Abstract
Aims
The aims of the present study were, firstly, to evaluate long‐term trends in the occurrence and treatment of cardiovascular disease (CVD) risk factors and the occurrence of CVD events in children with type 1 diabetes mellitus (T1DM) and, secondly, to assess the determinants of undertreatment of CVD risk factors.
Methods
A retrospective cohort study was conducted in 3728 children (<19 years of age) with T1DM and up to 5 age‐ and gender‐matched diabetes‐free children (reference cohort) (n = 18 513) using data from the Clinical Practice Research Datalink (CPRD).
Results
Compared with diabetes‐free subjects, children with T1DM had significantly higher annual prevalence rates of CVD risk factors and cardiovascular (CV) medication use 20 years after the onset of diabetes (index date): hypertension: 35.2% vs. 11.4%, P < 0.001; hypercholesterolaemia: 66.7% vs. 7.14%, P < 0.001; and CV medication use: 37.0% vs. 3.6%, P < 0.001. The significant differences between prevalence rates in the two cohorts started from 1 year before the index date. Furthermore, 50% of the children in the T1DM cohort with hypertension and 53% with hypercholesterolaemia remained untreated with CV drugs for a period of 2–5 years during the 20‐year follow‐up. Age was the only determinant associated with undertreated hypertension in the T1DM cohort.
Conclusions
Children with T1DM had substantially higher prevalence rates of hypertension and hypercholesterolaemia from 1 year before up to 20 years after the onset of diabetes compared with nondiabetics. There is a substantial undertreatment of CVD risk factors with CV drugs. In children with T1DM, screening for CVD risk factors and adequate treatment are of the utmost importance to prevent CVD later in life.
Keywords: children, hypercholesterolaemia, hypertension, type 1 diabetes, undertreatment
What is Already Known about this Subject
In the paediatric population with type 1 diabetes (T1DM), the prevalence rates of cardiovascular disease (CVD) risk factors are higher than in children without T1DM.
The prevalence rates of cardiovascular (CV) medication use to treat these risk factors in children with T1DM are low.
There are limited follow‐up studies on the incidence and prevalence rates of CVD risk factors and their treatment in this population.
What this Study Adds
In a large population‐based study, with 20 years of follow‐up, the prevalence and incidence rates of hypertension, hypercholesterolaemia and CV medications were significantly higher in children with T1DM compared with a matched diabetes‐free reference cohort.
Higher prevalence rates of CVD risk factors and CV medications started from 1 year before the onset of diabetes and further increased during the follow‐up period.
A substantial number of diabetic children with hypertension (50%) and hypercholesterolaemia (53%) were undertreated for a period of 2–5 years during the 20‐year follow‐up. Age appeared to be associated with undertreated hypertension.
Introduction
Type 1 diabetes mellitus (T1DM) is associated with an increased risk of cardiovascular disease (CVD), which is evident in all age groups, including children 1, 2. Even as early as in childhood, T1DM is associated with vascular smooth muscle dysfunction and increased intima media thickness 3, 4, 5, 6. There is extensive evidence to support the role of atherosclerosis in the development of CVD; this starts in childhood and further increases throughout life 7. Although the pathogenesis of CVD begins in childhood, clinical manifestations of CVD are not common before adulthood 8.
Several studies have shown that children with T1DM are almost twice as likely to have CVD risk factors compared with the general population, although the use of CV medication to treat these risk factors is low 9, 10, 11, 12, 13, 14. Previous studies on CVD risk factors in T1DM have had some important limitations. Firstly, most of these studies used a cross‐sectional study design or had a relatively short follow‐up, without adequately taking into account the dynamics of the occurrence of CVD risk factors during ageing 10, 11, 12, 13, 15. Secondly, in most studies CVD risk factors such as body mass index (BMI), smoking status and family history of CVD were not evaluated.
Therefore, in the present study we aimed to calculate the long‐term trends in the prevalence and incidence rates of CVD risk factors, CVD events and use of CV medication before and after the onset of T1DM in children and adolescents, and to compare these with prevalence and incidence rates in a group of age‐and gender‐matched diabetes‐free children and adolescents. We also evaluated the percentages of untreated children with hypertension and hypercholesterolaemia, as well as determinants associated with undertreatment.
Methods
Data source
Data for the present study were obtained from the Clinical Practice Research Datalink (CPRD). CPRD data are collected from anonymized patient records from participating general practitioners (GPs) in the UK. The size of general practices (providing around 6.9% national coverage) and their geographical distribution in the CPRD database are largely representative of the population of England and Wales, and patients are broadly representative of the UK general population in terms of age, gender and ethnicity 16, 17.
Data available from the CPRD include patient demographic data, patient registration details, practice details, medication records (including medicines prescribed for patients), consultation details, clinical records, laboratory test results and referrals. Medication records in the CPRD are based on prescriptions and do not provide information regarding the filling of prescriptions. Ethics approval was granted by the CPRD Scientific and Ethical Advisory Group, and the protocol (15_133R2) was reviewed and approved by the Independent Scientific Advisory Committee for Medicines and Healthcare Products Regulatory Agency database research. Further details on the CPRD have been published elsewhere 16, 18, 19.
Study design and study population
Using a retrospective cohort study design, we identified all children and adolescents in the CPRD who started insulin therapy before they were 19 years of age (T1DM cohort) between 1 January 1988 and 4 November 2014. Within this period, patients were included in the study if they had a first‐ever diagnosis of diabetes and a first‐ever insulin prescription. Insulin use was defined as at least two prescriptions during the study period, and the date of the first insulin prescription was termed as the index date. Patients were excluded from the T1DM cohort if they had had a glucagon prescription before the index date and if the children had already ever used any type of oral antidiabetic agents. Furthermore, patients with a history of cystic fibrosis were excluded from the study.
Up to five diabetes‐free subjects (without a diagnosis of T1DM or any prescription of insulin before the index date and during the follow‐up), matched on the year of birth (age), gender, general practice and being registered in the CPRD on the index date, were sampled at random as a reference cohort. The index date for the reference cohort was set as the index date for the matched T1DM cohort. Baseline characteristics were recorded at the index date.
