Abstract
Objectives
To explore effects of disease prevalence adjustment on ambulatory care–sensitive hospitalization (ACSH) rates used for quality comparisons.
Data Sources/Study Setting
County‐level hospital administrative data on adults discharged from German hospitals in 2011 and prevalence estimates based on administrative ambulatory diagnosis data were used.
Study Design
A retrospective cross‐sectional study using in‐ and outpatient secondary data was performed.
Data Collection
Hospitalization data for hypertension, diabetes, heart failure, chronic obstructive pulmonary disease, and asthma were obtained from the German Diagnosis Related Groups (DRG) database. Prevalence estimates were obtained from the German Central Research Institute of Ambulatory Health Care.
Principal Findings
Crude hospitalization rates varied substantially across counties (coefficients of variation [CV] 28–37 percent across conditions); this variation was reduced by prevalence adjustment (CV 21–28 percent). Prevalence explained 40–50 percent of the observed variation (r = 0.65–0.70) in ACSH rates for all conditions except asthma (r = 0.07). Between 30 percent and 38 percent of areas moved into or outside condition‐specific control limits with prevalence adjustment.
Conclusions
Unadjusted ACSH rates should be used with caution for high‐stakes public reporting as differences in prevalence may have a marked impact. Prevalence adjustment should be considered in models analyzing ACSH.
Keywords: Ambulatory care–sensitive conditions, quality of care, small area analysis
Quality is a key factor to effective health care systems (Institute of Medicine 2001) and directly leads to an essential need for valid quality indicators. Development and implementation of appropriate quality indicators is not trivial and faces challenges worldwide (Lester, Hannon, and Campbell 2011; Rushforth et al. 2015; Saust et al. 2016). While in the German health care system a structured set of quality indicators is mandatory for all German hospitals, the use of indicators for outpatient care shows potential for improvement (Albrecht, Loos, and Otten 2013; de Cruppé et al. 2015; Hermes‐Moll et al. 2015).
In the last years, ambulatory care–sensitive hospitalizations (ACSHs) were brought into the debate as indicators for the assessment of ambulatory care in Germany (Freund, Heller, and Szecsenyi 2014; Sundmacher et al. 2015). Hospitalizations for the so‐called ambulatory care–sensitive conditions were first described by Weissmann, Gatsonis, and Epstein (1992) and are considered as potentially preventable through timely and effective ambulatory care (Billings et al. 1993). For example, appropriate disease management in chronic conditions like heart failure and hypertension, and rapid access to urgent care for acute conditions like urinary tract infections, may obviate the need for inpatient care.
The Agency for Healthcare Research and Quality in the United States (AHRQ) and the Organization for Economic Cooperation and Development (OECD) use population‐based rates of ACSH to evaluate ambulatory care performance in various health care systems (Agency for Healthcare Research and Quality 2007; Organization for Economic Cooperation and Development 2013). In Germany, the national Advisory Council on the Assessment of Developments in the Healthcare System recommends ACSH reporting on a small area scale. This recommendation includes population‐based outpatient care quality measurement to supplement indicators on hospital care. To maximize international comparability, the Council suggests use of existing OECD indicators (Advisory Council on the Assessment of Developments in the Healthcare System 2012).
Interpretation of these rates is complex and context dependent. Measurement of ACSH has been most often used for the evaluation of access to ambulatory care, especially in the United States (Bindman et al. 1995; Laditka, Laditka, and Probst 2009; Rosano et al. 2012; Saver et al. 2013). But ACSHs are also often considered as indicators of ambulatory care quality (Busby, Purdy, and Hollingworth 2015), especially in health care systems with universal access to primary care and full cost coverage like Germany (Purdy et al. 2009). Several aspects of quality have been linked to ACSH, for example, continuity of care, skill mix in practices (van Loenen et al. 2016), practice style (Orueta et al. 2015), intensity of monitoring (Freund et al. 2013), provision of specialist services for disease management (Saxena et al. 2006), or financial incentives to improve quality of care (Lippi Bruni, Nobilio, and Ugolini 2009). However, as ACSHs are not driven by a single factor, spatial variation in ACSH may also indicate inadequate structures or access barriers in settings with universal access to care (Ansari, Laditka, and Laditka 2006).
When analyzing variation in ACSH, it is important to consider such factors as socioeconomic status and disease prevalence (Ansari 2007; Faisst and Sundmacher 2014). Some authors have shown that disease prevalence may bias population‐based rates of ACSH in that more cases of a disease might lead to more hospitalizations related to that disease, independent of other predictors (Bindman et al. 1995; Laditka and Laditka 2004; Hossain and Laditka 2009), but influence of prevalence differs in dependence of the disease examined (Lui and Wallace 2011). It is significant that performance indicators should not be determined by factors that are not controlled by those whose performance is being measured (Giuffrida, Gravelle, and Roland 1999). This leads to a need for risk adjustment of ACSH before using them as comparative quality indicators.
Few studies have focused on the distribution and predictors of ACSH in Europe, and to our knowledge, no one has directly examined the influence of corresponding disease prevalence on ACSH rates (Burgdorf and Sundmacher 2014; Eggli et al. 2014; Freund, Heller, and Szecsenyi 2014; Thygesen et al. 2015). Our analysis therefore contributes to the existing literature in two ways: First, we use an easily applicable method for disease prevalence adjustment of chronic ambulatory care sensitive conditions and examine the variation in crude and adjusted rates with regard to random and systematic components of variation. Second, we show the effects of disease prevalence adjustment on the spatial distribution of ACSH rates in a Bismarck‐type health care system with free access to care for the first time.
Methods
Data Sources
Our study was an ecological study at the county level (n = 402 German counties and cities with county rights) using data from 2011. The study dataset for ACSH was developed from the German DRG (Diagnosis Related Groups) database, which includes the entire hospital discharge data from all 1,736 German hospitals with 17.38 million discharges (Statistisches Bundesamt 2013). This dataset is stored in the Federal Statistical Office and was accessed through remote execution. In our study, we included all hospitalizations among persons older than 14 years of age that met the indicator definitions as stated below. We excluded cases transferred directly from another hospital to avoid double counting of the same inpatient episode in different hospitals. Population data for calculation of rates were also obtained from the Federal Statistical Office. For disease prevalence adjustment, the Central Research Institute of Ambulatory Health Care in Germany (Zi) provided small area estimates based on nationwide ambulatory diagnosis data from 2011. Disease coding is mandatory in German ambulatory care. The Zi calculated the number of patients with ambulatory visits for the relevant diagnosis (numerator) in relation to all patients with ambulatory visits (denominator) in 2011. To make sure the diagnosis is chronic, the Zi only counted those patients in the numerator who went to ambulatory visits with the relevant diagnosis in at least two quarters of the evaluation period.
