Abstract
Thrombospondin‐1 (TSP‐1), a matricellular protein and one of the first endogenous anti‐angiogenic molecules identified, has long been considered a potent modulator of human diseases. While the therapeutic effect of TSP‐1 to suppress cancer was investigated in both research and clinical settings, the mechanisms of how TSP‐1 is regulated in cancer remain elusive, and the scientific answers to the question of whether TSP‐1 expressions can be utilized as diagnostic or prognostic marker for patients with cancer are largely inconsistent. Moreover, TSP‐1 plays crucial functions in angiogenesis, inflammation and tissue remodelling, which are essential biological processes in the progression of many cardiovascular diseases, and therefore, its dysregulated expressions in such conditions may have therapeutic significance. Herein, we critically analysed the literature pertaining to TSP‐1 expression in circulating blood and pathological tissues in various types of cancer as well as cardiovascular and inflammation‐related diseases in humans. We compare the secretion rates of TSP‐1 by different cancer and non‐cancer cells and discuss the potential connection between the expression changes of TSP‐1 and vascular endothelial growth factor (VEGF) observed in patients with cancer. Moreover, the pattern and emerging significance of TSP‐1 profiles in cardiovascular disease, such as peripheral arterial disease, diabetes and other related non‐cancer disorders, are highlighted. The analysis of published TSP‐1 data presented in this review may have implications for the future exploration of novel TSP‐1‐based treatment strategies for cancer and cardiovascular‐related diseases.
Keywords: angiogenesis, cancer, cardiovascular disease, matricellular, peripheral arterial disease, thrombospondin‐1
List of Main Topics.
Thrombospondin‐1 (TSP‐1), a matricellular protein, is often induced at sites of injury and tissue remodelling, and it is secreted by circulating, stromal and parenchymal cells at very different quantities.
The major cell‐surface receptors for TSP‐1, namely CD36, CD47 and integrins, participate in important cellular processes including apoptosis, angiogenesis, blood flow, phagocytosis, migration and immune regulation.
TSP‐1 is known to inhibit angiogenesis and endothelial cell survival.
Cancer patient data on TSP‐1 in blood and cancerous tissues suggest that the patterns of TSP‐1 expression compared to non‐cancer controls are highly heterogeneous across different cancer types and that the correlations between TSP‐1 levels in patients and survival are also cancer type‐specific.
Human TSP‐1 protein expression in circulating blood is uniformly up‐regulated in a variety of cardiovascular and inflammatory diseases and is often associated with worse patient outcomes.
1. INTRODUCTION
Complex multicellular organisms require surface adhesion and a three‐dimensional support system. The extracellular matrix provides these necessary biomechanical structural elements.1 The generation and maintenance of this enveloping structural system are cooperatively regulated by a variety of cellular activities and signals. A specialized group of secreted proteins interface with and bind to the matrix and the cells contained by the matrix to act as intermediates to alter cell response primarily from outside‐in. This class of proteins has been termed matricellular proteins, and it represents an expanding group of molecules that includes over nine subfamilies of proteins that are increasingly recognized as playing important roles in homoeostasis and recently in diseases.2 As the name implies matricellular proteins function to span the area between the cell surface and the matrix scaffold. Also important to the understanding of these molecules is that they do not impart strength to the matrix themselves, instead they can regulate matrix metabolism to alter the biomechanical properties of the extracellular matrix.
A quintessential and founding member of this group is thrombospondin‐1 (TSP‐1), a protein first identified in the particulate fraction of thrombin‐activated platelets3 and this fact being incorporated in its name. Like many secreted proteins post‐development, TSP‐1 is minimally detectable in health but rapidly up‐regulated with injury and persists in chronic diseases, being found in the parenchyma as well as fluid compartments including the blood,4 urine5 and cerebrospinal fluid.6 TSP‐1 is trimeric, with each monomer about 130‐150 kD, but the secreted protein is heavily modified by glycosylation and weighs over 450 kD (Figure 1).7 Secreted TSP‐1 directly transduces signals through binding via discrete domains to cell‐surface receptors including but not limited to CD47, CD36 and integrins.8 Indirectly, TSP‐1 regulates cell signalling through binding to other molecules such as enzymes and growth factors.9, 10 Consequently, the manifold roles of TSP‐1 in modulating cell functions are concentration‐ and cell type‐specific. Nonetheless, some trends have emerged. From the perspective of pharmacology, high concentrations of TSP‐1 check the cell cycle in primary cells to impede self‐renewal and proliferation11 and can, at certain concentrations, induce cell death.12, 13 These effects arise, in part, from the ability of TSP‐1 to limit pro‐growth signals and several key elements of metabolism.14 Another essential feature of TSP‐1 is its ability to control tissue repair and remodelling in response to injury and stress, a property enhanced by its important function to increase transforming growth factor beta (TGF‐beta) activity.15 In the central nervous system, TSP‐1 is expressed and secreted by astrocytes and is a promoter of synapse formation as well as neuronal proliferation and differentiation.16 During immune activation, TSP‐1 has a supportive role and can increase the activation of inflammatory cells including monocytes,17 dendritic cells,18 macrophages19 and T cells.20 Conversely, in other settings such as during the resolution of inflammation, TSP‐1 may act to suppress inflammation.21, 22 A fundamental and well‐established property of TSP‐1 is to limit endothelial cell (EC)‐mediated angiogenesis by inhibiting the activity of vascular endothelial growth factor (VEGF)23 and the pleiotropic signals of the gasotransmitter nitric oxide (NO).24 In this way, TSP‐1 adversely impacts angiogenesis and blood flow25 and modulates blood pressure by limiting NO‐mediated vasorelaxation.26 In this latter capacity, TSP‐1 is now recognized to alter cardiovascular responses in general.25, 27 In the light of its role to retard angiogenesis, TSP‐1 is shown to suppress tumour growth and is often found down‐regulated in the tumour microenvironment coincident with accelerated tumour invasiveness.28 Increases in circulating TSP‐1 expression have also been found to be positively correlated with patient survival in some cancers.29, 30 Expectantly, TSP‐1‐derived drugs have been developed in the interest of inhibiting angiogenesis and treating cancer.31, 32 However, the effect of TSP‐1 in cancer is not simple, and it has been reported that TSP‐1 can also support tumour growth and spread.33 Interest has been growing in TSP‐1 as a possible biomarker and an important contributor to human diseases, particularly in age‐related and metabolic diseases.34, 35 At the same time, TSP‐1 and several of its cell‐surface receptors, notably CD36 and CD47, have and continue to be pursued as therapeutic targets.36, 37
Figure 1.
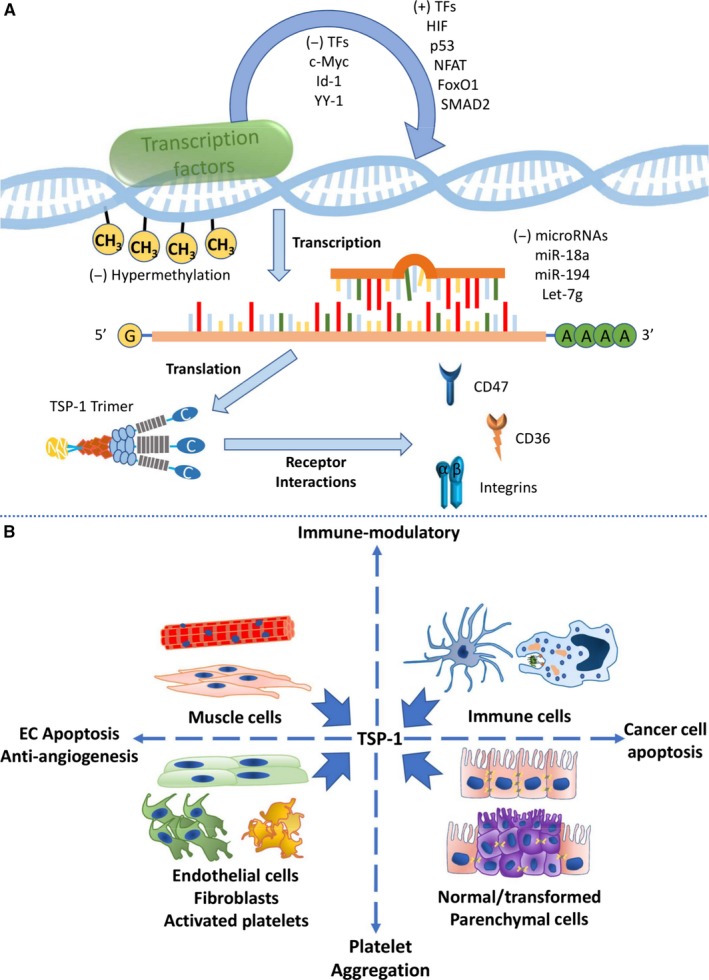
Regulation of thrombospondin‐1 (TSP‐1) at multiple levels and TSP‐1 secretion by different cells. (A) TSP‐1 gene transcription is regulated by multiple transcription factors and gene methylation status; TSP‐1 mRNA can be targeted by several microRNAs, and TSP‐1 protein which usually exists in trimers interacts with several cell‐surface receptors. (B) Various types of cells in humans produce and secrete TSP‐1, which can potently regulate many important cellular processes. Note that not all TSP‐1 receptors and functions are depicted here (see Conclusions and Future Considerations for more details)
Renewed appreciation of TSP‐1 in the pathophysiology of diseases has encouraged research into the expression of this protein in different cells and body compartments. However, a systematic characterization and quantification of published TSP‐1 expression data in diseases, both amount and rate of production, has not been previously approached. Such an analysis is important, and it can provide insight into the sometimes conflicting activity of this molecule and serve to guide therapeutic interventions that target pathways mediated by TSP‐1; it is also important for systems biology computational studies of TSP‐1.38, 39, 40 Herein, we present the results of a systematic multilevel (cell, fluid, tissue) characterization of TSP‐1 concentrations in people in health and disease, specifically in cancer and cardiovascular diseases, derived from analysis of the current literature. Interestingly, strong patterns emerge. In cancer, TSP‐1 expression is surprisingly heterogeneous to the point of forestalling prediction as to its role in many of these cases. Conversely, in inflammatory and cardiovascular diseases, TSP‐1 expression levels show a persistent trend to be significantly elevated compared to non‐diseased subjects. The correlation between dysregulated TSP‐1 expressions in diseases and patient outcomes is also discussed. These results emphasize the role of the protein, beyond the sphere of cancer, as indicative of promoting disease and as a possible therapeutic index.