To ensure that all subjects were active within their general practice, they were required to have had at least 12 months' valid history in the CPRD before and after the index date. Subjects were followed until the end of the study period (4 November 2015), death or the date of transfer out of the practice – whichever came first.
CVD, risk factors and treatment
The presence of CVD risk factors, including hypertension, hypercholesterolaemia, poor glucose level [measured by glycosylated haemoglobin (HbA1c) level] and family history of CVD before and after the index date, was identified by read codes (information available from the corresponding author on request).
Hypertension was identified by a GP diagnosis (based on read codes) and/or systolic (SBP) and diastolic blood pressure (DBP) values [having an elevated blood pressure (BP) (SBP ≥140 mmHg and DBP ≥90 mmHg) three times during follow‐up] and/or by antihypertensive medication use (based on product codes) 20, 21.
Hypercholesterolaemia was identified based on GP diagnoses and/or lipid values [low‐density lipoprotein cholesterol ≥100 mg dl–1 (2.6 mmol l–1) and/or total cholesterol ≥200 mg dl–1 (5.2 mmol l–1)] or the use of lipid‐lowering medication 21, 22.
When the first‐time hypertension or hypercholesterolaemia occurred between the second year prior to the index date up to 20 years after the index date, we assumed that this risk factor remained present during the rest of the follow‐up.
Poor glycaemic control was defined as HbA1c >7.5% (58 mmol mol–1), and is a determinant for CVD risk factors, including hypertension 21, 23. Any record of HbA1c >7.5% (58 mmol/mol–1) during the years after the index date was defined as poor glycaemic control.
Ever family history of CVD was identified by the GP's recording.
Smoking habits in children during the complete follow‐up were categorized as ever vs. never smokers or unknown.
Obesity was defined as BMI ≥95th percentile and categorized as ever vs. never obese or unknown. We calculated age‐ and gender‐adjusted BMI percentiles (for children aged 2–20 years at the time of BMI measurement) using height and weight measures as defined by the Centers for Disease Control and Prevention standardized gender‐ and age‐specific growth charts (http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm).
Treatment with CV medication was identified using CPRD product codes (information available from the corresponding author on request).
CV medication was defined as an ever‐recorded prescription for CV medication categorized into the following groups: nitrates, antihypertensive medications [diuretics, beta‐blockers, angiotensin‐converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs) and calcium channel blockers], lipid‐lowering medications (statins, nicotinic acid, fibrates and bile acid sequestrants) and antithrombotic agents. Children and adolescents who received at least one prescription for a CV medication, regardless of refills, during the study period were defined as CV medication users; if a patient received CV medications in the first year after the index date but not in the second and the third year, this patient was classified as a CV medication user only during the first year after the index date.
CVD events were identified by CPRD diagnosis. Any record of stable/unstable angina pectoris, myocardial infarction, heart failure, stroke, atrial fibrillation and/or peripheral artery disease during the follow‐up was defined as a CVD event.
Undertreatment of hypertension and hypercholesterolaemia
To evaluate undertreatment of hypertension and hypercholesterolaemia, we used the International Society for Pediatric and Adolescent Diabetes Clinical Practice Consensus Guidelines 2014 Compendium: Microvascular and macrovascular complications in children and adolescents 21. Children in our study were classified as being undertreated if they were identified as hypertensive without at least one antihypertensive agent in their medication regimen during the same year after the index date. For hypercholesterolaemia, we used the equivalent definition. For the evaluation of determinants of undertreatment of hypertension and hypercholesterolaemia, age, gender and family history of CVD were considered. Owing to the high rates of missing values, we did not use the data on BMI and smoking status as possible determinants of medication undertreatment.
Statistical analysis
Descriptive statistics were used to summarize the characteristics of children in both the T1DM and the reference cohorts. The annual prevalence rates for hypertension, hypercholesterolaemia and CV medication use in the period between 2 years before and up to 20 years after the index date were calculated. To compare the rates of CVD risk factors and CV medication use between the two cohorts, chi‐squared tests were used. To compare cumulative incidence rates of events in the two cohorts, Kaplan–Meier survival analyses were used. For this analysis, the date of the first diagnosis of a CVD event or a CVD risk factor, or the date of the first prescription of a CV medication after the index date (t = 0) was counted as the first event. Prevalent cases at the index date (t = 0) were excluded from the denominator. In subgroup analyses, the rates were stratified by age (being split into four bands: 0–4, 5–9, 10–14 and 15–18 years) and gender. In the T1DM cohort, children undertreated for hypertension and hypercholesterolaemia were further stratified by the number of years of being undertreated (being split into four bands: ≤1, 2–5, 6–10 and ≥11 years). Chi‐squared statistics and the Fisher's exact test were used to test determinants. All statistical analyses were carried out using statistical package R version 3.2.3 and SPSS 23.0 (IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp).
Results
A total of 22 241 children met the inclusion criteria. Table 1 shows the demographic features of 3728 children aged younger than 19 years with diagnosed diabetes and at least two insulin prescriptions (T1DM cohort), and 18 513 age‐and gender‐matched diabetes‐free peers (reference cohort). The mean age [standard deviation (SD)] at the index date was almost 11 (4.5) years. Males comprised over half of the population (54.8%) in both cohorts. The median duration of follow‐up [interquartile range (IQR)] was almost 6 (3–10) years in both cohorts.
Table 1.
