Abstract
Aims
Published data on long‐term adherence and persistence with adalimumab (Humira®) in clinical practice are scarce and often limited to selected patient populations. This study assessed adherence with adalimumab across different indications and identified correlates and outcomes of poor adherence.
Methods
We analysed data originating from the electronic database of Maccabi Healthcare Services (MHS) that includes 2.1 million enrolees. We randomly selected patients with at least one dispense of adalimumab since it was included in the local health basket in Israel in 2008 until the end of 2013. Patients with the following indications (n = 1339) were included: Crohn's disease (CD), ulcerative colitis (UC), rheumatoid arthritis (RA), psoriatic arthritis (PSA), ankylosing spondylitis (AS) and psoriasis. Adherence with therapy was assessed by the medication possession ratio (MPR) during the follow‐up period.
Results
Good adherence (MPR ≥ 80%) was observed among 80% of study patients and was associated with lower risk for ≥1 hospitalization per year of follow‐up (adjusted‐OR = 1.94, 95% CI:1.15–3.28). Patients with AS and CD persisted on adalimumab therapy the most, reaching median use of 27.0 and 26.7 months, respectively. Half (52.4%) of the patients discontinued treatment during a mean (SD) follow‐up of 3.07 (1.71) years. High socioeconomic status was associated with lower risk for discontinuation (adjusted‐HR = 0.74; 0.60–0.91). UC and concomitant prednisolone use were associated with increased risk for treatment discontinuation (HR = 1.31; 1.00–1.72, and HR = 1.40; 1.17–1.68, respectively).
Conclusion
Our results indicate encouraging persistence and adherence with adalimumab of patients with inflammatory conditions.
Keywords: Adalimumab, adherence, compliance, persistence, rheumatoid arthritis
What is Already Known about this Subject
‘Real life’ studies across different medical disciplines show poor adherence rates to drug therapy.
Many studies have shown that low adherence rates to therapy results in overall poor clinical outcomes. This pattern has also been demonstrated in multiple rheumatic conditions.
What this Study Adds
The vast majority of patients with different inflammatory conditions show extremely high rates of adherence to therapy with adalimumab (Humira) reaching rates above 80%.
Higher rates of adherence were associated with lower rates of hospitalizations in patients treated with adalimumab (Humira).
Introduction
Immune‐mediated inflammatory disorders include a large group of conditions of unknown aetiology that are clinically diverse. They affect millions of people worldwide, including 5–7% of the population of Western societies. These patients have various comorbidities, which strongly impact their quality of life and longevity 1, 2, 3, 4, 5, 6, 7.
Despite their clinical heterogeneity, many of these immune‐mediated disorders share common inflammatory pathways, which trigger dysregulation of the normal immune response 8, 9, 10, 11. Tumour necrosis factor‐alpha (TNF‐α) serves as a key regulator of innate immunity and plays an important role in the regulation of Th1 immune responses against microbiological pathogens. However, dysregulated TNF‐α can also contribute to numerous pathological conditions. This discovery heralded a new era of targeted and highly effective therapies for rheumatoid arthritis (RA) and subsequently for other chronic inflammatory disorders such as inflammatory bowel disease (IBD), psoriasis and spondyloarthopathies.
Anti‐TNF agents, either in the form of a neutralizing monoclonal antibody or as a soluble TNF receptor blocker, induce biological and cellular mechanisms that revolutionized the clinical management of these inflammatory disorders 12, 13, 14. Treatment with anti‐TNF medications brought about significant therapeutic improvements in many prospective studies and randomized control trials (RCTs) for several immune‐mediated conditions 1, 10, 12, 13. Nevertheless, the successful results observed in RCTs are usually achieved with a high degree of medication adherence, not necessarily representing real‐world patients' behaviour.
Treatment adherence, defined as the extent to which patients take medications prescribed by their healthcare providers, depends on many factors including the type of medication, concomitant drug use and familial and medical support 15, 16.
Several factors affect the levels of compliance with biological therapy in Israel that differentiate them from other medications. The co‐payment of the patients (‘out of pocket’ costs) is not negligible, and is about $75 a month. Given the high costs and profits of biological therapy, in order to preserve high adherence rates, all the pharmaceutical companies hire nurses that are in continuous contact with the patients either directly or by a third party (dependent on the company's regulations). By and large, in Israel, patients are instructed to self‐administer the medication, yet a minority of them seeks the assistance of nurses in the communities' clinics or of family members.
The parenteral mode of administration also impacts adherence rates; the fact that the medication is injected subcutaneously mandates periodical encounters with health professionals even in the case of self‐use. This, by nature, improves the persistence and adherence rates. Mohr et al. 17 have found that for injectable treatments, self‐administration is associated with better compliance than when given by healthcare providers and family members. Previous adherence studies in different indications such as gout and fibromyalgia have also found that single individuals are less adherent than those who live with family members 15, 16, 18.
Any deviation from the prescribed drug regimen may lead to treatment failure and disease or symptom recurrence, as well as increased healthcare costs 19. A large multicentre, prospective, observational cohort study of RA patients in the UK found that inadequate adherence to biologics resulted in worse clinical outcomes compared to those of adherent counterparts 20. A retrospective study based on an American healthcare database of Crohn's disease patients showed that low adherence to adalimumab treatment was associated with increased medical costs and hospitalizations 21.
The definitions of ‘adherent’ and ‘non‐adherent’ and methods for measuring adherence lack consensus 15, 22. A recent systematic review of over 20 studies 23 analysing adherence to biologic therapies and associated factors for a variety of inflammatory conditions reported diverse results across studies. This was partially due to the wide variability in the definition of adherence, study design and measurement methods.
The current research characterized and evaluated adherence and persistence with adalimumab treatment, which is the first fully human monoclonal antibody directed against TNFα. It is given subcutaneously once every two weeks for a broad variety of immune‐mediated disorders. Our study investigated the factors associated with compliance of a ‘real‐life’ population of patients with a variety of immune‐mediated inflammatory conditions.
Methods
Study sample and data collection
We conducted a retrospective cohort study using the database of Maccabi Healthcare Services (MHS); the second largest healthcare provider in Israel. The MHS patient database is contained within a central electronic medical record system, with over 18 years of longitudinal data on a stable population of approximately 2.1 million people. We received the approval of the MHS ethics board to conduct this study (approval number (36/2013).
