Abstract
Aims
Several studies have reported the under‐representation of women in clinical trials, thereby challenging the external validity of the benefit/risk assessments of launched drugs. Our aim was to determine the extent to which women have been included in clinical trials used for drug registration and to analyse the fraction of women participating in phases I, II and III.
Methods
We conducted cross‐sectional, structured research into publicly available registration dossiers of Food and Drug Administration (FDA)‐approved drugs that are prescribed frequently. Furthermore, we analysed compounds with high hepatic clearance and a known gender‐related difference in drug response. In a sensitivity analysis, we compared figures with US disease prevalence data.
Results
For 38 of the initial 137 drugs (28%), sufficient data were reported and publicly available. For these drugs, 185 479 trial participants were included, of whom 47% were female and 44% were male; gender was not reported for 9% of participants. However, the number of female participants varied with the phase of the trial, with 22% females in phase I trials vs. 48% and 49%, respectively, in phase II and III trials. When compared with US disease prevalence data, 10 drugs (26%) had a greater than 20% difference between the proportion of females affected with the disease compared with representation in clinical trials.
Conclusions
From these publicly available data, there was no evidence of any systematic under‐representation of women in clinical trials.
Keywords: adverse event, clinical trials, disease prevalence, efficacy, gender, gender subgroup analysis, safety
What is Already Known about this Subject
Several studies have reported under‐representation of women in clinical trials, thereby challenging the external validity of benefit/risk assessments of launched drugs.
Physiological differences in women and the presentation of disease symptoms may give rise to differences in the clinical outcome of medications. Therefore, it is paramount to evaluate the inclusion of women in clinical trials of newly approved drug molecules, and the possible effect size variation, for a proper regulatory review.
What this Study Adds
A structured, cross‐sectional review of publicly available registration data of clinical trials at the Food and Drug Administration was performed for the most frequently prescribed drug classes.
No evidence was found of any systemic significant under‐representation of women in clinical trials.
During the clinical development phase, the proportion of women participating in trials increased gradually, from 22% in phase I to over 48% for phase II/III trials.
Introduction
The lack of gender and racial diversity in clinical trials has long been a matter of controversy. Traditionally, studies have been carried out in predominantly Caucasian countries, using mainly adult white males, especially for phase I clinical trials investigating tolerability, clinical pharmacology, dose‐related side effects and early evidence of efficacy 1. Historically, women of child‐bearing age have been discouraged from participation in phase I trials because of undue risk to fetal development, especially after the thalidomide scandal of the 1960s 2. This approach seems plausible and responsible in early clinical drug development, to avoid teratogenic effects in studies of compounds of which the vast majority will never reach the market anyway. However, public pressure has spurred detailed research on the participation of women in clinical drug trials, with an emphasis on later‐stage studies – i.e. phase II and III trials.
In an analysis of new drug applications (NDAs) submitted in the 1980s and 1990s, it was shown that women were under‐represented for analysis of some of these drugs 3. Subsequently, in 1993, the Food and Drug Administration (FDA) published the Guideline for the Study and Evaluation of Gender Differences in the Clinical Evaluation of Drugs 4. This guideline called for increased participation of females in clinical studies, and for gender‐specific analysis to discern in greater detail any gender difference in drug effect(s). In 2001, an updated analysis revealed that in 36 NDAs that were granted FDA market authorization, the proportion of female participants had increased 4, 5. In the latter US General Accounting Office report 5, across all the clinical trials, 52% of the study participants were women, 39% were men and 9% percent were not identified by gender. Meinert et al. performed a detailed analysis of the composition of study populations in reported clinical trials and argued that the idea that women were under‐represented in clinical trials was exaggerated 6. The analysis showed that in the period 1966–1975, a combination of male/female participants was present in 41% of trials, and this had increased to 55% over the period 1996–1998. A subsequent analysis comprised a review of all 724 clinical trials published in five major journals in 1985, 1990 and 1995. These trials were analysed for the numbers of female/male participants, and showed that the total percentage of female participants was 60%, and the percentage of women in mixed‐gender trials was approximately 40%. It can thus be concluded that women have been involved in the majority of clinical trials since the 1960s, even if the ratio has not been equal. Importantly, women‐only studies were over‐represented in cancer studies, even when excluding prostate and breast cancer 6.
