Abstract
Aim
The aim of this paper is to clarify the inconsistent findings in the association between antidepressant use and the risk of epithelial ovarian cancer (EOC).
Methods
This study is a meta‐analysis of observational studies retrieved from the PubMed, EMBASE, and Web of Science databases prior to August 15, 2017. Two researchers independently screened studies and extracted study characteristics and risk estimates. The odds ratios (OR) and 95% confidence intervals (CI) of EOC risk were summarized using an inverse variance weighted random‐effects model. Heterogeneity between studies was assessed with the I 2 statistic.
Results
Eight case–control studies involving 7878 EOC cases and 73 913 controls were identified. Compared with non‐use, use of antidepressants was not significantly associated with EOC risk (summarized OR = 1.10, 95% CI: 0.91–1.32, I 2 = 74.4%). Similar null results were also observed in the use of selective serotonin reuptake inhibitors (OR = 1.04, 95% CI = 0.80–1.35), tricyclic antidepressants (OR = 1.01, 95% CI = 0.79–1.30), and other antidepressant drugs (OR = 0.91, 95% CI = 0.74–1.12). Subgroup analyses of study characteristics, stratified by the type of control subjects, geographic location, exposure assessment, number of cases, and adjustment for potential confounders, showed that the ORs were broadly consistent across strata. The OR per 1 year‐increment of duration was 0.99 (95% CI = 0.94–1.05, I 2 = 40.0%, P = 0.154). Additionally, the OR for the greatest intensity of antidepressant use compared with never use was 0.82 (95% CI = 0.70–0.98, I 2 = 0%, P = 0.489). Furthermore, no evidence of publication bias was detected through Funnel plots as well as Egger's and Begg's tests.
Conclusions
There is no association between antidepressant use and EOC risk. Further prospective studies are warranted to confirm these findings.
Keywords: antidepressant, meta‐analysis, observational studies, ovarian cancer, systematic review
What is Already Known about this Subject
Antidepressants are widely prescribed to treat depression and anxiety disorders that may become chronic conditions among women.
Epidemiological studies have yielded inconsistent results on the correlation between antidepressant use and ovarian cancer risk.
What this Study Adds
Women who take antidepressants are not at an increased risk of ovarian cancer.
Since these findings were generated on the basis of case–control studies, further large‐scale prospective cohort studies are warranted.
Introduction
Epithelial ovarian cancer (EOC) is the second most frequently occurring female reproductive malignancy and causes more deaths than any other type of gynaecological cancer, accounting for approximately 240 000 new diagnoses and 150 000 deaths worldwide in 2012 1. Recent studies have suggested that hormones and reproductive status are the predominant risk and protective factors for this disease 2, 3, 4.
Antidepressants are widely prescribed to treat depression and anxiety disorders that may become chronic conditions among women 5. Of the more than 27 million people currently taking antidepressants in the United States, most are women 6, as they are twice as likely as men to be diagnosed with a major depressive disorder and up to three times more likely to be diagnosed with a dysthymic disorder 7.
Rodent experiments have shown that two distinct chemical classes of antidepressants, selective serotonin reuptake inhibitors (SSRIs) and tricyclics (TCAs), may have a growth‐promoting effect on various types of tumours, including mammary tumours, fibrosarcoma, and melanoma 8, 9, 10. Specially, these drugs may affect levels of dopamine or norepinephrine, leading to increased levels of gonadotropins and a subsequent greater risk of EOC 11. However, since some studies have found antineoplastic properties or no effects of SSRIs and TCAs on cancer growth 7, 12, 13, evidence from experimental studies remains controversial. However, the concerns raised by a number of simulated epidemiologic investigations have questioned the possible association between antidepressant use and various types of cancer 14, 15, 16, 17, 18, 19, including EOC 20. Similarly, the evidence from epidemiological studies has been inconsistent 5, 11, 20, 21, 22, 23, 24, 25.
A 2011 meta‐analysis by Cosgrove et al. 7 reported that there might be a modest increase in the risk of EOC with the use of antidepressants, especially SSRIs. However, these findings were based on a combination of studies of breast and ovarian cancers, which is not ideal given that such an association could differ between these two entities. In view of this meta‐analysis, several relevant studies with large sample sizes have been published 5, 21. For example, a recent case–control study conducted in Denmark involving 4103 EOC patients and 58 706 population‐based controls suggested that use of SSRIs was associated with a decreased risk of EOC 21. However, no recent meta‐analysis has comprehensively summarized the evidence of a dose–response relationship between the duration of antidepressant use and EOC risk. Therefore, considering these inconsistencies, the aim of the present updated meta‐analysis of observational studies was to evaluate the association between antidepressant use and the risk of EOC.
Methods
Data sources and search strategy
The present systematic review and meta‐analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses statement 26. We carried out a systematic search of the PubMed (https://www.ncbi.nlm.nih.gov/pubmed/), EMBASE (https://www.elsevier.com/solutions/embase‐biomedical‐research), and Web of Science (https://login.webofknowledge.com) databases (inception to August 15, 2017) for observational studies using the following combined key words and medical subject heading terms without language restriction: “antidepressants,” “serotonin reuptake inhibitors,” “SSRI,” “fluoxetine,” “paroxetine,” “citalopram,” “escitalopram,” “sertraline,” “fluvoxamine,” “mianserin,” “desipramine,” “clomipramine,” “lofepramine,” “nortriptyline,” “protriptyline,” “imipramine,” “tricyclics,” “MAO inhibitors,” “venlafaxine,” “amitriptyline,” “MAOI,” “isocarboxazid,” “moclobemide,” “phenelzine,” “tranylcypromine,” “selegiline,” “iproniazid,” “TCAs,” “ovarian,” “ovary,” “cancer,” “tumour,” “neoplasms,” and “carcinoma” 27, 28, 29. Furthermore, the references of the included studies and review articles were screened for relevant articles 30.