Baseline characteristics of patients in the type 1 diabetes (T1DM) and reference cohorts
| T1DM cohort (n = 3728) | Reference cohort (n = 18 513) | ||
|---|---|---|---|
| Gender, n (%) | Females | 1685 (45.2) | 8375 (45.2) |
| Males | 2043 (54.8) | 10 138 (54.8) | |
| Age at index date, n (%) | 0–4 years | 494 (13.3) | 2240 (12.1) |
| 5–9 years | 1052 (28.2) | 5212 (28.2) | |
| 10–14 years | 1434 (38.5) | 7331 (39.6) | |
| 15–19 years | 748 (20.0) | 3730 (20.1) | |
| Age at index date (years), mean (SD) | 10.7 (4.5) | 10.9 (4.4) | |
| BMI available, n (%) | 2967 (79.6) | 5276 (28.5) | |
| Unknown BMI, n (%) | 761 (20.4) | 13 237 (71.5) | |
| BMI percentile category (aged 2–20 years at time of measurement) a , n (%) | Overweight (≥85th to 95th percentile) | 488 (13.1) | 984 (5.3) |
| Obese (≥95th percentile) | 328 (8.8) | 1491 (8.1) | |
| BMI category (aged >20 years at time of measurement), n (%) | Overweight (≥25–30 kg m–2) | 11 (0.30) | 131 (0.71) |
| Obese (≥30 kg m–2) | 9 (0.24) | 99 (0.53) | |
| Smoking status during follow‐up, n (%) | Ever‐smoker | 251 (6.7) | 1599 (8.6) |
| Never‐smoker | 2234 (59.9) | 7645 (41.3) | |
| Unknown | 1243 (33.3) | 9269 (50.1) | |
| Family history of CVD | 182 (4.9) | 834 (4.5) | |
| Number of participants in each year of the study period, before the index date | 2 years | 3726 | 18 506 |
| 1 year | 3728 | 18 513 | |
| Number of participants in each year of the study period, after the index date | 1 year | 3728 | 18 513 |
| 2 years | 3727 | 18 506 | |
| 3 years | 3302 | 16 358 | |
| 4 years | 2910 | 14 227 | |
| 5 years | 2535 | 12 324 | |
| 6 years | 2198 | 10 664 | |
| 7 years | 1896 | 9207 | |
| 8 years | 1635 | 7823 | |
| 9 years | 1400 | 6621 | |
| 10 years | 1180 | 5507 | |
| 11 years | 963 | 4470 | |
| 12 years | 732 | 3450 | |
| 13 years | 571 | 2660 | |
| 14 years | 436 | 2064 | |
| 15 years | 326 | 1550 | |
| 16 years | 226 | 1065 | |
| 17 years | 146 | 759 | |
| 18 years | 109 | 570 | |
| 19 years | 82 | 419 | |
| 20 years | 54 | 280 | |
| Median follow‐up, years (IQR) | After the index date | 6.1 (3.3–10.2) | 6.0 (3.2–9.9) |
BMI, body mass index; CVD, cardiovascular disease; IQR, interquartile range; SD, standard deviation
The Centers for Disease Control and Prevention growth‐chart data set has not included children younger than 2 years of age in the BMI percentile calculation; however, none of the children younger than 2 years of age had BMI data available in the present study. Index date is the time of first insulin prescription
In the T1DM cohort, obesity (at any time during follow‐up) was found in 8.8% of children aged 2–20 years (BMI ≥95th percentile) and in 0.24% aged older than 20 years (BMI ≥30 mg kg–2). The prevalence rates of obesity in the reference cohort were 8.1% and 0.53%, respectively. Compared with children in the T1DM cohort, those in the reference cohort were more likely to have ever smoked (8.6% vs. 6.7%; P < 0.001) during the years before and after the index date. Approximately 5% of both study cohorts had a family history of CVD.
Time trends in CVD risk factors
The annual prevalence rates of CVD risk factors in the T1DM cohort compared with the reference cohort are shown in Figure 1A, B. The prevalence rates of hypertension and hypercholesterolaemia were significantly higher in the T1DM cohort compared with the reference cohort after the index date. The prevalence rates of hypertension in the T1DM cohort compared with the reference cohort started from 0.27% vs. 0.18% (P = 0.29) in the second year before the onset of diabetes to 35.2% vs. 11.4% (P < 0.001) in the 20th year after the index date. In the first year before the onset of diabetes, there was already a significantly higher prevalence rate of hypertension in the T1DM cohort (0.64% vs. 0.34%; P = 0.007). The prevalence rate of hypercholesterolaemia was 0.13% in the T1DM cohort vs. 0.03% in the reference cohort (P < 0.001) in the second year before the onset of diabetes, and the difference in this rate between the cohorts increased further during follow‐up: 66.7% vs. 7.14% (P < 0.001) in 20th year after the index date.
Figure 1.
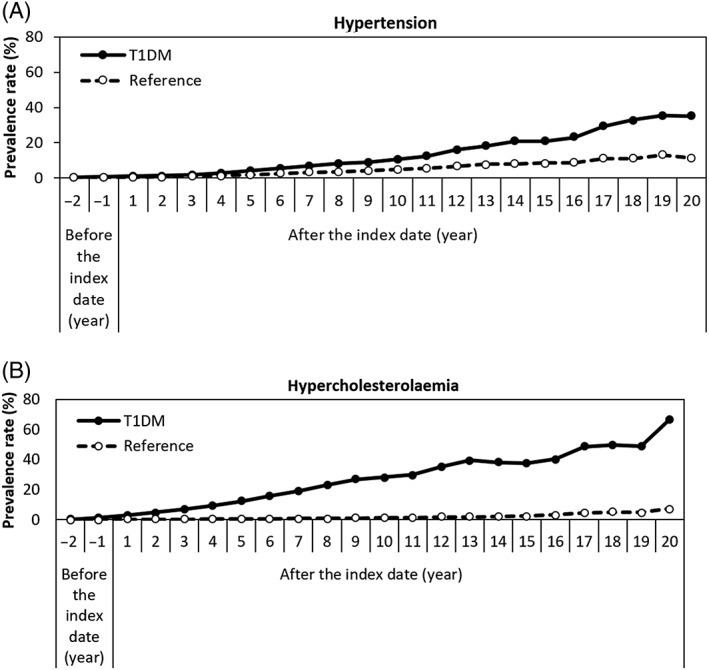
Prevalence rates of (A) hypertension and (B) hypercholesterolaemia, comparing the type 1 diabetes (T1DM) and reference cohorts. Index date is the time of first insulin prescription
The time to hypertension and to hypercholesterolaemia occurrence for the T1DM and reference cohorts is presented in Figure S1A, B. The probability of developing hypertension (log‐rank P < 0.001) or hypercholesterolaemia (log‐rank P < 0.001) was significantly higher in children with T1DM compared with children in the reference cohort. Age‐stratified prevalence rates of hypertension and hypercholesterolaemia in the T1DM cohort and the reference cohort are presented in Figure S2A–D. In both cohorts, children aged 10–14 years and 15–18 years at the index date had a higher risk of both hypertension and hypercholesterolaemia compared with younger children.
