Abstract
Aims
Inappropriate use of antibiotics is one of the most important factors contributing to the emergence of drug resistant pathogens. The purpose of this study was to measure the clinical impact of antimicrobial stewardship programme (ASP) interventions on hospitalized patients at the Intensive care unit at Palestinian Medical Complex.
Methods
A prospective audit with intervention and feedback by ASP team within 48–72 h of antibiotic administration began in September 2015. Four months of pre‐ASP data were compared with 4 months of post‐ASP data. Data collected included clinical and demographic data; use of antimicrobials measured by defined daily doses, duration of therapy, length of stay, readmission and all‐cause mortality.
Results
Overall, 176 interventions were made the ASP team with an average acceptance rate of 78.4%. The most accepted interventions were dose optimization (87.0%) followed by de‐escalation based on culture results with an acceptance rate of 84.4%. ASP interventions significantly reduces antimicrobial use by 24.3% (87.3 defined daily doses/100 beds vs. 66.1 defined daily doses/100 beds P < 0.001). The median (interquartile range) of length of stay was significantly reduced post ASP [11 (3–21) vs. 7 (4–19) days; P < 0.01]. Also, the median (interquartile range) of duration of therapy was significantly reduced post‐ASP [8 (5–12) days vs. 5 (3–9); P = 0.01]. There was no significant difference in overall 30‐day mortality or readmission between the pre‐ASP and post‐ASP groups (26.9% vs. 23.9%; P = 0.1) and (26.1% vs. 24.6%; P = 0.54) respectively.
Conclusions
Our prospective audit and feedback programme was associated with positive impact on antimicrobial use, duration of therapy and length of stay.
Keywords: antibiotic use, antimicrobials, intensive care unit, stewardship
What is Already Known about this Subject
Inappropriate use of antibiotic has been associated with increased resistance, morbidity and hospital stay.
Antimicrobial stewardship programmes (ASPs) aim to improve patient safety and outcomes whilst reducing adverse effects associated with antimicrobial use
What this Study Adds
This study identified the current patterns of antibiotic prescribing and the impact of an ASP on these practices.
ASP interventions are effective to manage the antimicrobial prescription according the local guidelines
The ASP team can effectively participate in health education to promote the rational use of antimicrobial agents
Introduction
Much has been written about antimicrobial resistance (AR) as an important factor in both patient‐safety and public‐health 1. Reports of AR bacterial infections are growing, and the ability of pharmaceutical industry to generate new classes of antibiotics is limited 2, 3. Factors that are known to contribute to AR include the extent of antimicrobial exposure and consumption of antibiotics in a population 4, 5, 6, 7. A recent meta‐analysis demonstrated a link between primary care physicians prescribing of antibiotics to AR in pathogens causing respiratory, urinary and skin infections 6.
The search for a way to improve antimicrobial prescribing practices has been addressed by the implementation of antimicrobial stewardship programmes (ASP) to control AR 7. ASP includes measures to promote the appropriate use of antimicrobials. Such measures include: educational programmes for all clinical staff to ensure competency; evidence‐based optimal treatment for routine infections; communication of issues related to antimicrobial use to stakeholders; and finally monitor the impact on change in clinical practice 8, 9, 10, 11. Ultimately, the aims of ASP are to improve efficacy, minimize adverse effects and limit AR. Infections caused by susceptible organisms are easier to treat than those caused by resistant organisms that may have poor clinical outcome (morbidity and mortality), extended hospital stay and higher cost 12.
Two core ASP strategies have been adopted by the Infectious Diseases Society of America to reduce the inappropriate use of antimicrobials: prospective audit and feedback interventions 11, 13. The main attribute of prospective audit and feedback strategy is that acceptance of recommendations is voluntary; as such doctors maintain their prescribing autonomy 14. It is therefore more acceptable to doctors and less likely to be opposed. In fact, due to the feedback mechanism, this type of intervention may be considered educational.
