Abstract
Background
Clinical signs of urinary tract disease in dogs often lead to prescription of antibiotics. Appropriate diagnostic work‐up could optimize treatment and reduce the risk of inappropriate use of antibiotics.
Hypothesis/Objectives
To describe and evaluate the impact of diagnostic work‐up on decision to treat (DTT) and choice of antibiotic treatment (COT) for dogs presenting with clinical signs of urinary tract disease.
Animals
One hundred and fifty‐one dogs presenting to 52 Danish veterinary practices.
Methods
Prospective, observational study. Clinical signs, diagnostic work‐up, and prescriptions were recorded. Urine samples were submitted to a reference laboratory for quantitative bacterial culture (QBC) and susceptibility testing. The laboratory results were used as reference for assessing the appropriateness of DTT and COT.
Results
In the majority of dogs, veterinarians performed dipstick (99%), microscopic examination of urine (80%) and bacterial culture (56%). Fifty‐one percent of dogs had urinary tract infection (UTI) based on reference QBC. Appropriate DTT was made for 62% of the dogs, while 36% were over‐prescribed and 2% under‐prescribed. Inappropriate use of second‐line agents was found in 57% of the UTI cases. Performing microscopy—but not culture—significantly impacted DTT (P = 0.039) while no difference was seen in COT (P = 0.67). The accuracy of in‐house microscopy and culture were 64.5 and 77%, respectively.
Conclusions and Clinical Importance: Over‐prescription of antibiotics was common among dogs with suspected UTI, regardless of the diagnostic work‐up performed. Test inaccuracy under practice conditions and incoherence between diagnostic test results and decision‐making both explained inappropriate and unnecessary use of antibiotics.
Keywords: Antibiotic prescription, Bacterial culture, Dog, Microscopy, Primary practice, Urinary tract infection
Abbreviations
- AST
antibiotic susceptibility testing
- CFU
colony‐forming units
- COT
choice of treatment
- DTT
decision to treat with antibiotics
- IQR
interquartile range
- QBC
quantitative bacterial culture
- TMS
potentiated sulfonamides
- UTI
urinary tract infection
Antimicrobial resistance is of increasing concern in both human and veterinary medicine, causing an increased focus on antibiotic stewardship.1 An important goal of antibiotic stewardship is to decrease inappropriate prescription of antibiotics.2, 3 Retrospective studies on patterns of antibiotic use in companion animals show that in the majority of cases prescription of antibiotics is not preceded by proper diagnostic testing, potentially leading to over‐treatment with antibiotics.4, 5 In human medicine, 90% of antibiotics are prescribed in primary healthcare.6, 7 and probably the same applies for primary companion animal practice. Genitourinary tract infections are the second to third most common reason for antimicrobial use in dogs, representing approximately 12% of the total prescriptions of oral antibiotics.8, 9 Dogs with a tentative diagnosis of urinary tract infection (UTI) are frequently treated with antibiotics.10 However, clinical signs of lower urinary tract disease are unspecific, and diagnostic testing is necessary to correctly identify dogs with actual infection.11 Some studies have investigated the accuracy of diagnostic tools for diagnosing UTI,12, 13, 14, 15, 16 but few have validated their use in primary veterinary practice,12 and none have investigated the impact of diagnostics on clinical decision‐making.
The aim of this study was to describe current veterinary management of suspected UTI in dogs, and to investigate the impact of different diagnostic work‐up strategies on prescription of antibiotics among veterinary practitioners in Denmark.
We hypothesized (i) that performing microscopy or bacterial culture as part of the diagnostic work‐up for UTI improves the proportion of appropriate decisions to treat dogs with antibiotics, and (ii) that performing culture with antimicrobial susceptibility testing (AST) leads to an appropriate choice of antibiotic.
Materials and Methods
Design and Setting
The study was designed as a prospective multicenter observational cohort study in Danish veterinary practices. A thorough description of the protocol was published earlier17 and the study is reported according to STROBE‐Vet guidelines.18 During 2014, 241 primary practices covering all 5 Danish regions were invited to participate through meetings, by email or telephone. Practices participated voluntarily with a small financial compensation for each dog enrolled. Basic information concerning the practices was collected at recruitment (number of veterinarians, proportion of companion animal patients, and availability of diagnostic tests).
The study was approved by the Ethical Administrative Committee at the University Hospital of Companion Animals (case number 2014–8). Informed written consent was obtained from all dog owners before participating in the study.
Data Collection
Dogs were recruited between May 2014 and November 2016. On the day of consultation, dogs' signalment, clinical history, diagnostic procedures performed, results thereof, tentative diagnosis, and prescribed treatments were registered on a standardized recording sheet. The tentative diagnosis was categorized as (i) not a UTI, (ii) uncomplicated UTI, or (iii) complicated UTI, defined by the presence of an underlying structural, neurological, or functional abnormality.
Follow‐up on all patients was carried out by the primary investigator (Sørensen) by telephone or email to collect missing registrations, compare prescriptions with the local electronic patient record and obtain information about changes in the prescribed treatment after the consultation. If in‐house culture was performed, the result of the culture was also retrieved (positive or negative growth).
Owners were asked to fill out a diary reporting the presence of clinical signs and administration of treatment during the 10 days after the consultation. Both recording sheets and client diaries were posted to the primary investigator when completed.
Study Population
Dogs of any age, sex, and breed, presenting with one or more clinical or paraclinical signs of urinary tract disease (dysuria, pollakiuria, stranguria, hematuria, or malodorous urine) were eligible to participate in the study. Exclusion criteria were (i) presence of severe systemic illness or chronic disease (eg, diabetes mellitus or hyperadrenocorticism), (ii) antibiotic treatment within 3 weeks before the consultation, (iii) inability to collect a urine sample for reference culture, and (iv) previous participation in the study.
