Abstract
Adrenomedullin (ADM) 2/intermedin (IMD) is a short peptide that belongs to the CGRP superfamily. Although it shares receptors with CGRP, ADM and amylin, ADM2 has significant and unique functions in the cardiovascular system. In the past decade, the cardiovascular effect of ADM2 has been carefully analysed. In this review, progress in understanding the effects of ADM2 on the cardiovascular system and its protective role in cardiometabolic diseases are summarized.
Linked Articles
This article is part of a themed section on Spotlight on Small Molecules in Cardiovascular Diseases. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v175.8/issuetoc
Abbreviations
- AAC
abdominal aortic constriction
- ACS
acute coronary syndrome
- ADM
adrenomedullin
- AMPK
AMP‐activated protein kinase
- AMY
amylin
- CRLR
calcitonin receptor‐like receptor
- ER
endoplasmic reticulum
- I/R
ischaemia/reperfusion
- RAMP
receptor activity‐modifying protein
- SHR
spontaneously hypertensive rat
- TAC
transverse aortic contraction
- VSMC
vascular smooth muscle cell
Introduction
Adrenomedullin (ADM) 2 is a secreted peptide that was discovered in 2004 by two groups (Roh et al., 2004; Takei et al., 2004). It is one of the members of the ADM family which are all found in fish but, in mammals, only three peptides ‐ ADM, ADM2 and ADM5 ‐ have been found (Takei et al., 2008). These ADM peptides are themselves a part of the CGRP superfamily. ADM2 is also called intermedin (IMD), owing to its high expression in the intermediate lobe of the pituitary. Both terms, ‘ADM2’ and ‘IMD’, are widely used in the literature. However, because IMD was used as the former name for α‐melanocyte stimulating hormone, we will use the terms ADM2, ADM2/IMD1–53, ADM2/IMD1–47 and ADM2/IMD1–40 in this review to avoid confusion.
ADM2 shares receptors with CGRP, ADM and amylin (AMY) (Hay et al., 2005) and is widely expressed throughout the body. Recently, progress has been made on the physiological roles of ADM2 in cardiovascular homeostasis and as a protective agent against cardiometabolic diseases. In this review, the structure and expression of ADM2 and the pharmacology of ADM2, in the context of the cardiovascular system and cardiometabolic diseases, are summarized.
ADM2 and ADM2 receptors
The structure of ADM2
The Adm2 gene is located on the distal arm of human chromosome 22q13.33 and encodes a pre‐pro‐peptide containing 148 amino acid residues with a signal sequence at the N terminus (Chang et al., 2004). ADM2 belongs to the CGRP superfamily, which includes α‐CGRP, β‐CGRP, calcitonin receptor‐stimulating peptide, AMY, ADM and ADM5 (Hong et al., 2012). ADM2 shares the same structural characteristics with the other family members, including an intramolecular ring of six amino acids residues flanked by a disulfide bond and a putative amidation signal at the C terminus (Takei et al., 2004).
Sequence alignment has shown that ADM2 shares only 28% sequence identity with its closest paralogue, ADM. However, ADM2 is highly conserved in the orthologues of different species. The mature human ADM2 shares over 60% similarity with fish and 87% identity with the rodent peptides (Roh et al., 2004). The property of high conservation in orthologues and low homology in paralogues suggests that ADM2 has a significant and unique function in the body.
The fragments of ADM2
By identifying the conserved arginine residues at the N terminus, three potential active cleavage fragments have been found and are believed to mediate most functions of ADM2. They are the peptides ADM2/IMD1–53, with cleavage at Arg94‐His95 (Yang et al., 2005), ADM2/IMD1–47, with cleavage at Arg100‐Thr101 and ADM2/IMD1–40, with cleavage at Arg107‐Val108 (Roh et al., 2004) (Figure 1). Because the antibodies against ADM2 cannot distinguish between the different cleavage fragments, the actual form of ADM2 in vivo remains a mystery. Although the presence of ADM2/IMD1–47 has been demonstrated by gel filtration chromatographic analysis and HPLC (Morimoto et al., 2007; Wong et al., 2013), the presence of ADM2/IMD1–53 and ADM2/IMD1–40 still requires confirmation.
Figure 1.
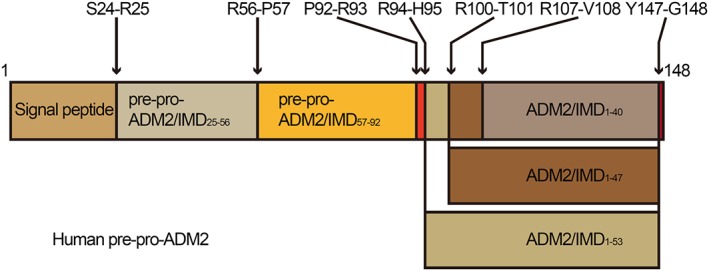
Diagram of human pre‐pro‐ADM2, showing the fragments derived from processing of the precursor at the putative cleavage sites indicated.
ADM2/IMD1–40 is more potent in stimulating cAMP generation in vitro and inhibiting food intake in vivo than ADM2/IMD1–47. However, the hypotensive, heart rate‐raising and gastric‐emptying effects of ADM2/IMD1–47 are greater than those of ADM2/IMD1–40 , after i.p. administration (Roh et al., 2004). The hypertensive effects of ADM2/IMD1–47 following i.c.v. injection are weaker than those of ADM2/IMD1–53 (Ren et al., 2006). An exonic insertion/deletion polymorphism (rs3840963) that may influence the generation of ADM2/IMD1–53 has been found to be associated with renal dysfunction, hypertension and cerebrovascular disease in Japanese individuals (Hirose et al., 2011), suggesting different effects of the ADM2 fragments in vivo. However, different laboratories have chosen to use different types of ADM2 fragments to study their functions and, regrettably, some groups have not provided sufficient information on the ADM2 fragments that they use. ADM2 fragment information should be provided to allow a proper comparison of the function, receptor affinity and subcellular signalling of the different ADM2 fragments.
The expression of ADM2
ADM2 is widely expressed throughout the body and is highly expressed in the circulatory system, that is, in the heart and vasculature; the reproductive system, that is, in the ovaries, placenta and uterus; the kidneys; the digestive system, that is, in the submaxillary gland, pancreas, oesophagus, stomach, small intestine, colon and rectum; and the adipose tissue (Roh et al., 2004; Takei et al., 2004; Morimoto et al., 2007; Chauhan et al., 2009; Wong et al., 2013). In contrast to the high tissue expression of ADM2, the plasma levels of ADM2 are relatively low, approximately 100 ‐ 200 pg·mL−1 (Taylor et al., 2005a; Morimoto et al., 2007). The factor (s) determining the plasma levels of ADM2 is unknown. Neutral endopeptidase, which can cleave CGRP and ADM, might also degrade plasma ADM2 (Bell and McDermott, 2008).
ADM2 may act in an autocrine or paracrine manner, regulating organ perfusion and hormone secretion. In the iodine‐deficient rat, expression of ADM2 is up‐regulated by thyroid‐stimulating hormone in the thyroid follicular cells. ADM2 locally dilates the thyroid inter‐follicular capillaries, facilitating thyroid hormone synthesis and goitre formation (Nagasaki et al., 2014). ADM2 is also up‐regulated in the intermediate lobe of the pituitary by oestrogen. ADM2 acts as a prolactin‐releasing factor, participating in the oestrogen‐induced release of prolactin from the anterior pituitary (Lin Chang et al., 2005). However, ADM2 is elevated in the plasma of pregnant women, exerting a hypotensive effect (Chauhan et al., 2007). Low plasma levels of ADM2 are closely associated with pre‐eclampsia (Chauhan et al., 2016), suggesting an endocrine effect of ADM2, under specific conditions.
