Abstract
Background and Purpose
Our recent studies show that the reduced activity of epithelial sodium channels (ENaC) in endothelial cells accounts for the adaptation of vasculature to salt in Sprague–Dawley rats. The present study examines a hypothesis that enhanced ENaC activity mediates the loss of vasorelaxation in Dahl salt‐sensitive (SS) rats.
Experimental Approach
We used the cell‐attached patch‐clamp technique to record ENaC activity in split‐open mesenteric arteries. Western blot and immunofluorescence staining were used to evaluate the levels of aldosterone, ENaC, eNOS and NO. Blood pressure was measured with the tail‐cuff method and the artery relaxation was measured with the wire myograph assay.
Key Results
High‐salt (HS) diet significantly increased plasma aldosterone and ENaC activity in the endothelial cells of Dahl SS rats. The endothelium‐dependent artery relaxation was blunted by HS challenge in these rats. Amiloride, a potent blocker of ENaC, increased both phosphorylated eNOS and NO and therefore prevented the HS‐induced loss of vasorelaxation. As, in SS rats, endogenous aldosterone was already elevated by HS challenge, exogenous aldosterone did not further elevate ENaC activity in the rats fed with HS. Eplerenone, a mineralocorticoid receptor antagonist, attenuated the effects of HS on both ENaC activity and artery relaxation.
Conclusions and Implications
These data suggest that HS diet blunts artery relaxation and causes hypertension via a pathway associated with aldosterone‐dependent activation of ENaC in endothelial cells. This pathway provides one of the mechanisms by which HS causes hypertension in Dahl SS rats.
Linked Articles
This article is part of a themed section on Spotlight on Small Molecules in Cardiovascular Diseases. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v175.8/issuetoc
Abbreviations
- ENaC
epithelial sodium channels
- HS
high salt
- MAs
mesenteric arteries
- MR
mineralocorticoid receptor
- NS
normal salt
- NTG
nitroglycerin
- PO
open probability
- PSS
physiological saline solution
- SBP
systolic blood pressure
- SD rat
Sprague–Dawley rat
- SR rat
salt‐resistant rat
- SS rat
salt‐sensitive rat
Tables of Links
| TARGETS |
|---|
| Ligand‐gated ion channels a |
| Epithelial sodium channels (ENaC) |
| Enzymes b |
| Akt |
| eNOS |
These Tables list key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,bAlexander et al., 2015a,b).
Introduction
Excess dietary salt intake is an essential risk factor for the development and progression of cardiovascular disease (He and MacGregor, 2007; Bibbins‐Domingo et al., 2010), which is typically attributed to increases in blood pressure (De Wardener and MacGregor, 2002; Meneton et al., 2005). Several lines of evidence including epidemiological observations, animal studies and clinical trials have consistently shown a causal relation between dietary salt intake and hypertension (Taubes, 1998; Bibbins‐Domingo et al., 2010). However, the mechanisms by which high dietary salt raises blood pressure are complex with the involvement of the kidney, the CNS and the vasculature. In the kidney, the role of epithelial sodium channels (ENaC) in regulating blood pressure has been extensively studied. Excess salt intake elevates ENaC activity in the principal cells of renal cortical collecting ducts, leading to increased Na+ reabsorption and water retention, and eventually causes salt‐sensitive hypertension (Aoi et al., 2007; Kakizoe et al., 2009). High salt (HS) raises the levels of hydrogen peroxide in the kidney of Dahl salt‐sensitive (SS) rats (Taylor and Cowley, 2005) and we have shown that hydrogen peroxide stimulated ENaC in distal nephron cells (Ma, 2011; Zhang et al., 2013). Earlier studies have also suggested that ATP, vasopressin, endothelin‐1 and epidermal growth factor contribute to salt‐sensitive hypertension by stimulating ENaC (Nicco et al., 2001; Ahn et al., 2004; Ma and Eaton, 2005; Pochynyuk et al., 2008; Pavlov et al., 2013). Recently, angiotensin II has been shown to induce hypertension by stimulating ENaC via (pro)renin receptors (Peng et al., 2017). Therefore, the underlying mechanism involves several signalling molecules (Ma et al., 2007; Liu et al., 2013; Pavlov and Staruschenko, 2016).
In the CNS, ENaC also plays an important role in regulating blood pressure (Leenen, 2010; Takahashi et al., 2011). The ENaC consists of three major subunits, α, β and γ (Canessa et al., 1994). It usually requires all three subunits to form functional channels with spontaneous activity (Fyfe and Canessa, 1998). However, only the α and the β subunits, but not the γ, are abundantly expressed in the brain (Amin et al., 2005). We and others have shown that either the α subunit alone or with another subunit can also form a functional channel, even without any spontaneous activity (Kizer et al., 1997; Ma et al., 2004). These studies suggest that stoichiometrically different population of ENaC may regulate blood pressure by altering cerebrospinal fluid, interstitial Na+ concentration and neuronal excitation. Although the ENaC in the brain may not have any spontaneous activity, it can be regulated by aldosterone (Wang et al., 2016) and even mediate the development of hypertension in Liddle Syndrome (Van Huysse et al., 2012).
In the vasculature, little is known how ENaC is involved in salt‐sensitive hypertension, even though it has been long known that vascular smooth muscle and endothelial cells express ENaC (Golestaneh et al., 2001; Jernigan et al., 2008). It is well known that endothelial cells play a very important role in the regulation of vascular tone (Luscher et al., 1992). In vitro studies from cultured endothelial cells also show that slight elevation of extracellular sodium induces cell swelling and plasma membrane stiffening and that these effects are dependent on both aldosterone and the functional ENaC (Oberleithner et al., 2004; Oberleithner et al., 2007). These studies together suggest that ENaC may mediate HS‐induced endothelial dysfunction.
Our recent report shows that, in Sprague–Dawley (SD) rats, HS intake decreases plasma aldosterone and ENaC activity and consequently enhances endothelium‐dependent relaxation to allow the vasculature to adapt to HS challenge (Liu et al., 2015). Conversely, in Dahl SS rats, enhanced ENaC activity may result in loss of this adaptation. Therefore, the present study was designed to determine whether ENaC in endothelial cells was activated by dietary salt and mediated salt‐sensitive hypertension.
Methods
Animals
All animal care and experimental procedures were approved by the Harbin Medical University Animal Supervision Committee. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath et al., 2010; McGrath and Lilley, 2015).
A total number of 210 normotensive male Dahl SS rats weighing 220 ~ 240 g was used. These rats were randomly assigned into two groups (105 rats in each group) and were fed either a normal salt (NS) diet (NS, 0.3% NaCl, w/w for 3 weeks) or HS diet (HS, 8% NaCl, w/w for 3 weeks). The systolic blood pressure (SBP) of SS rats was measured in conscious rats by the tail‐cuff method (BP 98A, Softon, Tokyo, Japan). Then, the rats were killed, and blood was collected from the main abdominal artery for the measurement of plasma aldosterone. The heart and whole kidney were quickly excised and weighed in cold (4°C) buffer. Sixty rats of each assigned group were used for primary culture of the mesenteric artery (MA) endothelial cells. Western blots were carried out using these cells to detect the levels of α, β and γ‐ENaC and the phosphorylation of eNOS and Akt. Immunofluorescence experiments were performed to detect NO production. The second‐order MAs isolated from 40 rats of each assigned group were prepared to test the vasodilation and to record single‐channel activity of ENaC. Five rats of each assigned group were used to examine the expression levels of α, β and γ‐ENaC in MAs by immunofluorescence staining.