Study selection criteria
The included studies were limited to (1) observational studies that (2) defined the non‐exposed group as women who did not use any kind of antidepressant, and (3) provided adjusted risk estimates (e.g., odds ratio [OR], relative risk, or risk ratios with 95% confidence intervals [CIs]) or provided data allowing the calculation of the risk estimates and 95% CIs of the association between antidepressant use and EOC risk. Review articles, systematic reviews, meta‐analyses, commentaries, editorials and meeting abstracts, as well as those with other study designs (e.g., descriptive or ecological studies) were excluded from analysis. The duration of antidepressant use was defined as the day supply of the prescription 5. The dose of antidepressant use was defined as the daily dose of the prescription. The intensity of antidepressant use was defined as the daily dose divided by the duration of use in days 21. The selection and exclusion of studies were reviewed by two investigators (Y.‐L.H. and S.G.). Disagreements were resolved by consensus with a third author (J.‐M.Q.).
Data extraction and quality assessment
Two researchers (Y.‐L.H. and S.G.) independently retrieved the following general study information, where available, from all included studies: author, publication year, geographic location, study design, prevalence of antidepressant use, numbers of cases and controls, exposure category and measurement, outcome with risk estimates and 95% CIs, and adjusted/matched factors.
As all of the included articles were case–control studies, two researchers (Y.‐L.H. and S.G.) independently used the Newcastle–Ottawa quality assessment scale for case–control studies to assess the risk of bias 31, 32, 33, 34, 35. Subsequently, the studies were considered at a low risk of bias if they achieved a full rating in at least two categories of selection, comparability, or outcome assessment 36.
Statistical analysis
Since the absolute risk of EOC is low and all of the included articles were case–control studies, all results are reported as the OR for simplicity 37. For studies that separately reported results for the use of SSRIs, TCAs, and other drugs, but not in combination, inverse variance weighted fixed effects meta‐analysis was first used to generate an OR value of the overall study level of antidepressant use before random‐effects meta‐analysis 37. Heterogeneity among studies was quantified with the I 2 statistic 32, 38 and visually depicted using a Galbraith plot 39. An I 2 statistic of ≤50% was considered to indicate less heterogeneity among the included studies. For all analyses, overall summary estimates were calculated using inverse variance weighted random‐effects meta‐analysis. Individual OR estimates and summary estimates are displayed graphically with forest plots. Pre‐specified subgroup analyses were conducted according to type of control subjects (population‐based vs. hospital‐based), geographical location (US vs. non‐US), exposure measurement (database vs. questionnaire), median number of cases (≥550 vs. <550), and adjustment for potential confounders (age, reproductive factors, and use of other non‐antidepressant drugs). Furthermore, the study‐specific OR was summarized for each 1‐year‐increment for the duration of antidepressant use. A study‐specific trend reflecting correlated log OR values across different durations of drug use was computed using the generalized least‐squares trend estimation method developed by Greenland et al. 40 and Orsini et al. 41. Additionally, a potential nonlinear dose–response relationship between the duration of antidepressant use and EOC risk was modelled using restricted cubic splines with three knots at fixed percentiles (10%, 50% and 90%, respectively) of the distribution of exposure. An overall P‐value was calculated by showing that these two regression coefficients simultaneously equalled zero. The P‐value of nonlinearity was calculated by showing that the coefficient of the second spline equalled zero. The details of this method for calculating P‐values are described elsewhere 42, 43.
The following information was required and used to conduct the dose–response meta‐analysis 44, 45, 46, 47: (1) the distribution of cases and controls, and the risk and variance estimates for at least three quantitative exposure categories; (2) the median or mean level of these exposures in each category (if reported by ranges, mean levels were calculated by averaging the lower and upper boundaries; if the lowest category was open ended, the lowest boundary was considered to be zero; if the highest category was open ended, the open‐ended interval length was assumed to be the same as the adjacent interval). Finally, six studies met the specifications when using these techniques and were included in the dose–response analysis of duration of antidepressant use and EOC risk. Given that only two studies met the criteria of intensity of antidepressant use and EOC risk, dose–response analysis was not conducted.
The sequential exclusion strategy proposed by Patsopoulos et al. 48 was used to examine whether the overall estimates were influenced by the substantial heterogeneity observed. Studies that accounted for the largest share of heterogeneity were sequentially and cumulatively excluded until I 2 was <50%. We then examined whether the OR estimates were consistent 36. Evidence of publication bias was examined using funnel plots. Additionally, funnel plot asymmetry was further confirmed with the Egger's 49 and Begg's 50 tests (P ≤ 0.10). All statistical analyses were conducted using Stata 12.1 software (StataCorp LP, College Station, TX, USA).
Nomenclature of ligands
Key ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 51.
Results
Characteristics of the retrieved studies
Of a total of 202 studies that were reviewed, 189 (93.6%) were excluded after screening of the titles and abstracts. After full text review of the remaining 13 (6.4%) studies, eight published studies of 7878 EOC cases and 73 913 controls detailing the associations between antidepressant use and EOC risk were included for analysis (Figure 1).
Figure 1.