The expression of ADM2 is up‐regulated by oestrogen in the rat pituitary (Lin Chang et al., 2005), thyroid‐stimulating hormone in the rat thyroid (Nagasaki et al., 2014), hypoxia in the human lungs (Pfeil et al., 2009), endoplasmic reticulum (ER) stress in HeLa cells (Kovaleva et al., 2016) and hypercholesterolaemia in the rat aorta (Meng et al., 2016). However, the regulation of ADM2 transcription, translation, processing, modification and secretion has not been carefully studied. ADM2 is a target gene of oestrogen receptors (Lin Chang et al., 2005), hypoxia‐inducible factor 1α (Pfeil et al., 2009) and ATF‐4 (Kovaleva et al., 2016). The binding elements for AP‐2, SP1, progesterone receptor, FOXF1, GATA‐binding factor 1 and WT1, which are transcriptional factors for stress, reproduction and embryonic development, have also been found in the human and mouse Adm2 promoter (Pfeil et al., 2009). Therefore, ADM2 primarily acts as a stress and reproduction‐inducible gene.
The receptors of ADM2
ADM2 activates the downstream signalling of calcitonin receptor‐like receptor (CRLR) and calcitonin receptor (CTR) (Hay et al., 2005). CRLR and CTR are responsive to ADM2 when associated with the receptor activity‐modifying proteins (RAMPs). RAMPs interact with CRLR and CTR, facilitating the trafficking of CRLR to the cell membrane and modulating its ligand preferences (Qi and Hay, 2010). There are three of these accessory proteins, RAMP1, RAMP2 and RAMP3, in cells. CTR and CRLR interact with different RAMPs, displaying different ligand selectivity. CRLR/RAMP1 is the CGRP receptor; CRLR/RAMP2 and CRLR/RAMP3 are the two ADM receptors (AM1 and AM2); CTR/RAMP1, CTR/RAMP2 and CTR/RAMP3 are the AMY receptors (AMY1, AMY2 and AMY3) (Alexander et al., 2015a).
The receptor affinity of ADM2 is distinct from those of the other agonist peptides, CGRP, ADM and AMY. By comparing the agonist potencies and accumulation of cAMP in HEK‐293T and COS‐7 cells transfected with different CRLR or CTR complexes, the receptors of ADM2 have been identified on the basis of receptor binding affinity: AM2 receptor ≥ CGRP receptor > AM1 receptor ≥ AMY1 receptor > AMY3 receptor. ADM2 displays a different pharmacological profile from CGRP, ADM or AMY (Hong et al., 2012). These results suggest that although it shares receptors with other family members, ADM2 acts in a distinct manner partly on the basis of different receptor affinity. In addition, the expression of ADM2 receptors in different organs also affects the function of ADM2. CRLR, RAMP1 and RAMP2 are highly expressed in the heart and vascular, but the expression of RAMP3 is relatively low (Bell and McDermott, 2008). As a result, although the AM2 receptor has the highest affinity for ADM2, most of the cardiovascular effect of ADM2 are mediated by CGRP receptors and AM1 receptors.
The pharmacological effects of ADM2 acting on CGRP receptors and AM1/2 receptors have been widely studied. However, the outcomes of ADM2 acting through AMY1/3 receptors have not been explored. Furthermore, there is a possibility that there is another, unknown, receptor for ADM2, because ADM2/IMD17–47, a truncated and amidated ADM2 fragment that was believed to be a functional antagonist of ADM2, does not function as a full antagonist. Instead, ADM2/IMD17–47 acts as an inverse agonist in the CNS (White and Samson, 2007). The combined use of antagonists of CGRP receptors, AM1/2 receptors and AMY1/2/3 receptors only partly blocked the action of ADM2 in rat spinal cord cells (Owji et al., 2008). Therefore, the actions of ADM2 through the AMY1/3 receptors and the unknown receptor(s) need further investigation.
The signalling bias of ADM2
CRLR and CTR belong to the secretin family (class B) GPCR (Alexander et al., 2015a). GPCRs couple to different G protein, initiating different signal pathway. RAMPs not only alter the ligand affinity but also modify the G protein selectivity of receptors (Weston et al., 2015). Gαs is the main signal pathway activated by CRLR‐ and CTR‐based receptors (Hong et al., 2012), but RAMPs influence the biased activation of CRLR and CTR in a ligand‐dependent manner. In HEK‐293 cells treated with ADM2/IMD1–47, the CGRP receptors display a bias toward Gαs signalling; AM1 receptors shows a bias toward Gαq signalling; and AM2 receptors show bias toward Gαs signalling (Weston et al., 2016) (Figure 2).
Figure 2.
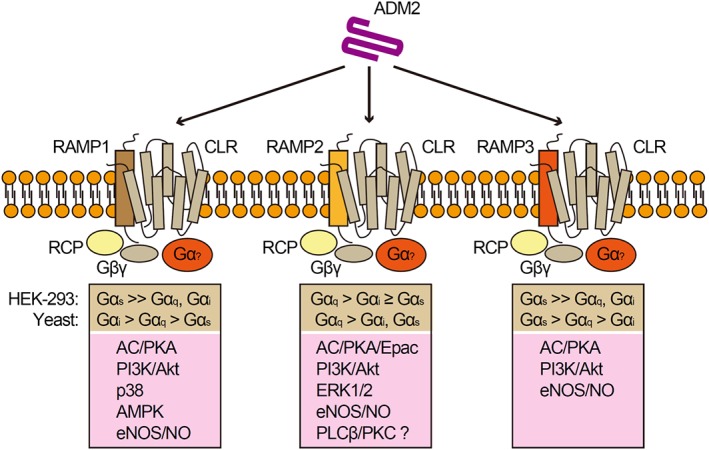
Diagram showing the biased activation of CGRP receptors and AM1/2 receptors by ADM2, as well as the downstream signal pathway. RCP, receptor component protein.
However, when HEK‐293 cells are treated with CGRP, ADM or ADM2, the G protein selectivity of CRLR is also changed. Now, CGRP receptors displays a bias toward Gαs signalling in response to CGRP and change to a Gαi bias, in response to ADM. AM1 receptors shows a bias toward Gαs when treated with CGRP and ADM. AM2 receptors show bias toward Gαs in response to ADM and change to Gαq in response to CGRP (Weston et al., 2016). Therefore, the biased activation of CRLR by different peptides is another mechanism underlying the differences in effects between ADM2, CGRP and ADM.
Cardiovascular effects of ADM2
ADM2 and haemodynamics
ADM2 has a regulatory effect on systemic haemodynamics. The i.v. infusion of ADM2/IMD1–47 increases the blood flow and decreases the vascular resistance of the heart, lungs, kidneys, liver, stomach, small intestine, adrenal glands and testes (Fujisawa et al., 2007), more potently than ADM (Jolly et al., 2009). The modified cardiac function, vasodilation, renal function and neuroendocrine effect are involved in the haemodynamic regulation by ADM2 (Figure 3). The renal and neuroendocrine effects of ADM2 are discussed here, and the cardiovascular effects of ADM2 will be discussed in the following sections.
Figure 3.
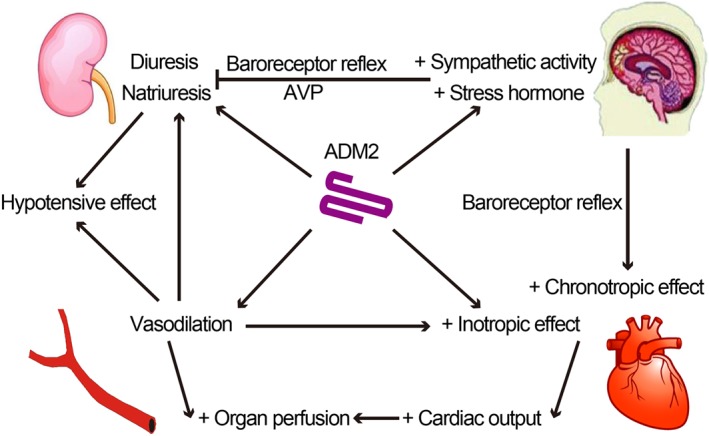
Diagram showing the direct and indirect regulatory effects of ADM2 on cardiovascular homeostasis. ADM2 exerts vasodilator effects, increasing the perfusion of heart and kidneys. ADM2 also has a positive inotropic effect. The enhanced myocardial perfusion, induced by ADM2, augments myocardial contractility indirectly. In addition, the baroreceptor reflex, activated by hypotension, exerts a positive chronotropic effect. Thus, ADM2 increases the cardiac output and organ perfusion. ADM2 has diuretic and natriuretic effect on kidney. Taken together, the vasodilator, diuretic and natriuretic effects of ADM2 decrease the BP. However, ADM2 in the CNS increases sympathetic output and arginine‐vasopressin (AVP) secretion, which may counteract the diuretic and natriuretic effect, increasing the BP.
