Abstract
Treatment for Angelman syndrome is currently limited to symptomatic interventions. A mouse model of Angelman syndrome has reduced calcium/calmodulin-dependent kinase II activity due to excessive phosphorylation of specific threonine residues, leading to diminished long-term potentiation. In a rat model of Parkinson disease, levodopa reduced phosphorylation of various proteins, including calcium/calmodulin-dependent kinase II. Further studies demonstrated that Angelman syndrome mice treated with levodopa performed better on rotarod testing than untreated Angelman syndrome mice. We conducted a multi-center double-blind randomized placebo-controlled one-year trial of levodopa / carbidopa with either 10 or 15 mg/kg/day of levodopa in children with Angelman syndrome. The outcome of this intervention was assessed using either the Bayley Scales of Infant Development or the Mullen Scales of Early Learning, as well as the Vineland Adaptive Behavior Scales, and the Aberrant Behavior Checklist. Of the 78 participants enrolled, 67 participants received study medication (33 on levodopa, 34 on placebo), and 55 participants (29 on levodopa, 26 on placebo) completed the 1-year study. There were no clinically or statistically significant changes in any of the outcome measures over a 1-year period comparing the levodopa and placebo groups. The number of adverse events reported, including the more serious adverse events, was similar in both groups, but none were related to treatment with levodopa. Our data demonstrate that levodopa is well-tolerated by children with Angelman syndrome. However, in the doses used in this study, it failed to improve their neurodevelopment or behavioral outcome.
Keywords: Clinical Trial, Developmental Disabilities, Inborn Genetic Diseases, Calcium-Calmodulin-Dependent Protein Kinase Type 2, Angelman syndrome, Levodopa, UBE3A, randomized controlled trial, rare disease
INTRODUCTION
Angelman syndrome (AS) is a neurodevelopmental disorder characterized by global developmental delay, intellectual disability, epilepsy, ataxia, and tremors. The disorder results from the loss of the maternally-inherited copy of UBE3A due to either a deletion of the AS critical region on the maternally-inherited copy of chromosome 15 (i.e., deletion-positive AS) or other molecular mechanisms. About 70% – 75% of individuals with AS have deletion-positive AS and the remaining have deletion-negative AS [Bird, 2014]. Previous studies have shown that children with AS who are deletion-positive tend to be more developmentally delayed and are more likely to have seizures than those who are deletion-negative [Gentile et al., 2010; Tan et al., 2011]. Treatment for AS is currently limited to developmental interventions and symptomatic treatment for complications including seizures, sleep disturbances, and hyperactivity [Tan and Bird, 2016]. A previous study showed that a mouse model of AS had diminished calcium/calmodulin-dependent kinase II (CaMKII) activity associated with increased phosphorylation at the threonine residues at positions 286, 305, and 306 (i.e., Thr286, Thr305, Thr306 respectively) of this enzyme [Weeber et al., 2003]. Mice that are homozygous for a mutation that prevents autophosphorylation at Thr286 have impaired hippocampal long-term potentiation (LTP), which suggests that autophosphorylation of CaMKII Thr286 is critical for synaptic plasticity [Giese et al., 1998]. On the other hand, autophosphorylation of Thr305 and Thr306 prevents further binding of the calcium/calmodulin complex to CaMKII and blocks the binding of CaMKII to its substrates [Blitzer et al., 2005; Colbran and Brown, 2004], so excessive phosphorylation at these residues impairs LTP, and hence learning [Elgersma et al., 2002]. A mouse model of AS genetically engineered to prevent autophosphorylation of Thr305 and Thr306 in CaMKII had normal CaMKII activity in the hippocampus, normal hippocampal-dependent learning, and normal spatial learning [van Woerden et al., 2007]. These findings suggest that the LTP and learning deficits in AS mice may be reversed by regulating the phosphorylation of CaMKII.
In a Parkinson disease rat model, phosphorylation of CaMKII Thr286 and a threonine residue (Thr75) in regulatory subunit 1B of protein phosphatase 1 was pathologically increased. Treatment with levodopa reversed the excess phosphorylation at these threonine residues [Brown et al., 2005]. These data suggest that levodopa can reverse or decrease the phosphorylation of at least some of the enzymes that are involved in synaptic function, although the mechanism(s) of action remains unknown.