Although this study showed that a large percentage of studies have been performed with a more or less equal mix of participants, the numbers of participants in these studies may not have been sufficient to result in enough power to discern any gender differences in outcomes accurately 6. This question was specifically addressed for NIH‐funded research 7. Analysis of these trials showed that mixed‐gender trials had an even mix of male (49%) and female (49%) participants. For all studies, including male‐only and female‐only trials, 57% of trial participants were female, 42% were male and 1% were of unknown gender. This, again, suggests an even distribution between male and female participants, at least for the NIH‐funded research 7. Recently, Cochrane meta‐analyses were conducted to investigate gender‐based subgroup differences in randomized controlled trials (RCTs) 8. In the latter study, a total of 311 RCTs, 162 including both genders, 46 with males only, 103 with females only, were analysed, of which only 9% had a statistically significant gender–treatment interaction; this led the authors to conclude that these interactions occurred only slightly more frequently than would be expected by chance.
By contrast, several other studies have reported an under‐representation of females in clinical trials (e.g. cancer, vascular surgery and cardiovascular trials 9, 10, 11, 12, 13), with the general media continuing to report that large gender gaps are still putting women at a disadvantage 14. This reporting has had serious effects on policy, with large amounts of money being allocated to study gender differences in healthcare 15. However, while there have been several thorough analyses on trials, as reported in scientific journals and the Cochrane Database of Systematic Reviews, the publicly available data from registration authorities have not been assessed recently.
Therefore, the present study investigated the extent to which women have been included in clinical trials used for drug registration. As no review of data of approval dossiers was available, we performed a structured, cross‐sectional study of the publicly available registration dossiers of FDA‐approved and frequently prescribed drugs, and also compared these to disease prevalence between the genders. Furthermore, we analysed quantitative and qualitative differences in drug effect and safety between men and women in these clinical trials.
Materials and methods
A cross‐sectional, structured study was performed of publicly available registration dossiers of FDA‐approved drugs that are frequently prescribed on‐label for the 10 most frequently occurring drug classes, as reported by the IMS health report 16. The following drug classes were selected: antidepressants, lipid regulators, narcotic analgesics, antidiabetics, angiotensin‐converting enzyme inhibitors, beta blockers, respiratory agents, antiulcerants, diuretics and antiepileptics 16. Furthermore, we analysed compounds with high hepatic clearance or with a known gender‐related difference in drug response. The selection category for each drug is specified in Table 1, while the total list of drugs used in these analyses is presented in Table S1.
Table 1.
Characteristics of analysed drugs and corresponding disease prevalence data. US prevalence data were retrieved from the US Centers for Disease Control and Prevention, or otherwise specified in the table
| Drug | Year approved | Disease state | Prevalence data year | Reason for inclusion |
|---|---|---|---|---|
| Atorvastatin | 1996 | Hypercholesterolaemia | 2000 | Most utilized drug class |
| Celecoxib | 1998 | Antirheumatic | 2005 | Hepatically cleared |
| Citalopram | 1998 | Depression | 2000 | Most utilized drug class |
| Desvenlafaxine | 2008 | Depression | 2010 | Most utilized drug class |
| Dexlansoprazole | 2009 | GERD | 1997 32 | Most utilized drug class |
| Duloxetine | 2004 | Depression | 2000 | Most utilized drug class |
| Empagliflozin | 2014 | Diabetes | 2012 | Most utilized drug class |
| Eplerenone | 2002 | Heart failure | 2000 33 | Most utilized drug class |
| Escitalopram | 2001 | Depression | 2000 | Most utilized drug class |
| Esmolol | 1986 | Hypertension | 1991 | Most utilized drug class |
| Esomeprazole | 2001 | GERD | 1997 32 | Most utilized drug class |
| Exenatide‐4 | 2005 | Diabetes | 2005 | Most utilized drug class |