ADM2 has a hypotensive effect, but the potency comparisons have produced variable results. The overall rank of the hypotensive potency is ADM2/IMD1–53 > ADM > ADM2/IMD1–47 = CGRP > ADM2/IMD1–40, which can be blocked by CGRP receptor antagonist (Roh et al., 2004; Taylor et al., 2005a; Jolly et al., 2009). However, other studies have reported a different potency ranking for the hypotensive effect: ADM2/IMD1–47 = ADM2/IMD1–40 ≥ ADM = ADM2/IMD1–53 (Takei et al., 2004; Pan et al., 2005; Fujisawa et al., 2006; Ren et al., 2006). This discrepancy may be due to the different doses of ADM2 used by different laboratories and to the fact that both CGRP receptors and AM1/2 receptors are involved in the hypotensive effect. The former group, mentioned above, used a lower dose of ADM2 (1 nmol·kg−1, i.v. or 50 nmol, i.p.), which acted mainly through CGRP receptors. The other group used a high dose of ADM2 (3 nmol·kg−1, 10 nmol·kg−1 or 150 nmol, i.v.), which could mediate its hypotensive effects via AM1/2 receptors.
The kidney is an important organ in regulating blood volume and osmotic pressure. Infusion of ADM2/IMD1–47 into the renal artery, increases renal blood flow and urine flow. ADM2/IMD1–47 infusion promotes urinary Na+ excretion but does not affect the GFR, and it is less potent than ADM (Fujisawa et al., 2004), indicating that ADM2 exerts direct diuretic and natriuretic effects. However, other studies have observed decreased urine flow and Na+ excretion after the systemic administration of ADM2/IMD1–47 (Takei et al., 2004; Charles et al., 2006). This effect is probably due to the hypotensive action of ADM2 that overcomes the direct effect on the kidneys. The systemic administration of ADM2/IMD1–47 increases renal sympathetic nerve activity and is partly reversed by baroreceptor denervation. The plasma levels of both hypertensive, anti‐diuretic hormones, such as renin, aldosterone and vasopressin, and hypotensive, diuretic hormones, such as atrial natriuretic peptide and brain natriuretic peptide, were increased after i.v. infusion of ADM2/IMD1–47 (Charles et al., 2006), suggesting that the renal action of ADM2 is a consequence of direct and indirect effects.
In contrast to the effects of peripheral infusion of ADM2, i.c.v. administration of ADM2/IMD1–53 raised BP and was more potent than the administration of ADM2/IMD1–47, CGRP or ADM (Ren et al., 2006). Microinjection of ADM2 into the nucleus tractus solitarii (NTS) increased mean arterial pressure and renal sympathetic nerve activity through a pathway involving AM1/2 receptors, cAMP and PKA (Li et al., 2013). The hypertensive effect of i.c.v. administration of ADM2/IMD1–47 was abolished by α‐adrenoceptor antagonists in a CGRP receptor‐dependent manner (Taylor et al., 2005a). These results suggest that ADM2 increases BP by increasing sympathetic nerve activity in the CNS.
The i.c.v. injection of ADM2/IMD1–47 raised plasma levels of vasopressin, adrenocorticotropin and corticosterone, actions which may also be involved in the hypertensive effect of ADM2 in the CNS. The increased plasma levels of adrenocorticotropin and corticosterone, induced by ADM2/IMD1–47, were abolished by a corticotropin releasing factor antagonist, suggesting involvement of the hypothalamo‐pituitary‐adrenal axis (Taylor and Samson, 2005b). However, ADM2 administered to the paraventricular nucleus decreases the renal sympathetic nerve activity and BP via a pathway involving AM1/2 receptors, NOS and NO (Zhou et al., 2014), indicating that ADM2 in different encephalic regions has different function.
ADM2 and cardiac function
ADM2 increases cardiac output in vivo. ADM2/IMD1–47 and ADM2/IMD1–53 exert positive inotropic effects in vivo (Charles et al., 2006; Fujisawa et al., 2007) and in isolated hearts (Yang et al., 2005). The administration of ADM2/IMD1–47 in vivo also mildly elevated heart rate, an effect that can be blocked by antagonism at nicotinic ACh receptors and baroreceptor denervation, indicating that the increased heart rate is partially due to the baroreceptor reflex in response to hypotension (Abdelrahman and Pang, 2006; Fujisawa et al., 2006). ADM2/IMD1–47 directly enhances cardiac contraction through increasing intracellular Ca2+ in PKC‐ and PKA‐dependent pathways and is more potent than CGRP but less potent than ADM (Dong et al., 2006). In anaesthetized pigs, ADM2/IMD1–47 has a positive inotropic effect and increases coronary blood flow. The effect of ADM2/IMD1–47 is mediated by CGRP/AM1/2 receptors‐NO and the autonomic nervous system through β2‐adrenoceptors (Grossini et al., 2009).
However, other studies have indicated that ADM2 has a negative effect on cardiac function. The administration of ADM2/IMD1–47 or ADM2/IMD1–40 in vivo has a negative inotropic effect (Pan et al., 2005). This contradiction may be due to the contribution of NO. In cultures of cardiomyocytes, ADM2/IMD1–47 has a positive inotropic effect through the cAMP‐Ca2+ pathway, but in isolated hearts, ADM2/IMD1–47 has an opposite effect in an NO‐dependent manner (Munzel et al., 2011). The negative inotropic effect of ADM2/IMD1–47 depends on the endothelium through the CGRP/AM1/2 receptor‐eNOS‐cGMP pathway. ADM2/IMD1–47 exerts a direct, positive inotropic effect through the CGRP receptor‐cAMP pathway, in the absence of endothelium (Pires et al., 2012). Furthermore, in animal models of transverse aortic contraction (TAC) and L‐NAME administration, whose endothelium or eNOS is dysfunctional, the negative inotropic effect of ADM2/IMD1–47 is abolished (Pires et al., 2013).
ADM2 and vasodilation
Increased organ perfusion, decreased peripheral vascular resistance and hypotensive effects of ADM2 are partially due to the vasodilation. ADM2/IMD1–40 and ADM2/IMD1–47 are equally potent in relaxing pre‐constricted thoracic aortic rings (Pan et al., 2005). ADM2/IMD1–53 relaxes aortic rings more potently than ADM. ADM2 increases cNOS activity and NO generation by increasing SLC7A1 and SLC7A2 expression and arginine uptake (Yang et al., 2006). In coronary arterial rings, ADM2 induced vasodilation through CGRP receptors (Kobayashi et al., 2004). In coronary endothelial cells, ADM2/IMD1–47 increased the phosphorylation of eNOS through a CGRP/AM1/2 receptor‐PKA‐ERK/p38/Akt pathway. Furthermore, the opening of Kir6 and KCa1.1 channels and the β2‐adrenoceptors are also involved in NO generation induced by ADM2/IMD1–47 (Grossini et al., 2009). In isolated rat lungs and pulmonary arterial rings, ADM2/IMD1–47 dilates the pulmonary vasculature in an endothelial‐dependent manner through the CGRP/AM1/2 receptor‐NO‐cGMP/PKG‐KCa1.1 pathway, more potently than ADM and CGRP (Kandilci et al., 2008). In addition to dilating vascular tissue in an endothelial‐dependent manner, ADM2 may also have a direct effect on vascular smooth muscle cells (VSMCs). CRLR and RAMP1, 2 and 3 are expressed in VSMCs. In VSMC‐specific RAMP2 transgenic mice, the ADM‐induced vasodilator effect is enhanced (Tam et al., 2006). ADM2 may also relax VSMCs via mechanisms similar to those of CGRP and ADM.
