Abstract
Currently, nutraceuticals do not have a specific definition distinct from those of other food‐derived categories, such as food supplements, herbal products, pre‐ and probiotics, functional foods, and fortified foods. Many studies have led to an understanding of the potential mechanisms of action of pharmaceutically active components contained in food that may improve health and reduce the risk of pathological conditions while enhancing overall well‐being. Nevertheless, there is a lack of clear information and, often, the claimed health benefits may not be properly substantiated by safety and efficacy information or in vitro and in vivo data, which can induce false expectations and miss the target for a product to be effective, as claimed. An officially shared and accepted definition of nutraceuticals is still missing, as nutraceuticals are mostly referred to as pharma‐foods, a powerful toolbox to be used beyond the diet but before the drugs to prevent and treat pathological conditions, such as in subjects who may not yet be eligible for conventional pharmaceutical therapy. Hence, it is of utmost importance to have a proper and unequivocal definition of nutraceuticals and shared regulations. It also seems wise to assess the safety, mechanism of action and efficacy of nutraceuticals with clinical data. A growing demand exists for nutraceuticals, which seem to reside in the grey area between pharmaceuticals and food. Nonetheless, given specific legislation from different countries, nutraceuticals are experiencing challenges with safety and health claim substantiation.
Keywords: claims, health, labels, nutraceuticals, regulation, regulatory
Introduction
There is a growing overlap between conventional food (including beverages) and food supplements, including energy bars and teas or liquids. This overlap becomes even wider when we consider functional foods and nutraceuticals. What could be considered a functional food under a given set of circumstances may be named a dietary supplement, medical food, food for special dietary use or nutraceutical under different circumstances, depending on its ingredients (active components) and the claims reported on its label 1.
While the definition of a food supplement is quite clear and understandable (see Table 1), the definition of a nutraceutical is still in the grey area between food, food supplements and pharmaceuticals. The term nutraceutical, a syncretic neologism of the words nutrient and pharmaceutical, was originally coined by Stephen DeFelice, who defined nutraceuticals as “food or part of a food that provides medical or health benefits, including the prevention and/or treatment of a disease” 2. This concept has been proposed as a modern approach to food science, and the area of possible use has been defined as beyond the diet, but before the drugs 3, 4. Notwithstanding this concept, the definition of nutraceuticals and a legitimate assessment of their potential in medicine are still contradictory and far from being shared and accepted worldwide 5.
Table 1.
Some definitions for food‐derived products
| Food supplement (United States Government Office, 1994) | A product (other than tobacco) in the form of a capsule, powder, softgel or gelcap intended to supplement the diet to enhance health that bears or contains one or more of the following dietary ingredients: a vitamin, mineral, amino acid, or other botanical or dietary substance. | United States Food and Drug Administration (FDA). Dietary Supplement Health and Education Act (DSHEA). U.S. Department of Health and Human Services. 1994. United States. Public Law 103–417, available at FDA Website: http://www.fda.gov. [42] |
| Food supplement (EU, 2002) | Food product whose purpose is to supplement the normal diet and which consists of a concentrated source of nutrients or other substances with nutritional effects or physiological, single or in combination, marketed in dosed formulations, such as capsules, tablets, tablets or pills, designed to be taken in small individual quantities measured. | EU Directive 2002/46/EC [17] |
| Phytochemical (Bloch and Thomson, 1995) | Substances found in edible fruit and vegetables that can be ingested daily (in quantities of grams) by humans and that exhibit a potential to favourably modulate human metabolism to prevent cancer and other diseases (isoflavones, resveratrol, garlic allyl sulphides, tomato lycopene, onion quercetin etc.). | Bloch A, Thomson CA. Position of The American Dietetic Association (phytochemicals and functional foods). J Am Diet Assoc 1995; 95: 493–496. [74] |
| Nutraceuticals (De Felice, 1995) | Food or part of food that provides medical or health benefits, including the prevention and/or treatment of a disease. | DeFelice SL. The nutraceutical revolution: its impact on food industry R&D. Trends Food Sci Technol 1995; 6: 59–61. [2] |
| Nutraceuticals (Zeisel, 1999; DSHEA, 1994) | A diet supplement that delivers a concentrated form of a biologically active component of food in a nonfood matrix to enhance health. | Zeisel SH. Regulation of “Nutraceuticals”. Science. 1999: 285; 1853–5. Food and Drug Administration, FDA, Dietary Supplement Health and Education Act of 1994 (DSHEA), United States. [75] |
| Nutraceuticals (Brower, 1998) | Any substance that is a food or a part of a food and is able to induce medical and health benefits, including the prevention and treatment of disease. | Brower V. Nat Biotechnol 1998; 16: 728. [76] |
| Nutraceuticals (Merriam Webster Dictionary, 2015) | A foodstuff (as a fortified food or dietary supplement) that provides health benefits in addition to its basic nutritional. | Merriam‐Webster Online Dictionary. 2015. Merriam‐Webster Inc., P.O. Box 281, Springfield, MA 01102, United States. [77] |
| Nutraceuticals (ENA, 2016) | Nutritional products that provide health and medical benefits, including the prevention and treatment of disease. | European Nutraceutical Association (ENA). 2016. Science behind Nutraceuticals. In E. N. Association (Ed.), (Vol. 2016). 594 Basel, Switzerland. [78] |
| Functional food (Zeisel, 1999) | Nutrient consumed as part of a normal diet but delivering one or more active ingredients (that have physiological effects and may enhance health) within the food matrix. | Zeisel SH. Regulation of “Nutraceuticals”. Science 1999: 285; 1853–5. Food and Drug Administration, FDA, Dietary Supplement Health and Education Act of 1994 (DSHEA), United States. [79] |
| Functional food (Diplock et al., 1999) | Product which is shown in a satisfactory manner that, in addition to adequate nutritional effects, induces beneficial effects on one or more target functions of the organism, significantly improving the health status and welfare or reducing the risk of disease. | Diplock A, Aggett P, Ashwell M, Bornet F, Fern E, Roberfroid M. The European Commission concerted action on functional foods science in Europe (FUFOSE). Scientific concepts of functional foods in Europe. Consensus document. Br J Nutr 1999; 81: S1–S27. [80] |
| Functional food (Hardy, 2000) | Any food or ingredient that has a positive impact on an individual's health, physical performance, or state of mind, in addition to its nutritive value. | Hardy G. Nutraceuticals and functional foods: introduction and meaning. Nutrition 2000; 16: 688–689. [81] |
| Food for medical use (EU, 1999) | Complete nutritional food with a formulation of nutrients standards, which may constitute the sole source nutrition for the person to whom it is addressed. Or alternatively: complete nutritional food with a formulation of nutrient adapted to a specific disease, disorder or medical condition, which may constitute the only source of nutrition for the person to whom it is addressed. Or alternatively: nutritionally incomplete food with a formulation standard nutrients or adapted for a specific disease, disorder or medical condition, which it is not suitable to be used as the only source of nutrition. | EU Directive 1999/21/ EC. [82] |
The nutraceutical definition often overlaps with the accepted definition of food supplements 6, 7, and the rationale behind the use of these products is becoming more and more important in their distinction.
Nutraceuticals are continuously developed and have quickly spread worldwide 8. Existing contradictory information in the field is generating confusion about the possible effective use of these products, which are available on the market and in pharmacies. Food supplements, as per their micronutrient content, can be used to improve the health of individuals in need. In general, many of the health claims that are currently associated with food supplements, pro‐ and prebiotics, herbal products, and functional foods may not properly be substantiated by scientific data on their safety, efficacy and effect on health and/or pathological conditions.