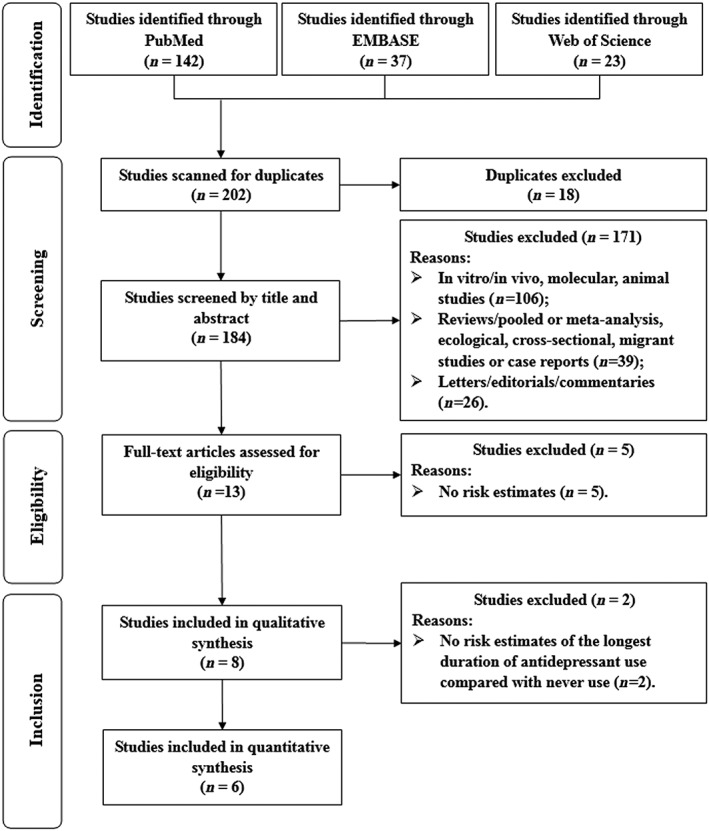
Selection of studies for inclusion in this meta‐analysis
Supplementary Table S1 summarizes the characteristics of the eight included studies 5, 11, 20, 21, 22, 23, 24, 25. Notably, each was published between 1984 and 2017 and all employed a case–control study design. Of these, six included population‐based controls 5, 11, 21, 22, 23, 25 and two had hospital‐based controls 20, 24. The number of cases in each study ranged from 150 20 to 4103 21, and the number of controls ranged from 250 20 to 58 706 21. Furthermore, five and three studies used questionnaires 11, 20, 22, 24, 25 and databases 5, 21, 23, respectively, to collect information regarding the duration of drug exposure. The majority of studies were conducted in North America (n = 5), Europe (n = 2), and Asia (n = 1), and were adjusted for potentially important confounders, such as age (n = 7), reproductive factors (n = 3), and use of other drugs (n = 3). On the basis of the Newcastle–Ottawa quality assessment scale, six studies 5, 11, 21, 22, 23, 25 were judged to be at a low risk of bias (Supplementary Table S2).
Ever antidepressant medication use and EOC risk
All eight included studies, involving 7878 EOC cases and 73 913 controls, examined ever antidepressant use and EOC risk. The pooled OR was 1.10 (95% CI = 0.91–1.32) (Figure 2). High heterogeneity was observed (I 2 = 74.4%, P < 0.001). The study by Coogan et al. was the farthest one from the fitted values, which contributed the most to the heterogeneity (Figure 3). Null results were also observed for the use of SSRIs (OR = 1.04, 95% CI = 0.80–1.35), TCAs (OR = 1.01, 95% CI = 0.79–1.30) and use of other non‐antidepressant drugs (OR = 0.91, 95% CI = 0.74–1.12) (Table 1 and Supplementary Figure S1). Subgroup analyses of the study characteristics, stratified by the type of control subjects, geographic location, exposure assessment, number of cases, and adjustment for potential confounders, showed that the OR of outcomes were broadly consistent across strata (Table 1). The significance of the meta‐regression analysis result for whether adjustment for age was observed might be attributed to a single study that only provided data for crude OR calculations 20. Funnel plots as well as Egger's and Begg's tests showed no evidence of publication bias (Supplementary Figure S2). When we sequentially excluded the studies that contributed the largest amount to heterogeneity until I 2 was less than 50%, the pooled ORs for outcomes (OR = 1.00, 95% CI = 0.86–1.16, I 2 = 48.4%) were similar to the original estimates. Of note, after excluding the study by Coogan et al. 24, the result was robust but with moderate heterogeneity. Additionally, sensitivity analysis of the studies retrieved from the databases with less information bias as well as those using population‐based controls was also conducted. Furthermore, the study by Tzonou et al. 20 was excluded because the authors failed to control for age in their primary analysis. Therefore, only three studies 5, 21, 23 met the inclusion criteria. The summarized OR was 0.93 (95% CI = 0.84–1.02), which was consistent with the main finding.
Figure 2.
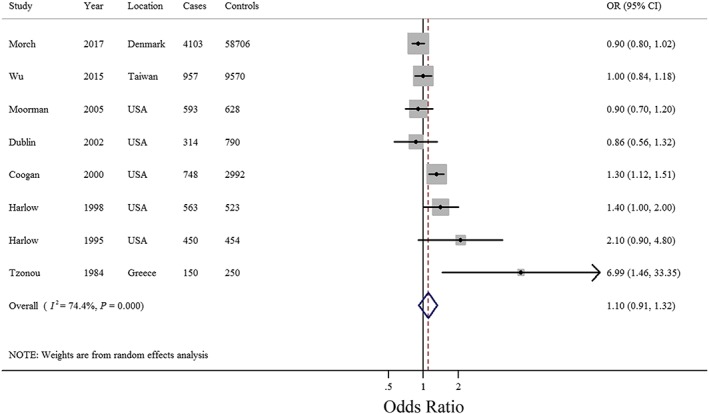
Forest plot (random‐effects model) of antidepressant use and epithelial ovarian cancer risk (ever vs. never use). The squares indicate study‐specific odds ratio (ORs) (size of the square reflects the study‐specific statistical weight), the horizontal lines indicate 95% confidence intervals (CIs), and the diamond indicates the summary OR estimate with its 95% CI. CI, confidence intervals; OR, odds ratio
Figure 3.
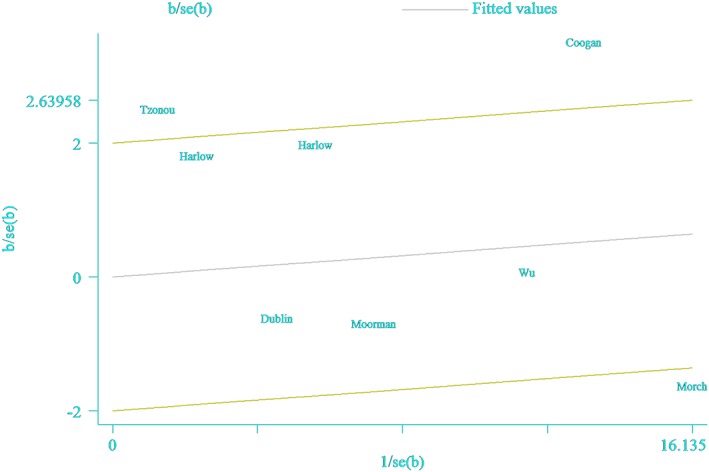
Galbraith plot corresponding to the relationship between antidepressant use and epithelial ovarian cancer risk (ever vs. never use)
Table 1.