2. TSP‐1 SECRETION FROM PARENCHYMA AND STROMA
Thrombospondin‐1 is reported to be expressed and secreted by a variety of normal cell types in human including ECs, fibroblasts, muscle cells, immune cells, platelets (and megakaryocytes) as well as transformed parenchymal cells in many types of cancer (Figure 1).41, 42, 43 Secreted TSP‐1 proteins play key roles in the regulation of angiogenesis and immune response, both of which are critical processes in the progression of tumours and cardiovascular diseases. However, specific cell types exist in quite different numbers within the whole tissue. Therefore, a direct, quantitative comparison of the absolute TSP‐1 secretion rates by different parenchymal and stromal cells will introduce new insight into the research of heterogeneity in the tumour microenvironment and other diseases.
Beginning at the cell level, a literature search was conducted and TSP‐1 secretion rates from different types of cells were analysed (Table 1).44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54 Interestingly, secretion rates of TSP‐1 protein from the stromal components (eg fibroblasts, ECs) are at least one to two orders of magnitude greater than those rates from cancer cells. Among different types of stromal cells, ECs (represented by human umbilical vein ECs) produce and secrete TSP‐1 proteins at very high rates. ECs usually occupy a fairly small portion (1%‐2%) of the total cells in a tissue, but this percentage can be significantly higher in some highly vascularized tissues such as in certain tumours, lungs and hearts.55, 56 In this sense, modulating EC‐specific pathways (eg via transcription factors, receptor activation and microRNAs) that regulate TSP‐1 production and secretion may substantially influence the overall TSP‐1 abundance in the tissue environment and vasculature. In contrast, the many types of cancer cells examined, except for glioma, Kaposi's sarcoma and prostate cancer cells, contribute relatively insignificant amounts of TSP‐1 on a rate per million cells basis. Analyses of tumour sample immunohistochemical staining confirm the high TSP‐1 expression and localization in the tumour stroma rather than in the tumour cells.44, 57, 58 In addition, published data of TSP‐1 mRNA expressions across various cell lines show a qualitatively similar trend compared with the secretion rates presented in Table 1.59 Still, production and secretion rates of TSP‐1 would depend on various factors including the density of cultured cells and also the appropriate stimuli which are present in tissue environments in vivo but may not be contained in the culture media; therefore, the differences between cell‐specific secretion rates outlined here should be interpreted in both quantitative and qualitative manners. Further studies and measurements are needed to elucidate the potential correlations between in vitro (summarized in this review) and in vivo TSP‐1 production capacities in the different cell types.
Table 1.
TSP‐1 secretion rates from different cancer and non‐cancer cell types
| Human cell type (cell line) | TSP‐1 protein secretion rate (ng/106 cells/24 h) | References |
|---|---|---|
| Pancreatic cancer (AsPC‐1; Colo‐357; Panc‐1; T3M4) | 276; 61; 90; 94 | 44 |
| Glioma (T98G; U251; A172; KG‐1‐C; TM2; YMG1; YMG2; YMG3; YMG4; YMG5) | 2431; 275; 59; 43; 475; 69; 1081; 1450; 126; 250 | 45 |
| Breast cancer (YMB‐1) | 3 | 45 |
| Breast cancer (T47D; BT‐474)a | 3; 3 | 46 |
| Lung cancer (A549) | 20 | 45 |
| Gastric cancer (NUGC‐4) | 31 | 45 |
| Hepatic cancer (HLF) | 89 | 45 |
| Colon cancer (Colo‐201) | 3 | 45 |
| Prostate cancer (PC3) | 610 | 45 |
| Melanoma (DFB) | 8 | 45 |
| Neuroblastoma (IMR‐32) | 4 | 45 |
| B‐Chronic lymphocytic leukaemia (B cells from patients) | 9 | 47 |
| Promyelocytic leukaemia (NB4; HL‐60)b | 55; 40 | 48 |
| Kaposi's sarcoma (IST‐KS XVI; IST‐KS VIII; IST‐KS XI; IST‐KS IV) | 6500; 3400; 4400; 8500 | 51 |
| Human foreskin fibroblast | 474 | 44 |
| Human foreskin fibroblastc | 15 700 | 49 |
| Human foreskin fibroblastc | 3333 | 50 |
| Human foetal lung fibroblast (GM1604)c | 5800 | 49 |
| Endothelial cell (HUVEC)c | 21 000 | 49 |
| Endothelial cell (HUVEC) | 19 500 | 51 |
| Endothelial cell (HUVEC)c | 49 000 | 52 |
| Human aortic smooth muscle cellc | 9467 | 50 |
| Human dendritic celld | 10 153; 3053 | 53 |
| Human retinal glial cell (MIO‐M1) | 125 | 54 |
Unit conversion is implemented by assuming 150 pg of total protein per cell.60
Cells are treated with all‐trans retinoid acid.
Study did not specify that TSP data are limited to TSP‐1.
Cells are treated with ATP and prostaglandin E2, respectively. Values are rounded to the nearest integer.
3. QUANTITATIVE TSP‐1 EXPRESSION PROFILES IN PATHOLOGICAL CONDITIONS
3.1. Human cancers
The potential of circulating and tissue TSP‐1 protein as diagnostic or prognostic markers for cancers, given its anti‐angiogenic and pro‐apoptotic properties, has been studied extensively. Table 2 summarizes the quantitatively measured TSP‐1 protein levels in the plasma, serum, platelet and tissue of individuals with various cancer conditions.61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85 Most measured values of plasma TSP‐1 protein levels are in the range of a few hundred to a few thousand ng/mL, indicating that physiological TSP‐1 concentrations are relatively low in the circulation.86 It should be noted that data on soluble plasma and serum TSP‐1 may be confounded by varying degrees of platelet activation that serve as a reservoir of pre‐formed TSP‐1 in alpha granules during sample acquisition and processing.42 Nonetheless, it is worth noting that a large portion of the plasma TSP‐1 data actually indicate an up‐regulation of TSP‐1 in patients with cancer compared to normal controls, especially in breast cancer, which might be considered counterintuitive to the well‐established anti‐angiogenic property of TSP‐1. In the case of breast cancer (general disease and not in the context of any specific subtypes), four separate studies have found a consistent, significant increase in plasma or tissue TSP‐1 protein in patients with cancer compared to healthy controls.67, 71, 83, 87 A strong positive correlation between plasma and intratumoural TSP‐1 is observed, and patients with lymph node metastasis have significantly higher plasma TSP‐1 compared to lymph node‐negative patients. Intratumoural TSP‐1 expression is also positively correlated with microvessel density, suggesting a pro‐angiogenic role of TSP‐1 in breast cancer.67 The up‐regulation of tissue TSP‐1 expression in breast cancer is further supported by TSP‐1 mRNA and immunohistochemical data.88, 89 In non‐small cell lung cancers, significantly lower plasma and serum levels of TSP‐1 have been observed in patients,75 and higher baseline serum TSP‐1 levels are found associated with increased overall survival in patients receiving treatments.76 In colon cancer, conflicting results of plasma TSP‐1 in patients versus controls have been reported.72, 73 In pancreatic cancer, serum TSP‐1 is down‐regulated in patients with cancer.78, 79 In glioblastoma, no significant difference is observed in serum TSP‐1 levels in patients compared to healthy subjects, but higher pre‐surgery serum TSP‐1 is prognostic of longer survival in patients after tumour resection.80 Overall, these data suggest that the cancer‐driven regulation of TSP‐1 expression in humans may be highly dependent on the specific cancer types and clinical stage and deserves further investigation. Given the high TSP‐1 secretion rates in stromal cells, the potential for cancer cells to up‐ and down‐regulate stromal cell TSP‐1 production to favour cancer growth and metastasis should also be considered in future TSP‐1 studies.
Table 2.