Another point to consider is the method for implementation and evaluation of such programmes. Some of the options include; the selection of audit cases based on surgical or medical fields and/or based on pre‐defined antibiotics. Monitoring for consumption can be done in the form of defined daily doses (DDD) or days of therapy 15, 16. This may identify high prescription areas and maximize the impact of interventions.
To date, there has been no programme or evaluation of ASP in Palestinian hospitals. Hence, in this study, the aim is to evaluate the impact of ASP on the following outcomes: (i) antibiotic consumption; (ii) duration of therapy; (iii) length of hospital stay; (iv) intervention acceptance rate; (v) readmission within 30 days of discharge; and (vi) mortality within 30 days of ASP audit.
Methodology
Patients and setting
This was a single‐centre, prospective, pre‐ and postintervention study at Palestinian Medical Complex (PMC) in Ramallah. The PMC consists of five hospitals; Ramallah Public Hospital; Al‐Sheikh Zayed Hospital; National Center for Blood Diseases; Bahrain Pediatrics Hospital; and Kuwaiti Specialized Surgery Hospital. The PMC has 214 beds. It provides a wide range of services, including neonatal care, maternity care, internal medicine, paediatrics, general surgery and cardiovascular surgery.
All patients admitted to the intensive care unit (ICU) and administered any antimicrobial drug were included in the study. A review of the ASP database was conducted, for interventions made between September 2015 and December 2015.
Description of the ASP
The ASP team, consisting of an infectious diseases physician, clinical microbiologist, clinical pharmacists at the 12‐bed ICU, drew up new antibiotic guidelines for empirical treatment of common infections. Evidence for these guidelines was drawn from international published guidelines and was adapted to Palestinian Medical complex microbial susceptibility patterns. The clinical pharmacists performed the primary review, screened cases for appropriateness and made therapeutic recommendations; for example, dosage optimization, or switch from intravenous to oral antibiotics. These recommendations were reviewed by the ASP team on the 2nd, 4th and 7th days, allowing for bacterial culture to be processed with recommendations for de‐escalation, change, dose adjustment of antibiotics where appropriate (Figure 1). Criteria used to determine if antimicrobial was inappropriately prescribed include:
If hospital antibiotic guidelines were not followed without reasonable explanation;
If empiric treatment was less than optimal per guideline including dose level, dose duration or dosing regimen;
If cultures results indicate that a narrower‐spectrum antibiotic may be more appropriate;
If culture results show that there was no infection and an alternative reason for the fever is identified.
Figure 1.
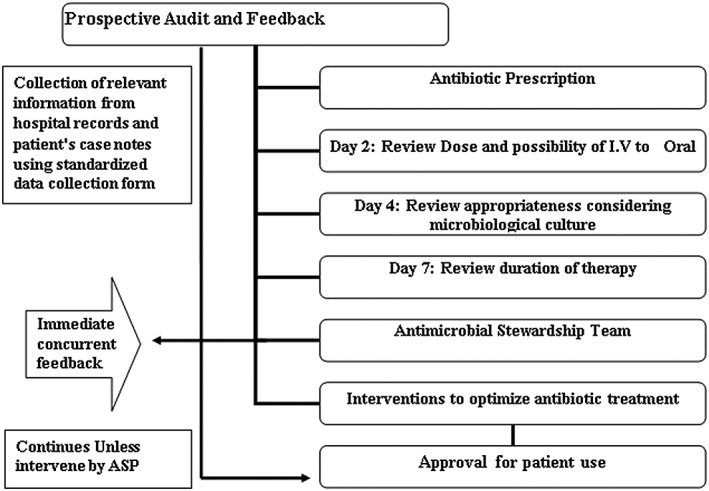
Schematic diagram of the antimicrobial stewardship programme prospective audit with immediate concurrent feedback workflow
The prospective audit with intervention and feedback made by the ASP team within 48–72 h of antibiotic administration began in September 2015. Four months of pre‐ASP data were compared with 4 months of post‐ASP data. Data collected included clinical and demographic data; as well as use of antimicrobials which was calculated using DDD.
