Abstract
Background
Diarrhea associated with parvovirus infection is common in dogs. Supportive care is the mainstay of treatment, but recovery may be prolonged and mortality rate can be high. Modification of the intestinal bacterial microbiota has been promising in human and veterinary medicine as an adjunctive treatment of various enteric diseases.
Objectives
To investigate the safety and efficacy of fecal microbiota transplantation (FMT) on the clinical recovery of puppies with acute hemorrhagic diarrhea syndrome.
Animals
Sixty‐six puppies with parvovirus infection were evaluated at 2 veterinary hospitals.
Methods
Randomized clinical trial. Puppies were randomly distributed into 2 groups: standard treatment (STD) and standard treatment + FMT (STD + FMT). The STD puppies (n = 33) received only treatment with IV fluids and antimicrobials and the STD + FMT puppies (n = 33) received FMT in addition to standard treatment. For FMT, 10 g of feces from a healthy dog diluted in 10 mL of saline were administered rectally 6‐12 hours post‐admission.
Results
Among survivors, treatment with FMT was associated with faster resolution of diarrhea (P < .001) and shorter hospitalization time (P = .001; median, 3 days in STD + FMT; median, 6 days in STD) compared to standard treatment. Mortality in STD was 36.4% (12/33) as compared to 21.2% (7/33) in puppies treated with FMT, but there was no statistical difference between groups (P = .174). Polymerase chain reaction indicated that all animals carried canine parvovirus, strain CPV‐2b.
Conclusions
Fecal microbiota transplantation in parvovirus‐infected puppies was associated with faster resolution of diarrhea.
Keywords: bacteriotherapy, diarrhea, dog, FMT, microbiome, stool transplant
Abbreviations
- CPV
canine parvovirus
- FMT
fecal microbiota transplantation
- PCR
polymerase chain reaction
- STD
standard
- TMS
trimethoprim‐sulfa
1. INTRODUCTION
Diarrhea can lead to serious and life‐threatening conditions and can have many causes, including infectious agents, such as bacteria, parasites, and viruses. Infectious gastrointestinal disease is common and can be severe in puppies, with canine parvovirus (CPV) being a leading cause of illness and death in some populations.1 Regardless of etiology, early detection, and management are important to decrease mortality rates that can be very high, especially in young animals.2
The gastrointestinal tract of dogs is colonized by an immense population of microorganisms, termed the microbiota, which is composed of bacteria, Archaea, fungi, viruses, and protozoa.3 The intestinal microbiota benefits the host, acting as a defense barrier against enteropathogens, regulating the immune system, digesting complex fibers, providing nutritional support for enterocytes, and stimulating gastrointestinal motility.4, 5 Alterations in the intestinal microbiota have been associated with various diseases in numerous species, including acute and chronic gastrointestinal diseases in dogs.6, 7, 8
Because alterations of the microbiota have been associated with disease, measures to restore or optimize the microbiota are of interest therapeutically. Probiotics and prebiotics have been used with limited success. Much attention has been paid recently in human medicine to fecal microbial transplantation (FMT), whereby fecal suspensions from a healthy donor are administered to an individual with disease. Most work in humans has involved recurrent Clostridium difficile infection, and FMT has resulted in high cure rates.9, 10, 11, 12
Fecal microbiota transplantation has received limited study in dogs.13, 14 Encouraging results were reported for the treatment of chronic diarrhea in dogs in a pilot study, and the clinical response was consistent with significant changes in fecal microbiota after treatment.15
Our objectives were to evaluate the effectiveness and safety of FMT in puppies with acute hemorrhagic diarrhea syndrome.
2. MATERIAL AND METHODS
2.1. Experimental design
All dogs <1 year of age suffering from acute hemorrhagic diarrhea syndrome admitted to 2 veterinary hospitals, without restriction of sex and breed, were enrolled in the study. Sixty‐six dogs were evaluated from July 2015 to August 2016 at the Universidade Estadual de Londrina and Universidade Philadelphia, both located in the city of Londrina, Paraná state, Brazil. The study was approved by the Ethics Committee on the Use of Animals of the 2 Institutions (numbers 128/2015 and 010/2015, respectively). The animals' owners consented to participate in the study by signing an Informed Consent Form for Research.