The study sample comprised a stratified, random sample of all patients in the database with at least one dispense of adalimumab between 1 January 2008, the date of its inclusion in the national dispensary in Israel, and 31 December 2013. Patients were stratified according to the disease for which adalimumab was prescribed, defined as the last relevant diagnosis reported in the medical record prior to first dispense. Within each stratum, the sample size was randomly determined within a fixed interval of 100–350 patients (to accommodate MHS policy of confidentiality regarding the numbers of patient per indications). Patients were then randomly selected for the study. Diseases included Crohn's disease (CD), ulcerative colitis (UC), RA, psoriatic arthritis (PSA), ankylosing spondylitis (AS), and psoriasis. Since juvenile idiopathic arthritis was included in covered health services only in 2010, the total number of patients with dispensed adalimumab was low. Thus, this indication was not included in the study. Date of first dispense of adalimumab was defined as the index date. Follow‐up concluded on 31 December 2013.
Using the patients' unique 9‐digit national identification numbers, data extracted from the database included demographics (age, sex, district of residence and date of immigration), BMI, smoking status, medication dispenses, visits to primary and secondary clinics, and hospitalization dates. Disease information was also extracted from MHS automated patient registries, including diabetes mellitus, chronic kidney disease (CKD), hypertension (HTN), cancer, and cardiovascular disease registries. These registries are automatically updated daily according to strict computerized algorithms 24, 25. Concomitant pharmacological treatments were defined as at least two dispenses within 180 days from the index date and included azathioprine, methotrexate, hydroxychloroquine, sulfasalazine, leflunomide, mercapto‐purine and prednisone. Patients with less than 90 days of follow‐up or less than 1 year enrolment in MHS prior to first dispense of adalimumab were excluded (Figure 1).
Figure 1.
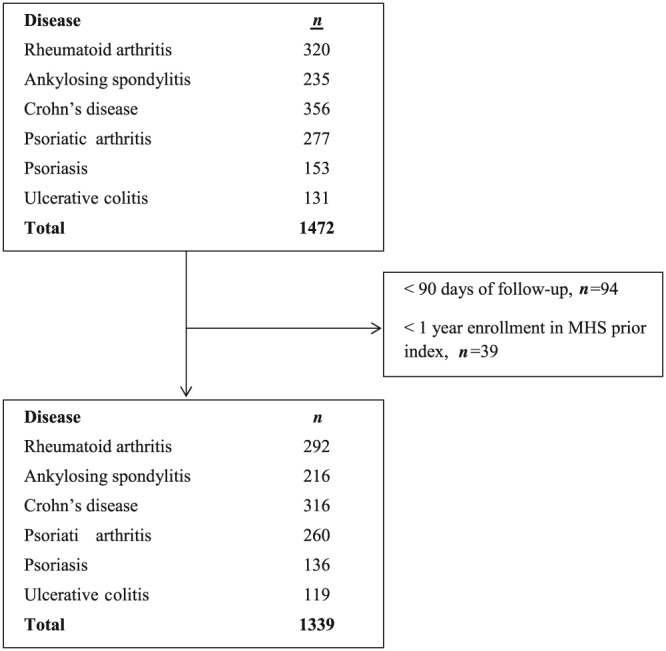
Disposition of patients throughout the study phases. Patients with <90 days of follow‐up or <1 year enrolment in MHS prior first dispense of adalimumab were excluded
Socioeconomic status (SES) was defined according to the poverty index of the member's enumeration area, as defined during the Israeli national census in 2008. The poverty index is based on several parameters, including household income, education, crowding, material conditions, and car ownership. It ranges from 1 to 20, based on cluster analysis, with 1 being the lowest and 20 being the highest SES level 26.
Compliance with treatment
We evaluated adherence with medical therapy using the Medication Possession Ratio (MPR), which reflects the ratio of the number of treatment days dispensed and the total number of days from first dispense to the last supply day of the last dispense in the follow‐up period or until discontinuation of 180 days or more. Patients were followed until 31 December 2013; however, an additional 180 days were assessed for dispenses of adalimumab to allow for evaluation of gap in treatment of 180 days or more for all study patients. Adherence was defined as high (MPR ≥ 80%), intermediate (20% < MPR < 80%) or low (MPR < 20%), or as a dichotomous variable, MPR ≥80% (adherent) vs. MPR <80% (non‐adherent). Persistence was measured by the duration from initiation to discontinuation of therapy, defined as a gap of 180 days or more between dispenses. Concomitant use of methotrexate among RA patients was also assessed. This was defined as at least two dispenses (one in the 180 days before and one following the first dispense of adalimumab). It should be underlined that even in the event of patients who chose to be injected by a health professional, it remained their responsibility to dispense the medications. Therefore, the mode of administration did not affect the adherence or persistence rates that were investigated in this study.
Statistical analysis
Mean ± standard deviation (SD) or medians ± inter‐quantile range (IQR) and proportions were calculated for continuous and categorical variables, respectively. Continuous baseline patient characteristics were compared between study groups using the Mann–Whitney or Kruskal–Wallis test, as appropriate. Categorical characteristics and MPR levels were compared using the Chi‐square test. Correlation between categorical variables was assessed using the Spearman correlation coefficient. Kaplan–Meier methods were used to construct disease‐specific curves for time to treatment discontinuation and the log‐rank test was used to evaluate statistical significance. Multivariable Cox proportional hazards regression models were used to estimate hazard ratios (HR) and 95% confidence intervals (95% CI) for time to treatment discontinuation. The proportional hazards assumption was examined visually using Schoenfeld residuals plotted over time, and found to be reasonably fulfilled for all covariates. Multivariate logistics regression models were used to estimate odds ratios (OR) and 95% CIs for factors associated with adherence (MPR ≥ 80%) vs. non‐adherence (MPR < 80%). In addition, various measures of health services utilization during follow‐up were compared between adherent and non‐adherent patients using multivariate logistic regression models, for dichotomous measures, and multivariate generalized linear models with gamma distribution and log‐link function, for continuous measures. Selection of covariates for inclusion in all final regression models was based on the minimal Akaike information criterion and clinical relevance. In sensitivity analysis, only patients with at least 2 years of follow‐up were analysed. Analyses were done using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp, Armonk, NY).
Nomenclature of targets and ligands
Key ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal fo data from the IUPHAR/BPS Guide to PHARMACOLOGY 27.