The claims are mainly unsubstantiated due to a lack of studies on possible mechanisms of action and a lack of in vivo research confirming the claimed beneficial health effects on specific pathological conditions. Another key aspect is related to the data reported in the literature, which mainly comes from in vitro studies focused on single food constituents (micronutrients); these studies are based on the assumption that micronutrients can be considered safe (or generally recognized as so) because they are derived from commonly used food or food components 8, 9. Safety is of utmost importance, as there is also the possibility of contaminants of inorganic and organic origins in these products 10, 11. Moreover, the ingredients themselves may cause health problems, and proper information on possible unwanted side effects should be provided on the label. From our point of view, it is imperative to have more information on safety and efficacy based on clinical studies rather than only in vitro studies, and it is also important to reach a better understanding of the mechanism of action and bioavailability of these products. The first step in assessing the action of nutraceuticals is to distinguish them from the other food‐derived products, particularly food supplements, which are not always included in daily dietary habits in the presence of a specific need 12.
This exercise may require: (i) appropriate target identification; (ii) safety assessment; (iii) a clear understanding of the mechanism of action; (iv) efficacy assessment substantiated by clinical studies; (v) an evaluation of possible unwanted side effects; and (vi) an evaluation of possible interactions with other products (e.g. food, food supplements and drugs). We think that a different approach for nutraceutical concept assessment, use and definition is needed, and the existing assessment methodology for pharmaceutical products may be considered as a starting point.
Resolving the discrepancies between the different accepted definitions of nutraceuticals and their regulatory aspects will be a significant challenge. An outline of the lack of shared regulations regarding nutraceuticals as pharma‐foods is needed 13; nutraceuticals are considered to be in the grey area after diet, but before drugs. Therefore, it is important to clearly identify their specificity in view of their possible use and utility in the pharmaceutical arena.
State of the art and future trends
The first step in food regulation in Europe was established in 1997 with the Green Paper on Food Law, and it was followed by the White Paper on Food Safety in 2000, which outlined the need for new and improved legislation in the field 14. These papers led to the General Food Law Regulation [Regulation (EC) No. 178/2002 of the European Parliament and of the Council 2002], which established the principle and foresaw the creation of an independent organization called the European Food Safety Authority (EFSA) with the specific task of giving scientific advice based upon scientific assessment of the beneficial health effects and associated risks related to food intake 15.
The EFSA mission also includes food supplements, whereas the responsibility for risk management and communication was taken on by the European Commission and Parliament. The risk assessment and risk evaluation can also be included as a part of the EFSA mandate if it follows a specific request made by the European Commission and Parliament from a European Member State or stakeholder. This step should then be followed by specific action from local national authorities, who take the suggestions on board and propose appropriate legislation and regulation in the field. The mission of the EFSA is to “provide the basis for the assurance of a high level of protection of human health and consumer interest in relation to food, considering the diversity in the supply of food, including traditional products, while ensuring the effective functioning of the internal market. It establishes common principles and responsibilities, the means to provide a strong science base, efficient organizational arrangements and procedures to underpin decision‐making in matters of food and feed safety.” This statement applies to all foodstuffs, and these general principles also cover foods with added functional properties (e.g. functional foods, food supplements, herbal products, pre‐ and probiotics, and dietetic foods); hence, they could also include nutraceuticals. The European Council Regulation (EC) No. 178/2002 defines food or foodstuff as “any substance or product, whether processed, partially processed or unprocessed, intended to be, or reasonably expected to be ingested by humans” 15. According to the abovementioned definition, medicinal products cannot be considered food, and this seems to suggest that one possible tool to decide whether medicinal or food regulations apply to a specific product may be identified in the medicinal regulation area. Nonetheless, food derivatives with specific beneficial effects on health, such as functional foods and nutraceuticals, can be considered similar to medicinal products if their micronutrient content is considered. Medicinal products are defined by Directive 2001/83/EC of the European Parliament, which was amended by Directive 2004/27/EC of the Council 16. This Directive, among others, addressed the requirements and procedures for marketing authorization of medicinal products for human use. Medicinal products are defined as “any substance or combination of substances presented as having properties for treating or preventing disease in human beings, or any substance or combination of substances that may be used in or administered to human beings either with a view to restoring, correcting or modifying physiological functions by exerting a pharmacological, immunological or metabolic action, or to making a medical diagnosis”.
Section 2.2 of Directive 2004/27/EC clarifies that “In cases of doubt, where, taking into account all its characteristics, a product may fall within the definition of a ‘medicinal product’ and within the definition of a product covered by other Community legislation, the provisions of this Directive shall apply” 16.
In our opinion, nutraceuticals require an appropriate description and specific classification. Nevertheless, while nutraceuticals should always have specific added health value for the prevention or treatment of pathological conditions, food supplements may not be required to have this characteristic. Notwithstanding the differences existing between them that were outlined previously, Article 2 of Directive 2002/46/EC (Directive 2002/46/EC of the European Parliament and of the Council 2002) defines only food supplements as “foodstuffs, the purpose of which is to supplement the normal diet and which are concentrated sources of nutrients or other substances with a nutritional or physiological effect, alone or in combination, marketed in dose form, namely, forms such as capsules, pastilles, tablets, pills and other similar forms, sachets of powder, ampoules of liquids, drop dispensing bottles, and other similar forms of liquids and powders designed to be taken in measured small unit quantities”, without mentioning nutraceuticals 17.
Food supplements can contain substances with a physiological effect other than nutritional value and so can nutraceuticals, according to our view. A product must be available in a concentrated form and should be taken in the proper pharmaceutical form to fall under Directive 2002/46/EC.
Nutraceuticals can be marketed with same pharmaceutical form of food supplements. We instead propose a different classification approach for these products to differentiate them from food supplements 17. The current European Directive 2002/46/EC on food supplements mainly focuses inter alia on vitamins and minerals as supplements and foresees that maximum amounts of vitamins and minerals must consider the upper safe levels established by scientific risk assessment considering different consumer groups and the intake of vitamins and minerals derived from other dietary sources. For nutraceuticals, a different approach based on beneficial health effects may be more appropriate considering the pharmacological effect of these food derivatives. Prior to 2002, food supplements were subjected to national regulations, which set the legislation framework and risk assessment management. Regarding food supplements, the European Responsible Nutrition Alliance (ERNA) suggested a risk management model for determining maximum risk levels for different nutrient intakes based on the population safety index in 2004 18. In 2006, ERNA contributed to the establishment of guidelines for nutrition and health claim regulations, suggesting these guidelines as a tool for companies to provide correct communication about the many health benefits of vitamins, minerals, omega 3 fatty‐acids etc. 19.
The key aspect here is that food supplements and nutraceuticals are both considered to be derived foodstuffs, which means that, in many cases, the precautionary principle valid for food is applied to food supplements, and the term nutraceutical is used for products available on the market without proper assessment of their beneficial health effects, except in cases specifically provided in the food supplement legislation itself. This arrangement does not completely fix the issue of borderline definitions and classifications between medicinal products, food supplements and nutraceuticals 15.
It would hence be advisable that the European Commission or authorities in charge explore the possibility of including nutraceuticals in their Directives or Regulations by defining a new category that differentiates them from food supplements and pharmaceuticals. This area is hence not completely regulated in Europe nor in the USA. In particular, no effective, shared regulatory system exists in the USA regarding medical or health claims for nutraceuticals, even though these products have entered mainstream science and medicine and the consumer marketplace. This lack of rules negatively influences the market for these products, can generate confusion among consumers, and eventually, can lead to possible misuse.