Summary risk estimates of the association between antidepressant drug use and ovarian cancer risk (ever vs. never use)
| No. of studies | Summary OR 95% CI | I 2 (%) a | P h b | P h c | |
|---|---|---|---|---|---|
| Antidepressant drugs | 8 | 1.10 (0.91–1.32) | 74.4 | <0.001 | |
| SSRIs | 4 | 1.04 (0.80–1.35) | 62.9 | 0.04 | |
| TCAs | 4 | 1.01 (0.79–1.30) | 56.6 | 0.08 | |
| Others | 3 | 0.91 (0.74–1.12) | 0 | 0.56 | |
| Subgroup analyses of antidepressant drugs | |||||
| Type of control subjects | 0.17 | ||||
| Population based | 6 | 1.00 (0.86–1.16) | 48.4 | 0.08 | |
| Hospital based | 2 | 2.50 (0.50–12.46) | 77.3 | <0.001 | |
| Geographic location | 0.50 | ||||
| US | 5 | 1.16 (0.92–1.47) | 61.1 | 0.04 | |
| Non‐US | 3 | 1.00 (0.77–1.29) | 72.7 | <0.001 | |
| Exposure assessment | 0.13 | ||||
| Database | 3 | 0.93 (0.84–1.02) | 0 | 0.58 | |
| Questionnaire | 5 | 1.32 (0.98–1.78) | 68.0 | 0.01 | |
| Number of cases | 0.95 | ||||
| <550 | 4 | 1.10 (0.90–1.36) | 70.0 | 0.02 | |
| ≥550 | 4 | 1.27 (0.75–2.15) | 82.4 | <0.001 | |
| Risk of bias | 0.17 | ||||
| Low | 6 | 1.00 (0.86–1.16) | 48.4 | 0.08 | |
| High | 2 | 2.50 (0.50–12.46) | 77.3 | <0.001 | |
| Adjustment for potential confounders | |||||
| Age | 0.08 | ||||
| Yes | 7 | 1.07 (0.90–1.27) | 72.2 | <0.001 | |
| No | 1 | 6.99 (1.46–33.35) | N/A | N/A | |
| Reproductive factors | 0.78 | ||||
| Yes | 3 | 1.23 (0.79–1.90) | 77.8 | 0.01 | |
| No | 5 | 1.07 (0.85–1.36) | 72.9 | 0.01 | |
| Other non‐antidepressant drugs use | 0.78 | ||||
| Yes | 3 | 1.23 (0.79–1.90) | 77.8 | 0.01 | |
| No | 5 | 1.07 (0.85–1.36) | 72.9 | 0.01 | |
CI, confidence interval; N/A, not available; OR, odds ratio; SSRIs, selective serotonin reuptake inhibitors; TCAs, tricyclic antidepressants.
I 2 statistic was used to quantify the magnitude between‐study heterogeneity, and assigned values of 50% or less, 51–75%, and 76% or more for low, moderate and high heterogeneity, respectively.
P value for heterogeneity within each subgroup. A two‐tailed P < 0.1 was considered statistically significant.
P value for heterogeneity between subgroups with meta‐regression analysis. A two‐tailed P < 0.1 was considered statistically significant.
Duration of antidepressant use and EOC risk
Six studies 5, 21, 22, 23, 24, 25 involving 7165 EOC cases and 71 644 controls provided risk estimates for the longest duration of antidepressant use compared with never use. The pooled OR was 0.89 (95% CI = 0.66–1.19) with low heterogeneity (I 2 = 35.0%, P = 0.174) (Supplementary Figure S3). Additionally, of the five studies 5, 21, 22, 23, 25 included for dose–response analysis of duration, the summary OR per year was 0.99 (95% CI = 0.94–1.05) with low heterogeneity (I 2 = 40.0%) (Figure 4). There was no evidence of a nonlinear association between the parity number and gastric cancer risk (P for nonlinearity = 0.144).
Figure 4.
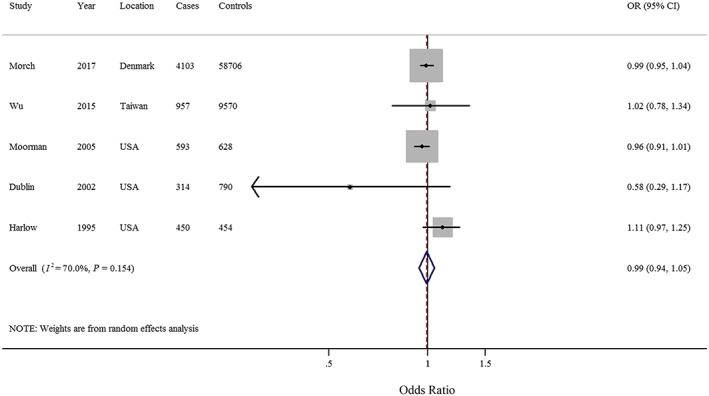
Forest plot (random‐effects model) of dose–response analysis of the duration (per 1‐year increment) of antidepressant use and epithelial ovarian cancer risk. The squares indicate study‐specific odds ratio (size of the square reflects the study‐specific statistical weight), the horizontal lines indicate 95% confidence intervals (CIs), and the diamond indicates the summary OR estimate with its 95% CI. CI, confidence intervals; OR, odds ratio
Intensity of antidepressant use and EOC risk
Two studies 21, 23 involving 4417 EOC cases and 59 496 controls examined the intensity of antidepressant use and EOC risk. The pooled OR was 0.79 (95% CI = 0.62–1.02) with low heterogeneity (I 2 = 17.4%, P = 0.271) (Supplementary Figure S4).
Discussion
The present updated meta‐analysis with a large sample size (involving 7878 EOC cases and 73 913 controls) failed to detect a significant association between antidepressant use and the risk of EOC. These findings were consistent among different antidepressants, including SSRIs and TCAs. Null results were observed throughout the subgroup analyses stratified by study characteristics.