Results
A total of 1339 patients were followed for a mean (SD) of 3.07 (1.71) years. Ages ranged from 5.5 to 91.5 years. Patients with AS, psoriasis and CD were significantly younger than the others (Table 1). Most RA patients were female, while the majority of AS patients were male. In addition, more individuals who ever smoked were reported among patients with AS and psoriasis compared to other disorders. Lower BMI ratios were recorded among patients with IBD, while patients with psoriasis and psoriatic arthritis (PSA) had higher BMIs.
Table 1.
Characteristics of study patients by indication (n = 1339)
| Characteristic | RA (n = 292) % (n) | AS (n = 216) % (n) | CD (n = 316) % (n) | PsA (n = 260) % (n) | Psoriasis (n = 136) % (n) | UC (n = 119) % (n) | Total (n = 1339) % (n) | P‐value |
|---|---|---|---|---|---|---|---|---|
| Age (mean, SD) | 53.0 (14.4) | 36.1 (15.4) | 41.7 (14.2) | 47.6 (12.7) | 33.7 (12.8) | 44.1 (11.5) | 43.3 (15.1) | <0.001 |
| Female sex | 81.2% (237) | 34.3% (74) | 50.9% (161) | 53.8% (140) | 38.2% (52) | 55.5% (66) | 54.5% (730) | <0.001 |
| SES score (1–20 scale; mean, SD) | 12.5 (4.0) | 12.4 (4.1) | 12.9 (4.1) | 12.4 (4.2) | 12.4 (4.4) | 13.4 (3.7) | 12.5 (4.0) | 0.198 |
| Missing SES score | 12.3% (36) | 13.4% (29) | 17.4% (55) | 15.8% (41) | 13.2% (18) | 16.8% (20) | 14.9% (199) | 0.513 |
| District | ||||||||
| Centre | 63.4% (185) | 65.7% (142) | 67.1% (212) | 66.2% (172) | 72.1% (98) | 73.9% (88) | 67.0% (897) | 0.030 |
| North | 25.0% (73) | 20.8% (45) | 14.6% (46) | 19.2% (50) | 14.7% (20) | 15.1% (18) | 18.8% (252) | |
| South | 11.6% (34) | 13.4% (29) | 18.4% (58) | 14.6% (38) | 13.2% (18) | 10.9% (13) | 14.2% (190) | |
| Ever smoked | 20.5% (60) | 23.6% (51) | 19.0% (60) | 16.2% (42) | 23.5% (32) | 18.5% (22) | 19.9% (267) | |
| BMI (kg m −2 ) | ||||||||
| <25 | 18.2% (53) | 14.4% (31) | 44.9% (142) | 14.2% (37) | 14.7% (20) | 36.1% (43) | 24.3% (326) | <0.001 |
| 26–30 | 30.5% (89) | 32.9% (71) | 19.0% (60) | 32.3% (84) | 30.1% (41) | 21.8% (26) | 27.7% (371) | |
| >30 | 29.8% (87) | 28.7% (62) | 6.6% (21) | 36.5% (95) | 27.2% (37) | 16.8% (20) | 24.0% (322) | |
| Unknown | 21.6% (63) | 24.1% (52) | 29.4% (93) | 16.9% (44) | 27.9% (38) | 25.2% (30) | 23.9% (320) | |
| Clinical history | ||||||||
| Cardiovascular disease | 13.7% (40) | 7.9% (17) | 3.5% (11) | 7.7% (20) | 8.1% (11) | 5.9% (7) | 7.9% (106) | <0.001 |
| Diabetes | 11.3% (33) | 6.5% (14) | 2.5% (8) | 16.5% (43) | 9.6% (13) | 7.6% (9) | 9.0% (120) | <0.001 |
| HTN | 31.5% (92) | 17.1% (37) | 7.9% (25) | 30.0% (78) | 18.4% (25) | 9.2% (11) | 20.0% (268) | <0.001 |
| CKD | 12.7% (37) | 6.0% (13) | 3.2% (10) | 6.5% (17) | 5.1% (7) | 5.0% (6) | 6.7% (90) | <0.001 |
| History of cancer | 6.5% (19) | 0.5% (1) | 5.4% (17) | 4.6% (12) | 2.2% (3) | 5.9% (7) | 4.4% (59) | 0.016 |
| Utilization of healthcare services in baseline year, median (IQR) | ||||||||
| Primary care visits | 22.5 (15–36) | 17 (11–27) | 20 (13–31) | 18 (12–29) | 13.5 (7.5–23) | 28 (16–37) | 20 (12–31) | <0.001 |
| Secondary care visits | 9 (5–14) | 9 (5–13) | 6 (2–10) | 8 (4–14) | 9 (4–13) | 6 (2–13) | 8 (4–13) | <0.001 |
| ≥1 Hospitalization | 21.9% (64) | 17.1% (37) | 49.1% (155) | 16.5% (43) | 22.8% (31) | 48.7% (58) | 29.0% (388) | <0.001 |
| Number of inpatient days among hospitalized patients (n = 388) | 4.5 (2–10.5) | 4 (2–9) | 5 (2–10) | 4 (1–9) | 7 (4–16) | 7 (4–12) | 5 (2–11) | 0.015 |
Crohn's disease (CD), ulcerative colitis (UC), rheumatoid arthritis (RA), psoriatic arthritis (pSA), ankylosing spondylitis (AS)
During the year prior to the day of inclusion in the study, patients with IBD were hospitalized more often compared to other patients, with almost half hospitalized at least once (Table 1). Except for psoriasis patients, of whom 65% had at least ten encounters with their primary care physician, 80–89% of patients in all other disease groups visited their primary‐care physician ten or more times during the baseline year, with a median of >15 visits for most groups. In addition, 30–48% had at least ten encounters with a secondary‐care physician with a median of 6–9 in the various groups.
Only half of RA patients who received adalimumab were also treated with methotrexate. Among RA patients given adalimumab, 42.1% were co‐treated with prednisone. Lower, but significant percentages of UC and CD patients were treated with steroids during the first 6 months following the initiation of adalimumab (18.4% and 14.9%, respectively) (Supplementary Table S1). As expected, mercaptopurine was given primarily to patients with IBD (12% to patients with CD and 10.9% to those with UC).