Nutraceuticals that demonstrate specific pharmacological activities can claim beneficial health effects for pathological conditions, and if they are considered vegetal supplements (in cases of vegetal origin), they can be covered by legislation for food supplements. This stipulation could, however, imply the need to obtain authorization for a health claim from EFSA in Europe, as a cause–effect relationship must be substantiated by clinical data. An EFSA positive opinion would be necessary to assess and recognize their potential use and hence market the nutraceutical as a pharma‐food, demonstrating the large difference that exists between nutraceuticals and food supplements. Regulation EC 1924/2006 on nutrition and health claims made on food was issued to harmonize the laws and different legislations among Member States to guarantee safety and efficacy and to simultaneously protect the internal market by providing proper, understandable information to consumers 20. In accordance with this Regulation, any health or nutritional claims on food must be approved from EFSA before marketing. Health claims are generally related to nutritional profiles, the overall nutritional composition of a foodstuff and the presence of nutrients that have been scientifically recognized as having a beneficial effect on health. National regulations often apply in the absence of specific shared international rules concerning recommendations or any endorsements by national medical associations. This scheme outlines the need for a supra national legal framework for claims and to provide correct information to consumers. Nutraceuticals could be effectively used to prevent and cure some illnesses, and consequently their correct use and proper information on claims substantiated by clinical data are of utmost importance. Nutraceuticals are generally reported to have good safety profile with few unwanted side effects and high bioavailability 21. Their application area ranges from metabolic syndrome and inflammation control to Alzheimer's disease 4, 22, 23, 24, 25, 26, 27.
It must, however, be noted again that current European legislation does not mention the term nutraceutical. Nutraceuticals could be considered within the Foods for Particular Nutritional Uses (PARNUTS) regulatory framework (Directive 89/398/EEC 1989), which includes foods for special medical purposes and food intended for particular nutritional needs, once their safety and efficacy have been properly assessed by in vitro and in vivo studies 28.
This aspect was first outlined in 2002 when the Foundation for Innovation in Medicine guided by Stephen DeFelice launched the idea to establish a shared regulatory framework for nutraceuticals: the Nutraceutical Research and Education Act, which envisioned the creation of a worldwide Nutraceutical Commission specifically dedicated to the review and approval of nutraceuticals and the development of programmes stimulating clinical research in this field with the aim of substantiating all health claims with clinical data 29. The realization of these actions and proper worldwide availability of information could: (i) be beneficial for individuals; (ii) be beneficial for research in this area; (iii) help lower the cost of pharmacological treatment for the National Health Systems by the adoption of prevention mechanisms, including nutraceuticals in the daily diet regimen; and (iv) realize a proactive approach towards preventing, rather than curing, pathological conditions, many of which may arise from improper diet and lifestyle (e.g. metabolic syndrome). The simplest proactive preventive approach could be recognized by changing lifestyle and dietary habits and increasing physical activity, which have been suggested by all national health disease prevention plans. This outcome is, however, not always easy to achieve in populations and remains to be completed.
Efficacy, together with safety, is to be considered of utmost importance for nutraceuticals. European Council Regulation (EC) No. 178/2002 on food introduced a precautionary principle in the risk management process in 2002. Risk analysis in authorization procedures and claims approval may also be relevant for nutraceuticals, food supplements and functional foods 15.
When possible harmful health effects are identified, but not fully scientifically substantiated, the precautionary principle protects the rights of individuals, industry and organizations with the necessity of reducing the risk of affecting the health of humans, the environment, plants and animals.
The first step of safety assessment was established in 1997 with Regulation EC No. 258/97 of the European Parliament and of the Council on novel foods and novel food ingredients and was followed by European Council and Commission Regulation EC No. 1852/2001 30, 31.
Both recognized the need to have a safety assessment through a shared procedure before placing food supplement on the market. This procedure was demanded by the Member States and is still far from being completed. In November 2015, Regulation EU 2015/2283 of the European Parliament and of the Council on novel foods revised the rules to define and place novel foods on the market within the European Union, amending Regulation EU No. 1169/2011 of the European Parliament and of the Council 32.
Novel foods appear, as per their definition, in Article 3, and are described as “food consisting of, isolated from or produced from plants or their parts, except when the food has a history of safe food use within the Union and is consisting of, isolated from or produced from a plant or a variety of the same species obtained by traditional propagating practices which have been used for food production within the Union before 15 May 1997, or nontraditional propagating practices which have not been used for food production within the Union before 15 May 1997, where those practices do not give rise to significant changes in the composition or structure of the food affecting its nutritional value, metabolism or level of undesirable substances”.
They are further defined as “food consisting of, isolated from or produced from animals or their parts, except for animals obtained by traditional breeding practices which have been used for food production within the Union before 15 May 1997 and the food from those animals has a history of safe food use within the Union”.
The same Regulation also includes “food used exclusively in food supplements within the Union before 15 May 1997, where it is intended to be used in foods other than food supplements as defined in point (a) of Article 2 of Directive 2002/46/EC”. The same consideration might be considered valid for foods that have a “history of safe food use in a third country”, suggesting that the safety of the food in question has been confirmed from continued use for at least 25 years in the diet of a significant number of people in at least one‐third of the country. Nutraceuticals are still not mentioned in the 2015 Regulation, notwithstanding their potential medicinal use to prevent/cure some pathological conditions.
It may be feasible to apply current legislation for pharmaceuticals regarding efficacy, safety and health effect assessment to nutraceuticals with the same restrictions. Another possible approach could be to start by reconsidering Directive 1999/21/EC, which defines processed or formulated products that are intended for the dietary management of individuals with disrupted metabolism, or in special physiological conditions, and must be prescribed by a medical doctor and used under their supervision as PARNUTS 33. PARNUTS legislation could be adopted to draw a possible scheme for nutraceutical validation with the aim of clearly distinguishing them from food supplements and/or from functional foods intended for particular nutritional or clinical uses.
According to Directive 89/398/EEC, PARNUTS should have a special composition for their claimed nutritional/medical purposes and should be clearly different from foodstuffs for dietary use 28. These products should be addressed to individuals who do not qualify for pharmacological treatment and who could benefit from alternative treatment given their proven efficacy. In this case, the possible target population could be quite wide considering the growing impact on health due to improper lifestyles, particularly metabolic syndrome, which includes different pathological health conditions, such as hypertriglyceridaemia, hypertension, hypercholesterolaemia, type II diabetes and obesity 34, 35.
The need for a scientific rationale
The proper starting point for nutraceutical concept assessment should begin with a sharp distinction between nutraceuticals and food supplements and with the identification of an appropriate epidemiological target. Food supplements have been already defined as previously reported.
Regarding nutraceuticals, we propose the following definition: (i) for food of vegetal origin, a nutraceutical is the phytocomplex; and (ii) for food of animal origin, a nutraceutical is the pool of secondary metabolites. Both are concentrated and administered in the proper pharmaceutical form. They are capable of providing beneficial health effects, including the prevention and/or the treatment of a disease.
Table 1 collects some of the most common definitions of these products together with other food‐derived products. Many of them belong to the food supplement category, which also includes herbal products; these food supplements are often miscategorized with nutraceuticals. All of these productions, including pre‐ and probiotics, functional foods, and herbal supplements, claim beneficial effects on human health.
Food supplements are often confused with functional food, which, as per their definition, are food‐derived products (or food with the addition of one or more active compounds) that are included as part of a normal diet with the aim of obtaining beneficial health effects. These supplements and functional foods can compensate and/or can have a beneficial effect due to the addition of specific components if there is a lack of one micro‐ or macronutrient in the body. They may not have any proven pharmacological effect 36.
Probiotics and prebiotics are live bacteria generally used to help gastrointestinal conditions and specialized plant fibres that nourish the good bacteria already present in the large bowel or colon (gut microbiota), respectively. Neither has a specific effect on pathological health conditions, but they can both be effective in helping good bacteria grow 3. It can be observed that this definition partially overlaps with the food supplement definition, creating confusing information.
Medicinal products are well‐defined for specific indications. They must follow specific legislation on efficacy, safety, production and use in therapy to be authorized and marketed. The dose and mechanism of action must be identified in detail, as must possible undesired side effects and pharmacokinetics depending on the dose and on the method of administration. Each step is clearly designed and addressed before a new drug can be considered for the market. Once a medicinal product is placed on the market, its benefit/risk ratio continues to be assessed throughout its entire lifespan.
The question is, why do nutraceuticals not follow a similar procedure for assessing their safety, mechanism of action and efficacy before and after they are marketed? Nutraceuticals contain many active substances extracted from a vegetal matrix (as a phytocomplex) or from animal origin that are concentrated and administered in suitable pharmaceutical form, and as per their definition, they must have a proven pharmacological effect and rationale to be used in a pathological condition (in addition to their nutritional value), unlike food supplements and other food‐derived substances, including herbal derivatives.