Patients were assigned to 2 groups: standard treatment (STD; n = 33) receiving only standard treatment and FMT (STD + FMT; n = 33) receiving FMT in addition to standard treatment. Standard treatment consisted of IV fluid administration of polyionic isotonic solutions (lactated Ringer's or 0.9% sodium chloride with 30 mEq/L of potassium chloride added) at an infusion rate ranging from 50 to 150 mL/kg q24h, as well as anti‐emetics (ondansetron, 0.2 mg/kg q8h IV or maropitant, 1 mg/kg q24h SC), gastric protectant (ranitidine, 3 mg/kg q12h SC), and IV antibiotics (cephalothin, 30 mg/kg q12h or trimethoprim‐sulfa [TMS] 30 mg/kg q12h, and metronidazole 25 mg/kg q24h). Treatment, including antibiotic treatment, was started in all patients at the time of admission and therefore between 6 and 12 hours before FMT. The safety of FMT was assessed by the presence or absence of discomfort during and after the procedure and by monitoring of vital parameters.
Age, sex, alimentary habits, previous episodes of diarrhea, vaccination, and deworming history were obtained from the owners. After admission, physical examinations were performed daily (temperature, heart and respiratory rates, posture, level of consciousness, and appetite). Feces also were evaluated daily until the time of discharge, and classified as liquid (diarrhea), pasty, normal, or absent.
2.2. Collection and processing of biological samples
On admission, blood samples were collected from all patients in EDTA tubes for a CBC. The first fecal sample from each patient was obtained by spontaneous defecation and refrigerated in a conventional refrigerator at 6°C for a maximum of 48 hours for parasitological examinations. A rectal swab also was collected and stored in plastic tubes at −80°C for detection of CPV.
Feces for the FMT procedure were obtained by spontaneous defecation from a healthy 6‐year‐old American Pit Bull Terrier (donor) resident at the Universidade Philadelphia's kennel. The donor dog was clinically normal, fed exclusively a commercial cooked diet (PremieR Formula—Adults, Premier Pet, São Paulo, Brazil), was current on vaccinations and deworming and had not received antimicrobials or had an episode of vomiting or diarrhea in the last 6 months. Complete blood count, serum biochemical profile, and fecal parasitology tests were normal. In addition, the dog was negative for parvovirus, distemper virus, and Erlichia canis, based on PCR of blood.
Donor feces were harvested daily for 2 weeks, divided into 10‐g aliquots and frozen at −20°C. Consecutive collection was performed to minimize normal variations of the donor's microbiota that might have occurred over time, so that all patients received FMT containing a similar microbiota.
2.3. Sample analysis
The Willis, Hoffman Pons and Janer, modified Faust, and modified Ziehl‐Neelsen methods were used for fecal parasitology.
Extraction of nucleic acid, PCR for detection of CPV, and sequencing of amplified products were performed as previously described.16
2.4. Fecal microbiota transplantation
For FMT, 10 g of the donor's feces was diluted in 10 mL of 0.9% sodium chloride solution, which was mixed and aspirated in a 20‐mL syringe, connected to a urethral catheter, introduced anally, and the contents deposited in the proximal portion of the rectum. Sedation or anesthesia was not used.
The animal was maintained in lateral recumbency for 2 minutes, with the pelvis raised to approximately 45° from the surface, to aid in the diffusion of the transplanted contents by gravity. The FMT was performed between 6 and 12 hours post‐admission and repeated every 48 hours until resolution of the diarrhea or a total of 5 applications.
2.5. Statistical analysis
Age, sex, body weight, and CBC data at baseline were compared using a Student's t test to ensure similarity between animals assigned to STD or STD + FMT.
The mortality between groups (STD versus STD + FMT) and resolution of diarrhea within each time interval (0 to 48 hours; between 48 and 96 hours; > 96 hours after admission) were compared by the Chi‐Square test. The number of days in hospital was compared between groups by the non‐parametric Mann–Whitney Test, using a statistical software (Minitab 16, Minitab Inc, Pennsylvania, United States.)