Indicators for Ambulatory Care–Sensitive Hospitalizations
The Primary Care Indicators are a subset of the OECD Health Care Quality Indicators and include rates for ACSH (Marshall, Leatherman, and Mattke 2004). Those OECD indicators are based on specifications developed by the US Agency for Healthcare Research and Quality (AHRQ) (Agency for Healthcare Research and Quality 2007). Indicator specifications from OECD and AHRQ differ in parts, for example, in age of population count. In our study, we used OECD definitions and included five indicators for ACSH: hospital admission rates for heart failure (HF), hypertension, chronic obstructive pulmonary disease (COPD), diabetes, and asthma. The complete indicator definitions are available online (Organization for Economic Co‐operation and Development 2015a). The numerator of all indicators relies on a count of all patients with hospital admissions because of a defined principal diagnosis in a county, while the population count of this region forms the denominator.
We adapted indicator definitions to the German Modification of the International Statistical Classification of Diseases and Related Health Problems 10th revision (ICD 10 GM) and counted all cases that met the inclusion criteria on county level. For data privacy reasons, the Federal Statistical Office blinded sums less than three. However, the total number of all anonymized cases was still available. We divided the total number of anonymized cases by the number of counties which were affected by blinding. The result was the average number of cases in blinded counties, which we used as estimation for all blinded counties. Finally, we estimated crude hospitalization rates (not sex‐specific) for all German counties using population data in the denominator. We neither performed age nor sex standardization of the ACSH rates because prevalence data are not stratified by age or sex.
Disease Prevalence Adjustment
For disease prevalence adjustment, a method from the OECD was adopted, which was originally developed for the adjustment of Patient Safety Indicator rates by the number of secondary diagnoses (Drösler et al. 2012). According to this method, ordinary least‐squares unweighted regression models were estimated. The outcome variables in these models were the county‐level crude rates of ACSH, while the associated disease prevalence estimates acted as predictor variables. These models led to estimated county‐specific residuals, which were linearly transformed into an adjusted rate with the mean value from the crude rates. This approach is based on the assumption that the prevalence of a disease is linearly related to the hospitalizations with this disease. We verified this assumption through visual inspection of scatter plots (not displayed).
To explore the complex relationship of prevalence and ACSH with socioeconomic factors, we determined Pearson product–moment correlation coefficients (Pearson's r) between prevalence, average household income per county, and hospitalization rates, both crude and adjusted. Additionally, we estimated crude ordinary least‐squares regression models to evaluate differences in influence of disease prevalence on ACSH rates in four quartile‐based income strata.
Funnel Plots
Spatial variation in area‐level hospitalization rates can be explained from two perspectives: variation due to chance and variation due to systematic influence of known or unknown factors. In statistical process control, variation by chance is known as “common cause variation,” while variation related to some extrinsic predictors is known as “special cause variation” (Mohammed et al. 2001). Funnel plots are one application of statistical process control that has been used in public health and health services research (Lack and Gerhardinger 2009; Dover and Schopflocher 2011; Morton et al. 2011). Starting with a target that determines the expected value of an indicator and an a priori defined probability distribution, control limits are calculated (Spiegelhalter 2005a). Those control limits can be interpreted as prediction intervals; indicator results within the control limits are consistent with common cause variation, whereas results beyond these limits differ significantly from the estimated distribution and are consistent with special cause variation. Therefore, funnel plots act as statistical tests for every county based on the null hypothesis that the indicators follow the specific probability distribution.
In our analysis, we constructed and compared funnel plots for the indicators to determine the number of counties with special cause variation before and after disease prevalence adjustment. Following Spiegelhalter's suggestion for cross‐sectional data, we used a county's population, which is the denominator of ACSH rates, as a measure of precision, and defined the target as proportion of the sum of hospitalizations to the sum of population per county, respectively. We also obtained control limits from the inverse Binomial distribution (Spiegelhalter 2005a>). A usual method for calculation of the distribution‐based limits is to use two‐sigma limits, which is approximately equivalent to a 95 percent confidence interval, but there might be concerns regarding multiple statistical testing (Benneyan, Lloyd, and Plsek 2003; Coory, Duckett, and Sketcher‐Baker 2007). To address these problems, we constructed a second, broader interval for each indicator based on a 95 percent confidence interval with Bonferroni correction (Abdi 2007). Accordingly, our control limits are roughly four‐sigma limits for 402 counties. Funnel plots were calculated in SAS 9.4 (SAS Institute Inc., Cary, NC, USA); geographical maps were plotted in RegioGraph 2011.
Results
Table 1 shows measures of central tendency and statistical dispersion of disease prevalence as well as crude and adjusted rates for chronic ACSH. The coefficient of variation was between 0.22 and 0.30 for all prevalence estimates and between 0.28 and 0.37 for all crude ACSH rates. Pearson's r between each crude ACSH rate and the corresponding disease prevalence at the county level were between 0.65 and 0.70, except asthma (r = 0.07). Regarding this Pearson's r, we omitted prevalence adjustment for asthma.
Table 1.
Characteristics of Disease Prevalence and Population Rates of Ambulatory Care–Sensitive Hospitalizations on County Level in Germany
| Number of Counties (n) | Median | Mean | Standard Deviation | Coefficient of Variation (%)a | Min | 25th Percentile | 75th Percentile | Max | |
|---|---|---|---|---|---|---|---|---|---|
| Disease prevalence (%) | |||||||||
| Hypertension | 402 | 23.8 | 25.2 | 5.5 | 22 | 13.5 | 21.7 | 27.3 | 39.7 |
| Diabetes | 402 | 8.9 | 9.6 | 2.3 | 24 | 5.7 | 8.0 | 10.4 | 16.8 |
| Heart failure | 402 | 2.7 | 2.9 | 0.9 | 30 | 1.4 | 2.3 | 3.4 | 5.8 |
| COPD | 402 | 4.4 | 4.4 | 1.0 | 23 | 1.9 | 3.6 | 5.0 | 7.8 |
| Asthma | 402 | 4.8 | 4.8 | 0.9 | 18 | 2.5 | 4.2 | 5.3 | 7.7 |
| Crude rates (per 100,000)b | |||||||||
| Hypertension | 402 | 311.0 | 320.7 | 119.1 | 37 | 83.0 | 232.1 | 391.1 | 774.2 |
| Diabetes | 402 | 250.2 | 276.7 | 98.4 | 36 | 103.9 | 204.7 | 336.5 | 656.1 |
| Heart failure | 402 | 501.9 | 524.7 | 149.0 | 28 | 226.0 | 410.4 | 619.2 | 1043.0 |
| COPD | 402 | 258.5 | 267.8 | 84.6 | 32 | 122.9 | 201.8 | 314.0 | 638.6 |
| Asthma | 402 | 19.4 | 20.3 | 8.8 | 43 | 1.4 | 14.5 | 23.9 | 53.5 |
| Adjusted rates (per 100,000)c | |||||||||
| Hypertension | 402 | 318.9 | 320.7 | 90.7 | 28 | 20.2 | 261.8 | 344.2 | 616.9 |
| Diabetes | 402 | 267.8 | 276.7 | 70.4 | 25 | 112.7 | 231.0 | 281.1 | 597.1 |
| Heart failure | 402 | 515.9 | 524.7 | 110.2 | 21 | 272.2 | 452.2 | 539.2 | 911.0 |
| COPD | 402 | 266.6 | 267.8 | 64.0 | 24 | 81.5 | 230.7 | 278.1 | 562.6 |
The coefficient of variation is a relative measure of dispersion of a frequency distribution. It is defined as the ratio of the standard deviation to the mean.