Data collections and outcomes
Compliance with or rejection of ASP recommendations was determined via review of patient's medical record/chart at 24 and 48 h after antibiotic recommendation. During the study period, hospital pharmacy records were used to obtain drug prescription data for the audited antibiotics. DDD/100 beds for each drug or drug category prescribed monthly were calculated following the World Health Organization Anatomical Therapeutic Chemical classification system 17. All recommendations made by ASP team were recorded on a standardized form.
Ethics
The study was approved by Palestinian Medical Complex ethical committee. Informed consent was deemed unnecessary since ASP constituted routine clinical practice and medical record analysis was analysed anonymously.
Data analysis
All statistical calculations were analysed using SPSS version 16.0 (SPSS Inc., Chicago, IL). Data were expressed as the mean ± standard deviation for continuous variables, and unpaired Student t test was performed to determine differences between mean values. For noncontinuous variables data were expressed as median [interquartile range (IQR)]. Mann–Whitney test was performed; for categorical variables, data were expressed as number and percentage and were analysed by chi‐square test or Fisher exact test, as appropriate.
Results
Demographics and comorbid conditions
There were no statistically significant differences in terms of age, number of comorbid conditions, previous hospitalization and previous antibiotic use between the pre‐ASP group and post‐ASP group (Table 1). Patients in the post‐ASP period had more episodes of sepsis and respiratory infections. Types of infection were defined based on the International Classification ICD‐10 17.
Table 1.
Patient demographics and comorbid conditions by period
| Demographic characteristics | Pre‐ASP period (n = 115) | Post‐ASP period (n = 142) | P |
|---|---|---|---|
| Mean age (years) | 68.4 (15.3) | 70.1 (16.6) | 0.35 |
| Male sex | 55 (47.8) | 82 (57.7) | 0.01 |
| Previous hospitalization within 3 months | 53 (46.1) | 68 (47.9) | 0.43 |
| Previous antibiotic use within 3 months | 79 (68.7) | 90 (63.4) | 0.17 |
| Comorbid conditions | <0.01 | ||
| No comorbidities | 9 (7.8) | 21 (14.8) | |
| 1–2 | 47 (40.8) | 65 (45.8) | |
| 3–4 | 43 (37.4) | 44 (30.9) | |
| >5 | 16 (14.0) | 12 (8.5) | |
| Median (IQR) | 4 (0–13) | 4 (0–12) | |
| Respiratory infection | 44 (38.3) | 62 (43.7) | <0.01 |
| Sepsis | 27 (23.5) | 29 (20.4) | <0.01 |
| Genitourinary | 10 (8.7) | 19 (13.4) | <0.01 |
| Skin, joint bone, soft tissue | 11 (9.6) | 14(9.9) | 0.88 |
| Infection of the central nervous system | 9 (7.8) | 10 (7.0) | 0.68 |
| Infection of cardiovascular system | 4 (3.5) | 6 (4.2) | 0.28 |
| Others | 10 (8.6) | 2 (1.4) | <0.01 |
Data are mean ± standard deviation, median (interquartile range, IQR), or number (%) of patients
Differences assessed by Fisher's exact or χ2 test (categorical data), t test (continuous data) as appropriate
ASP, antimicrobial stewardship programme
Interventions
During the post‐ASP period, a total of 356 antimicrobial prescriptions for 142 patients were revised during a 4‐month period. Of these, 49.4% were considered inappropriate in 101 patients. The majority of interventions recommended by ASP team were: de‐escalating 64 (36.4%); discontinue antibiotics 53 (30.1%); and intravenous to oral switch intervention 32 (18.2%). Overall, 176 interventions were made by ASP team with an average acceptance rate of 78.4% by the ICU team. The most accepted interventions were dose optimization based on pharmacokinetic and dynamics of antibiotics with an acceptance rate of 87.0% followed by de‐escalation based on culture results with an acceptance rate of 84.4% (Table 2). Changing the antibiotic was the most common reason for non‐acceptance, another key reason was the ICU team did not view the recommendations; therefore, the recommendations were no longer applicable.
Table 2.