Urine Sampling and Reference Culture
The method for urine collection was chosen by the veterinarians. After in‐house diagnostic procedures, excess urine was sent by the postal service to the reference laboratory (SUND Vet Diagnostik, University of Copenhagen, Denmark) for quantitative bacterial culture (QBC) and AST, according to the Clinical and Laboratory Standards Institute, as previously reported.17, 19 Samples collected by cystocentesis were transported unpreserved in sterile silicone‐coated clot tubes,1 while samples collected by catheter or voiding were transported in boric acid‐preserved urine transportation tubes.2 Dogs were defined as having UTI if reference QBC reached the currently accepted thresholds for clinically relevant11, 20: ≥1,000 colony‐forming units (CFU)/mL for cystocentesis samples, ≥10,000 CFU/mL for male catheter samples, and ≥100,000 CFU/mL for female catheter samples as well as for all voided samples. If swarming of Proteus sp. on agar plates made quantification impossible, growth was regarded as clinically important. Results of reference QBC were not available for the participating veterinarians during the study period, but were used as the reference standard for the study. Laboratory technicians were blinded to the collection method, diagnostic results, and diagnosis made by the primary veterinarians.
Outcomes
The primary outcomes were appropriate decision to treat (DTT) and appropriate choice of antibiotic treatment (COT). The first treatment decision (first DTT) refers to the decision made during the consultation. The final treatment decision (final DTT) refers to the decision made in the days after the consultation. Appropriate DTT was defined as prescribing antibiotics in case of clinically relevant bacteriuria and not prescribing antibiotics in case of sterile or growth below clinical relevance on reference QBC. Appropriate COT was defined as (i) prescribing antibiotics to which the pathogen was in vitro susceptible (according to reference QBC), and (ii) choosing recommended first‐line agents over second‐line agents. The recommended first‐line agents in Denmark are amoxicillin or potentiated sulfonamides (TMS).21
Secondary outcomes were accuracy of the in‐house diagnostic tests, clinical cure rates on Day 4 after consultation, and number of days to clinical cure.
Study Size
A sample size calculation was performed with SAS Enterprise Guide v6.13 based on the assumptions: (i) in‐house point‐of‐care diagnostic tools (ie, urine dipstick, microscopy, or both) were exclusively used in 80% of the cases, (ii) an appropriate DTT was made for 55% of the dogs without culture performed, and (iii) appropriate DTT was made for 70% of the dogs in which culture was performed. Intra‐class correlation of 0.2, α = 0.05 and β = 0.20. Based on those assumptions, a total of 800 dogs from 100 veterinary practices were required.
Data Management and Statistical Analysis
Descriptive statistics were reported as proportions, mean (±SD), or median (interquartile range [IQR]). The statistical analysis comparing the impact of microscopy or bacterial culture on DTT was performed in 2 steps. First, possible predictors for the use of microscopy and culture were investigated individually in 2 multilevel logistic regression models with practices as a random effect. A significance level of 10% (P < 0.1) was applied for evaluation of possible predictors in order not to exclude potential confounders. The tested predictors were: (i) presenting clinical signs and duration thereof, (ii) information on previous diagnostic test results, and (iii) availability of diagnostic tools (Supporting information Table S1).
Second (main analysis), the likelihood ratio test statistics were applied to identify the best‐fitting logistic regression model for the diagnostic work‐up prediction of DTT, adjusted for the significant predictors from the first model. Hypothesized causal structure among variables is shown in Figure 1.
Figure 1.
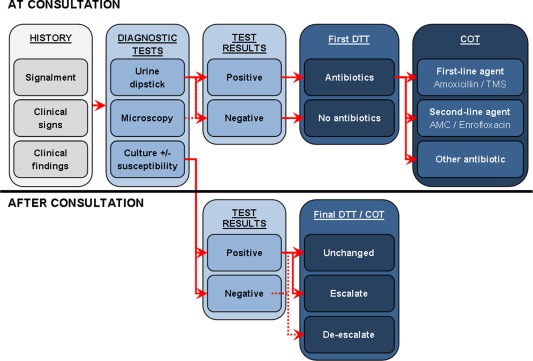
Hypothesized causal structure among variables relevant for prescription decisions. Optimal causal structure among variables leading to different prescription decisions depending on availability of test results at consultation or after consultation. Escalation is defined as decision change from no antibiotic to antibiotic treatment. De‐escalation is defined as decision change from antibiotic to no antibiotic. AMC, amoxicillin with clavulanate potassium; COT, choice of treatment; DTT, decision to treat with antibiotics; TMS, potentiated sulphonamides.
Sensitivity, specificity, predictive values, and likelihood ratios were calculated for microscopic and culture test results obtained in‐house compared to the reference QBC. The chi‐square test was applied to test difference in clinical cure rate proportions between groups.
An alpha level of 0.05 was used for statistical significance in the main analysis, and 2‐sided 95% confidence levels were calculated where appropriate. All analysis was performed in RStudio v1.1.1384 by the lme4 package for regression analysis.22
Results
Participating Practices
A total of 96 practices were enrolled, of which 52 actively participated in the study. Of these, 15 were located in the Capital Region of Denmark, 8 in Region Zealand, 5 in the Region of Northern Denmark, 13 in the Region of Central Denmark, and 11 in the Region of Southern Denmark. The majority of practices were medium‐sized with 2–4 veterinarians employed (n = 41), and most were pure companion animal practices (n = 45). The diagnostic tools available for veterinarians are shown in Table 1.
Table 1.
Diagnostic tools available in the 52 participating Danish veterinary practices.
| Diagnostic Tool | Number of Practices | Proportion (%) |
|---|---|---|
| Urine dipstick | 52 | 100 |
| Refractometer | 51 | 98 |
| Light microscope | 52 | 100 |
| Sediment staining | 38 | 73 |
| Bacterial culture in‐house | 36 | 69 |
| Susceptibility testing in‐house | 30 | 58 |
| Blood agar | 21 | 59a |
| Uricult/Uricult Trio | 9 | 25a |
| Mueller‐Hinton agar | 6 | 17a |
| FlexicultVet | 17 | 47a |
| Neo‐Sensitabs | 23 | 64a |
Uricult or Uricult Trio, Orion Diagnostica Oy, Finland/Denmark; Flexicult Vet, SSI Diagnostica A/S, Hillerød, Denmark; Neo‐Sensitabs, Rosco Diagnostica A/S, Taastrup, Denmark.