Although we noticed no significant result of the subgroup analysis of exposure assessment, the point estimate was relatively different (0.93 for database vs. 1.32 for questionnaire) and no heterogeneity was observed in studies using prescription record databases. Compared with the use of a questionnaire, using computerized prescription data to collect information might have eliminated recall bias, minimized selection bias, and provided a longer study period 21. However, it must be recognized that pharmacy data also are imperfect and therefore insufficient to determine drug compliance 22. Similar situations were also observed in the subgroup analyses stratified by exposure assessment for SSRIs and TCAs (data not shown). However, we could not rule out the possibility of limited inclusion of these two kinds of drugs in the studies (n = 4). Furthermore, misclassification of exposure status might still occur. If incorrect classification from these sources occurred similarly in both the case and control groups, thus creating bias, the risk estimates would tend to be biased towards the null 5, 52. Consequently, the neutral association between antidepressant use and EOC risk might be partly attributed to these aforementioned issues. However, compared to misclassification of exposure status, reporting/information bias (cases recalling exposures of use of antidepressants because of their disease) as well as controls not trying to find an explanation for their disease may possibly overestimate the aforementioned association.
The prevalence of antidepressant users varied among countries and studies, which might have resulted in the slightly stronger point estimate after summarizing the studies from the United States only. For example, in a study conducted in the US, Dublin et al. 23 reported that approximately 23.3% of the control group used antidepressants. By comparison, Harlow et al. 25 reported that only 2.2% of 454 population‐based controls from the US used antidepressants, which was far less than that in a study conducted in Denmark by Morch et al. 21 who reported that 17.1% of 58 706 population‐based controls used antidepressants. A similar situation was also observed for use of SSRIs and TCAs.
Even though the exact biological mechanism underlying the association of antidepressant with EOC risk remains unclear, several potential hypotheses have been proposed. Several studies of rodents and goldfish have indicated that certain dopaminergic medications affect the release of gonadotropins and prolactin 24, 53, 54, 55, 56, which may play a role in ovarian carcinogenesis. Human studies have also shown that stimulation of dopaminergic receptors is involved in the release of luteinizing hormone, follicle‐stimulating hormone, and prolactin 11, 57, 58, 59. Increases in endogenous norepinephrine have been shown to induce gonadotropin secretion as well 54, 60. Fluoxetine, a type of SSRI, was demonstrated to induce apoptosis of ovarian carcinoma OVCAR‐3 cells 61. Additionally, other studies have shown that sertraline and paroxetine can inhibit HT29 cells and induce alterations in apoptosis‐related proteins, stimulating programmed death of tumour cells 62. Citalopram has also been suggested to control tumour growth by reducing the malignant cell cycle 63 and activating the immune system leading to apoptosis of tumour cells 12. On the other hand, the possibility that antidepressants may exhibit a biphasic effect, characterized by ‘low‐dose stimulation and high‐dose inhibition’ of neoplastic cell proliferation, has been suggested 5, 7. Some in vitro studies of rodents have indicated that antidepressant use can both promote and inhibit tumour growth 24, 64. Considering that the evidence from biological studies are both limited and speculative, future experimental studies are certainly warranted.
An important strength of the present study was the large sample size. As compared with a previous meta‐analysis conducted in 2011 7, the present study also searched the EMBASE database. Furthermore, we included two studies 5, 21 published in the past 5 years comprising 5060 EOC patients and 68 276 controls. Hence, the numbers of patients and controls (n = 2668 and 5387, respectively) were far greater than included in the 2011 meta‐analysis. Additionally, we included one study 20 that was missed in the previous meta‐analysis, though only crude OR data were provided. Notably, we investigated the aforementioned association in numerous subgroup analyses stratified by study characteristics.
Nonetheless, there were also some limitations to the present study that should be addressed. First, all of the included studies were case–control studies. As compared with prospective studies, case–control studies are more susceptible to bias (e.g., recall bias, selection bias) due to their nature. However, no recent prospective study has reported the association between antidepressant use and EOC risk according to our search strategy. Secondly, we could not rule out the influence of depression besides that of antidepressant therapy. Huang et al. 65 reported that a state of depression was associated with an increased risk of EOC on the basis of two large prospective cohort studies. Only one study had adjusted for comorbid medical and psychiatric illnesses in their primary analysis 5. Considering that severely depressed patients are more likely to be treated with antidepressants, they might just be a surrogate marker for the depression‐related incidence of EOC 5. Furthermore, since TCAs are used predominantly for manifest depression, whereas SSRIs are used for variety of other or ‘off‐label’ indications, we cannot exclude the possibility that the specific indications for use of SSRIs and TCAs may have differentially influenced the associations of EOC risk with SSRI and TCA use. However, a limited number of the included studies elaborated on this issue and presented primary analysis data restricted to women taking some kind of antidepressant.
Thirdly, previous studies found a tendency of a decreasing risk of EOC with increasing intensity of antidepressant use, which was defined as the daily dose divided by the duration of use in days, but not with increasing duration 21. However, as limited studies (n = 2) provided risk estimates of the intensity of antidepressant use 21, 23 and none of the included studies focused on the dose of antidepressant use, further studies are warranted to investigate this possible association as well as to confirm the findings of the present study. Furthermore, as a limited number of studies included in our analysis provided sufficient data for dose–response analysis, meta‐analysis of the intensity of antidepressant use and EOC was not conducted. Notably, only three studies 11, 21, 23 carried out subgroup analyses by histological type; therefore, future studies are needed.
Lastly, although the primary analyses of the included studies were adjusted for several known risk factors for EOC, we could not adjust for all potential confounders in this meta‐analysis nor rule out the possibility of residual confounding factors. For example, nulliparity and infertility may be associated with not only EOC, but also antidepressant use 11. However, only four studies 11, 21, 23, 25 considered these factors and just one 23 conducted subgroup analysis subdivided by parity. Therefore, future studies should investigate this association after careful and thorough adjustment for potential confounders.
In conclusion, the results of the present updated meta‐analysis involving the largest sample size to date and mostly included comprehensive observational studies showed no association between antidepressant use and the risk of EOC. Further prospective studies are warranted to confirm these findings.
