Urinary and faecal N‐methylhistamine concentrations do not serve as markers for mast cell activation or clinical disease activity in dogs with chronic enteropathies. Acta Vet Scand. 2014;56:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heilmann RM, Grellet A, Allenspach K, et al. Association between fecal S100A12 concentration and histologic, endoscopic, and clinical disease severity in dogs with idiopathic inflammatory bowel disease. Vet Immunol Immunopathol. 2014;158:156–166. [DOI] [PubMed] [Google Scholar]
- 16. Heilmann RM, Volkmann M, Otoni CC, et al. Fecal S100A12 concentration predicts a lack of response to treatment in dogs affected with chronic enteropathy. Vet J. 2016;215:96–100. [DOI] [PubMed] [Google Scholar]
- 17. Sattasathuchana P, Grützner N, Lopes R, et al. Stability of 3‐bromotyrosine in serum and serum 3‐bromotyrosine concentrations in dogs with gastrointestinal diseases. BMC Vet Res. 2015;11:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sattasathuchana P, Allenspach K, Lopes R, et al. Evaluation of serum 3‐bromotyrosine concentrations in dogs with steroid‐responsive diarrhea and food‐responsive diarrhea. J Vet Intern Med. 2017;31:1056–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heilmann RM. Evaluation of Canine S100A12 and sRAGE as Novel Disease Markers in Dogs with Inflammatory Bowel Disease [PhD Thesis], College Station, TX: Texas A&M University; 2015. [Google Scholar]
- 20. Grellet A, Heilmann RM, Lecoindre P, et al. Fecal calprotectin concentrations in adult dogs with chronic diarrhea. Am J Vet Res. 2013;74:706–711. [DOI] [PubMed] [Google Scholar]
- 21. Otoni CC, Heilmann RM, García‐Sancho M, et al. Serologic and fecal markers in prediction of acute disease course in canine chronic enteropathies. J Vet Intern Med. 2012;26(3):768 (abstract). [Google Scholar]
- 22. Wilke VL, Nettleton D, Wymore MJ, et al. Gene expression in intestinal mucosal biopsy specimens obtained from dogs with chronic enteropathy. Am J Vet Res. 2012;73:1219–1229. [DOI] [PubMed] [Google Scholar]
- 23. Rhodes B, Fürnrohr BG, Vyse TJ. C‐reactive protein in rheumatology: biology and genetics. Nat Rev Rheumatol. 2011;7:282–289. [DOI] [PubMed] [Google Scholar]
- 24. Heilmann RM, Toresson L, Berghoff N, Grützner N, Suchodolski JS, Steiner JM. Intra‐individual variability of fecal calprotectin concentrations in dogs with chronic gastrointestinal disease. J Vet Intern Med. 2011;25(3):694–695 (abstract). [Google Scholar]
- 25. ClinCalc . ClinCalc Sample size calculator. http://clincalc.com/stats/samplesize.aspx. Accessed June 10, 2017.
- 26. Heilmann RM, Guard BC, Weber K, Suchodolski JS, Steiner JM. Development and analytical validation of an enzyme‐linked immunosorbent assay for the quantification of canine calprotectin in serum and feces from dogs. J Vet Intern Med. 2011;25(3):693 (abstract). [Google Scholar]
- 27. Berghoff N, Suchodolski JS, Steiner JM. Assessment of stability and determination of a reference range for canine C‐reactive protein in serum. J Vet Intern Med. 2006;20(3):790 (abstract). [Google Scholar]
- 28. Heilmann RM, Cranford S, Ambrus A, et al. Validation of an enzyme‐linked immunosorbent assay (ELISA) for the measurement of canine S100A12. Vet Clin Pathol. 2016;45:135–147. [DOI] [PubMed] [Google Scholar]
- 29. Valenzuela C. Two solutions for estimating odds ratios with zeros. Rev Med Chil. 1993;121:1441–1444. [PubMed] [Google Scholar]
- 30. Texas A&M University . Texas A&M University Gastrointestinal Laboratory reference interval for serum cobalamin. http://vetmed.tamu.edu/gilab/service/assays/b12folate. Accessed July 13, 2017.
- 31. Texas A&M University . Texas A&M University Gastrointestinal Laboratory reference interval for serum folate. http://vetmed.tamu.edu/gilab/service/assays/b12folate. Accessed July 13, 2017.