Primary culture of rat mesenteric artery endothelial cells
The primary cultures of endothelial cells from rat MAs were carried out as previously described (Liu et al., 2015). Briefly, SS rats receiving heparin were anaesthetised with 10% chloral hydrate (4 mL·kg−1). The abdomen was opened, and the heart was perfused with sterile and chilled physiological saline solution (PSS) to remove circulating blood from blood vessels. PSS contained (in mM) 137 NaCl, 5.4 KCl, 0.05 CaCl2, 0.4 KH2PO4, 0.4 Na2HPO4, 4.4 NaHCO3 and 10 HEPES (pH 7.4 with HCl). The mesenteric vascular bed was dissected out, and all the vein branches of the mesenteric bed were rapidly excised under a dissecting microscope. The remaining arterial branches were digested with 0.2 mg·mL−1 collagenase I for 1 h at 37°C with mild shaking. Detached endothelial cells were collected by centrifugation, re‐suspended in 20% FBS‐DMEM and then cultured in gelatin‐coated petri dishes. Two hours later, non‐adherent adherent cells were removed and the adherent endothelial cells were cultured at 37°C with 5% CO2 for 3–5 days. These cells were used for experiments without further cell passage.
In situ patch‐clamp recording
In situ patch‐clamp recordings of ENaC single‐channel current were performed using intact vascular endothelium as previously described (Liu et al., 2015). Briefly, second‐ to third‐order branches of MA were dissected and the arterial segment were placed on a 5 × 5 mm cover glass coated with L‐polylysine and then transferred to a chamber mounted on an inverted Nikon microscope, allowing direct access to the endothelial cell layer. Patch pipettes of resistance 6 ~ 10 MΩ were pulled from borosilicate glass capillaries with a Sutter P‐97 horizontal puller. The bath and the pipette solutions contained (in mM): 135 NaCl, 4.5 KCl, 1 MgCl2, 1 CaCl2, 5 HEPES and 5 Na‐HEPES (pH 7.4 with NaOH).
Single‐channel ENaC currents were recorded in the cell‐attached configuration with an Axon Multiclamp 200B amplifier (Axon Instruments, Foster City, CA, USA) at room temperature (22–24°C). The single‐channel currents were recorded at least over 30 min immediately after gigaseal formation. For most experiments, the data were acquired by application of 0 mV to patch pipettes and were sampled at 5 kHz and low‐pass filtered at 1 kHz with Clampex 10.2 software (Molecular Devices, Sunnyvale, CA, USA). Prior to analysis, the single‐channel traces were further filtered at 30 Hz. Open probability (P O) was calculated as follow: P O = NP O/N, where N (N was estimated by the current amplitude histogram) represents the apparent number of active channels in the patch.
Wire myograph studies
The vasodilation of isolated MA rings was measured using an isometric myograph (Danish Myo Technology, Aarhus, Denmark), as previously described (Yang et al., 2010; Liu et al., 2015). After rats were killed, MAs were removed and dissected in oxygenated ice‐cold PSS. Changes in isometric tone of MAs (second order) were recorded in wire myograph equilibrated in PSS at 37°C and bubbled with a mixture of 5% CO2 in 95% O2. In some rings, the endothelium was removed by gentle rubbing of the intimal surface with a hair. After measurement of the passive‐tension internal circumference characteristics, the tension was set to an estimated in vivo internal circumference. After a 60 min stabilization period, KPSS (PSS containing 60 mM K+) was added to the chambers and washed out with PSS until a reproducible maximal contraction was achieved. Endothelium‐dependent and ‐independent relaxation was measured by testing concentration‐responses to cumulative addition of ACh or nitroglycerin (NTG) in rings precontracted with phenylephrine (10 μM) . Some rings were incubated with ENaC blocker amiloride (0.5 μM) for 10 min or an aldosterone receptor blocker eplerenone (10 μM) for 1 h before assessing their relaxation response to ACh and NTG.
Immunofluorescence staining
The MA segments were fixed with 4% paraformaldehyde for 2 h followed by 20% sucrose at 4°C overnight, embedded into optimal cutting temperature solution and cut at 6 μm thickness with freezing microtome (Leica Biosystem, Germany). Arterial sections were then permeated with 0.25% Trixiton X‐100 and blocked with 1% BSA 30 min prior to incubation with primary antibody. For double staining, we double‐labelled the tissues with antibodies against CD31, an endothelial cell marker (Sigma‐Aldrich, USA) and ENaC (α, β or γ‐ENaC, StressMarq, Canada) at 4°C overnight, followed by corresponding secondary fluorescence antibodies for 1 h. Hoechst 33 342 (10 μM) was used to stain nuclei for 5 min. All slides were imaged using a confocal microscope (Olympus, Fluoview1000, Japan). Identical acquisition settings were used for all images.
Western blotting
Protein samples prepared from endothelial cells homogenates were analysed by electrophoresis with 10% SDS‐PAGE and transferred to nitrocellulose membranes using a Trans‐blot unit (Bio‐Rad Laboratories) for 1.5 h at 250 mA. Membranes were blocked with 5% (w/v) BSA in TBS (pH 7.4) containing 0.1% (v/v) Tween 20 (TBST) for 1 h at room temperature (22–24°C). The membrane was incubated with the primary antibodies against α‐ENaC (StressMarq, Canada), β‐ENaC (Sigma, Germany), γ‐ENaC (StressMarq, Canada), phospho‐Akt (Cell Signalling Technology, USA), Akt (Cell Signalling Technology, USA), phospho‐eNOS (Cell Signalling Technology, USA), eNOS (Abcam, USA), β‐actin (Santa Cruz Biotechnology, USA) or GAPDH (Santa Cruz Biotechnology, USA) overnight at 4°C. After washing with TBST, blots were incubated for 1 h at room temperature with the corresponding secondary antibodies (1:10 000). Membranes were finally washed with TBS‐T and the protein bands were detected by ECL kit (Invitrogen, Carlsbad, CA) and scanned densitometry (Bio‐Rad, USA).
Measurement of NO production in the endothelial cells of mesenteric artery
NO production in endothelial cells of MA was assessed by measuring the fluorescence of 4‐amino‐5‐methylamino‐2′,7′‐difluorofluorescein diacetate (DAF‐FM diacetate; Life Technology, Rockford, IL), a specific NO probe. Briefly, DAF‐FM diacetate (10 μM) was added to the endothelial cells for 1 h. Labelled cells including those treated with amiloride were washed twice in modified PBS before analysis by confocal microscopy. NO levels were assessed by fluorescence intensity.
Data and statistical analysis
The study design and analysis conformed to the recent guidance on experimental design and analysis (Curtis et al., 2015). Results represent means ± SEM from different groups. Concentration–response curves were analysed by non‐linear regression followed by Student's t‐test and compared between two groups. Comparisons were made between two groups by using unpaired Student's t‐test. P < 0.05 was accepted as statistically significant.
Materials
The following compounds were supplied by Sigma‐Aldrich (St. Louis, MO.): ACh, aldosterone, amiloride, eplerenone, NTG and phenylephrine. Hoechst33342 was supplied by Invitrogen.