Competing Interests
There are no competing interests to declare.
Contributors
Y.‐L.H. and S.G. designed the research, analyzed the data, and wrote the draft manuscript. All authors conducted the study search, read, reviewed and approved the final manuscript. S.G. had primary responsibility for final content.
Supporting information
Table S1 Characteristics of observational studies of antidepressant drug use and epithelial ovarian cancer risk
Table S2 Quality assessment of the included case–control studies using the Newcastle–Ottawa quality assessment scale
Figure S1 Test for publication bias of antidepressant use and epithelial ovarian cancer risk through Begg's funnel plot. OR, odds ratio; SE, standard error. The circles alone are real studies. The vertical lines represent the summary effect estimates, and the dashed lines represent pseudo‐95% confidence interval limits
Figure S2 Forest plot (random‐effects model) of antidepressant use and epithelial ovarian cancer risk by types of drug (ever vs. never use). The squares indicate study‐specific odd ratios (size of the square reflects the study‐specific statistical weight), the horizontal lines indicate 95% CIs, and the diamond indicates the summary OR estimate with its 95% CI. CI, confidence intervals; OR, odds ratio; SSRIs, selective serotonin reuptake inhibitors; TCAs, tricyclic antidepressants
Figure S3 Forest plot (random‐effects model) of antidepressant use and epithelial ovarian cancer risk (longest duration vs. never use). The squares indicate study‐specific ORs (size of the square reflects the study‐specific statistical weight), the horizontal lines indicate 95% CIs, and the diamond indicates the summary OR estimate with its 95% CI. CI, confidence intervals; OR, odds ratio
Figure S4 Forest plot (random‐effects model) of antidepressant use and epithelial ovarian cancer risk (strongest intensity vs. never use). The squares indicate study‐specific ORs (size of the square reflects the study‐specific statistical weight), the horizontal lines indicate 95% CIs, and the diamond indicates the summary OR estimate with its 95% CI. CI, confidence intervals; OR, odds ratio
Huo, Y.‐L. , Qiao, J.‐M. , and Gao, S. (2018) Association between antidepressant medication use and epithelial ovarian cancer risk: a systematic review and meta‐analysis of observational studies. Br J Clin Pharmacol, 84: 649–658. doi: 10.1111/bcp.13498.
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. Luan NN, Wu QJ, Gong TT, Vogtmann E, Wang YL, Lin B. Breastfeeding and ovarian cancer risk: a meta‐analysis of epidemiologic studies. Am J Clin Nutr 2013; 98: 1020–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gong TT, Wu QJ, Vogtmann E, Lin B, Wang YL. Age at menarche and risk of ovarian cancer: a meta‐analysis of epidemiological studies. Int J Cancer 2013; 132: 2894–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang HP, Trabert B, Murphy MA, Sherman ME, Sampson JN, Brinton LA, et al Ovarian cancer risk factors by histologic subtypes in the NIH‐AARP Diet and Health Study. Int J Cancer 2012; 131: 938–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu CS, Lu ML, Liao YT, Lee CT, Chen VC. Ovarian cancer and antidepressants. Psychooncology 2015; 24: 579–584. [DOI] [PubMed] [Google Scholar]
- 6. Olfson M, Marcus SC. National patterns in antidepressant medication treatment. Arch Gen Psychiatry 2009; 66: 848–856. [DOI] [PubMed] [Google Scholar]
- 7. Cosgrove L, Shi L, Creasey DE, Anaya‐McKivergan M, Myers JA, Huybrechts KF. Antidepressants and breast and ovarian cancer risk: a review of the literature and researchers' financial associations with industry. Plos One 2011; 6: e18210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brandes LJ, Arron RJ, Bogdanovic RP, Tong J, Zaborniak CL, Hogg GR, et al Stimulation of malignant growth in rodents by antidepressant drugs at clinically relevant doses. Cancer Res 1992; 52: 3796–3800. [PubMed] [Google Scholar]
- 9. Hilakivi‐Clarke L, Wright A, Lippman ME. DMBA‐induced mammary tumor growth in rats exhibiting increased or decreased ability to cope with stress due to early postnatal handling or antidepressant treatment. Physiol Behav 1993; 54: 229–236. [DOI] [PubMed] [Google Scholar]
- 10. Iishi H, Tatsuta M, Baba M, Taniguchi H. Enhancement by the tricyclic antidepressant, desipramine, of experimental carcinogenesis in rat colon induced by azoxymethane. Carcinogenesis 1993; 14: 1837–1840. [DOI] [PubMed] [Google Scholar]
- 11. Harlow BL, Cramer DW, Baron JA, Titus‐Ernstoff L, Greenberg ER. Psychotropic medication use and risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev 1998; 7: 697–702. [PubMed] [Google Scholar]
- 12. Frick LR, Rapanelli M. Antidepressants: influence on cancer and immunity? Life Sci 2013; 92: 525–532. [DOI] [PubMed] [Google Scholar]
- 13. Sternbach H. Are antidepressants carcinogenic? A review of preclinical and clinical studies. J Clin Psychiatry 2003; 64: 1153–1162. [PubMed] [Google Scholar]
- 14. Lee HC, Chiu WC, Wang TN, Liao YT, Chien IC, Lee Y, et al Antidepressants and colorectal cancer: a population‐based nested case–control study. J Affect Disord 2017; 207: 353–358. [DOI] [PubMed] [Google Scholar]
- 15. Lin CF, Chan HL, Hsieh YH, Liang HY, Chiu WC, Huang KY, et al Endometrial cancer and antidepressants: a nationwide population‐based study. Medicine (Baltimore) 2016; 95: e4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hsieh YH, Chiu WC, Lin CF, Chan HL, Liang HY, Lee Y, et al Antidepressants and gastric cancer: a nationwide population‐based nested case–control study. Plos One 2015; 10: e143668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chan HL, Hsieh YH, Lin CF, Liang HY, Huang KY, Chiu WC, et al Invasive cervical cancer and antidepressants: a nationwide population‐based study. Medicine (Baltimore) 2015; 94: e1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Friedman GD, Schwalbe J, Achacoso N, Meng MV, Kroenke CH, Habel LA. Antidepressants and testicular cancer. Cancer Causes Control 2014; 25: 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eom CS, Park SM, Cho KH. Use of antidepressants and the risk of breast cancer: a meta‐analysis. Breast Cancer Res Treat 2012; 136: 635–645. [DOI] [PubMed] [Google Scholar]