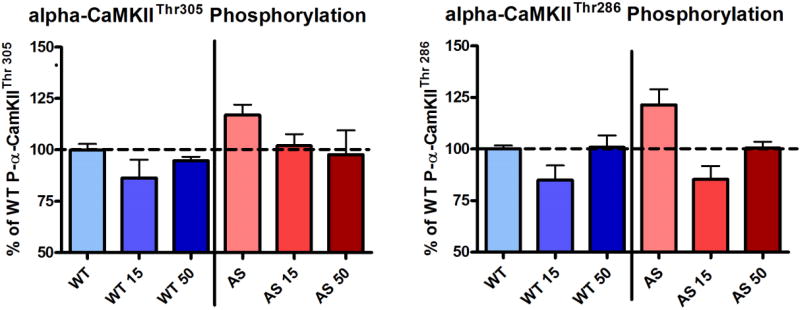
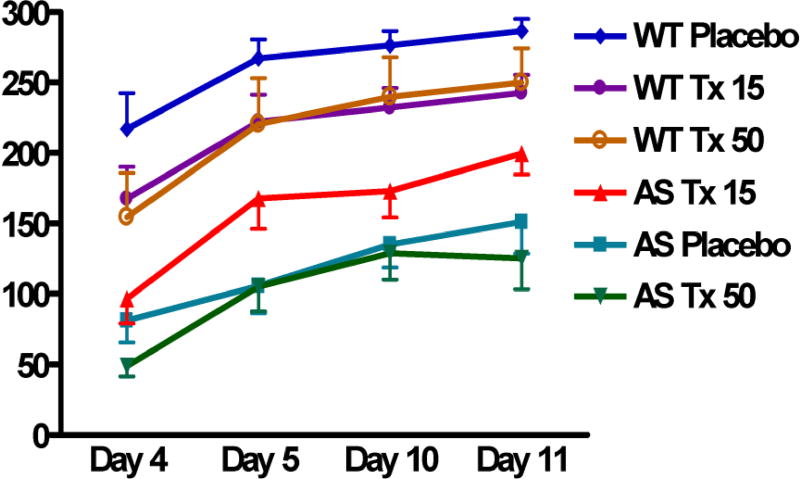
We (KFH, EJW) demonstrated that treatment of AS mice with levodopa (with carbidopa) resulted in normalization of phosphorylation levels at both Thr286 and Thr305 of CaMKII in hippocampal homogenates (Figure 1). When their motor learning abilities were assessed using the rotarod, AS mice that had been treated with 15 mg/kg/day of levodopa for at least four days performed better than untreated AS mice, but no improvement was seen in those treated with 50 mg/kg/day of levodopa (Figure 2).
Figure 1.
Western blot analysis on hippocampal homogenates from wild type (WT) and Angelman syndrome (AS) mice using phospho-specific antibodies against calcium/calmodulin-dependent kinase II (CaMKII) threonine residues at amino acid positions 286 and 305 (i.e., Thr286 and Thr305) showed that treatment with 15 mg/kg/day (WT 15, AS 15) and 50 mg/kg/day (WT 50, AS 50) of levodopa for seven days resulted in a reduction in the amount of phosphorylation at both Thr305 and Thr286 residues, and the effects were greater in the mice treated with low-dose than those with high-dose levodopa (n=5 in each group). [Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1552-4833.]
Figure 2.
Rotarod testing of wild type (WT) and Angelman syndrome (AS) mice 4, 5, 10, and 11 days after being treated with placebo (WT Placebo, AS Placebo), 15 mg/kg/day of levodopa (WT Tx 15, AS Tx 15), and 50 mg/kg/day of levodopa (WT Tx 50, AS Tx 50). On each day, each mouse underwent three trials (“attempts”) on a rotarod, accelerating from 5 to 40 rpm over a five minute period, with each trial separated by approximately 45 minutes. The duration (in seconds) that each mouse is able to remain on the rotarod, i.e., “latency” is on the Y axis (n=6 in each group). [Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1552-4833.]
There is one published report on the use of levodopa in two individuals with deletion-positive AS – a 23-year-old man with a 4-year history of episodic resting tremor, cogwheel rigidity, and bradykinesia, and a 43-year-old woman with a 25-year history of episodic tremor and cogwheel rigidity. The tremor and rigidity in both individuals resolved following treatment with 200 mg per day of levodopa in the man and 500 mg per day of levodopa in the woman [Harbord, 2001]. These observations led to our hypothesis that treatment with levodopa could improve neurodevelopment and tremors in children with AS.
MATERIALS AND METHODS
Determination of Levodopa Dosage for Clinical Trial
Notwithstanding the mouse data and the anecdotal reports described above, the oral dose of levodopa that would be optimal for use in this clinical trial was unclear. Previous studies have reported using levodopa to treat children with dopa-responsive dystonia and tetrahydrobiopterin deficiency at dosages ranging from 1 to 18 mg/kg/day [de Rijk-Van Andel et al., 2000; Mittal et al., 2001]. For the treatment of Parkinson disease, the levodopa dosage typically ranges from 100 to 800 mg per day, up to 2,000 mg per day, and therapeutic effects are typically observed when the plasma levodopa concentration is maintained between 1 and 3 µg/ml [LeWitt, 2015] (Personal communication, John Nutt, MD, Oregon Health & Science University). In a 70 kg adult, this would be equivalent to a levodopa dose ranging from 1.4 to 11 mg/kg/day, with a maximum dose of 29 mg/kg/day.