Adherence to treatment
The MPR of adalimumab among patients of all inflammatory conditions analysed in this study was 80% or more in approximately 80% of the patients with no statistically significant differences between conditions (Figure 2). Patients with low compliance (MPR < 80%) were 2 years younger on average and had fewer visits to primary‐ and secondary‐care clinics in the baseline year compared to those with high compliance (MPR ≥ 80%) (Table 2). Concomitant use of methotrexate was lower among patients with low vs. high MPR (13.5% vs. 21.7%, respectively, P = 0.046). After adjusting for age, sex, disease group, smoking status and length of follow‐up, the association of age with high MPR remained significant (OR = 1.01 per 1‐year increment, 95% CI: 1.00–1.03, P = 0.012). In addition, compared with RA patients, psoriatic arthritis patients were significantly less likely to be compliant (OR = 0.56; 0.33–0.97, P = 0.038). No other differences were observed between high and low compliant patients (data not presented).
Figure 2.
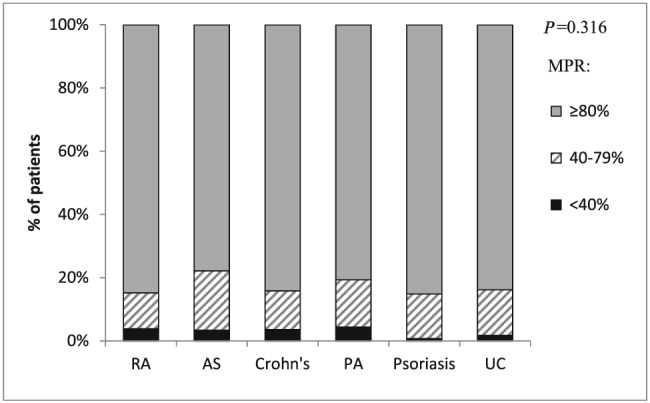
Medication possession ratio from first dispense to last supply day of last dispense in the study follow‐up period or till discontinuation of 180 days or more according to indication (n = 1279*)* Four patients hospitalized >20% of the follow‐up period and 56 patients with a single dispense were excluded. RA, rheumatoid arthritis; AS, ankylosing spondylitis; PA, psoriatic arthritis; UC, ulcerative colitis
Table 2.
Characteristics of adherent and non‐adherent patients (n = 1279)
| Characteristic | Adherent patients MPR ≥ 80% (n = 1059) % (n) | Non‐adherent patients MPR < 80% (n = 220) % (n) | P‐value |
|---|---|---|---|
| Age (mean, SD) | 43.6 (15.3) | 41.7 (13.6) | 0.079 |
| Female sex | 54.8 (580) | 55.0 (121) | 1.0 |
| SES score (1–20 scale; mean, SD) | 11.0 (5.8) | 10.2 (5.9) | 0.074 |
| Missing SES score | 14.5 (154) | 17.3 (38) | 0.302 |
| District | 0.734 | ||
| Centre | 67.4 (714) | 69.1 (152) | |
| North | 19.1 (202) | 16.8 (37) | |
| South | 13.5 (143) | 14.1 (31) | |
| Ever smoked | 19.3 (204) | 24.1 (53) | 0.125 |
| BMI (kg m −2 ) | 0.695 | ||
| <25 | 24.5 (259) | 22.3 (49) | |
| 26–30 | 28.0 (297) | 26.8 (59) | |
| >30 | 24.3 (257) | 24.1 (53) | |
| Unknown | 23.2 (246) | 26.8 (59) | |
| Disease | 0.273 | ||
| Rheumatoid arthritis | 19.5 (43) | 22.5 (238) | |
| Ankylosing spondylitis | 20.5 (45) | 14.9 (158) | |
| Crohn's disease | 21.4 (47) | 24.1 (255) | |
| Psoriatic arthritis | 21.8 (48) | 18.9 (200) | |
| Psoriasis | 9.1 (20) | 10.7 (113) | |
| Ulcerative Colitis | 7.7 (17) | 9.0 (95) | |
| Clinical history | |||
| Cardiovascular disease | 8.2 (87) | 5.0 (11) | 0.136 |
| Diabetes | 9.3 (98) | 8.2 (18) | 0.708 |
| Hypertension | 20.8 (220) | 15.0 (33) | 0.062 |
| Chronic kidney disease | 6.8 (72) | 4.5 (10) | 0.276 |
| History of cancer | 5.0 (53) | 1.8 (4) | 0.057 |
| Utilization of healthcare services in baseline year, median (IQR) | |||
| Primary care visits | 20.0 (12.0, 31.0) | 17.0 (11.0, 27.0) | 0.020 |
| Secondary care visits | 8.0 (4.0, 13.0) | 7.0 (3.75, 12.0) | 0.046 |
| ≥1 Hospitalization | 29.3 (310) | 20.5 (55) | 0.174 |
| Number of inpatient days among hospitalized patients (n = 365) | 5.5 (2; 11) | 5 (1;9) | 0.275 |
The association between adherence with adalimumab treatment and utilization of healthcare services during the follow‐up period was assessed among patients followed for at least 180 days. The median number of annual primary care visits among adherent and non‐adherent patients was 17.8 vs. 15.2 and of the number of annual specialist visits was 6.7 vs. 5.0, respectively (Table 3). After adjusting for multiple covariates, non‐adherent patients had 1 annual primary‐care visit and 0.16 secondary‐care visits less than adherent patients. Moreover, non‐adherent patients had a higher odds ratio of 1.94 (95% CI: 1.15–3.28) for being hospitalized at least once per year of follow‐up (Table 3).
Table 3.
Multivariate adjusted comparison of health services utilization in adherent vs. non‐adherent patients (n = 1217a)
| Measure | Adherent patients MPR ≥ 80% (n = 1005) Median (IQR) | Non‐adherent patients MPR < 80% (n = 212) Median (IQR) | P‐value | Adjusted difference between non‐adherent vs. adherent patients (95% CI) | P‐value |
|---|---|---|---|---|---|
| Follow‐up (years) | 3.07 (1.74; 4.46) | 3.42 (1.99; 5.07) | 0.007 | ||
| Number of primary care physician visits per year | 17.8 (11.3; 28.2) | 15.2 (9.2; 25.0) | 0.002 | −1.0 (−0.18; −0.01) | 0.028 |
| Number of specialist visits per year among patients with at least one visit in follow‐up (n = 1198) | 6.7 (3.6; 11.0) | 5.0 (3.0; 8.3) | <0.001 | −0.16 (−0.27; −0.06) | 0.030 |
| ≥1 hospitalization per year during follow‐up, % (n) Odds ratio for ≥1 vs. <1 | 9.9% (99) | 11.8% (25) | 0.396 | 1.94 (1.15; 3.28) | 0.013 |
| Number of inpatient days per year among patients with ≥1 hospitalization in follow‐up (n = 476) | 2.0 (0.8; 4.9) | 2.0 (1.0; 4.5) | 0.846 | −0.15 (−0.43; −0.14) | 0.307 |
4 patients were hospitalized >20% of the follow‐up period; 56 patients with a single dispense and 62 patients with less than 180 days follow‐up were excluded.