- 20. Tzonou A, Day NE, Trichopoulos D, Walker A, Saliaraki M, Papapostolou M, et al The epidemiology of ovarian cancer in Greece: a case–control study. Eur J Cancer Clin Oncol 1984; 20: 1045–1052. [DOI] [PubMed] [Google Scholar]
- 21. Morch LS, Dehlendorff C, Baandrup L, Friis S, Kjaer SK. Use of antidepressants and risk of epithelial ovarian cancer. Int J Cancer 2017; 141: 2197–2203. [DOI] [PubMed] [Google Scholar]
- 22. Moorman PG, Berchuck A, Calingaert B, Halabi S, Schildkraut JM. Antidepressant medication use [corrected] and risk of ovarian cancer. Obstet Gynecol 2005; 105: 725–730. [DOI] [PubMed] [Google Scholar]
- 23. Dublin S, Rossing MA, Heckbert SR, Goff BA, Weiss NS. Risk of epithelial ovarian cancer in relation to use of antidepressants, benzodiazepines, and other centrally acting medications. Cancer Causes Control 2002; 13: 35–45. [DOI] [PubMed] [Google Scholar]
- 24. Coogan PF, Rosenberg L, Palmer JR, Strom BL, Stolley PD, Zauber AG, et al Risk of ovarian cancer according to use of antidepressants, phenothiazines, and benzodiazepines (United States). Cancer Causes Control 2000; 11: 839–845. [DOI] [PubMed] [Google Scholar]
- 25. Harlow BL, Cramer DW. Self‐reported use of antidepressants or benzodiazepine tranquilizers and risk of epithelial ovarian cancer: evidence from two combined case–control studies (Massachusetts, United States). Cancer Causes Control 1995; 6: 130–134. [DOI] [PubMed] [Google Scholar]
- 26. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang TN, Gao SY, Shen ZQ, Li D, Liu CX, Lv HC, et al Use of selective serotonin‐reuptake inhibitors in the first trimester and risk of cardiovascular‐related malformations: a meta‐analysis of cohort studies. Sci Rep 2017; 7: 43085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shen ZQ, Gao SY, Li SX, Zhang TN, Liu CX, Lv HC, et al Sertraline use in the first trimester and risk of congenital anomalies: a systemic review and meta‐analysis of cohort studies. Br J Clin Pharmacol 2017; 83: 909–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gao SY, Wu QJ, Zhang TN, Shen ZQ, Liu CX, Xu X, et al Fluoxetine and congenital malformations: a systematic review and meta‐analysis of cohort studies. Br J Clin Pharmacol 2017; 83: 2134–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu QJ, Yang Y, Vogtmann E, Wang J, Han LH, Li HL, et al Cruciferous vegetables intake and the risk of colorectal cancer: a meta‐analysis of observational studies. Ann Oncol 2013; 24: 1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Available at http://www.ohri.ca/programs/clinical_epidemiological/oxford.asp (last accessed 21 November 2017).
- 32. Wu QJ, Wu L, Zheng LQ, Xu X, Ji C, Gong TT. Consumption of fruit and vegetables reduces risk of pancreatic cancer: evidence from epidemiological studies. Eur J Cancer Prev 2016; 25: 196–205. [DOI] [PubMed] [Google Scholar]
- 33. Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta‐analysis. BMJ 2016; 355: i5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ben Q, Sun Y, Chai R, Qian A, Xu B, Yuan Y. Dietary fiber intake reduces risk for colorectal adenoma: a meta‐analysis. Gastroenterology 2014; 146: 689–699. [DOI] [PubMed] [Google Scholar]
- 35. Aune D, Saugstad OD, Henriksen T, Tonstad S. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta‐analysis. JAMA 2014; 311: 1536–1546. [DOI] [PubMed] [Google Scholar]
- 36. Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta‐analysis. BMJ 2016; 354: i4482. [DOI] [PubMed] [Google Scholar]
- 37. Hou R, Wu QJ, Gong TT, Jiang L. Dietary fat and fatty acid intake and epithelial ovarian cancer risk: evidence from epidemiological studies. Oncotarget 2015; 6: 43099–43119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 39. Yao X, Tian Z. Saturated, monounsaturated and polyunsaturated fatty acids intake and risk of pancreatic cancer: evidence from observational studies. Plos One 2015; 10: e130870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose–response data, with applications to meta‐analysis. Am J Epidemiol 1992; 135: 1301–1309. [DOI] [PubMed] [Google Scholar]
- 41. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta‐analysis for linear and nonlinear dose–response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 2012; 175: 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bagnardi V, Zambon A, Quatto P, Corrao G. Flexible meta‐regression functions for modeling aggregate dose–response data, with an application to alcohol and mortality. Am J Epidemiol 2004; 159: 1077–1086. [DOI] [PubMed] [Google Scholar]
- 43. Royston P. A strategy for modelling the effect of a continuous covariate in medicine and epidemiology. Stat Med 2000; 19: 1831–1847. [DOI] [PubMed] [Google Scholar]