The same trends were shown by Kaplan–Meier analysis, in which the probability of hypertension (log‐rank P < 0.001) and hypercholesterolaemia (log‐rank P < 0.001) after the index date was shown to be much higher in children with T1DM aged 15–18 years (at index date) compared with younger children (Figure S3A, B).
In the T1DM cohort, trends in the prevalence rates of hypertension were similar in males and females for most of the time during follow‐up. By contrast, in the reference cohort, females were more likely to have hypertension. Females in the T1DM cohort were significantly more likely to have hypercholesterolaemia, while no significant difference was observed between males and females in the reference cohort (Figure S4A–D).
The prevalence rate for an HbA1c level >7.5% (58 mmol mol–1) started from 58% 1 year after the onset of diabetes, followed by a sharp increase to 80% by the end of the fifth year. After that, it fluctuated slightly, and was almost 80% at the end of the follow‐up (the average rate during the follow‐up period was 77%) (Figure 2).
Figure 2.
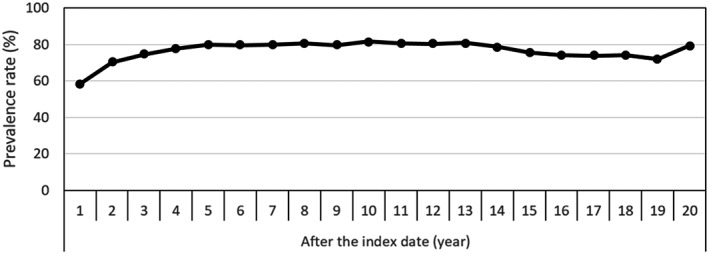
Prevalence rates of poor glycaemic control (glycosylated haemoglobin >7.5% or >58 mmol mol–1 at least once during each follow‐up year) among the type 1 diabetes cohort. Index date is the time of the first insulin prescription
Children aged 10–14 years and 15–18 years at the index date were significantly more likely to have poor glycaemic control during the follow‐up period compared with children in younger age groups. No significant difference was found between males and females regarding glycaemic control during the follow‐up (data not shown).
Time trends in CVD events and medication use
The number of children with CVD events during a maximum follow‐up of 20 years was extremely low in both the T1DM (n = 5) and the reference (n = 9) cohorts. The Kaplan–Meier survival curves for CVD events showed no significant difference between the two cohorts (log‐rank P = 0.07).
The prevalence rates of antihypertensive medications and lipid‐lowering medications are shown in Figure 3A, B. Compared with the reference cohort, children in the T1DM cohort were treated with antihypertensive medication and lipid‐lowering drugs significantly more often over the follow‐up period; the highest rates were found at the end of follow‐up for both antihypertensive medication (25.9% vs. 3.2%; P < 0.001) and for lipid‐lowering drugs (24.1% vs. 0.0%; P < 0.001). ACEIs and statins were the most commonly used CV medications in patients with T1DM. The prevalence rates of ACEIs in the T1DM and the reference cohorts started at the same rate (0.03%) in the second year before the index date and increased to 18.5% vs. 1.1% (P < 0.001) at the end of follow‐up. Statins were also prescribed significantly more often in diabetic children compared with the children in the reference cohort. Prevalence rates started from 0.0% vs. 0.01%, (P = 0.65) at the second year before the index date and increased to 24.1% vs. 0.0% (P < 0.001) at the 20th year after the index date (Figure S5A, B).
Figure 3.
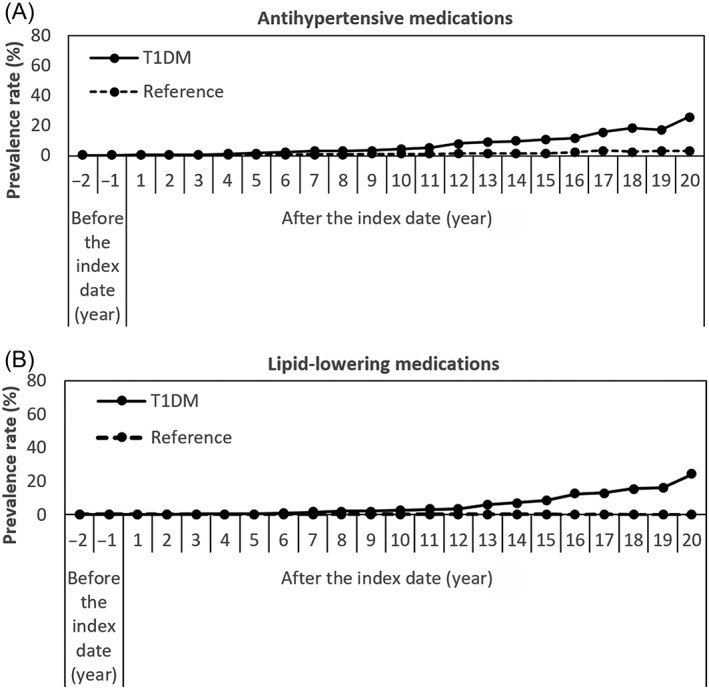
Prevalence rates of (A) antihypertensive and (B) lipid‐lowering medications comparing type 1 diabetes and the reference cohorts. Index date is the time of the first insulin prescription
The annual prevalence rate of CV medication use in the T1DM cohort compared with the reference cohort started from 0.40% vs. 0.18% (P = 0.009) in the second year before the index date and increased to 37.0% vs. 3.6% (P < 0.001) in the 20th year after the index date (Figure S6). The corresponding Kaplan–Meier analysis after the index date showed a significantly higher risk of CV medication use in the T1DM cohort compared with the reference cohort (log‐rank P < 0.001).