Proportion of practices among those with culture available (n = 36).
Availability of different diagnostic tools in the participating veterinary practices that included dogs presenting with clinical signs of urinary tract disease.
Participating Dogs
A total of 181 dogs were enrolled during the study period. Figure 2 shows the number of eligible dogs enrolled, reasons for exclusion from analysis, and diagnostic work‐up pursued by the veterinarians. The 151 dogs included in the final analysis represented 58 different breeds, with Labrador retrievers (n = 18), mixed‐breed dogs (n = 16), and Cocker Spaniels (n = 10) being most prevalent. The study included 110 female dogs (72.8%) of which 42 were neutered, 40 male dogs (26.5%) of which 6 were neutered, and 1 dog of unknown sex (0.6%). The dogs had a mean age of 6.0 years (SD ± 4.0), and a mean weight of 21.7 kg (SD ± 13.9, n = 141).
Figure 2.
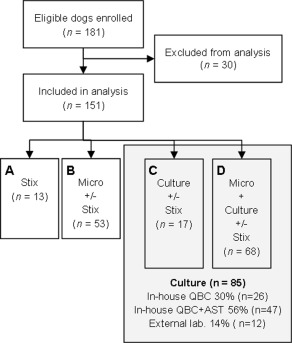
Flowchart of enrolled dogs with suspected urinary tract infection. Flowchart showing number of enrolled dogs with clinical signs of urinary tract disease, dogs excluded from analysis, and diagnostic work‐up pursued by the veterinarians in Danish veterinary practices. Reasons for exclusion were: (i) no recording sheet received (n = 7), (ii) no reference urine sample received at the reference laboratory (n = 2), (iii) no inclusion criteria present at consultation (n = 14), (iv) former participation in the study (n = 2), (v) urine transport time to laboratory > 6 days (n = 4), and (vi) antibiotic treatment before the consultation (n = 1). AST, antimicrobial susceptibility testing; lab, laboratory; Micro, microscopy; QBC, quantitative bacterial culture; Stix, urine dipstick analysis.
Clinical signs had been present for a median of 3.3 days (IQR 2;7, n = 142), and pollakiuria was the most commonly reported clinical sign. The clinical presentation and registrations at consultation are shown in Table 2.
Table 2.
Clinical signs and findings registered at consultation.
| All Dogs (n = 151) (%) | |
|---|---|
| Clinical presentation | |
| Dysuria | 32 (21.2) |
| Pollakiuria | 120 (79.5) |
| Hematuria | 75 (49.7) |
| Stranguria | 23 (15.2) |
| Malodorous urine | 2 (1.3) |
| Periuria | 74 (49.0) |
| Licking outer genitalia | 37 (24.5) |
| Incontinence | 26 (17.2) |
| Lethargic | 19 (12.6) |
| Painful back or abdomen | 19 (12.6) |
| Hyporexia/anorexia | 11 (7.3) |
| Increased temperature (>39.3°C) | 6 (4.0) |
| Urine collection method | |
| Cystocentesis | 27 (17.9) |
| Catheter | 16 (10.6) |
| Voided | 108 (71.5) |
| Tentative diagnosis | |
| Uncomplicated UTI | 113 (74.8) |
| Complicated UTI | 19 (12.6) |
| No UTI | 17 (11.3) |
| No diagnosis | 2 (1.3) |
UTI, urinary tract infection; QBC, quantitative bacterial culture (reference); IQR, interquartile range.
Registered presenting clinical signs and clinical findings at consultation of dogs included in the analysis. Numbers are n (%).
Reference QBC
Clinically relevant bacteriuria (ie, UTI) was detected in 51% of the cases (77/151) with 95% (73/77) being monocultures. Table 3 shows the distribution of the 81 bacterial isolates. Thirteen (10/77) and six (5/77) percent of isolates were resistant to amoxicillin and TMS, respectively. Only 3 of the 42 Escherichia coli isolates (7%) displayed resistance to a first‐line antibiotic (amoxicillin [n = 1], TMS [n = 1], or both [n = 1]). The median transportation time to the reference laboratory was 2 days (IQR 1;4, range 0–6 days).
Table 3.
Bacterial species isolated (n = 81) from 77 urine samples obtained from dogs with confirmed UTI.
| Uropathogens | ||
|---|---|---|
| Bacterial Genus/Species | Dominating Number (%) n = 77 | Secondary Number n = 4 |
| Escherichia coli | 42 (55) | 1 |
| Proteus mirabilis | 15 (20) | |
| Staphylococcus pseudintermedius | 11 (14) | |
| Other Staph. sp. | 2 (3) | 1 |
| Streptococcus canis | 1 | |
| Other Strep. sp. | 2 (3) | |
| Enterococcus faecalis and E. faecium | 1 (1) | 1 |
| Klebsiella oxytoca | 1 (1) | |
| Acinetobacter sp. | 2 (3) | |
| Others | 1 (1) | |
Diagnostic Work‐up
Urine dipstick analysis was performed in all but 2 cases (149/151), and overall 80.1% (121/151) included microscopic examination of urine and 56% (85/151) included culture in the diagnostic work‐up. Diagnostic work‐up pursued by the veterinarians is shown in Figure 2.