Types of interventions recommended by the antimicrobial stewardship programme team
| Intervention | n (%) | Accepted, n (%) |
|---|---|---|
| De‐escalation based on culture results | 64 (36.4) | 54 (84.4) |
| Discontinue antibiotic | 53 (30.1) | 41 (77.4) |
| Dose optimization | 23 (13.1) | 20 (87.0) |
| Intravenous‐to‐oral switch | 32 (18.2) | 21 (65.6) |
| Adding an antibiotic | 4 (2.3) | 2 (50.0) |
| Total | 176 (100) | 138 (78.4) |
Antibiotic use
There were seven drugs in the drug use 90% segment selected for audit out of 21 drugs prescribed in the ICU (Figure 2). Overall utilization was reduced by 24.3% (87.3 DDD/100 beds vs. 66.1 DDD/100 beds; P < 0.001), specifically driven by third generation cephalosporins, carbapenems and fluroquinolones. Ceftriaxone use was reduced by 34.2% (18.4 DDD/100 beds vs. 12.1 DDD/100 beds; P < 0001), piperacillin/tazobactam use decreased by 17.7% (12.4 DDD/100 beds vs. 10.2 DDD/100 beds; P < 0.001), and meropenem use decreased by 22.2 (10.8 DDD/100 vs. 8.4 DDD/100 beds; P < 0.001).
Figure 2.

Antimicrobial use pre‐ and post‐antimicrobial stewardship programme intervention
Clinical outcomes
The median (IQR) lengths of hospital stay before and after implementation of the ASP were 11 (3–21) days and 7 (4–19) days, respectively (P < 0.01). Also, there was a statistically significant difference in duration of therapy between the median (IQR) of pre‐ASP group [8 (5–12) days] and the post‐ASP group [5 (3–9) days; P = 0.01]. There was no significant difference in overall mortality between the pre‐ASP group (31, 26.9%) and the post‐ASP group (34, 23.9%; P = 0.1).
Regarding readmission, independent samples t test showed no significant differences between pre‐ and post‐ASP (Table 3). Of the 84 surviving patients in the pre‐ASP group 30 (26.1%) were re‐admitted within 30 days of discharge and 17 (14.8%) were re‐admitted within 60 days of discharge. Of the 108 surviving patients in the post‐ASP, 35 (24.6%) were re‐admitted within 30 days and 18 (12.7%) within 60 days of discharge (P = 0.54, P = 0.28 respectively).
Table 3.
Outcomes: post‐antimicrobial stewardship programme (ASP) period compared with pre‐ASP period
| Outcome | Pre‐ASP period (n = 115) | After‐ASP period (n = 142) | P‐Value |
|---|---|---|---|
| Duration of therapy (days), median (IQR) a | 8 (5–12) | 5 (3–9) | 0.01* |
| Length of stay (days), median (IQR) a | 11 (3–21) | 7 (4–19) | 0.01* |
| 30‐day readmission b | 30 (26.1) | 35 (24.6) | 0.54 |
| 60‐day readmission b | 17 (14.8) | 18 (12.7) | 0.28 |
| 30‐day all‐cause mortality b | 31 (26.9) | 34 (23.9) | 0.10 |
Mann–Whitney U test,
Student t test,
significant P < 0.05
IQR, interquartile range
Discussion
Little is known about the impact of implementing an ASP and monitoring of antimicrobial prescribing in Palestinian hospitals. Moreover, most drug related problems in hospitals are caused by anti‐infective medications 18. To the best of our knowledge this study was the first in West Bank to focus on the clinical impact of implementing ASP on patient clinical outcomes. One important core strategy of ASP, the prospective audit and feedback strategy will probably be the most widely implemented in view of its clear advantages particularly with regards to lack of opposition from prescribers 19. One of the desirable targets of an ASP is to control inappropriate and overuse of broad spectrum antibiotics. It is estimated that 50% of antimicrobial use in hospitals is inappropriate 20; our study confirms this number since 49.4% of antibiotic prescriptions were deemed to require intervention.