Results
HS diet elevates blood pressure and plasma aldosterone in SS rats
Our previous studies showed that HS diet elevates SBP in SD rats but also reduces plasma aldosterone, allowing the rats to adapt to HS challenge (Liu et al., 2015). Here, we show that in Dahl SS rats, HS diet increased SBP (Figure 1A; n = 20; P < 0.05) whereas the SBP remained at normal levels in the rats on NS diet (Figure 1A; n = 20; P > 0.05). Unlike the response of SD rats, plasma aldosterone levels were not reduced, but elevated in SS rats (Figure 1B). These observations indicate that the secretion of aldosterone in SS rats is up‐regulated by dietary salt. HS diet had no effect on the ratio of heart weight and body weight (HW/BW) (Figure 1C) but significantly increased the ratio of kidney weight and body weight (KW/BW) (Figure 1C), indicating that the kidney is the major target of HS challenge. The paradoxical elevation of plasma aldosterone may account for loss of adaptation to HS in SS rats.
Figure 1.
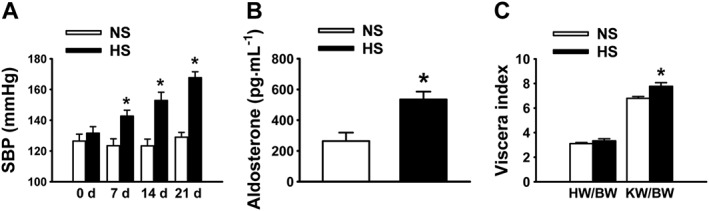
HS diet significantly increases SBP and plasma aldosterone levels of SS rats. (A) SBP in SS rats fed with either NS or HS diet. SBP was measured on days 7, 14 and 21. *P < 0.05, significantly different from NS; n = 20. (B) Plasma aldosterone levels in SS rats fed with either NS or HS diet. Plasma aldosterone levels were measured at day 21. *P < 0.05, significantly different from NS; n = 20. (C) HS diet had no effect on heart weight as demonstrated by the HW/BW index, but significantly increased kidney weight as demonstrated by changes in the KW/BW index of SS rats, compared with NS diet. *P < 0.05 significantly different from NS; n = 10.
HS diet enhances ENaC activity and expression in endothelial cells of SS rats
Recent studies suggest that the activity of ENaC in the distal nephron is up‐regulated by dietary sodium in SS rats (Pavlov et al., 2013). We showed that HS diet also stimulates ENaC in endothelial cells of SD rats but that the stimulation disappeared when the rats were continuously fed HS diet (Liu et al., 2015). To test whether HS‐induced ENaC activity can be sustained in SS rats, in situ cell‐attached patch‐clamp recording of ENaC single‐channel current in intact endothelial cells was performed, as previously reported (Climent et al., 2011; Liu et al., 2015). Our results showed that ENaC P O was significantly increased in SS rats fed HS diet for 3 weeks (Figure 2A, B), which was reduced by 0.5 μM amiloride.
Figure 2.
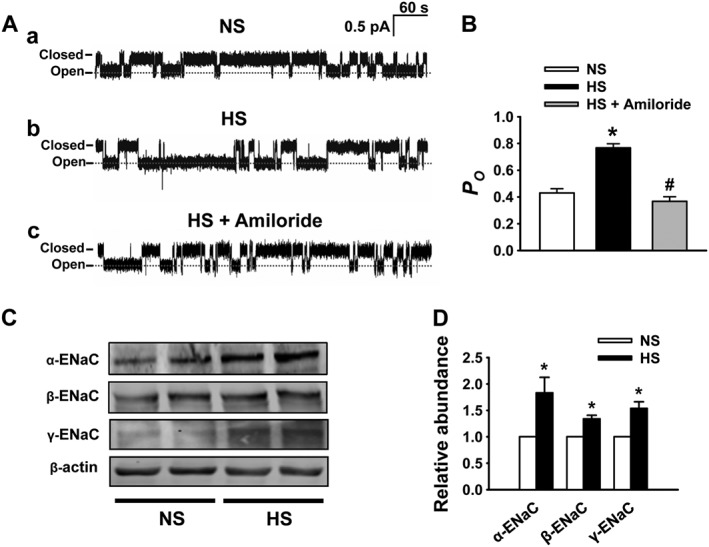
HS diet increases the activity and expression of ENaC in endothelial cells. (A) Representative ENaC single‐channel current detected, using split‐opened artery technique, in intact endothelial cells from SS rats fed with either NS or HS diet in the absence or in the presence of 0.5 μM amiloride. (B) Summarized ENaC P O obtained from single‐channel recordings as shown in (A), showing that P O was significantly increased after HS challenge. *P < 0.05, significantly different from NS; # P < 0.05, significantly different from HS; n = 7. (C) Western blots of α, β and γ‐ENaC in endothelial cells under indicated different experimental conditions. (D) Summary of normalized α, β and γ‐ENaC expression levels, showing that HS significantly increased the expression levels of all ENaC subunits. *P < 0.05, significantly different from NS; n = 6.
We have previously shown that α‐ENaC expression is reduced in response to HS challenge, probably due to a decreased plasma aldosterone (Liu et al., 2015). As HS diet paradoxically elevates plasma aldosterone in SS rats (shown in Figure 1B), it may affect the expression of ENaC in endothelial cells. Therefore, we assessed α‐, β‐ and γ‐ENaC protein in endothelial cells using Western blot and immunofluorescence labelling. As shown in Figure 2C and D, Western blot showed that α‐, β‐ and γ‐ENaC expression levels were significantly increased in endothelial cells freshly isolated from MA of SS rats on HS for 3 weeks. These results suggest that ENaC expression is up‐regulated by HS diet, which is consistent with the elevation of ENaC activity. We also examined the expression and distribution of ENaC in endothelial cells attached to the artery by immunofluorescence staining of ENaC. These results also demonstrated that α‐, β‐ and γ‐ENaC in endothelial cells of SS rats were elevated by HS diet (Figure 3A–D).
Figure 3.
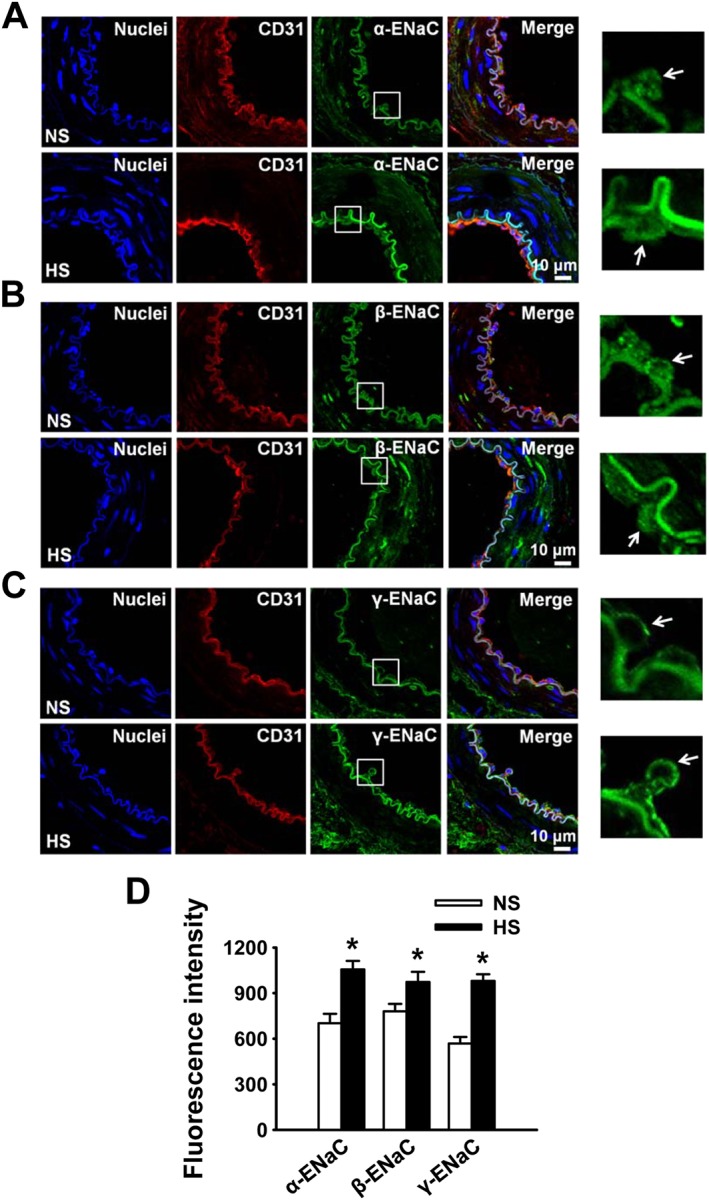
HS diet increases expression of ENaC in endothelial cells. (A–C) Representative immunofluorescence images showing α, β and γ‐ENaC expression in endothelial cells attached to the artery from SS rats on either NS or HS diet. Arrows point to endothelial cells. (D) Summarized mean fluorescent intensities of endothelial cells, which represent the levels of ENaC subunits. *P < 0.05, significantly different from NS; n = 5.