Identification of predictors for performing in‐house microscopy or culture are shown in Supporting information Table S1. Possible predictors for performing microscopy with a 10% level of significance were the number of days with clinical signs before consultation (OR 1.13 [1.01;1.26], P = 0.027), pollakiuria (OR 3.75 [0.94;14.97], P = 0.061), incontinence (OR 0.25 [0.06;1.13], P = 0.072), licking of the outer genitalia (OR 4.90 [0.98;24.60], P = 0.053), and a temperature above 39.3°C (OR 0.06 [0.003;1.38], P = 0.078). Significant predictors for performing culture were pollakiuria (OR 3.31 [0.98;11.22], P = 0.053), and licking of the outer genitalia (OR 3.03 [0.87;10.48], P = 0.080). The result of sediment analysis (absence or presence of bacteria, pyuria, or both) did not predict the use of culture.
Prescribed Treatment
Seventy‐nine percent (119/151) of dogs were empirically treated with antibiotics on the day of consultation (first DTT), while a total of 85% (129/151) were treated after the consultation (final DTT). Treatment was changed for 32 dogs, of which 88% (28/32) were escalated from no antibiotics to antibiotic treatment, or from a first‐line to a second‐line agent, while for 13% of the dogs (4/32) the treatment was de‐escalated. The reasons for changing treatment strategy were: (i) re‐evaluation after obtaining culture results in 59% (19/32), (ii) a change from injection therapy on the day of consultation to oral therapy with a different antibiotic class in 25% (8/32), and (iii) persistent clinical signs in 3% (1/32). In 13% (4/32) a reason for the change was not recorded. Prescribed median doses were: (i) 11 (range 5–47) mg/kg twice daily of amoxicillin (19 dogs), (ii) 13 (range 6–30) mg/kg twice daily of amoxicillin and clavulanate potassium (79 dogs), (iii) 20 (range 12–32) mg/kg twice daily of TMS (7 dogs) and (iv) 4 (range 1–8) mg/kg once daily of enrofloxacin (6 dogs). The median duration of antibiotic therapy was 10 days (range 2–30 days) among the 125 dogs for which this variable was recorded. Twenty‐eight dogs (18.5%, 28/151) were treated with nonsteroidal anti‐inflammatory drugs (NSAID) alone or in combination with antibiotics.
Appropriateness of Clinical Decision‐Making
Overall, appropriate final DTT occurred in 61.6% of cases (93/151) with over‐prescription of antibiotics occurring in 36% (55/151) and under‐prescription of antibiotics occurring in 2% (3/151) of the dogs. Of the 77 confirmed UTI cases, 96% (74/77) were appropriately treated with antibiotics, while 4% (3/77) were undertreated. Of the 74 cases without UTI, 26% (19/74) were not prescribed antibiotics (appropriate DTT) and 74% (55/74) were prescribed antibiotics (inappropriate DTT).
The logistic regression model with all 4 diagnostic work‐up groups pursued (Fig 2) was tested against a model without interaction between performing microscopy and culture. The likelihood ratio statistics showed no significant difference between the models (Chi‐square = 1.14, df = 1, P = 0.28). The interaction was therefore removed from the final model.
Proportions of appropriate final DTT, over‐treatment, and under‐treatment in relation to diagnostic procedures are shown in Figure 3. The adjusted analysis shows a significant increase in the likelihood of appropriate DTT when microscopy was performed (OR 2.57, P = 0.039), while no effect was found for performance of culture (OR 1.20, P = 0.62) (Table 4).
Figure 3.
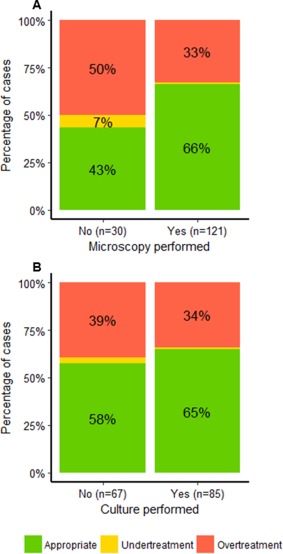
Proportion of different final decisions to treat (DTT) when performing microscopy (A) or culture (B). Decision to treat in dogs suspected of urinary tract infection in Danish private veterinary practices. Appropriate final DTT was: 1) prescribing antibiotics in the presence of clinically relevant bacteriuria on reference quantitative bacterial culture (QBC) or 2) not prescribing antibiotics in the absence of clinically relevant bacteriuria on reference QBC. Under‐treatment was not prescribing antibiotics when clinically relevant bacteriuria was found on reference QBC. Over‐treatment was prescribing antibiotics when sterile or clinically unimportant bacteriuria was found on reference QBC. (A) Proportions in cases including microscopy in the diagnostic work‐up (n = 121) compared to cases without microscopy included (n = 30). (B) Proportions in cases including bacterial culture in the diagnostic work‐up (n = 85) compared to cases without culture included (n = 67).
Table 4.
The association of the diagnostic work‐up with the appropriateness of the final treatment decision.
| Unadjusted (n = 151) | Adjusted (n = 144) | |||||
|---|---|---|---|---|---|---|
| Model | OR | 95% CI | P | OR | 95% CI | P |
| No microscopy (n = 30) | 1.00 | 1.00 | ||||
| Microscopy (n = 121) | 2.57 | 1.14–5.82 | 0.023 | 2.57 | 1.05–6.32 | 0.039 |
| No Culture (n = 66) | 1.00 | 1.00 | ||||
| Culture ± susceptibility (n = 85) | 1.32 | 0.67–2.58 | 0.42 | 1.20 | 0.58–2.51 | 0.62 |
CI, confidence interval; OR, estimated odds ratio.
Odds Ratios (OR) for making an appropriate final treatment decision (DTT) when performing microscopy or culture jointly estimated from a multivariable logistic regression model with practice as a random effect. The ORs are estimated unadjusted and adjusted for days with clinical signs, pollakiuria, increased temperature, incontinence, and licking of outer genitalia.
For the 77 dogs with confirmed UTI on reference culture, an appropriate COT was made in 36% of the cases (28/77). Inappropriate second‐line agents were prescribed in 57% of cases (44/77), and prescription of first‐line agents to which reference culture showed in vitro resistance were prescribed in 6% (5/77) of the cases (under‐treatment). Diagnostic work‐up included in‐house AST of isolates from 39% of the dogs with UTI (30/77). As shown in Figure 4, no significant difference in the proportion of appropriate COT could be demonstrated between dogs with and without AST performed (OR= 0.77 [0.23:2.60], P = 0.67).