The age‐stratified prevalence and cumulative incidence rates of CV medication use showed that increasing age was associated with a higher risk of CV medication use. Children with T1DM in the 15–18‐year age group at the index date were most likely to receive these medications during follow‐up (Figure S7A, B).
Neither the prevalence nor the incidence rates of CV medication use differed between males and females in the T1DM cohort, whereas in the reference cohort, females were significantly more likely than males to use CV medication during the follow‐up (data not shown).
Undertreatment of hypertension or hypercholesterolaemia in children with T1DM
In children with hypertension (based on the physician's diagnosis and/or BP measurement and/or use of antihypertensive medication) (n = 294), 205 (70%) were undertreated for at least 1 year after the index date (Figure S8A). Chi‐squared statistics showed that age was significantly associated with undertreatment in this group (P = 0.04) (Table S1). In the undertreated children (n = 205), stratified analyses by the number of years of being undertreated showed that 16 children (7.8%) were untreated for a period of at least 11 years during the follow‐up. The majority (50%) of undertreated children in this group did not take antihypertensive medication for a period of 2–5 years (Table S2).
In children with hypercholesterolaemia, 721 out of 739 (98%) diabetic children who were diagnosed with hypercholesterolaemia (based on the physician's diagnosis and/or laboratory test results and/or use of lipid‐lowering medications) were not treated pharmacologically for at least 1 year during follow‐up (Figure S8B). Age, gender and family history of CVD were not significantly associated with undertreatment of hypercholesterolaemia (Table S1). In the undertreated group (n = 721), 43 (5.9%) children were shown to be undertreated for hypercholesterolaemia for at least 11 years during the follow‐up. Most children (53%) were undertreated for a period ranging between 2 years and 5 years (Table S2).
The number of patients being treated with medications for hypertension or hypercholesterolaemia were 14/14 and 1/4, respectively, at the end of follow‐up.
Discussion
To the best of our knowledge, the present study provided the first large, population‐based study, with a long follow‐up, to quantify the rates of CVD risk factors, CVD events and CV medication use in children with T1DM. Children with T1DM had significantly higher prevalence rates of hypertension and hypercholesterolaemia, and used more CV medication compared with a matched diabetes‐free reference cohort in the period after the onset of diabetes. However, as expected, the percentage of children diagnosed with CVD events was low (and not different) in both cohorts. The significantly higher prevalence rates of hypertension, hypercholesterolaemia and use of CV medications started as early as 1 year before the onset of diabetes, and further increased during the 20‐year follow‐up. The prevalence rates of hypertension were similar among males and females in both cohorts. Females were more likely to have hypercholesterolaemia in the T1DM cohort and to use CV medication in the reference cohort. Older children (15–18 years at index date) were more likely to have hypertension and hypercholesterolaemia compared with younger peers. Our data indicated that a substantial number of diabetic children with hypertension (50%) and hypercholesterolaemia (53%) were undertreated for a period of 2–5 years during the 20‐year follow‐up. Age was the only determinant that appeared to be associated with undertreated hypertension in the T1DM cohort.
Although the prevalence rates of CVD risk factors prior to the index date were very low in the T1DM cohort, these rates were significantly higher than in the reference cohort. However, it is not yet clear what causes the higher prevalence rates of hypertension and hypercholesterolaemia in the year prior to the onset of diabetes; we speculate that beta‐cell destruction or the underlying factors causing this destruction before the clinical presentation of diabetes could be possible reasons 24. In a previous study using the PHARMO database in the Netherlands, we also showed that the use of CV medications was increased in children and adolescents as early as 1 year prior to the onset of T1DM 25. Further research is warranted to interpret and understand the increased risk of CVD risk factors prior to the clinical onset of T1DM.
In our study, to categorize patients as having hypertension or hypercholesterolaemia, three different recorded conditions/values were required. For instance, hypertension was identified by a GP diagnosis and/or SBP and DBP values [having an elevated BP (SBP ≥140 and DBP ≥90 mmHg) on three occasions during follow‐up] and/or by use of antihypertensive medication. As hypertension and hypercholesterolaemia are chronic conditions, we assumed that they persisted after diagnosis.
The higher prevalence rates of hypertension and hypercholesterolaemia in diabetic children compared with the reference cohort was in line with the results of previous studies 10, 11, 12, 13. Previous studies also showed an increased risk of CVD abnormalities (e.g. carotid intima‐media thickness and increased arterial stiffness) in children with T1DM 3, 4, 5. Elevated HbA1c in diabetic patients plays a role in the pathogenesis of CVD risk factors, particularly hypertension 26, and predicts long‐term CVD outcomes 21, 27, 28. The evaluation of glycaemic control in the T1DM cohort showed that 77% of the children had an HbA1c level above 7.5% (58 mmol mol–1) at least once during follow‐up. Previous studies have also shown that a relatively low percentage of children and adolescents with T1DM attained target HbA1c levels 1, 9. Although this is obviously undesirable, we realize that there are many reasons for a disturbance in glycaemic control, such as infections, diet changes, stress and lifestyle changes 29, 30, 31.
Recently, researchers showed that, compared with males, adolescent females with T1DM had a significantly worse CVD risk profile (higher BMI, HbA1c and cholesterol level) 32. In our study, in the T1DM cohort, there were no clear differences between males and females with respect to hypertension; however, females were significantly more likely to have hypercholesterolaemia during the follow‐up.
Our study showed that only 5% of both T1DM and reference cohorts had a family history of CVD. This low prevalence rate is probably due to the young age of the parents of patients in this population.
The high percentages of children with T1DM undertreated for a period of 2–5 years for hypertension (50%) and hypercholesterolaemia (53%) in our study was in line with previous findings 10, 11, 12, 13, 14, 15. For instance, in paediatric diabetes clinics next to underdiagnosis, undertreatment of hypertension was reported 14. In addition, Zgibor et al. showed that CVD risk factors, particularly hypercholesterolaemia, are not adequately treated in patients with childhood onset of T1DM 15. This is an issue of concern because hypertension and hypercholesterolaemia are the most important modifiable risk factors for CVD events 33, 34. Current guidelines 20, 21, 28, 35 highlight the importance of the screening, diagnosis and treatment of hypertension and hypercholesterolaemia in children with T1DM.