The adoption of nutraceuticals in daily diets may help prevent the onset of pathological conditions by possibly delaying or avoiding the need to use pharmaceuticals in subjects who qualify for an alternative, nonpharmacological approach to a health condition.
The starting point for identifying and testing a nutraceutical should be making a proper therapeutic hypothesis, such as hypothesis that is coherent and supportable in the modulation of a target capable of producing a beneficial health therapeutic effect. The specific target must then be defined with all the available scientific data.
Each step must be identified and properly considered, and know‐how, experience and expertise are considered essential to reach the target.
The first step to assess therapeutic efficacy should be based on positive evidence from clinical data. This step should include the contribution of different professionals and different expertise, ranging from food chemistry and food safety to structure–activity studies, with the aim of assessing the mechanism of action, nutritional aspects, pharmacology, pharmacokinetics and pharmacodynamics of each nutraceutical.
The utmost importance of the clinical aspects of any study concerning nutraceuticals as pharma‐foods (e.g. in vivo clinical trials) or any possible interactions between food and/or drugs assumed together with nutraceuticals is not to be underestimated 9, 37. Medical doctor involvement in these studies is a crucial step for proper evaluation of both safety and efficacy assessment.
Figure 1 outlines the differences between food supplements and nutraceuticals, stressing the efficacy/safety requirements for these products. Figure 2 outlines a proposed scheme that describes the necessary steps to consider when identifying and developing a new nutraceutical.
Figure 1.
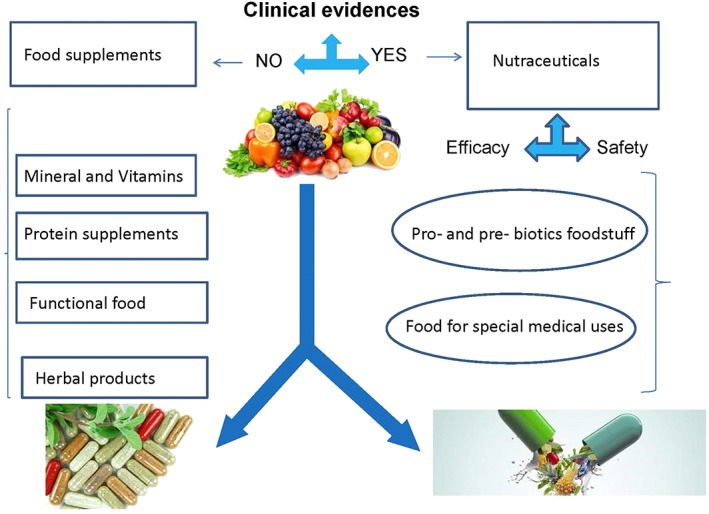
Differences between food supplements and nutraceuticals
Figure 2.
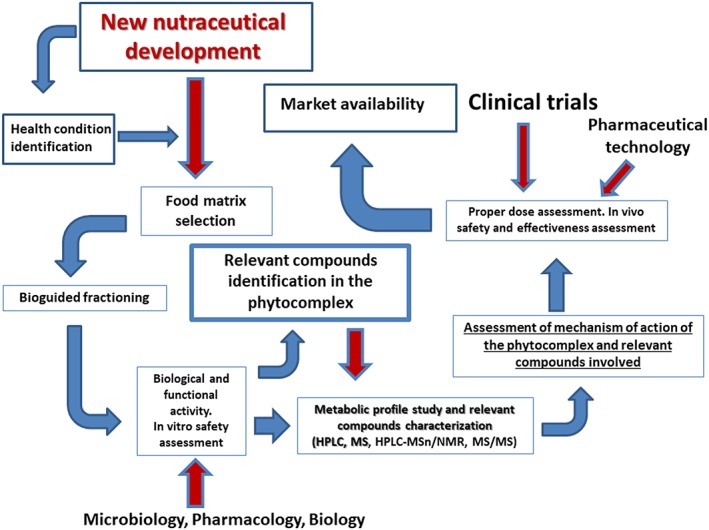
The necessary steps to consider when developing a new nutraceutical
It should be noted that the necessary time to develop a new nutraceutical is reduced compared to time required to develop a new drug, considering the natural origin of the constituents of a nutraceutical and that a nutraceutical is formed by many natural substances (not by a single substance, as in a drug).
Priority steps are indicated. The first step of utmost importance is the identification of the heath condition to address, followed by an assessment of safety and efficacy with clinical trials and a complete and accurate study/assessment of the mechanism of action. The absence of unwanted side effects and efficacy are crucial steps prior to submitting an application to EFSA in Europe (or to the equivalent authority in other countries) for a health claim to be authorized and advertised on the product label before advertising the product or putting it on the market.
The nutritional claims permitted according to the European legislation for food are defined and listed in the Annex of Regulation (EC) No. 1924/2006, which was ultimately amended by European Regulation (EU) No. 1047/2012. The health claims for food supplements refer to the nutrient, food or food category or substance, address the conditions of use and restrictions, and describe the health relationship (EU Register of health claims). The admitted claims are generally indicated as helps to or develop or stimulate a beneficial effect, and they often refer to substances or pre‐ or probiotics, not to a phytocomplex itself, as promoting a beneficial health effect 20, 38.
In contrast, a completely different scenario exists for food supplements (concentrated sources of nutrients or other substances) added to a normal diet that can have a nutritional or physiological effect. Directive 2002/46/EC proposed harmonized rules on these products with the aim of protecting consumers against potential health risks and avoiding misleading information 17. With respect to the safety of food supplements, the Directive lays down a harmonized list of vitamins and minerals that may be added for nutritional purposes in food supplements (in Annex I of the Directive), whereas Annex II of the same Directive contains a list of permitted sources from which the vitamins and minerals can be manufactured.
A different approach is needed to harmonize health claims about nutraceuticals, which should be considered a new category to recognize their potential as preventive agents in some health conditions and to differentiate them from food supplements. This approach, which is based on scientific evidence substantiated by safety and clinical efficacy tests, follows a step‐by‐step pathway as outlined in Figure 2.
A proposal for a regulatory framework for nutraceuticals
All the above‐mentioned food‐derived products are considered generally safe; however, in Japan, functional foods are defined according to their use of natural ingredients, whereas in the United States, they can also contain ingredients produced with biotechnology 39. One possible source of confusing information could be administration of the pharmaceutical form – pills, tablets and capsules – which can be the same for food supplements and nutraceuticals.
However, while nutraceuticals, in our opinion, should have proven clinical efficacy, beneficial health effects, greater or improved bioavailability and safety beyond their nutritional value, all the other abovementioned food‐derived products may not need to have a specific effect on any health condition substantiated by clinical trials.
The lack of a shared legislation is big challenge for nutraceutical globalization because the existence of different regulations can generate confusion and also give a somewhat dissimilar definition of products that are present in different countries.
Active substances, which can either be extracted from plants as phytocomplexes or can be of animal origin, can create a very promising nutraceutical toolbox that is useful for promoting health, preventing disease, or offering general medicinal properties, given their proven clinical efficacy when they are concentrated and administered in a suitable pharmaceutical form 4. This category encompasses food supplements, vitamin‐ and/or mineral‐based formulations, herbal supplements, and animal origin products.
The main focus on food supplement legislation has thus far addressed their safety and labelling, whereas less emphasis has been given to product claims and intended use of these supplements than for pharmaceuticals. This last aspect is accomplished through good manufacturing practice regulations, which should also be enforced. The terms nutraceuticals and food supplements are often used without noting the difference between them 40, 41.
A clear and shared regulation system allowing the identification and classification of these products at an international level that clearly indicates requirements for quality, efficacy, mechanism of action and safety could benefit potential consumers as well as the industry. Obtaining health claims approval could also represent a growing challenge for stakeholders because nutraceuticals are currently in a grey area between pharmaceuticals and medicinal food.