Figure 4.
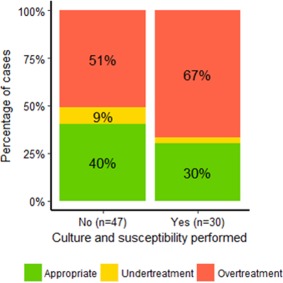
Proportions of different choices of treatment (COT) when performing susceptibility testing. Choice of antibiotic treatment in 77 dogs with confirmed urinary tract infections (UTI) from private veterinary practices in Denmark. Appropriate COT was: (i) prescribing antibiotics to which the pathogen was in vitro susceptible (according to reference QBC), and (ii) choosing recommended first‐line agents over second‐line agents. Recommended first‐line agents in Denmark are amoxicillin or potentiated sulphonamides. Proportions in cases including culture and susceptibility testing in the diagnostic work‐up (n = 30) compared to cases without susceptibility testing included (n = 47), P = 0.67.
Accuracy of Diagnostic Tests Performed In‐House
When comparing results from diagnostic tests performed in‐house to the reference QBC, the overall accuracy of microscopic bacteriuria was 64.5%, and the accuracy of bacterial culture (growth or no growth) was 77%. Sensitivity was high as only 1 negative in‐house culture showed significant growth on reference QBC. However, a high proportion of positive in‐house cultures were observed despite negative reference QBC (13 discordant results). Thirty‐one positive and 17 negative in‐house cultures were in accordance with the reference QBC. Sensitivities and specificities are shown in Table 5.
Table 5.
In‐house diagnostic tests performance compared to reference quantitative bacterial culture at Sund Vet Diagnostik.
| Microscopy (n = 121) | Culture (n = 62) | |||
|---|---|---|---|---|
| Bacteriuria | Pyuria | Bacteriuria, pyuria, or both | Growth/no growth | |
| Sensitivity |
0.70 (47/(47 + 20)) [0.58;0.67] |
0.61 (41/(41 + 26)) [0.49;0.73] |
0.84 (56/(56 + 11)) [0.73;0.92] |
0.97 (31/(31 + 1)) [0.84;1.00] |
| Specificity |
0.57 (31/(31 + 23)) [0.43;0.71] |
0.48 (26/(26 + 28)) [0.34;0.62] |
0.39 (21/(21 + 33)) [0.26;0.53] |
0.57 (17/(17 + 13)) [0.37;0.75] |
| LR+ | 1.65 [1.16;2.33] | 1.18 [0.86;1.63] | 1.37 [1.08; 1.73] | 2.24 [1.48;3.38] |
| LR− | 0.52 [0.34;0.80] | 0.81 [0.54;1.21] | 0.42 [0.22;0.80] | 0.06 [0.01;0.39] |
| Accuracy | 64.5% | 55.4% | 63.6% | 77% |
LR+/LR−, positive and negative likelihood ratio; QBC, quantitative bacterial culture.
Calculated sensitivity, specificity, likelihood ratios and overall accuracy of in‐house diagnostic results from veterinary practice compared to reference QBC. Brackets are proportions and 95% confidence intervals.
Coherence between In‐House Test Results and Decision‐Making
Test results on in‐house microscopy, culture, or both were registered in 129 dogs. Veterinarians treated 20.9% (27/129) of dogs despite no microscopic evidence of bacteriuria or a negative culture in‐house (over‐treatment), while 2.3% (3/129) were under‐treated, leaving 76.7% (99/129) that were treated in coherence with in‐house test results. According to reference QBC, 36.4% (55/151) dogs were over‐treated. Forty‐six cases thereof had in‐house test results registered, 37% (17/46) with no evidence of bacteriuria or positive cultures and thus treated incoherent to in‐house test results. Table 6 illustrates the treatment decisions made for the 62 dogs for which in‐house culture results were registered.
Table 6.
Treatment decisions made for 62 dogs with in‐house culture results registered.
| First DTT | In‐House Culture Result | Final DTT | Coherence |
|---|---|---|---|
|
Empiric antibiotics prescribed (n = 43) |
Positive (n = 32) |
Unchanged (n = 32) |
100% (32/32) |
|
Negative (n = 11) |
Unchanged (n = 8) |
27% (3/11) | |
|
De‐escalate (n = 3) | |||
|
No antibiotics prescribed (n = 19) |
Positive (n = 12) |
Escalate (n = 12) |
100% (12/12) |
|
Negative (n = 7) |
Unchanged (n = 7) |
100% (7/7) |
Treatment decisions made by veterinarians during consultation first treatment decision (first DTT), their in‐house culture results and the final treatment decision (final DTT) made after culture results were available. Escalation is defined as decision change from no antibiotic to antibiotic treatment. De‐escalation is defined as decision change from antibiotic to no antibiotic. Coherence is the proportion of final treatment decisions after the result of in‐house culture results: (i) prescription of antibiotics to dogs with positive culture results and (ii) no antibiotics prescribed to dogs with negative culture results.
Clinical Cure
One hundred and one owners returned the diary. Fifty‐three percent (54/101) of these had confirmed UTI on reference QBC. Overall, clinical cure rate 4 days after the consultation was 69% (65/94). Median time from consultation to clinical cure was 2 days (IQR 0;4) for both UTI cases (IQR 0;6) and non‐UTI cases (IQR 0;4). The overall rate of treatment failure (clinical signs still present 10 days after the consultation) was 7% (7/94).