As patients with T1DM are at excessive risk of CVD 8, the high proportion of diabetic children who are not treated for hypertension and hypercholesterolaemia indicates an area for improving care in this population. Implementation of guidelines on the management of CVD risk factors in diabetic patients should therefore be reinforced. A recent meta‐analysis showed the beneficial effect of BP‐lowering treatment on CVD morbidity in adults with diabetes 36, but there have been few studies in children.
We used the CPRD database, which is a population‐based database with high‐quality data collection. Children in the T1DM cohort were all diagnosed with diabetes and treated with insulin, and are probably representative of all children with T1DM in the UK. The estimated number of children and young people under the age of 19 years with diabetes in the UK was 42 000 in 2013–2014, with the vast majority (around 95%) of this population having T1DM 37.
Despite the positive aspects of using the CPRD for the presents study, there were some limitations that should be addressed. An important limitation is the possibility of ascertainment bias, in which CVD risk factors were more often diagnosed and treated in the T1DM cohort owing to increased screening in this population compared with the reference cohort. In our CPRD dataset, the number of children examined at least once for BP or plasma lipid levels during the follow‐up period was 3026/3728 (81%) and 2116/3728 (57%) in the T1DM cohort and 6679/18513 (36%) and 789/18513 (4%) in the reference cohort. Patients in the T1DM cohort had BP or lipid measurements approximately every 7–10 months, while those in the reference cohort underwent these measurements approximately every 10–14 months.
The completeness of follow‐up was another limitation in the present study; most children were lost to follow‐up, with only 1.5% remaining in the study until the end of the follow‐up period. In the CPRD, the loss to follow‐up can be explained by study participants leaving the practice area. This magnitude of loss to follow‐up had two important consequences for our results. Firstly, our effect estimates at the end of follow‐up could not be precise, and, secondly, observing a CVD event in patients at an older age was only possible in a small part of the population. A lack of information on the indications for prescribed medications was another important issue, which might have led to misclassification bias. For instance, in addition to CVD events, beta‐blockers can also be indicated for anxiety before school examinations or driving tests. Moreover, although the relatively high prevalence rate of ACEI use in children with T1DM is in line with available guidelines for diabetes in children and adolescents 20, 21, 28, 35, hypertension is not the only indication for the use of these drugs. A previous study showed that almost 36% of children with T1DM used ACEI or ARB medications to treat microalbuminuria 38. Unfortunately, it was not possible to test the influence of race/ethnicity on prevalence rates of CVD risk factors in our study population; only 27.1% of all patients in the CPRD (1990–2012) have their ethnicity recorded 39. Previous studies have shown that the prevalence rates of CVD risk factors vary according to race/ethnicity 40. Another limitation was the large amount of missing information about smoking, height and weight (and therefore BMI), and the family history of CVD. This limited us in the evaluation of the prevalence rates of these risk factors, and also of determinants of CVD risk factors. Furthermore, it limited us in the classification of hypertension, as hypertension in children is defined as a SBP and/or DBP is ≥95th percentile for the corresponding age, gender and height group. Defining hypertension based on a GP diagnosis (based on read codes), and/or SBP and DBP values [an elevated BP (SBP ≥140 mmHg and DBP ≥90 mmHg) three times during follow‐up] and/or antihypertensive medication use (based on product codes) might lead to an underestimation of hypertension in this population. More importantly, the data for the present study were collected from 1988 to 2014 – a time span during which the guidelines and treatment recommendations on hypertension and hypercholesterolaemia in children and adolescents changed. Earlier guidelines did not recommend treatment with antihypertensive and lipid‐lowering drugs before the age of 18 years, and the recommended duration of lifestyle changes (diet and exercise) before considering the initiation of medication was much longer. Furthermore, hypertension in children is often caused by special conditions (secondary hypertension) which may also have influenced our findings of undertreatment 41. Finally, the lack of information on lifestyle modifications and dietary changes did not allow us to evaluate the first treatment steps for hypertension and hypercholesterolaemia.
In summary, our findings confirmed that children with T1DM are at increased risk for hypertension and hypercholesterolaemia both before and after the onset of diabetes, and that, according to recent guidelines, there is a substantial undertreatment of hypertension and hypercholesterolaemia.
Competing Interests
There are no competing interests to declare. No funding source had any role in the design and conduct of the study; the collection, management, analysis and interpretation of the data; or the preparation, review or approval of the manuscript.
Contributors
F.A. contributed to the study design, analysed the data and wrote the manuscript. P.S., A.B. and A.H.M. contributed to the study design and the discussion, and edited the manuscript.
Supporting information
Figure S1 Time to (A) hypertension and B) hypercholesterolaemia comparing the type 1 diabetes and the reference cohorts. Index date is the time of the first insulin prescription
Figure S2 Index date age‐stratified prevalence rates of (A,B) hypertension and (C,D) hypercholesterolaemia comparing the type 1 diabetes and the reference cohorts. Index date is the time of the first insulin prescription
Figure S3 Time to (A) hypertension and B) hypercholesterolaemia in the type 1 diabetes cohort comparing different age categories. Index date is the time of the first insulin prescription
Figure S4 Gender‐stratified prevalence rates of (A,B) hypertension and (C,D) hypercholesterolaemia comparing the type 1 diabetes and the reference cohorts. Index date is the time of the first insulin prescription
Figure S5 Prevalence rates of (A) angiotensin‐converting enzyme inhibitors and (B) statin use comparing type 1 diabetes and the reference cohorts. Index date is the time of the first insulin prescription
Figure S6 Prevalence rate of cardiovascular medication use comparing the type 1 diabetes and the reference cohorts. Index date is the time of the first insulin prescription
Figure S7 Index date age‐stratified prevalence rates of cardiovascular medication use in (A) the type 1 diabetes cohort and (B) the reference cohort. Index date is the time of the first insulin prescription
Figure S8 Undertreated (A) hypertension and (B) hypercholesterolaemia (undertreatment for at least 1 year) in the type 1 diabetes cohort
Table S1 Determinants of children undertreated (no medication for at least 1 year) for hypertension and hypercholesterolaemia in the type 1 diabetes cohort
Table S2 Stratified analyses in children undertreated for hypertension and hypercholesterolaemia in the type 1 diabetes cohort, by the number of years of undertreatment
Ahmadizar, F. , Souverein, P. , de Boer, A. , and Maitland‐van der Zee, A. H. (2018) Undertreatment of hypertension and hypercholesterolaemia in children and adolescents with type 1 diabetes: long‐term follow‐up on time trends in the occurrence of cardiovascular disease, risk factors and medications use. Br J Clin Pharmacol, 84: 776–785. doi: 10.1111/bcp.13482.
