Abstract
Aims
Iron deficiency anaemia frequently complicates inflammatory bowel disease (IBD) in children and adults. Oral iron may exacerbate gastrointestinal symptoms and absorption may be insufficient in intestinal inflammation. Even where oral iron is successful, repletion of iron stores can be unacceptably slow. Intravenous iron compounds were in the past associated with serious adverse reactions and historically were considered a last resort in children. New generation preparations have a safer profile in adults, although reluctance to use them in children may persist, where safety data are lacking. We investigate the safety and efficacy of ferric carboxymaltose and iron sucrose in children.
Methods
We retrospectively identified all children with IBD who received parenteral iron over a 38‐month period in a single regional referral centre. Safety, tolerability and adverse events were established by case note review. Efficacy was assessed by change in haematinic indices pre‐ and post‐treatment.
Results
Forty‐one children (18 male; median age 14 years, range 3–17) received a total of 104 iron infusions. Of these, 44% (18) had Crohn's disease; 56% (23) ulcerative colitis. Thirty‐five received ferric carboxymaltose, seven iron sucrose and one both. Three children developed mild rash post infusion which resolved quickly with chlorphenamine. Mean increase in haemoglobin was 2.5 g dl–1 (0.3–5.8). Iron levels increased by a mean of 8.4 g dl–1 (1–25), transferrin saturation by 16.2% (2–47). Transferrin decreased by 0.84 g dl–1 (0.3–3.4).
Conclusions
New generation parenteral iron preparations are safe, well tolerated and efficacious in children with iron deficiency anaemia and IBD.
Keywords: ferric carboxymaltose, intravenous iron, iron deficiency anaemia, iron sucrose, paediatric
What is Already Known about this Subject
Iron deficiency anaemia (IDA) is a common complication of inflammatory bowel disease (IBD) and a significant contributor to morbidity.
IDA should be actively treated in IBD.
Parenteral iron is effective and relatively safe in adults.
What this Study Adds
The intravenous iron preparations ferric carboxymaltose (FCM) and iron sucrose are effective treatments for IDA in children with IBD.
The safety profiles of FCM and iron sucrose are favourable, and similar in children to that in adults.
FCM, with its easier administration schedule and requirement for fewer infusions, is appropriate and safe in children at least as young as 12 years.
Introduction
Anaemia is one of the most common extraintestinal complications of inflammatory bowel disease (IBD). It is more common in children than adults 1, affecting an estimated 70% of children with IBD 2, and occurs more frequently in Crohn's disease (CD) than ulcerative colitis 3. Anaemia in IBD is multifactorial and closely associated with IBD activity. The leading cause is iron deficiency anaemia (IDA) followed by anaemia of chronic disease. ID results from gastrointestinal blood loss, poor dietary intake and reduced iron bioavailability. Iron malabsorption is a contributing factor especially in CD affecting the small bowel 4. Nearly 90% of children with IBD have ID with or without associated anaemia 1. It is increasingly recognized that IDA is a significant contributor to morbidity in IBD.
Chronic IDA in children causes fatigue, impairs cognitive development and is associated with lower intelligence quotients, attention deficit and other behavioural disorders 5. Immune regulation and growth are also negatively impacted 6. These effects are apparent in ID even in the absence of anaemia. Adult IBD patients who are anaemic report low quality of life indices and there is a marked improvement in this when haemoglobin (Hb) is corrected in adults with IBD 7. For these reasons, early recognition and prompt and effective treatment of ID(A) are of paramount importance in IBD care.
Oral iron supplementation may be considered first line therapy, although issues with tolerance and adherence to prolonged treatment courses often impair therapeutic success. Blood transfusion as a therapy for IDA carries costs and risks, which are well recognized. Newer intravenous (IV) iron preparations with a more favourable side effect profile have become available in the last decade including iron sucrose (IS) and ferric carboxymaltose (FCM), which have been shown to be safe and effective in adults 8, 9, 10. Only a small number of studies on the safety and efficacy of modern IV iron products have been conducted in children. Most of these studied IS not FCM and concerned small numbers of children with chronic kidney disease 11, 12, 13, 14. More recently investigators have shown encouraging safety and efficacy of IS in 24 children with IBD and IDA 15.