Pearson's r between ACSH rate and disease prevalence was 0.65 for hypertension, 0.70 for diabetes, 0.67 for heart failure, 0.65 for COPD, and 0.07 for asthma.
We did not perform disease prevalence adjustment for asthma because of a low Pearson product–moment correlation coefficient between ACSH and prevalence estimators.
Standard deviations were lower after disease prevalence adjustment for all indicators. Before adjustment, hypertension showed the highest coefficient of variation while heart failure showed the lowest. Disease prevalence adjustment did not alter this pattern, but all coefficients of variation were lower after adjustment (Table 1). Hypertension and COPD showed lower minimum rates after disease prevalence adjustment, whereas minima of diabetes and heart failure were higher. All maxima were lower after adjustment for disease prevalence.
We found significant negative correlation coefficients between disease prevalence and household income (Pearson's r from −0.57 to −0.35) and between crude ACSH rates and household income (−0.48 to −0.37). We found no significant correlation coefficients between adjusted ACSH rates and household income except for CHF (r = −0.25) (data not shown). Regression analyses for influence of prevalence on ACSH rates showed no significant differences between income strata for all indicators (data not shown).
Funnel Plots
The funnel plots of all indicators before and after disease prevalence adjustment can be found in Figure 1. The plots show the population of a county on the x‐axis and the crude or adjusted rate of ACSH on the y‐axis. Control intervals show limits of common cause variation, both before and after Bonferroni correction. For better illustration, we excluded counties with very high population counts of 1 million or more (Munich, Cologne, Hamburg, and Berlin) from the funnel plots, but they are retained in the tables and maps. For all indicators, the number of counties beyond control limits (in either direction) decreases through disease prevalence adjustment. The variation also decreases visibly, while the target, which is represented as a straight line in the funnel plots, slightly increases for all indicators.
Figure 1.
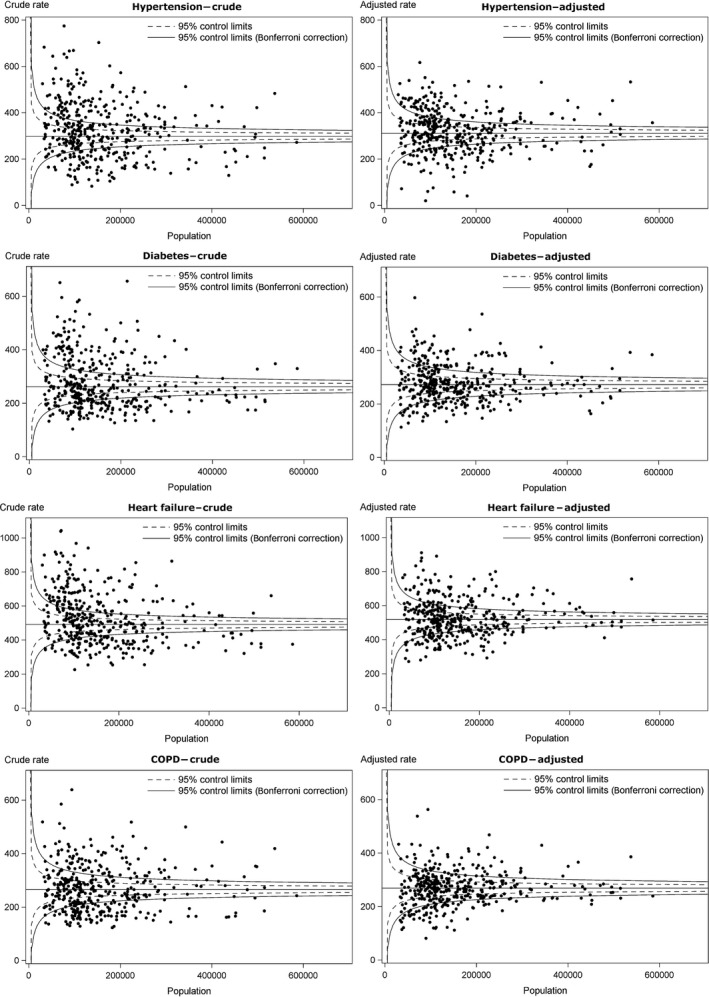
Funnel Plots for Crude and Disease Prevalence‐Adjusted Rates of Ambulatory Care–Sensitive Hospitalizations, on County Level 2011 (n = 402)
Exact numbers of counties beyond control limits can be obtained from Table 2. This table also shows how many counties changed their position in funnel plots, for example, from above control limits to below or between them. Counties which were above control limits and are now in between limits or below them, or counties which were in between limits and changed to below limits, are referred to as improved counties through disease prevalence adjustment. This applies vice versa for worsened counties. Regarding hypertension, 63 counties improved and 58 counties worsened in funnel plots (Table 2). Therefore, overall 121 counties were affected through adjustment. This is about 30 percent of all 402 German counties. Regarding diabetes (38 percent), heart failure (36 percent), and COPD (36 percent), these proportions are even higher.
Table 2.
Number of Counties in Funnel Plots Regarding Crude and Adjusted Rates of Ambulatory Care–Sensitive Hospitalizations
| Number of Counties below 95% Interval (Bonferroni Correction) | Number of Counties above 95% Interval (Bonferroni Correction) | Number of Improved Counties after Adjustmenta | Number of Worsened Counties after Adjustmentb | |
|---|---|---|---|---|
| Crude rates (per 100,000) | ||||
| Hypertension | 113 | 133 | ||
| Diabetes | 116 | 119 | ||
| Heart failure | 111 | 138 | ||
| COPD | 114 | 95 | ||
| Adjusted rates (per 100,000) | ||||
| Hypertension | 98 | 105 | 63 | 58 |
| Diabetes | 85 | 80 | 78 | 76 |
| Heart failure | 95 | 85 | 87 | 57 |
| COPD | 79 | 63 | 71 | 74 |
Counties that were above/in between control limits before adjustment and are in between/below control limits after adjustment.
Counties that were below/in between control limits before adjustment and are in between/above control limits after adjustment.
Maps
Figure 2 shows the spatial variation of counties beyond control limits before and after disease prevalence adjustment of hypertension and diabetes hospitalization rates. High crude rates of hypertension and diabetes admissions were observed in eastern states as well as in central Germany and parts of western Germany, whereas low crude rates were observed in southwestern Germany. After adjustment, relatively few eastern counties remained above the control limits, and relatively few southwestern counties remained below those limits.
Figure 2.