Circulating and tissue TSP‐1 protein levels in healthy (control) versus cancer subjects
| Cancer type studied | Plasma TSP‐1 (control) (ng/mL) | Plasma TSP‐1 (patient) (ng/mL) | P value of difference | No. of subjects (C; P) | References |
|---|---|---|---|---|---|
| Mixed | N/A | 54 (R = 7‐551) | N/A | N/A; 50 | 61 |
| Mixed | 399 (SEM = 61) | 491 (SEM = 66) | .3 | 43; 43 | 62 |
| Mixed a | 440 (IQR = 270‐559) | 850 (IQR = 493‐1336) | <.01 | 20; 24 | [ 63 ] |
| Mixed | 31 (IQR = 25‐34) | 73 (IQR = 34‐84) | <.001 | 12; 20 | [ 64 ] |
| AML a | 121 (IQR = 65‐181) | 11 (IQR = 7‐15) | <.01 | 12; 17 | [ 65 ] |
| GI, breast, lunga | 365 | 1095, 730, 1095 | N/A | 20; (22, 18, 17) | 66 |
| EBC, ABC | 221 (IQR = 175‐247) | 484, 588 (IQR = 344‐877, 430‐952) | <.05, <.001 | 36; (71, 66) | [ 67 ] |
| Breast | N/A | 280 (SEM = 53) | N/A | N/A; 12 | 68 |
| Breast | 396 (SD = 103) | 419 (SD = 102) | .45 | 65; 37 | 69 |
| Breast (metastatic) | 543 (IQR = 504‐967) | 2255 (IQR = 681‐4553) | .07 | 16;8 | 70 |
| Breast a | 190 (SD = 42) | 2482 (SD = 4095) | <.0001 | 31; 23 | [ 71 ] |
| Colon (dukes stage A, B, C, D) a | 124 (SD = 63) | 286, 389, 781, 1017 (SD = 211, 234, 589, 668) | <.05 for Stage B, C, D | 20; (42, 24, 21, 28) | [ 72 ] |
| Colon | 1698 (IQR = 1437‐2703) | 328 | <.001 | 36; 33 | [ 73 ] |
| Colon | 539 (SD = 389) | 412 (SD = 367) | NS | 84; 35 | 74 |
| NSCLC | 4167 (IQR = 3585‐5472) | 2500 | .004 | 46; 21 | [ 75 ] |
| Cancer type studied | Serum TSP‐1 (control) (μg/mL) | Serum TSP‐1 (patient) (μg/mL) | P value of difference | No. of subjects (C; P) | References |
|---|---|---|---|---|---|
| NSCLC | 108 (IQR = 49‐225) | 35 | .012 | 46; 21 | [ 75 ] |
| NSCLC | 48 (SD = 10) | 45 (SD = 16) | .3 | 60; 60 | 76 |
| NSCLC | 180 (R = 110‐201) | 177 (R = 97‐206) | .158 | 18; 40 | 77 |
| Pancreatic | 11 | 8 | <.0001 | 30; 34 | [ 78 ] |
| Pancreatic a | 16 | 14 | <.01 | 227; 333 | [ 79 ] |
| Glioblastoma | 67 (SD = 37) | 78 (SD = 26) | .37 | 9; 23 | 80 |
| HCC | N/A | 17 (IQR = 11‐23) | N/A | N/A; 60 | 81 |
| Cancer type studied | Platelet TSP‐1 (control) (ng/106 platelets) | Platelet TSP‐1 (patient) (ng/106 platelets) | P value of difference | No. of subjects (C; P) | References |
|---|---|---|---|---|---|
| Breast | 27 (IQR = 24‐59) | 29 (IQR = 22‐78) | .821 | 65; 37 | 69 |
| NSCLC | 9 (IQR = 2‐22) | 15 (IQR = 3‐58) | .092 | 68; 68 | 82 |
| Colon | 34 (R = 14‐100) | 35 (R = 17‐139) | .88 | 84; 35 | 74 |
| Mixed | 38 (IQR = 28‐45) | 29 (IQR = 18‐38) | .005 | 43; 43 | [ 62 ] |
| Cancer type studied | Tissue TSP‐1 (control/benign) (μg/g of total protein) | Tissue TSP‐1 (patient/cancer) (μg/g of total protein) | P value of difference | No. of subjects (C; P) | References |
|---|---|---|---|---|---|
| Breast a | 22 | 317 | <.000001 | 15; 101 | [ 83 ] |
| Breasta | N/A | 6 (IQR = 4‐10) | N/A | N/A; 166 | 84 |
| Adrenocortical | 142 (R = 40‐390) | 69 (R = 8‐344) | <.01 | 18; 13 | [ 85 ] |
| HCC | N/A | 9 (IQR = 5‐16) | N/A | N/A; 60 | 81 |
Mean/median values are shown in the left of the 2nd and 3rd columns; variations of measurements are shown in the right. Statistical analysis results are shown in the 4th column.
R, range; SEM, standard error of the mean; IQR, interquartile range; SD, standard deviation; N/A, not applicable; NS, non‐significant; AML, acute myeloid leukaemia; GI, gastrointestinal; EBC, early breast cancer; ABC, advanced breast cancer; NSCLC, non‐small cell lung cancer; HCC, hepatocellular carcinoma.
Study did not specify that TSP data are limited to TSP‐1. Values are rounded to the nearest integer. Rows with P values smaller than .05 (indicating statistical significance of the difference observed) are bolded.
3.2. Cardiovascular diseases
In contrast to cancer, angiogenesis is often impaired and therefore desired in many age‐related and cardiovascular diseases, especially in ischaemic vascular diseases such as coronary artery disease (CAD) and peripheral arterial disease (PAD). One hypothesis offered to explain the pathophysiology of CAD and PAD is that anti‐angiogenic factors (eg TSP‐1) may be highly up‐regulated in the ischaemic tissue, in addition to the insufficient induction of pro‐angiogenic factors (eg VEGF, NO).39, 90 To date, only a few studies have explored this hypothesis and confirmed the increase in plasma and skeletal muscle TSP‐1 in PAD91, 92, 93 and CAD94 patients. Related to this, blockade of TSP‐1/CD47 signalling can enhance ischaemic tissue survival in experimental PAD models.95 Further, TSP‐1 protein levels in the plasma are significantly elevated in patients who suffer from other cardiovascular and inflammatory diseases, as well as diseases that are commonly accompanied by cardiovascular complications,96 including diabetes97 and sickle cell disease4, 98 (Table 3). Marked up‐regulations of TSP‐1 have been observed in the various organs and tissues of patients with diabetes and also in animal models of diabetes.99 This may be in part secondary to the known effects of high glucose on TSP‐1 production.100 In terms of disease outcome, strong negative correlations between plasma TSP‐1 protein levels and patient survival have been observed for pulmonary hypertension, acute ischaemic stroke and end‐stage renal disease, all conditions characterized by vasculopathy.101, 102, 103 Interestingly, on the other hand, thrombospondin proteins including TSP‐1 are involved in the unfolded protein response (also known as the endoplasmic reticulum stress response), and they are found to be induced and exert protective effects following myocardial injury in animal models, which adds another layer of complexity to the functions of the up‐regulated TSP‐1 in cardiovascular diseases.104
Table 3.
Circulating and tissue TSP‐1 levels in healthy (control) versus cardiovascular/CV‐related disease subjects
| CV‐related disease studied | Plasma TSP‐1 (control) (ng/mL) | Plasma TSP‐1 (patient) (ng/mL) | P value of difference | No. of subjects (C; P) | References |
|---|---|---|---|---|---|
| PAD | 218 | 476 | <.0001 | 184; 330 | [ 91 ] |
| PAD | 176 (SEM = 58) | 160 (SEM = 62) | NS | 17; 17 | 92 |
| Pulmonary hypertension | 82 (SD = 16) | 1114 (SD = 136) | <.05 | 19; 93 | [ 101 ] |
| Sickle cell disease | 239 (IQR = 125‐344) | 303 (IQR = 187‐939) | .056 | 17; 27 | 4 |
| Sickle cell disease | 491 (R = 331‐723) | 536 (R = 333‐1107) | NS | 8; 14 | 98 |
| Ischaemic stroke | 146 (SD = 50) | 571 (SD = 226) | <.001 | 150; 192 | [ 102 ] |
| Type I diabetes a | 91 (SEM = 14) | 137 (SEM = 14) | <.05 | 15; 30 | [ 97 ] |
| Vasculitis a | 59 (SD = 29) | 791 (SD = 1412) | .0002 | 33; 20 | [ 96 ] |
| CAD and DM | 518 (SD = 127) | 579 (SD = 106) | <.01 | 108; 103 | [ 94 ] |
| CV‐related disease studied | Interstitial TSP‐1 (control) (ng/mL) | Interstitial TSP‐1 (patient) (ng/mL) | P value of difference | No. of subjects (C; P) | References |
|---|---|---|---|---|---|
| PAD (muscle dialysate) | 54 (SEM = 24) | 219 (SEM = 70) | <.05 | 7; 6 | [ 92 ] |
| Healthy (muscle dialysate) | 100 (SEM = 18) | N/A | N/A | 8; N/A | 132 |
| CV‐related disease studied | Proximal/healthy tissue (AU) | Distal/ischaemic tissue (AU) | P value of difference | No. of subjects (C; P) | References |
|---|---|---|---|---|---|
| Tissue TSP‐1 mRNA in amputated limbs from CLI patients | 2 (SEM = 0.8) | 21 (SEM = 3.9) | .0001 | 13 | [ 93 ] |
Mean/median values are shown in the left of the 2nd and 3rd columns; variations of measurements are shown in the right. Statistical analysis results are shown in the 4th column.
R, range; SEM, standard error of the mean; IQR, interquartile range; SD, standard deviation; N/A, not applicable; NS, non‐significant; DM, diabetes mellitus; CLI, critical limb ischaemia; AU, arbitrary units.
Study did not specify that TSP data are limited to TSP‐1. Values are rounded to the nearest integer (except for values less than 1). Values are for TSP‐1 protein levels unless otherwise noted. Rows with P values smaller than .05 (indicating statistical significance of the difference observed) are bolded.