Here we describe our 38‐month experience of FCM and IS in children with IBD with a focus on safety and efficacy.
Methods
All children with IBD who received IV iron in a single regional referral centre between April 2013 and May 2016 were retrospectively identified via pharmacy records. Inclusion criteria were therefore IBD and IDA. In most cases, oral iron was used as first‐line treatment for IDA. IV iron was used in the following circumstances: (i) patient reported side effects with oral iron; (ii) non‐resolution of IDA with 3 months of oral iron; (iii) physician assessment of severity of either IBD or IDA contraindicates trial of oral iron. Anaemia was defined as Hb below reference range for age and sex according to the World Health Organization 16. ID was diagnosed by the combination of low serum iron, low mean red cellular volume, high transferrin and low iron saturation. Ferritin falls in ID, but rises in the acute phase 17, complicating its interpretation as a marker for IDA in IBD. Ferritin was therefore defined as low in the context of C‐reactive protein (CRP; ferritin <30 if CRP <10 and ferritin <100 if CRP >10). Children with severe atopy or allergies or history of prior anaphylaxis were excluded from the study, and were not offered IV iron therapy.
Choice of iron preparation was based primarily on patient age, with those aged under 12 years receiving IS and those over 12 years receiving FCM. Demographic data, disease status, iron preparation type, dose and number of infusions were recorded. Dosage was calculated as a function of body weight and Hb, as per manufacturers' recommended dosing schedules. Iron preparations were infused according to British National Formulary guidelines. FCM infusions were administered over 15–30 min, and IS over 50–70 min. All patients completed a 2‐h inpatient observation period postinfusion, during which vital signs were recorded at regular intervals.
Adverse reactions were identified by detailed review of written case notes, nursing observations, and nursing, pharmacy and medical electronic records. Efficacy was assessed by pre‐ and post‐treatment iron, transferrin saturation, ferritin, transferrin and Hb levels. Changes in CRP and erythrocyte sedimentation rate (ESR) were also recorded.
The study was approved and registered as a Clinical Audit by the Local Quality Governance Department.
Results
Forty‐one children with IBD received 48 infusion episodes (104 IV iron infusions) over the study period. 23 children were female and 18 male. Mean age was 13.5 years, ranging from 3 to 17 years. Eighteen had CD (44%) and 23 ulcerative colitis (56%). Thirty‐five patients received 58 FCM infusions. Five of these 35 went on to require a second FCM infusion episode at a later date during the study period, resulting in 40 FCM infusion episodes in total. Thus, FCM was given at an average of 1.5 infusions per patient episode. Seven patients received 46 IS infusions; one of these required a further IS infusion episode during the study period. Thus, IS was given at an average of 5.8 infusions per patient episode. One patient initially completed a course of IS infusions and 11 months later received FCM as she moved age group. The doses ranged from 500 to 1000 mg per infusion for FCM and 70 to 100 mg per infusion for IS. Where an individual received a second infusion episode, the median time between episodes was 12.5 months (range 7–21).
All children aged >12 years received FCM. Patients under 12 years received IS, which, although also off licence, is in line with greater past paediatric experience and widespread paediatric practice in the UK. Choice of iron preparation for children between their 12th and 13th birthdays was made on a case by case basis. Three 12‐year‐olds received FCM and two IS.