Nonetheless, while the current European regulations (see EC Regulation No. 1924/2006 of the European Parliament and of the Council, recently updated by EU Regulation 2015/2283) define food categories and include a definition of food supplements, they do not officially mention or recognize the term nutraceutical 20, 32.
According to this vision, the EFSA does not make any distinction between food supplements and nutraceuticals for beneficial health claim applications for new products. Any claim authorization is strongly conditioned by the availability of clinical data in order substantiate its efficacy. In a similar way, the Dietary Supplement Health and Education Act (DSHEA, 1994) defined dietary supplements as a category of food, as did the US Food and Drug Administration (FDA), which regulates dietary supplements with updates according to the Food, Drug and Cosmetic Act (FD&C Act, 2014) per Section 413 (d) of the FD&C Act, 21 U.S.C. 350b (d) 42, 43.
Regulatory rules in different countries
The milestone for assessing the safety and use of food was set by the United Nations Food and Agricultural Organization (FAO) and the World Health Organization (WHO) in the Codex Alimentarius (FAO/WHO 1992). This collection of documents contains general internationally recognized guidelines that establish rules for producing and marketing foodstuffs and their derivatives. Many countries use these guidelines, which define health claims in terms of: (i) nutrient function; (ii) enhanced function; and (iii) reduction of risk 44, 45.
Specifically, the nutrient function claim, which is defined as “the claim that describes the physiological role of the nutrient in growth, development, and the normal function of the body”, does not explicitly refer to beneficial health effects of a nutrient or of a combination of micronutrients 44. It can be observed that nutraceutical (e.g. phytocomplexes) and food supplement (e.g. combinations of micronutrients) regulatory systems seem to evolve very slowly compared to the growing number of products that are available on the market worldwide.
In the USA, the FDA, which focuses on safety aspects and food supplements, acknowledges the term nutraceutical and applies a different set of regulations to them than those of conventional foods and drugs. As per the Dietary Supplement Health and Education Act established in 1994 (DSHEA), it is the manufacturer's responsibility to ensure that a nutraceutical is safe before it is marketed. The Food and Drug Administration Modernization Act of 1997 contains sections that enable health claims and nutrient content claims on food labelling to be authorized based on an authoritative statement from the Academy of Sciences or other federal authorities after notifying the FDA at least four months before the introduction of the supplement on the market 42.
While the FDA is authorized to act against any unsafe product on the market, the EFSA does not have the same mandate. EFSA must authorize/approve in detail any health claim before it is considered at a national or European level prior to being put on the market following a specific request from Member States, European Parliament or stakeholders. Following the EFSA opinion, each State can decide independently to set specific approval regulations and/or authorization. In the USA, however, manufacturers and other stakeholders do not have to register their products with the FDA because there is no need to obtain FDA approval and/or authorization before producing or selling food supplements or nutraceuticals. Surveillance activity is relegated to governmental agencies, and manufacturers are responsible for ensuring that information reported on the product label is true and not misleading 46. In Canada, nutraceuticals are regulated more like a drug than as a food category 47.
Other countries have specific legislation; for example Indian legislation does not ascribe any specific legal status to nutraceuticals. The government of India established the Food Safety and Standards Act (FSSA) in 2006 to introduce a legislation system. FSSA does not separate functional foods, nutraceuticals, and dietary supplements; instead, each is indicated as food for a special dietary application. It considers products with beneficial health claims to be similar to food without any statements about nutraceuticals with clinical trial results. In 2015, India notified the World Trade Organization of a draft regulation for nutraceuticals and foods for special diets and medical purposes. The draft regulation defined these categories based on ingredients, labelling, additives, contaminants, and health and nutritional claims. The draft regulation also determined the criteria for the manufacturing and sale of these categories of foods and recommended doses or consumption levels. The new regulation, namely, Food Safety and Standards for Food for Health Supplements, Nutraceuticals, Food for Special Dietary Use, Food for Special Medical Purposes, Functional Food and Normal Food Regulations 2016, is based on and framed from Section 22 of the FSSA. The full text of the Regulation can be accessed from the FSSA website (http://www.fssai.gov.in/), and the Food Safety and Standard Act No. 34 2006 can be accessed from the FSSA web portal (http://www.fssai.gov.in/portals/0/pdf/food‐act.pdf).
Japan, by contrast, was among the first countries to face the issue of regulating food supplements and foodstuff by issuing the Foods for Specified Health Use (FOSHU) based on a voluntary request from stakeholders for approval 48, 49. This legislation, originally set in 1991, evolved into the 2003 Health Promotion Law 50.
Possible approval is available for food with beneficial health activities even if these activities are not substantiated with scientific evidence if the product meets the level of FOSHU requirements (safety, nutritionally appropriate ingredient content, etc.). Even food without an assessed and defined mechanism of effectiveness for its function, namely, qualified FOSHU and standardized FOSHU, can be considered. Any reduction of disease risk claim is allowed if the reduction of disease risk is clinically and nutritionally established in one ingredient.
In general, many countries, such as Australia or China, regulate nutraceuticals simply as a category of food, and the national regulations valid for food apply 51, 52, 53, 54. A simple registration‐based approach has been adopted by some countries, such as Colombia, Brazil and Argentina 55. A notification‐based approach addressed to the local competent authority is valid in Mexico and Chile. Nevertheless, other countries, such as Brazil, China and Taiwan, have stricter requirements, and prior to registration, a complete animal or human clinical study is required 46. Based on this information, it is foreseeable that a safety assessment and complete clinical study may be necessary before any nutraceutical or any ingredient is put on the market. Moreover, a health claim should be authorized and attributed only after a complete clinical study is proposed to the appropriate authority for approval with the aim of substantiating its safety and efficacy with respect to the claimed beneficial health effect based on an understanding of the mechanism of action and the absence of undesired side effects.
Dose and safety: the paradigm of red yeast rice
Increases in cardiovascular disease have been further correlated with improper dietary habits, leading to an increase in morbidity and mortality and outlining the need for a different approach to the management of hypercholesterolemia to avoid the possible unwanted side effects of drugs, such as widely used statins 13. This aim has led to the use of red yeast rice (RYR). RYR is a food obtained from fermentation with the red yeast of Monascus purpureus, and the content of its bioactive component monacolin K allowed it to be used for people with moderate dyslipidaemia.
Monacolin K (chemically identical to lovastatin, a drug used to control hypercholesterolaemia), which has been recognized as a key component in cholesterol reduction, has been validated as the source of its hypocholesterolaemic effect. A recent study on the safety profile of RYR conducted within the Italian Surveillance System of Natural Health Products on possible unwanted side effects due to consumption observed cases of myalgia and/or an increase in creatine phosphokinase, liver injury and gastrointestinal reactions, and, in some cases, hospitalization 56.
The potential outcomes of myopathies and liver injury raise the hypothesis that the safety profile of RYR is similar to that of statins 57, 58. For this reason, accurate monitoring of RYR and other food constituents should be promoted to fully characterize their risk profile, thereby helping regulatory bodies take appropriate actions.
Another risk factor is represented by the possible presence of secondary toxic metabolites, such as the mycotoxin citrinin, whereas the variable statin content in different red yeast rice‐based food supplements generated a controversial scenario in which dose inconsistency and co‐occurrence of the toxin citrinin resulted in criticism of its dietary supplementation use 59.
Citrinin frequently contaminates foodstuff as do other secondary metabolites produced by microfungi present in, for example, milk 60. Exposure to citrinin, produced by microfungi of the Monascus species (Monascus purpureus and Monascus ruber) and Penicillium species (Penicillium citrinum, Penicillium expansum, Penicillium radicicola and Penicillium verrucosum), can cause nephrotoxicity and a strong oxidative stress 61. The maximum level of this contaminant has been determined by Regulation EU No. 519/2014 of the European Commission in March 2014, which set the maximum acceptable level of citrinin as 2000 μg kg–1 in RYR food supplements 62.