To determine an appropriate dose of levodopa for our double-blind placebo-controlled trial, we first conducted an open-label ‘dose-finding’ trial in children with AS between the ages of 4 and 12 years using the traditional 3+3 design [Storer, 1989] with levodopa dosages at 2 mg/kg per day, 5 mg/kg per day, 10 mg/kg/day, and 15 mg/kg/day (ClinicalTrials.gov Identifier: NCT00829439). None of the six participants who received less than 10 mg/kg/day of levodopa achieved a plasma concentration above 1 µg/ml. Three out of five of patients who received 10 mg/kg/day, and two out of four who received 15 mg/kg/day, achieved a plasma concentration between 1 µg/ml and 2.7 µg/ml. The remaining four participants on either 10 or 15 mg/kg/day of levodopa had plasma concentrations below 1 µg/ml. Following discussions with members of the Data and Safety Monitoring Board (DSMB), the present trial was initiated using a levodopa dose of 15 mg/kg/day. Because levodopa is decarboxylated to dopamine in peripheral tissues, it is usually administered concurrently with a peripheral decarboxylase inhibitor (e.g., carbidopa) to minimize the release of dopamine outside of the central nervous system and to reduce the systemic side effects. We administered levodopa and carbidopa in a 4:1 ratio.
Participants and Study Sites
The participants in this study were recruited through the AS parent support groups and through referrals from professional colleagues. The eligibility criteria were: (i) molecular diagnosis of AS that included cytogenetic or molecular testing to determine whether the participant has a chromosomal deletion in the AS critical region, (ii) age between 4 and 12 years (before the 13th birthday), (iii) not on levodopa or other dopamine agonist in the two weeks prior to enrollment, (iv) not on any other investigational products in preceding 3 months prior to enrollment, and (v) absence of another co-morbid condition that could affect neurodevelopment. We selected this specific age range because the typical AS phenotype is often not yet established in younger children and puberty may confound the developmental and behavioral changes in older children. In addition, because the rate of developmental progress declines with age, studying a relatively young and narrow age range improves our ability to demonstrate a clinically significant positive impact on behavioral and cognitive outcome in these patients.
Each participant was evaluated throughout the study at one of seven study sites: Boston Children’s Hospital, Rady Children’s Hospital San Diego, Greenwood Genetic Center, Texas Children’s Hospital, Monroe Carell Jr. Children's Hospital at Vanderbilt, Cincinnati Children’s Hospital Medical Center, and UCSF Benioff Children's Hospital, after ethics approval by the respective institutional review boards. Participants were evaluated at baseline and at approximately 12 months after they started taking the study medication.
Randomization
Each participant had an equal chance of being randomized to either levodopa or placebo. Only the site pharmacists and study statistician were aware of the treatment assignment.
Study Medication
Generic immediate-release combined levodopa 100 mg / carbidopa 25 mg tablets were compounded into capsules by Pelham Community Pharmacy (Waltham, MA). Placebo capsules contained corn starch and microcrystalline cellulose, as well as yellow coloring and flavoring matching those of the generic levodopa / carbidopa.
Each participant was initially assigned to receive up to 15 mg/kg/day of the study medication in three divided doses. Participants on 15 mg/kg/day who had an adverse event could have their dosage reduced to 10 mg/kg/day at the discretion of the site PI. Each participant was on the assigned study medication for a total of approximately 12 months.
Developmental and Behavioral Outcome Measures
Each participant was evaluated at the baseline and the 12-month visit by a child psychologist or psychiatrist using three developmental instruments: (i) either the Bayley Scales of Infant and Toddler Development, Third Edition (BSID-III) or for participants who exceeded specific scores on the BSID-III, the Mullen Scales of Early Learning (Mullen) (ii) the Vineland Adaptive Behavior Scales, Second Edition (VABS-II), and (iii) the Aberrant Behavior Checklist, Community version (ABC), all of which except the Mullen are developmental instruments that have previously been used in this population [Gentile et al., 2010; Peters et al., 2004].
The BSID-III provides a quantitative assessment of cognitive, language, and motor functioning. [Bayley, 2005]. The Mullen provides an objective assessment of the gross motor, fine motor, expressive language, receptive language, and visual reception in children with developmental ages from birth to 68 months [Mullen, 1995]. The VABS-II is a structured parental report measure that assesses skills in daily living, socialization, communication, and motor abilities, as well as the severity of maladaptive behaviors [Sparrow et al., 2005]. The ABC uses a 58-item checklist of symptoms to assess behavioral problems in children and adults with intellectual disability [Aman and Singh, 1994]. We chose outcome measures that were meaningful to caregivers in the AS community, and measures that we thought were most likely to demonstrate a potential benefit given the results of levodopa treatment in AS mice.
Secondary Outcome Measures
The caregivers were asked specifically about the presence or absence of tremors in the three months prior to each visit, as well as the attention span of the participant both when s/he is engaged in an activity of interest and during “normal (routine) daily activities”. The attention span of each participant was recorded on an ordinal scale: less than 5 seconds, 5 – 59 seconds, 1 – 4 minutes, 5 – 10 minutes, 11 – 30 minutes, 31 – 60 minutes, and more than 1 hour.
Statistical Analyses
Our target sample size for this study was 90 participants (n=45 in each arm), which would have given us 80% power to detect a standardized effect size of 0.60, assuming a type I (i.e., “false positive”) error rate of 5%.
Means with standard deviations for continuous variables and frequencies for categorical variables are reported. Demographic and clinical characteristics were compared between the treatment arms using independent two-sample t-test for continuous variables and chi-square test without Yates continuity correction for categorical variables.