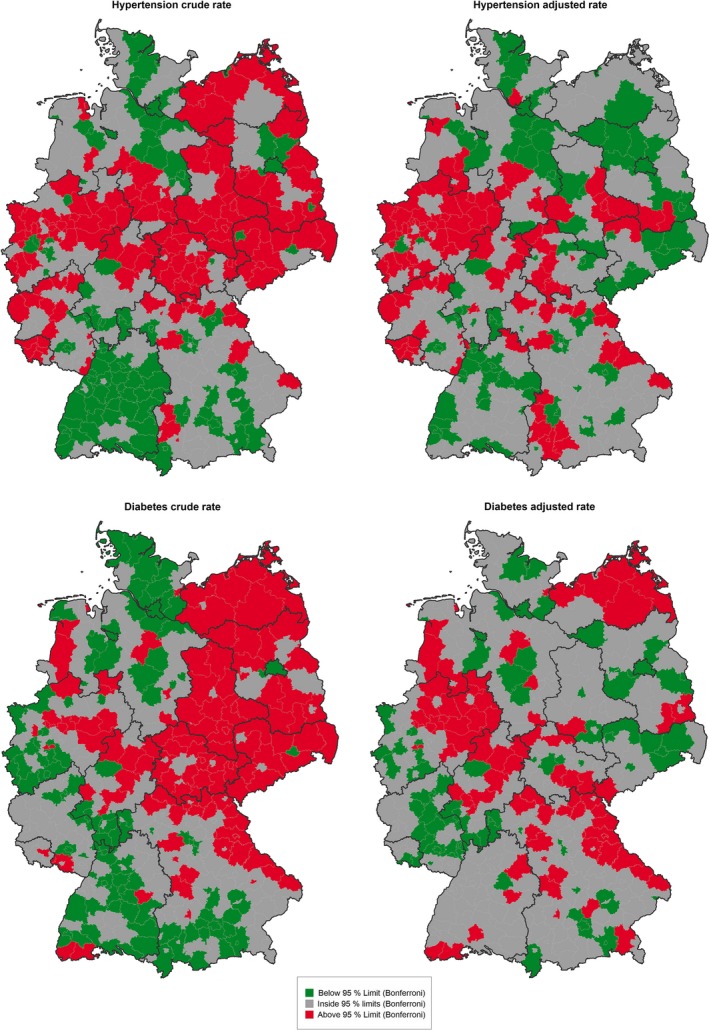
Maps of Counties with Special Cause Variation in Hospitalizations for Hypertension and Diabetes, Crude and Disease Prevalence‐Adjusted, 2011 [Color figure can be viewed at wileyonlinelibrary.com]
Figure 3 shows the effects of disease prevalence adjustment on HF and COPD hospitalization rates. Clusters of high HF rates are found mainly in eastern Germany. After adjustment, some of these counties fall between the control limits; however, this indicator still shows a heterogeneous pattern in large parts of Germany after adjustment. High hospitalization rates for COPD can be found predominantly in western Germany, while in the south a larger cluster of counties with low hospitalization rates can be seen. After disease prevalence adjustment, fewer counties in western Germany are above the control intervals and the majority of counties in the south lie between them.
Figure 3.
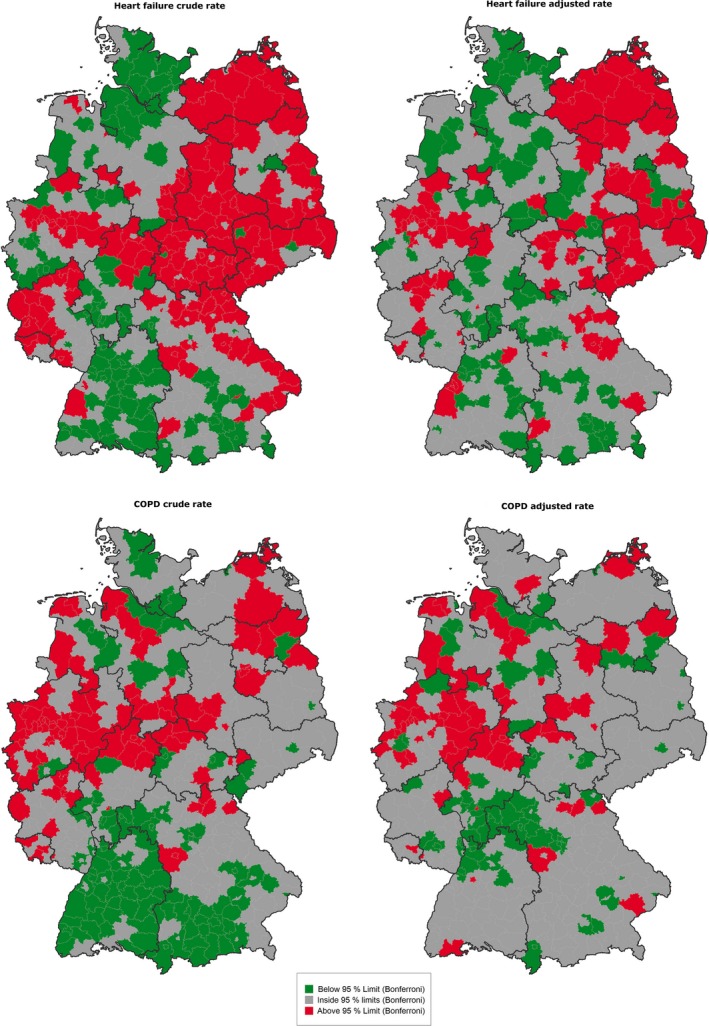
Maps of Counties with Special Cause Variation in Hospitalizations for Heart Failure (HF) and Chronic Obstructive Pulmonary Disease (COPD), Crude and Disease Prevalence‐Adjusted, 2011 [Color figure can be viewed at wileyonlinelibrary.com]
Discussion
We analyzed the effects of disease prevalence adjustment on hospitalization rates for four chronic ambulatory care–sensitive conditions across 402 cities and counties in Germany. For this purpose, we used an adjustment method that we adopted from the OECD. Crude ACSH rates were highly correlated (r = 0.65–0.70) with disease prevalence for all four of these conditions, but not for asthma (r = 0.07). Adjusting for disease prevalence reduced the coefficient of variation from 37 to 28 percent for hypertension, from 36 to 25 percent for diabetes, from 28 to 21 percent for HF, and from 32 to 24 percent for COPD. Funnel plots showed considerable variation in ACSH rates before and also after adjustment, which could not be explained by chance. However, prevalence adjustment reduced the number of counties both above and below the control limits, and changed the geographical pattern of ACSH rates across Germany. Specifically, the number of areas above the control limits dropped with adjustment from 133 to 105 for hypertension, from 119 to 80 for diabetes, from 138 to 85 for HF, and from 95 to 63 for COPD. The number of areas below the control limits dropped with adjustment from 113 to 98 for hypertension, from 116 to 85 for diabetes, from 111 to 95 for HF, and from 114 to 79 for COPD.
Our crude ACSH rates are supported by previous national studies. Burgdorf and Sundmacher showed the regional variation in crude HF hospitalization rates in 2008 and found high rates in many counties that we identified as affected by special cause variation (Burgdorf and Sundmacher 2014). Naumann and colleagues showed the distribution of age‐standardized hospitalization ratios for selected ambulatory care–sensitive conditions in 2006–2009 and found similar results to our crude ACSH rates, especially for diabetes (Naumann, Augustin, and Sundmacher 2014).