Consumption of restricted antibiotics was significantly reduced after implementation of ASP. Previous studies have shown that ASPs have consistently been effective in reducing prescriptions of restricted antibiotics 21, 22, 23, 24. Our study showed that consumption of all seven audited antimicrobials decreased due to ASP intervention. Ceftriaxone consumption was noticeably high at baseline and ASP intervention resulted in a significant decrease in its use. This may be attributed to the fact that approximately 40% of patients in the study suffered from respiratory diseases and chest infections. Based on local and international management standards on pneumonia, it is likely that the empirical therapy doctors selected was a cephalosporin 25, 26. Other broad‐spectrum antibiotics that had significant reductions in consumption include fluoroquinolones and carbapenems. Out of the five interventions evaluated in this study, de‐escalation of antibiotic based on culture results antibiotic were the most commonly encountered, if no resistant organism (e.g. Pseudomonas aeruginosa, Acinetobacter spp. or methicillin‐resistant Staphylococcus aureus).
It was observed from this study that the ICU physicians were more likely to accept ASP interventions; however, approximately 22% of the ASP interventions were rejected by the physicians. The main reason for rejection is changing the antibiotic. This may be expected considering that many physicians have personal preferences with no clear reasons indicated. Another possible reason for rejection of the recommendation may be the fact that some of the cases developed complications. In such situations, physicians may opt to be cautious and reject ASP interventions that may deviate from their opinions. Moreover, physicians are not keen to change antibiotics despite microbiology results suggesting that narrower‐spectrum antibiotics can be prescribed, as the patients had responded to the initial empiric antibiotics.
It should be noted that our recommendations were made through written notes. On some occasions, the ICU team did not view the recommendations within the specified 48 h; therefore, the recommendations were no longer applicable and were considered a rejection. One consideration may be that direct face‐to‐face communication with the providers may have had a greater impact or acceptance. One of the more common interventions made by ASP was to switch to oral therapy intervention, which has two main benefits: first, it reduces the cost of treatment for the patients as it is less expensive than parenteral antibiotic; second, it has fewer incidences of catheter‐related infections and may lead to a shorter hospital stay.
A decrease in both length of hospital stay and duration of therapy after implementation of the ASP was observed. However, the relationship between the decrease in hospital stay and the decrease in antimicrobial consumption is not clear. The study cannot distinguish if the decrease in antimicrobial consumption is caused by the shorter hospital stay or if ASP intervention resulted in better treatment and shorter hospitalization duration or duration of therapy. Many ASP studies have shown a reduction in hospital stay 27. Many potential confounders affect the overall hospital stay and it is difficult to determine the impact of ASP precisely. Future research needs to focus on hospital stay as it appears to be an important measure of the impact of an ASP.
Regarding mortality and re‐admissions, our findings are similar to most studies, which have demonstrated little to no impact of prospective audit and feedback ASPs on mortality 28, 29, 30 and 30‐day readmission 29, 31. This may be because of the large number of factors that may affect clinical response and outcomes. As ASPs continue to evolve, they may include some of the confounding factors such as resistance rate, drug costs, and total costs of care. Addition of those factors may allow for better characterization of the relationship between ASP and mortality and/or re‐admissions.
The results of our study are in line with other programmes that studied the impact of ASP in critical care and showed that ASP interventions were associated with shorter duration of antibiotic therapy, less inappropriate antimicrobial use 32.
In general, ASPs studies have consistently shown a reduction in the average length of hospital stay, infection‐related re‐admissions and 14‐day re‐infection rate. By contrast, assessment of 30‐day mortality has shown little or no difference 33. The reason for such observation appears to be that many factors affect mortality (and length of hospitalization) while the effect of intervention by antimicrobial stewardship (i.e. shortened duration of therapy or choice of a narrower‐spectrum antibiotic) appears negligible.
This study showed that ASP can be introduced successfully into hospitals in low‐ and middle‐income countries with limited human resources, which never practiced stewardship before 34, 35. Pharmacists would need to be trained in monitoring antibiotic use, and would be allowed time in partnership with other healthcare professionals according to the size of hospital 36.