3. RESULTS
Thirty‐four patients (STD, 17; STD + FMT, 17) were admitted to the Universidade Estadual de Londrina teaching hospital and 32 (STD, 16; STD + FMT, 16) to the Universidade Philadelphia during the trial period. The characterization of animals assigned to STD and STD + FMT is presented in Table 1. At admission, no statistical differences were found between groups in body weight (P = .238), breed (mixed versus pure, P = .125), leukocyte count (P = .381), plasma total protein concentration (P = .494), and PCV (P = .131; Table 1). Only age was statistically different between the 2 populations (P = .004). Twenty‐eight puppies from STD and 25 from STD + FMT received TMS and metronidazole as antimicrobial treatment, and 5 puppies from STD and 8 from STD + FMT were treated with cefalotin and metronidazole. Supporting Information Table 1 contains individual information about each dog.
Table 1.
Description of 2 populations of puppies with acute hemorrhagic diarrhea syndrome at admission to a veterinary hospital assigned to receive standard treatment (STD) or standard treatment with fecal microbiota transplantation (STD + FMT) administered rectally between 6 and 12 hours post‐admission
| P value | STD | STD + FMT | |
|---|---|---|---|
| .004 | Age (months) | ||
| Mean (SD) a | 3.67 (1.86) | 5.18 (2.54) | |
| Median | 3.00 | 4.00 | |
| Range | 2–9 months | 2–12 months | |
| .238 | Body weight (kg) | ||
| Mean (SD) | 5.03 (4.54) | 5.74 (3.43) | |
| Median | 2.95 | 4.50 | |
| Range | 0.85‐16.80 | 1.00–14.30 | |
| .457 | Sex | ||
| Male | 51.52% | 60.60% | |
| Female | 48.48% | 39.40% | |
| .125 | Breed | ||
| Pure breed | 72.72% | 54.55% | |
| Mixed breed | 27.28% | 45.45% | |
| CBC at admission | |||
| % of puppies with leukopenia | .392 | 93.33% | 87.87% |
| Leucocytes mean (SD) (×106/L) | .381 | 2759.09 (2539.30) | 2962.12 (2864.74) |
| PCV mean/(SD) (%) | .131 | 39.71 (10.20) | 42.47 (9.59) |
| Total protein mean/(SD) (g/dL) | .494 | 6.30 (0.98) | 6.29 (1.36) |
| Canine parvovirus infection | |||
| Positive | 100% | 100% | |
aStatistically different between STD and STD + FMT.
SD: Standard deviation.
The FMT was considered safe because none of the puppies assigned to the STD + FMT group developed any clinical abnormalities that were attributable to the procedure. The use of a fourth or fifth application was not required (mean number of procedures per dog, 1.82; standard deviation [SD], 0.68; range, 1–3).
The mortality rate in dogs treated with FMT was 21.2% (7/33) and 36.4% (12/33) for STD (P = .174). Interestingly, among survivors, only 4.8% (1/21) of patients in the STD group, but 61.5% (16/26) in the STD + FMT group, had resolution of diarrhea within 48 hours of hospitalization (P < .001; Figure 1). Nearly, 43% of dogs from STD (9/21) and 30.8% (8/26) from STD + FMT showed improvement of diarrhea between 48 and 96 hours (P = .3912), and 52.38% (11/21) from STD and 7.7% (2/26) from STD + FMT (P < .001) had improved fecal consistency after 96 hours of hospitalization (Figure 1).
Figure 1.
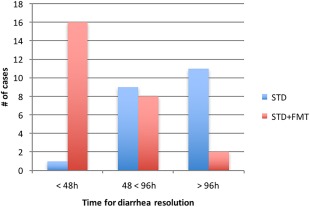
Time for resolution of diarrhea in survivor puppies with parvovirus infection treated with fecal microbiota transplantation (STD + FMT) compared to standard treatment (STD)
Dogs from the STD + FMT group spent statistically fewer days in the hospital (P < .001) compared to those in the STD group (median, 3.00; range, 1–6; mean, 3.31; SD, 1.49 days in the STD + FMT group; median, 6.00; range, 2–15; mean, 5.57; SD, 2.76 days in the STD group).
The prevalence of CPV in the studied population was 100% (66/66), and the molecular characterization of the virus indicated that 100% of the positive samples consisted of strain CPV‐2b.