Discussion
Only half of the dogs with clinical signs of lower urinary tract disease had confirmed UTI on reference QBC, and a high frequency of over‐prescription of antibiotics was observed in this study. Performing microscopy impacted the proportion of appropriate final DTT, while no impact could be demonstrated for culture. However, the proportion of inappropriate antibiotic treatment in dogs was high regardless of the diagnostic work‐up pursued. Including AST in the diagnostic work‐up did not influence more appropriate COT in the dogs with confirmed UTI on reference QBC, as the empiric prescription of unnecessary second‐line agents was high in both groups.
The primary strength of the study is that it was conducted in primary practice and was prospective in design, contrary to previous publications on prescription habits.4, 5 The study includes the entire decision‐making process from choice of diagnostic work‐up to treatment decisions, and thus contributes valuable information on current strategies for diagnosing and treating UTI in dogs in practice settings.
The veterinarians who participated in the study did so voluntarily and so could be more interested in quality improvement and in reducing inappropriate prescriptions than colleagues who did not volunteer, as is known from the human literature.23, 24 It could therefore be argued that our results are conservative and might underestimate the true proportion of over‐treatment.
A major challenge related to the multi‐centric research setting of this study was the transport of samples for reference QBC. Despite ordering next‐day delivery service, 65 (43.0%) samples had a postal delivery time of more than 2 days. The consequences of this are unknown, but delayed delivery might have resulted in both false positives and false negative culture results as previously shown.25, 26 The average transportation time was, however, similar across all groups (data not shown).
Surprisingly, only a few dogs were diagnosed without microscopy, which resulted in very few dogs in some diagnostic groups and induced a risk of type II error in the study with regard to DTT. Initial power analysis was calculated based on estimated proportions, as no previous studies were available. The actual difference in proportions observed in this study was greater than initially estimated and a higher proportion of dogs had culture performed as part of the diagnostic work‐up.
The veterinarians expect high prevalence of UTI in the presence of clinical signs, as 87.4% had a tentative diagnosis of UTI at consultation. However, the UTI prevalence of approximately 50% found in this study is comparable to other studies reporting prevalence of 38–65% in dogs with clinical signs.20, 27, 28, 29 In our study, over‐treatment of dogs without bacterial UTI accounted for the majority of inappropriate decisions. One study reported that confirmation of infection was sought in only 17.5% of antibiotic‐treated dogs, and that 38.4% had no documentation of infection at all.4 Interestingly, we found 35% over‐treatment despite performance of in‐house culture, showing that decision‐making is not merely a matter of performing diagnostic tests: other factors such as in‐house test accuracy, timing of treatment, and risk‐avoidance attitudes are of importance.
The validity of microscopy in our study, with an overall accuracy of up to 64.5%, was lower than the 97–98% previously reported.14, 15, 16, 29 However, in former studies microscopy was interpreted by experienced technicians or diplomats at referral hospitals, and not by veterinary nurses and veterinarians under busy daily conditions. The accuracy of in‐house bacterial culture in this study was also lower than previously reported with respect to growth or no growth.12, 13, 30 At least 2 factors might cause this difference: (i) urine collection method and (ii) transportation time to the reference laboratory. Cystocentesis was the main collection method in the previous studies, in contrast to our study in which the majority of samples were collected by voluntary voiding. False positive microscopy and culture results are more likely when voided urine samples are used, as it is especially important to quantify growth in voided samples to avoid misinterpreting contaminating growth. It was recently demonstrated that 24% (23/94) of voided canine urine samples had contaminating growth,20 even with immediate culture. Transportation time to the reference laboratory was longer than expected in a large proportion of the samples in this study, and it cannot be excluded that in‐house culture results were more reliable in some of these cases. Therefore, the accuracy of in‐house testing in practice might be higher than reported by this study, as some false positive in‐house results could in fact represent false negative reference culture results. However, in our study, the accuracy of in‐house culture only improved slightly if samples with >48 hours of transport were disregarded (79% overall accuracy, data not shown), and test inaccuracy was not the only explanation for the over‐treatment of antibiotics in dogs without UTI.
A simple but important explanation of incoherence between culture results and decision‐making is the order of events. For example, most dogs were prescribed empiric antibiotics at the time of consultation, and de‐escalation of treatment after negative in‐house culture results was only seen in approximately one third of the negative cases (Table 6). In contrast, there was complete coherence between test results and the final treatment decision in the few cases (19) where antibiotic treatment was withheld pending the culture results. The importance of the order of test‐reading and initiation of treatment is emphasized by the fact that only microscopy—the only point‐of‐care test, but also the least accurate in‐house test method—impacted the proportion of appropriate final DTT. Strategic use of culture as a decision‐making tool can involve withholding antibiotic therapy until test results (growth/no growth) are available. This strategy is feasible when test results are available within 24 hours (in‐house testing) and when clinical signs are not severe, and might potentially reduce over‐prescription of antibiotics in culture‐negative dogs. Clinical decision‐making is a complex process for which several possible diagnostic and treatment biases exist. The fact that antibiotic treatment was prescribed, or continued, despite negative in‐house test results could indicate the presence of confirmation bias. This is a tendency to selectively gather and interpret evidence that confirms a suspected diagnosis and ignore evidence that might disconfirm it.31 Risk avoidance (ie, regret bias) is another possible explanation for the lack of adherence to test results, as the anticipated regret caused by adverse reactions to antibiotic treatment is less important than the regret of clinical worsening if treatment is not provided.31