The prospective audit and feedback methodology allows for a team‐based approach to patient care with a focus on both individual patient outcomes and global hospital outcomes. Here, practitioners initiate therapy, and the ASP team intervenes only in select cases. These programmes address both over‐ and under‐treatment. This method allows the ASP team to interact directly with prescribers to tailor specific antibiotic therapy for each patient.
Practitioners may be more receptive to the stewardship programme when suggestions are not only limited to a reduction of antibiotic usage but are also focused on optimal patient care 37.
Implementation of an ASP in a Palestinian hospital over a relatively short time achieved results that appear consistent with other publications in this field of science 33, 34, 35, 36. This finding is particularly interesting, considering that most hospitals do not have a clinical pharmacist with formal training in infectious diseases to provide antimicrobial consultation. Other practices that may improve antimicrobial use may include: implementation of antimicrobial guide with empiric treatment recommendations. Certainly, the addition of routine education programmes for health care professional may improve overall antimicrobial use and provide update on new treatment options.
Limitations
There are several limitations to our study. The study design pre‐ and postintervention is associated with a number of inherent limitations, including the potential for confounding bias.
The ASP study duration was 4 months; it is possible that this study duration is not long enough to characterize the full effect of ASP. In addition, the study attempted to provide accurate definitions for outcomes and potential confounders, yet misclassification bias may still affect the results. Finally, it is unclear how changes in antimicrobial consumption may affect resistance rates and clinical outcomes.
Competing Interests
There are no competing interests to declare.
The authors thank Dr Ahmad Bitawi, Head of the PMC for facilitating this study and use of their database.
Approval for the study was granted by Ethical research committee at the Palestinian Medical Complex Requirement for consent was waived because the data were analyzed anonymously.
Contributors
M.R.K., H.O.H. and M.G.S. jointly conceived designed and coordinate the study. M.R.K. and H.O.H. critically revised the manuscript for important intellectual content. M.A.N., A.A.D., M.B.K. and A.M.K. contributed to data acquisition and analyses and critically revised the manuscript for important intellectual content. M.B.K. wrote code, ran the model and analysed output data. M.G.S. and H.O.H. edited the manuscript and provided conceptual advice. All authors read and approved the final manuscript.
Khdour, M. R. , Hallak, H. O. , Aldeyab, M. A. , Nasif, M. A. , Khalili, A. M. , Dallashi, A. A. , Khofash, M. B. , and Scott, M. G. (2018) Impact of antimicrobial stewardship programme on hospitalized patients at the intensive care unit: a prospective audit and feedback study. Br J Clin Pharmacol, 84: 708–715. doi: 10.1111/bcp.13486.
References
- 1. Tacconelli E, Cataldo MA, Dancer SJ, De Angelis G, Falcone M, Frank U, et al ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug‐resistant Gram‐negative bacteria in hospitalized patients. Clin Microbiol Infect 2014; 20 (Suppl. 1): 1–55. [DOI] [PubMed] [Google Scholar]
- 2. Finch R. Innovation—drugs and diagnostics. J Antimicrob Chemother 2007; 60 (Suppl 1): i79–i82. [DOI] [PubMed] [Google Scholar]
- 3. Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, et al Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 2009; 48: 1–12. [DOI] [PubMed] [Google Scholar]
- 4. London N, Nijsten R, Mertens P, van der Bogaard A, Stobberingh E. Effect of antibiotic therapy on the antibiotic resistance of faecal Escherichia coli in patients attending general practitioners. J Antimicrob Chemother 1994; 34: 239–246. [DOI] [PubMed] [Google Scholar]