The impact of disease prevalence on ACSH rates has been examined in previous studies. Often proxy measures have been used for underlying disease prevalence, for example, death rates from the related disease (Laditka, Laditka, and Probst 2005), self‐reported presence of a disease (Ansari, Laditka, and Laditka 2006), or a mixed measure of demand for health services, consisting of adjustment by age, sex, severity of illness, and socioeconomic measures (Ansari et al. 2003). While some authors found prevalence to be an independent predictor of ACSH in multivariable analyses (Bindman et al. 1995), others failed to show statistically significant associations in multivariable analyses (Ansari, Laditka, and Laditka 2006), even when there were associations in bivariable analyses (Laditka, Laditka, and Probst 2005). Lui and Wallace showed substantial differences in spatial distribution of hospitalization for HF in California after adjustment for disease prevalence, which is consistent with our results. On the other hand, they observed only slight changes in the spatial distribution of hypertension (Lui and Wallace 2011). This inconsistency may reflect differences between the German and US health care system: age–sex‐standardized hypertension admission rates in 2011–2012 were over four times higher in Germany than in the United States (242.1 vs. 57.3 per 100,000 population), whereas age–sex‐standardized HF admission rates were only 45 percent higher in Germany (616.5 vs. 423.9 per 100,000 population)1. We assume that the impact of disease prevalence on ACSH rates may depend on further regional differences in between countries and health care systems. Although we found no evidence in stratified analyses that household income biased influence of prevalence on ACSH, socioeconomic factors in general may have an impact on both prevalence and hospitalization rates and should therefore be considered.
Some authors argue that the influence of disease prevalence and other patient characteristics should not be taken into consideration while measuring ACSH, because the accessibility and quality of ambulatory care may influence disease prevalence through prevention activities (Nedel et al. 2011). However, this argument rests in untenable assumptions about the ability of ambulatory care providers to modify the genetic, social, environmental, and behavioral determinants of disease, and to provide equally effective care as the prevalence of disease increases, despite resource constraints (Laditka and Laditka 2006). We promote a more conservative understanding, where differences in disease prevalence cannot be fully attributed to quality of care or access problems but also depend on external factors that are unapproachable through health care (Ansari, Laditka, and Laditka 2006).
Crude rates may find application for particular questions, for example, to evaluate the extent of a health problem in the absence of prevalence data (Giuffrida, Gravelle, and Roland 1999). Crude rates may also be prior to adjusted rates for special policy implications which aim to address a health problem in its entirety, that is, not just to improve outpatient care quality but also decrease the amount of disease prevalence itself and the part of hospitalizations, which cannot be prevented through better quality in ambulatory care. It should not be forgotten that the relation of ACSH, predictors of ACSH, and disease prevalence is complex. While ACSH are also affected by demographic, socioeconomic, environmental, lifestyle, and psychological factors (Ansari 2007), most of these factors may also influence disease prevalence.
Strategies to reduce ACSH should be based on all predictors of ACSH at both the system level and the individual level, as others have discussed nationally and internationally (Schreiber and Zielinski 1997; Freund et al. 2013; Faisst and Sundmacher 2014). Following Spiegelhalter's framework, common cause variation is inherent to the average care process and can be reduced by process improvement, whereas reducing special cause variation requires identification and elimination of systematic influence factors (Mohammed et al. 2001). Among these systematic factors, some are internal and others are external to the health care system (Faisst and Sundmacher 2014). Examples of internal factors related to the accessibility and quality of care are ambulatory care structures, but also inpatient care structures that may increase ACSH rates through supplier‐induced demand (Ansari 2007; Corallo et al. 2014). Disease prevalence may be viewed as an external factor, similar to socioeconomic characteristics, which should be considered through appropriate risk adjustment when using ACSH rates as performance indicators.
Limitations
There are some limitations in our study resulting from the data sources and methods we used. First, our data for disease prevalence and hospitalizations were estimated at the county level. The population varies substantially across German counties and cities with county rights from about 34,000 in the smallest county to over 3 million inhabitants in the German capital city of Berlin. Disease prevalence and hospitalizations might show different relationships in smaller‐scale areas. For example, Berlin consists of many districts with different populations regarding socioeconomic factors, cultural background, personal lifestyle, and many other characteristics that may affect hospital utilization. County‐level measures may mask such differences.
Our disease prevalence data came from the Central Research Institute of Ambulatory Health Care in Germany and are based on outpatient disease coding. We included all cases with outpatient visits in at least two quarters of the investigation period to make sure that the disease is chronic. As one visit per quarter is necessary for drug prescription in Germany, this criteria covers all patients with disease in more severe stages. But there may be an underestimation of prevalence in diseases like diabetes, where medication is not indispensable in less severe stages.
Compared to inpatient settings, where ICD‐coded data are closely reviewed for billing purposes, ICD code assignment in ambulatory care is not regularly monitored. The comparable claims‐based data system in the United States, Medicare's Chronic Condition Data Warehouse, has been reported to capture only 69 percent of beneficiaries with diabetes, 63 percent with heart disease, and 24 percent with COPD (Gorina and Kramarow 2011). However, several factors suggest that the German chronic condition data may be superior to its American equivalent. First, utilization of ambulatory care in Germany is very high. According to OECD statistics, the mean number of physician visits per person in 2013 was 9.9 in Germany versus 4.0 in the United States (Organization for Economic Co‐operation and Development 2015b). In 2012, a national survey of 19,294 participants found that about 87 percent of the German adult population had visited a primary care physician in the previous 12 months (Robert‐Koch‐Institut 2014). However, while there were no differences in the use of outpatient services between age groups for women, and between education levels or regions for both sexes, the authors found significant differences between women and men (91 percent vs. 84 percent) and between old and middle‐aged men (91 percent vs. 80 percent) (Robert‐Koch‐Institut 2014). This might indicate an underestimation of disease prevalence for men. For further validation, we compared our estimates with previously published prevalence data from other sources.
Our country‐level prevalence estimates are quite similar to recent patient‐reported, survey‐based estimates for hypertension (24.3 percent vs. 28.4, respectively) and diabetes (9.3 percent vs. 8.9 percent, respectively) (Robert‐Koch‐Institut 2014). The spatial distributions in this study were examined in seven German regions, which were originally developed for market research. More than 19,000 study participants were distributed almost equally on these regions (Robert‐Koch‐Institut 2014). We calculated ACSH rates for these regions and found very similar spatial distributions for hypertension and diabetes. This may show a high validity of our prevalence estimates in these seven regions, as the survey covers a representative sample of the adult German population, but disparities on a smaller scale may be masked. Published prevalence data for COPD in Germany vary from 1.3 to 13.2 percent (Aumann and Prenzler 2013); our overall prevalence estimate is within that range (4.3 percent). For HF, Ohlmeier and colleagues found a lower prevalence (1.8 percent) in 2006 than we did in 2011 (2.7 percent), which might be attributable to the different years and data sources (Ohlmeier et al. 2015). To our knowledge, there are no published data on the spatial distribution of COPD and HF prevalence in Germany.