HS diet blunts endothelium‐dependent artery relaxation by stimulating ENaC
We have previously shown that in SD rats, reduced ENaC activity induced by HS diet mediates endothelium‐dependent artery relaxation (Liu et al., 2015). Therefore, we hypothesized that elevated ENaC activity induced by HS diet in SS rats may blunt endothelium‐dependent artery relaxation. Indeed, the present study shows that HS diet did cause loss of endothelium‐dependent artery relaxation. Precontraction of the arterial ring was induced by application of 10 μM phenylephrine in all the similar experiments. As shown in Figure 4A and B, HS diet blunted endothelium‐dependent artery relaxation induced by ACh in SS rats. To determine the possible role of ENaC in this relaxation, we pre‐incubated the artery with an ENaC blocker, amiloride, for 10 min before measuring ACh‐induced relaxation. The data show that blockade of ENaC enhanced the relaxation induced by ACh in rats on both NS and HS diet (Figure 4A, B). However, in the rats on HS diet, blockade of ENaC caused a quick rebound of the relaxation induced by a high dose of ACh (1 μM), indicating that HS diet not only reduces artery relaxation but also causes loss of relaxation stability. To avoid the accumulation of different concentrations of ACh, which could induce instability of MA relaxation, single applications (0.1 or 1 μM) of ACh were used to test the artery relaxation in rats on NS or HS diet. The data show that single doses of ACh also induced reduction and instability of relaxation in MA rings from HS treated rats (Figure 4C, D) and that blocking ENaC in MA rings enhanced the ACh‐induced relaxation in HS diet group. Moreover, amiloride also prevented the unstable relaxation induced by 0.1 or 1 μM ACh. These data suggest that ENaC plays an important role in controlling vascular tone, especially in SS rats on HS diet.
Figure 4.
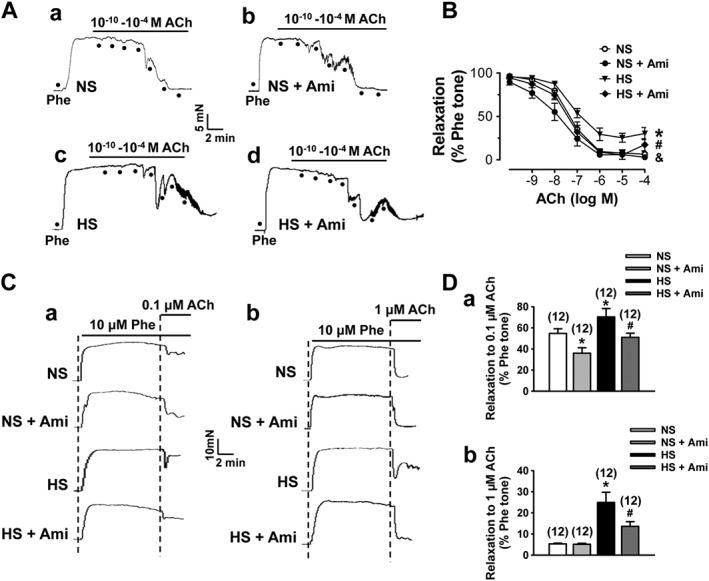
HS diet blunts endothelium‐dependent artery relaxation. (A) Representative traces of artery relaxation induced by gradually accumulated ACh in SS rats fed with either NS or HS diet in the absence (a or c) or in the presence of 0.5 μM amiloride (Ami; b or d). The first dot indicates application of 10−10 M ACh to the precontracted (by 10 μM phenylephrine; Phe) arterial ring, whereas the following dots indicate the concentrations of ACh were increased to 10−9, 10−8, 10−7, 10−6, 10−5 and 10−4 M respectively. (B) Summary of relaxation in response to different doses of ACh obtained from the experiments shown in (A). *P < 0.05, significant differences between NS and HS; & P < 0.05, significant differences between NS and NS + Ami; # P < 0.05, significant differences between HS and HS + Ami; n = 6 for each group. (C) Representative traces of artery relaxation induced by ACh at 0.1 μM (a) or 1 μM (b) in SS rats fed with either NS or HS diet, without and with treatment with 0.5 μM Ami. (D) Summary of artery relaxation induced by single dose of ACh under the conditions described in (C). *P < 0.05, significantly different from NS; # P < 0.05, significantly different from HS; n = 12.
HS diet does not affect endothelium‐independent artery relaxations in SS rats
We and others have shown that ENaC is not only expressed in endothelial cells but also in smooth muscle cells (Jernigan et al., 2008; Liu et al., 2015). Therefore, the possible involvement of smooth muscle ENaC in regulating endothelium‐independent relaxation was determined by exposing the isolated artery to NTG. The data show that there was no significant difference in NTG‐induced, endothelium‐independent relaxation, regardless of the diet. To further rule out the possible involvement of ENaC expressed in vascular smooth muscle cells, we also performed the experiments to test whether blocking ENaC with amiloride can affect NTG‐induced endothelium‐independent relaxation. The data show that amiloride did not alter NTG‐induced artery relaxation (Figure 5A, B). There was no ACh‐induced relaxation observed in the artery when its endothelium was removed. Removal of the endothelial cell layer did not affect NTG‐induced vascular relaxation. More importantly, amiloride affected neither ACh‐ nor NTG‐induced relaxation in the endothelium‐denuded arteries (Figure 5C–F). These results suggest that only ENaC in the endothelium, not in the vascular smooth muscle cells, mediated the loss of vascular relaxation induced by HS challenge.
Figure 5.