- 5. Hillier S, Roberts Z, Dunstan F, Butler C, Howard A, Palmer S. Prior antibiotics and risk of antibiotic‐resistant community‐acquired urinary tract infection: a case–control study. J Antimicrob Chemother 2007; 60: 92–99. [DOI] [PubMed] [Google Scholar]
- 6. Davey P, Brown E, Fenelon L, Finch R, Gould I, Hartman G, et al Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2005; 4: CD003543. [DOI] [PubMed] [Google Scholar]
- 7. Shah RC, Shah P. Antimicrobial stewardship in institutions and office practices. Indian J Pediatr 2008; 75: 815–820. [DOI] [PubMed] [Google Scholar]
- 8. Evans RS, Pestotnik SL, Classen DC, Clemmer TP, Weaver LK, Orme Jr JF, et al A computer‐assisted management program for antibiotics and other antiinfective agents. N Engl J Med 1998; 338: 232–238. [DOI] [PubMed] [Google Scholar]
- 9. Finley RL, Collignon P, Larsson DG, McEwen SA, Li XZ, Gaze WH, et al The scourge of antibiotic resistance: the important role of the environment. Clin Infect Dis 2013; 57: 704–710. [DOI] [PubMed] [Google Scholar]
- 10. Laxminarayan R, Heymann DL. Challenges of drug resistance in the developing world. BMJ 2012; 344: e1567. [DOI] [PubMed] [Google Scholar]
- 11. Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, et al Implementing an antibiotic stewardship program: guidelines by the infectious diseases society of America and the society for healthcare epidemiology of America. Clin Infect Dis 2016; 62: e51e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ashiru‐Oredope D, Sharland M, Charani E, McNulty C, Cooke J. Improving the quality of antibiotic prescribing in the NHS by developing a new Antimicrobial Stewardship Programme: Start Smart—Then Focus. J Antimicrob Chemother 2012; 67 (Suppl 1): i51–i63. [DOI] [PubMed] [Google Scholar]
- 13. Nicolle LE. Antimicrobial stewardship in long term care facilities: what is effective? Antimicrob Resist Infect Control 2014; 3: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cisneros JM, Neth O, Gil‐Navarro MV, Lepe JA, Jimenez‐Parrilla F, Cordero E, et al Global impact of an educational antimicrobial stewardship programme on prescribing practice in a tertiary hospital centre. Clin Microbiol Infect 2014; 20: 82–88. [DOI] [PubMed] [Google Scholar]
- 15. Monnet DL, Mölstad S, Cars O. Defined daily doses of antimicrobials reflect antimicrobial prescriptions in ambulatory care. J Antimicrob Chemother 2004; 53: 1109–1111. [DOI] [PubMed] [Google Scholar]
- 16. Polk RE, Fox C, Mahoney A, Letcavage J, MacDougall C. Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin Infect Dis 2007; 44: 664–670. [DOI] [PubMed] [Google Scholar]
- 17. Steindel SJ. International classification of diseases, clinical modification and procedure coding system: descriptive overview of the next generation HIPAA code sets. J Am Med Inform Assoc 2010; 17: 274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khdour MR, Jarab AS, Adas HO, Samaro EZ, Mukattash TL, Hallak HO. Identification of drug‐related problems: a prospective study in two general hospitals. Curr Clin Pharmacol 2012; 7: 276–281. [DOI] [PubMed] [Google Scholar]
- 19. Charani E, Edwards R, Sevdalis N, Alexandrou B, Sibley E, Mullett D, et al Behavior change strategies to influence antimicrobial prescribing in acute care: a systematic review. Clin Infect Dis 2011; 53: 651–662. [DOI] [PubMed] [Google Scholar]
- 20. Dellit TH, Owens RC, McGowan JE Jr, Gerding DN, Weinstein RA, Burke JP, et al Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007; 44: 159–177. [DOI] [PubMed] [Google Scholar]
- 21. Bosso JA, Drew RH. Application of antimicrobial stewardship to optimise management of community acquired pneumonia. Int J Clin Pract 2001; 65: 775–783. [DOI] [PubMed] [Google Scholar]