ADM2 and vascular permeability
ADM2 has differing effects on vascular permeability depending on the vascular bed studied. In cultures of coronary microvascular endothelial cells, ADM2/IMD1–53 increases permeability, resulting from the derangement of actin cytoskeleton and loss of VE‐cadherin through the CGRP receptor‐cAMP/PKA pathway. With human umbilical vein endothelial cells, ADM2/IMD1–53 decreases cell layer permeability through an AM1 receptor‐cAMP/Epac/PKA pathway, leading to the stabilization of adherence junctions and the relaxation of endothelial cells (Aslam et al., 2011; Aslam et al., 2012). In cerebral endothelial cells, ADM2 decreases basal and oxidative stress‐induced hyperpermeability and endothelial apoptosis in a cAMP/NO‐dependent manner (Chen et al., 2006). In pulmonary microvascular endothelial cells, ADM2/IMD1–47 inhibits basal, pressure‐ and thrombin‐induced hyperpermeability through the CGRP/AM1/2 receptor‐PKA pathway, improving ventilator‐induced pulmonary injury (Pfeil et al., 2009; Muller‐Redetzky et al., 2012).
ADM2 and angiogenesis
In an ischaemic hind limb model, ADM2/IMD1–40 increases blood perfusion and capillary and arteriole density in ischaemic limbs. ADM2/IMD1–40 promotes endothelial migration and tube formation in an NO‐dependent manner through the CGRP/AM1/2 pathway, facilitating angiogenesis (Smith et al., 2009). ADM2 directly activates phosphorylation of VEGFR‐2 through CGRP/AM1 receptors, which is an additional pro‐angiogenesis mechanism (Albertin et al., 2010). However, another study has shown that ADM2/IMD1–40 stabilizes endothelial networks, thus promoting lumen formation, anastomosis and inhibiting sprouting. ADM2/IMD1–40 induces endothelial cell quiescence by antagonizing VEGF function through the AM1 receptor‐ERK/Akt pathway (Zhang et al., 2012a). Further studies have shown that ADM2/IMD1–40 inhibits VEGF‐induced excessive sprouting by strengthening cell–cell contacts mediated by VE‐cadherin (Xiao et al., 2015). Despite conflicting observations, ADM2 generally has a positive effect on angiogenesis.
ADM2 and cardiometabolic diseases
The metabolic syndrome comprises a series of symptoms, including obesity, insulin resistance, dyslipidemia, hyperglycaemia and hypertension, which is primarily caused by the over‐expansion of adipose tissue (Ordovas and Corella, 2008). Patients with metabolic syndrome also have an increased risk for cardiovascular disease. The adverse effect of the metabolic syndrome contributes to the development of atherosclerosis, cardiac infarction, cardiac hypertrophy and heart failure (Maruthur et al., 2014). Recently, a series of studies have shown that ADM2 not only inhibits cardiovascular disease directly but also indirectly has a protective effect against metabolic syndrome, improving the risk factors for cardiovascular diseases (Figure 4).
Figure 4.
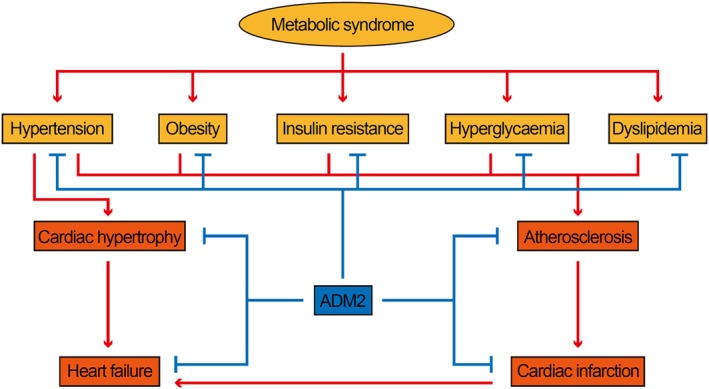
Diagram showing the protective effect of ADM2 on cardiometabolic disease. The symptoms of the metabolic syndrome are risk factors for cardiovascular disease. ADM2 not only inhibits cardiovascular disease directly, but also indirectly has a protective effect against metabolic syndrome, improving the risk factors for cardiovascular diseases.
ADM2 and the metabolic syndrome
ADM2 inhibits metabolic syndrome by targeting adipose tissue. ADM2 is expressed in adipose tissue and is down‐regulated during obesity in mice (Zhang et al., 2016a). The plasma level of ADM2 is associated negatively with the body mass index and HOMA‐IR index in humans, suggesting a decreased ADM2 level during obesity and insulin resistance (Lv et al., 2016; Zhang et al., 2016b). By using adipose‐specific ADM2/IMD1–53 transgenic mice, ADM2/IMD1–53 has been shown to have a protective effect against high‐fat diet‐induced obesity and insulin resistance in mice (Zhang et al., 2016a).
The protective effect of ADM2 is partly due to the modified mitochondrial function. ADM2 attenuates the loss of mitochondria and the decreased mitochondrial membrane potential induced by oxidative stress in cerebral endothelial cells (Chen et al., 2006). ADM2/IMD1–53 enhances mitochondrial respiration and energetic expenditure in adipocytes through promoting beige cell biogenesis in vitro and in vivo. The thermogenic effect of ADM2/IMD1–53 is mediated in CGRP receptor‐AMP‐activated protein kinase (AMPK)‐PGC1α‐ and CGRP receptor‐p38‐dependent manner in adipocytes (Lv et al., 2016; Zhang et al., 2016a).
The activation of M2 macrophages induced beige cell biogenesis through secreting catecholamines (Qiu et al., 2014). ADM2/IMD1–53 reverses the M1/M2 imbalance in adipose tissue in an AMPK‐dependent manner. The ADM2/IMD1–53‐induced activation of adipose M2 macrophages induces beiging indirectly and ameliorates adipose inflammation (Lv et al., 2016; Pang et al., 2016). Noradrenaline released by M2 macrophages has an anti‐inflammatory effect on adipose macrophages through a β2‐adrenoceptor‐cAMP‐HDAC4‐dependent pathway (Luan et al., 2014), which may be involved in the improvement of adipose inflammation. CGRP/AM1/2 receptors are all expressed in macrophages, but the anti‐inflammatory effect of ADM2 is only mediated by CGRP receptors (Soultanova et al., 2016). Therefore, the protective effect of ADM2 on adipose tissue is partly mediated by modifying adipose macrophages.
Adipocytes are antigen presenting cells, expressing class II MHC and initiating adaptive immune response during obesity (Deng et al., 2013). ADM2/IMD1–53 down‐regulates adipocyte class II MHC expression in a CGRP receptor‐cAMP‐Blimp1‐dependent manner (Zhang et al., 2016b). Besides, CGRP reduces class II MHC expression in dendritic cells (Carucci et al., 2000), suggesting that ADM2 may inhibit class II MHC expression in different antigen‐presenting cells through CGRP receptors.
ADM2 and atherosclerosis
ADM2 protects blood vessels from atherosclerosis. ApoE−/− mice treated with ADM2/IMD1–53 displays decreased atherosclerotic lesions. ADM2/IMD1–53 inhibits macrophage foam cell formation through decreasing the uptake of modified LDLs by CD36 and class A scavenger receptor (Dai et al., 2012; Dai et al., 2014). Another study also showed that ADM2 improved atherosclerosis by increasing plasma HDL cholesterol and decreasing plasma total and LDL cholesterol levels in high‐fat diet‐fed ApoE−/− mice (Zhang et al., 2012b).
ADM2 and myocardial infarction
The levels of ADM2 and its receptors are increased during myocardial infarction. Clinical data have shown that the plasma level of ADM2 is elevated in patients with acute coronary syndrome (ACS) and is positively correlated with the severity of coronary stenosis and myocardial infarction (Lv et al., 2013; Qin et al., 2013). ADM2 may be a potential prognostic biomarker for ST‐segment elevation or non‐ST‐segment elevation ACS (Tang et al., 2014; Li et al., 2016). In myocardial ischaemia/reperfusion (I/R) models, the levels of ADM2, CRLR and RAMP2/3 in plasma and the non‐ischaemic area are increased, but in the ischaemic area, ADM2 is down‐regulated (Zhang et al., 2009; Teng et al., 2013).