Only 5.1% (3/59) of patients had positive results on fecal parasitology: 2 with Toxocara spp. (STD and STD + FMT) and 1 with Entamoeba spp. (STD).
4. DISCUSSION
We report for the first time the use of FMT in puppies with gastroenteritis caused by parvovirus infection. The procedure proved to be safe with no adverse effects on the animals studied, which is in agreement with other studies.14, 15 No discomfort was identified during the procedure with the volume used, and there was no need for physical restraint, sedation, or analgesia.
A striking difference in resolution of diarrhea within the first 48 hours of hospitalization was evident with FMT in the dogs that survived (61% in STD + FMT versus 5% in STD). The rapid response is beneficial from numerous standpoints. Cost can be a limiting factor in the treatment of diarrhea in puppies, and a faster response can decrease treatment time and the likelihood of euthanasia for economic reasons. The rapid response also means that typically only 1 FMT procedure is required per patient, decreasing the cost and effort. Although diarrhea in puppies is very different from recurrent C. difficile infection in humans, the rapidity of response noted here is consistent with various studies in humans that noted high cure rates, often with a single treatment.12 More rapid resolution of diarrhea likely was the main reason associated with the faster clinical recovery and fewer days in the hospital observed in treated animals. In addition to decreasing cost and euthanasia for economic reasons, shorter hospital stays also decrease the risk of hospital‐associated infections and other complications.
Parvovirus infects crypt cells from the intestinal villi decreasing absorption and increasing permeability.17 Currently, the mechanism of how FMT resolves clinical sign in dogs with virally‐mediated diarrhea is uncertain. It can be hypothesized that diarrhea is perpetuated by bacterial dysbiosis caused by inflammation, hypersecretion, and hypermotility and changes in osmolality and pH.18 Although scientific evidence about FMT is scarce, response to the procedure is likely related to reconstitution of the intestinal microbiota and its corresponding metabolites.6, 7 Evaluation of the microbiota has been performed in a small number of FMT studies in humans and in 1 study in puppies,14 but was not done in our study. Further evaluation of the mechanism of FMT response in CPV or other conditions would improve the understanding of the pathophysiology of these diseases and perhaps lead to refinements in FMT to provide a more specific, standardized approach.
Mortality rates in puppies with parvovirus infection can reach 36% in dogs receiving standard treatment and up to 91% in untreated animals.19 In our study, both STD and STD + FMT groups responded well to standard treatment, but there were almost twice as many deaths in the STD group. Although this result did not achieve statistical significance, it indicates the potential benefits of adding this easy, low cost, and practical treatment.
It was not possible to fulfill all of the clinical trials standard guidelines (eg, blinding, placebo treatment) in our study. Animals assigned to the STD + FMT group were slightly older than controls. Although this could have an impact on their capacity for recovery, it is unlikely that this difference (1.5 months on average) could be responsible for the differences observed in diarrhea resolution between the groups. Further studies evaluating larger and less diverse populations are necessary.
It is important to emphasize that FMT alone was not assessed, and the importance of standard supportive care for the treatment of CPV or other acute diarrheic diseases in puppies must not be overlooked.
5. CONCLUSIONS
Fecal microbiota transplantation was associated with more rapid clinical recovery and decreased time of hospitalization in survivor puppies with acute hemorrhagic diarrhea caused by CPV.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supplemental Table 1
Pereira GQ, Gomes LA, Santos IS, Alfieri AF, Weese JS, Costa MC. Fecal microbiota transplantation in puppies with canine parvovirus infection. J Vet Intern Med. 2018;32:707–711. https://doi.org/10.1111/jvim.15072
This study was performed at “Universidade Estadual de Londrina” and “Universidade Philadelphia”, Londrina, Paraná state, Brazil. There was no support from funding agencies or private companies. Results were presented as a research abstract at the 2016 ANCLIVEPA Congress, Goiania, Brazil.