| Ezetimibe | 2002 | Hypercholesterolaemia | 2000 | Most utilized drug class |
| Formoterol | 2001 | Asthma | 2001 | Most utilized drug class |
| Insulin aspart | 2000 | Diabetes | 2000 | Most utilized drug class |
| Insulin degludec | 2015 | Diabetes | 2012 | Most utilized drug class |
| Irbesartan | 1997 | Hypertension | 2001 | Most utilized drug class |
| Lansoprazole | 1995 | GERD | 1997 32 | Most utilized drug class |
| Levetiracetam | 1999 | Epilepsy | 1990 | Most utilized drug class |
| Liraglutide | 2010 | Diabetes | 2012 | Most utilized drug class |
| Milnacipran | 2009 | Depression | 2010 | Most utilized drug class |
| Mometasone | 2005 | Asthma | 2005 | Most utilized drug class |
| Montelukast | 1998 | Asthma | 2001 | Most utilized drug class |
| Olanzapine | 1996 | Schizophrenia | 2005 34 | Most utilized drug class |
| Olodaterol | 2014 | COPD | 2013 | Most utilized drug class |
| Oxcarbazepine | 2000 | Epilepsy | 1990 | Most utilized drug class |
| Palonosetron | 2003 | Chemotherapy‐induced nausea & vomitinga | 2010 35 | Hepatically cleared + known gender difference |
| Pantoprazole | 2000 | GERD | 1997 32 | Most utilized drug class |
| Pioglitazone | 1999 | Diabetes | 2000 | Most utilized drug class |
| Rabeprazole | 1999 | GERD | 1997 32 | Most utilized drug class |
| Rosuvastatin | 2003 | Hypercholesterolaemia | 2005 | Most utilized drug class |
| Rufinamide | 2008 | Epilepsy | 2010 | Most utilized drug class |
| Saxagliptin | 2009 | Diabetes | 2005 | Most utilized drug class |
| Sitagliptin | 2006 | Diabetes | 2005 | Most utilized drug class |
| Tiotropium | 2004 | COPD | 2013 | Most utilized drug class |
| Topiramate | 1996 | Epilepsy | 1990 | Most utilized drug class |
| Vigabatrin | 2009 | Epilepsy | 2010 | Most utilized drug class |
| Zolpidem | 1992 | Insomnia | 2000 36 | Most utilized drug class + known gender difference 37 |
COPD, chronic obstructive pulmonary disease; GERD, gastroesophageal reflux disease
Drug use data obtained from the Netherlands for ondansetron and granisetron
Extraction of gender data
Using the FDA drug database, initial approval documents were searched for all drugs selected. These were evaluated for availability of gender data; only drugs for which information on gender data was available were included in the analyses. Data on the year of marketing approval, gender of participants by clinical phase, and qualitative subgroup analyses were extracted. Both clinical efficacy and safety data were extracted as well as pharmacokinetic data. When phase I and phase II trials were not explicitly distinguished in the FDA file, then these clinical pharmacology studies were combined.
Disease prevalence data
US disease prevalence data were collected via the Centers for Disease Control and Prevention (https://www.cdc.gov). If these data were unavailable, the sources were used as referenced in Table 1 and supplemented by drug use data. For one drug, there were no data available on either US disease prevalence or US drug use, so data on drug use in the Netherlands were used instead. Prevalence data were obtained for periods as close as possible to the year of drug marketing approval (see Table 1). The average time between approval and reported prevalence data was 3.8 years, and ranged from 0 to 12 years. Data on gender were stratified by phase of the clinical trial.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 17.
Results
Drug characteristics
An initial pool of 137 drugs (Appendix 1) was narrowed down to 46 drugs for which review data were available. From these 46, eight drugs could not be included owing to a lack of clinical phase data in the review documents. Of the 38 remaining drugs, 55% (n = 21) had been approved between 2000 and 2009, and only two drugs (5%) had been approved before the publication of the 1993 FDA guidelines on gender inclusion. Thirty‐two per cent of the drugs had been approved between 1990 and 1999, and 10% of drugs during or after 2010. Most of the drugs were selected because of their inclusion in a drug class that was in the top 10 most utilized 16 while only three drugs were included because of known differences in pharmacokinetics or pharmacodynamics (Table 1). The disease states for which the drugs were intended included various indications. Diabetes was the most prevalent disease indication (21%), followed by epilepsy, depression and gastroesophageal reflux disease, each with an incidence of 13% (Table 1).