- 44. Liu Y, Zhang T, Wu Q, Jiao Y, Gong T, Ma X, et al Relationship between initiation time of adjuvant chemotherapy and survival in ovarian cancer patients: a dose–response meta‐analysis of cohort studies. Sci Rep 2017; 7: 9461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen J, Gong TT, Wu QJ. Parity and gastric cancer risk: a systematic review and dose–response meta‐analysis of prospective cohort studies. Sci Rep 2016; 6: 18766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gong TT, Li D, Wu QJ, Wang YZ. Cholesterol consumption and risk of endometrial cancer: a systematic review and dose–response meta‐analysis of observational studies. Oncotarget 2016; 7: 16996–17008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wu QJ, Gong TT, Wang YZ. Dietary fatty acids intake and endometrial cancer risk: a dose–response meta‐analysis of epidemiological studies. Oncotarget 2015; 6: 36081–36097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between‐study heterogeneity in meta‐analysis: proposed metrics and empirical evaluation. Int J Epidemiol 2008; 37: 1148–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 51. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH, et al The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 2016; 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Greenland S, Lash TL. Bias analysis In: Modern Epidemiology, 3rd edn, eds Rothman KJ, Greenland S, Lash TL. Philadelphia, PA: Lippincott Williams & Wilkins, 2008; 346–381. [Google Scholar]
- 53. Sloley BD, Kah O, Trudeau VL, Dulka JG, Peter RE. Amino acid neurotransmitters and dopamine in brain and pituitary of the goldfish: involvement in the regulation of gonadotropin secretion. J Neurochem 1992; 58: 2254–2262. [DOI] [PubMed] [Google Scholar]
- 54. Bedran‐de‐Castro JC, Petrovic SL, McCann SM. Involvement of beta‐adrenergic receptors in the differential release of gonadotropins in acutely orchidectomized rats. Braz J Med Biol Res 1990; 23: 1025–1027. [PubMed] [Google Scholar]
- 55. Kannisto P, Owman C, Walles B. Involvement of local adrenergic receptors in the process of ovulation in gonadotrophin‐primed immature rats. J Reprod Fertil 1985; 75: 357–362. [DOI] [PubMed] [Google Scholar]
- 56. Aguilar E, Ranchal A, Aguilar R, Pinilla L. Gonadotropin and prolactin secretion in prepubertal female rats treated with 8‐hydroxy‐2‐(di‐n‐propylamino) tetralin. J Neural Transm Gen Sect 1993; 94: 165–173. [DOI] [PubMed] [Google Scholar]
- 57. Boesgaard S, Hagen C, Hangaard J, Andersen AN, Eldrup E. Pulsatile gonadotropin secretion and basal prolactin levels during dopamine D‐1 receptor stimulation in normal women. Fertil Steril 1991; 55: 281–286. [DOI] [PubMed] [Google Scholar]
- 58. Donnelly PJ, Dailey RA. Effects of dopamine, norepinephrine and serotonin on secretion of luteinizing hormone, follicle‐stimulating hormone and prolactin in ovariectomized, pituitary stalk‐transected ewes. Domest Anim Endocrinol 1991; 8: 87–98. [DOI] [PubMed] [Google Scholar]
- 59. Greenspan SL, Sparrow D, Rowe JW. Dopaminergic regulation of gonadotropin and thyrotropin hormone secretion is altered with age. Horm Res 1991; 36: 41–46. [DOI] [PubMed] [Google Scholar]
- 60. Chang JP, Van Goor F, Acharya S. Influences of norepinephrine, and adrenergic agonists and antagonists on gonadotropin secretion from dispersed pituitary cells of goldfish, Carassius auratus. Neuroendocrinology 1991; 54: 202–210. [DOI] [PubMed] [Google Scholar]
- 61. Lee CS, Kim YJ, Jang ER, Kim W, Myung SC. Fluoxetine induces apoptosis in ovarian carcinoma cell line OVCAR‐3 through reactive oxygen species‐dependent activation of nuclear factor‐kappaB. Basic Clin Pharmacol Toxicol 2010; 106: 446–453. [DOI] [PubMed] [Google Scholar]
- 62. Gil‐Ad I, Zolokov A, Lomnitski L, Taler M, Bar M, Luria D, et al Evaluation of the potential anti‐cancer activity of the antidepressant sertraline in human colon cancer cell lines and in colorectal cancer‐xenografted mice. Int J Oncol 2008; 33: 277–286. [PubMed] [Google Scholar]
- 63. Xia Z, Bergstrand A, DePierre JW, Nassberger L. The antidepressants imipramine, clomipramine, and citalopram induce apoptosis in human acute myeloid leukemia HL‐60 cells via caspase‐3 activation. J Biochem Mol Toxicol 1999; 13: 338–347. [DOI] [PubMed] [Google Scholar]
- 64. Steingart AB, Cotterchio M. Do antidepressants cause, promote, or inhibit cancers? J Clin Epidemiol 1995; 48: 1407–1412. [DOI] [PubMed] [Google Scholar]
- 65. Huang T, Poole EM, Okereke OI, Kubzansky LD, Eliassen AH, Sood AK, et al Depression and risk of epithelial ovarian cancer: results from two large prospective cohort studies. Gynecol Oncol 2015; 139: 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Characteristics of observational studies of antidepressant drug use and epithelial ovarian cancer risk
Table S2 Quality assessment of the included case–control studies using the Newcastle–Ottawa quality assessment scale
Figure S1 Test for publication bias of antidepressant use and epithelial ovarian cancer risk through Begg's funnel plot. OR, odds ratio; SE, standard error. The circles alone are real studies. The vertical lines represent the summary effect estimates, and the dashed lines represent pseudo‐95% confidence interval limits
Figure S2 Forest plot (random‐effects model) of antidepressant use and epithelial ovarian cancer risk by types of drug (ever vs. never use). The squares indicate study‐specific odd ratios (size of the square reflects the study‐specific statistical weight), the horizontal lines indicate 95% CIs, and the diamond indicates the summary OR estimate with its 95% CI. CI, confidence intervals; OR, odds ratio; SSRIs, selective serotonin reuptake inhibitors; TCAs, tricyclic antidepressants
Figure S3 Forest plot (random‐effects model) of antidepressant use and epithelial ovarian cancer risk (longest duration vs. never use). The squares indicate study‐specific ORs (size of the square reflects the study‐specific statistical weight), the horizontal lines indicate 95% CIs, and the diamond indicates the summary OR estimate with its 95% CI. CI, confidence intervals; OR, odds ratio
Figure S4 Forest plot (random‐effects model) of antidepressant use and epithelial ovarian cancer risk (strongest intensity vs. never use). The squares indicate study‐specific ORs (size of the square reflects the study‐specific statistical weight), the horizontal lines indicate 95% CIs, and the diamond indicates the summary OR estimate with its 95% CI. CI, confidence intervals; OR, odds ratio