Safety
Two of 35 patients who received FCM developed rash after completing their first infusion, giving a reaction rate of 5.7%. Both reactions were mild. In one patient, facial and upper limb urticarial rash appeared 30 min post infusion, with a 20% drop in systolic blood pressure to 80 mmHg on a single measurement, which resolved rapidly. Symptoms resolved after a single dose of IV chlorphenamine with no further complications. Subsequent doses of FCM were not given and the patient continued on oral iron therapy. Another patient developed upper limb rash 1 h postinfusion with no systemic compromise. She received a single dose of IV chlorphenamine, after which the rash resolved. She did not require a second dose of FCM as adequate increment in Hb was achieved with single dose. One patient of the seven who received IS developed facial itching and mild eye puffiness 15 min into her first infusion, giving a reaction rate of 14%. There was no systemic compromise and symptoms resolved with single dose of IV chlorphenamine.
Efficacy
Of the 40 patient episodes of FCM, 18 received a second dose 1–4 weeks later. Of the 22 episodes in which single dose was given, three did not attend for their scheduled second infusion, in one case the second dose was cancelled because of adverse reaction, one was less than 35 kg – as per administration guidelines – and 17 achieved adequate increment in Hb with single dose. Hb was recorded preinfusion in all 48 infusion episodes and postinfusion in 44/48 cases. Mean Hb pre‐infusion was 9.3 (range 7.3–12.1) g dl–1. Mean Hb post‐treatment was 11.8, an increase of 2.5 per patient (P < 0.0001). Post‐treatment Hb was measured 1–16 weeks after infusion; although data were available 1–4 weeks after infusion in 43/48 episodes. There was significant improvement in all haematinic indices following infusion (Table 1 and Figure 1), and in 30/48 episodes (62.5%), Hb normalized for age and sex.
Table 1.
Haematinic indices pre‐ and post‐treatment. Data were imported into STATA version 14. Descriptive statistics: mean, standard deviation (SD), minimum and maximum were calculated for pre‐ and post‐treatment values of haemoglobin (Hb), transferrin saturation, CRP, ESR, transferrin, ferritin and iron. Paired sample Student t tests for paired differences were calculated for pre‐ and post‐treatment values for each variable. Hb, transferrin, transferrin saturation, Fe and ESR: P < 0.0001. Ferritin: P < 0.0002. CRP: P = 0.94
| n | Mean | SD | Min | Max | ||
|---|---|---|---|---|---|---|
| Index | 48 | 13.52 | 2.88 | 3 | 17 | |
| Hb | Pre | 48 | 9.26 | 1.01 | 7.3 | 12.1 |
| Post | 44 | 11.8 | 1.28 | 9.3 | 15 | |
| Transferrin saturation | Pre | 44 | 4.66 | 2.91 | 1 | 15 |
| Post | 40 | 20.95 | 11.02 | 3 | 47 | |
| CRP | Pre | 20 | 8.87 | 15.47 | .2 | 65.0 |
| Post | 19 | 8.66 | 12.10 | .2 | 46.0 | |
| ESR | Pre | 16 | 37.50 | 23.67 | 5.0 | 112.0 |
| Post | 18 | 22.06 | 17.80 | 2.0 | 73.0 | |
| Transferrin | Pre | 42 | 3.06 | .50 | 2.3 | 4.0 |
| Post | 35 | 2.24 | .42 | 1.7 | 4.0 | |
| Ferritin | Pre | 30 | 11.3 | 11.88 | 2 | 56 |
| Post | 23 | 146.43 | 133.08 | 10 | 446 | |
| Iron | Pre | 47 | 3.38 | 1.78 | 1 | 9 |
| Post | 40 | 11.78 | 5.21 | 3 | 25 | |
Figure 1.