Another limitation is that the German DRG database does not allow linkage of records at the patient level. Quality deficiencies in hospitals could have led to readmissions, which would have been indistinguishable from admissions in our analysis, but would not reflect on ambulatory care quality or access.
Even after prevalence adjustment, the conjunction between ACSH and quality in ambulatory care may not be clear. For example, Magán et al. (2011) found a positive association between ACSH and physician workload in crude analysis, but not after adjustment for socioeconomic variables, which may itself be related to prevalence. Others found that the provision of specialist clinics can lower mortality‐adjusted admission rates, but not in all ambulatory care–sensitive conditions (Saxena et al. 2006). More research is needed to evaluate the suitability of ACSH as quality indicator after prevalence adjustment.
Regarding funnel plots, Spiegelhalter described the problem of overdispersion, meaning that more counties fall outside control limits than would be expected from the defined probability distribution (Spiegelhalter 2005b). In our analysis, this overdispersion might be related to inadequate risk adjustment, which could lead to exaggeration of ambulatory care quality and access problems. Additional risk adjustment besides disease prevalence could be explored in future studies, but it was omitted in this analysis to avoid masking variation that could be attributable to the effects of disease prevalence (Flowers 2007).
Conclusion
The AHRQ Prevention Quality Indicators (PQIs) for chronic ACSH have been endorsed by the National Quality Forum in the United States2 and adapted for international comparisons in the OECD's Health Care Quality Indicators Program. Within the United States, they have been adopted for public comparisons across states by AHRQ's National Healthcare Quality Report,3 for public comparisons across counties by at least 15 states, and for monitoring state Medicaid programs and Accountable Care Organizations by the Centers for Medicare & Medicaid Services (CMS).4 The US Measure Applications Partnership is encouraging consideration of PQI composites5 for the physician Merit‐Based Incentive Payment System (MIPS) that was recently authorized by the Medicare Access & CHIP Reauthorization Act of 2015 (MACRA).6 These applications of ACSH indicators are somewhat controversial (Davies et al. 2011), largely due to concerns about the adequacy of risk adjustment using only age and sex, in the absence of information about the prevalence of comorbid conditions. We tested this concern using data from 402 German counties and cities with county rights, and we found that at least 40 percent of the variation in country‐level ACSH rates for diabetes, heart failure, hypertension, and COPD could be explained by the prevalence of the corresponding chronic condition (r = 0.65–0.70). However, less than 5 percent of the variation in county‐level ACSH rates for asthma could be explained by the prevalence of asthma, at least based on ambulatory visit data (r = 0.07). We also found clear spatial differences between crude rates and prevalence‐adjusted rates for the first four ACSH indicators in Germany. Unadjusted area‐level ACSH rates should therefore be interpreted with caution. Although our analysis was limited to Germany, the same confounding problem might apply to comparisons between countries, or between counties or states in the United States (or other large countries). ACSH should not be used for the evaluation of performance in ambulatory care without considering variation in disease prevalence. But even after adjustment, ACSH rates still vary more than can be explained by random processes. Additional research is needed to assess the impact of efforts to prevent ACSH as a means to reduce health care costs while improving patient outcomes.
Supporting information
Appendix SA1: Author Matrix.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: This research was supported by the Niederrhein University of Applied Sciences. The authors thank the Central Research Institute of Ambulatory Health Care in Germany for the provision of disease prevalence data on a small‐area scale.
Disclosures: None.
Disclaimers: None.
Notes
References
- Abdi, H . 2007. “Bonferroni and Sidak Corrections for Multiple Comparisons” In Encyclopedia of Measurement and Statistics, edited by Salkind N. J., pp. 103–7. Thousand Oaks, CA: SAGE. [Google Scholar]
- Advisory Council on the Assessment of Developments in the Healthcare System . 2012. “Competition at the Interfaces Between Inpatient and Outpatient Healthcare” [accessed September 20, 2016]. Available at http://www.svr-gesundheit.de/fileadmin/user_upload/Gutachten/2012/Kurzfassung-eng_formatiert.pdf
- Agency for Healthcare Research and Quality . 2007. “AHRQ Quality Indicators – Guide to Prevention Quality Indicators. Version 3.1” [accessed September 20, 2016]. Available at http://www.qualityindicators.ahrq.gov/Downloads/Modules/PQI/V31/pqi_guide_v31.pdf
- Albrecht, M. , Loos S., and Otten M.. 2013. “Cross‐Sectoral Quality Assurance in Ambulatory Care.” Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 107 (8): 528–33. [DOI] [PubMed] [Google Scholar]
- Ansari, Z. 2007. “The Concept and Usefulness of Ambulatory Care Sensitive Conditions as Indicators of Quality and Access to Primary Health Care.” Australian Journal of Primary Health 13 (3): 91–110. [Google Scholar]
- Ansari, Z. , Laditka J. N., and Laditka S. B.. 2006. “Access to Health Care and Hospitalization for Ambulatory Care Sensitive Conditions.” Medical Care Research and Review 63 (6): 719–41. [DOI] [PubMed] [Google Scholar]
- Ansari, Z. , Barbetti T., Carson N. J., Auckland M. J., and Cicuttini F.. 2003. “The Victorian Ambulatory Care Sensitive Conditions Study: Rural and Urban Perspectives.” Sozial‐Und Präventivmedizin/Social and Preventive Medicine 48 (1): 33–43. [DOI] [PubMed] [Google Scholar]
- Aumann, I. , and Prenzler A.. 2013. “Epidemiology and Costs of COPD in Germany – A Systematic Literature Search to Prevalence, Incidence and Health Care Costs.” Der Klinikarzt 42 (04): 168–72. [Google Scholar]
- Benneyan, J. C. , Lloyd R. C., and Plsek P. E.. 2003. “Statistical Process Control as a Tool for Research and Healthcare Improvement.” Quality and Safety in Health Care 12 (6): 458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings, J. , Zeitel L., Lukomnik J., Carey T. S., Blank A. E., and Newman L.. 1993. “Impact of Socioeconomic Status on Hospital use in New York City.” Health Affairs 12 (1): 162–73. [DOI] [PubMed] [Google Scholar]