The analysis of citrinin in RYR as a dietary supplement and traditional medicine carried out using a citrinin immuno‐affinity column for sample clean‐up before LC analysis with fluorescence detection enabled analysis of citrinin with high degree of accuracy (limit of quantitation for citrinin in red yeast rice was 10 μg kg–1, and limit of detection was 3 μg kg–1), which confirmed the presence of this contaminant in trace amounts 63.
A recent systematic analysis of 10 randomized controlled trials involving 905 Chinese subjects with dyslipidaemia compared the use of RYR (monacolin K) and simvastatin. The results confirmed that they have similar effects on elevated lipid levels, suggesting that the use of RYR as an alternative to simvastatin is not supported by current evidence, excluding patients intolerant to statins 64.
The German Federal Institute for Drugs and Medical Devices warned about the use of RYR, and the food safety European regulation requirements for food supplements containing active compounds established that the monacolin K content should be considered a drug at levels higher than 5 mg day–1 due to existing scientific data supporting a pharmacological effect at this dose 65. This regulation differs from the EFSA 2011 opinion in which the limit of monacolin K from RYR was set at 10 mg day–1 for the maintenance of normal blood levels of low density lipoprotein cholesterol concentration 66.
This value has, however, been considered acceptable in many European countries even though it has been observed that lovastatin (monacolin K) can cause undesired side effects, e.g. kidney problems or muscle issues; thus, RYR products can also cause the same health issues, depending on the dose and considering the higher bioavailability of red yeast rice‐based food supplements, as pure lovastatin 67. Recently, the Conseil Superior de la Sante of Belgium advised that the exact amount of monacolin K in RYR is highly variable, ranging from 3 to 30 mg daily depending on the brand, representing a health risk due to the lack of appropriate information to its users 68.
By contrast, the US FDA issued a warning on these RYR products in 2007. According to this warning, the content of monacolin K should be considered a drug at levels higher than 5 mg day–1 due to existing scientific data that support a pharmacological effect at this dose, as recently also confirmed by the German expert panel of the Bundesamt für Verbraucherschutz und Lebensmittelsicherheit (BVL) und das Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM) 65. This report confirms the opinion of the Autorité française de Sécurité sanitaire de l'Alimentation, de l'Environnement et du Travail, which seems to suggest that high values of monacolin K in RYR products should be considered with caution due to possible monacolin K content variability 69. The possible contamination from co‐occurrence of the toxic metabolite citrinin generates an additional disputable scenario in which dose inconsistency and safety issues suggest caution against the use and need for shared regulation assessment to guarantee safety, content and dose‐related efficacy. The establishment of a regulatory framework would help avoid this scenario. Innovative, cross‐sectorial strategies may contribute directly or indirectly to improving the quality of life making our health and social care systems more efficient and sustainable. Adherence to adequate lifestyles and behaviour is important to improve quality of life and prevent adverse health outcomes 70.
Multitude of good practices developed throughout the European Union favours a comprehensive and multidimensional scaling‐up strategy at European level. For this reason, would be important to identify strategies to implement a scale‐up and to develop synergies 71.
Conclusion
Based on the above discussion, in our personal opinion, a restructuring of the entire regulatory framework of dietary supplements in view of the role of nutraceuticals is deemed necessary to first give credit to their different purposes and definition, and second, to assess their specific role in the prevention and treatment of pathological conditions, supporting their potential medical use in prevention and therapy only when proven by sound scientific and clinical data.
The specificity of nutraceuticals would need a premarket approval system substantiated by scientific data (in vivo clinical trials) showing their complete safety and efficacy profile. The regulatory approach could be based on the one adopted for drugs, which is stricter and more complex. The likelihood of this occurring in the foreseeable future is unfortunately quite low. However, some measures could result in improvements of the existing situation.
It may be reasonable for national competent authorities to ask manufacturers to provide data on the safety, efficacy and mechanism of action supporting any claims contained on the labels of their products, especially when the term nutraceutical is used.
Strict guidelines would be needed and shared at international level to ensure that proper information is used to substantiate any health claims on the product label and detailed in the recommendation/safety guidelines, which should be included in the packaged products.
Moreover, relevant information and data should be related to the complex of substances forming a nutraceutical and should not refer to a single substance (or a mixture of different substances) of natural origin with physiological activity and/or beneficial health effects, highlighting the difference between food supplements and nutraceuticals.
It is indeed obvious that the current situation regarding nutraceuticals is far from satisfactory; our aim is to stimulate a fruitful debate on this subject. We hope that our proposals generate comments and useful objections from other experts in this field. This manuscript could be a starting point for a discussion on these products, even if DeFelice has decided to take a step backwards on his original classification of nutraceutical products. Based on this decision, we still suggest adopting a global approach to rethink a new/amended regulatory framework.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 72, and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 73.
Competing Interests
There are no competing interests to declare.
The views expressed in this work by Silvia Miriam Cammarata, Giacomo Capone and Luca Pani are personal and may not be understood or quoted as being made on behalf of or reflecting the position of AIFA, EMA or of any of their committees or working parties.
Santini, A. , Cammarata, S. M. , Capone, G. , Ianaro, A. , Tenore, G. C. , Pani, L. , and Novellino, E. (2018) Nutraceuticals: opening the debate for a regulatory framework. Br J Clin Pharmacol, 84: 659–672. doi: 10.1111/bcp.13496.
References
- 1. Borchers AT, Keen CL, Gerswin ME. The basis of structure/function claims of nutraceuticals. Clin Rev Allergy Immunol 2016; 51: 370–382. [DOI] [PubMed] [Google Scholar]
- 2. DeFelice SL. The nutraceutical revolution: its impact on food industry R&D. Trends Food Sci Technol 1995; 6: 59–61. [Google Scholar]
- 3. El Sohaimy SA. Functional foods and nutraceuticals‐modern approach to food science. World Appl Sci J 2012; 20: 691–708. [Google Scholar]
- 4. Santini A, Novellino E. Nutraceuticals: beyond the diet before the drugs. Curr Bioact Compd 2014; 10: 1–12. [Google Scholar]
- 5. Aronson JK. Defining nutraceuticals: neither nutritious nor pharmaceutical. Br J Clin Pharmacol 2017; 83: 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Finley JW. The nutraceutical revolution: Emerging vision or broken dream? Understanding scientific and regulatory concerns. Clin Res Regul Aff 2016; 33: 1–3. [Google Scholar]
- 7. Volpe G, Sotis G. Nutraceuticals: definition and epidemiological rationale for their use in clinical practice. High Blood Press Cardiovasc Prev 2015; 22: 199–201. [DOI] [PubMed] [Google Scholar]
- 8. Pinto da Costa J. A current look at nutraceuticals – key concepts and future prospects. Trends Food Sci Technol 2017; 62: 68–78. [Google Scholar]
- 9. Gupta RC. Nutraceuticals: Efficacy, Safety and Toxicity. Boston, MA: Academic Press, 2016. [Google Scholar]
- 10. Filipiak‐Szok A, Kurzawa M, Szłyk EJ. Determination of toxic metals by ICP‐MS in Asiatic and European medicinal plants and dietary supplements. Trace Elem Med Biol 2015; 30: 54–58. [DOI] [PubMed] [Google Scholar]
- 11. Fernando G, Hernández AF, Martín‐Domingo MC. Toxic contamination of nutraceuticals and food ingredients In: Nutraceuticals: Efficacy, Safety and Toxicity, ed Gupta RC. Boston, MA: Academic Press; Chapter 58, 2016; 1–13. [Google Scholar]
- 12. Dickinson A, Blatman J, El‐Dash N, Franco JC. Consumer usage and reasons for using dietary supplements: report of a series of surveys. J Am Coll Nutr 2014; 33: 176–182. [DOI] [PubMed] [Google Scholar]
- 13. Santini A, Novellino E. Nutraceuticals in hypercholesterolaemia: an Overview. Br J Pharmacol 2017; 174: 1450–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. White paper on Food Safety . 2000. Available at http://ec.europa.eu/dgs/health_food‐safety/library/pub/pub06_en.pdf (last accessed 31 March 2017).