- 32. Volkmann M, Steiner JM, Fosgate GT, et al. Chronic diarrhea in dogs – retrospective study in 136 cases. J Vet Intern Med. 2017;31:1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jones J, Loftus EV Jr, Panaccione R, et al. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn's disease. Clin Gastroenterol Hepatol. 2008;6:1218–1224. [DOI] [PubMed] [Google Scholar]
- 34. Panes J, Jairath V, Levesque BG. Advances in use of endoscopy, radiology, and biomarkers to monitor inflammatory bowel disease. Gastroenterology. 2017;152:362–373. [DOI] [PubMed] [Google Scholar]
- 35. Ricanek R, Brackmann S, Perminow G, et al. Evaluation of disease activity in IBD at the time of diagnosis by the use of clinical, biochemical, and fecal markers. Scand J Gastroenterol. 2011;46:1081–1091. [DOI] [PubMed] [Google Scholar]
- 36. Hoekman DR, Diederen K, Koot BG, et al. Relationship of clinical symptoms with biomarkers of inflammation in pediatric inflammatory bowel disease. Eur J Pediatr. 2016;175:1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vieira A, Fang CB, Rolim EG, et al. Inflammatory bowel disease activity assessed by fecal calprotectin and lactoferrin: correlation with laboratory parameters, clinical, endoscopic and histological indexes. BMC Res Notes. 2009;2:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Poullis AP, Zar S, Sundaram KK, et al. A new, highly sensitive assay for C‐reactive protein can aid the differentiation of inflammatory bowel disorders from constipation‐ and diarrhea‐predominant functional bowel disorders. Eur J Gastroenterol Hepatol. 2002;14:409–412. [DOI] [PubMed] [Google Scholar]
- 39. Carney PC, Ruaux CG, Suchodolski JS, Steiner JM. Biological variability of C‐reactive protein and specific pancreatic lipase immunoreactivity (Spec cPL) in apparently healthy dogs. J Vet Intern Med. 2011;25:825–830. [DOI] [PubMed] [Google Scholar]
- 40. Nakamura M, Takahashi M, Ohno K, et al. C‐reactive protein concentration in dogs with various diseases. J Vet Med Sci. 2008;70:127–131. [DOI] [PubMed] [Google Scholar]
- 41. Heilmann RM, Suchodolski JS, Steiner JM. Development and analytic validation of a radioimmunoassay for the quantification of canine calprotectin in serum and feces from dogs. Am J Vet Res. 2008;69:845–853. [DOI] [PubMed] [Google Scholar]
- 42. Schoepfer AM, Vavricka S, Zahnd‐Straumann N, et al. Monitoring inflammatory bowel disease activity: clinical activity is judged to be more relevant than endoscopic severity or biomarkers. J Crohns Colitis. 2012;6:412–418. [DOI] [PubMed] [Google Scholar]
- 43. Burri E, Beglinger C, von Felten S, Lehmann FS. Fecal calprotectin and the clinical activity index are both useful to monitor medical treatment in patients with ulcerative colitis. Dig Dis Sci. 2015;60:485–491. [DOI] [PubMed] [Google Scholar]
- 44. Theede K, Holck S, Ibsen P, et al. Level of fecal calprotectin correlates with endoscopic and histologic inflammation and identifies patients with mucosal healing in ulcerative colitis. Clin Gastroenterol Hepatol. 2015;13:1929–1936. [DOI] [PubMed] [Google Scholar]
- 45. Zittan E, Kelly OB, Kirsch R, et al. Low fecal calprotectin correlates with histological remission and mucosal healing in ulcerative colitis and colonic Crohn's disease. Inflamm Bowel Dis. 2016;22:623–630. [DOI] [PubMed] [Google Scholar]
- 46. Bunn SK, Bisset WM, Main MJ, Golden BE. Fecal calprotectin as a measure of disease activity in childhood inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2001;32:171–177. [DOI] [PubMed] [Google Scholar]
- 47. Canani RB, Terrin G, Rapacciuolo L, et al. Fecal calprotectin as reliable non‐invasive marker to assess the severity of mucosal inflammation in children with inflammatory bowel disease. Dig Liver Dis. 2008;40:547–553. [DOI] [PubMed] [Google Scholar]
- 48. Guardiola J, Lobatón T, Rodríguez‐Alonso L, et al. Fecal level of calprotectin identifies histologic inflammation in patients with ulcerative colitis in clinical and endoscopic remission. Clin Gastroenterol Hepatol. 2014;12:1865–1870. [DOI] [PubMed] [Google Scholar]
- 49. Ehrchen JM, Sunderkötter C, Foell D, et al. The endogenous Toll‐like receptor 4 agonist S100A8/A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol. 2009;86:557–566. [DOI] [PubMed] [Google Scholar]