- Bindman, A. B. , Grumbach K., Komaromy M., Vranizan K., Lurie N., Billings J., and Stewart A.. 1995. “Preventable Hospitalizations and Access to Health Care.” Journal of the American Medical Association 274 (4): 305. [PubMed] [Google Scholar]
- Burgdorf, F. , and Sundmacher L.. 2014. “Potentially Avoidable Hospital Admissions in Germany — An Analysis of Factors Influencing Rates of Ambulatory Care Sensitive Hospitalizations.” Deutsches Ärzteblatt International 111 (13): 215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busby, J. , Purdy S., and Hollingworth W.. 2015. “A Systematic Review of the Magnitude and Cause of Geographic Variation in Unplanned Hospital Admission Rates and Length of Stay for Ambulatory Care Sensitive Conditions.” BMC Health Services Research 15 (1): 689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coory, M. , Duckett S., and Sketcher‐Baker K.. 2007. “Using Control Charts to Monitor Quality of Hospital Care with Administrative Data.” International Journal for Quality in Health Care 20 (1): 31–9. [DOI] [PubMed] [Google Scholar]
- Corallo, A. N. , Croxford R., Goodman D. C., Bryan E. L., Srivastava D., and Stukel T. A.. 2014. “A Systematic Review of Medical Practice Variation in OECD Countries.” Health Policy 114 (1): 5–14. [DOI] [PubMed] [Google Scholar]
- de Cruppé, W. , Kleudgen S., Diel F., Burgdorf F., and Geraedts M.. 2015. “Feasibility of 48 Quality Indicators in Ambulatory Care in Germany: A Cross‐Sectional Observational Study.” Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 109 (9–10): 682–94. [DOI] [PubMed] [Google Scholar]
- Davies, S. , McDonald K. M., Schmidt E., Schultz E., Geppert J., and Romano P. S.. 2011. “Expanding the Uses of AHRQʼs Prevention Quality Indicators.” Medical Care 49 (8): 679–85. [DOI] [PubMed] [Google Scholar]
- Dover, D. C. , and Schopflocher D. P.. 2011. “Using Funnel Plots in Public Health Surveillance.” Population Health Metrics 9 (1): 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drösler, S. E. , Romano P. S., Tancredi D. J., and Klazinga N. S.. 2012. “International Comparability of Patient Safety Indicators in 15 OECD Member Countries: A Methodological Approach of Adjustment by Secondary Diagnoses.” Health Services Research 47 (1 pt 1): 275–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggli, Y. , Desquins B., Seker E., and Halfon P.. 2014. “Comparing Potentially Avoidable Hospitalization Rates Related to Ambulatory Care Sensitive Conditions in Switzerland: The Need to Refine the Definition of Health Conditions and to Adjust for Population Health Status.” BMC Health Services Research 14 (1): 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faisst, C. , and Sundmacher L.. 2014. “Ambulatory Care‐Sensitive Conditions: An International Overview with Conclusions for a German Catalogue.” Das Gesundheitswesen 77: 168–77. [DOI] [PubMed] [Google Scholar]
- Flowers, J. 2007. Technical Briefing 2: Statistical Process Control Methods in Public Health Intelligence. York: Association of Public Health Obervatories. [Google Scholar]
- Freund, T. , Heller G., and Szecsenyi J.. 2014. “Hospitalisations for Ambulatory Care Sensitive Conditions in Germany.” Zeitschrift für Evidenz, Fortbildung und Qualität im Gesundheitswesen 108 (5–6): 251–7. [DOI] [PubMed] [Google Scholar]
- Freund, T. , Campbell S. M., Geissler S., Kunz C. U., Mahler C., Peters‐Klimm F., and Szecsenyi J.. 2013. “Strategies for Reducing Potentially Avoidable Hospitalizations for Ambulatory Care‐Sensitive Conditions.” Annals of Family Medicine 11 (4): 363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrida, A. , Gravelle H., and Roland M.. 1999. “Measuring Quality of Care With Routine Data: Avoiding Confusion Between Performance Indicators and Health Outcomes.” British Medical Journal 319 (7202): 94–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorina, Y. , and Kramarow E. A.. 2011. “Identifying Chronic Conditions in Medicare Claims Data: Evaluating the Chronic Condition Data Warehouse Algorithm.” Health Services Research 46 (5): 1610–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes‐Moll, K. , Baumann W., Zimmermann A., Kleeberg U., Geraedts M., and Schmitz S.. 2015. “Feasibility Analysis of Quality Indicators for Measuring the Quality of Outpatient Oncology Care Using Data from Patient Records.” Gesundheitsökonomie & Qualitätsmanagement 20 (01): 36–42. [Google Scholar]
- Hossain, M. M. , and Laditka J. N.. 2009. “Using Hospitalization for Ambulatory Care Sensitive Conditions to Measure Access to Primary Health Care: An Application of Spatial Structural Equation Modeling.” International Journal of Health Geographics 8 (1): 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine . 2001. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press. [PubMed] [Google Scholar]
- Lack, N. , and Gerhardinger U.. 2009. “Comparison of Quality by Means of Funnel Plots — A Plea for a Uniform Methodology.” Zeitschrift für Evidenz, Fortbildung und Qualität im Gesundheitswesen 103 (8): 536–41. [DOI] [PubMed] [Google Scholar]
- Laditka, J. N. , and Laditka S. B.. 2004. “Insurance Status and Access to Primary Health Care.” Journal of Health & Social Policy 19 (2): 81–100. [DOI] [PubMed] [Google Scholar]
- Laditka, J. N. , and Laditka S. B.. 2006. “Race, Ethnicity and Hospitalization for Six Chronic Ambulatory Care Sensitive Conditions in the USA.” Ethnicity & Health 11 (3): 247–63. [DOI] [PubMed] [Google Scholar]
- Laditka, J. N. , Laditka S. B., and Probst J. C.. 2005. “More May Be Better: Evidence of a Negative Relationship Between Physician Supply and Hospitalization for Ambulatory Care Sensitive Conditions.” Health Services Research 40 (4): 1148–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laditka, J. N. , Laditka S. B., and Probst J. C.. 2009. “Health Care Access in Rural Areas: Evidence That Hospitalization for Ambulatory Care‐Sensitive Conditions in the United States may Increase with the Level of Rurality.” Health & Place 15 (3): 761–70. [DOI] [PubMed] [Google Scholar]
- Lester, H. E. , Hannon K. L., and Campbell S. M.. 2011. “Identifying Unintended Consequences of Quality Indicators: A Qualitative Study.” BMJ Quality & Safety 20 (12): 1057–61. [DOI] [PubMed] [Google Scholar]
- Lippi Bruni, M. , Nobilio L., and Ugolini C.. 2009. “Economic Incentives in General Practice: The Impact of Pay‐for‐Participation and Pay‐for‐Compliance Programs on Diabetes Care.” Health Policy (Amsterdam, Netherlands) 90(2–3): 140–8. [DOI] [PubMed] [Google Scholar]
- van Loenen, T. , Faber M. J., Westert G. P., and Van den Berg M. J.. 2016. “The Impact of Primary Care Organization on Avoidable Hospital Admissions for Diabetes in 23 Countries.” Scandinavian Journal of Primary Health Care 34 (1): 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui, C. K. , and Wallace S. P.. 2011. “A Common Denominator: Calculating Hospitalization Rates for Ambulatory Care‐Sensitive Conditions in California.” Preventing Chronic Disease 8 (5, A103): 1–14. [PMC free article] [PubMed] [Google Scholar]