To determine whether statistically significant changes in the developmental and behavioral outcome measures between the levodopa and placebo arms were observed over the treatment period, we performed generalized estimating equations with an unstructured covariance matrix to account for inter-correlations within measurements on the same participant over time. We modeled each outcome variable as a function of treatment (i.e., levodopa versus placebo), visit (i.e., baseline versus 12-month follow-up) and the treatment-by-visit interaction; the interaction term tests whether the effect of levodopa treatment over time is significantly greater than that of the placebo group. As the number of participants with deletion-negative AS was too small for sub-group analysis, the data were not analyzed according to the deletion status of the participants. We performed per-protocol analysis because with only two study visits, we could not impute missing values for the participants who failed to complete the final study visit.
All analyses were performed using IBM SPSS Statistics (version 22). Two-sided p-values less than 0.05, after Bonferroni correction for multiple comparisons within each developmental instrument, were considered significant.
This study was registered on ClinicalTrials.gov prior to the enrollment of the first participant (ClinicalTrials.gov Identifier: NCT01281475).
RESULTS
A total of 78 participants were recruited over a 2-year 9-month period. Eleven participants did not receive study medication for one of the following reasons: (i) the caregivers decided to withdraw them from the study prior to, or during, the initial study visit (n=8), (ii) the participant failed to meet eligibility criteria (n=2), or (iii) other unspecified reason (n=1). Therefore, 67 participants were prescribed the study medication – 33 received levodopa and 34 received placebo. The two groups were evenly matched in terms of sex, age, genetic sub-classes, and a history of seizures, which we used as a surrogate of disease severity (Supplementary Table S1). Of these 67 participants, 29 (88%) of those who were prescribed levodopa and 26 (76%) of those who were prescribed placebo completed the 1-year study. Four participants in the levodopa group withdrew, or were withdrawn, from the study due to: (i) adverse event (hyperexcitability and reduced attention span compared to baseline) (n=1); (ii) non-compliance with study medication (n=2); and (iii) lost to follow-up (n=1). In the placebo group, five participants withdrew due to adverse events (one each of: hallucinations, increased nocturnal awakening, excessive lethargy and somnolence, new type of seizure and tremors, and involuntary extrapyramidal movements).
All subsequent analyses were performed on the remaining 55 participants who completed the study. The baseline characteristics of the two treatment arms were similar (Table I and Supplementary Table S2).
Table I.
Baseline developmental and behavioral outcome measures in participants who completed the study
| Levodopa (n=29) | Placebo (n=26) | p value | |
|---|---|---|---|
| BSID-III Age Equivalent in months: Mean (± SD) | |||
| Cognitive scale | 19.0 (± 7.9) | 18.3 (± 6.4) | 0.75 |
| Receptive Communication | 16.0 (± 8.5) | 15.0 (± 8.2) | 0.64 |
| Expressive Communication | 9.7 (± 4.0) | 8.7 (± 3.8) | 0.32 |
| Fine Motor | 17.8 (± 10.4) | 15.7 (± 7.8) | 0.40 |
| Gross Motor | 16.8 (± 6.0)* | 15.8 (± 5.0) | 0.52 |
| VABS-II Standard Scores: Mean (± SD) | |||
| Communication | 48.9 (± 9.1) | 46.9 (± 7.4) | 0.39 |
| Daily Living Skills | 53.2 (± 10.2) | 50.3 (± 10.9) | 0.33 |
| Socialization | 57.5 (± 10.8) | 54.9 (± 10.9) | 0.37 |
| Motor Skills | 55.5 (± 5.1)* | 53.9 (± 7.1) | 0.33 |
| Adaptive Behavior Composite | 51.7 (± 9.3) | 49.8 (± 8.3) | 0.44 |
| ABC Raw scores: Mean (± SD) | n=28 | n=24 | |
| Irritability | 9.1 (± 9.3) | 4.8 (± 4.6) | 0.05 |
| Lethargy | 4.7 (± 4.6) | 2.7 (± 3.3) | 0.07 |
| Stereotypy | 5.1 (± 3.7) | 3.0 (± 3.6) | 0.04 |
| Hyperactivity | 19.5 (± 12.2) | 15.5 (± 10.8) | 0.22 |
| Tremors in last 3 months | 16 (55%) | 15 (58%) | 0.85 |
n=28 in the Levodopa group
Abbreviations: BSID-III – Bayley Scales of Infant and Toddler Development, Third Edition; VABS-II – Vineland Adaptive Behavior Scales, Second Edition; ABC – Aberrant Behavior Checklist, Community Version
Levodopa failed to improve neurodevelopment and behavior
As shown in Tables II to IV, the changes in the neurodevelopmental outcomes were neither statistically nor clinically significant between the two treatment arms. In fact, there appeared to be a slower rate of improvement across almost all domains in the levodopa compared with the placebo arm.
Table II.