- 50. Røseth AG, Schmidt PN, Fagerhol MK. Correlation between faecal excretion of indium‐111‐labelled granulocytes and calprotectin, a granulocyte marker protein, in patients with inflammatory bowel disease. Scand J Gastroenterol. 1999;34:50–54. [DOI] [PubMed] [Google Scholar]
- 51. Willard M, Moore G, Denton B, et al. Effect of tissue processing on assessment of endoscopic intestinal biopsies in dogs and cats. J Vet Intern Med. 2010;24:84–89. [DOI] [PubMed] [Google Scholar]
- 52. Bastan I, Robinson NA, Ge XN, et al. Assessment of eosinophil peroxidase as a potential diagnostic and prognostic marker in dogs with inflammatory bowel disease. Am J Vet Res. 2017;78:36–41. [DOI] [PubMed] [Google Scholar]
- 53. Schreiner NMS, Gaschen F, Gröne A, et al. Clinical signs, histology, and CD3‐positive cells before and after treatment of dogs with chronic enteropathies. J Vet Intern Med. 2008;22:1079–1083. [DOI] [PubMed] [Google Scholar]
- 54. Dandrieux J, Bornand V, Doherr M, et al. Evaluation of lymphocyte apoptosis in dogs with inflammatory bowel disease. Am J Vet Res. 2008;69:1279–1285. [DOI] [PubMed] [Google Scholar]
- 55. García‐Sancho M, Rodríguez‐Franco F, Sainz A, et al. Evaluation of clinical, macroscopic, and histopathologic response to treatment in nonhypoproteinemic dogs with lymphocytic‐plasmacytic enteritis. J Vet Intern Med. 2007;21:11–17. [DOI] [PubMed] [Google Scholar]
- 56. Sanchez‐Muñoz F, Dominguez‐Lopez A, Yamamoto‐Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. 2008;14:4280–4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jergens AE, Sonea IM, O'Connor AM, et al. Intestinal cytokine mRNA expression in canine inflammatory bowel disease: a meta‐analysis with critical appraisal. Comp Med. 2009;59:153–162. [PMC free article] [PubMed] [Google Scholar]
- 58. Heilmann RM, Suchodolski JS. Is inflammatory bowel disease in dogs and cats associated with a Th1 or Th2 polarization? Vet Immunol Immunopathol .168:131–134. [DOI] [PubMed] [Google Scholar]
- 59. German AJ, Hall EJ, Day MJ. Immune cell populations within the duodenal mucosa of dogs with enteropathies. J Vet Intern Med. 2001;15:14–25. [DOI] [PubMed] [Google Scholar]
- 60. Luckschander N, Hall JA, Gaschen F, et al. Activation of nuclear factor‐kappaB in dogs with chronic enteropathies. Vet Immunol Immunopathol. 2010;133:228–236. [DOI] [PubMed] [Google Scholar]
- 61. Burgener IA, König A, Allenspach K, et al. Upregulation of toll‐like receptors in chronic enteropathies in dogs. J Vet Intern Med. 2008;22:553–560. [DOI] [PubMed] [Google Scholar]
- 62. McMahon LA, House AK, Catchpole B, et al. Expression of Toll‐like receptor 2 in duodenal biopsies from dogs with inflammatory bowel disease is associated with severity of disease. Vet Immunol Immunopathol. 2010;135:158–163. [DOI] [PubMed] [Google Scholar]
- 63. D'Angelo F, Felley C, Frossard JL. Calprotectin in daily practice: where do we stand in 2017? Digestion. 2017;95:293–301. [DOI] [PubMed] [Google Scholar]
- 64. Erdmann C, Heilmann RM. Diagnostic and therapeutic approach to chronic inflammatory enteropathies in dogs. Tierarztl Prax Ausg K Kleintiere Heimtiere 2017;45:317–327. [DOI] [PubMed] [Google Scholar]
- 65. Guidi L, Marzo M, Andrisani G, et al. Faecal calprotectin assay after induction with anti‐tumour necrosis factor α agents in inflammatory bowel disease: prediction of clinical response and mucosal healing at one year. Dig Liver Dis. 2014;46:974–979. [DOI] [PubMed] [Google Scholar]
- 66. Boschetti G, Garnero P, Moussata D, et al. Accuracies of serum and fecal S100 proteins (calprotectin and calgranulin C) to predict the response to TNF antagonists in patients with Crohn's disease. Inflamm Bowel Dis. 2015;21:331–336. [DOI] [PubMed] [Google Scholar]
- 67. Røseth AG, Kristinsson J, Fagerhol MK, et al. Faecal calprotectin: a novel test for the diagnosis of colorectal cancer? Scand J Gastroenterol. 1993;28:1073–1076. [DOI] [PubMed] [Google Scholar]
- 68. Van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta‐analysis. BMJ 2010;341:c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jergens AE, Evans RB, Ackermann M, et al. Design of a simplified histopathologic model for gastrointestinal inflammation in dogs. Vet Pathol. 2014;51:946–950. [DOI] [PubMed] [Google Scholar]