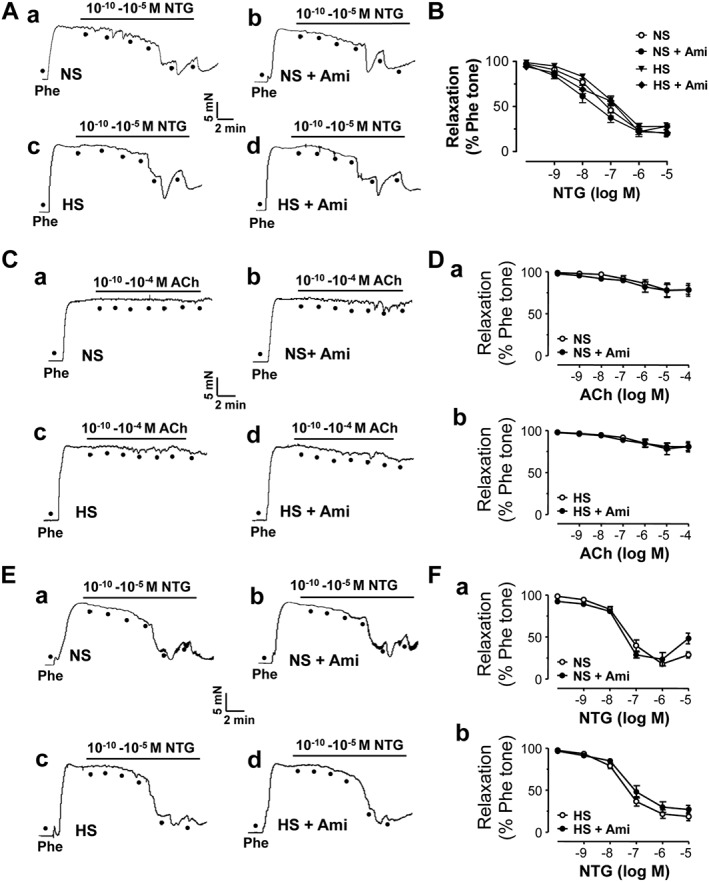
HS diet does not affect endothelium‐independent relaxation. (A) Arterial rings from SS rats fed with either NS or HS diet were precontracted by 10 μM phenylephrine (Phe). Relaxation was induced by cumulative treatment with NTG. Responses are shown from rings without (a or c) or with 0.5 μM amiloride (Ami; b or d). The first dot indicates application of 10−10 M NTG to the precontracted artery ring, whereas the following dots indicate the concentrations of NTG were increased to 10−9, 10−8, 10−7, 10−6 and 10−5 respectively. (B) Summary of artery relaxation induced by different doses of NTG (n = 9). (C) Relaxation of endothelium‐removed artery from SS rats fed with either NS or HS diet in response to cumulative doses of ACh, as described in the legend of Figure 4. (D) Summary of artery relaxation in response to different doses of ACh when the endothelium was removed (n = 9 for each data point). (E) Relaxation of endothelium‐removed artery from SS rats fed with either NS or HS in response to NTG. (F) Summary of artery relaxation in response to different doses of NTG when the endothelium was removed (n = 9 for each data point).
ENaC mediates HS‐reduced eNOS activity and NO production
To determine whether ENaC controls artery relaxation by altering NO bioavailability, eNOS phosphorylation and NO production in endothelial cells were measured. The data show that eNOS phosphorylation at Ser1177 and Akt phosphorylation at Ser473 were significantly decreased in the endothelial cells isolated from SS rats on HS diet, but not on NS diet. The decrease was reversed by blocking ENaC with amiloride (Figure 6A–D). Consistent with reduced eNOS activity, NO levels were also significantly decreased in the endothelial cells isolated from the rats on HS diet, but not on NS diet. This HS‐induced reduction of NO was also reversed by amiloride (Figure 6E, F). These results suggest that HS diet reduces eNOS activity and NO production by stimulating ENaC and therefore increases vascular tone.
Figure 6.
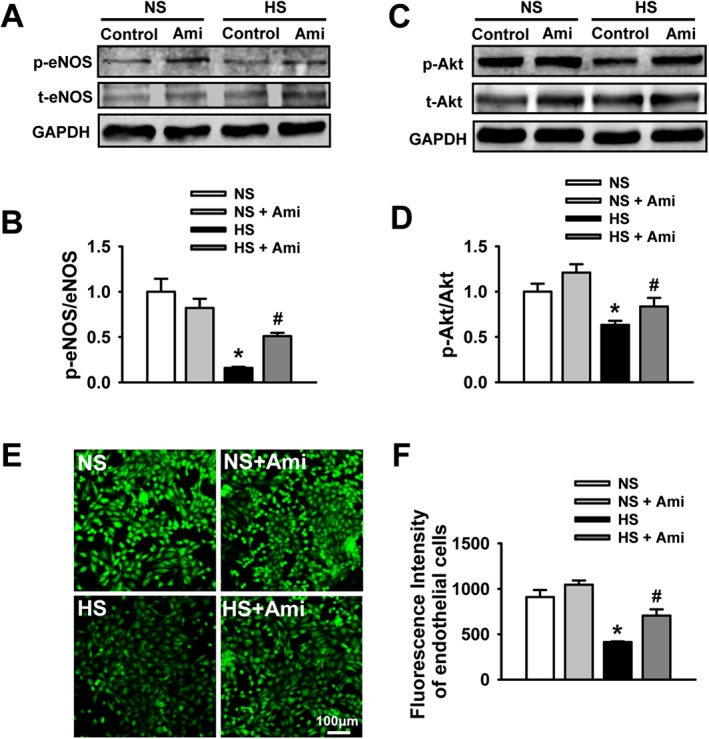
HS diet reduces eNOS phosphorylation and NO production. (A) Western blots of eNOS in isolated arteries with intact epithelium under indicated experimental conditions. (B) Summary of the levels of phosphorylated eNOS (p‐eNOS) after blocking ENaC with 0.5 μM amiloride (Ami) in SS rats fed with either NS or HS diet. (C) Western blots of Akt in isolated arteries with intact epithelium under indicated experimental conditions. (D) Summary of the levels of phosphorylated Akt (p‐Akt) after blocking ENaC with 0.5 μM amiloride in SS rats fed with either NS or HS. *P < 0.05, significantly different from NS; #P < 0.05, significantly different from HS; n = 6. (E) Intracellular NO levels detected with a membrane permeable fluorescent probe, DAF‐FMDA, under indicated conditions in endothelial cells. (F) Summary of fluorescence results from (E). *P < 0.05, significantly different from NS; #P < 0.05, significantly different from HS; n = 6.
HS diet stimulates ENaC by elevating aldosterone
To determine whether aldosterone accounts for the increased ENaC activity in SS rats on HS diet, the isolated arteries were incubated for 1 h with either aldosterone (10 nM) or eplerenone (10 μM, a specific aldosterone receptor antagonist). As shown in Figure 7A and B, exogenous aldosterone significantly increased ENaC P O in endothelial cells from the rats fed with NS diet, but did not further elevate ENaC P O from the rats in which ENaC is highly activated by already elevated endogenous aldosterone. Furthermore, blockade of aldosterone receptors with eplerenone significantly reduced ENaC P O in the rats fed with HS diet, but not NS diet (Figure 7C, D). These results suggest that the high level of activation of ENaC in endothelial cells can be attributed to the raised plasma aldosterone, induced by HS diet.
Figure 7.