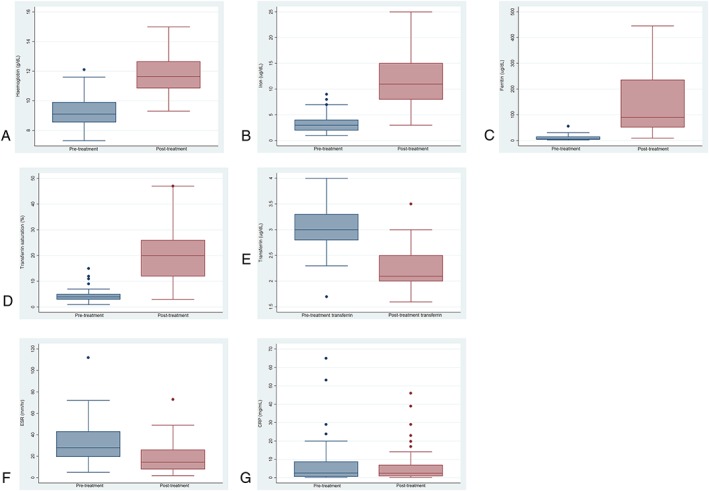
(A) Hb pre‐ and post‐treatment [g dl–1; normal ranges (NR) differ with age and sex]. P < 0.0001. (B) Iron pre‐ and post‐treatment (NR 7–27 μg dl–1). P < 0.0001. (C) Ferritin pre‐ and post‐treatment (NR 7–140 μg dl–1). P < 0.0002. (D) Transferrin saturation pre‐ and post‐treatment (NR 16–55%). P < 0.0001. (E) Transferrin pre‐ and post‐treatment (NR 1.7–3.4 g l–1). P < 0.0001. (F) Erythrocyte sedimentation rate (ESR; mm h–1) P < 0.0001. (G) C‐reactive protein (CRP; mg ml–1) P = 0.94 pre‐ and post‐treatment
Disease severity (as represented by ESR) did not correlate significantly with either pretreatment Hb, increment in Hb after iron infusion or likelihood of achieving normalization of the anaemia (Table 2). Thus, there is no suggestion from the current study that efficacy of iron infusion is affected by disease severity.
Table 2.
Hameoglobin (Hb) concentrations (g l–1) pre‐ and post‐treatment, stratified according to disease activity as measured by pretreatment ESR. Percentage (and patient number) who achieved normalization of Hb for age and sex are given for each ESR group
| ESR ≤ 20 (n = 15) | ESR 20–50 (n = 28) | ESR > 50 (n = 5) | |||
|---|---|---|---|---|---|
| Pre Hb | Post Hb | Pre Hb | Post Hb | Pre Hb | Post Hb |
| Mean 9.2 | Mean 11.8 | Mean 9.2 | Mean 11.9 | Mean 9.7 | Mean 11.5 |
| Normal in 73% (n = 11/15) | Normal in 57% (n = 16/28) | Normal in 60% (n = 3/5) | |||
Discussion
Published evidence strongly supports both proactive treatment of IDA in IBD, and the use of newer IV iron preparations, which are efficacious with fewer doses and less allergenic than their predecessors. As in many other areas of paediatric practice, treatment of children has relied upon the extrapolation of adult research. Here we contribute safety and efficacy data for IV iron therapy in paediatric IBD.
Oral ferrous supplements are oxidized in the gut, produce activated hydroxyl radicals that affect the mucosa 18 and may provoke gastrointestinal symptoms including bloating, nausea and pain, which can be exacerbated in patients with IBD 19. In inflammatory diseases, iron absorption is reduced and increased hepcidin, an acute phase protein, plays a key role. In health, hepcidin levels rise with total body iron stores and protect against iron overload. Hepcidin is upregulated in systemic inflammatory states by interleukins‐6 and ‐1, and impairs absorption of oral iron via multiple mechanisms 20. Adherence to oral iron therapy, particularly in adolescent IBD patients, is frequently suboptimal 21. Even in the adherent patient, several months of oral supplementation may be required to achieve target Hb.
The first IV iron introduced in the 1930s, an iron oxyhydroxide complex 22, had major side effects and was only indicated in rare circumstances 23. In the 1950s, a high‐molecular weight iron dextran was developed in which the iron oxyhydroxide complex was surrounded by a shell of dextran polymers 24. This improved its bioavailability and side effect profile; however, allergic reactions were frequent 25. Gradually, low‐molecular weight iron dextran compounds became available, which caused fewer adverse reactions 26. Later, ferric gluconate was introduced and was safer than iron dextran 27.