- 15. Regulation EC No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. Available at http://eur‐lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32002R0178:EN:NOT. (last accessed March 2017).
- 16. Directive 2004/27/EC of the European Parliament and of the Council of 31 March 2004 amending Directive 2001/83/EC on the Community code relating to medicinal products for human use. Off J Eur Union 2004; L136/34–L136/57. Available at http://eur‐lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2004:136:0034:0057:en:PDF last accessed March 2017. [Google Scholar]
- 17. Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements. Off J Eur Communities 2002; L183/51–L183/57 Available at http%3A%2F%2Feur-lex.europa.eu%2Flegal-content%2FEN%2FTXT%2FPDF%2F%3Furi%3DCELEX%3A32002L0046%26amp%3Bfrom%3DENhttp%3A%2F%2Feur-lex.europa.eu%2Flegal-content%2FEN%2FTXT%2FPDF%2F%3Furi%3DCELEX%3A32002L0046%26amp%3Bfrom%3DEN (last accessed March 2017). [Google Scholar]
- 18. ERNA. European Responsible Nutrition Alliance . Vitamin and mineral supplements: a risk management model. Brussels. Belgium: Available at www.erna.org: ERNA, 2004. last accessed March 2017. [Google Scholar]
- 19. ERNA. European Responsible Nutrition Alliance . 2006. Discussion document on the setting of maximum and minimum amounts of vitamins and minerals in foodstuffs. Available at https://ec.europa.eu/food/sites/food/files/safety/docs/labelling_nutrition‐supplements‐responses‐erna_en.pdf. (last accessed 31 March 2017).
- 20. Regulation EC No 1924/2006 of the European Parliament and of the Council of 30 December 2006 on nutrition and health claims made on foods. 2006. Available at http%3A%2F%2Feur-lex.europa.eu%2Flegal-content%2FEN%2FTXT%2FPDF%2F%3Furi%3DCELEX%3A02006R1924-20121129%26amp%3Bqid%3D1479119886705%26amp%3Bfrom%3Dit (last accessed March 2017).
- 21. Augustin MA, Sanguansri L. Challenges and solutions to incorporation of nutraceuticals in foods. Annu Rev Food Sci Technol 2015; 6: 463–477. [DOI] [PubMed] [Google Scholar]
- 22. Abuajah CA, Ogbonna A, Osuji C. Functional components and medicinal properties of food: a review. J Food Sci Technol 2015; 52: 2522–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dadhania VP, Trivedi PP, Vikram A, Tripathi DN. Nutraceuticals against neurodegeneration: a mechanistic insight. Curr Neuropharmacol 2016; 14: 627–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Olaiya CO, Soetan KO, Esan AM. The role of nutraceuticals, functional foods and value added food products in the prevention and treatment of chronic diseases. Afr J Food Sci 2016; 10: 185–193. [Google Scholar]
- 25. Yang N, Kaarunya Sampathkumar K, Joachim Loo SC. Recent advances in complementary and replacement therapy with nutraceuticals in combating gastrointestinal illnesses. Clin Nutr 2016; 36: 968–979. [DOI] [PubMed] [Google Scholar]
- 26. Wildman R, Keller M. Nutraceuticals and functional foods In: Handbook of Nutraceuticals and Functional Foods, 2nd edn, ed Wildman REC. Boca Raton: CRC press, 2016. [Google Scholar]
- 27. Santini A, Tenore GC, Novellino E. Nutraceuticals: a paradigm of proactive medicine. Eur J Pharm Sci 2017; 96: 56–61. [DOI] [PubMed] [Google Scholar]
- 28. Directive of the Council 89/389/EEC . Foodstuff intended for particular nutritional use. 1989. Available at http%3A%2F%2Feur-lex.europa.eu%2Flegal-content%2FEN%2FTXT%2FHTML%2F%3Furi%3DURISERV%3Al21100%26amp%3Bfrom%3DEN (last accessed March 2017).
- 29. De Felice S. FIM Rationale and Proposed Guidelines for the Nutraceutical Research & Education Act – NREA. FIM's 10th Nutraceutical Conference November 10–11, 2002. The Waldorf‐Astoria, New York City, USA. http://www.fimdefelice.org/archives/arc.researchact.html (last accessed March 2017).
- 30. Regulation EC No 258/97 of the European parliament and of the council of 27 January 1991 concerning novel foods and novel food ingredients. Off J Eur Communities L43/1–L43/6 Available at http%3A%2F%2Feur-lex.europa.eu%2Flegal%2520content%2FEN%2FTXT%2FPDF%2F%3Furi%3DCELEX%3A31997R0258%26amp%3Bfrom%3Den (last accessed March 2017). [Google Scholar]
- 31. Commission Regulation EC No 1852/2001 of 20 September 2001 laying down detailed rules for making certain information available to the public and for the protection of information submitted pursuant to European Parliament and Council Regulation (EC) No 258/97. Off J Eur Communities 2001; L253/17–L253/18. [Google Scholar]
- 32. Regulation EU 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001. Off J Eur Union 2015; L327/1–L32722. [Google Scholar]
- 33. Commission Directive 1999/21/EC . On dietary foods for special medical purposes. Off J European Communities 1999; L91/29. [Google Scholar]
- 34. O'Neill S, O'Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev 2015; 16: 1–12. [DOI] [PubMed] [Google Scholar]
- 35. Dragsbæk K, Neergaard JS, Laursen JM, Hansen HB, Christiansen H, Beck‐Nielsen H, et al Metabolic syndrome and subsequent risk of type 2 diabetes and cardiovascular disease in elderly women: challenging the current definition. Medicine 2016; 95: e4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rautiainen S, Manson JE, Lichtenstein AH, Sesso HD. Dietary supplements and disease prevention – a global overview. Nat Rev Endocrinol 2016; 1: 407–420. [DOI] [PubMed] [Google Scholar]
- 37. McCarville JL, Caminero A, Verdu EF. Novel perspectives on therapeutic modulation of the gut microbiota. Ther Adv Gastroenterol 2016; 9: 580–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Commission Regulation (EU) No 1047/2012 of 8 November 2012 amending Regulation (EC) No 1924/2006 with regard to the list of nutrition claims. Off J Eur Union 2012; L 310/36. [Google Scholar]
- 39. Bagchi D. Nutraceutical and Functional Food Regulations in the United States and Around the World. Boston, MA: Academic Press, 2008; 115–364. [Google Scholar]
- 40. Voinea L, Popescu DV, Negrea MT. Good practices in educating and informing the new generation of consumers on organic foodstuffs. Amfiteatru Economic 2015; 17: 488–506. [Google Scholar]
- 41. Nally JD. Good manufacturing practices for pharmaceuticals, Sixth edn. New Vernon, NJ: CRC Press, 2016. [Google Scholar]
- 42. DSHEA . 1994. United States Food and Drug Administration (FDA). Dietary Supplement Health and Education Act (DSHEA). U.S. Department of Health and Human Services. United States. Public Law 103–417. http://www.fda.gov; https://ods.od.nih.gov/About/DSHEA_Wording.aspx. (last accessed March 2017).
- 43. FD&C Act. Food, Drug and Cosmetic Act, United States . 2014. Available at http://legcounsel.house.gov/Comps/Federal%20Food,%20Drug,%20And%20Cosmetic%20Act.pdf (accessed March 2017).
- 44. FAO/WHO Joint Codex Alimentarius Commission . Codex Alimentarius. Rome: Food and Agriculture Organization of the United Nations, 1992. [Google Scholar]
- 45. Codex Alimentarius Commission . Available at http://www.fao.org/fao‐who‐codexalimentarius/en/ (last accessed 31 March 2017).