References
- 1. Brunvand L, Fugelseth D, Stensaeth KH, Dahl‐Jorgensen K, Margeirsdottir HD. Early reduced myocardial diastolic function in children and adolescents with type 1 diabetes mellitus a population‐based study. BMC Cardiovasc Disord 2016; 16: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McVeigh GE, Gibson W, Hamilton PK. Cardiovascular risk in the young type 1 diabetes population with a low 10‐year, but high lifetime risk of cardiovascular disease. Diabetes Obes Metab 2013; 15: 198–203. [DOI] [PubMed] [Google Scholar]
- 3. Lamotte C, Iliescu C, Libersa C, Gottrand F. Increased intima‐media thickness of the carotid artery in childhood: a systematic review of observational studies. Eur J Pediatr 2011; 170: 719–729. [DOI] [PubMed] [Google Scholar]
- 4. Heilman K, Zilmer M, Zilmer K, Lintrop M, Kampus P, Kals J, et al Arterial stiffness, carotid artery intima‐media thickness and plasma myeloperoxidase level in children with type 1 diabetes. Diabetes Res Clin Pract 2009; 84: 168–173. [DOI] [PubMed] [Google Scholar]
- 5. Urbina EM, Dabelea D, D'Agostino RB Jr, Shah AS, Dolan LM, Hamman RF, et al Effect of type 1 diabetes on carotid structure and function in adolescents and young adults: the SEARCH CVD study. Diabetes Care 2013; 36: 2597–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van der Heyden JC, Birnie E, Bovenberg SA, Cabezas MC, van der Meulen N, Mul D, et al Do traditional cardiovascular risk factors solely explain intima‐media thickening in youth with type 1 diabetes? J Diabetes Complications 2016; 30: 1137–1143. [DOI] [PubMed] [Google Scholar]
- 7. Hong YM. Atherosclerotic cardiovascular disease beginning in childhood. Korean Circ J 2010; 40: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Ferranti SD, de Boer IH, Fonseca V, Fox CS, Golden SH, Lavie CJ, et al Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care 2014; 37: 2843–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wood JR, Miller KM, Maahs DM, Beck RW, DiMeglio LA, Libman IM, et al T1D exchange clinic network. Most youth with type 1 diabetes in the T1D exchange clinic registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes clinical guidelines. Diabetes Care 2013; 36: 2035–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maahs DM, Wadwa RP, McFann K, Nadeau K, Williams MR, Eckel RH, et al Longitudinal lipid screening and use of lipid‐lowering medications in pediatric type 1 diabetes. J Pediatr 2007; 150: 146–150. [DOI] [PubMed] [Google Scholar]
- 11. Schwab KO, Doerfer J, Hecker W, Grulich‐Henn J, Wiemann D, Kordonouri O, et al Spectrum and prevalence of atherogenic risk factors in 27,358 children, adolescents, and young adults with type 1 diabetes: cross‐sectional data from the German diabetes documentation and quality management system (DPV). Diabetes Care 2006; 29: 218–225. [DOI] [PubMed] [Google Scholar]
- 12. Margeirsdottir HD, Larsen JR, Brunborg C, Overby NC, Dahl‐Jorgensen K, Norwegian Study Group for Childhood Diabetes . High prevalence of cardiovascular risk factors in children and adolescents with type 1 diabetes: a population‐based study. Diabetologia 2008; 51: 554–561. [DOI] [PubMed] [Google Scholar]
- 13. Steigleder‐Schweiger C, Rami‐Merhar B, Waldhor T, Frohlich‐Reiterer E, Schwarz I, Fritsch M, et al Prevalence of cardiovascular risk factors in children and adolescents with type 1 diabetes in Austria. Eur J Pediatr 2012; 171: 1193–1202. [DOI] [PubMed] [Google Scholar]
- 14. Nambam B, DuBose SN, Nathan BM, Beck RW, Maahs DM, Wadwa RP, et al T1D exchange clinic network. Therapeutic inertia: underdiagnosed and undertreated hypertension in children participating in the T1D exchange clinic registry. Pediatr Diabetes 2016; 17: 15–20. [DOI] [PubMed] [Google Scholar]
- 15. Zgibor JC, Wilson RR, Orchard TJ. Has control of hypercholesterolemia and hypertension in type 1 diabetes improved over time? Diabetes Care 2005; 28: 521–526. [DOI] [PubMed] [Google Scholar]
- 16. Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, et al Data resource profile: clinical practice research Datalink (CPRD). Int J Epidemiol 2015; 44: 827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garcia Rodriguez LA, Perez Gutthann S. Use of the UK general practice research database for pharmacoepidemiology. Br J Clin Pharmacol 1998; 45: 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the general practice research database: a systematic review. Br J Clin Pharmacol 2010; 69: 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Williams T, van Staa T, Puri S, Eaton S. Recent advances in the utility and use of the general practice research database as an example of a UK primary care data resource. Ther Adv Drug Saf 2012; 3: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Flynn JT, Daniels SR, Hayman LL, Maahs DM, McCrindle BW, Mitsnefes M, et al Update: ambulatory blood pressure monitoring in children and adolescents: a scientific statement from the American Heart Association. Hypertension 2014; 63: 1116–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Donaghue KC, Wadwa RP, Dimeglio LA, Wong TY, Chiarelli F, Marcovecchio ML, et al ISPAD clinical practice consensus guidelines 2014. Microvascular and macrovascular complications in children and adolescents. Pediatr Diabetes 2014; 15 (Suppl. 20): 257–269. [DOI] [PubMed] [Google Scholar]
- 22. Wilson DP, McNeal C, Blackett P. Pediatric dyslipidemia: recommendations for clinical management. South Med J 2015; 108: 7–14. [DOI] [PubMed] [Google Scholar]
- 23. NICE clinical guidelines – CG15: type 1 diabetes: diagnosis and management of type 1 diabetes in children, young people and adults. Available at http://www.nice.org.uk/CG15 (last accessed 23 March 2016).