- 22. Gyssens IC, Kern WV, Livermore DM. The role of antibiotic stewardship in limiting antibacterial resistance among hematology patients. Haematologica 2013; 98: 1821–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bauer KA, West JE, Balada‐Llasat JM, Pancholi P, Stevenson KB, Goff DA. An antimicrobial stewardship program's impact with rapid polymerase chain reaction methicillin‐resistant Staphylococcus aureus/S. aureus blood culture test in patients with S. aureus bacteremia. Clin Infect Dis 2010; 51: 1074–1080. [DOI] [PubMed] [Google Scholar]
- 24. Hayashi Y, Paterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis 2011; 52: 1232–1240. [DOI] [PubMed] [Google Scholar]
- 25. Marshall BM, Levy SB. Food animals and antimicrobials: impacts on human health. Clin Microbiol Rev 2011; 24: 718–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Niederman MS, Mandell LA, Anzueto A, Bass JB, Broughton WA. Campbell GD, et al. Guidelines for the management of adults with community‐acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med 2001; 163: 1730–1754. [DOI] [PubMed] [Google Scholar]
- 27. Gums JG, Yancey RW Jr, Hamilton CA. A Randomized, prospective study measuring outcomes after antibiotic therapy intervention by a multidisciplinary consult team. Pharmacotherapy 1999; 19: 1369–1377. [DOI] [PubMed] [Google Scholar]
- 28. Lesprit P, Landelle C, Brun‐Buisson C. Clinical impact of unsolicited post‐prescription antibiotic review in surgical and medical wards: a randomized controlled trial. Clin Microbiol Infect 2013; 19: E91–E97. [DOI] [PubMed] [Google Scholar]
- 29. Camins BC, King MD, Wells JB, Googe HL, Patel M, Kourbatova EV, et al Impact of an antimicrobial utilization program on antimicrobial use at a large teaching hospital: a randomized controlled trial. Infect Control Hosp Epidemiol 2009; 30: 931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Masia M, Matoses C, Padilla S, Murcia A, Sanchez V, Romero I, et al Limited efficacy of a nonrestricted intervention on antimicrobial prescription of commonly used antibiotics in the hospital setting: results of a randomized controlled trial. Eur J Clin Microbiol Infect Dis 2008; 27: 597–605. [DOI] [PubMed] [Google Scholar]
- 31. Standiford HC, Chan S, Tripoli M, Weekes E, Forrest GN. Antimicrobial stewardship at a large tertiary care academic medical center: cost analysis before, during, and after a 7‐year program. Infect Control Hosp Epidemiol 2012; 33: 338–345. [DOI] [PubMed] [Google Scholar]
- 32. Kaki R, Elligsen M, Walker S, Simor A, Palmay L, Daneman N. Impact of antimicrobial stewardship in critical care: a systematic review. J Antimicrob Chemother 2011; 66: 1223–1230. [DOI] [PubMed] [Google Scholar]
- 33. Davey P, Brown E, Fenelon L, Finch R, Gould I, Hartman G, et al Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2005; CD003543. [DOI] [PubMed] [Google Scholar]
- 34. Goff DA, Kullar R, Goldstein EJ, Gilchrist M, Nathwani D, Cheng AC, et al A global call from five countries to collaborate in antibiotic stewardship: united we succeed, divided we might fail. Lancet Infect Dis 2016; 17: e56–e63. [DOI] [PubMed] [Google Scholar]
- 35. Brink AJ, van den Bergh D, Mendelson M, Richards GA. Passing the baton to pharmacists and nurses: new models of antibiotic stewardship for South Africa? S Afr Med J 2016; 106: 947–948. [DOI] [PubMed] [Google Scholar]
- 36. Brink AJ, Messina AP, Feldman C, Richards G, Becker PJ, Goff DA, et al Antimicrobial stewardship across 47 South African hospitals: an implementation study. Lancet Infect Dis 2016; 16: 1017–1025. [DOI] [PubMed] [Google Scholar]
- 37. Rimawi RH, Mazer MA, Siraj DS, Gooch M, Cook PP. Impact of regular collaboration between infectious diseases and critical care practitioners on antimicrobial utilization and patient outcome. Crit Care Med 2013; 41: 2099–2107. [DOI] [PubMed] [Google Scholar]