ADM2 has a protective role in ischaemic cardiac injury. ADM2/IMD1–40 ameliorates I/R‐induced inhibition of cardiac function and decreases plasma levels of MDA and LDH (Zhang et al., 2009). ADM2/IMD1–53 protects cardiomyocytes against I/R‐induced injury by inhibiting ER stress, oxidative stress and apoptosis in a PI3K/Akt‐ and ERK‐dependent pathway (Song et al., 2009; Teng et al., 2011; Zhao et al., 2012). In isolated hearts, ADM2/IMD1–40, ADM2/IMD1–47 and ADM2/IMD1–53 ameliorated the I/R‐induced myocardial injury and decreased cardiac function (Yang et al., 2014). Further studies have shown that ADM2/IMD1–47 protects cardiomyocytes and cardiac microvascular endothelium from I/R‐induced injury via AM1 receptors (Bell et al., 2012; Bell et al., 2016a).
ADM2 and cardiac hypertrophy
ADM2 and its receptors are up‐regulated during cardiac hypertrophy in different animal models. In the cardiomyocytes of spontaneously hypertensive rats (SHRs), the expression of ADM2, CRLR and RAMP1/3 are markedly increased (Bell et al., 2008a). The plasma level of ADM2 is also elevated and is negatively correlated with the systolic BP of SHRs (Zeng et al., 2009). In TAC or abdominal aortic constriction (AAC)‐induced hypertensive rats, the expression of ADM2, CRLR and RAMP1/3 are increased in cardiac tissues (Chen et al., 2013; Lu et al., 2015). In a NO deficient hypertensive rat, myocardial expression of ADM2, CRLR and RAMP1/2/3 are also increased, which was abolished by treatment with antihypertensive drugs (Bell et al., 2008b).
ADM2/IMD1–40 ameliorates TAC‐induced cardiac hypertrophy, fibrosis, dysfunction and apoptosis (Chen et al., 2013). ADM2/IMD1–53 improves AAC‐induced cardiac hypertrophy and apoptosis by inhibiting ER stress in an AMPK‐dependent manner (Lu et al., 2015). An in vitro study showed that ADM2/IMD1–40 inhibits angiotensin II‐ or isoprenaline‐induced hypertrophy and apoptosis via promoting autophagy in a cAMP/PKA‐ and ERK‐dependent manner in cardiomyocytes (Chen et al., 2013). ADM2/IMD1–53 also inhibits angiotensin II‐induced proliferation, collagen production and phenotype conversion of myocardial fibroblasts in a CGRP/AM1/2 receptor‐cAMP/PKA‐dependent pathway, more potently than ADM (Yang et al., 2009).
ADM2 and heart failure
Heart failure is a common outcome of cardiac hypertrophy and myocardial infarction. Microinjection of ADM2 into the hypothalamic paraventricular nucleus decreases cardiac sympathetic afferent reflex through AM1/2 receptors and improves heart failure indirectly (Gan et al., 2014). In addition, ADM2/IMD1–53 inhibits myocardial fibrosis directly through down‐regulation of TGFβ (Wei et al., 2015). Therefore, ADM2 has a protective effect on heart failure.
In rat left coronary ligation‐induced heart failure models, the expression of ADM2, CRLR and RAMP1/2/3 are increased in cardiac tissues (Hirose et al., 2008). Besides, the expression of ADM2 and ADM are also increased in leukocytes of patients with chronic heart failure (Cabiati et al., 2014), suggesting that both ADM and ADM2 may act as a plasma biomarker for heart failure. ADM has been shown to be an independent predictor of death for heart failure (Yuyun et al., 2015). The plasma level of ADM2 is also increased in patients with heart failure (Bell et al., 2016b). Similar to ADM, it is difficult to accurately determine serum ADM2 level because of the binding of receptors, the short half‐life, the existence of binding proteins and the technical difficulties. However, the mid‐regional pro‐ADM45–92, generated during the processing of ADM, is stable and easily detected. It has been shown to predict a favourable clinical course in cardiac resynchronization therapy‐induced heart failure (Arrigo et al., 2017). Pre‐pro‐ADM2/IMD25–56 and pre‐pro‐ADM2/IMD57–92 have also been identified in human plasma and may act as biomarkers for heart failure (Bell et al., 2016b) (Figure 1).
Perspective
ADM2 is a short secretory peptide and has a regulatory effect on cardiovascular homoeostasis. ADM2 is an attractive drug candidate for the treatment of cardiometabolic disease, but there are still several challenges preventing the clinical application of ADM2. Although there are three possible splicing fragments of pre‐pro‐ADM2 with biological activity, the details of the generation of ADM2 fragments and their relative amounts in vivo are not yet known. In addition, the receptor affinity, the activation of signal pathway and the biological effect of different ADM2 fragments have still to be defined. ADM2 is widely expressed throughout the body tissues, but the plasma levels of ADM2 are relatively low. The half‐life of plasma ADM2 needs to be established, along with the enzyme(s) responsible for its degradation. Most of the physiological or pathophysiological effects of ADM2 are mediated by CGRP receptors and AM1/2 receptors. However, the lack of specific antagonists for the AM1 and AM2 receptors impede the further distinction of the different receptor subtypes. Non‐peptide antagonists specifically against AM1 and AM2 receptors are needed. Additionally, although AMY1/3 receptors have been identified as receptors of ADM2, the actions of ADM2 through AMY1/3 receptors has not been studied.
The plasma level of ADM2 is closely associated with different cardiometabolic diseases. Therefore, the plasma level of ADM2 or of pre‐pro‐ADM2 fragments may be used as putative biomarkers for cardiometabolic disease. Although ADM2 is a putative drug candidate against cardiometabolic diseases, the haemodynamic effect of ADM2, especially the positive inotropic, hypotensive and vasodilatory effects, may compromise its safety. Results have suggested that the different function of ADM2 may be mediated by different receptors and different signalling pathways. Therefore, ADM2 analogues that show bias towards specific ADM2 receptors may be developed as novel drugs for cardiometabolic diseases.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015a,b,c,d).
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31230035 to X.W. and 81470557 to M.J.X).
Zhang, S.‐Y. , Xu, M.‐J. , and Wang, X. (2018) Adrenomedullin 2/intermedin: a putative drug candidate for treatment of cardiometabolic diseases. British Journal of Pharmacology, 175: 1230–1240. doi: 10.1111/bph.13814.