A total of 185 479 subjects participated in the drug trials reported in the retrieved registration dossiers. Of the entire population, 47% of trial participants were female, 44% were male and gender information were missing from 9%, as presented in Table 2. Thus, gender data were available for 91% of the trial subjects included in the registration dossiers. Differences in the proportions of female participation were apparent when the data were broken down into the different drug development phases (Table 2). Twenty‐two per cent of the participants in phase I trials, 48% of those in phase II trials and 49% of those in phase III trials were female. Gender distribution was similar for phase I trials and combined I/II studies (25% female), and the latter category had the highest percentage (31%) of participants of unknown gender (Table 2).
Table 2.
Proportion of women in clinical studies, according to development phase
| Development phase | Number of drugs | Female participation | Percentage | Unknown gender: proportion | Unknown gender: percentage |
|---|---|---|---|---|---|
| Phase I | 9 | 788/3600 | 22% | 798/4398 | 18% |
| Phase II | 9 | 3477/7268 | 48% | 987/8255 | 12% |
| Combined phase I/II | 29 | 3024/11 881 | 25% | 5344/17 225 | 31% |
| Phase III | 38 | 71 049/145 296 | 49% | 10 305 /155 601 | 7% |
| Total | 38 | 78 338/168 045 | 47% | 17 434 /185 479 | 9% |
All the drug trial data were compared with the disease prevalence data that were available, which were often from the same time period as the market authorization. The proportions of females in the trial populations ranged from 25% (topiramate) to 87% (milnacipran), while the prevalence of disease in women for the drugs' indication ranged from 45% for rosuvastatin to 78% for zolpidem. The difference between the proportion of females with the disease and the proportion of females in the clinical trials ranged between 0.7% and 29% for saxagliptin and topiramate, respectively (Figure 1B). Ten drugs had a greater than 20% difference between the proportion of females with the disease and the proportion of females in the clinical trials. For the majority of drugs, this percentage was similar (Figure 1).
Figure 1.

Bar graph representing the percentages of females participating in clinical trials (green bars) vs. the proportion of females with the disease, and the proportion for whom no gender was reported (yellow). The drugs are listed alphabetically, from A–L (A) and Li–Z (B)
In addition to the quantitative data retrieved, qualitative gender subgroup analyses were available for 95% of the drugs studied. For 50% of the investigated drugs, gender subgroup analyses were available with regard to efficacy. Subgroup analyses were available for both efficacy and safety for 21% of drugs, and for safety alone for 24% of drugs (Figure 2).
Figure 2.

Pie chart representing the outcomes of the qualitative gender analysis. Subgroup analysis of the retrieved drug data was performed for efficacy (purple), safety (green), and efficacy and safety (yellow). For a small proportion, no subgroup analysis was performed (red)
For 82% of the drugs for which a subgroup analysis on efficacy by gender was performed, no difference was found between men and women. Eleven per cent of the drugs were found to be more efficacious in males, and 7% were found to be more efficacious in females (Figure 3). Of the drugs that showed gender differences in efficacy, only one had a difference that was greater than 10% – palonosetron, which is indicated for chemotherapy‐induced nausea and vomiting. When used for highly emetogenic chemotherapy, this drug had 90% efficacy in males and 67% efficacy in females. For moderately emetogenic chemotherapy, the efficacy in males and females were 67% and 52%, respectively.
Figure 3.

Pie chart representing the outcome of the qualitative efficacy analysis, with the green area representing no difference between men and women; the yellow area representing higher efficacy in women than in men; and the blue area representing higher efficacy in men than in women
The gender‐specific safety analysis showed that for 53% of the drugs, females reported more side effects than males (Figure 4), although the nature of these side effects was generally identical and the differences were small. Only zolpidem showed a large difference in the frequency of side effects, with women reporting twice as many adverse events as men. For the other drugs, the difference in the frequency of side effects ranged from 5% to 13%, or were not reported.