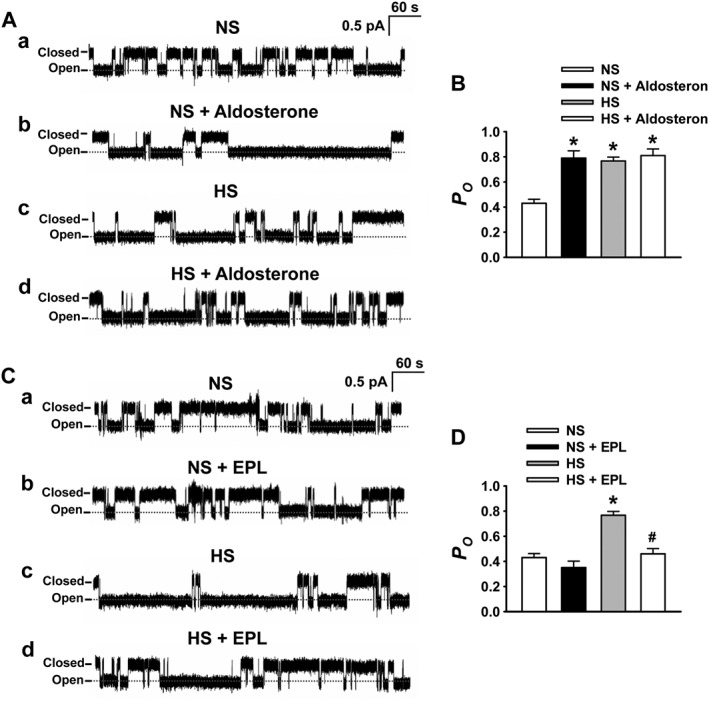
Blockade of aldosterone receptors prevents HS‐induced ENaC activity in endothelial cells. (A) Representative ENaC single‐channel current recorded in intact endothelial cells attached to MA in SS rats fed with either NS or HS diet, without (a or c) or with treatment with 10 nM aldosterone (b or d). (B) Summarized P O obtained from the single‐channel recordings as shown in (A). *P < 0.05, significantly different from NS; n = 9. (C) Representative ENaC single‐channel current recorded in intact endothelial cells attached to the artery from SS rats fed with either NS or HS diet without (a or c) or with giving 10 μM eplerenone (EPL; b or d). (D) Summarized P O obtained from the single‐channel recordings as shown in (C). *P < 0.05, significantly different from NS; # P < 0.05, significantly different from HS; n = 9.
HS diet blunts endothelium‐dependent artery relaxation by elevating aldosterone
We next determined the effects of blockade of aldosterone on ACh‐induced artery relaxation. The data show that HS diet blunted ACh‐induced artery relaxations (Figure 8). Incubation of the isolated artery with eplerenone (10 μM) for 1 h reversed HS‐induced reduction of artery relaxation (Figure 8A–C). As shown in Figure 8D–E, eplerenone also enhanced the artery relaxation induced by a high dose of ACh in the rats fed with HS diet. These data suggest that aldosterone plays a key role in HS‐induced ENaC activity and loss of endothelium‐dependent artery relaxation in SS rats.
Figure 8.
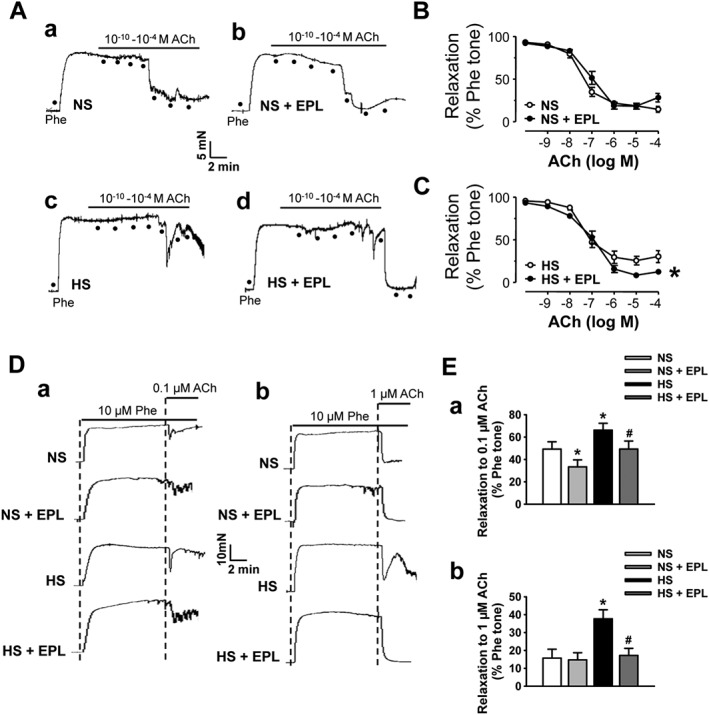
Blockade of aldosterone receptors prevents endothelium‐dependent artery relaxation reduced by HS in SS rats. (A) Artery relaxation induced by cumulative treatment with ACh in rats fed with either NS or HS diet without (a and c) or with 10 μM eplerenone (EPL; b and d). (B and C) Summary of relaxation in response to either 0.1 μM ACh (a) or 1 μM ACh (b) without and with 10 μM eplerenone. *P < 0.05, significantly different from HS; n = 6. (D) Representative traces of artery relaxation induced by ACh at 0.1 μM (a) or 1 μM (b) in SS rats fed with either NS or HS diet without and with giving 10 μM eplerenone. (E) Summary of artery relaxation under the conditions described in (D). *P < 0.05, significantly different from NS; # P < 0.05, significantly different from HS; n = 18).
Discussion
Our data indicate that endothelial ENaC is regulated by HS intake in SS rats and that this regulation may play an important role in dietary salt‐induced endothelial dysfunction in salt‐sensitive hypertension. HS significantly elevates ENaC expression and activity in endothelial cells, leading to the reduction of eNOS activity and NO production, and therefore blunts the endothelium‐dependent relaxation in SS rats. ENaC play an important role in HS‐induced loss of vascular relaxation in SS rats because blockade of ENaC can reverse the decreased eNOS activity and NO production caused by HS intake in these rats. Although amiloride can also block the Na+‐H+ exchanger, it has much higher binding affinity to ENaC than the Na+‐H+ exchanger (Weinman and Reuss, 1982). However, the role of ENaC described here can be further determined by specific knockout of ENaC in the vasculature. It appears that aldosterone acts as an initial signalling molecule for HS‐induced endothelial dysfunction because HS diet increases plasma aldosterone levels in SS rats and blockade of aldosterone receptors markedly inhibits ENaC activity and prevents the loss of artery relaxation induced by HS intake. However, to our knowledge, it is unknown why HS intake elevates plasma aldosterone in SS rats. Nevertheless, our results strongly suggest that ENaC is one crucial determinant of endothelium‐dependent artery relaxation, especially in SS rats.
Although HS intake may affect endothelium‐dependent relaxation through various pathways, the dysregulation of the NO system is the main signalling pathway involved in controlling vascular tone, by targeting endothelial cells (Feletou and Vanhoutte, 2006). Studies in humans and animal models have shown that HS diet causes an increase in plasma Na+ concentration and a small increase in Na+ concentration (from 137 to 142 mM) can suppress eNOS activity, leading to loss of NO dependent vasodilation (Li et al., 2009; Suckling et al., 2012). The elevated plasma Na+ may pass through active ENaC to inhibit eNOS phosphorylation via a pathway associated with PI3K and Akt (Perez et al., 2009). Here, we show that, without functional ENaC, HS diet may not cause loss of NO dependent vasodilation. This implies that the ENaC blocker amiloride may treat salt‐sensitive hypertension by restoring NO‐dependent vasodilation. Although vascular smooth muscle cells also express ENaC (Jernigan et al., 2008; Liu et al., 2015), our data show that blockade of ENaC did not alter ACh‐ and NTG‐induced relaxation of endothelium‐denuded arteries, indicating that the ENaC in smooth muscle cells may not be targeted by HS diet, but is regulated by hydrostatic pressure to mediate myogenic constriction, as reported previously (Jernigan et al., 2008).