The preferred empiric treatment among veterinarians was amoxicillin with clavulanate potassium as also reported by other studies,4, 5, 32. This is incoherent with the Danish national guidelines, which recommends the use of un‐potentiated amoxicillin or TMS.21 The majority of prescribed doses were in accordance with current guidelines. However, the duration of treatment was longer than recommended by the national Danish guidelines as well as international guidelines.11, 21 Uropathogen resistance to recommended first‐line antibiotics in our population was low, with only 5% of E. coli displaying in vitro resistance to ampicillin and 5% to TMS, making the use of clavulanate inappropriate in the majority of cases. Markedly higher levels have previously been published from Danish diagnostic laboratories reporting resistance to ampicillin and TMS in 20–22.2% and 19.2% of canine uropathogenic E. coli, respectively.33, 34 The results of our study suggest that susceptibility patterns from reference laboratories represent more complicated recurrent cases and might not be representative of the susceptibility patterns among pathogens commonly encountered in primary practice. In our study, AST did not lead to a more appropriate use of antibiotics. The in‐house AST results were not recorded, therefore it is unknown if inappropriate COT was caused by low accuracy of in‐house AST methods or incoherence with test results, but it was evident that a large proportion of AST tools available in the clinics were not validated for urine cultures in practice. It should be emphasized that even though AST testing did not impact the COT in our study, this is not necessarily applicable to other settings where resistance levels are markedly different. In settings with high levels of resistance toward common first‐line agents, local resistance surveillance and individual tailored treatment based on susceptibility reports is essential.35
Conclusion
Our study investigated the validity and impact of different diagnostic work‐ups on medical decision‐making for dogs suspected of UTI under daily practice conditions. Only half of the dogs had confirmed UTI, and our study demonstrated a high level of over‐prescription of antibiotics in dogs with clinical signs of urinary tract disease, regardless of the diagnostic work‐up performed. Test accuracy of culture and microscopy was lower than previously reported but was not the sole explanation for over‐prescription of antibiotics. The study revealed incoherence between decision‐making and diagnostic test results, as antibiotic treatment was often initiated before receiving culture test results and continued even in the event of negative culture. Unnecessary use of second‐line agents like amoxicillin with clavulanate potassium was common, and the use of susceptibility testing did not lead to de‐escalation. This highlights the need for multifaceted interventions to reduce inappropriate use of antibiotics in dogs with clinical signs of lower urinary tract disease. Interventions should include (i) education of practice personnel in use and interpretation of currently available diagnostic tests, (ii) introduction of treatment strategies involving withholding or de‐escalation of antibiotic treatment in response to test results, and (iii) promotion of first‐line agents for empiric treatment. Furthermore, novel non‐culture‐based point‐of‐care tests for improving diagnosis of UTI, and rational decision‐making in primary veterinary practice are greatly needed to reduce unnecessary prescription of antibiotics.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article:
Table S1. Predictors for performing microscopy and culture in dogs with suspected UTI (n=151).
Acknowledgments
The authors thank Kirsten M. Hoelmkjær, Cathrine Agger, Martina Ekström and Line Gaarsdahl for assistance in recruitment of practices and follow‐up on included dogs. We also thank all practices involved for their valuable contribution of data from primary veterinary practice. This study was funded by (i) University of Copenhagen Research Center for Control of Antibiotic Resistance (UC‐CARE, www.uc-care.ku.dk), (ii) Fondet for sygdomsbekæmpelse hos vore familiedyr, and (iii) Vetfond.
Conflict of Interest Declaration
Authors declare no conflict of interest.
Off‐Label Antimicrobial Declaration
Authors declare no off‐label use of antimicrobials.
Institutional Animal Care and Use Committee (IACUC) or Other Approval Declaration
Authors declare no IACUC or other approval was needed.
Private veterinary practices in Denmark and coordinated by University of Copenhagen. Preliminary results were presented at the 26th ECVIM‐CA Congress as oral abstracts ESVNU‐6 and ECVNU‐7 in September 2016.
Footnotes
BD Vacutainer Serum Tubes, #369032, BD Denmark, Lyngby, Denmark
BD Vacutainer Urine Tubes, #364959, BD Denmark, Lyngby, Denmark
Copyright © 2013 by SAS Institute Inc, Cary, NC
Copyright © 2009–2016 RStudio Inc, Boston, MA
References
- 1. Guardabassi L, Prescott JF. Antimicrobial stewardship in small animal veterinary practice: From theory to practice. Vet Clin North Am Small Anim Pract 2015;45:361–376. [DOI] [PubMed] [Google Scholar]
- 2. Davey P, Marwick CA, Scott CL, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2017;2:CD003543. [DOI] [PMC free article] [PubMed]
- 3. Kaki R, Elligsen M, Walker S, et al. Impact of antimicrobial stewardship in critical care: A systematic review. J Antimicrob Chemother 2011;66:1223–1230. [DOI] [PubMed] [Google Scholar]
- 4. Wayne A, McCarthy R, Lindenmayer J. Therapeutic antibiotic use patterns in dogs: Observations from a veterinary teaching hospital. J Small Anim Pract 2011;52:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weese JS. Investigation of antimicrobial use and the impact of antimicrobial use guidelines in a small animal veterinary teaching hospital: 1995–2004. J Am Vet Med Assoc 2006;228:553–558. [DOI] [PubMed] [Google Scholar]
- 6. Goossens H, Ferech M, Vander Stichele R, et al. Outpatient antibiotic use in Europe and association with resistance: A cross‐national database study. Lancet 365:579–587. [DOI] [PubMed] [Google Scholar]
- 7.DANMAP. Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. SSI/DTU‐Vet/DTU‐Food; 2015. 144 p. ISSN 1600‐2032.