Changes in the Bayley Scales of Infant Development, Third Edition (BSID-III) mean age equivalents after one year of treatment with levodopa or placebo, and the relative difference in the changes between these two groups. A positive “difference in gains” suggests that either the gain (i.e., improvement) in that skill was greater in the levodopa group than the control group, or the reduction (i.e., loss) of that skill was less in the levodopa group compared to the control group.
| BSID-III Age equivalent: Mean ± SD |
Levodopa (n=29) | Placebo (n=26) | Difference in Gains (Levodopa - Placebo) |
Difference in Gains (Generalized Estimating Equation) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Initial | 1-year | Gain in 1 year |
Initial | 1-year | Gain in 1 year |
95% CI | p value | ||
| Cognitive scale | 19.0 (± 8.0) | 19.1 (± 7.2) | 0.1 | 18.3 (± 6.4) | 19.1 (± 8.5) | 0.8 | −0.6 | (−2.57, 1.31) | 0.52 |
| Receptive Communication* | 16.0 (± 8.5) | 16.0 (± 9.0) | 0.0 | 15.0 (± 8.2) | 16.9 (± 9.0) | 1.9 | −2.0 | (−4.19, 0.535) | 0.13 |
| Expressive Communication | 9.7 (± 4.0) | 9.9 (± 4.0) | 0.2 | 8.7 (± 3.8) | 9.1 (± 4.5) | 0.4 | −0.2 | (−1.62, 1.19) | 0.76 |
| Fine Motor** | 17.8 (± 10.4) | 18.1 (± 10.3) | 0.4 | 15.7 (± 7.8) | 17.6 (± 8.9) | 1.9 | −1.6 | (−3.57, 1.40) | 0.39 |
| Gross Motor*** | 16.8 (± 6.0) | 18.8 (± 8.0) | 2.1 | 15.8 (± 5.0) | 17.4 (± 6.5) | 1.6 | 0.5 | (−1.59, 1.88) | 0.87 |
BSID-III Receptive Communication - Levodopa at 1-year: n=28
BSID-III Fine Motor - Levodopa at 1-year: n=28
BSID-III Gross Motor - Placebo at 1-year: n=25; Levodopa at Baseline & at 1-year: n=28
Table IV.
Changes in the Vineland Adaptive Behavior Scales, Second Edition (VABS-II) age equivalents in months after one year of treatment with levodopa or placebo, and the relative difference in the changes between these two groups. A positive “difference in gains” suggests that either the gain (i.e., improvement) in that skill was greater in the levodopa group than the control group, or the reduction (i.e., loss) of that skill was less in the levodopa group compared to the control group.
| VABS-II Age equivalent: Mean ± SD |
Levodopa (n=29) | Placebo (n=26) | Difference in Gains (Levodopa - Placebo) |
Difference in Gains (Generalized Estimating Equation) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Initial | 1-year | Gain in 1 year |
Initial | 1-year | Gain in 1 year |
95% C.I. | p value | ||
| Communication - Receptive | 19.7 (± 12.4) | 23.1 (± 18.1) | 3.4 | 18.6 (± 12.2) | 21.6 (± 11.5) | 3.0 | 0.4 | (−5.48, 6.30) | 0.89 |
| Communication - Expressive | 11.1 (± 4.0) | 11.9 (± 4.3) | 0.8 | 10.2 (± 3.5) | 12.1 (± 4.2) | 1.9 | −1.1 | (−2.42, 0.16) | 0.09 |
| Communication - Written | 13.3 (± 17.8) | 16.7 (± 19.6) | 3.4 | 8.1 (± 15.4) | 13.3 (± 17.8) | 5.2 | −1.7 | (−7.58, 4.17) | 0.57 |
| Daily Living Skills - Personal | 21.2 (± 10.6) | 23.4 (± 11.2) | 2.2 | 21.9 (± 10.2) | 25.3 (± 12.5) | 3.4 | −1.2 | (−3.71, 1.37) | 0.37 |
| Daily Living Skills – Domestic | 21.9 (± 21.4) | 22.8 (± 23.8) | 1.0 | 20.5 (± 22.7) | 23.5 (± 22.1) | 3.0 | −2.0 | (−7.27, 3.21) | 0.45 |
| Daily Living Skills – Community | 22.0 (± 13.6) | 24.0 (± 16.9) | 2.0 | 19.5 (± 13.8) | 24.2 (± 18.1) | 4.7 | −2.8 | (−8.85, 3.32) | 0.37 |
| Socialization - Interpersonal | 15.1 (± 8.3) | 15.9 (± 8.4) | 0.7 | 14.7 (± 7.6) | 16.7 (± 8.8) | 1.9 | −1.2 | (−3.83, 1.44) | 0.37 |
| Socialization - Play / Leisure | 19.2 (± 12.0) | 21.3 (± 13.9) | 2.1 | 17.2 (± 8.5) | 20.6 (± 11.9) | 3.4 | −1.3 | (−6.46, 3.89) | 0.63 |
| Socialization – Coping | 18.2 (± 15.1) | 19.0 (± 13.5) | 0.8 | 20.8 (± 13.9) | 23.1 (± 15.4) | 2.3 | −1.5 | (−6.76, 3.73) | 0.57 |
| Motor Skills* - Gross Motor | 19.3 (± 7.4) | 22.0 (± 8.1) | 2.7 | 19.0 (± 7.8) | 22.1 (± 10.4) | 3.0 | −0.3 | (−2.90, 2.25) | 0.80 |
| Motor Skills* - Fine Motor | 24.0 (± 10.6) | 24.1 (± 11.9) | 0.1 | 22.6 (± 11.5) | 24.6 (± 12.1) | 2.0 | −1.9 | (−4.56, 0.79) | 0.17 |
VABS-II – Placebo at Baseline & 1-year: n=25; Levodopa at Baseline: n=28, Levodopa at 1-year: n=27
Although the levodopa arm appeared to have a slight improvement in maladaptive behaviors as measured by the ABC, the differences were not clinically apparent. Moreover, the levodopa arm had slightly higher (but not clinically or statistically significant) scores across all maladaptive domains at baseline compared to the placebo arm (Table V), and the scores after one year of treatment were very similar in both arms across all domains, so this apparent improvement may have been due to a ‘regression to the mean’.