All models were adjusted for follow‐up days, age, sex, disease. Additional factors were included in different models according to their contribution to the model's fit. Among these factors were socio‐demographics, comorbidities, baseline medications, region of residence, smoking and BMI
Persistence with treatment
About half (52.4%) of the study patients stopped adalimumab treatment for 180 days or more; this number included discontinuations. No correlation was found between low adherence and treatment discontinuation (r = 0.031, P = 0.259). Baseline characteristics of persistent adalimumab users and of those who discontinued treatment are shown in Supplementary Table S2. Median time to discontinuation of therapy differed across medical conditions. It was shortest for RA (16 months) and longest for AS (27 months) (Figure 3). UC had shorter median drug survival than did CD (Figure 4). Among RA patients, concomitant treatment with methotrexate was associated with higher persistence rates throughout the years; however, this difference was not statistically different compared to patients who were treated with adalimumab as monotherapy (Figure 5).
Figure 3.
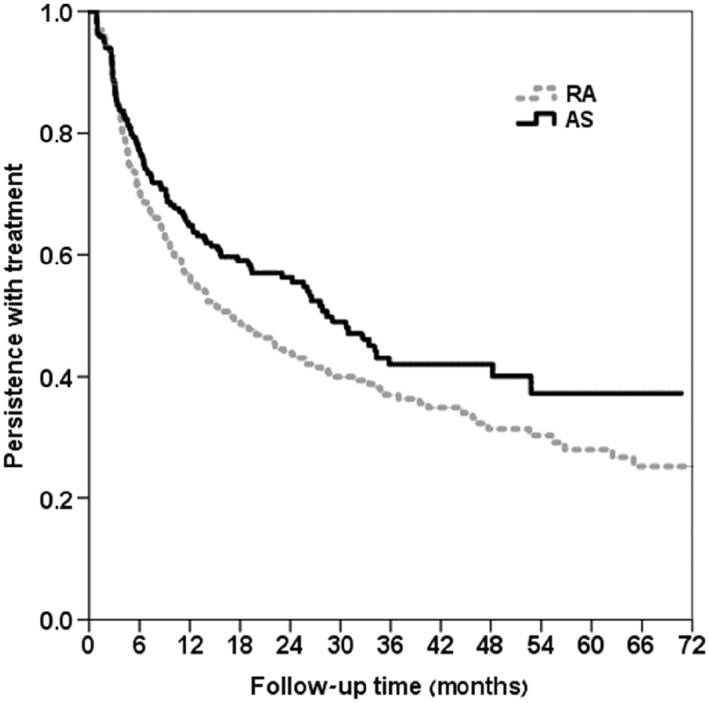
Kaplan–Meier plots of time to discontinuation by indication (RA vs. AS, n = 507) P‐value from log rank test = 0.040. RA, rheumatoid arthritis; AS, ankylosing spondylitis
Figure 4.
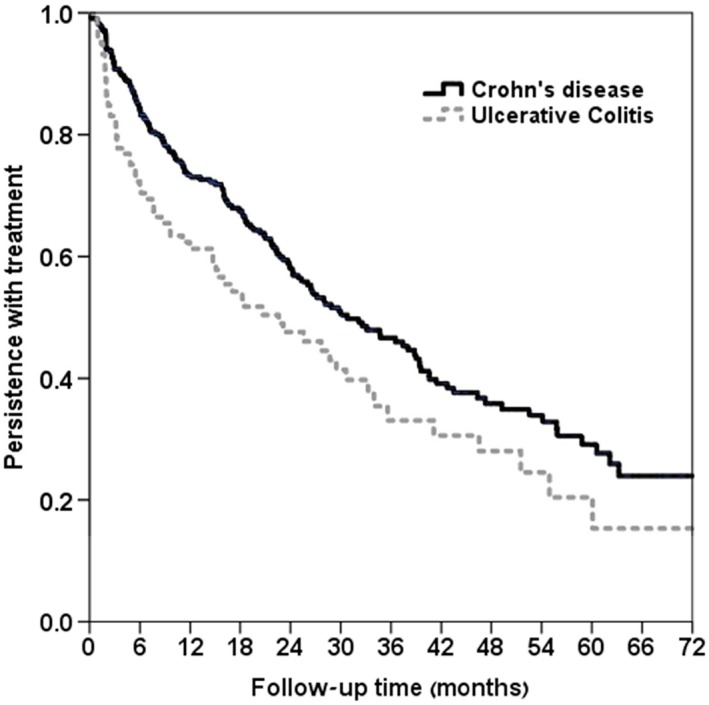
Kaplan–Meier plot of time to discontinuation in IBD (Crohn vs. UC, n = 433) P‐value from log rank test = 0.011
Figure 5.
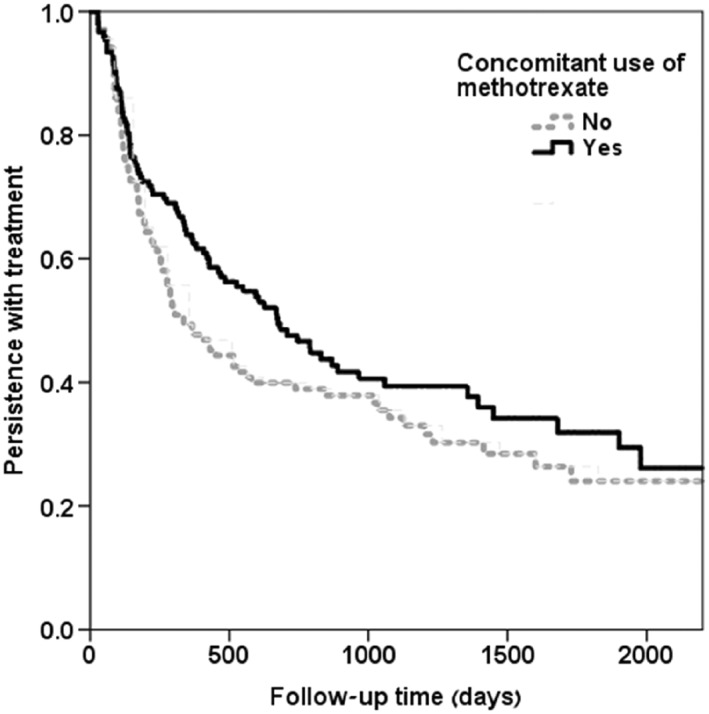
Kaplan–Meier plots of time to discontinuation of adalimumab in RA patients by concomitant methotrexate use; (methotrexate users n = 153, nonusers of methotrexate n = 138, total n = 291) P‐value from log rank test = 0.114
Table 4 shows mutually adjusted associations between patient factors and persistence with adalimumab. Compared to low SES, high SES was associated with longer drug persistence, while concomitant steroid use was associated with earlier cessation of adalimumab. UC patients were at higher risk for drug discontinuation than patients with other indications.