In 2000, IS came into clinical use; its incidence of anaphylaxis was lower at 0.002%, compared to 0.04% with ferric gluconate and 0.6–2.3% with high‐molecular weight iron dextran 28. The biggest drawback of IS is its low maximum single administrable dose, resulting in multiple separate infusions to reach therapeutic dose. Finally, in 2007 FCM became available with a license in Europe for patients older than 14 years. A single dose of 1000 mg of iron can be given, and administered relatively rapidly, over 15–60 min 29, with no requirement for test dose, thus reducing hospital attendance times and costs.
Of the 40 children in our series who received 104 infusions of IV iron none experienced major or lasting adverse event. In our series, FCM was associated with a 5.7% mild reaction rate (rash) and IS with a 14% mild reaction rate (itch and eye swelling). These numbers do not allow comparative conclusions to be drawn between the two iron preparations in terms of safety; however, we provide evidence that parenteral iron and in particular FCM is safe and well tolerated in children.
We show that IV iron is a highly efficacious treatment for IDA in children with IBD, inducing clinically and statistically significant increments in Hb, Fe and transferrin saturation, and reductions in transferrin and ESR. The reduction in ESR was not paralleled by significant reduction in CRP and reflects resolution of IDA rather than change in IBD activity.
As well as its lower reported incidence of anaphylaxis, FCM offers a less onerous dosing schedule, no requirement for test dose and shorter infusion time in contrast to IS. In our series we demonstrate safety of FCM in children at least as young as 12 years. This is an off‐label use of this medication (age <14 years). The British Society of Paediatric Gastroenterology Hepatology and Nutrition (BSPGHAN) has published iron therapy guidelines in which it is simply noted that parenteral iron preparations are not licenced in children aged <14 years 30. Other investigators have more recently demonstrated safety of FCM in even younger age groups, not specific to IBD 31.
We have selected lower risk cases by excluding those with a personal history of severe allergy, atopy or anaphylaxis to other agents. Whilst the safety profile of parenteral iron in children is probably comparable to that in adults, oral iron remains a safer and less costly option, and should be trialled in the first instance in most cases, where IDA is mild to moderate, IBD activity is quiescent and there is no history of intolerance.
We acknowledge the limitations of this study associated with its retrospective design and heterogeneous clinical nature. Whilst prospective randomized trial design is needed in the field, the current study provides supporting evidence for the safety and efficacy of new‐generation IV iron preparations in the treatment of IBD‐associated IDA in children.
Competing Interests
J.F. is on the advisory board with Janssen and has received travel sponsorship with Falk. J.M.E.F. has received travel sponsorship with Falk.
The study was approved as a registered clinical audit by the Chelsea and Westminster and West Middlesex University Hospitals Clinical Governance Department reference LA187.
Contributors
M.P. acquired and interpreted the data and drafted the paper. D.P. acquired the data and revised the manuscript. R.K.L. performed the statistical analyses and revised the manuscript. E.G. acquired the data. K.S. revised the manuscript. J.M.E.F. interpreted the data and revised the manuscript. J.E. designed the study, interpreted the data and co‐wrote the paper. All authors approved the final version of the manuscript and are accountable for the accuracy and integrity of the work.
Papadopoulos, M. , Patel, D. , Korologou‐Linden, R. , Goto, E. , Soondrum, K. , Fell, J. M. E. , and Epstein, J. (2018) Safety and efficacy of parenteral iron in children with inflammatory bowel disease. Br J Clin Pharmacol, 84: 694–699. doi: 10.1111/bcp.13493.