REFERENCES
- 1. Pinto LD, Streck AF, Goncalves KR, et al. Typing of canine parvovirus strains circulating in Brazil between 2008 and 2010. Virus Res. 2012;165:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Spibey N, Greenwood NM, Sutton D, et al. Canine parvovirus type 2 vaccine protects against virulent challenge with type 2c virus. Vet Microbiol. 2008;128:48–55. [DOI] [PubMed] [Google Scholar]
- 3. Suchodolski JS. Diagnosis and interpretation of intestinal dysbiosis in dogs and cats. Vet J. 2016;215:30–37. [DOI] [PubMed] [Google Scholar]
- 4. Karst SM. The influence of commensal bacteria on infection with enteric viruses. Nat Rev Microbiol. 2016;14:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sunvold GD, Fahey GCJ, Merchen NR, et al. Dietary fiber for dogs. IV. In vitro fermentation of selected fiber sources by dog fecal inoculum and in vivo digestion and metabolism of fiber‐supplemented diets. J Anim Sci. 1995;73:1099–1109. [DOI] [PubMed] [Google Scholar]
- 6. Allenspach K, House A, Smith K, et al. Evaluation of mucosal bacteria and histopathology, clinical disease activity and expression of Toll‐like receptors in German shepherd dogs with chronic enteropathies. Vet Microbiol. 2010;146:326–335. [DOI] [PubMed] [Google Scholar]
- 7. Honneffer JB, Minamoto Y, Suchodolski JS. Microbiota alterations in acute and chronic gastrointestinal inflammation of cats and dogs. WJG. 2014;20:16489–16497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Minamoto Y, Dhanani N, Markel ME, et al. Prevalence of Clostridium perfringens, Clostridium perfringens enterotoxin and dysbiosis in fecal samples of dogs with diarrhea. Vet Microbiol. 2014;174:463–473. [DOI] [PubMed] [Google Scholar]
- 9. Jalanka J, Mattila E, Jouhten H, et al. Long‐term effects on luminal and mucosal microbiota and commonly acquired taxa in faecal microbiota transplantation for recurrent Clostridium difficile infection. BMC Med. 2016;14:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khanna S, Vazquez‐Baeza Y, Gonzalez A, et al. Changes in microbial ecology after fecal microbiota transplantation for recurrent C. difficile infection affected by underlying inflammatory bowel disease. Microbiome. 2017;5:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weingarden A, Gonzalez A, Vazquez‐Baeza Y, et al. Dynamic changes in short‐ and long‐term bacterial composition following fecal microbiota transplantation for recurrent Clostridium difficile infection. Microbiome. 2015;3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53:994–1002. [DOI] [PubMed] [Google Scholar]
- 13. Chaitman J, Jergens AE, Gaschen F, et al. Commentary on key aspects of fecal microbiota transplantation in small animal practice. Vet Med Res Rep. 2016;7:71–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burton EN, O'Connor E, Ericsson AC, Franklin CL. Evaluation of fecal microbiota transfer as treatment for postweaning diarrhea in research‐colony puppies. J Am Assoc Lab Anim Sci. 2016;55:582–587. [PMC free article] [PubMed] [Google Scholar]
- 15. Weese JS, Costa MC, Webb JA. Preliminary Clinical and microbiome assessment of stool transplantation in the dog and cat. Proceedings of the 2013 ACVIM Forum. J Vet Intern Med. 2013;27:705. [Google Scholar]
- 16. Hong C, Decaro N, Desario C, et al. Occurrence of canine parvovirus type 2c in the United States. J Vet Diagn Invest. 2007;19:535–539. [DOI] [PubMed] [Google Scholar]
- 17. Mccaw DL, Hoskins JD. Canine viral enteritis In: Greene CE, ed. Infectious Diseases of the Dog and Cat. 3rd ed. St Louis: Saunders Elsevier; 2006:63–70. [Google Scholar]
- 18. Heilmann RM, Guard MM, Steiner JM, et al. Fecal markers of inflammation, protein loss, and microbial changes in dogs with the acute hemorrhagic diarrhea syndrome (AHDS). J Vet Emerg Crit Care (San Antonio). 2017;27:586–589. [DOI] [PubMed] [Google Scholar]
- 19. Otto CM, Drobatz KJ, Soter C. Endotoxemia and tumor necrosis factor activity in dogs with naturally occurring parvoviral enteritis. J Vet Intern Med. 1997;11:65–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supplemental Table 1