Table 4.
Factors associated with time to a discontinuation of ≥180 days of adalimumab treatment: multivariate Cox proportional hazards regression modela (n = 1335)
| Variable | Hazard Ratio | 95% CI | P‐value |
|---|---|---|---|
| Female sex | 1.14 | 0.98–1.33 | 0.089 |
| Age (per 1 year increment) | 1.01 | 1.00–1.01 | 0.010 |
| SES tertile (1–20 scale) | |||
| Low (1–10) | 1.00 (ref.) | ||
| Intermediate (11–14) | 0.89 | 0.73–1.09 | 0.257 |
| High (15–20) | 0.74 | 0.60–0.91 | 0.002 |
| Unknown | 0.72 | 0.56–0.92 | 0.015 |
| Immigrated after 1988 | 0.84 | 0.68–1.04 | 0.106 |
| Ulcerative colitis vs. other diseases | 1.32 | 1.02–1.71 | 0.034 |
| Concomitant use of prednisone | 1.46 | 1.22–1.75 | <0.001 |
Discontinuation was defined as ≥180‐day gap in days of supply. Four patients hospitalized >20% of the follow‐up period were excluded from analysis.
Discussion
Deciphering patterns of drug behaviour is difficult given the inconsistency in definitions and the lack of a uniform methodology for defining adherence. This issue has not been sufficiently explored in patients with inflammatory conditions treated with biological therapies 28. We conducted a retrospective analysis of drug behaviour using ‘real‐life’ electronic medical record data of patients treated with adalimumab for various inflammatory conditions. In contrast to many publications underlining the low persistence rates of drug therapy in various medical disciplines, we found extremely high and even surprising rates; reaching 80% across the different inflammatory diseases we studied 15, 16, 18.
The current study demonstrates that patients relate to treatment with adalimumab differently than they do to other medications. Tkacz et al. 29 retrospectively analysed adherence to adalimumab, etanercept and golimumab among 3892 patients with RA. Overall, patients treated with golimumab, (which is administered subcutaneously once a month), were the most adherent group compared with both adalimumab and etanercept, with MPR > 80% among 82% vs. 71% and 62%, respectively (P < 0.001). The authors speculated that better adherence to golimumab was attributable to more active disease among these patients prior to therapy and due to the more convenient dosing schedule. In contrast, Borah et al. 30 retrospectively analysed adherence (measured by MPR) and persistence with 1532 adalimumab, 2099 etanercept, and 261 golimumab patients with RA enrolled in a large healthcare organization. Patients were divided into current biological users and those naïve to biologic treatment (at baseline). Unadjusted adherence rates for naïve and non‐naïve patients were 63% and 70% for adalimumab and 65% and 73% for etanercept, respectively.
We found that variables such as age, gender, education, smoking and socioeconomic status were not related to adherence. These associations vary between studies: while some report older patients to have better adherence, others showed that younger patients were more persistent 31.
Since out study encompassed all ethnicities and socioeconomic strata, we believe that it provides a better understanding of drug compliance in Israel. In addition, age groups and different comorbidities that are often excluded from RCTs were also included in our study. The intermediate age group (25–34 years) was found to be less compliant, while the presence of comorbidities (additional inflammatory or cardiovascular diseases) was associated with higher adherence rates. Previous reports support the logic that the more patients are accustomed to taking medications for coexisting disorders, the higher the chances that their adherence rates will be higher and durable for additional medications 16. This pattern was also exhibited in the current study (Table 2).
Interestingly, higher rates of adherence were associated in our study with significantly shorter hospitalizations, a finding that has pertinent medical and financial significance.
Similar to previous reports in RA patients, concomitant use of adalimumab with methotrexate was associated with a higher degree of adherence 32, 33. The size of the sample was not big enough to show statistical significance, yet this trend was of no surprise given the known synergistic effects of combining methotrexate and biologicals (particularly anti‐TNF agents), especially in RA 34, 35, 36, 37, 38.
Persistence rates to adalimumab and the continuous decline in use of the drug observed over time in our study are in accordance with those of previous reports. Koncs et al. 39 reviewed 13 retrospective studies regarding persistence to infliximab, etanercept and adalimumab among RA patients. Persistence decreased over time in all studies, ranging from 65% to 87% after 12 months, to 41% to 56% after 48 months. In their review, none of the medications presented superiority over the others. Data from the nationwide Danish DANBIO registry including 764 patients with PSA treated with adalimumab, etanercept or infliximab, demonstrated drug survival rates of 70% in the first year and 57% after 2 years. The crude retention rates were similar among all the anti‐TNFs 40.
Several medical conditions had higher persistence rates than others, such as AS over RA (Figure 4) and CD over UC (Figure 5). This clearly shows that certain conditions are treated more effectively and result in greater improvement with adalimumab than others, as persistence serves as a surrogate marker for effectiveness. On the other hand, these observations might also reflect the limited options of alternative therapy in non‐RA inflammatory conditions, since, at the time the study was conducted, the anti‐IL‐17 monoclonal antibody, secukinumab, and the anti‐IL‐12 and IL‐23 monoclonal antibody, ustekinumab, were not available.
Using Kaplan–Meier plots, we observed several factors influencing drug survival. We found a negative association between female sex and persistence, which might derive from women's tendency to prioritize domestic issues over health, but this is only a conjecture. Age disparities demonstrating better survival rates among the youngest age group could be because they receive support from parents that emphasizes the importance of persisting in proper drug use.