3.3. Correlations between TSP‐1 and VEGF
Paired data on quantitative TSP‐1 and VEGF protein expressions in cancers and PAD are shown in Table 4. While the trend of TSP‐1 expression in cancers remains elusive, VEGF levels (plasma, serum, platelet, tissue) tend to be up‐regulated in most cases.105 Published data so far have not suggested any correlation between circulating levels of VEGF and TSP‐1 in patients with cancer (Table 4), but elevated circulating VEGF expression alone is a well‐established prognostic marker of decreased patient survival in several types of cancer.106 Some previous studies have tried to elucidate the potential relationship between VEGF and TSP‐1 expressions within tumours. Although inverse correlation between tumour VEGF and TSP‐1 expressions has been suggested in prostate and endometrial cancers,57, 107 it may not always be the case, at least in bladder cancer, gastric cancer and hepatocellular carcinoma patients in which TSP‐1 protein levels are shown to be positively correlated with VEGF protein levels in the tumour tissue.81, 108, 109 In PAD studies, so far no correlations between TSP‐1 and VEGF levels in patients have been suggested, although both proteins are found up‐regulated in the patient plasma, and muscle interstitial TSP‐1, but not VEGF, is significantly induced in PAD patients which may indicate a potential imbalance between pro‐angiogenic and anti‐angiogenic factors in the ischaemic tissue.91, 92, 110, 111 Feedforward and feedback interactions between VEGF and TSP‐1 through direct intersection and with the gasotransmitter NO likely also complicate these expression patterns.23, 38 Moreover, the interplay between VEGF and TSP‐1 exists not only in angiogenic pathways, as both VEGF and TSP‐1 are shown to be regulators of the immune system.19, 20, 112
Table 4.
Circulating and tissue TSP‐1/VEGF protein levels in healthy (control) versus disease subjects
| Disease studied | Plasma TSP‐1 (control, patient) (ng/mL) | Plasma VEGF (control, patient) (pg/mL) | No. of subjects (C; P) | References |
|---|---|---|---|---|
| Cancer (mixed) | 399, 491 NS | 7, 44 P = .003 | 43; 43 | 62 |
| Breast cancer | 396, 419 NS | 53, 54 NS | 65; 37 | 69 |
| Breast cancer (metastatic) | 543, 2255 NS | 12, 29 P = .001 | 16; 17 | 70 |
| Colon cancer | 1698, 328 P < .001 | 2, 48 P < .001 | 36; 33 | 73 |
| Colon cancer | 539, 412 NS | 53, 40 NS | 84; 35 | 74 |
| PAD | 218, 476 P < .0001 | 14, 17 NS | 184; 330 | 91 |
| Disease studied | Serum TSP‐1 (control, patient) (μg/mL) | Serum VEGF (control, patient) (pg/mL) | No. of subjects (C; P) | References |
|---|---|---|---|---|
| NSCLC | 108, 35 P = .012 | 249, 452 P < .001 | 46; 21 | 75 |
| NSCLC | 48, 45 NS | 147, 408 P < .0001 | 60; 60 | 76 |
| NSCLC | 180, 177 NS | 189, 266 NS | 18; 40 | 77 |
| Disease studied | Platelet TSP‐1 (control, patient) (ng/106 platelets) | Platelet VEGF (control, patient) (pg/106 platelets) | No. of subjects (C; P) | References |
|---|---|---|---|---|
| Breast cancer | 27, 29 NS | 0.9, 2 P < .001 | 65; 37 | 69 |
| NSCLC | 9, 15 NS | 22, 41 P = .041 | 68; 68 | 82 |
| Colon cancer | 34, 35 NS | 0.6, 1.3 P < .0001 | 84; 35 | 74 |
| Cancer (mixed) | 38, 29 P = .005 | 0.6, 1.4 P < .0001 | 43; 43 | 62 |
| Disease studied | Tissue TSP‐1 (control/benign, patient/cancer) (μg/g of total protein) | Tissue VEGF (control/benign, patient/cancer) (ng/g of total protein) | No. of subjects (C; P) | References |
|---|---|---|---|---|
| Adrenocortical cancer | 142, 69 P < .01 | 44, 404 P < .001 | (18; 13), (15; 12) | 85 |
| Disease studied | Interstitial TSP‐1 (control, patient) (ng/mL) | Interstitial VEGF (control, patient) (pg/mL) | No. of subjects (C; P) | References |
|---|---|---|---|---|
| PAD | 54, 219 P < .05 | 55, 63 NS | (7; 6), (16; 16) | 92 |
Mean/median values are shown in the left of the 2nd and 3rd columns; statistical analysis results are shown in the right. P values greater than .05 are denoted as NS (non‐significant), and observations with P < .05 are bolded. Values are rounded to the nearest integer (except for values less than 2).
4. CONCLUSIONS AND FUTURE CONSIDERATIONS
Herein, we systematically reviewed the literature characterizing human TSP‐1 expressions in the circulation and in tissues, its cell type‐specific secretion and its significance and correlations with outcomes in human diseases. This analysis is driven by quantitative data and demonstrated some interesting findings. In cancer, TSP‐1 expression patterns in general are quite variable and, thus, appear to have limited prognostic or diagnostic value, although in specific cancer types (eg breast cancer), TSP‐1 up‐regulation is relatively consistent across independent datasets and is associated with malignancy and metastasis. In addition, a similar paradox in expression patterns pertains to TSP‐1 and VEGF in general cancer conditions. Contrary to the situation in cancer, TSP‐1 expression is uniformly up‐regulated in cardiovascular and inflammatory diseases and is associated with worse outcomes, albeit the studies testing the latter hypothesis are of limited number. Interpretation of these results should be tempered as expression data may not distinguish between changes in TSP‐1 production/secretion and uptake/degradation/cleavage. Although TSP‐1 in blood could likely have an effect on circulating inflammatory cells, red blood cells and platelets to increase inflammation, adhesion and aggregation, since TSP‐1 binds to various components in the extracellular matrix, its up‐ and down‐regulations as measured in circulating blood may not reflect the actual changes of TSP‐1 abundance and its functional activities in the matrix. A further limitation is that very few quantitative data on tissue TSP‐1 in diseases are available in the literature, and it should be pointed out that the protein expression levels may not correlate with activation of downstream signalling. Finally, being obtained from human subjects, the majority of these expression data lack time course analysis that could contribute to a better understanding of the changes noted. These caveats aside, the literature data collected and presented in our quantitative analysis remain the first of their kind and should serve to guide future basic and translational research.
Specific domains of TSP‐1 interact with different proteins and non‐protein molecules in the extracellular matrix, and the affinity Kd values of these interactions are mostly in the nmol L−1 range.9, 113 The cell‐surface receptors that TSP‐1 interacts with also have important therapeutic value in cancer and cardiovascular diseases. CD36 is a low‐affinity receptor which recognizes the type I repeats of TSP‐1 and is reported to be an activator of the apoptotic pathways in ECs and some cancer cells.114, 115, 116 The first TSP‐1‐based therapeutic (ABT‐510) was developed based on this interaction more than a decade ago. However, it failed to demonstrate clinical efficacy against metastatic cancers and did not move forward beyond phase II trials.117, 118 Similar to ABT‐510, several other peptides that are derived from the type I repeats were shown to be anti‐angiogenic in vitro.119, 120 In recent years, CD47, a high‐affinity receptor which interacts with the C‐terminal domain of TSP‐1, has garnered attention.121 Accumulating evidence has suggested that the TSP‐1/CD47 axis could be a promising therapeutic target in cancer as well as in cardiovascular diseases.32, 36 Besides its well‐established anti‐angiogenic and anti‐proliferative effects when engaged with TSP‐1,23 CD47 also participates in immune suppression of macrophages and is found widely overexpressed in different types of cancer.122 As a self‐recognition signal, CD47 on cancer cells associates with SIRPα (signal regulatory protein alpha) on macrophages and inhibits phagocytic activities. Interestingly, TSP‐1 also interacts with SIRPα expressed on non‐phagocytic cells; however, the potential role of how TSP‐1 modulates the CD47/SIRPα axis during macrophage activation remains unclear.32, 123 Another class of TSP‐1 receptors is the integrins that recognize the RGD motifs (Arg‐Gly‐Asp) of TSP‐1,124 and intuitively, TSP‐1 can facilitate the adhesion of various cells, including cancer cells, to the extracellular matrix.125 Other major factors that TSP‐1 interacts with include heparin, CD148, syndecan‐1, calreticulin/LRP‐1 complex (low‐density lipoprotein receptor‐related protein 1), and it has also been suggested that TSP‐1 may trigger pro‐survival and pro‐migratory functions in cells through binding with some of its receptors.9, 45, 126 Indeed, few studies have tracked TSP‐1 protein expression concurrent with its receptor level expression. In terms of studying how TSP‐1‐mediated signal transduction contributes to diseases, it will be important to track both the ligand TSP‐1 as well as its specific receptors in cell and tissue compartments.
Besides diseases, many natural biological processes can also contribute to the endogenous regulation of TSP‐1. Preclinical studies in mice have demonstrated age‐associated up‐regulation of TSP‐1 in kidney,127 heart128 and skin.129 The fact that both TSP‐1 and CD47 are significantly induced in the skin of aged mice and negatively act on blood flow may imply a deleterious role of TSP‐1/CD47 axis in ageing and ageing‐related complications.95 In addition, diabetes‐induced up‐regulation of TSP‐1 may contribute to ageing‐related vascular rarefaction in the hearts of leptin‐resistant mice, and loss of TSP‐1 expression can attenuate this pattern.130 Exercise is another factor that seems to control TSP‐1 dynamics. Confirmed in both mice and humans, TSP‐1 expression is greatly increased in skeletal muscles following active training and this is accompanied by an increase in VEGF expression.131, 132 Interestingly, a delay is observed between TSP‐1 induction and VEGF induction (VEGF induction preceding TSP‐1), which suggests endogenous feedback mechanisms through timely regulation of pro‐ and anti‐angiogenic factors to sustain adequate but not excessive angiogenesis and blood flow during and after exercise.133 Gestation status can also affect TSP‐1 expression in the uterus, in which TSP‐1 expression was shown to increase over the last few weeks before labour and peak during labour,134 putatively where its role to promote platelet activation by resisting NO‐mediated effects on platelets135 and blood vessels26 may have beneficial effects to limit haemorrhage.