Figure 4.
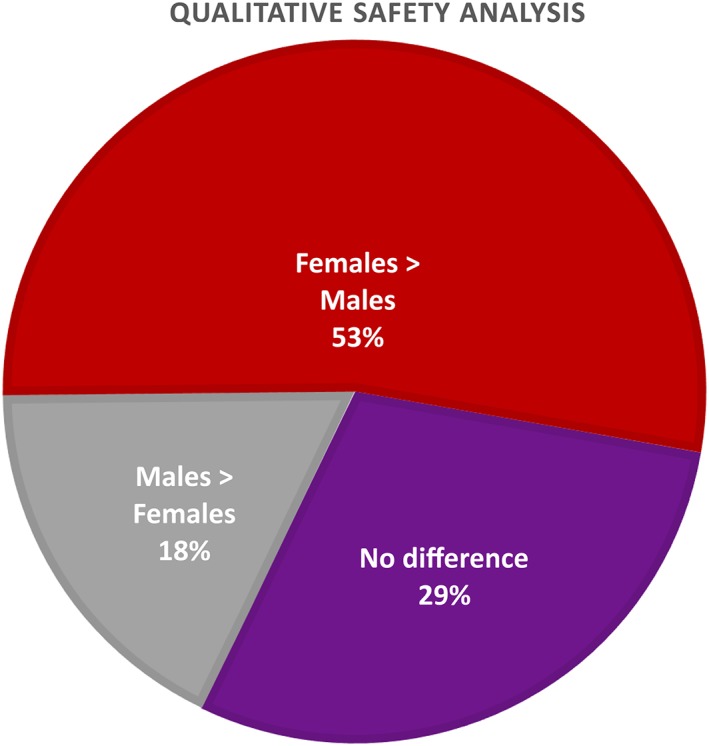
Pie chart representing the outcome of the qualitative safety analysis, with the purple area representing no difference between men and women, the red area representing more adverse events reported in women than in men, and the grey area representing more adverse events reported in men than in women
Only a few drugs showed different side effect profiles between the genders. Insulin degludec showed an increased risk of cardiovascular‐related events in males, with no such increase in females. Tiotropium was associated with urinary side effects only in males, with a higher risk of dry mouth, pharyngitis, and upper respiratory tract infections in females.
Discussion
Our data showed that, overall, women are studied in adequate proportions, and that some type of gender subgroup analysis is performed for most drugs that are approved. The subgroup analyses on efficacy showed that the majority of drugs are equally effective in males and females. While there was a higher proportion of females with side effects compared with males, these differences were relatively small, and likely to be of little clinical significance. It is important to realize that gender difference is one of many variables that cause variability in drug response for efficacy and/or safety in any target population. Other factors include weight, age, genotype, phenotype, ethnicity, hormonal status, fasting conditions, polymorphisms of metabolizing enzymes, receptor expression and sensitivity, co‐medication interactions, co‐morbidities, pregnancy status, gut microbiome status 18, 19, 20, 21, 22. Many of these factors are known to induce substantially more variability than gender if they are distributed heterogeneously in the target population 21, 22. For instance, weight is often correlated closely with drug distribution and can vary by as much as double in Western populations, thereby giving rise to substantial differences in drug response when a similar oral dose is taken, especially in obese patients 23. Furthermore, children and the elderly are often under‐represented in registration trials, although guidelines advise that these groups are investigated 24, 25, 26. Therefore, it is crucial for governmental bodies and funders to prioritize research based on a rational approach. Although division of the human population by gender is easy, the expected variability in response does not seem to outweigh the costs and efforts needed when demanding a more stringent approach in drug registration as indeed suggested by our review. Other sources of variability, such as body weight and genetic polymorphism in drug metabolizing enzymes, cause substantially more clinically relevant issues and should be dealt with first in the attempt to move to personalized medicine 22, 27, 28.