The classical action of aldosterone is control of ENaC expression and activity via mineralocorticoid receptor (MR)‐associated pathways only in the epithelia of kidneys (Aoi et al., 2007). However, recent studies suggest that aldosterone also regulates ENaC expressed in endothelial cells (Golestaneh et al., 2001; Kusche‐Vihrog et al., 2008). The effects of aldosterone on endothelial function can be either reversed by the ENaC‐specific blocker amiloride or prevented by spironolactone, a competitive MR antagonist (Kusche‐Vihrog et al., 2008). Moreover, both aldosterone and high extracellular sodium can alter endothelial function (Li et al., 2009; Druppel et al., 2013). We and others have shown that HS diet suppresses plasma aldosterone in SD rats but increases plasma aldosterone levels in Dahl SS rats (Morizane et al., 2012; Liu et al., 2015). In SD rats, HS intake enhances ACh‐induced artery relaxation by reducing ENaC activity, due to a decreased plasma aldosterone (Liu et al., 2015). Conversely, here we show that in SS rats, HS intake blunts the artery relaxation by stimulating ENaC due to an increased plasma aldosterone. Furthermore, the increased ENaC activity in endothelial cells causes a reduction of NO production and consequently elevates vascular tone. Therefore, the differences in the regulation of plasma aldosterone and ENaC activity between SD and Dahl SS rats may respectively account for salt adaptation and salt‐sensitive hypertension.
Consistent with our data, Morizane et al. (2012) also had shown that HS diet increased plasma aldosterone levels in SS rats but completely suppressed plasma aldosterone in salt‐resistant (SR) rats. However, the response of plasma aldosterone levels to salt loading in SS rats is still a matter of debate. For instance, Matsukawa et al. (1993) found that HS diet reduced plasma aldosterone in both SS and SR rats. It has been argued that the controversial results may be due to the different feeding methods or breeding facilities (Morizane et al., 2012). Thus, the role of aldosterone in the pathophysiology of salt‐sensitive hypertension needs to be further investigated.
In conclusion, our present experiments have shown that dietary HS intake significantly elevates blood pressure in SS rats via a new pathway associated with increased ENaC activity in endothelial cells, which controls vascular tone in an NO‐dependent manner. Although the present study has not tested whether amiloride can lower blood pressure in SS rats, previous studies have already shown that HS diet dramatically increased the mean arterial pressure of Dahl SS rat and amiloride or benzamil (an analogue of amiloride) significantly reduced the level of salt‐induced hypertension (Kakizoe et al., 2009; Pavlov et al., 2013). Therefore, this study may provide useful information for the eventual development of drugs to treat salt‐sensitive hypertension.
Author contributions
Z.‐R.W. and Y.‐Y.S. performed experiments and analysed data; H.‐B.L. analysed data and drafted the manuscript; Q.‐Q.H., Y.‐X.L., W.‐W.Z., C.‐J.Y., X.‐Y.L., M.‐M.W. and B.‐L.S. performed partial experiments and analysed data; Z.‐Y.Y. and J.‐J.M. provided animal model; Z.‐R.Z. and H.‐P.M. designed experiments and revised the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
This work was supported by Key Project of Chinese National Program for Fundamental Research and Development (973 Program 2014CB542401 to Z.Z.), National Natural Science Foundation of China (81320108002 and 91639202 to Z.Z. and 81300191 to H. L.). This work was also partially supported by a grant from National Institutes of Health (NIH) (R01 DK 100582 to H.‐P.M.).
Wang, Z.‐R. , Liu, H.‐B. , Sun, Y.‐Y. , Hu, Q.‐Q. , Li, Y.‐X. , Zheng, W.‐W. , Yu, C.‐J. , Li, X.‐Y. , Wu, M.‐M. , Song, B.‐L. , Mu, J.‐J. , Yuan, Z.‐Y. , Zhang, Z.‐R. , and Ma, H.‐P. (2018) Dietary salt blunts vasodilation by stimulating epithelial sodium channels in endothelial cells from salt‐sensitive Dahl rats. British Journal of Pharmacology, 175: 1305–1317. doi: 10.1111/bph.13817.
References
- Ahn D, Ge Y, Stricklett PK, Gill P, Taylor D, Hughes AK et al. (2004). Collecting duct‐specific knockout of endothelin‐1 causes hypertension and sodium retention. J Clin Invest 114: 504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Peters JA, Kelly E, Marrion N, Benson HE, Faccenda E et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Ligand‐gated ion channels. Br J Pharmacol 172: 5870–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin MS, Wang HW, Reza E, Whitman SC, Tuana BS, Leenen FH (2005). Distribution of epithelial sodium channels and mineralocorticoid receptors in cardiovascular regulatory centers in rat brain. Am J Physiol Regul Integr Comp Physiol 289: R1787–R1797. [DOI] [PubMed] [Google Scholar]
- Aoi W, Niisato N, Sawabe Y, Miyazaki H, Tokuda S, Nishio K et al. (2007). Abnormal expression of ENaC and SGK1 mRNA induced by dietary sodium in Dahl salt‐sensitively hypertensive rats. Cell Biol Int 31: 1288–1291. [DOI] [PubMed] [Google Scholar]
- Bibbins‐Domingo K, Chertow GM, Coxson PG, Moran A, Lightwood JM, Pletcher MJ et al. (2010). Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med 362: 590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD et al. (1994). Amiloride‐sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367: 463–467. [DOI] [PubMed] [Google Scholar]
- Climent B, Zsiros E, Stankevicius E, de la Villa P, Panyi G, Simonsen U et al. (2011). Intact rat superior mesenteric artery endothelium is an electrical syncytium and expresses strong inward rectifier K+ conductance. Biochem Biophys Res Commun 410: 501–507. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wardener HE, MacGregor GA (2002). Sodium and blood pressure. Curr Opin Cardiol 17: 360–367. [DOI] [PubMed] [Google Scholar]
- Druppel V, Kusche‐Vihrog K, Grossmann C, Gekle M, Kasprzak B, Brand E et al. (2013). Long‐term application of the aldosterone antagonist spironolactone prevents stiff endothelial cell syndrome. FASEB J 27: 3652–3659. [DOI] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM (2006). Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture). Am J Physiol Heart Circ Physiol 291: H985–1002. [DOI] [PubMed] [Google Scholar]
- Fyfe GK, Canessa CM (1998). Subunit composition determines the single channel kinetics of the epithelial sodium channel. J Gen Physiol 112: 423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golestaneh N, Klein C, Valamanesh F, Suarez G, Agarwal MK, Mirshahi M (2001). Mineralocorticoid receptor‐mediated signaling regulates the ion gated sodium channel in vascular endothelial cells and requires an intact cytoskeleton. Biochem Biophys Res Commun 280: 1300–1306. [DOI] [PubMed] [Google Scholar]
- He FJ, MacGregor GA (2007). Salt, blood pressure and cardiovascular disease. Curr Opin Cardiol 22: 298–305. [DOI] [PubMed] [Google Scholar]
- Jernigan NL, LaMarca B, Speed J, Galmiche L, Granger JP, Drummond HA (2008). Dietary salt enhances benzamil‐sensitive component of myogenic constriction in mesenteric arteries. Am J Physiol Heart Circ Physiol 294: H409–H420. [DOI] [PubMed] [Google Scholar]