References
- Abdelrahman AM, Pang CC (2006). Effect of intermedin/adrenomedullin‐2 on venous tone in conscious rats. Naunyn Schmiedebergs Arch Pharmacol 373: 376–380. [DOI] [PubMed] [Google Scholar]
- Albertin G, Sorato E, Oselladore B, Mascarin A, Tortorella C, Guidolin D (2010). Involvement of vascular endothelial growth factor signaling in CLR/RAMP1 and CLR/RAMP2‐mediated pro‐angiogenic effect of intermedin on human vascular endothelial cells. Int J Mol Med 26: 289–294. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Catterall WA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Voltage‐gated ion channels. Br J Pharmacol 172: 5904–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015d). The Concise Guide to PHARMACOLOGY 2015/16: Catalytic receptors. Br J Pharmacol 172: 5979–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo M, Truong QA, Szymonifka J, Rivas‐Lasarte M, Tolppanen H, Sadoune M et al. (2017). Mid‐regional pro‐atrial natriuretic peptide to predict clinical course in heart failure patients undergoing cardiac resynchronization therapy. Europace: European Pacing, Arrhythmias, and Cardiac Electrophysiology: Journal of the Working Groups on Cardiac Pacing, Arrhythmias, and Cardiac Cellular Electrophysiology of the European Society of Cardiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M, Gunduz D, Schuler D, Li L, Sharifpanah F, Sedding D et al. (2011). Intermedin induces loss of coronary microvascular endothelial barrier via derangement of actin cytoskeleton: role of RhoA and Rac1. Cardiovasc Res 92: 276–286. [DOI] [PubMed] [Google Scholar]
- Aslam M, Pfeil U, Gunduz D, Rafiq A, Kummer W, Piper HM et al. (2012). Intermedin (adrenomedullin2) stabilizes the endothelial barrier and antagonizes thrombin‐induced barrier failure in endothelial cell monolayers. Br J Pharmacol 165: 208–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell D, McDermott BJ (2008). Intermedin (adrenomedullin‐2): a novel counter‐regulatory peptide in the cardiovascular and renal systems. Br J Pharmacol 153 (Suppl 1): S247–S262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell D, Campbell M, Ferguson M, Sayers L, Donaghy L, O'Regan A et al. (2012). AM(1)‐receptor‐dependent protection by intermedin of human vascular and cardiac non‐vascular cells from ischaemia‐reperfusion injury. J Physiol 590 (Pt 5): 1181–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell D, Campbell M, McAleer SF, Ferguson M, Donaghy L, Harbinson MT (2016a). Endothelium‐derived intermedin/adrenomedullin‐2 protects human ventricular cardiomyocytes from ischaemia‐reoxygenation injury predominantly via the AM(1) receptor. Peptides 76: 1–13. [DOI] [PubMed] [Google Scholar]
- Bell D, Gordon BJ, Lavery A, Megaw K, Kinney MO, Harbinson MT (2016b). Plasma levels of intermedin (adrenomedullin‐2) in healthy human volunteers and patients with heart failure. Peptides 76: 19–29. [DOI] [PubMed] [Google Scholar]
- Bell D, Zhao Y, McCoy FP, Devine A, McDermott BJ (2008a). Expression of the counter‐regulatory peptide intermedin is augmented in the presence of oxidative stress in hypertrophied cardiomyocytes. Cell Physiol Biochem 21: 409–420. [DOI] [PubMed] [Google Scholar]
- Bell D, Zhao YY, Devine AB, McDermott BJ (2008b). Influence of atenolol and nifedipine on nitric‐oxide deficient cardiomyocyte hypertrophy and expression of the cardio‐endocrine peptide intermedin and its receptor components. Cell Physiol Biochem 21: 203–214. [DOI] [PubMed] [Google Scholar]
- Cabiati M, Sabatino L, Svezia B, Caruso R, Verde A, Caselli C et al. (2014). Adrenomedullin and intermedin gene transcription is increased in leukocytes of patients with chronic heart failure at different stages of the disease. Peptides 55: 13–16. [DOI] [PubMed] [Google Scholar]
- Carucci JA, Ignatius R, Wei Y, Cypess AM, Schaer DA, Pope M et al. (2000). Calcitonin gene‐related peptide decreases expression of HLA‐DR and CD86 by human dendritic cells and dampens dendritic cell‐driven T cell‐proliferative responses via the type I calcitonin gene‐related peptide receptor. J Immunol 164: 3494–3499. [DOI] [PubMed] [Google Scholar]
- Chang CL, Roh J, Hsu SY (2004). Intermedin, a novel calcitonin family peptide that exists in teleosts as well as in mammals: a comparison with other calcitonin/intermedin family peptides in vertebrates. Peptides 25: 1633–1642. [DOI] [PubMed] [Google Scholar]
- Charles CJ, Rademaker MT, Richards AM (2006). Hemodynamic, hormonal, and renal actions of adrenomedullin‐2 in normal conscious sheep. Endocrinology 147: 1871–1877. [DOI] [PubMed] [Google Scholar]
- Chauhan M, Balakrishnan M, Vidaeff A, Yallampalli U, Lugo F, Fox K et al. (2016). Adrenomedullin2 (ADM2)/intermedin (IMD): a potential role in the pathophysiology of preeclampsia. J Clin Endocrinol Metab 101: 4478–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan M, Ross GR, Yallampalli U, Yallampalli C (2007). Adrenomedullin‐2, a novel calcitonin/calcitonin‐gene‐related peptide family peptide, relaxes rat mesenteric artery: influence of pregnancy. Endocrinology 148: 1727–1735. [DOI] [PubMed] [Google Scholar]
- Chauhan M, Yallampalli U, Dong YL, Hankins GD, Yallampalli C (2009). Expression of adrenomedullin 2 (ADM2)/intermedin (IMD) in human placenta: role in trophoblast invasion and migration. Biol Reprod 81: 777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Wang X, Tong M, Wu D, Wu S, Chen J et al. (2013). Intermedin suppresses pressure overload cardiac hypertrophy through activation of autophagy. PLoS One 8: e64757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Kis B, Hashimoto H, Busija DW, Takei Y, Yamashita H et al. (2006). Adrenomedullin 2 protects rat cerebral endothelial cells from oxidative damage in vitro. Brain Res 1086: 42–49. [DOI] [PubMed] [Google Scholar]
- Dai XY, Cai Y, Mao DD, Qi YF, Tang C, Xu Q et al. (2012). Increased stability of phosphatase and tensin homolog by intermedin leading to scavenger receptor A inhibition of macrophages reduces atherosclerosis in apolipoprotein E‐deficient mice. J Mol Cell Cardiol 53: 509–520. [DOI] [PubMed] [Google Scholar]
- Dai XY, Cai Y, Sun W, Ding Y, Wang W, Kong W et al. (2014). Intermedin inhibits macrophage foam‐cell formation via tristetraprolin‐mediated decay of CD36 mRNA. Cardiovasc Res 101: 297–305. [DOI] [PubMed] [Google Scholar]
- Deng T, Lyon CJ, Minze LJ, Lin J, Zou J, Liu JZ et al. (2013). Class II major histocompatibility complex plays an essential role in obesity‐induced adipose inflammation. Cell Metab 17: 411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong F, Taylor MM, Samson WK, Ren J (2006). Intermedin (adrenomedullin‐2) enhances cardiac contractile function via a protein kinase C‐ and protein kinase A‐dependent pathway in murine ventricular myocytes. J Appl Physiol 101: 778–784. [DOI] [PubMed] [Google Scholar]
- Fujisawa Y, Nagai Y, Miyatake A, Miura K, Nishiyama A, Kimura S et al. (2007). Effects of adrenomedullin 2 on regional hemodynamics in conscious rats. Eur J Pharmacol 558: 128–132. [DOI] [PubMed] [Google Scholar]
- Fujisawa Y, Nagai Y, Miyatake A, Miura K, Shokoji T, Nishiyama A et al. (2006). Roles of adrenomedullin 2 in regulating the cardiovascular and sympathetic nervous systems in conscious rats. Am J Physiol Heart Circ Physiol 290: H1120–H1127. [DOI] [PubMed] [Google Scholar]
- Fujisawa Y, Nagai Y, Miyatake A, Takei Y, Miura K, Shoukouji T et al. (2004). Renal effects of a new member of adrenomedullin family, adrenomedullin2, in rats. Eur J Pharmacol 497: 75–80. [DOI] [PubMed] [Google Scholar]