To our knowledge, the present study was the first structured investigation conducted on gender differences in trial participation based on publicly available FDA registration data. Moreover, our study investigated female participation by clinical drug development phase and compared with the total proportions of females studied to gain marketing approval. Most studies chose to look at just one phase, at total numbers or at a random assortment of phase I–III studies without classifying them according to the drug approved 6, 8, 10. Overall, a fairly even proportion of women (47%) were included in the clinical trials on the drugs included in our review. While there was a large difference in gender participation in early‐phase drug development trials, with only 22% of subjects in phase I trials being female, a trend towards higher levels of female participation in later phases of these trials was observed, as also demanded by regulators, including the European Medicines Agency (EMA) 29 and the FDA 4. Of note, phase I trials are carried out in much smaller populations than the large, later‐stage clinical trials that are typically designed for efficacy and safety. In addition, the impact of these studies on the eventual use of the medication is limited. Indeed, when enough females are included in the pivotal clinical trials to detect differences in efficacy and safety between men and women, the occasional larger gap between female representation and the prevalence data is of less importance in phase I trials. Of the drugs selected, esmolol was the only one for which a large sample of women was not included in the clinical trial, and for which no gender analysis was performed. Seventy‐four per cent of gender data were not reported for this drug. However, for a drug that has been on the market for over three decades and for which close monitoring during (in‐hospital) use is mandatory, it is unlikely that further research will result in new gender‐specific recommendations for use.
A limitation of our study was that it was based on a relatively small sample of drugs (38), so selection bias could not be excluded. However, these 38 drugs represented a sample of frequently prescribed compounds of the most utilized therapeutic classes in the US, and 185 479 trial participants were included in our analyses. Although it could be argued that the situation would be completely different for drugs that are used to a lesser extent, this is at present speculative. Further research could include a comparison between registration data and actual prescribing patterns from a healthcare database for less frequently used drugs, to address this potential difference. A similar analysis could and should be performed for more, or all, drugs available, before concluding that females are under‐represented. Such an exercise would be far cheaper and quicker than spending public money to solve an unsubstantiated research question. It is also remarkable that various FDA reports had missing information; as noted, 9% of gender data overall were missing. This may have had an impact on our findings, but would have been unlikely to alter the overall conclusion. In line with other recent studies, we found that subgroup analysis of gender is not fully reported, with the majority (79%) of reports lacking either safety or efficacy analysis (Figure 2) 30, 31. In addition, there is no way to ascertain whether all data submitted to the FDA are available in the online published review. Some information had been redacted because of confidentiality considerations. While gender data were not always included in the published FDA data online, it is unclear whether the FDA would have had access to these originally. In general, the issue of missing data in public reports should not be considered synonymous with market authorization based on incomplete data submission. Obviously, it would have been better if these data, when available, had been included in documents that are in the public domain. We suggest that publication of the gender composition of all clinical trials of new drugs, regardless of the phase of development, should be mandatory.
In conclusion, we found no evidence of any systematic under‐representation of women. This conclusion is in line with that of the FDA, EMA and a recent Cochrane meta‐analysis 8. Such research, and the present analysis, may help in future decision making about performing drug research focused on gender differences for new and existing compounds.
Competing Interests
There are no competing interests to declare.
Supporting information
Table S1 Initial drug list
Labots, G. , Jones, A. , de Visser, S. J. , Rissmann, R. , and Burggraaf, J. (2018) Gender differences in clinical registration trials: is there a real problem?. Br J Clin Pharmacol, 84: 700–707. doi: 10.1111/bcp.13497.
References
- 1. Fisher JA, Kalbaugh CA. Challenging assumptions about minority participation in US clinical research. Am J Public Health 2011; 101: 2217–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parekh A, Fadiran EO, Uhl K, Throckmorton DC. Adverse effects in women: implications for drug development and regulatory policies. Expert Rev Clin Pharmacol 2011; 4: 453–466. [DOI] [PubMed] [Google Scholar]
- 3. US General Accounting Office . Women'S Health: FDA needs to ensure more study of gender differences in prescription drug testing. 1992. Available at http://archive.gao.gov/d35t11/147861.pdf (last accessed 26 January 2018).