Previous studies reported prolonged drug survival in RA with concomitant MTX + anti‐TNF treatment, including adalimumab 41. It seems that this combination reduces the immunogenicity of the anti‐TNFs in RA as well in Crohn's disease, SpA and possibly psoriasis 42.
The strength of the current study derives from the characteristics of the data which reflect actual experience, without selection bias or randomization of the recipients. Patients were included regardless of their age, sex, socioeconomic status or other comorbidities, which may confound the outcomes of persistence and compliance. Our data therefore presents true ‘drug behaviours’, which by definition differs from the artificial environment of RCTs.
The main limitation of this study is that we used administrative and computerized data, which lacked more accurate clinical information regarding patient outcomes. We were unable to retrieve the reasons for drug discontinuation of each subject and whether it was related to loss of efficacy or due to safety issues. Nevertheless, it is evident that the high rates of persistence with adalimumab shown in this study clearly indicate high efficacy and a satisfactory safety profile of the medication across a wide range of clinical conditions.
Competing Interests
There are no competing interests to declare.
The study was supported by a research grant from Abbvie. The authors thank AbbVie Biotherapeutics for their financial support by providing an educational grant.
Supporting information
Table S1 Oral medications that were taken concomitantly to therapy with adalimumab*
Table S2 Characteristics of patients who persisted with treatment and those who discontinued treatment (n = 1335)
Gendelman, O. , Weitzman, D. , Rosenberg, V. , Shalev, V. , Chodick, G. , and Amital, H. (2018) Characterization of adherence and persistence profile in a real‐life population of patients treated with adalimumab. Br J Clin Pharmacol, 84: 786–795. doi: 10.1111/bcp.13494.
References
- 1. Dahan S, Shor DB, Comaneshter D, Tekes‐Manova D, Shovman O, Amital H, et al All disease begins in the gut: celiac disease co‐existence with SLE. Autoimmun Rev 2016; 15: 848–853. [DOI] [PubMed] [Google Scholar]
- 2. Farhi A, Cohen AD, Shovman O, Comaneshter D, Amital H, Amital D. Bipolar disorder associated with rheumatoid arthritis: a case‐control study. J Affect Disord 2016; 189: 287–289. [DOI] [PubMed] [Google Scholar]
- 3. Gendelman O, Mahroum N, Comaneshter D, Rotman‐Pikielny P, Cohen AD, Amital H, et al Hepatitis B carrier state among SLE patients: case‐control study. Immunol Res 2017; 65: 257–261. [DOI] [PubMed] [Google Scholar]
- 4. Kuek A, Hazleman BL, Ostor AJ. Immune‐mediated inflammatory diseases (IMIDs) and biologic therapy: a medical revolution. Postgrad Med J 2007; 83: 251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shor DB, Dahan S, Comaneshter D, Cohen AD, Amital H. Does inflammatory bowel disease coexist with systemic lupus erythematosus? Autoimmun Rev 2016; 15: 1034–1037. [DOI] [PubMed] [Google Scholar]
- 6. Tiosano S, Farhi A, Watad A, Grysman N, Stryjer R, Amital H, et al Schizophrenia among patients with systemic lupus erythematosus: population‐based cross‐sectional study. Epidemiol Psychiatr Sci 2017; 26: 424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Twig G, Livneh A, Vivante A, Afek A, Shamiss A, Derazne E, et al Mortality risk factors associated with familial Mediterranean fever among a cohort of 1.25 million adolescents. Ann Rheum Dis 2014; 73: 704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Keat A, Bennett AN, Gaffney K, Marzo‐Ortega H, Sengupta R, Everiss T. Should axial spondyloarthritis without radiographic changes be treated with anti‐TNF agents? Rheumatol Int 2017; 37: 327–336. [DOI] [PubMed] [Google Scholar]
- 9. Martelli L, Olivera P, Roblin X, Attar A, Peyrin‐Biroulet L. Cost‐effectiveness of drug monitoring of anti‐TNF therapy in inflammatory bowel disease and rheumatoid arthritis: a systematic review. J Gastroenterol 2017; 52: 19–25. [DOI] [PubMed] [Google Scholar]
- 10. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011; 365: 2205–2219. [DOI] [PubMed] [Google Scholar]
- 11. Yamauchi PS, Bissonnette R, Teixeira HD, Valdecantos WC. Systematic review of efficacy of anti‐tumor necrosis factor (TNF) therapy in patients with psoriasis previously treated with a different anti‐TNF agent. J Am Acad Dermatol 2016; 75: 612–618. [DOI] [PubMed] [Google Scholar]
- 12. Atzeni F, Sarzi‐Puttini P, Botsios C, Carletto A, Cipriani P, Favalli EG, et al Long‐term anti‐TNF therapy and the risk of serious infections in a cohort of patients with rheumatoid arthritis: comparison of adalimumab, etanercept and infliximab in the GISEA registry. Autoimmun Rev 2012; 12: 225–229. [DOI] [PubMed] [Google Scholar]
- 13. Ben‐Horin S, Kopylov U, Chowers Y. Optimizing anti‐TNF treatments in inflammatory bowel disease. Autoimmun Rev 2014; 13: 24–30. [DOI] [PubMed] [Google Scholar]
- 14. Feldmann M, Maini SR. Role of cytokines in rheumatoid arthritis: an education in pathophysiology and therapeutics. Immunol Rev 2008; 223: 7–19. [DOI] [PubMed] [Google Scholar]
- 15. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005; 353: 487–497. [DOI] [PubMed] [Google Scholar]
- 16. Zandman‐Goddard G, Amital H, Shamrayevsky N, Raz R, Shalev V, Chodick G. Rates of adherence and persistence with allopurinol therapy among gout patients in Israel. Rheumatology (Oxford) 2013; 52: 1126–1131. [DOI] [PubMed] [Google Scholar]
- 17. Mohr DC, Cox D, Epstein L, Boudewyn A. Teaching patients to self‐inject: pilot study of a treatment for injection anxiety and phobia in multiple sclerosis patients prescribed injectable medications. J Behav Ther Exp Psychiatry 2002; 33: 39–47. [DOI] [PubMed] [Google Scholar]
- 18. Ben‐Ami Shor D, Weitzman D, Dahan S, Gendelman O, Bar‐On Y, Amital D, et al Adherence and persistence with drug therapy among fibromyalgia patients: data from a large health maintenance organization. J Rheumatol 2017; 44: 1499–1506. [DOI] [PubMed] [Google Scholar]