Table V.
Changes in the Aberrant Behavior Checklist – Community (ABC) raw scores after one year of treatment with levodopa or placebo, and the relative difference in the changes between these two groups. A positive “difference in gains” suggests that either the gain (i.e., worsening) in that behavior was greater in the levodopa group than in the control group, or the reduction (i.e., improvement) in that behavior was less in the levodopa group compared to the control group.
| Aberrant Behavior Checklist Raw Score: Mean ± SD |
Levodopa (n=29) | Placebo (n=26) | Difference in Gains (Levodopa - Placebo) |
Difference in Gains (Generalized Estimating Equation) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Initial | 1-year | Gain in 1 year |
Initial | 1-year | Gain in 1 year |
95% C.I. | p value | ||
| Irritability | 9.1 (± 9.3) | 7.3 (± 8.0) | −1.8 | 4.8 (± 6.0) | 6.6 (± 7.1) | 1.8 | −3.6 | (−6.85, 0.16) | 0.06 |
| Lethargy | 4.7 (± 4.6) | 4.5 (± 3.9) | −0.3 | 2.7 (± 3.3) | 5.2 (± 8.4) | 2.5 | −2.8 | (−6.37, 0.76) | 0.12 |
| Stereotypy | 5.1 (± 3.7) | 3.5 (± 3.7) | −1.6 | 3.0 (± 3.6) | 3.6 (± 3.9) | 0.7 | −2.2 | (−3.93, −0.33) | 0.02 |
| Hyperactivity | 19.5 (± 12.2) | 18.2 (± 9.9) | −1.3 | 15.5 (± 10.8) | 17.0 (± 10.4) | 1.5 | −2.8 | (−7.74, 1.66) | 0.20 |
Levodopa did not improve tremors
Among the 55 participants, 21 did not have tremors at baseline or after a year of treatment, and 23 had tremors at baseline that persisted after a year of treatment. Eight had tremors at baseline that resolved after a year of treatment – four in each arm, which suggests that levodopa at 15 mg/kg is ineffective for tremors in children with AS.
Levodopa had no impact on attention span, aggression, or bizarre behaviors
Although the caregivers of some participants reported an improvement in attention span, the cumulative data suggested no difference between the levodopa and placebo arms (Supplementary Tables S3 and S4).
While this study was underway, emerging data from an AS mouse model suggested that increased dopamine levels from levodopa might exacerbate abnormal behaviors including aggression [Riday et al., 2012]. Thus, we also analyzed outcome measures for behavior and aggression in our trial participants. Thirty participants did not have any aggression at baseline – 15 in each arm. At the one year visit, six of them were reported to have aggression – three in each arm. There were no changes in the ABC Irritability subscale, which can be a surrogate measure of aggression. Of the 21 participants (11 on levodopa, ten on placebo) without bizarre behaviors at baseline, five participants reported bizarre behaviors at the one year visit – two on levodopa, three on placebo. These findings suggested that levodopa did not increase the risk for such behaviors in AS.
Levodopa was well tolerated
All adverse events were categorized and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. Compared to those on placebo, participants on levodopa did not appear to have an excess number of adverse events (Supplementary Table S5), nor was there an excess of grade 3 adverse events (i.e., “Severe or medically significant but not immediately life-threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care activities of daily living”) (Supplementary Table S6). No participant had a grade 4 (“Life-threatening consequences; urgent intervention indicated”) or grade 5 (“Death related to AE”) adverse event. The only grade 3 adverse event that was deemed to have been “definitely or probably” related to the study medication was syncope in a subject treated with placebo. The majority of the adverse events among those on levodopa were deemed “probably or definitely unrelated” to the study medication.
DISCUSSION
This clinical trial arose from the observation that an increasing number of children with AS were being prescribed levodopa off-label based on the aforementioned pre-clinical studies and anecdotal reports suggesting possible benefit. As physician-scientists involved in the care of children with AS, we recognized that a randomized controlled clinical trial would be necessary to help parents and healthcare providers make informed decisions regarding the use of levodopa for AS. The study did not show that levodopa at 15 mg/kg/day had a clinically significant effect on the development or behavior of children with AS.