- 4. US Food and Drug Administration . Guideline for the study and evaluation of gender differences in the clinical evaluation of drugs; notice. Fed Regist 1993; 58: 39406–39416. [PubMed] [Google Scholar]
- 5. US General Accounting Office . Report to Congressional Requesters by United States General Accounting Office (GAO). Women sufficiently represented in new drug testing, but FDA oversight needs improvement. 2001. Available at https://www.gao.gov/new.items/d01754.pdf (last accessed 26 January 2018).
- 6. Meinert CL, Gilpin AK, Unalp A, Dawson C. Gender representation in trials. Control Clin Trials 2000; 21: 462–475. [DOI] [PubMed] [Google Scholar]
- 7. Pinn VW RC, Bates AC, Wagner R, Jarema K. Monitoring adherence to the NIH policy on the inclusion of women and minorities as subjects in clinical research (Comprehensive Report: Fiscal Year 2007 and 2008 Tracking Data). National Institutes of Health, Department of Health and Human Services; 2009.
- 8. Wallach JD, Sullivan PG, Trepanowski JF, Steyerberg EW, Ioannidis JP. Sex based subgroup differences in randomized controlled trials: empirical evidence from Cochrane meta‐analyses. BMJ 2016; 355: i5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoel AW, Kayssi A, Brahmanandam S, Belkin M, Conte MS, Nguyen LL. Under‐representation of women and ethnic minorities in vascular surgery randomized controlled trials. J Vasc Surg 2009; 50: 349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jagsi R, Motomura AR, Amarnath S, Jankovic A, Sheets N, Ubel PA. Under‐representation of women in high‐impact published clinical cancer research. Cancer 2009; 115: 3293–3301. [DOI] [PubMed] [Google Scholar]
- 11. Kwiatkowski K, Coe K, Bailar JC, Swanson GM. Inclusion of minorities and women in cancer clinical trials, a decade later: have we improved? Cancer 2013; 119: 2956–2963. [DOI] [PubMed] [Google Scholar]
- 12. Tsang W, Alter DA, Wijeysundera HC, Zhang T, Ko DT. The impact of cardiovascular disease prevalence on women's enrollment in landmark randomized cardiovascular trials: a systematic review. J Gen Intern Med 2012; 27: 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang Y, Carlin AS, Faustino PJ, Motta MI, Hamad ML, He R, et al Participation of women in clinical trials for new drugs approved by the food and drug administration in 2000‐2002. J Womens Health 2009; 18: 303–310. [DOI] [PubMed] [Google Scholar]
- 14. Llamas M. How the FDA let women down. Drugwatch, 2017. Available at https://www.drugwatch.com/fda‐let‐women‐down/. (last accessed 28 January 2018).
- 15. Anon . €12m allocated to researching gender differences in healthcare. Dutchnewsnl, 2016. Available at http://www.dutchnews.nl/news/archives/2016/03/e12m-allocated-to-researching-gender-differences-in-healthcare/ (last accessed 29 January 2018).
- 16. IMS Institute for Healthcare Informatics . The use of medicines in the United States: review of 2011. April 2012. Available at www.imshealth.com/ims/Global/Content/Insights/IMS%20Institute%20for%20Healthcare%20Informatics/IHII_Medicines_in_U.S_Report_2011.pdf (last accessed 28 September 2017).