The inconsistent pattern of TSP‐1 expression observed in different types of cancer is reflected in the equally controversial role of tumour TSP‐1 expression as a survival predictor. In accordance to its anti‐angiogenic property, high tumour expression of TSP‐1 is correlated with increased patient survival in colon,136 lung,137 bladder,138 ovarian,139 cervical140 and gastric cancer.109 However, high tumour tissue TSP‐1 is also associated with decreased survival in patients with hepatocellular carcinoma,81 breast cancer141 and melanoma.142 Along with the observation that high VEGF in cancer is usually associated with worse prognosis and increased metastasis,106 it is very likely that certain types of cancer (eg breast cancer) may have developed compensatory mechanisms to counteract the anti‐angiogenic pathways activated by TSP‐1.143, 144 Resistance to apoptosis and increased VEGF secretion in response to the hypoxic environments resulting from the TSP‐1‐induced loss of tumour vascularization are possible explanations, which hints future investigations of combination therapies that target both molecules in cancer treatments. Separate from cancer, anti‐angiogenic molecules such as TSP‐1 and VEGF165b have emerged as new promising targets in ischaemic vascular diseases (eg PAD).145, 146 Therapeutics that inhibit TSP‐1 as well as its downstream pathways through small molecule/RNAi‐based inhibitors, modulation of upstream transcription factors and TSP‐1 receptor antibodies or morpholino oligonucleotides could potentially be novel modes to accelerate muscle perfusion and regeneration, given that single‐agent gene therapies that deliver pro‐angiogenic factors (eg VEGF, FGF, HGF) so far have been unsuccessful clinically.147 Conversely, in the scenario of age‐related macular degeneration where TSP‐1 levels in the eye are greatly reduced, enhancing TSP‐1 expression may help to counteract the excessive angiogenesis and restore the balance between pro‐ and anti‐angiogenic factors.148
CONFLICT OF INTEREST
J.S.I. serves as Chair of the Scientific Advisory Board of Radiation Control Technologies, Inc. (RCTI, Garden City, NJ) and has equity interest in RCTI and Tioma Therapeutics (St. Louis, MO) that licensed CD47 technology for development. The other authors have declared that no competing interests exist.
Zhao C, Isenberg JS, Popel AS. Human expression patterns: qualitative and quantitative analysis of thrombospondin‐1 under physiological and pathological conditions. J Cell Mol Med. 2018;22:2086–2097. https://doi.org/10.1111/jcmm.13565
Funding information
This work was supported by NIH grants R01 HL101200, R01 CA138264 and R21 HL122721 (ASP); 2P01HL103455, R01 HL108954 and 1R01 HL112914 (J.S.I.). This work was also supported by the Institute for Transfusion Medicine, the Hemophilia Center of Western Pennsylvania and the Heart, Lung, Blood and Vascular Medicine Institute of the University of Pittsburgh School of Medicine (J.S.I.).
REFERENCES
- 1. Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216‐1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murphy‐Ullrich JE, Sage EH. Revisiting the matricellular concept. Matrix Biol. 2014;37:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baenziger NL, Brodie GN, Majerus PW. Isolation and properties of a thrombin‐sensitive protein of human platelets. J Biol Chem. 1972;247:2723‐2731. [PubMed] [Google Scholar]
- 4. Novelli EM, Kato GJ, Ragni MV, et al. Plasma thrombospondin‐1 is increased during acute sickle cell vaso‐occlusive events and associated with acute chest syndrome, hydroxyurea therapy, and lower hemolytic rates. Am J Hematol. 2012;87:326‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mas VR, Mas LA, Archer KJ, et al. Evaluation of gene panel mRNAs in urine samples of kidney transplant recipients as a non‐invasive tool of graft function. Mol Med. 2007;13:315‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen Q, Ye ZN, Liu JP, et al. Elevated cerebrospinal fluid levels of thrombospondin‐1 correlate with adverse clinical outcome in patients with aneurysmal subarachnoid hemorrhage. J Neurol Sci. 2016;369:126‐130. [DOI] [PubMed] [Google Scholar]
- 7. Misenheimer TM, Mosher DF. Calcium ion binding to thrombospondin 1. J Biol Chem. 1995;270:1729‐1733. [DOI] [PubMed] [Google Scholar]
- 8. Lawler PR, Lawler J. Molecular basis for the regulation of angiogenesis by thrombospondin‐1 and ‐2. Cold Spring Harb Perspect Med. 2012;2:a006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Resovi A, Pinessi D, Chiorino G, Taraboletti G. Current understanding of the thrombospondin‐1 interactome. Matrix Biol. 2014;37:83‐91. [DOI] [PubMed] [Google Scholar]
- 10. Belotti D, Capelli C, Resovi A, Introna M, Taraboletti G. Thrombospondin‐1 promotes mesenchymal stromal cell functions via TGFbeta and in cooperation with PDGF. Matrix Biol. 2016;55:106‐116. [DOI] [PubMed] [Google Scholar]
- 11. Gao Q, Chen K, Gao L, Zheng Y, Yang YG. Thrombospondin‐1 signaling through CD47 inhibits cell cycle progression and induces senescence in endothelial cells. Cell Death Dis. 2016;7:e2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mirochnik Y, Kwiatek A, Volpert OV. Thrombospondin and apoptosis: molecular mechanisms and use for design of complementation treatments. Curr Drug Targets. 2008;9:851‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ren B, Song K, Parangi S, et al. A double hit to kill tumor and endothelial cells by TRAIL and antiangiogenic 3TSR. Cancer Res. 2009;69:3856‐3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miller TW, Soto‐Pantoja DR, Schwartz AL, et al. CD47 receptor globally regulates metabolic pathways that control resistance to ionizing radiation. J Biol Chem. 2015;290:24858‐24874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sweetwyne MT, Murphy‐Ullrich JE. Thrombospondin1 in tissue repair and fibrosis: TGF‐beta‐dependent and independent mechanisms. Matrix Biol. 2012;31:178‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lu Z, Kipnis J. Thrombospondin 1—a key astrocyte‐derived neurogenic factor. FASEB J. 2010;24:1925‐1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Narizhneva NV, Razorenova OV, Podrez EA, et al. Thrombospondin‐1 up‐regulates expression of cell adhesion molecules and promotes monocyte binding to endothelium. FASEB J. 2005;19:1158‐1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tabib A, Krispin A, Trahtemberg U, et al. Thrombospondin‐1‐N‐terminal domain induces a phagocytic state and thrombospondin‐1‐C‐terminal domain induces a tolerizing phenotype in dendritic cells. PLoS ONE. 2009;4:e6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kirsch T, Woywodt A, Klose J, et al. Endothelial‐derived thrombospondin‐1 promotes macrophage recruitment and apoptotic cell clearance. J Cell Mol Med. 2010;14:1922‐1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bergstrom SE, Uzunel M, Talme T, Bergdahl E, Sundqvist KG. Antigen‐induced regulation of T‐cell motility, interaction with antigen‐presenting cells and activation through endogenous thrombospondin‐1 and its receptors. Immunology. 2015;144:687‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhao Y, Xiong Z, Lechner EJ, et al. Thrombospondin‐1 triggers macrophage IL‐10 production and promotes resolution of experimental lung injury. Mucosal Immunol. 2014;7:440‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Velasco P, Huegel R, Brasch J, et al. The angiogenesis inhibitor thrombospondin‐1 inhibits acute cutaneous hypersensitivity reactions. J Invest Dermatol. 2009;129:2022‐2030. [DOI] [PubMed] [Google Scholar]
- 23. Kaur S, Martin‐Manso G, Pendrak ML, Garfield SH, Isenberg JS, Roberts DD. Thrombospondin‐1 inhibits VEGF receptor‐2 signaling by disrupting its association with CD47. J Biol Chem. 2010;285:38923‐38932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Isenberg JS, Ridnour LA, Perruccio EM, Espey MG, Wink DA, Roberts DD. Thrombospondin‐1 inhibits endothelial cell responses to nitric oxide in a cGMP‐dependent manner. Proc Natl Acad Sci USA. 2005;102:13141‐13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rogers NM, Seeger F, Garcin ED, Roberts DD, Isenberg JS. Regulation of soluble guanylate cyclase by matricellular thrombospondins: implications for blood flow. Front Physiol. 2014;5:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bauer EM, Qin Y, Miller TW, et al. Thrombospondin‐1 supports blood pressure by limiting eNOS activation and endothelial‐dependent vasorelaxation. Cardiovasc Res. 2010;88:471‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Isenberg JS, Qin Y, Maxhimer JB, et al. Thrombospondin‐1 and CD47 regulate blood pressure and cardiac responses to vasoactive stress. Matrix Biol. 2009;28:110‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lawler J. Thrombospondin‐1 as an endogenous inhibitor of angiogenesis and tumor growth. J Cell Mol Med. 2002;6:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rouanne M, Adam J, Goubar A, et al. Osteopontin and thrombospondin‐1 play opposite roles in promoting tumor aggressiveness of primary resected non‐small cell lung cancer. BMC Cancer. 2016;16:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cymbaluk‐Ploska A, Chudecka‐Glaz A, Pius‐Sadowska E, Machalinski B, Menkiszak J. Thrombospondin‐I concentrations behavior in plasma of patients with ovarian cancer. Cancer Biomark. 2017;20:31‐39. [DOI] [PubMed] [Google Scholar]