- Gan XB, Sun HJ, Chen D, Zhang LL, Zhou H, Chen LY et al. (2014). Intermedin in the paraventricular nucleus attenuates cardiac sympathetic afferent reflex in chronic heart failure rats. PLoS One 9: e94234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossini E, Molinari C, Mary DA, Uberti F, Caimmi PP, Vacca G (2009). Intracoronary intermedin 1‐47 augments cardiac perfusion and function in anesthetized pigs: role of calcitonin receptors and beta‐adrenoreceptor‐mediated nitric oxide release. J Appl Physiol 107: 1037–1050. [DOI] [PubMed] [Google Scholar]
- Hay DL, Christopoulos G, Christopoulos A, Poyner DR, Sexton PM (2005). Pharmacological discrimination of calcitonin receptor: receptor activity‐modifying protein complexes. Mol Pharmacol 67: 1655–1665. [DOI] [PubMed] [Google Scholar]
- Hirose T, Totsune K, Mori N, Morimoto R, Hashimoto M, Nakashige Y et al. (2008). Increased expression of adrenomedullin 2/intermedin in rat hearts with congestive heart failure. Eur J Heart Fail 10: 840–849. [DOI] [PubMed] [Google Scholar]
- Hirose T, Totsune K, Nakashige Y, Metoki H, Kikuya M, Ohkubo T et al. (2011). Influence of adrenomedullin 2/intermedin gene polymorphism on blood pressure, renal function and silent cerebrovascular lesions in Japanese: the Ohasama study. Hypertens Res 34: 1327–1332. [DOI] [PubMed] [Google Scholar]
- Hong Y, Hay DL, Quirion R, Poyner DR (2012). The pharmacology of adrenomedullin 2/intermedin. Br J Pharmacol 166: 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly L, March JE, Kemp PA, Bennett T, Gardiner SM (2009). Mechanisms involved in the regional haemodynamic effects of intermedin (adrenomedullin 2) compared with adrenomedullin in conscious rats. Br J Pharmacol 157: 1502–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandilci HB, Gumusel B, Lippton H (2008). Intermedin/adrenomedullin‐2 (IMD/AM2) relaxes rat main pulmonary arterial rings via cGMP‐dependent pathway: role of nitric oxide and large conductance calcium‐activated potassium channels (BK(Ca)). Peptides 29: 1321–1328. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Liu YJ, Gonda T, Takei Y (2004). Coronary vasodilatory response to a novel peptide, adrenomedullin 2. Clin Exp Pharmacol Physiol 31 (Suppl 2): S49–S50. [DOI] [PubMed] [Google Scholar]
- Kovaleva IE, Garaeva AA, Chumakov PM, Evstafieva AG (2016). Intermedin/adrenomedullin 2 is a stress‐inducible gene controlled by activating transcription factor 4. Gene 590: 177–185. [DOI] [PubMed] [Google Scholar]
- Li P, Shi L, Han Y, Zhao Y, Qi Y, Wang B (2016). Prognostic value of plasma intermedin level in patients with non‐st‐segment elevation acute coronary syndrome. Medicine 95: e3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Sun HJ, Han Y, Wang JJ, Zhang F, Tang CS et al. (2013). Intermedin enhances sympathetic outflow via receptor‐mediated cAMP/PKA signaling pathway in nucleus tractus solitarii of rats. Peptides 47C: 1–6. [DOI] [PubMed] [Google Scholar]
- Lin Chang C, Roh J, Park JI, Klein C, Cushman N, Haberberger RV et al. (2005). Intermedin functions as a pituitary paracrine factor regulating prolactin release. Mol Endocrinol 19: 2824–2838. [DOI] [PubMed] [Google Scholar]
- Lu WW, Zhao L, Zhang JS, Hou YL, Yu YR, Jia MZ et al. (2015). Intermedin1‐53 protects against cardiac hypertrophy by inhibiting endoplasmic reticulum stress via activating AMP‐activated protein kinase. J Hypertens 33: 1676–1687. [DOI] [PubMed] [Google Scholar]
- Luan B, Goodarzi MO, Phillips NG, Guo X, Chen YD, Yao J et al. (2014). Leptin‐mediated increases in catecholamine signaling reduce adipose tissue inflammation via activation of macrophage HDAC4. Cell Metab 19: 1058–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Y, Zhang SY, Liang X, Zhang H, Xu Z, Liu B et al. (2016). Adrenomedullin 2 enhances beiging in white adipose tissue directly in an adipocyte‐autonomous manner and indirectly through activation of M2 macrophages. J Biol Chem 291: 23390–23402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Z, Wu K, Chen X, Zhang X, Hong B (2013). Plasma intermedin levels in patients with acute myocardial infarction. Peptides 43: 121–125. [DOI] [PubMed] [Google Scholar]
- Maruthur NM, Gudzune K, Hutfless S, Fawole OA, Wilson RF, Lau BD et al. (2014). Avoiding weight gain in cardiometabolic disease: a systematic review. Journal of Obesity 2014: 358919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q, Shi D, Feng J, Su Y, Long Y, He S et al. (2016). Hypercholesterolemia up‐regulates the expression of intermedin and its receptor components in the aorta of rats via inducing the oxidative stress. Ann Clin Lab Sci 46: 5–17. [PubMed] [Google Scholar]
- Morimoto R, Satoh F, Murakami O, Totsune K, Suzuki T, Sasano H et al. (2007). Expression of adrenomedullin2/intermedin in human brain, heart, and kidney. Peptides 28: 1095–1103. [DOI] [PubMed] [Google Scholar]
- Muller‐Redetzky HC, Kummer W, Pfeil U, Hellwig K, Will D, Paddenberg R et al. (2012). Intermedin stabilized endothelial barrier function and attenuated ventilator‐induced lung injury in mice. PLoS One 7: e35832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzel G, Schlier A, Schreckenberg R, Abdallah Y, Schluter KD (2011). Rat intermedin1‐47 does not improve functional recovery in postischemic hearts. Naunyn Schmiedebergs Arch Pharmacol 384: 535–542. [DOI] [PubMed] [Google Scholar]
- Nagasaki S, Fukui M, Asano S, Ono K, Miki Y, Araki S et al. (2014). Induction of adrenomedullin 2/intermedin expression by thyroid stimulating hormone in thyroid. Mol Cell Endocrinol 395: 32–40. [DOI] [PubMed] [Google Scholar]
- Ordovas JM, Corella D (2008). Metabolic syndrome pathophysiology: the role of adipose tissue. Kidney International (Suppl): S10–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owji AA, Chabot JG, Dumont Y, Quirion R (2008). Adrenomedullin‐2/intermedin induces cAMP accumulation in dissociated rat spinal cord cells: evidence for the existence of a distinct class of sites of action. J Mol Neurosci 35: 355–361. [DOI] [PubMed] [Google Scholar]
- Pan CS, Yang JH, Cai DY, Zhao J, Gerns H, Yang J et al. (2005). Cardiovascular effects of newly discovered peptide intermedin/adrenomedullin 2. Peptides 26: 1640–1646. [DOI] [PubMed] [Google Scholar]
- Pang Y, Li Y, Lv Y, Sun L, Zhang S, Li Y et al. (2016). Intermedin restores hyperhomocysteinemia‐induced macrophage polarization and improves insulin resistance in mice. J Biol Chem 291: 12336–12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeil U, Aslam M, Paddenberg R, Quanz K, Chang CL, Park JI et al. (2009). Intermedin/adrenomedullin‐2 is a hypoxia‐induced endothelial peptide that stabilizes pulmonary microvascular permeability. Am J Physiol Lung Cell Mol Physiol 297: L837–L845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires AL, Pinho M, Alves BS, Pinho S, Sena C, Seica RM et al. (2013). Reverse myocardial effects of intermedin in pressure‐overloaded hearts: role of endothelial nitric oxide synthase activity. J Physiol 591: 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires AL, Pinho M, Sena CM, Seica R, Leite‐Moreira AF (2012). Intermedin elicits a negative inotropic effect in rat papillary muscles mediated by endothelial‐derived nitric oxide. Am J Physiol Heart Circ Physiol 302: H1131–H1137. [DOI] [PubMed] [Google Scholar]
- Qi T, Hay DL (2010). Structure‐function relationships of the N‐terminus of receptor activity‐modifying proteins. Br J Pharmacol 159: 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin YW, Teng X, He JQ, Du J, Tang CS, Qi YF (2013). Increased plasma levels of intermedin and brain natriuretic peptide associated with severity of coronary stenosis in acute coronary syndrome. Peptides 42: 84–88. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM et al. (2014). Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 157: 1292–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren YS, Yang JH, Zhang J, Pan CS, Yang J, Zhao J et al. (2006). Intermedin 1‐53 in central nervous system elevates arterial blood pressure in rats. Peptides 27: 74–79. [DOI] [PubMed] [Google Scholar]