- 17. Southan CSJ, Benson HE, Faccenda E, Pawson AJ, Alexander SPH, et al The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Evans WE, Johnson JA. Pharmacogenomics: the inherited basis for interindividual differences in drug response. Annu Rev Genomics Hum Genet 2001; 2: 9–39. [DOI] [PubMed] [Google Scholar]
- 19. Kalow W, Tang BK, Endrenyi L. Hypothesis: comparisons of inter‐ and intra‐individual variations can substitute for twin studies in drug research. Pharmacogenetics 1998; 8: 283–289. [DOI] [PubMed] [Google Scholar]
- 20. Rissmann R, Dubois EA, Franson KL, Cohen AF. Concept‐based learning of personalized prescribing. Br J Clin Pharmacol 2012; 74: 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, et al Gut microbiome influences efficacy of PD‐1‐based immunotherapy against epithelial tumors. Science 2017; eaan3706. [DOI] [PubMed] [Google Scholar]
- 22. Wilkinson GR. Drug metabolism and variability among patients in drug response. N Engl J Med 2005; 352: 2211–2221. [DOI] [PubMed] [Google Scholar]
- 23. Knibbe CA, Brill MJ, van Rongen A, Diepstraten J, van der Graaf PH, Danhof M. Drug disposition in obesity: toward evidence‐based dosing. Annu Rev Pharmacol Toxicol 2015; 55: 149–167. [DOI] [PubMed] [Google Scholar]
- 24. Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race‐, sex‐, and age‐based disparities. JAMA 2004; 291: 2720–2726. [DOI] [PubMed] [Google Scholar]
- 25. Vitale C, Fini M, Spoletini I, Lainscak M, Seferovic P, Rosano GM. Under‐representation of elderly and women in clinical trials. Int J Cardiol 2017; 232: 216–221. [DOI] [PubMed] [Google Scholar]
- 26. EC. REGULATION No 1901/2006 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL on medicinal products for paediatric use and amending Regulation (EEC) No 1768/92, Directive 2001/20/EC, Directive 2001/83/EC and Regulation (EC) No 726/2004. 2006.
- 27. SEARCH Collaborative Group , Link E, Parish S, Armitage J, Bowman L, Heath S, et al SLCO1B1 variants and statin‐induced myopathy – a genomewide study. N Engl J Med 2008; 359: 789–799. [DOI] [PubMed] [Google Scholar]
- 28. Shuldiner AR, O'Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, et al Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA 2009; 302: 849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. European Medicines Agency . ICH: gender considerations in the conduct of clinical trials. 2005.
- 30. Geller SE, Koch A, Pellettieri B, Carnes M. Inclusion, analysis, and reporting of sex and race/ethnicity in clinical trials: have we made progress? J Womens Health 2011; 20: 315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nolan MR, Nguyen TL. Analysis and reporting of sex differences in phase III medical device clinical trials – how are we doing? J Womens Health 2013; 22: 399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. El‐Serag HB, Petersen NJ, Carter J, Graham DY, Richardson P, Genta RM, et al Gastroesophageal reflux among different racial groups in the United States. Gastroenterology 2004; 126: 1692–1699. [DOI] [PubMed] [Google Scholar]
- 33. Writing Group Members , Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al Heart Disease and Stroke Statistics – 2016 update: a report from the American Heart Association. Circulation 2016; 133: e38–360. [DOI] [PubMed] [Google Scholar]
- 34. National Institute for Mental Health . Schizophrenia. Available at https://www.nimh.nih.gov/health/statistics/prevalence/schizophrenia.shtml (last accessed 11 September 2017).
- 35. Hilarius DL, Kloeg PH, van der Wall E, van den Heuvel JJ, Gundy CM, Aaronson NK. Chemotherapy‐induced nausea and vomiting in daily clinical practice: a community hospital‐based study. Support Care Cancer 2012; 20: 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Souza Lopes C, Robaina Jaqueline Rodrigues and Rotenberg Lúcia. Epidemiology of insomnia: prevalence and risk factors, can't sleep? Issues of being an insomniac. Sahoo DS, ed, 2012. Available at https://www.intechopen.com/books/can-t-sleep-issues-of-being-an-insomniac/epidemiology-of-insomnia-prevalence-and-risk-factors (last accessed 29 January 2018).
- 37. Greenblatt DJ, Harmatz JS, von Moltke LL, Wright CE, Durol AL, Harrel‐Joseph LM, et al Comparative kinetics and response to the benzodiazepine agonists triazolam and zolpidem: evaluation of sex‐dependent differences. J Pharmacol Exp Ther 2000; 293: 435–443. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Initial drug list