- 19. Dusing R. Compliance in hypertension therapy. What works in ‘forgetfulness’? MMW Fortschr Med 2001; 143: 41–42. [PubMed] [Google Scholar]
- 20. Bluett J, Morgan C, Thurston L, Plant D, Hyrich KL, Morgan AW, et al Impact of inadequate adherence on response to subcutaneously administered anti‐tumour necrosis factor drugs: results from the Biologics in Rheumatoid Arthritis Genetics and Genomics Study Syndicate cohort. Rheumatology (Oxford) 2015; 54: 494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kane SV, Chao J, Mulani PM. Adherence to infliximab maintenance therapy and health care utilization and costs by Crohn's disease patients. Adv Ther 2009; 26: 936–946. [DOI] [PubMed] [Google Scholar]
- 22. Pasma A, van't Spijker A, Hazes JM, Busschbach JJ, Luime JJ. Factors associated with adherence to pharmaceutical treatment for rheumatoid arthritis patients: a systematic review. Semin Arthritis Rheum 2013; 43: 18–28. [DOI] [PubMed] [Google Scholar]
- 23. Lopez‐Gonzalez R, Leon L, Loza E, Redondo M, Garcia de Yebenes MJ, Carmona L. Adherence to biologic therapies and associated factors in rheumatoid arthritis, spondyloarthritis and psoriatic arthritis: a systematic literature review. Clin Exp Rheumatol 2015; 33: 559–569. [PubMed] [Google Scholar]
- 24. Chodick G, Heymann AD, Shalev V, Kookia E. The epidemiology of diabetes in a large Israeli HMO. Eur J Epidemiol 2003; 18: 1143–1146. [DOI] [PubMed] [Google Scholar]
- 25. Shalev V, Chodick G, Goren I, Silber H, Kokia E, Heymann AD. The use of an automated patient registry to manage and monitor cardiovascular conditions and related outcomes in a large health organization. Int J Cardiol 2011; 152: 345–349. [DOI] [PubMed] [Google Scholar]
- 26. Burck L, Tsibel N. Characterization and classification of geographical units by the socio‐economic level of the population. Central Bureau of Statistics: Jerusalem. [Special Publication], publication No. 1‐1‐2013.
- 27. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH, et al The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blum MA, Koo D, Doshi JA. Measurement and rates of persistence with and adherence to biologics for rheumatoid arthritis: a systematic review. Clin Ther 2011; 33: 901–913. [DOI] [PubMed] [Google Scholar]
- 29. Tkacz J, Ellis L, Bolge SC, Meyer R, Brady BL, Ruetsch C. Utilization and adherence patterns of subcutaneously administered anti‐tumor necrosis factor treatment among rheumatoid arthritis patients. Clin Ther 2014; 36: 737–747. [DOI] [PubMed] [Google Scholar]
- 30. Borah BJ, Huang X, Zarotsky V, Globe D. Trends in RA patients' adherence to subcutaneous anti‐TNF therapies and costs. Curr Med Res Opin 2009; 25: 1365–1377. [DOI] [PubMed] [Google Scholar]
- 31. Salt E, Rayens MK, Frazier SK. Predictors of perceived higher quality patient‐provider communication in patients with rheumatoid arthritis. J Am Assoc Nurse Pract 2014; 26: 681–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tang B, Rahman M, Waters HC, Callegari P. Treatment persistence with adalimumab, etanercept, or infliximab in combination with methotrexate and the effects on health care costs in patients with rheumatoid arthritis. Clin Ther 2008; 30: 1375–1384. [DOI] [PubMed] [Google Scholar]
- 33. Zhang J, Xie F, Delzell E, Yun H, Lewis JD, Haynes K, et al Impact of biologic agents with and without concomitant methotrexate and at reduced doses in older rheumatoid arthritis patients. Arthritis Care Res (Hoboken) 2015; 67: 624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, et al The PREMIER study: a multicenter, randomized, double‐blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 2006; 54: 26–37. [DOI] [PubMed] [Google Scholar]
- 35. Burmester GR, Rigby WF, van Vollenhoven RF, Kay J, Rubbert‐Roth A, Kelman A, et al Tocilizumab in early progressive rheumatoid arthritis: FUNCTION, a randomised controlled trial. Ann Rheum Dis 2016; 75: 1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Edwards JC, Szczepanski L, Szechinski J, Filipowicz‐Sosnowska A, Emery P, Close DR, et al Efficacy of B‐cell‐targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 2004; 350: 2572–2581. [DOI] [PubMed] [Google Scholar]
- 37. Klareskog L, van der Heijde D, de Jager JP, Gough A, Kalden J, Malaise M, et al Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double‐blind randomised controlled trial. Lancet 2004; 363: 675–681. [DOI] [PubMed] [Google Scholar]
- 38. Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, et al Infliximab (chimeric anti‐tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet 1999; 354: 1932–1939. [DOI] [PubMed] [Google Scholar]
- 39. Koncz T, Pentek M, Brodszky V, Ersek K, Orlewska E, Gulacsi L. Adherence to biologic DMARD therapies in rheumatoid arthritis. Expert Opin Biol Ther 2010; 10: 1367–1378. [DOI] [PubMed] [Google Scholar]
- 40. Glintborg B, Ostergaard M, Dreyer L, Krogh NS, Tarp U, Hansen MS, et al Treatment response, drug survival, and predictors thereof in 764 patients with psoriatic arthritis treated with anti‐tumor necrosis factor alpha therapy: results from the nationwide Danish DANBIO registry. Arthritis Rheum 2011; 63: 382–390. [DOI] [PubMed] [Google Scholar]
- 41. Du Pan SM, Dehler S, Ciurea A, Ziswiler HR, Gabay C, Finckh A. Comparison of drug retention rates and causes of drug discontinuation between anti‐tumor necrosis factor agents in rheumatoid arthritis. Arthritis Rheum 2009; 61: 560–568. [DOI] [PubMed] [Google Scholar]
- 42. Jani M, Barton A, Warren RB, Griffiths CE, Chinoy H. The role of DMARDs in reducing the immunogenicity of TNF inhibitors in chronic inflammatory diseases. Rheumatology (Oxford) 2014; 53: 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Oral medications that were taken concomitantly to therapy with adalimumab*
Table S2 Characteristics of patients who persisted with treatment and those who discontinued treatment (n = 1335)