- Kakizoe Y, Kitamura K, Ko T, Wakida N, Maekawa A, Miyoshi T et al. (2009). Aberrant ENaC activation in Dahl salt‐sensitive rats. J Hypertens 27: 1679–1689. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizer N, Guo XL, Hruska K (1997). Reconstitution of stretch‐activated cation channels by expression of the alpha‐subunit of the epithelial sodium channel cloned from osteoblasts. Proc Natl Acad Sci U S A 94: 1013–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusche‐Vihrog K, Sobczak K, Bangel N, Wilhelmi M, Nechyporuk‐Zloy V, Schwab A et al. (2008). Aldosterone and amiloride alter ENaC abundance in vascular endothelium. Pflugers Arch 455: 849–857. [DOI] [PubMed] [Google Scholar]
- Leenen FH (2010). The central role of the brain aldosterone‐“ouabain” pathway in salt‐sensitive hypertension. Biochim Biophys Acta 1802: 1132–1139. [DOI] [PubMed] [Google Scholar]
- Li J, White J, Guo L, Zhao X, Wang J, Smart EJ et al. (2009). Salt inactivates endothelial nitric oxide synthase in endothelial cells. J Nutr 139: 447–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HB, Zhang J, Sun YY, Li XY, Jiang S, Liu MY et al. (2015). Dietary salt regulates epithelial sodium channels in rat endothelial cells: adaptation of vasculature to salt. Br J Pharmacol 172: 5634–5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HB, Zhang J, Xin SY, Liu C, Wang CY, Zhao D et al. (2013). Mechanosensitive properties in the endothelium and their roles in the regulation of endothelial function. J Cardiovasc Pharmacol 61: 461–470. [DOI] [PubMed] [Google Scholar]
- Luscher TF, Boulanger CM, Dohi Y, Yang ZH (1992). Endothelium‐derived contracting factors. Hypertension 19: 117–130. [DOI] [PubMed] [Google Scholar]
- Ma HP (2011). Hydrogen peroxide stimulates the epithelial sodium channel through a phosphatidylinositide 3‐kinase‐dependent pathway. J Biol Chem 286: 32444–32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma HP, Al‐Khalili O, Ramosevac S, Saxena S, Liang YY, Warnock DG et al. (2004). Steroids and exogenous gamma‐ENaC subunit modulate cation channels formed by alpha‐ENaC in human B lymphocytes. J Biol Chem 279: 33206–33212. [DOI] [PubMed] [Google Scholar]
- Ma HP, Chou CF, Wei SP, Eaton DC (2007). Regulation of the epithelial sodium channel by phosphatidylinositides: experiments, implications, and speculations. Pflugers Arch 455: 169–180. [DOI] [PubMed] [Google Scholar]
- Ma HP, Eaton DC (2005). Acute regulation of epithelial sodium channel by anionic phospholipids. J Am Soc Nephrol 16: 3182–3187. [DOI] [PubMed] [Google Scholar]
- Matsukawa N, Nonaka Y, Higaki J, Nagano M, Mikami H, Ogihara T et al. (1993). Dahl's salt‐resistant normotensive rat has mutations in cytochrome P450(11 beta), but the salt‐sensitive hypertensive rat does not. J Biol Chem 268: 9117–9121. [PubMed] [Google Scholar]
- McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL (2010). Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol 160: 1573–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA (2005). Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev 85: 679–715. [DOI] [PubMed] [Google Scholar]
- Morizane S, Mitani F, Ozawa K, Ito K, Matsuhashi T, Katsumata Y et al. (2012). Biphasic time course of the changes in aldosterone biosynthesis under high‐salt conditions in Dahl salt‐sensitive rats. Arterioscler Thromb Vasc Biol 32: 1194–1203. [DOI] [PubMed] [Google Scholar]
- Nicco C, Wittner M, DiStefano A, Jounier S, Bankir L, Bouby N (2001). Chronic exposure to vasopressin upregulates ENaC and sodium transport in the rat renal collecting duct and lung. Hypertension 38: 1143–1149. [DOI] [PubMed] [Google Scholar]
- Oberleithner H, Ludwig T, Riethmuller C, Hillebrand U, Albermann L, Schafer C et al. (2004). Human endothelium: target for aldosterone. Hypertension 43: 952–956. [DOI] [PubMed] [Google Scholar]
- Oberleithner H, Riethmuller C, Schillers H, MacGregor GA, de Wardener HE, Hausberg M (2007). Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci U S A 104: 16281–16286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov TS, Levchenko V, O'Connor PM, Ilatovskaya DV, Palygin O, Mori T et al. (2013). Deficiency of renal cortical EGF increases ENaC activity and contributes to salt‐sensitive hypertension. J Am Soc Nephrol 24: 1053–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov TS, Staruschenko A (2016). Involvement of ENaC in the development of salt‐sensitive hypertension. Am J Physiol Renal Physiol. https://doi.org/10.1152/ajprenal.00427.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng K, Lu X, Wang F, Nau A, Chen R, Zhou SF et al. (2017). Collecting duct (pro)renin receptor targets ENaC to mediate angiotensin II‐induced hypertension. Am J Physiol Renal Physiol 312: F245–F253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez FR, Venegas F, Gonzalez M, Andres S, Vallejos C, Riquelme G et al. (2009). Endothelial epithelial sodium channel inhibition activates endothelial nitric oxide synthase via phosphoinositide 3‐kinase/Akt in small‐diameter mesenteric arteries. Hypertension 53: 1000–1007. [DOI] [PubMed] [Google Scholar]
- Pochynyuk O, Bugaj V, Rieg T, Insel PA, Mironova E, Vallon V et al. (2008). Paracrine regulation of the epithelial Na+ channel in the mammalian collecting duct by purinergic P2Y2 receptor tone. J Biol Chem 283: 36599–36607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suckling RJ, He FJ, Markandu ND, MacGregor GA (2012). Dietary salt influences postprandial plasma sodium concentration and systolic blood pressure. Kidney Int 81: 407–411. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Yoshika M, Komiyama Y, Nishimura M (2011). The central mechanism underlying hypertension: a review of the roles of sodium ions, epithelial sodium channels, the renin‐angiotensin‐aldosterone system, oxidative stress and endogenous digitalis in the brain. Hypertens Res 34: 1147–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubes G (1998). The (political) science of salt. Science 281: 898–901, 903‐897. [DOI] [PubMed] [Google Scholar]
- Taylor NE, Cowley AW Jr (2005). Effect of renal medullary H2O2 on salt‐induced hypertension and renal injury. Am J Physiol Regul Integr Comp Physiol 289: R1573–R1579. [DOI] [PubMed] [Google Scholar]
- Van Huysse JW, Amin MS, Yang B, Leenen FH (2012). Salt‐induced hypertension in a mouse model of Liddle syndrome is mediated by epithelial sodium channels in the brain. Hypertension 60: 691–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HW, Huang BS, Chen A, Ahmad M, White RA, Leenen FH (2016). Role of brain aldosterone and mineralocorticoid receptors in aldosterone‐salt hypertension in rats. Neuroscience 314: 90–105. [DOI] [PubMed] [Google Scholar]
- Weinman SA, Reuss L (1982). Na+−H+ exchange at the apical membrane of Necturus gallbladder. Extracellular and intracellular pH studies. J Gen Physiol 80: 299–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Luo Z, Ma S, Wong WT, Ma L, Zhong J et al. (2010). Activation of TRPV1 by dietary capsaicin improves endothelium‐dependent vasorelaxation and prevents hypertension. Cell Metab 12: 130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Chen S, Liu H, Zhang B, Zhao Y, Ma K et al. (2013). Hydrogen sulfide prevents hydrogen peroxide‐induced activation of epithelial sodium channel through a PTEN/PI(3,4,5)P3 dependent pathway. PLoS One 8: e64304. [DOI] [PMC free article] [PubMed] [Google Scholar]