- 8. Rantala M, Huovinen P, Holso K, et al. Survey of condition‐based prescribing of antimicrobial drugs for dogs at a veterinary teaching hospital. Vet Rec 2004;155:259–262. [DOI] [PubMed] [Google Scholar]
- 9. De Briyne N, Atkinson J, Pokludova L, et al. Antibiotics used most commonly to treat animals in Europe. Vet Rec 2014;175:325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Radford AD, Noble PJ, Coyne KP, et al. Antibacterial prescribing patterns in small animal veterinary practice identified via SAVSNET: The small animal veterinary surveillance network. Vet Rec 2011;169:310–318. [DOI] [PubMed] [Google Scholar]
- 11. Weese JS, Blondeau JM, Boothe D, et al. Antimicrobial use guidelines for treatment of urinary tract disease in dogs and cats: Antimicrobial guidelines working group of the international society for companion animal infectious diseases. Vet Med Int 2011;2011:263768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guardabassi L, Hedberg S, Jessen LR, et al. Optimization and evaluation of Flexicult((R)) Vet for detection, identification and antimicrobial susceptibility testing of bacterial uropathogens in small animal veterinary practice. Acta Vet Scand 2015;57:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ybarra WL, Sykes JE, Wang Y, et al. Performance of a veterinary urine dipstick paddle system for diagnosis and identification of urinary tract infections in dogs and cats. J Am Vet Med Assoc 2014;244:814–819. [DOI] [PubMed] [Google Scholar]
- 14. Way LI, Sullivan LA, Johnson V, et al. Comparison of routine urinalysis and urine Gram stain for detection of bacteriuria in dogs. J Vet Emerg Crit Care 2013;23:23–28. [DOI] [PubMed] [Google Scholar]
- 15. O'Neil E, Horney B, Burton S, et al. Comparison of wet‐mount, Wright‐Giemsa and Gram‐stained urine sediment for predicting bacteriuria in dogs and cats. Canad Vet 2013;54:1061–1066. [PMC free article] [PubMed] [Google Scholar]
- 16. Swenson CL, Boisvert AM, Kruger JM, et al. Evaluation of modified Wright‐staining of urine sediment as a method for accurate detection of bacteriuria in dogs. J Am Vet Med Assoc 2004;224:1282–1289. [DOI] [PubMed] [Google Scholar]
- 17. Cordoba G, Sørensen TM, Holm A, et al. Exploring the feasibility and synergistic value of the One Health approach in clinical research: Protocol for a prospective observational study of diagnostic pathways in human and canine patients with suspected urinary tract infection. Pilot Feasibility Stud 2015;1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sargeant JM, O'Connor AM, Dohoo IR, et al. Methods and processes of developing the strengthening the reporting of observational studies in epidemiology‐veterinary (STROBE‐Vet) statement. J Food Prot 2016;79:2211–2219. [DOI] [PubMed] [Google Scholar]
- 19. CLSI . Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, Approved Standard, 4th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2013.
- 20. Sørensen TM, Jensen AB, Damborg P, et al. Evaluation of different sampling methods and criteria for diagnosing canine urinary tract infection by quantitative bacterial culture. Vet J 2016;216:168–173. [DOI] [PubMed] [Google Scholar]
- 21. Jessen LR, Damborg P, Spohr A, et al. Antibiotic Use Guidelines for Companion Animal Practice [Antibiotikavejledning til familiedyr]. Copenhagen, Denmark: Den Danske Dyrlægeforenings Sektion for Hund; 2012.
- 22. Bates D, Mächler M, Bolker B, et al. Fitting linear mixed‐effects models using lme4. J Stat Soft 2015;67:48. [Google Scholar]
- 23. Strandberg EL, Ovhed I, Troein M, et al. Influence of self‐registration on audit participants and their non‐participating colleagues. A retrospective study of medical records concerning prescription patterns. Scand J Prim Health Care 2005;23:42–46. [DOI] [PubMed] [Google Scholar]
- 24. Akkerman AE, Kuyvenhoven MM, Verheij TJ, et al. Antibiotics in Dutch general practice: Nationwide electronic GP database and national reimbursement rates. Pharmacoepidemiol Drug Saf 2008;17:378–383. [DOI] [PubMed] [Google Scholar]
- 25. Padilla J, Osborne CA, Ward GE. Effects of storage time and temperature on quantitative culture of canine urine. J Am Vet Med Assoc 1981;178:1077–1081. [PubMed] [Google Scholar]
- 26. Rowlands M, Blackwood L, Mas A, et al. The effect of boric acid on bacterial culture of canine and feline urine. J Small Anim Pract 2011;52:510–514. [DOI] [PubMed] [Google Scholar]
- 27. Windahl U, Holst BS, Nyman A, et al. Characterisation of bacterial growth and antimicrobial susceptibility patterns in canine urinary tract infections. BMC Vet Res 2014;10:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cetin C, Senturk S, Kocabiyik AL, et al. Bacteriological examination of urine samples from dogs with symptoms of urinary tract infection. Turk J Vet Anim Sci 2003;27:1225–1229. [Google Scholar]
- 29. Brloznik M, Sterk K, Zdovc I. Prevalence and resistance patterns of canine uropathogens in regard to concurrent diseases. Berl Munch Tierarztl Wochenschr 2016;129:340–350. [PubMed] [Google Scholar]
- 30. Olin SJ, Bartges JW, Jones RD, et al. Diagnostic accuracy of a point‐of‐care urine bacteriologic culture test in dogs. J Am Vet Med Assoc 2013;243:1719–1725. [DOI] [PubMed] [Google Scholar]
- 31. Bornstein BH, Emler AC. Rationality in medical decision making: A review of the literature on doctors' decision‐making biases. J Eval Clin Pract 2001;7:97–107. [DOI] [PubMed] [Google Scholar]
- 32. Black DM, Rankin SC, King LG. Antimicrobial therapy and aerobic bacteriologic culture patterns in canine intensive care unit patients: 74 dogs (January‐June 2006). J Vet Emerg Crit Care 2009;19:489–495. [DOI] [PubMed] [Google Scholar]
- 33. Pedersen K, Pedersen K, Jensen H, et al. Occurrence of antimicrobial resistance in bacteria from diagnostic samples from dogs. J Antimicrob Chemother 2007;60:775–781. [DOI] [PubMed] [Google Scholar]
- 34. Damborg PP. Antibiotikaresistens blandt kliniske bakterieisolater fra hunde og katte i 2013 og 2014: den hidtil største undersøgelse af resistensforekomsten i danske kæledyr. Dansk Veterinaertidsskrift 2015;98:26–31. [Google Scholar]
- 35. Lulich JP, Osborne CA. Urine culture as a test for cure: Why, when, and how?. Vet Clin North Am Small Anim Pract 2004;34:1027–1041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article:
Table S1. Predictors for performing microscopy and culture in dogs with suspected UTI (n=151).