- 31. Rusnati M, Urbinati C, Bonifacio S, Presta M, Taraboletti G. Thrombospondin‐1 as a paradigm for the development of antiangiogenic agents endowed with multiple mechanisms of action. Pharmaceuticals (Basel). 2010;3:1241‐1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jeanne A, Schneider C, Martiny L, Dedieu S. Original insights on thrombospondin‐1‐related antireceptor strategies in cancer. Front Pharmacol. 2015;6:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kazerounian S, Yee KO, Lawler J. Thrombospondins in cancer. Cell Mol Life Sci. 2008;65:700‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matsuo Y, Tanaka M, Yamakage H, et al. Thrombospondin 1 as a novel biological marker of obesity and metabolic syndrome. Metabolism. 2015;64:1490‐1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roberts DD, Kaur S, Isenberg JS. Regulation of cellular redox signaling by matricellular proteins in vascular biology, immunology, and cancer. Antioxid Redox Signal. 2017;27:874‐911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Soto‐Pantoja DR, Stein EV, Rogers NM, Sharifi‐Sanjani M, Isenberg JS, Roberts DD. Therapeutic opportunities for targeting the ubiquitous cell surface receptor CD47. Expert Opin Ther Targets. 2013;17:89‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Russell S, Duquette M, Liu J, Drapkin R, Lawler J, Petrik J. Combined therapy with thrombospondin‐1 type I repeats (3TSR) and chemotherapy induces regression and significantly improves survival in a preclinical model of advanced stage epithelial ovarian cancer. FASEB J. 2015;29:576‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bazzazi H, Isenberg JS, Popel AS. Inhibition of VEGFR2 activation and its downstream signaling to ERK1/2 and calcium by thrombospondin‐1 (TSP1): in silico investigation. Front Physiol. 2017;8:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhao C, Isenberg JS, Popel AS. Transcriptional and post‐transcriptional regulation of thrombospondin‐1 Expression: a computational model. PLoS Comput Biol. 2017;13:e1005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rohrs JA, Sulistio CD, Finley SD. Predictive model of thrombospondin‐1 and vascular endothelial growth factor in breast tumor tissue. NPJ Syst Biol Appl. 2016;2:16030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lopez‐Dee Z, Pidcock K, Gutierrez LS. Thrombospondin‐1: multiple paths to inflammation. Mediators Inflamm. 2011;2011:296069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zaslavsky A, Baek KH, Lynch RC, et al. Platelet‐derived thrombospondin‐1 is a critical negative regulator and potential biomarker of angiogenesis. Blood. 2010;115:4605‐4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Italiano JE Jr, Richardson JL, Patel‐Hett S, et al. Angiogenesis is regulated by a novel mechanism: pro‐ and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111:1227‐1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Qian X, Rothman VL, Nicosia RF, Tuszynski GP. Expression of thrombospondin‐1 in human pancreatic adenocarcinomas: role in matrix metalloproteinase‐9 production. Pathol Oncol Res. 2001;7:251‐259. [DOI] [PubMed] [Google Scholar]
- 45. Naganuma H, Satoh E, Asahara T, et al. Quantification of thrombospondin‐1 secretion and expression of alphavbeta3 and alpha3beta1 integrins and syndecan‐1 as cell‐surface receptors for thrombospondin‐1 in malignant glioma cells. J Neurooncol. 2004;70:309‐317. [DOI] [PubMed] [Google Scholar]
- 46. Hyder SM, Liang Y, Wu J, Welbern V. Regulation of thrombospondin‐1 by natural and synthetic progestins in human breast cancer cells. Endocr Relat Cancer. 2009;16:809‐817. [DOI] [PubMed] [Google Scholar]
- 47. Kay NE, Bone ND, Tschumper RC, et al. B‐CLL cells are capable of synthesis and secretion of both pro‐ and anti‐angiogenic molecules. Leukemia. 2002;16:911‐919. [DOI] [PubMed] [Google Scholar]
- 48. Bruel A, Touhami‐Carrier M, Thomaidis A, Legrand C. Thrombospondin‐1 (TSP‐1) and TSP‐1‐derived heparin‐binding peptides induce promyelocytic leukemia cell differentiation and apoptosis. Anticancer Res. 2005;25:757‐764. [PubMed] [Google Scholar]
- 49. Jaffe EA, Ruggiero JT, Leung LK, Doyle MJ, McKeown‐Longo PJ, Mosher DF. Cultured human fibroblasts synthesize and secrete thrombospondin and incorporate it into extracellular matrix. Proc Natl Acad Sci USA. 1983;80:998‐1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Raugi GJ, Mumby SM, Abbott‐Brown D, Bornstein P. Thrombospondin: synthesis and secretion by cells in culture. J Cell Biol. 1982;95:351‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Taraboletti G, Benelli R, Borsotti P, et al. Thrombospondin‐1 inhibits Kaposi's sarcoma (KS) cell and HIV‐1 Tat‐induced angiogenesis and is poorly expressed in KS lesions. J Pathol. 1999;188:76‐81. [DOI] [PubMed] [Google Scholar]
- 52. Mosher DF, Doyle MJ, Jaffe EA. Synthesis and secretion of thrombospondin by cultured human endothelial cells. J Cell Biol. 1982;93:343‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Marteau F, Gonzalez NS, Communi D, Goldman M, Boeynaems JM, Communi D. Thrombospondin‐1 and indoleamine 2,3‐dioxygenase are major targets of extracellular ATP in human dendritic cells. Blood. 2005;106:3860‐3866. [DOI] [PubMed] [Google Scholar]
- 54. Eichler W, Yafai Y, Wiedemann P, Reichenbach A. Angiogenesis‐related factors derived from retinal glial (Muller) cells in hypoxia. NeuroReport. 2004;15:1633‐1637. [DOI] [PubMed] [Google Scholar]
- 55. van Beijnum JR, Rousch M, Castermans K, van der Linden E, Griffioen AW. Isolation of endothelial cells from fresh tissues. Nat Protoc. 2008;3:1085‐1091. [DOI] [PubMed] [Google Scholar]
- 56. Pinto AR, Ilinykh A, Ivey MJ, et al. Revisiting cardiac cellular composition. Circ Res. 2016;118:400‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kwak C, Jin RJ, Lee C, Park MS, Lee SE. Thrombospondin‐1, vascular endothelial growth factor expression and their relationship with p53 status in prostate cancer and benign prostatic hyperplasia. BJU Int. 2002;89:303‐309. [DOI] [PubMed] [Google Scholar]
- 58. Miyanaga K, Kato Y, Nakamura T, et al. Expression and role of thrombospondin‐1 in colorectal cancer. Anticancer Res. 2002;22:3941‐3948. [PubMed] [Google Scholar]
- 59. Thul PJ, Akesson L, Wiking M, et al. A subcellular map of the human proteome. Science. 2017;356:pii: eaal3321. [DOI] [PubMed] [Google Scholar]
- 60. Finka A, Goloubinoff P. Proteomic data from human cell cultures refine mechanisms of chaperone‐mediated protein homeostasis. Cell Stress Chaperones. 2013;18:591‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Liu Y, Starr MD, Brady JC, et al. Modulation of circulating protein biomarkers following TRC105 (anti‐endoglin antibody) treatment in patients with advanced cancer. Cancer Med. 2014;3:580‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wiesner T, Bugl S, Mayer F, Hartmann JT, Kopp HG. Differential changes in platelet VEGF, Tsp, CXCL12, and CXCL4 in patients with metastatic cancer. Clin Exp Metastasis. 2010;27:141‐149. [DOI] [PubMed] [Google Scholar]
- 63. Nathan FE, Hernandez E, Dunton CJ, et al. Plasma thrombospondin levels in patients with gynecologic malignancies. Cancer. 1994;73:2853‐2858. [DOI] [PubMed] [Google Scholar]
- 64. Starlinger P, Moll HP, Assinger A, et al. Thrombospondin‐1: a unique marker to identify in vitro platelet activation when monitoring in vivo processes. J Thromb Haemost. 2010;8:1809‐1819. [DOI] [PubMed] [Google Scholar]
- 65. Ozatli D, Kocoglu H, Haznedaroglu IC, et al. Circulating thrombomodulin, thrombospondin, and fibronectin in acute myeloblastic leukemias. Haematologia (Budap). 1999;29:277‐283. [PubMed] [Google Scholar]
- 66. Tuszynski GP, Smith M, Rothman VL, et al. Thrombospondin levels in patients with malignancy. Thromb Haemost. 1992;67:607‐611. [PubMed] [Google Scholar]
- 67. Byrne GJ, Hayden KE, McDowell G, et al. Angiogenic characteristics of circulating and tumoural thrombospondin‐1 in breast cancer. Int J Oncol. 2007;31:1127‐1132. [DOI] [PubMed] [Google Scholar]
- 68. Holmes CE, Jasielec J, Levis JE, Skelly J, Muss HB. Initiation of aspirin therapy modulates angiogenic protein levels in women with breast cancer receiving tamoxifen therapy. Clin Transl Sci. 2013;6:386‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Han H, Cao FL, Wang BZ, Mu XR, Li GY, Wang XW. Expression of angiogenesis regulatory proteins and epithelial‐mesenchymal transition factors in platelets of the breast cancer patients. ScientificWorldJournal. 2014;2014:878209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Brostjan C, Bayer A, Zommer A, et al. Monitoring of circulating angiogenic factors in dendritic cell‐based cancer immunotherapy. Cancer. 2003;98:2291‐2301. [DOI] [PubMed] [Google Scholar]