- Roh J, Chang CL, Bhalla A, Klein C, Hsu SY (2004). Intermedin is a calcitonin/calcitonin gene‐related peptide family peptide acting through the calcitonin receptor‐like receptor/receptor activity‐modifying protein receptor complexes. J Biol Chem 279: 7264–7274. [DOI] [PubMed] [Google Scholar]
- Smith RS Jr, Gao L, Bledsoe G, Chao L, Chao J (2009). Intermedin is a new angiogenic growth factor. Am J Physiol Heart Circ Physiol 297: H1040–H1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JQ, Teng X, Cai Y, Tang CS, Qi YF (2009). Activation of Akt/GSK‐3beta signaling pathway is involved in intermedin(1‐53) protection against myocardial apoptosis induced by ischemia/reperfusion. Apoptosis 14: 1299–1307. [DOI] [PubMed] [Google Scholar]
- Soultanova A, Mikulski Z, Pfeil U, Grau V, Kummer W (2016). Calcitonin peptide family members are differentially regulated by LPS and inhibit functions of rat alveolar NR8383 macrophages. PLoS One 11: e0163483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44 (D1): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei Y, Hashimoto H, Inoue K, Osaki T, Yoshizawa‐Kumagaye K, Tsunemi M et al. (2008). Central and peripheral cardiovascular actions of adrenomedullin 5, a novel member of the calcitonin gene‐related peptide family, in mammals. J Endocrinol 197: 391–400. [DOI] [PubMed] [Google Scholar]
- Takei Y, Inoue K, Ogoshi M, Kawahara T, Bannai H, Miyano S (2004). Identification of novel adrenomedullin in mammals: a potent cardiovascular and renal regulator. FEBS Lett 556: 53–58. [DOI] [PubMed] [Google Scholar]
- Tam CW, Husmann K, Clark NC, Clark JE, Lazar Z, Ittner LM et al. (2006). Enhanced vascular responses to adrenomedullin in mice overexpressing receptor‐activity‐modifying protein 2. Circ Res 98: 262–270. [DOI] [PubMed] [Google Scholar]
- Tang B, Zhong Z, Shen HW, Wu HP, Xiang P, Hu B (2014). Intermedin as a prognostic factor for major adverse cardiovascular events in patients with ST‐segment elevation acute myocardial infarction. Peptides 58: 98–102. [DOI] [PubMed] [Google Scholar]
- Taylor MM, Bagley SL, Samson WK (2005a). Intermedin/adrenomedullin‐2 acts within central nervous system to elevate blood pressure and inhibit food and water intake. Am J Physiol Regul Integr Comp Physiol 288: R919–R927. [DOI] [PubMed] [Google Scholar]
- Taylor MM, Samson WK (2005b). Stress hormone secretion is altered by central administration of intermedin/adrenomedullin‐2. Brain Res 1045: 199–205. [DOI] [PubMed] [Google Scholar]
- Teng X, Bian Y, Cai Y, Duan X, Yuan F, Du J et al. (2013). Downregulation of endogenous intermedin augmented myocardial injury in rats ischemic/reperfusion. Horm Metab Res 45: 206–212. [DOI] [PubMed] [Google Scholar]
- Teng X, Song J, Zhang G, Cai Y, Yuan F, Du J et al. (2011). Inhibition of endoplasmic reticulum stress by intermedin(1‐53) protects against myocardial injury through a PI3 kinase‐Akt signaling pathway. J Mol Med (Berl) 89: 1195–1205. [DOI] [PubMed] [Google Scholar]
- Wei P, Yang XJ, Fu Q, Han B, Ling L, Bai J et al. (2015). Intermedin attenuates myocardial infarction through activation of autophagy in a rat model of ischemic heart failure via both cAMP and MAPK/ERK1/2 pathways. Inte0rnational journal of clinical and experimental pathology 8: 9836–9844. [PMC free article] [PubMed] [Google Scholar]
- Weston C, Lu J, Li N, Barkan K, Richards GO, Roberts DJ et al. (2015). Modulation of glucagon receptor pharmacology by receptor activity‐modifying protein‐2 (RAMP2). J Biol Chem 290: 23009–23022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston C, Winfield I, Harris M, Hodgson R, Shah A, Dowell SJ et al. (2016). Receptor activity‐modifying protein‐directed G protein signaling specificity for the calcitonin gene‐related peptide family of receptors. J Biol Chem 291: 21925–21944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MM, Samson WK (2007). Intermedin 17‐47 does not function as a full intermedin antagonist within the central nervous system or pituitary. Peptides 28: 2171–2178. [DOI] [PubMed] [Google Scholar]
- Wong CW, O WS, Tang F (2013). Intermedin in rat uterus: changes in gene expression and peptide levels across the estrous cycle and its effects on uterine contraction. Reprod Biol Endocrinol 11: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F, Wang LJ, Zhao H, Tan C, Wang DN, Zhang H et al. (2015). Intermedin restricts vessel sprouting by inhibiting the loosening of endothelial junction. Biochem Biophys Res Commun 458: 174–179. [DOI] [PubMed] [Google Scholar]
- Yang JH, Cai Y, Duan XH, Ma CG, Wang X, Tang CS et al. (2009). Intermedin 1‐53 inhibits rat cardiac fibroblast activation induced by angiotensin II. Regul Pept 158: 19–25. [DOI] [PubMed] [Google Scholar]
- Yang JH, Jia YX, Pan CS, Zhao J, Ouyang M, Yang J et al. (2005). Effects of intermedin(1‐53) on cardiac function and ischemia/reperfusion injury in isolated rat hearts. Biochem Biophys Res Commun 327: 713–719. [DOI] [PubMed] [Google Scholar]
- Yang JH, Pan CS, Jia YX, Zhang J, Zhao J, Pang YZ et al. (2006). Intermedin1‐53 activates L‐arginine/nitric oxide synthase/nitric oxide pathway in rat aortas. Biochem Biophys Res Commun 341: 567–572. [DOI] [PubMed] [Google Scholar]
- Yang SM, Liu J, Li CX (2014). Intermedin protects against myocardial ischemia‐reperfusion injury in hyperlipidemia rats. Genetics and Molecular Research: GMR 13: 8309–8319. [DOI] [PubMed] [Google Scholar]
- Yuyun MF, Narayan HK, Ng LL (2015). Prognostic significance of adrenomedullin in patients with heart failure and with myocardial infarction. Am J Cardiol 115: 986–991. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Yuan Y, Wang X, Wu HM, Fan L, Qi YF et al. (2009). Upregulated expression of intermedin and its receptor in the myocardium and aorta in spontaneously hypertensive rats. Peptides 30: 391–399. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhang SY, Jiang C, Li Y, Xu G, Xu MJ et al. (2016a). Intermedin/adrenomedullin 2 polypeptide promotes adipose tissue browning and reduces high‐fat diet‐induced obesity and insulin resistance in mice. Int J Obes (Lond) 40: 852–860. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Jiang W, Liu JY, Li Y, Chen CL, Xin HB et al. (2009). Intermedin is upregulated and has protective roles in a mouse ischemia/reperfusion model. Hypertens Res 32: 861–868. [DOI] [PubMed] [Google Scholar]
- Zhang SY, Lv Y, Zhang H, Gao S, Wang T, Feng J et al. (2016b). Adrenomedullin 2 improves early obesity‐induced adipose insulin resistance by inhibiting the class II MHC in adipocytes. Diabetes 65: 2342–2355. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang LJ, Xiao F, Wei Y, Ke W, Xin HB (2012a). Intermedin: a novel regulator for vascular remodeling and tumor vessel normalization by regulating vascular endothelial‐cadherin and extracellular signal‐regulated kinase. Arterioscler Thromb Vasc Biol 32: 2721–2732. [DOI] [PubMed] [Google Scholar]
- Zhang X, Gu L, Chen X, Wang S, Deng X, Liu K et al. (2012b). Intermedin ameliorates atherosclerosis in ApoE null mice by modifying lipid profiles. Peptides 37: 189–193. [DOI] [PubMed] [Google Scholar]
- Zhao L, Peng DQ, Zhang J, Song JQ, Teng X, Yu YR et al. (2012). Extracellular signal‐regulated kinase 1/2 activation is involved in intermedin1‐53 attenuating myocardial oxidative stress injury induced by ischemia/reperfusion. Peptides 33: 329–335. [DOI] [PubMed] [Google Scholar]
- Zhou YB, Sun HJ, Chen D, Liu TY, Han Y, Wang JJ et al. (2014). Intermedin in paraventricular nucleus attenuates sympathetic activity and blood pressure via nitric oxide in hypertensive rats. Hypertension 63: 330–337. [DOI] [PubMed] [Google Scholar]