- 46. Hasler CM. In: Regulation of Functional Foods and Nutraceuticals: A Global Perspective, ed Hasler CM. Ames, IA: Blackwell Publishing, 2008; 389. [Google Scholar]
- 47. L'abbé MR, Dumais L, Chao E, Junkins B. Health claims on foods in Canada. J Nutr 2008; 138: 1221–1227. [DOI] [PubMed] [Google Scholar]
- 48. Shimizu T. Health claims on functional foods: the Japanese regulations and an international comparison. Nutr Res Rev 2003; 16: 241–252. [DOI] [PubMed] [Google Scholar]
- 49. Saito M. Role of FOSHU (food for specified health uses) for healthier life. Yakugaku Zasshi 2007; 127: 407–416. [DOI] [PubMed] [Google Scholar]
- 50. Yamada K, Sato‐Mito N, Nagata J, Umegaki K. Health claim evidence requirements in Japan. J Nutr 2008; 138: 1192–1198. [DOI] [PubMed] [Google Scholar]
- 51. Tee ES, Tamin S, Ilyas R, Ramos A, Tan WL, Lai DK, et al Current status of nutrition labelling and claims in the South‐East Asian region: are we in harmony? Asia Pac J Clin Nutr 2002; 11: 80–86. [DOI] [PubMed] [Google Scholar]
- 52. Tapsell LC. Evidence for health claims: a perspective from the Australia–New Zealand region. J Nutr 2008; 138: 1206–1209. [DOI] [PubMed] [Google Scholar]
- 53. Umegaki K. Positive and negative aspects of food with health claims in Japan. J Nutr Sci Vitaminol 2015; 61: S133–S135. [DOI] [PubMed] [Google Scholar]
- 54. Yang Y. Scientific substantiation of functional food health claims in China. J Nutr 2008; 138: 1199–1205. [DOI] [PubMed] [Google Scholar]
- 55. Mansour M. 22 Codex and its competitors: the future of the global regulatory and trading regime for food and agricultural products In: Regulation of Functional Foods and Nutraceuticals: A Global Perspective, ed Hasler CM. Ames, IA: Blackwell Publishing, 2008; 377–389. [Google Scholar]
- 56. Mazzanti G, Moro PA, Raschi E, Da Cas R, Menniti‐Ippolito F. Adverse reactions to dietary supplements containing red yeast rice: assessment of cases from the Italian surveillance system. Br J Clin Pharmacol 2017; 83: 894–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lapi F, Gallo E, Bernasconi S, Vietri M, Menniti‐Ippolito F, Raschetti R, et al Myopathies associated with red yeast rice and liquorice: spontaneous reports from the Italian Surveillance System of Natural Health Products. Br J Clin Pharmacol 2008; 66: 572–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Menniti‐Ippolito F, Mazzanti G, Santuccio C, Moro PA, Calapai G, Firenzuoli F, et al Surveillance of suspected adverse reactions to natural health products in Italy. Pharmacoepidemiol Drug Saf 2008; 17: 626–635. [DOI] [PubMed] [Google Scholar]
- 59. Patel S. Functional food red yeast rice (RYR) for metabolic syndrome amelioration: a review on pros and cons. World J Microbiol Biotechnol 2016; 32: 32–87. [DOI] [PubMed] [Google Scholar]
- 60. Santini A, Raiola A, Ferrantelli V, Giangrosso G, Macaluso A, Bognanno M, et al Aflatoxin M1 in raw, UHT milk and dairy products in Sicily (Italy). Food Addit Contam B 2013; 6: 181–186. [DOI] [PubMed] [Google Scholar]
- 61. Vanacloig‐Pedros E, Proft M, Pascual‐Ahuir A. Different toxicity mechanisms for citrinin and ochratoxin A revealed by transcriptomic analysis in yeast. Toxins 2016; 8: 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Regulation UE n. 519/2014 of the European Commission, amending Regulation (EC) No 1881/2006 as regards maximum levels of the contaminant citrinin in food supplements based on rice fermented with red yeast Monascus purpureus . March 2014.
- 63. Marley E, Brown P, Leeman D, Donnelly C. Analysis of citrinin in cereals, red yeast rice dietary supplement, and animal feed by immunoaffinity column cleanup and LC with fluorescence detection. J AOAC Int 2016; 99: 1025–1031. [DOI] [PubMed] [Google Scholar]
- 64. Ong YC, Aziz Z. Systematic review of red yeast rice compared with simvastatin in dyslipidemia. Int J Clin Pharmacol Ther 2016; 41: 170–179. [DOI] [PubMed] [Google Scholar]
- 65. BVL/BfArM , Bundesamt für Verbraucherschutz und Lebensmittelsicherheit (BVL) und das Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM) . 2016. Available at http://www.bfarm.de/SharedDocs/Downloads/DE/Arzneimittel/Zulassung/ZulRelThemen/abgrenzung/stellungnahmen/201601.pdf;jsessionid=CBC18D6FB04E23CAB8BAE1E1B5BA240C.1_cid340?__blob=publicationFile&v=4 (last accessed 31 March 2017).
- 66. EFSA . Scientific Opinion. EFSA J 2011; 9: 2207–2228. [Google Scholar]
- 67. Venhuis BJ, van Hunsel F, van de Koppel S, Keizers PHJ, Jeurissen SMF, De Caste D. Pharmacologically effective red yeast rice preparations marketed as dietary supplements illustrated by a case report. Drug Test Anal 2016; 8: 3–4. [DOI] [PubMed] [Google Scholar]
- 68. Avis Du Conseil Superieur De La Sante N° 9312 . Compléments alimentaires à base de “levure de riz rouge”. 2016; 20 pp.
- 69. ANSES: Opinion of the French Agency for Food, Environmental and Occupational Health & Safety on the risks associated with the presence of “red yeast rice” in food supplements. Request No. 2012‐SA‐0228, 2014; 34 pp.
- 70. Illario M, Vollenbroek‐Hutten M, Molloy DW, Menditto E, Iaccarino G, Eklund P. Active and healthy ageing and independent living. J Aging Res 2015; ID: 542183; DOI: https://doi.org/10.1155/2015/542183; 2015: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bousquet J, Bewick M, Cano A, Eklund P, Fico G, Goswami N, et al Building bridges for innovation in ageing: synergies between action groups of the EIP on AHA. J Nutr Health Aging 2017; 21: 92–104. [DOI] [PubMed] [Google Scholar]
- 72. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH, et al The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: Towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E, et al The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 2017; 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bloch A, Thomson CA. Position of The American Dietetic Association (phytochemicals and functional foods). J Am Diet Assoc 1995; 95: 493–496. [DOI] [PubMed] [Google Scholar]
- 75. Zeisel SH. Regulation of “Nutraceuticals”. Science 1999; 285: 1853–1855. [DOI] [PubMed] [Google Scholar]
- 76. Brower V. Nature Biotechnology 1998; 16: 728–731. [DOI] [PubMed] [Google Scholar]
- 77. Merriam‐Webster Online Dictionary . 2015. Merriam‐Webster Inc., P.O. Box 281, Springfield, MA 01102, United States.
- 78. European Nutraceutical Association (ENA) . 2016. Science behind Nutraceuticals. In E. N. Association (Ed.), (Vol. 2016). 594 Basel, Switzerland.
- 79. Zeisel, S.H. Regulation of “Nutraceuticals” Food and Drug Administration, FDA, Dietary Supplement Health and Education Act of 1994 (DSHEA), United States. Science 1999, 285: 1853–1855. [DOI] [PubMed] [Google Scholar]
- 80. Diplock A, Aggett P, Ashwell M, Bornet F, Fern E, Roberfroid M. The European Commission concerted action on functional foods science in Europe (FUFOSE). Scientific concepts of functional foods in Europe. Consensus document. Br J Nutr 1999; 81: S1–27. [PubMed] [Google Scholar]
- 81. Hardy G. Nutraceuticals and functional foods: introduction and meaning. Nutrition 2000; 16: 688–689. [DOI] [PubMed] [Google Scholar]
- 82. EU Directive 1999/21/ EC .