- 24. Knip M, Simell O. Environmental triggers of type 1 diabetes. Cold Spring Harb Perspect Med 2012; 2: a007690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ahmadizar F, Fazeli Farsani S, Souverein PC, van der Vorst MM, de Boer A, Maitland‐van der Zee AH. Cardiovascular medication use and cardiovascular disease in children and adolescents with type 1 diabetes: a population‐based cohort study. Pediatr Diabetes 2016; 17: 433–440. [DOI] [PubMed] [Google Scholar]
- 26. Bower JK, Appel LJ, Matsushita K, Young JH, Alonso A, Brancati FL, et al Glycated hemoglobin and risk of hypertension in the atherosclerosis risk in communities study. Diabetes Care 2012; 35: 1031–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353: 2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. American Diabetes Association . Standards of medical care in diabetes‐2016 abridged for primary care providers. Clin Diabetes 2016; 34: 3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scottish Study Group for the Care of the Young Diabetic . Factors influencing glycemic control in young people with type 1 diabetes in Scotland: a population‐based study (DIABAUD2). Diabetes Care 2001; 24: 239–244. [DOI] [PubMed] [Google Scholar]
- 30. Galli‐Tsinopoulou A, Maggana I, Kyrgios I, Mouzaki K, Grammatikopoulou MG, Stylianou C, et al Association between magnesium concentration and HbA1c in children and adolescents with type 1 diabetes mellitus. J Diabetes 2014; 6: 369–377. [DOI] [PubMed] [Google Scholar]
- 31. Van Tilburg MA, McCaskill CC, Lane JD, Edwards CL, Bethel A, Feinglos MN, et al Depressed mood is a factor in glycemic control in type 1 diabetes. Psychosom Med 2001; 63: 551–555. [DOI] [PubMed] [Google Scholar]
- 32. Brown TL, Maahs DM, Bishop FK, Snell‐Bergeon JK, Wadwa RP. Influences of gender on cardiovascular disease risk factors in adolescents with and without type 1 diabetes. Int J Pediatr Endocrinol 2016; 2016: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ibsen H, Olsen MH, Wachtell K, Borch‐Johnsen K, Lindholm LH, Mogensen CE, et al Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: losartan intervention for endpoint reduction in hypertension study. Hypertension 2005; 45: 198–202. [DOI] [PubMed] [Google Scholar]
- 34. Nelson RH. Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care 2013; 40: 195–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. National Institute for Health and Care Excellence (UK), London 2015. [PubMed]
- 36. Brunstrom M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta‐analyses. BMJ 2016; 352: i717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. HQIP & RCPCH: National Pediatric Diabetes Audit 2013/14: report 1: care processes and outcomes. Available at: http://www.rcpch.ac.uk/system/files/protected/page/2014%20NPDA%20Report%201%202014%20FINAL.pdf (last accessed 19 July 2016).
- 38. Daniels M, DuBose SN, Maahs DM, Beck RW, Fox LA, Gubitosi‐Klug R, et al Factors associated with microalbuminuria in 7,549 children and adolescents with type 1 diabetes in the T1D exchange clinic registry. Diabetes Care 2013; 36: 2639–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mathur R, Bhaskaran K, Chaturvedi N, Leon DA, vanStaa T, Grundy E, et al Completeness and usability of ethnicity data in UK‐based primary care and hospital databases. J Public Health 2014; 36: 684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Frierson GM, Howard EN, DeFina LE, Powell‐Wiley TM, Willis BL. Effect of race and socioeconomic status on cardiovascular risk factor burden: the Cooper Center longitudinal study. Ethn Dis 2013; 23: 35–42. [PMC free article] [PubMed] [Google Scholar]
- 41. Anyaegbu EI, Dharnidharka VR. Hypertension in the teenager. Pediatr Clin North Am 2014; 61: 131–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Time to (A) hypertension and B) hypercholesterolaemia comparing the type 1 diabetes and the reference cohorts. Index date is the time of the first insulin prescription
Figure S2 Index date age‐stratified prevalence rates of (A,B) hypertension and (C,D) hypercholesterolaemia comparing the type 1 diabetes and the reference cohorts. Index date is the time of the first insulin prescription
Figure S3 Time to (A) hypertension and B) hypercholesterolaemia in the type 1 diabetes cohort comparing different age categories. Index date is the time of the first insulin prescription
Figure S4 Gender‐stratified prevalence rates of (A,B) hypertension and (C,D) hypercholesterolaemia comparing the type 1 diabetes and the reference cohorts. Index date is the time of the first insulin prescription
Figure S5 Prevalence rates of (A) angiotensin‐converting enzyme inhibitors and (B) statin use comparing type 1 diabetes and the reference cohorts. Index date is the time of the first insulin prescription
Figure S6 Prevalence rate of cardiovascular medication use comparing the type 1 diabetes and the reference cohorts. Index date is the time of the first insulin prescription
Figure S7 Index date age‐stratified prevalence rates of cardiovascular medication use in (A) the type 1 diabetes cohort and (B) the reference cohort. Index date is the time of the first insulin prescription
Figure S8 Undertreated (A) hypertension and (B) hypercholesterolaemia (undertreatment for at least 1 year) in the type 1 diabetes cohort
Table S1 Determinants of children undertreated (no medication for at least 1 year) for hypertension and hypercholesterolaemia in the type 1 diabetes cohort
Table S2 Stratified analyses in children undertreated for hypertension and hypercholesterolaemia in the type 1 diabetes cohort, by the number of years of undertreatment