References
- 1. Goodhand JR, Kamperidis N, Rao A, Laskaratos F, McDermott A, Wahed M, et al Prevalence and management of anaemia in children, adolescents and adults with IBD. Inflamm Bowel Dis 2012; 18: 513–519. [DOI] [PubMed] [Google Scholar]
- 2. Gerasimidis K, Barclay A, Papangelou A, Missiou D, Buchanan E, Tracey C, et al The epidemiology of anemia in pediatric inflammatory bowel disease: prevalence and associated factors at diagnosis and follow‐up and the impact of exclusive enteral nutrition. Inflamm Bowel Dis 2013; 19: 2411–2422. [DOI] [PubMed] [Google Scholar]
- 3. Bager P, Befrits R, Wikman O, Lindgren S, Moum B, Hjortswang H, et al The prevalence of anemia and iron deficiency in IBD outpatients in Scandinavia. Scand J Gastroenterol 2011; 46: 304–309. [DOI] [PubMed] [Google Scholar]
- 4. Bartels U, Pedersen NS, Jarnum S. Iron absorption and serum ferritin in chronic inflammatory bowel disease. Scand J Gastroenterol 1978; 13: 649–656. [DOI] [PubMed] [Google Scholar]
- 5. Agaoglu L, Torun O, Unuvar E, Sefil Y, Demir D. Effects of iron deficiency anemia on cognitive function in children. Arzneimittelforschung 2007; 57: 426–430. [DOI] [PubMed] [Google Scholar]
- 6. Reinisch W, Staun M, Bhandari S, Munoz M. State of the iron: how to diagnose and efficiently treat iron deficiency anemia in inflammatory bowel disease. J Crohns Colitis 2013; 7: 429–440. [DOI] [PubMed] [Google Scholar]
- 7. Wells CW, Lewis S, Barton JR, Corbett S. Effects of changes in hemoglobin level on quality of life and cognitive function in inflammatory bowel disease patients. Inflamm Bowel Dis 2006; 12: 123–130. [DOI] [PubMed] [Google Scholar]
- 8. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, et al FAIR‐HF Trial Investigators. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361: 2436–2448. [DOI] [PubMed] [Google Scholar]
- 9. Van Wyck DB, Mangione A, Morrison J, Hadley PE, Jehle JA, Goodnough LT. Large‐dose intravenous ferric carboxymaltose injection for iron deficiency anemia in heavy uterine bleeding: a randomized controlled trial. Transfusion 2009; 49: 2719–2728. [DOI] [PubMed] [Google Scholar]
- 10. Covic A, Mircescu G. The safety and efficacy of intravenous iron carboxymaltose in anaemic patients underoing haemodialysis: a multi‐centre, open‐label, clinical study. Nephrol Dial Transplant 2010; 25: 2722–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moorani KN, Asim S. Parenteral iron sucrose in iron deficiency anaemia of paediatric chronic kidney disease. J Ayub Med Coll Abbottabad 2011; 23: 47–50. [PubMed] [Google Scholar]
- 12. Pinsk V, Levy J, Moser A, Yerushalmi B, Kapelushnik J. Efficacy and safety of intravenous iron sucrose therapy in a group of children with iron deficiency anemia. Isr Med Assoc J 2008; 10: 335–338. [PubMed] [Google Scholar]
- 13. Goldstein SL, Morris D, Warady BA. Comparison of the safety and efficacy of 3 iron sucrose iron maintenance regimens in children, adolescents, and young adults with CKD: a randomized controlled trial. Am J Kidney Dis 2013; 61: 588–597. [DOI] [PubMed] [Google Scholar]
- 14. Laass MW, Straub S, Chainey S, Virgin G, Cushway T. Effectiveness and safety of ferric carboxymaltose treatment in children and adolescents with inflammatory bowel disease and other gastrointestinal diseases. BMC Gastroenterol 2014; 14: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Danko I, Weidkamp M. Correction of iron deficiency anemia with intravenous iron sucrose in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2016; 63: e107–e111. [DOI] [PubMed] [Google Scholar]
- 16. WHO . Iron deficiency anaemia: assessment, prevention, and control. A guide for programme managers. Available at http%3A%2F%2Fwww.who.int%2Fnutrition%2Fpublications%2Fen%2Fida_assessment_prevention_control.pdf (last accessed 16 January 2018).