- Magán, P. , Alberquilla Á., Otero Á., and Ribera J. M.. 2011. “Hospitalizations for Ambulatory Care Sensitive Conditions and Quality of Primary Care.” Medical Care 49 (1): 17–23. [DOI] [PubMed] [Google Scholar]
- Marshall, M. , Leatherman S., and Mattke S.. 2004. “Selecting Indicators for the Quality of Health Promotion, Prevention and Primary Care at the Health Systems Level in OECD Countries.: OECD Health Technical Papers No. 16” [accessed September 20, 2016]. Available at http://www.oecd.org/els/health-systems/33865865.pdf
- Mohammed, M. A. , Cheng K. K., Rouse A., and Marshall T.. 2001. “Bristol, Shipman, and Clinical Governance: Shewhart's Forgotten Lessons.” Lancet 357 (9254): 463–7. [DOI] [PubMed] [Google Scholar]
- Morton, A. , Mengersen K., Rajmokan M., Whitby M., Playford E. G., and Jones M.. 2011. “Funnel Plots and Risk‐Adjusted Count Data Adverse Events. A Limitation of Indirect Standardisation.” Journal of Hospital Infection 78 (4): 260–3. [DOI] [PubMed] [Google Scholar]
- Naumann, C. , Augustin U., and Sundmacher L.. 2014. “Ambulatory Care‐Sensitive Conditions in Germany: A Small Area Analysis (2006–2009).” Das Gesundheitswesen 77: e91–e105. [DOI] [PubMed] [Google Scholar]
- Nedel, F. B. , Facchini L. A., Bastos J. L., and Martin‐Mateo M.. 2011. “Conceptual and Methodological Aspects in the Study of Hospitalizations for Ambulatory Care Sensitive Conditions.” Ciência & Saúde Coletiva 16 (Suppl. 1): 1145–54. [DOI] [PubMed] [Google Scholar]
- Ohlmeier, C. , Mikolajczyk R., Frick J., Prütz F., Haverkamp W., and Garbe E.. 2015. “Incidence, Prevalence and 1‐Year All‐Cause Mortality of Heart Failure in Germany: A Study Based on Electronic Healthcare Data of More Than Six Million Persons.” Clinical Research in Cardiology 104 (8): 688–96. [DOI] [PubMed] [Google Scholar]
- Organization for Economic Co‐operation and Development . 2013. “Health at a Glance 2013 — OECD Indicators” [accessed September 20, 2016]. Available at http://www.oecd.org/els/health-systems/Health-at-a-Glance-2013.pdf
- Organization for Economic Co‐operation and Development . 2015a. “Definitions for Health Care Quality Indicators — 2014–2015 HCQI Data Collection” [accessed September 20, 2016]. Available at http://stats.oecd.org/wbos/fileview2.aspx?IDFile=62f94ae6-180c-4e4b-9a22-b030ddadfd35
- Organization for Economic Co‐operation and Development . 2015b. “Health at a Glance 2015 – OECD Indicators” [accessed September 20, 2016]. Available at http://www.oecd-ilibrary.org/docserver/download/8115071e.pdf?expires=1452097378%26id=id%26accname=guest%26checksum=CA0C758505DF9625C37EE7B380CB2841
- Orueta, J. F. , Garcia‐Alvarez A., Grandes G., and Nuno‐Solinis R.. 2015. “Variability in Potentially Preventable Hospitalisations: An Observational Study of Clinical Practice Patterns of General Practitioners and Care Outcomes in the Basque Country (Spain).” BMJ Open 5 (5): e007360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy, S. , Griffin T., Salisbury C., and Sharp D.. 2009. “Ambulatory Care Sensitive Conditions: Terminology and Disease Coding Need to Be More Specific to Aid Policy Makers and Clinicians.” Public Health 123 (2): 169–73. [DOI] [PubMed] [Google Scholar]
- Robert‐Koch‐Institut . 2014. Daten und Fakten: Ergebnisse der Studie “Gesundheit in Deutschland Aktuell 2012, pp. 135–137. Berlin: Robert‐Koch‐Institut. [Google Scholar]
- Rosano, A. , Loha C. A., Falvo R., van der Zee J., Ricciardi W., Guasticchi G., and de Belvis A. G.. 2012. “The Relationship between Avoidable Hospitalization and Accessibility to Primary Care: A Systematic Review.” European Journal of Public Health 23 (3): 356–60. [DOI] [PubMed] [Google Scholar]
- Rushforth, B. , Stokes T., Andrews E., Willis T. A., McEachan R., Faulkner S., and Foy R.. 2015. “Developing ‘High Impact’ Guideline‐Based Quality Indicators for UK Primary Care: A Multi‐Stage Consensus Process.” BMC Family Practice 16: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saust, L. T. , Monrad R. N., Hansen M. P., Arpi M., and Bjerrum L.. 2016. “Quality Assessment of Diagnosis and Antibiotic Treatment of Infectious Diseases in Primary Care: A Systematic Review of Quality Indicators.” Scandinavian Journal of Primary Health Care 34 (3): 258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saver, B. G. , Wang C.‐Y., Dobie S. A., Green P. K., and Baldwin L.‐M.. 2013. “The Central Role of Comorbidity in Predicting Ambulatory Care Sensitive Hospitalizations.” European Journal of Public Health 24 (1): 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena, S. , George J., Barber J., Fitzpatrick J., and Majeed A.. 2006. “Association of Population and Practice Factors with Potentially Avoidable Admission Rates for Chronic Diseases in London: Cross Sectional Analysis.” Journal of the Royal Society of Medicine 99 (2): 81–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber, S. , and Zielinski T.. 1997. “The Meaning of Ambulatory Care Sensitive Admissions: Urban and Rural Perspectives.” Journal of Rural Health 13 (4): 276–84. [DOI] [PubMed] [Google Scholar]
- Spiegelhalter, D. J. 2005a. “Funnel Plots for Comparing Institutional Performance.” Statistics in Medicine 24 (8): 1185–202. [DOI] [PubMed] [Google Scholar]
- Spiegelhalter, D. J. . 2005b. “Handling Over‐Dispersion of Performance Indicators.” Quality & Safety in Health Care 14 (5): 347–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistisches Bundesamt . 2013. “Grunddaten der Krankenhäuser 2011” [accessed September 20, 2016]. Available at https://www.destatis.de/DE/Publikationen/Thematisch/Gesundheit/Krankenhaeuser/GrunddatenKrankenhaeuser2120611117004.pdf?__blob=publicationFile
- Sundmacher, L. , Fischbach D., Schuettig W., Naumann C., Augustin U., and Faisst C.. 2015. “Which Hospitalisations Are Ambulatory Care‐Sensitive, to What Degree, and How Could the Rates Be Reduced? Results of a Group Consensus Study in Germany.” Health Policy 119 (11): 1415–23. [DOI] [PubMed] [Google Scholar]
- Thygesen, L. C. , Christiansen T., Garcia‐Armesto S., Angulo‐Pueyo E., Martinez‐Lizaga N., and Bernal‐Delgado E.. 2015. “Potentially Avoidable Hospitalizations in Five European Countries in 2009 and Time Trends from 2002 to 2009 Based on Administrative Data.” European Journal of Public Health 25 (suppl 1): 35–43. [DOI] [PubMed] [Google Scholar]
- Weissmann, J. S. , Gatsonis C., and Epstein A. M.. 1992. “Rates of Avoidable Hospitalization by Insurance Status in Massachusetts and Maryland.” Journal of the American Medical Association 286 (17): 2388–94. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.