Certain aspects of our study design potentially limited our findings. One possible reason for the failure to detect a significant difference in the neurodevelopmental progress in the levodopa-treated participants was insufficient sensitivity of the outcome measures used in this study to detect minor changes in development and behavior over a one-year period. However, these were the most appropriate developmental instruments that were available to us at the initiation of this clinical trial, and they have been used in other studies in this population. Additionally, the treatment dose may not have been appropriate for the outcomes we selected. As shown by our preclinical data, AS mice treated with “high dose” levodopa had no improvement in rotarod performance, while those that were administered “low dose” levodopa showed some improvement suggesting a very tight dosing range and possibly relative “over-dosing” in these study participants. However, the rates of adverse events were similar in both treatment arms, arguing against over-dosing, and raising the possibility of under-dosing.
In conclusion, treatment with 15 mg/kg/day of levodopa does not appear to be beneficial in children with AS between the ages of 4 and 12 years old. We believe that ultimately, a more effective treatment for AS is likely to come from therapies that restore UBE3A expression in neurons, although treatment of specific symptoms and complications may always be needed.
Supplementary Material
Table III.
Changes in the Vineland Adaptive Behavior Scales, Second Edition (VABS-II) standard scores after one year of treatment with levodopa or placebo, and the relative difference in the changes between these two groups. A positive “difference in gains” suggests that either the gain (i.e., improvement) in that skill was greater in the levodopa group than the control group, or the reduction (i.e., loss) of that skill was less in the levodopa group compared to the control group.
| VABS-II Standard Scores: Mean ± SD |
Levodopa (n=29) | Placebo (n=26) | Difference in Gains (Levodopa - Placebo) |
Difference in Gains (Generalized Estimating Equation) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Initial | 1-year | Gain in 1 year |
Initial | 1-year | Gain in 1 year |
95% C.I. | p value | ||
| Communication | 48.9 (± 9.1) | 48.2 (± 7.3) | −0.7 | 46.9 (± 7.4) | 47.7 (± 7.5) | 0.8 | −1.5 | (−4.17, 1.25) | 0.29 |
| Daily Living Skills | 53.2 (± 10.2) | 51.1 (± 10.6) | −2.1 | 50.3 (± 10.9) | 52.1 (± 10.2) | 1.8 | −3.8 | (−7.21, −0.47) | 0.03 |
| Socialization | 57.5 (± 10.8) | 56.6 (± 9.3) | −0.9 | 54.9 (± 10.9) | 56.3 (± 9.6) | 1.4 | −2.4 | (−5.88, 1.17) | 0.19 |
| Motor Skills* | 55.5 (± 5.1) | 54.4 (± 6.7) | −1.1 | 53.9 (± 7.1) | 54.4 (± 7.4) | 0.6 | −1.7 | (−3.26, 0.20) | 0.08 |
| Adaptive Behavior Composite** | 51.7 (± 9.3) | 51.8 (± 7.6) | 0.0 | 49.8 (± 8.3) | 51.0 (± 9.4) | 1.2 | −1.1 | (−4.22, 0.93) | 0.21 |
VABS-II – Placebo at Baseline & 1-year: n=25; Levodopa at Baseline: n=28, Levodopa at 1-year: n=27
VABS-II Adaptive Behavior – Placebo at 1-year: n=25; Levodopa at 1-year: n=28
Acknowledgments
The authors thank the following individuals and organizations whose support was crucial to this study:
All participants and their families, Terry Jo Bichell, and the Angelman Syndrome Foundation.
Study coordinators: Rachel Winograd, Fran Annese, Beverly Feldman, Amy Wilson, Stefanie Barnett, Selena Carpenter, Tori Schaefer, Bridget Crippen, Katherine Friedmann, Kaela O’Brien, and Janette Lawrence.
Study pharmacists: Rocco Anzaldi, David Bowling, Alan Arao, Scott Fields, Denise LaGory, Jennifer Lynds, Richard Malone, Tara McCartney, Bill Rogers, Michelle Stevenson, and John Storey.
Compounding pharmacists: Bhuren Patel, Aimee Drew, and the staff at Pelham Community Pharmacy.
Study statisticians: Al Ozonoff, Carly Milliren, Christine Powell, and Chao-Yu Guo.
Database support: James Gregoric and Qiaoli Chen.
Data monitors: Heidi Zeitz, Kerry McEnaney, Matthew Minahan, and Kudret Usmani.
FUNDING
This study was supported by: Food and Drug Administration Office of Orphan Products Development R01 FD003523, National Institute of Child Health and Human Development U54 HD061222 (awarded to Alan K. Percy), Clinical Investigator Training Program: Harvard/MIT Health Sciences and Technology – Beth Israel Deaconess Medical Center, in collaboration with Pfizer Inc. and Merck & Co. (to WHT), and Angelman Syndrome Foundation (to LMB).
Footnotes
The authors have no conflict of interest.