- 17. Gasche C, Berstad A, Befrits R, Beglinger C, Dignass A, Erichsen K. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm Bowel Dis 2007; 13: 1545–1553. [DOI] [PubMed] [Google Scholar]
- 18. Millar AD, Rampton DS, Blake DR. Effects of iron and iron chelation in vitro on mucosal oxidant activity in ulcerative colitis. Aliment Pharmacol Ther 14: 1163–1168. [DOI] [PubMed] [Google Scholar]
- 19. Erichsen K, Hausken T, Ulvik RJ, Svardal A, Berstad A, Berge RK. Ferrous fumarate deteriorated plasma antioxidant status in patients with Crohn disease. Scand J Gastroenterol 2003; 38: 543–548. [DOI] [PubMed] [Google Scholar]
- 20. Bergamaschi G, Di Sabatino A, Pasini A, Ubezio C, Costanzo F, Grataroli D, et al Intestinal expression of genes implicated in iron absorption and their regulation by hepcidin. Clin Nutr 2017; 36: 1427–1433. [DOI] [PubMed] [Google Scholar]
- 21. Greenley RN, Stephens KA, Nguyen EU, Kunz JH, Janas L, Goday P, et al Vitamin and mineral supplement adherence in pediatric inflammatory bowel disease. J Pediatr Psychol 2013; 38: 883–892. [DOI] [PubMed] [Google Scholar]
- 22. Ganz T, Nemeth E. Iron sequestration and anemia of inflammation. Semin Hematol 2009; 46: 387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Macdougall IC. Evolution of iv iron compounds over the last century. J Ren Care 2009; 35 (Suppl 2): 8–13. [DOI] [PubMed] [Google Scholar]
- 24. Auerbach M, Ballard H. Clinical use of intravenous iron. Administration efficacy and safety. Hematology Am Soc Hematol Educ Program 2010; 2010: 338–347. [DOI] [PubMed] [Google Scholar]
- 25. Cançado RD, Muñoz M. Intravenous iron therapy: how far have we come? Rev Bras Hematol Hemoter 2011; 33: 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fishbane S, Ungureanu VD, Maesaka JK, Kaupke CJ, Lim V, Wish J. The safety of intravenous iron dextran in hemodialysis patients. Am J Kidney Dis 1996; 28: 529–534. [DOI] [PubMed] [Google Scholar]
- 27. Faich G, Strobos J. Sodium ferric gluconate complex in sucrose: safer intravenous iron therapy than iron dextrans. Am J Kidney Dis 1999; 33: 464–470. [DOI] [PubMed] [Google Scholar]
- 28. Chertow GM, Mason PD, Vaage‐Nilsen O, Ahlmén J. Update on adverse drug events associated with parenteral iron. Nephrol Dial Transplant 2006; 21: 378–382. [DOI] [PubMed] [Google Scholar]
- 29. Lyseng‐Williamson KA, Keating GM. Ferric carboxymaltose: a review of its use in iron‐deficiency anaemia. Drugs 2009; 69: 739–756. [DOI] [PubMed] [Google Scholar]
- 30.Available at https%3A%2F%2Fbspghan.org.uk%2Fcontent%2Fnew-guidelines-management-iron-deficiency-anaemia-paediatric-ibd (last accessed 16 January 2018)
- 31. Powers JM, Shamoun M, McCavit TL, Adix L, Buchanan GR. Intravenous ferric carboxymaltose in children with iron deficiency anemia who respond poorly to oral iron. J Pediatr 2017; 180: 212–216. [DOI] [PubMed] [Google Scholar]