- 71. Hayden K, Tetlow L, Byrne G, Bundred N. Radioimmunoassay for the measurement of thrombospondin in plasma and breast cyst fluid: validation and clinical application. Ann Clin Biochem. 2000;37(Pt 3):319‐325. [DOI] [PubMed] [Google Scholar]
- 72. Yamashita Y, Kurohiji T, Tuszynski GP, Sakai T, Shirakusa T. Plasma thrombospondin levels in patients with colorectal carcinoma. Cancer. 1998;82:632‐638. [DOI] [PubMed] [Google Scholar]
- 73. Brostjan C, Gebhardt K, Gruenberger B, et al. Neoadjuvant treatment of colorectal cancer with bevacizumab: the perioperative angiogenic balance is sensitive to systemic thrombospondin‐1 levels. Clin Cancer Res. 2008;14:2065‐2074. [DOI] [PubMed] [Google Scholar]
- 74. Peterson JE, Zurakowski D, Italiano JE Jr, et al. VEGF, PF4 and PDGF are elevated in platelets of colorectal cancer patients. Angiogenesis. 2012;15:265‐273. [DOI] [PubMed] [Google Scholar]
- 75. Dudek AZ, Mahaseth H. Circulating angiogenic cytokines in patients with advanced non‐small cell lung cancer: correlation with treatment response and survival. Cancer Invest. 2005;23:193‐200. [DOI] [PubMed] [Google Scholar]
- 76. Fleitas T, Martinez‐Sales V, Vila V, et al. VEGF and TSP1 levels correlate with prognosis in advanced non‐small cell lung cancer. Clin Transl Oncol. 2013;15:897‐902. [DOI] [PubMed] [Google Scholar]
- 77. Tas F, Duranyildiz D, Soydinc HO, et al. Effect of maximum‐tolerated doses and low‐dose metronomic chemotherapy on serum vascular endothelial growth factor and thrombospondin‐1 levels in patients with advanced nonsmall cell lung cancer. Cancer Chemother Pharmacol. 2008;61:721‐725. [DOI] [PubMed] [Google Scholar]
- 78. Nie S, Lo A, Wu J, et al. Glycoprotein biomarker panel for pancreatic cancer discovered by quantitative proteomics analysis. J Proteome Res. 2014;13:1873‐1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Brand RE, Nolen BM, Zeh HJ, et al. Serum biomarker panels for the detection of pancreatic cancer. Clin Cancer Res. 2011;17:805‐816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Elstner A, Stockhammer F, Nguyen‐Dobinsky TN, et al. Identification of diagnostic serum protein profiles of glioblastoma patients. J Neurooncol. 2011;102:71‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Poon RT, Chung KK, Cheung ST, et al. Clinical significance of thrombospondin 1 expression in hepatocellular carcinoma. Clin Cancer Res. 2004;10:4150‐4157. [DOI] [PubMed] [Google Scholar]
- 82. Yao L, Dong H, Luo Y, Du J, Hu W. Net platelet angiogenic activity (NPAA) correlates with progression and prognosis of non‐small cell lung cancer. PLoS ONE. 2014;9:e96206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pratt DA, Miller WR, Dawes J. Thrombospondin in malignant and non‐malignant breast tissue. Eur J Cancer Clin Oncol. 1989;25:343‐350. [DOI] [PubMed] [Google Scholar]
- 84. Gasparini G, Toi M, Biganzoli E, et al. Thrombospondin‐1 and ‐2 in node‐negative breast cancer: correlation with angiogenic factors, p53, cathepsin D, hormone receptors and prognosis. Oncology. 2001;60:72‐80. [DOI] [PubMed] [Google Scholar]
- 85. de Fraipont F, El Atifi M, Gicquel C, Bertagna X, Chambaz EM, Feige JJ. Expression of the angiogenesis markers vascular endothelial growth factor‐A, thrombospondin‐1, and platelet‐derived endothelial cell growth factor in human sporadic adrenocortical tumors: correlation with genotypic alterations. J Clin Endocrinol Metab. 2000;85:4734‐4741. [DOI] [PubMed] [Google Scholar]
- 86. Bonnefoy A, Daenens K, Feys HB, et al. Thrombospondin‐1 controls vascular platelet recruitment and thrombus adherence in mice by protecting (sub)endothelial VWF from cleavage by ADAMTS13. Blood. 2006;107:955‐964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Suh EJ, Kabir MH, Kang UB, et al. Comparative profiling of plasma proteome from breast cancer patients reveals thrombospondin‐1 and BRWD3 as serological biomarkers. Exp Mol Med. 2012;44:36‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wong SY, Purdie AT, Han P. Thrombospondin and other possible related matrix proteins in malignant and benign breast disease. An immunohistochemical study. Am J Pathol. 1992;140:1473‐1482. [PMC free article] [PubMed] [Google Scholar]
- 89. Bertin N, Clezardin P, Kubiak R, Frappart L. Thrombospondin‐1 and ‐2 messenger RNA expression in normal, benign, and neoplastic human breast tissues: correlation with prognostic factors, tumor angiogenesis, and fibroblastic desmoplasia. Cancer Res. 1997;57:396‐399. [PubMed] [Google Scholar]
- 90. Zhao C, Popel AS. Computational model of microRNA control of HIF‐VEGF pathway: insights into the pathophysiology of ischemic vascular disease and cancer. PLoS Comput Biol. 2015;11:e1004612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Smadja DM, d'Audigier C, Bieche I, et al. Thrombospondin‐1 is a plasmatic marker of peripheral arterial disease that modulates endothelial progenitor cell angiogenic properties. Arterioscler Thromb Vasc Biol. 2011;31:551‐559. [DOI] [PubMed] [Google Scholar]
- 92. Hoier B, Walker M, Passos M, et al. Angiogenic response to passive movement and active exercise in individuals with peripheral arterial disease. J Appl Physiol (1985). 2013;115:1777‐1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Favier J, Germain S, Emmerich J, Corvol P, Gasc JM. Critical overexpression of thrombospondin 1 in chronic leg ischaemia. J Pathol. 2005;207:358‐366. [DOI] [PubMed] [Google Scholar]
- 94. Choi KY, Kim DB, Kim MJ, et al. Higher plasma thrombospondin‐1 levels in patients with coronary artery disease and diabetes mellitus. Korean Circ J. 2012;42:100‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Isenberg JS, Hyodo F, Pappan LK, et al. Blocking thrombospondin‐1/CD47 signaling alleviates deleterious effects of aging on tissue responses to ischemia. Arterioscler Thromb Vasc Biol. 2007;27:2582‐2588. [DOI] [PubMed] [Google Scholar]
- 96. McCrohan MB, Huang SW, Sleasman JW, Klein PA, Kao KJ. Plasma thrombospondin as an indicator of intravascular platelet activation in patients with vasculitis. Thromb Haemost. 1987;58:850‐852. [PubMed] [Google Scholar]
- 97. Bayraktar M, Dundar S, Kirazli S, Teletar F. Platelet factor 4, beta‐thromboglobulin and thrombospondin levels in type I diabetes mellitus patients. J Int Med Res. 1994;22:90‐94. [DOI] [PubMed] [Google Scholar]
- 98. Browne PV, Mosher DF, Steinberg MH, Hebbel RP. Disturbance of plasma and platelet thrombospondin levels in sickle cell disease. Am J Hematol. 1996;51:296‐301. [DOI] [PubMed] [Google Scholar]
- 99. Kong P, Cavalera M, Frangogiannis NG. The role of thrombospondin (TSP)‐1 in obesity and diabetes. Adipocyte. 2014;3:81‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wang S, Skorczewski J, Feng X, Mei L, Murphy‐Ullrich JE. Glucose up‐regulates thrombospondin 1 gene transcription and transforming growth factor‐beta activity through antagonism of cGMP‐dependent protein kinase repression via upstream stimulatory factor 2. J Biol Chem. 2004;279:34311‐34322. [DOI] [PubMed] [Google Scholar]
- 101. Kaiser R, Frantz C, Bals R, Wilkens H. The role of circulating thrombospondin‐1 in patients with precapillary pulmonary hypertension. Respir Res. 2016;17:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Gao JB, Tang WD, Wang HX, Xu Y. Predictive value of thrombospondin‐1 for outcomes in patients with acute ischemic stroke. Clin Chim Acta. 2015;450:176‐180. [DOI] [PubMed] [Google Scholar]
- 103. Huang CL, Jong YS, Wu YW, et al. Association of plasma thrombospondin‐1 level with cardiovascular disease and mortality in hemodialysis patients. Acta Cardiol Sin. 2015;31:113‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Lynch JM, Maillet M, Vanhoutte D, et al. A thrombospondin‐dependent pathway for a protective ER stress response. Cell. 2012;149:1257‐1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kut C, Mac Gabhann F, Popel AS. Where is VEGF in the body? A meta‐analysis of VEGF distribution in cancer. Br J Cancer. 2007;97:978‐985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Otrock ZK, Hatoum HA, Musallam KM, Awada AH, Shamseddine AI. Is VEGF a predictive biomarker to anti‐angiogenic therapy? Crit Rev Oncol Hematol. 2011;79:103‐111. [DOI] [PubMed] [Google Scholar]
- 107. Salvesen HB, Akslen LA. Significance of tumour‐associated macrophages, vascular endothelial growth factor and thrombospondin‐1 expression for tumour angiogenesis and prognosis in endometrial carcinomas. Int J Cancer. 1999;84:538‐543. [DOI] [PubMed] [Google Scholar]
- 108. Ioachim E, Michael MC, Salmas M, et al. Thrombospondin‐1 expression in urothelial carcinoma: prognostic significance and association with p53 alterations, tumour angiogenesis and extracellular matrix components. BMC Cancer. 2006;6:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Nakao T, Kurita N, Komatsu M, et al. Expression of thrombospondin‐1 and Ski are prognostic factors in advanced gastric cancer. Int J Clin Oncol. 2011;16:145‐152. [DOI] [PubMed] [Google Scholar]