References
- Aman MG, Singh NN. Aberrant Behavior Checklist, Residential /or Community Version. East Aurora, NY: Slosson Educational Publications, Inc.; 1994. [Google Scholar]
- Bayley N. Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III) San Antonio, TX: Harcourt Assessment, Inc.; 2005. [Google Scholar]
- Bird LM. Angelman syndrome: review of clinical and molecular aspects. Appl Clin Genet. 2014;7:93–104. doi: 10.2147/TACG.S57386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitzer RD, Iyengar R, Landau EM. Postsynaptic signaling networks: cellular cogwheels underlying long-term plasticity. Biol Psychiatry. 2005;57:113–119. doi: 10.1016/j.biopsych.2004.02.031. [DOI] [PubMed] [Google Scholar]
- Brown AM, Deutch AY, Colbran RJ. Dopamine depletion alters phosphorylation of striatal proteins in a model of Parkinsonism. Eur J Neurosci. 2005;22:247–256. doi: 10.1111/j.1460-9568.2005.04190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbran RJ, Brown AM. Calcium/calmodulin-dependent protein kinase II and synaptic plasticity. Curr Opin Neurobiol. 2004;14:318–327. doi: 10.1016/j.conb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- de Rijk-Van Andel JF, Gabreels FJ, Geurtz B, Steenbergen-Spanjers GC, van Den Heuvel LP, Smeitink JA, Wevers RA. L-dopa-responsive infantile hypokinetic rigid parkinsonism due to tyrosine hydroxylase deficiency. Neurology. 2000;55:1926–1928. doi: 10.1212/wnl.55.12.1926. [DOI] [PubMed] [Google Scholar]
- Elgersma Y, Fedorov NB, Ikonen S, Choi ES, Elgersma M, Carvalho OM, Giese KP, Silva AJ. Inhibitory autophosphorylation of CaMKII controls PSD association, plasticity, and learning. Neuron. 2002;36:493–505. doi: 10.1016/s0896-6273(02)01007-3. [DOI] [PubMed] [Google Scholar]
- Gentile JK, Tan WH, Horowitz LT, Bacino CA, Skinner SA, Barbieri-Welge R, Bauer-Carlin A, Beaudet AL, Bichell TJ, Lee HS, Sahoo T, Waisbren SE, Bird LM, Peters SU, et al. A neurodevelopmental survey of Angelman syndrome with genotype-phenotype correlations. J Dev Behav Pediatr. 2010;31:592–601. doi: 10.1097/DBP.0b013e3181ee408e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- Harbord M. Levodopa responsive Parkinsonism in adults with Angelman Syndrome. J Clin Neurosci. 2001;8:421–422. doi: 10.1054/jocn.2000.0753. [DOI] [PubMed] [Google Scholar]
- LeWitt PA. Levodopa therapy for Parkinson's disease: Pharmacokinetics and pharmacodynamics. Movement Disorders. 2015;30:64–72. doi: 10.1002/mds.26082. [DOI] [PubMed] [Google Scholar]
- Mittal R, Goraya JS, Basu S. Dopa-responsive dystonia. Indian Pediatr. 2001;38:1056–1058. [PubMed] [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Services, Inc.; 1995. [Google Scholar]
- Peters SU, Goddard-Finegold J, Beaudet AL, Madduri N, Turcich M, Bacino CA, et al. Cognitive and adaptive behavior profiles of children with Angelman syndrome. Am J Med Genet A. 2004;128:110–113. doi: 10.1002/ajmg.a.30065. [DOI] [PubMed] [Google Scholar]
- Riday TT, Dankoski EC, Krouse MC, Fish EW, Walsh PL, Han JE, Hodge CW, Wightman RM, Philpot BD, Malanga CJ. Pathway-specific dopaminergic deficits in a mouse model of Angelman syndrome. J Clin Invest. 2012;122:4544–4554. doi: 10.1172/JCI61888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales, Second Edition (Vineland-II) Upper Saddle River, NJ: Pearson Education, Inc.; 2005. [Google Scholar]
- Storer BE. Design and analysis of phase I clinical trials. Biometrics. 1989;45:925–937. [PubMed] [Google Scholar]
- Tan WH, Bacino CA, Skinner SA, Anselm I, Barbieri-Welge R, Bauer-Carlin A, Beaudet AL, Bichell TJ, Gentile JK, Glaze DG, Horowitz LT, Kothare SV, Lee HS, Nespeca MP, Peters SU, Sahoo T, Sarco D, Waisbren SE, Bird LM, et al. Angelman syndrome: Mutations influence features in early childhood. Am J Med Genet A. 2011;155A:81–90. doi: 10.1002/ajmg.a.33775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan WH, Bird LM. Angelman syndrome: Current and emerging therapies in 2016. American Journal of Medical Genetics Part C: Seminars in Medical Genetics. 2016;172:384–401. doi: 10.1002/ajmg.c.31536. [DOI] [PubMed] [Google Scholar]
- van Woerden GM, Harris KD, Hojjati MR, Gustin RM, Qiu S, de Avila Freire R, Jiang YH, Elgersma Y, Weeber EJ. Rescue of neurological deficits in a mouse model for Angelman syndrome by reduction of alphaCaMKII inhibitory phosphorylation. Nat Neurosci. 2007;10:280–282. doi: 10.1038/nn1845. [DOI] [PubMed] [Google Scholar]
- Weeber EJ, Jiang YH, Elgersma Y, Varga AW, Carrasquillo Y, Brown SE, Christian JM, Mirnikjoo B, Silva A, Beaudet AL, Sweatt JD, et al. Derangements of hippocampal calcium/calmodulin-dependent protein kinase II in a mouse model for Angelman mental retardation syndrome. J Neurosci. 2003;23:2634–2644. doi: 10.1523/JNEUROSCI.23-07-02634.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.