- 110. Findley CM, Mitchell RG, Duscha BD, Annex BH, Kontos CD. Plasma levels of soluble Tie2 and vascular endothelial growth factor distinguish critical limb ischemia from intermittent claudication in patients with peripheral arterial disease. J Am Coll Cardiol. 2008;52:387‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Makin AJ, Chung NA, Silverman SH, Lip GY. Vascular endothelial growth factor and tissue factor in patients with established peripheral artery disease: a link between angiogenesis and thrombogenesis? Clin Sci (Lond). 2003;104:397‐404. [DOI] [PubMed] [Google Scholar]
- 112. Voron T, Marcheteau E, Pernot S, et al. Control of the immune response by pro‐angiogenic factors. Front Oncol. 2014;4:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Rivera CG, Bader JS, Popel AS. Angiogenesis‐associated crosstalk between collagens, CXC chemokines, and thrombospondin domain‐containing proteins. Ann Biomed Eng. 2011;39:2213‐2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Choi SH, Tamura K, Khajuria RK, et al. Antiangiogenic variant of TSP‐1 targets tumor cells in glioblastomas. Mol Ther. 2015;23:235‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Greenaway J, Henkin J, Lawler J, Moorehead R, Petrik J. ABT‐510 induces tumor cell apoptosis and inhibits ovarian tumor growth in an orthotopic, syngeneic model of epithelial ovarian cancer. Mol Cancer Ther. 2009;8:64‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis‐dependent inhibition of neovascularization by thrombospondin‐1. Nat Med. 2000;6:41‐48. [DOI] [PubMed] [Google Scholar]
- 117. Markovic SN, Suman VJ, Rao RA, et al. A phase II study of ABT‐510 (thrombospondin‐1 analog) for the treatment of metastatic melanoma. Am J Clin Oncol. 2007;30:303‐309. [DOI] [PubMed] [Google Scholar]
- 118. Sims JN, Lawler J. Thrombospondin‐1‐based antiangiogenic therapy. J Ocul Pharmacol Ther. 2015;31:366‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Karagiannis ED, Popel AS. Anti‐angiogenic peptides identified in thrombospondin type I domains. Biochem Biophys Res Commun. 2007;359:63‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Karagiannis ED, Popel AS. Peptides derived from type I thrombospondin repeat‐containing proteins of the CCN family inhibit proliferation and migration of endothelial cells. Int J Biochem Cell Biol. 2007;39:2314‐2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Henkin J, Volpert OV. Therapies using anti‐angiogenic peptide mimetics of thrombospondin‐1. Expert Opin Ther Targets. 2011;15:1369‐1386. [DOI] [PubMed] [Google Scholar]
- 122. Liu X, Kwon H, Li Z, Fu YX. Is CD47 an innate immune checkpoint for tumor evasion? J Hematol Oncol. 2017;10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Yao M, Rogers NM, Csanyi G, et al. Thrombospondin‐1 activation of signal‐regulatory protein‐alpha stimulates reactive oxygen species production and promotes renal ischemia reperfusion injury. J Am Soc Nephrol. 2014;25:1171‐1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Lawler J, Weinstein R, Hynes RO. Cell attachment to thrombospondin: the role of ARG‐GLY‐ASP, calcium, and integrin receptors. J Cell Biol. 1988;107:2351‐2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. John AS, Rothman VL, Tuszynski GP. Thrombospondin‐1 (TSP‐1) stimulates expression of integrin alpha6 in human breast carcinoma cells: a downstream modulator of TSP‐1‐induced cellular adhesion. J Oncol. 2010;2010:645376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Sweetwyne MT, Pallero MA, Lu A, Van Duyn Graham L, Murphy‐Ullrich JE. The calreticulin‐binding sequence of thrombospondin 1 regulates collagen expression and organization during tissue remodeling. Am J Pathol. 2010;177:1710‐1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kang DH, Anderson S, Kim YG, et al. Impaired angiogenesis in the aging kidney: vascular endothelial growth factor and thrombospondin‐1 in renal disease. Am J Kidney Dis. 2001;37:601‐611. [DOI] [PubMed] [Google Scholar]
- 128. Cai H, Yuan Z, Fei Q, Zhao J. Investigation of thrombospondin‐1 and transforming growth factor‐beta expression in the heart of aging mice. Exp Ther Med. 2012;3:433‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Rogers NM, Roberts DD, Isenberg JS. Age‐associated induction of cell membrane CD47 limits basal and temperature‐induced changes in cutaneous blood flow. Ann Surg. 2013;258:184‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Gonzalez‐Quesada C, Cavalera M, Biernacka A, et al. Thrombospondin‐1 induction in the diabetic myocardium stabilizes the cardiac matrix in addition to promoting vascular rarefaction through angiopoietin‐2 upregulation. Circ Res. 2013;113:1331‐1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Olfert IM, Breen EC, Gavin TP, Wagner PD. Temporal thrombospondin‐1 mRNA response in skeletal muscle exposed to acute and chronic exercise. Growth Factors. 2006;24:253‐259. [DOI] [PubMed] [Google Scholar]
- 132. Hoier B, Passos M, Bangsbo J, Hellsten Y. Intense intermittent exercise provides weak stimulus for vascular endothelial growth factor secretion and capillary growth in skeletal muscle. Exp Physiol. 2013;98:585‐597. [DOI] [PubMed] [Google Scholar]
- 133. Olenich SA, Gutierrez‐Reed N, Audet GN, Olfert IM. Temporal response of positive and negative regulators in response to acute and chronic exercise training in mice. J Physiol. 2013;591:5157‐5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Morimoto T, Head JR, MacDonald PC, Casey ML. Thrombospondin‐1 expression in human myometrium before and during pregnancy, before and during labor, and in human myometrial cells in culture. Biol Reprod. 1998;59:862‐870. [DOI] [PubMed] [Google Scholar]
- 135. Isenberg JS, Romeo MJ, Yu C, et al. Thrombospondin‐1 stimulates platelet aggregation by blocking the antithrombotic activity of nitric oxide/cGMP signaling. Blood. 2008;111:613‐623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Maeda K, Nishiguchi Y, Yashiro M, et al. Expression of vascular endothelial growth factor and thrombospondin‐1 in colorectal carcinoma. Int J Mol Med. 2000;5:373‐378. [DOI] [PubMed] [Google Scholar]
- 137. Papadaki C, Mavroudis D, Trypaki M, et al. Tumoral expression of TXR1 and TSP1 predicts overall survival of patients with lung adenocarcinoma treated with first‐line docetaxel‐gemcitabine regimen. Clin Cancer Res. 2009;15:3827‐3833. [DOI] [PubMed] [Google Scholar]
- 138. Grossfeld GD, Ginsberg DA, Stein JP, et al. Thrombospondin‐1 expression in bladder cancer: association with p53 alterations, tumor angiogenesis, and tumor progression. J Natl Cancer Inst. 1997;89:219‐227. [DOI] [PubMed] [Google Scholar]
- 139. Alvarez AA, Axelrod JR, Whitaker RS, et al. Thrombospondin‐1 expression in epithelial ovarian carcinoma: association with p53 status, tumor angiogenesis, and survival in platinum‐treated patients. Gynecol Oncol. 2001;82:273‐278. [DOI] [PubMed] [Google Scholar]
- 140. Kodama J, Hashimoto I, Seki N, et al. Thrombospondin‐1 and ‐2 messenger RNA expression in invasive cervical cancer: correlation with angiogenesis and prognosis. Clin Cancer Res. 2001;7:2826‐2831. [PubMed] [Google Scholar]
- 141. Campone M, Valo I, Jezequel P, et al. Prediction of recurrence and survival for triple‐negative breast cancer (TNBC) by a protein signature in tissue samples. Mol Cell Proteomics. 2015;14:2936‐2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Straume O, Akslen LA. Expresson of vascular endothelial growth factor, its receptors (FLT‐1, KDR) and TSP‐1 related to microvessel density and patient outcome in vertical growth phase melanomas. Am J Pathol. 2001;159:223‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Fontana A, Filleur S, Guglielmi J, et al. Human breast tumors override the antiangiogenic effect of stromal thrombospondin‐1 in vivo. Int J Cancer. 2005;116:686‐691. [DOI] [PubMed] [Google Scholar]
- 144. Filleur S, Volpert OV, Degeorges A, et al. In vivo mechanisms by which tumors producing thrombospondin 1 bypass its inhibitory effects. Genes Dev. 2001;15:1373‐1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Chu LH, Vijay CG, Annex BH, Bader JS, Popel AS. PADPIN: protein–protein interaction networks of angiogenesis, arteriogenesis, and inflammation in peripheral arterial disease. Physiol Genomics. 2015;47:331‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Ganta VC, Choi M, Kutateladze A, Annex BH. VEGF165b modulates endothelial VEGFR1‐STAT3 signaling pathway and angiogenesis in human and experimental peripheral arterial disease. Circ Res. 2017;120:282‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Shimamura M, Nakagami H, Taniyama Y, Morishita R. Gene therapy for peripheral arterial disease. Expert Opin Biol Ther. 2014;14:1175‐1184. [DOI] [PubMed] [Google Scholar]
- 148. Uno K, Bhutto IA, McLeod DS, Merges C, Lutty GA. Impaired expression of thrombospondin‐1 in eyes with age related macular degeneration. Br J Ophthalmol. 2006;90:48‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]


